Summary
We describe a protocol to study inflammatory responses triggered by the mRNA-lipid nanoparticle (LNP) vaccine formulations in skin, muscle, and lung and the adaptive immune responses induced in the draining lymph nodes. Here, we will present how to deliver these reagents through intradermal, intramuscular, and intranasal routes, generating single-cell suspensions from the inoculated and target organs for downstream analyses.
For complete details on the use and execution of this protocol, please refer to Ndeupen et al. (2021) and (2022).
Subject areas: Health Sciences, Immunology
Graphical abstract
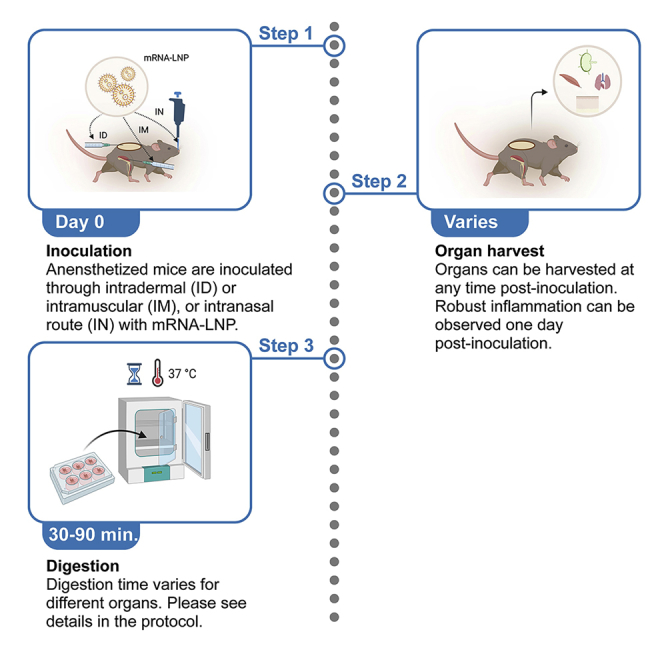
Highlights
-
•
Provides detailed instructions for delivery of mRNA-LNP through different routes in mice
-
•
Presents techniques to harvest and prepare single-cell suspensions from different organs
-
•
Provides an access point from which to study mRNA-LNP-induced immune responses
We describe a protocol to study inflammatory responses triggered by the mRNA-lipid nanoparticle (LNP) vaccine formulations in skin, muscle, and lung and the adaptive immune responses induced in the draining lymph nodes. Here, we will present how to deliver these reagents through intradermal, intramuscular, and intranasal routes, generating single-cell suspensions from the inoculated and target organs for downstream analyses.
Before you begin
As with any protocol, before you start a big experiment, make sure that you first become familiar with all the steps and techniques presented here. Practice and become an expert on the different methods, such as handling mice, anesthesia, shaving, intradermal, intramuscular, and intranasal inoculations, harvesting organs/tissues, prepare single cell suspensions/lysates, running and analyzing your samples using different machines and software. When you feel confident, then have a detailed experimental plan in place. Check the availability of all the necessary reagents to perform the experiments, and then prepare upfront everything needed for each step of this protocol.
Institutional permissions
Institutional Care and Use Committee at Thomas Jefferson University approved all mouse protocols. All the experiments conform to the relevant regulatory standards. Users will need to acquire permissions from their relevant institutions to perform animal experiments.
