Abstract
Motion management is essential in treatment planning of radiotherapy for breast cancer. This study assessed the movement of organs-at-risk and the breast using 4D magnetic resonance imaging (MRI). A self-gating respiration-resolved radial 3D gradient echo sequence was used. Five healthy volunteers were imaged at 1.5 T during free-breathing in supine position making use of a breast board. Median distances between heart and chest wall in axial views were 2.4 cm (range: 1.5 cm) and 3.0 cm (range: 1.7 cm) for end-of-exhale and end-of-inhale. 4D-MRI allowed organ delineation and might be a promising addition to novel RT planning for breast cancer patients.
Keywords: MR-in-RT, Breast MRI, 4D MRI, Self-gating MRI, Free-breathing
1. Introduction
With over 2 million cases in 2020 [1] breast cancer is the most common cancer among women worldwide. In recent years, in addition to computer tomography (CT) imaging, MRI soft tissue imaging has supported image--guided RT plans for breast cancer patients. Breast cancer patients are immobilized in supine and prone position for CT imaging [2], but diagnostic breast MRI is typically performed in prone position, which could lead to mismatch and registration errors when using MRI as well as CT images for radiation therapy (RT) planning. Overall, little attention has been given to including MR in the clinical workflow, [3], [4], [5]. Several recent studies [6], [7] have evaluated the influence of imaging modality and patient position in partial breast irradiation. However, none of these studies have proposed an organ motion assessment with 4D technic and 3D volume. The aim of this study was to evaluate the motion of breast and organs-at-risk (OAR) in supine position during free-breathing, without using external surrogates for respiration, but rather the scanner’s own 4D respiratory-self-gating (4D MRI) capability. Image quality for organ depiction and contouring was also assessed.
2. Material and methods
The study enrolled five female healthy volunteers with varying breast sizes (n = 5, age: 56 ± 9 years, weight: 68 ± 17 kg, height: 167 ± 7 cm). The study was conducted according to local regulations and had institutional review board approval. Written consent was obtained.
For immobilization we used an indexed Breast board (Qfix® Avondale, USA), a tabletop, and a coil holder (RT-4546 Access Supine MR Breast Device, RT-4546AA-01 Access Arm Support, RT-4546AW-01 Access Wrist Support) on a 1.5 T scanner (MAGNETOM Sola, Siemens Healthcare GmbH, Erlangen, Germany). Both arms were positioned overhead (Fig. 1 (a)). This setup reduced the distance between the patient’s surface and the 18-channel body array coil improving signal-to-noise ratio (SNR). No external surrogates were needed for recording the respiratory phases. No abdominal compression was used. The 18-channel body array coil was positioned without touching the subject. A 32-channel spine coil was positioned below the body.
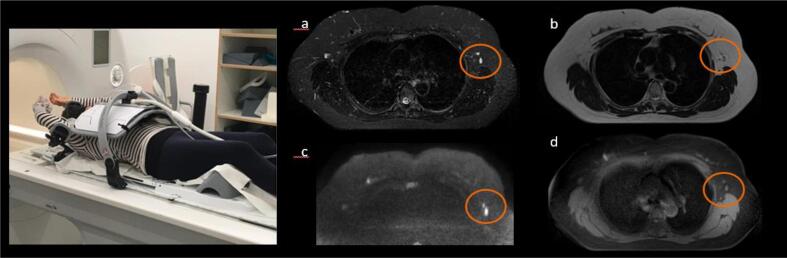
Fig. 1.
(left) Volunteer, in a 1.5 T scanner room, positioned supine with immobilization aids (table top, breast board, coil holder, knee cushion and arm holders) and body 18-channel coil. (right) Breast axial images in different contrasts - (a) T2w STIR, (b) T2w TSE, (c) RESOLVE DWI b800, (d) 4D MRI Bin7 - showing OAR such as heart, breast, lungs and lymph nodes in orange circles.
The volunteer setup required less than 5 min including tabletop setup and preparing coil holders. Free-breathing removed the necessity for DIBH training. Positioning the arms over the head reduced skin-coil distance, gained in higher SNR. A limitation was the bore diameter. With the 70 cm bore used, an angle of more than 30° for the arm holders led to contact with the bore.