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| CD45.2-PE (or other fluorochrome; 1–2 μg in 200 μL PBS/mouse) | BioLegend | Cat# 109807; RRID: AB_313444 |
| Biological samples | ||
| Skin, muscle, lung, and lymph nodes | mouse | N/A |
| Chemicals, peptides, and recombinant proteins | ||
| ACK lysing buffer | Fisher Scientific | Cat# A1049201 |
| Collagenase D | Sigma-Aldrich | Cat# 11088882001 |
| Collagenase XI | Sigma-Aldrich | Cat# C7657 |
| Different lipid nanoparticle formulations | Dr. Norbert Pardi/Acuitas | https://www.itmat.upenn.edu/engineered-mrna-and-targeted-nanomedicine-core.html |
| DNase | Sigma-Aldrich | Cat# D5025-150KU |
| EDTA | Sigma-Aldrich | Cat# E7889-100G |
| Fetal bovine serum (FBS) | Fisher Scientific | Cat# SH3007003 |
| HEPES | Sigma-Aldrich | Cat# H0887 |
| Hyaluronidase | Sigma-Aldrich | Cat# H3884 |
| Ketamine | ShopMedVet | Cat# RXKETAMINE |
| PBS | Fisher Scientific | Cat# 10-010-049 |
| RPMI-1640 | Fisher Scientific | Cat# 11-875-119 |
| Sodium azide | Sigma-Aldrich | Cat# S2002-25G |
| Xylazine | ShopMedVet | Cat# RXXYLAZINE |
| Experimental models: Organisms/strains | ||
| C57BL/6J mice We used six to twelve weeks old wildtype female and male mice for these protocols. However, the presented protocols will work with any mouse strain. |
The Jackson Laboratory | Stock No: 000664 |
| Other | ||
| 28G insulin syringes | Fisher Scientific | Cat# 14-826-79 |
| 40 μm cell strainers | Fisher Scientific | Cat# 22-363-547 |
| 50 mL conical tubes | Fisher Scientific | Cat# 05-539-13 |
| 6-well plates | Fisher Scientific | Cat# FB012927 |
| 96-well plates | Fisher Scientific | Cat# 12-565-501 |
| Centrifuges | N/A | N/A |
| CO2 incubator | N/A | N/A |
| CO2 incubator compatible shaker | N/A | N/A |
| Electric clipper | N/A | N/A |
| Flow cytometer | N/A | N/A |
| Micropipettes and serological pipettes | N/A | N/A |
| Single edge prep blade | QuickMedical | Cat# 74-0014 |
| Surgical tools (scissors and fine forceps) | N/A | N/A |
Materials and equipment
Ketamine/Xylazine (K/X) for anesthesia
| Reagent | Final concentration | Stock concentration | Add to 1 mL |
|---|---|---|---|
| Ketamine | 20 mg/mL | 100 mg/mL | 200 μL |
| Xylazine | 2 mg/mL | 100 mg/mL | 20 μL |
| PBS | Up to 1 mL (780 μL) |
The stocks are kept at 20°C–23°C. Prepare the working solution fresh. Inject 5 μL/g (0.1 mg ketamine and 0.01 mg xylazine) mouse.
Collagenase/hyaluronidase/DNase (CHD) digestion solution (Kashem and Kaplan, 2018)
| Reagent | Final concentration | Stock concentration | Add to 10 mL |
|---|---|---|---|
| Collagenase XI | 2.7 mg/mL | 27 mg | |
| Hyaluronidase | 0.25 mg/mL | 2.5 mg | |
| DNase | 0.1 mg/mL | 1 mg/mL | 1 mL |
| RPMI-1640 supplemented with 10% FBS and 10 mM HEPES | Up to 10 mL (9 mL) |
The digestion solution can be made fresh from powder or prepared upfront, aliquoted and stored frozen at −20°C–80°C. Good for at least 6 months.
Collagenase D digestion medium for lymph nodes
| Reagent | Final concentration | Stock concentration | Add to 2 mL |
|---|---|---|---|
| Collagenase D | 600 U/mL | 4,000 U/mL | 0.3 mL |
| DNase | 0.025 mg/mL | 1 mg/mL | 0.05 mL |
| RPMI-1640 supplemented with 10% FBS and 10 mM HEPES | Up to 2 mL (1.650 mL) |
Can be prepared upfront, aliquoted and stored frozen at −20°C–80°C. Good for at least 6 months.
Staining medium (SM)
| Reagent | Final concentration | Stock concentration | Add to 500 mL |
|---|---|---|---|
| FBS (or calf serum; CS) | 3% (v/v) | 100% (v/v) | 15 mL |
| EDTA | 5 mM | 0.5 M | 5 mL |
| Azide | 0.04% (w/v) | 10% (w/v) | 2 mL |
| PBS | Up to 500 mL (478 mL) |
Store at 4°C. Good for months.
Step-by-step method details
Intradermal route – shaving
Timing: Varies based on skills and number of mice being handled
The mice will be anesthetized and shaved.