We acquired a respiration-resolved radial 3D T1-weighted imaging (T1w) GRE sequence (termed 4D MRI [8]) during free-breathing (FB), and a Cartesian 3D T1 w gradient echo (GRE) sequence (volumetric interpolated breath-hold examination (VIBE) - T1w VIBE Dixon) during deep inspiration breath-holding (DIBH). The radial sequence involved a stack-of-stars trajectory with radial in-plane sampling and Cartesian partition encoding. The radial views were acquired using golden-angle ordering [9]. A respiratory self-gating signal was extracted from the central k-space samples and used as a respiratory surrogate signal [8], [10]. The 4D MRI protocol was optimized to reduce the streak artifacts inherent to radial acquisitions by adapting the number of radial views. The number of bins (phases) were also set to obtain overall good image quality for each phase, and an efficient description of breast, heart, liver, and lung motion [11].
Motion of the target volume and OAR was assessed were assessed over 7 phase bins, with 3500 radial views. 4D MRI was applied in axial orientation, from jugulum to liver, and coronal orientation, from nipple to posterior lung lobes (each with TE = 2.46 ms, TR = 3.6 ms, FA = 10°, FOV 420 × 420 mm, matrix 288 × 288, slice thickness 3 mm, 64 slices, TA = 8:35 min, slab-selective excitation, 890 Hz/pixel). In addition to the 7 bin images, an average image was reconstructed (Fig. 2 (c)).
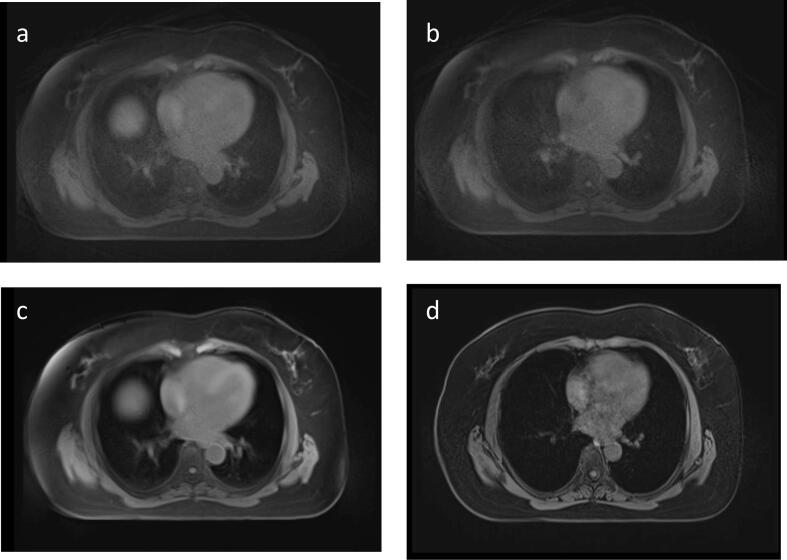
Fig. 2.
Axial views of depicted breast and OAR at different breathing phases. (a) 4D MRI bin 1 – end-of-exhale (b) 4D MRI bin 7 – end-of-inhale (c) 4D MRI average (sum of all radial views of 4D MRI) and (d) T1w VIBE Dixon in DIBH.
We performed breast diagnostic clinical routine protocols [12], [13], [14] including T2w, T2w short tau inversion recovery (STIR) and readout-segmented echo planar diffusion weighted imaging (RESOLVE DWI) [13], excluding T1w dynamic-contrast enhanced imaging, in free-breathing supine position. Since clinical routine protocols are more sensitive to motion artifacts, they were performed in the same order ∼ 10 mins after the subject was put in position, when they were breathing more quietly and regularly.
Organ motion was quantified by measuring the minimum distance between heart (OAR) and chest wall at end of exhale and end of inhale, and in DIBH [15]. In addition, the anterior-posterior and lateral relative OAR motion (OAR movement between inhale and exhale) for heart and chest wall were assessed [16].