-
1.Day -1. Anesthetize and shave the mice.
-
a.Anesthetize the mice by injecting 5 μL/g of K/X solution. Wait minimum of 5 min before you proceed to the next step.
-
b.Remove the hair from the dorsal part of the mice using electric clipper.
-
c.Spray the area lightly with 70% ethanol (sterile water can also be used).
-
d.Wet shave the area using a sterile razor blade (single edge prep blade).
-
a.
Note: It is possible to perform intradermal injections without wet shaving the mice, but it requires more advanced skills and practice.
Note: It is necessary to anesthetize mice for shaving. For this purpose, we prefer to use a combination of ketamine/xylazine, but as an alternative, isoflurane inhalation can also be applied. Animals can be overdosed with ketamine, so make sure you adjust the dose to weight and start slightly lower than the recommended dose.
Note: We recommend shaving the mice the day before immunization. This allows the skin to “rebalance” before the injection and speeds up the process of inoculation. This is critical because the LNP/mRNA-LNP is temperature-sensitive, and even if kept on ice, has a short half-life. Thus, if you have a lot of mice to inoculate, shaving them a day before becomes a critical step. The downside of this is that the mice will have to be anesthetized again at the time of inoculation with the vaccine.
Note: A video with the procedure is available upon request.
Intradermal inoculation
Timing: Varies based on skills and number of mice being handled
The mice will be intradermally injected.
-
2.Day 0. Inoculate the mice intradermally.
-
a.Using a 28-gauge syringe aspirate the necessary volume (20 μL/spot).
-
b.With two fingers, stretch the skin in the area to be inoculated, and at a very shallow angle with the bevel up, slowly penetrate the skin with the needle. Keep it parallel with the surface and make sure that the opening of the needle is at ∼2 mm into the skin before you inject. Successful intradermal injection of liquid will form a bubble that will stay visible for minutes, and no to minimal liquid will come out at the site of injection.
-
c.Label the site of injection with a fine permanent marker by drawing a small circle around it.
-
d.Repeat step b, if multiple injections to be performed on the same mouse.
-
a.
Note: It is necessary to anesthetize the mice for successful intradermal inoculation. Please see our note above for details. As with any procedure, make sure to record any deviation from the protocol, such as partial inoculation, too deep, etc.
Note: Do not exceed 20–25 μL/spot. The excess liquid causes physical damage to the tissue, so in general lower volumes are preferred.
Note: Accurate volumes are critical to minimize variability. We suggest using a syringe with minimal dead space, and even then, make sure to account for the dead space (needle) by filling up that space before measuring the required volume. If you do not want to rely on the accuracy of the syringe, then use a calibrated pipettor to measure the necessary volume and then dispense it on a clean piece of parafilm, then slowly aspirate with the syringe.
Note: The number of injected spots does not have to be 4. Our studies use this strategy to target several primary skin-draining lymph nodes (axillary, brachial, and inguinal). However, if you would like to limit the immune responses to a certain area, then inject there and leave the others undisturbed or for control purposes (Figure 1) (Ndeupen et al., 2022).
CRITICAL: The mRNA-LNPs and the empty LNPs must be kept on ice and injected ∼30 min post-thawing. The leftovers should be discarded. Do not refreeze these reagents. Their efficacy decreases by ∼50% with every freeze and thaw cycle.
Note: A video on intradermal inoculation is available upon request.
Figure 1.
Depicts different areas to inject to target specific lymph nodes
Green: left brachial and axillary lymph nodes. Red: right brachial and axillary lymph nodes. Yellow: left inguinal lymph node. Blue: right inguinal lymph node.
Harvesting skin samples and single-cell preparation
Timing: Varies based on skills and number of mice being handled
The mice will be euthanized, and single-cell suspensions prepared from the skin samples.
-
3.Day 1. Harvest the skin samples and generate single cell suspension.
-
a.Euthanize the mice following IACUC regulations.
-
b.Using a ruler draw 1 cm2 around the injection site and cut it out using a sharp scissor.
-
c.Transfer the harvested skin samples into 6-well plate containing 3 mL sterile PBS and keep them on ice until all the samples have been harvested.