3. Results
Supine positioning improved installation time and patient comfort compared to prone position. None of the volunteers reported discomfort and pain using the zero degree inclination of the breast board.
Distance between the spine coil and the patient body was higher in treatment position than in non-RT setup, due to the additional tabletop and breast board, but despite this, the combination with the anterior 18-channel body array coil allowed us to reach diagnostic quality imaging in T1w, T2w, and DWI contrasts, and to map the body contour.
We demonstrated reasonable image quality for potential lesion detection (Fig. 1 (right). We see the same number of lymph nodes over the different contrasts (Fig. 1 b). Diffusion weighted images were refined using the RESOLVE technic, and yielded comparable quality compared to prone acquisition, allowing depiction of lymph nodes in axillary regions.
With large z-coverage, for example from jugulum to liver, the axillary lymph nodes were clearly depicted for all volunteers (z-coverage was 192 mm, 420 mm, 216 mm, 239.3 mm, 259 mm, 190 mm respectively for 4D MRI transverse, 4D MRI coronal, T1w VIBE Dixon transverse in DIBH, T2w TSE transverse, T2w TSE STIR transverse, RESOLVE DWI transverse). Heart, ribs, breast, liver, pancreas, aorta, muscles, soft tissues, spleen, stomach, and spine could be contoured with 4D MRI, and with the additional contrasts.
OAR moved mainly in the head-feet direction. Across all volunteers, the median distance between heart and chest wall, measured in axial views including the nipple, was 2.4 cm, range: 1.5 cm, 3.0 cm, range: 1.7 cm and 4.0 cm; range: 3.8 cm for FB exhale, FB inhale and DIBH, respectively. Negligible chest movement was observed between FB exhale and FB inhale (median: 0.1 cm, range: 0.2 cm). The lateral R-L movement of the heart between inhale and exhale was negligible, at 0.3 cm (median; range: 1 cm) (Fig. 2 b and a). A slight lateral R-L movement of the heart of 1.1 cm (median; range: 2.2 cm) was observed between inhale and DIBH (Fig. 2 b and d). Chest wall movement between bin 1 and 7 was low. Visually, we also observed very little chest motion when volunteers were positioned and breathed freely. The same volunteer was scanned twice using the same setup with a one-day interval to check for physical variations. The average distance between heart and chest wall measured in axial views was 1.6 ± 0.5 cm, 1.9 ± 0.1 cm and 2.1 ± 0.8 cm for FB exhale, FB inhale and DIBH, respectively. Organ position and movement over two scans of the same volunteer were almost identical.
Because each phase bin included approximately 170 breathing cycles, 4D MRI strongly reduced the influence of irregular breathing in image quality, compared with 4D CT imaging. We also observed accurate depiction of the heart and chest, despite heart motion.
4. Discussion
A new supine position Workflow has been proposed and Breast and OAR motion have been evaluated, showing head-feet main direction.
We have shown that the 4D MRI is well adapted for MR-in-RT planning. It had overall good delineation of OAR in 4D MRI images [3], with coverage comparable to a CT examination. The analysis of the distance between the OAR showed a dominant head-feet motion in the chest and heart region when the breast (without surgery in our case) was not prone to large amplitude movements [17], [18], [19] in our proposed treatment setup.
Our free-breathing approach could satisfy the needs of partial breast irradiation strategies [18], [19], [20]. As has been shown [21], 4D MRI using T1w GRE sequences was useful for characterizing the position of marker clips. Moreover, it could help to guide irradiation of small cavities after tumor resection, allowing optimal treatment in free-breathing for patients who cannot maintain deep-inspiration breath-holds. Less flexible/mobile patients, for example, those who have undergone surgery, will likely experience difficulties keeping their arms straight up [12]. Overall, our approach may lead to reduced dose in the breast, reducing medium/long term toxicity. The introduction of 4D MRI could change breast treatment workflows as finding the proper slices for motion assessment is no longer necessary during the scan session in comparison with 2D MRI dynamic acquisition across phases of the respiratory cycle [18]. In combination with 4DCT or DIBH CT for planning, it potentially leads to optimal image registration for planning in one case and/or reduction of irradiation [7]. Due to the proximity of treated breast tissue to the heart, DIBH is commonly used for left breast cancer while right breast cancer can be irradiated in free-breathing, so 4D MRI could be an immediate and alternative imaging solution for right breast cancer.