-
d.Chop the skin samples in small pieces using a curved scissor or two razor blades on clean smooth surface, such as Petri-dish.
-
e.Transfer the chopped skin pieces to a 6-well plate containing 1 mL CHD digestion media/ 1 cm2 (4 mL in total for 4 spots) skin using curved forceps and disperse the skin pieces.
-
f.Incubate the plate in a 5% CO2 incubator at 37°C for 90 min under continuous shaking at 90 rpm.
-
g.To further break up the tissue, pipette the solution up and down forcefully using 5 mL serological pipette and filter through a 40 μm cell strainer into a 50 mL conical tube. You can use a syringe plunger to aid the filtration and tissue disruption.
-
h.Rinse the well and the strainer using 10 mL SM.
-
i.Spin @ 600 g at 4°C for 7 min.
-
j.Decant, resuspend in 1 mL of SM.
-
k.Count the cells and proceed to stain for flow cytometry.
-
a.
Note: If you are using transgenic mice, save a small piece from the tail of each mouse and store them at −80°C. This step will help you to confirm the genotype if the results are unexpected.
Note: The harvested skin samples can be snap frozen and used for genomic analyses, Luminex, histology, etc.
Note: The cell suspensions can be further used for sorting, in vitro restimulation, and other downstream analyses.
Note: Skin samples can be harvested at any time after injection. Robust inflammatory responses can be observed one day post inoculation that slowly resolves in 10–14 days.
Note: A video with the skin harvesting and chopping is available upon request.
Staining the cell suspension for flow cytometer
Timing: Varies based on skills and number of samples being handled
The cells will be stained for flow cytometer.
-
4.Staining for flow cytometry.
-
a.Distribute 2 × 106 cells/well in round bottom 96-well plate.
-
b.Spin @ 350 g at 4°C for 3 min.
-
c.Discard the supernatant using a multichannel pipettor.
-
d.Resuspend the cells in your antibody master mix (100 μL/well) and stain on ice in dark for 30–60 min.
-
e.Add 100 μL of SM and spin @ 350 g at 4°C for 3 min.
-
f.Discard the supernatant and repeat the washing step.
-
g.Resuspend the cells in 100 μL SM and transfer them into flow tubes and proceed to run them on flow cytometer. Please see corresponding flow cytometry staining panels here: (Ndeupen et al., 2021, 2022).
-
a.
Note: It is important to design flow panels adjusted to your flow machine.
Note: The stained cells can be stored 12–24 h at 4°C in SM without significant signal loss, but for longer storage fixation is recommended.
Note: Always save the leftover cells and store them at 4°C in SM. They can be stored for at least 24 h, and used to perform extra stains, troubleshooting, etc.
Note: The number of cells used for staining is not set in stone and can be adjusted to your needs. The staining volume is also flexible. The titration of the antibodies is recommended for the best outcome and efficient use of the reagents.
Intramuscular inoculation
Timing: Varies based on skills and number of mice being handled
The mice will be intramuscularly injected.
-
5.Day 0. Inoculate the mice intramuscularly.
-
a.Anesthetize the mice.
-
b.Pull and stretch mouse lower limb (left or right).
-
c.Position the needle at approximately 20°C angle relative to the thigh (left or right).
-
d.Insert the needle in the thigh using your dominant hand.
-
e.Palpate the thigh with your other hand to ensure that the needle has been successfully inserted.
-
f.Inject 10 μg of mRNA-LNP in 30 μL per mouse.
-
a.
Note: Increase in muscle volume indicates successful inoculation.
Note: As with intradermal inoculation we recommend anesthetizing the mice.
Note: A video on intramuscular inoculation is available upon request.
Harvesting muscle samples and single-cell preparation
Timing: Varies based on skills and number of mice being handled
The mice will be euthanized, and single-cell suspensions prepared from the muscle samples.
-
6.Day 1. Harvest the muscle samples and generate single cell suspension.
-
a.Euthanize the mice following IACUC regulations.
-
b.Spray the mouse with 70% alcohol and make a crosscut in the belly skin.
-
c.Peel the skin to expose the muscle completely.