The described setup and acquisition parameters yielded excellent image quality in supine position, making it possible to distinguish different breathing phases during postprocessing (on Siemens RT Image Suite, syngo.via VB40A, Siemens Healthcare GmbH, Erlangen, Germany). Reduced lung motion at the end of exhale might be the reason for sharper images in the corresponding bins. Staff training time is reduced because positioning using an indexed breastboard allows high reproducibility compared with self-made plastic foam setups.
Very promising was the depiction of lymph nodes in all phases of the 4D MRI images, giving valuable information for clinicians (Fig. 1 b). This applies especially to patients who have undergone partial surgery [21] but no lymph node resection. Further studies should assess the efficiency of MRI in immobilization setup to detect lymph nodes in the axillary region compared with the gold standard CT imaging [22], [23].
Compared with 4DCT imaging, 4D MRI gives oncologists excellent soft tissue contrast [3], [24] and similar z-coverage for dose planning, without compromising in OAR localization. A later study could compare margins in dose planning after contouring on MR images.
Similar free-breathing technics could drive future developments of synthetic CT algorithms in the breast region as Groot et al. recently started for treatment on MRI-linac system [25]. The feasibility for future evaluation of deposited dose and reduction of toxicity in OAR will be assessed.
An extension of this study with a range of patients, including women who have undergone surgery, would clarify the patients who would benefit from this approach for treatment planning compared with CT or 4DCT only workflow. It could include patients with limited breathhold capacity, and those unable to adopt prone position. This may increase the range of patient treatment options (dose, positioning, comfort), and modify the treatment planning workflow, reducing registration errors, costs and needs for CT or 4DCT in addition to 4D MRI. A full free-breathing workflow with 4D MRI will also influence dose planning.
In conclusion, we have demonstrated the usefulness of 4D MRI technic allowing supine patient positioning for treatment planning, which could be used for both breast and lung cancer patients. 4D MRI promises to play an important role in contouring organs and analyzing target volume movement and should have a positive impact on breast-cancer treatment.
Declaration of Competing Interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: All 4 co-authors are Siemens Healthcare GmbH employees.
Acknowledgements
This paper is part of a special issue that contains contributions originally submitted to the scientific meeting MR in RT, which was planned to take place 05/2020, organized by the German Research Center (DKFZ) in Heidelberg. We acknowledge funding by DKFZ for the publication costs of this special issue.
References
- 1.WHO (World Health Organization). International Agency for Research on Cancer. World Health Organization - cancer today. In: Organization. IAfRoCWH, editor.; 2021.
- 2.Kirby A.M., Evans P.M., Donovan E.M., Convery H.M., Haviland J.S., Yarnold J.R. Prone versus supine positioning for whole and partial-breast radiotherapy: A comparison of non-target tissue dosimetry. Radiother Oncol. 2010;96(2):178–184. doi: 10.1016/j.radonc.2010.05.014. [DOI] [PubMed] [Google Scholar]
- 3.Oar A., Liney G., Rai R., Deshpande S., Pan L., Johnston, et al. Comparison of four-dimensional computed tomography and magnetic resonance imaging in abdominal radiotherapy planning. Phys Imaging. Radiat Oncol. 2018;7(70–75) doi: 10.1016/j.phro.2018.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dundas K.L., Pogson E.M., Batumalai V., Boxer M.M., Yap M.L., Delaney G.P., et al. Australian survey on current practices for breast radiotherapy. J Med Imaging Radiat Oncol. 2015;59(6):736–742. doi: 10.1111/1754-9485.12348. [DOI] [PubMed] [Google Scholar]