-
d.Dissect the muscles.
-
e.Collect muscles in cold PBS until ready to proceed with all the samples.
-
f.Using a curved scissor or two razor blades carefully chop the muscles in small pieces.
-
g.Transfer tissue to a 10 mL Petri dish filled with digestion solution (use 10 mL for 2 g of tissue. Adjust the volume based on tissue weight).
-
h.Incubate in 5% CO2 incubator at 37°C for 45 min with continuous agitation (90 rpm).
-
i.Pipette up and down using a serological pipette and filter through a 40 μm cell strainer into a 50 mL conical tube.
-
j.Rinse the dish and the cell strainer with 10 mL of SM.
-
k.Centrifuge @ 600 g at 4°C for 7 min.
-
l.Discard the supernatant.
-
m.Resuspend the pellet in 1 mL of SM, count and proceed with downstream analyses.
-
a.
Note: Spraying the mice with ethanol or sterile water is optional but helps to keep the preparation clean and free of hair.
Intranasal inoculation
Timing: Varies based on skills and number of mice being handled
The mice will be intranasally inoculated.
-
7.Day 0. Anesthetize and inoculate the mice intranasally.
-
a.Anesthetize the mice.
-
b.With one hand hold the mice straight up.
-
c.Pipette 30 μL of reagent with 100 μL pipettor.
-
d.Slowly inoculate the mice through one of their nostrils.
-
e.Keep the mice in an upward position for a few seconds after inoculation and look for two contractions of the chest cavity.
-
f.Check for bubble formation or sign of distress (coughing or dysphagia).
-
a.
Note: To perform this technique, it is important to have a steady hand. Find a flat surface to use as support for your elbows.
CRITICAL: It is imperative to anesthetize mice before performing intranasal inoculation. If mice are not anesthetized, the inoculant will go through the esophagus rather than the respiratory route. The recommended inoculation time is about 8–10 min after injection with the anesthetic. It is also critical to keep the mice in upwards position: 1) the gravity will help the reagent to flow into the lungs; 2) it allows to observe the complete inhalation of the reagent by 2 contractions signs that happen a few seconds after inoculation (please see video for details).
Note: A video on intranasal inoculation is available upon request.
Harvesting lungs and single-cell preparation
Timing: Varies based on skills and number of mice being handled
The mice will be euthanized, and single-cell suspensions prepared from the lungs.
-
8.Day 1. Harvest the lungs and generate single cell suspension.
-
a.Inject the mice through tail vein with 1–2 μg of anti-CD45.2 or 1 (depends on congenic marker expressed) in 200–300 μL of sterile PBS.
-
b.Euthanize the mice 5 min post injection.
-
c.Spray the mouse with 70% alcohol and make a crosscut in the belly skin.
-
d.Peel the skin to expose the chest area.
-
e.Open the chest cavity and harvest the lungs in 6-well plate in sterile, cold PBS and keep them on ice until all the lungs have been harvested.
-
f.Cut the lungs in small pieces using curved scissor or two razor blades.
-
g.Transfer the samples into a 6-well plate containing 5 mL digestion media and incubate in 5% CO2 incubator at 37°C for 60 min with continuous agitation (90 rpm).
-
h.Pipette up and down using a serological pipette and filter through a 40 μm cell strainer into a 50 mL conical tube. Use syringe plunger to help the filtration.
-
i.Rinse the dish and the cell strainer with 10 mL of SM.
-
j.Centrifuge @ 600 g at 4°C for 7 min.
-
k.Discard the supernatant by aspirating carefully using a pipettor.
-
l.Resuspend the pellet in 1 mL ACK, quickly vortex and incubate at 20°C–23°C for 5 min.
-
m.Add 10 mL of SM and centrifuge @ 600 g at 4°C for 7 min.
-
n.Resuspend the pellet in 1 mL of SM, count the cells and proceed with the downstream analyses.
-
a.
CRITICAL: If discrimination between blood contaminants and immune cells found in the lungs’ parenchyma is important, then injecting the mice intravenously with a fluorescently labeled common leukocyte marker a few minutes before euthanasia becomes a critical step (Anderson et al., 2014).
Note: The cell pellet prepared from lung is quite loose, so we recommend aspirating the supernatant instead of discarding it by flipping the tube.
Note: Intranasal inoculation can also be used to study weight loss and distress caused by the exposure to the LNPs. LNP doses higher than 2.5 μg can cause significant distress to the mice and death.
Note: A video on lung harvesting and processing is available upon request.
Harvesting skin draining lymph nodes and generating single-cell suspension
Timing: Varies based on skills and number of mice being handled
Skin draining lymph nodes will be harvested and single-cell suspensions generated.
-
9.Harvest skin draining lymph nodes.
-
a.Euthanize the mice following IACUC regulations.
-
b.Spray the mouse with 70% alcohol and make a crosscut in the belly skin.
-
c.Pull the skin apart to expose the lymph nodes. Harvest axillary, branchial and inguinal LNs and place them in a 6-well plate with scratched bottom (please see note) containing 2 mL of collagenase D digestion medium.
-
d.Using the plunger of a 3 mL syringe to crush the lymph nodes against bottom of scratched well.
-
e.Incubate in 5% CO2 incubator at 37°C for 30 min with continuous shaking at 90 RPM.
-
f.Pipette up and down using a serological pipette and filter through a 40 μm cell strainer into a 50 mL conical tube.
-
g.Rinse the well and the cell strainer with 10 mL of SM.
-
h.Centrifuge @ 600 g at 4°C for 7 min.
-
i.Discard the supernatant.
-
j.Resuspend the pellet in 500 μL of SM and count the cells.
-
k.Proceed with staining.
-
a.
Note: Scratched bottom serves as a rough surface for mechanical grinding/disruption of the lymphoid organs. Scratching can be achieved using an ∼18-gauge needle. Even checkered pattern is preferred.
Note: We suggest day 7 and day 14 post inoculation, respectively to observe effector T and B cell responses.
Note: A video on preparing scratched surface, and harvesting skin draining lymph nodes are available upon request.
Expected outcomes
It is expected almost complete digestion with all the organs presented. Some of the epithelial layers, such as the skin’s epidermis, will not be digested efficiently with this enzyme cocktail. If required, we suggest separating and digesting the epidermal sheets using trypsin digestion first and then digesting the dermis with the enzyme cocktail presented here (Kashem and Kaplan, 2018). The following cell numbers are expected: Skin: 5 ± 1.41 × 105/1 cm2 (PBS) and 1 ± 0.31 × 106/1 cm2 (LNP). Muscle: 3.57 ± 0.69 × 106/1 mg (PBS) and 7.5 ± 0.95 × 106/1 mg (LNP). Lungs: 1.27 ± 0.20 × 107/lungs (PBS) and 8.92 ± 5.24 × 107/lungs (LNP). Lymph nodes: 5.5 ± 1.23 × 106/6 skin draining lymph nodes (PBS) and 1.43 ± 0.25 × 107/6 skin draining lymph nodes (LNP).
Limitations
To limit variability between preparations here we used the same digestion solution across most of the organs. Other protocols that are tailored and might aid more efficient recovery of the immune cells from a specific organ exist and should be consulted if needed (Bouladoux et al., 2017; Steinert et al., 2015).
Troubleshooting
Problem 1
No visible bubble is formed after intradermal injection, or the bubble disappears soon after injection. This indicates that the injection was too deep and likely ended up in a subdermal/subcutaneous area (step 2b).
Potential solution
Unlike the human skin, the mouse epidermis is only a few cell layers thick. Thus, it is imperative to use a fine needle for injection. Also, keep the needle with bevel up, and as soon as the needle’s tip breaks the epidermis, try to pull the skin up with the needle while pushing ahead slowly. Keep the needle close to the surface and visible all the time.
Problem 2
No visible inflammation at the site of inoculation (step 2d/critical), no significant leukocytic infiltrates detected by flow cytometry or other means.
Potential solution
Double check your notes and records on your mRNA-LNP stock. LNPs that do not contain ionizable lipid are not inflammatory. The potency of the regents not stored at appropriate temperature quickly decreases.
Problem 3
Inefficient digestion (step 3g).
Potential solution
Make sure to remove any extra fatty/connective tissue associated with the organs prior mince. Optimization of digestion time and digestion solution volumes might be needed.
Problem 4
A significant portion of the cells are lost during staining for flow cytometry (step 4f).
Potential solution
Make sure to use plates with round bottom wells for your staining. The round bottom wells allow the cells to form a pellet in the center of the well and thus provides space for the pipettor to safely remove the liquid without touching the pellet. Pay attention when resuspending the cell pellet. Always keep the pipette tip submerged in the liquid and pipette up ∼80%–90% of the whole volume and dispense it back. Following these steps will prevent air bubble formation, which is the primary route of cell loss.
Problem 5
If air bubbles are formed during intranasal inoculation (step 7f), that could indicate that: 1) the mouse is not anesthetized well (i.e., too early or too late after anesthetization); 2) both nostrils covered by the liquid and the mouse is choking.
Potential solution
Make sure to limit the inoculation to one of the nostrils. Slow down and use a pipettor to remove the bubbles. Keep mice in a straight-up position and observe for any sign of distress.
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact, Botond Z. Igyártó, botond.igyarto@jefferson.edu.
Materials availability
This study did not generate new unique reagents. The LNPs and mRNA-LNPs used are proprietary to Acuitas and are not commercially available. University of Pennsylvania has a core facility from which some of these reagents can be requested: https://www.itmat.upenn.edu/engineered-mrna-and-targeted-nanomedicine-core.html.
Acknowledgments
B.Z.I. is supported by NIH, R01AI146420 and faculty start-up funds.
Author contributions
Writing – Original Draft, S.N.; Writing – Review & Editing, S.N., Z.Q., and B.Z.I.
Declaration of interests
The authors declare no competing interests.
Contributor Information
Sonia Ndeupen, Email: snn008@jefferson.edu.
Zhen Qin, Email: zhen.qin@jefferson.edu.
Botond Z. Igyártó, Email: botond.igyarto@jefferson.edu.
Data and code availability
The published articles include all datasets generated or analyzed during this study (Ndeupen et al., 2021, 2022).
References
- Anderson K.G., Mayer-Barber K., Sung H., Beura L., James B.R., Taylor J.J., Qunaj L., Griffith T.S., Vezys V., Barber D.L., et al. Intravascular staining for discrimination of vascular and tissue leukocytes. Nat. Protoc. 2014;9:209–222. doi: 10.1038/nprot.2014.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouladoux N., Hennequin C., Malosse C., Malissen B., Belkaid Y., Henri S. In: Methods in Molecular Biology. ClausenJon Björn E., Laman Jon D., editors. Springer; 2017. Hapten-specific T cell-mediated skin inflammation: flow cytometry analysis of mouse skin inflammatory infiltrate; pp. 21–36. [DOI] [PubMed] [Google Scholar]
- Kashem S.W., Kaplan D.H. Isolation of murine skin resident and migratory dendritic cells via enzymatic digestion. Curr. Protoc. Immunol. 2018;121:e45. doi: 10.1002/cpim.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ndeupen S., Qin Z., Jacobsen S., Bouteau A., Estanbouli H., Igyártó B.Z. The mRNA-LNP platform’s lipid nanoparticle component used in preclinical vaccine studies is highly inflammatory. iScience. 2021;24:103479. doi: 10.1016/j.isci.2021.103479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ndeupen S., Bouteau A., Herbst C., Qin Z., Jacobsen S., Powers N.E., Hutchins Z., Kurup D., Diba L.Z., Watson M., et al. Langerhans cells and cDC1s play redundant roles in mRNA-LNP induced protective anti-influenza and anti-SARS-CoV-2 immune responses. PLoS Pathog. 2022;18:e1010255. doi: 10.1371/journal.ppat.1010255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinert E.M., Schenkel J.M., Fraser K.A., Beura L.K., Manlove L.S., Igyártó B.Z., Southern P.J., Masopust D. Quantifying memory CD8 T cells reveals regionalization of immunosurveillance. Cell. 2015;161:737–749. doi: 10.1016/j.cell.2015.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The published articles include all datasets generated or analyzed during this study (Ndeupen et al., 2021, 2022).