- 5.Berlangieri A., Elliott S., Wasiak J., Chao M., Foroudi F. Use of magnetic resonance image-guided radiotherapy for breast cancer: a scoping review. J Med Radiat Sci. 2022;69:122–133. doi: 10.1002/jmrs.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brown E., Dundas K., Surjan Y., Miller D., Lim K., Boxer M., et al. The effect of imaging modality (magnetic resonance imaging vs. Computed tomography) and patient position (supine vs. Prone) on target and organ at risk doses in partial breast irradiation. J Med Radiat Sci. 2021;68(2):157–166. doi: 10.1002/jmrs.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Groot Koerkamp M.L., van der Leij F., van 't Westeinde T., Philippens M.E.P., van den Bongard H.J.G.D., Houweling A.C. Prone vs. supine accelerated partial breast irradiation on an MR-Linac: A planning study. Radiother Oncol. 2021;165(193–199) doi: 10.1016/j.radonc.2021.11.001. [DOI] [PubMed] [Google Scholar]
- 8.Chandarana H., Block T.K., Rosenkrantz A.B., Lim R.P., Kim D., Mossa D.J., et al. Free-breathing radial 3d fat-suppressed T1- weighted gradient echo sequence: a viable alternative for contrast-enhanced liver imaging in patients unable to suspend respiration. Invest Radiol. 2011;46:648–653. doi: 10.1097/RLI.0b013e31821eea45. [DOI] [PubMed] [Google Scholar]
- 9.Winkelmann S., Schaeffter T., Koehler T., Eggers H., Doessel O. An optimal radial profile order based on the Golden Ratio for time-resolved MRI. IEEE Trans Med Imaging. 2007;26:68–76. doi: 10.1109/TMI.2006.885337. [DOI] [PubMed] [Google Scholar]
- 10.Batumalai V, Liney G, DeLaney GP, Rai R, Boxer M, Min M, et al. Assessment of MRI image quality for various setup positions used in breast radiotherapy planning. Radiother Oncol. 2016; 119:57–60. https://doi: 10.1016/j.radonc.2016.02.024. [DOI] [PubMed]
- 11.Grimm R, Bauer S, Kiefer B, Hornegger J, Block T. Optimal channel selection for respiratory self-gating signals. In Proceedings of the 21st Annual Meeting ISMRM 2013 Salt Lake City, Utah, USA (Abstract 3992).
- 12.Groot Koerkamp M.L., Vasmel J.E., Russell N.R., Shaitelman S.F., Anandadas C.N., Currey A., et al. Optimizing MR-Guided Radiotherapy for Breast Cancer Patients. Front Oncol. 2020;10:1107. doi: 10.3389/fonc.2020.01107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mann R.M., Cho N., Moy L. Breast MRI: State of the Art. Radiology. 2019;292:520–536. doi: 10.1148/radiol.2019182947. [DOI] [PubMed] [Google Scholar]
- 14.MR-integrated Workflows in Radiation Therapy (2018) published by Siemens Healthcare GmbH · Order No. A91MR-1100-95C-7600 · Printed in Germany · 6085 04180.2 · © Siemens Healthcare GmbH.
- 15.Henseler H., Ju X., Ray A.K. Correlation of centroid-based breast size, surface-based breast volume, and asymmetry-score-based breast symmetry in three-dimensional breast shape analysis. GMS Ger Plast Reconstr Aesthet Surg. 2016;6 doi: 10.3205/gpras000038. ISSN 2193–7052. [DOI] [Google Scholar]
- 16.Al-Ward S.M., Kim A., McCann C., Ruschin M., Cheung P., Sahgal A., et al. The development of a 4D treatment planning methodology to simulate the tracking of central lung tumors in an MRI-linac. J Appl Clin Med Phys. 2018;19:145–155. doi: 10.1002/acm2.12233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lowanichkiattikul C., Dhanachai M., Sitathanee C., Khachonkham S., Khaothong P. Impact of chest wall motion caused by respiration in adjuvant radiotherapy for postoperative breast cancer patients. SpringerPlus. 2016;5:144. doi: 10.1186/s40064-016-1831-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Price A.T., Kennedy W.R., Henke L.E., Brown S.R., Green O.L., Thomas M.A., et al. Implementing stereotactic accelerated partial breast irradiation using magnetic resonance guided radiation therapy. Radiother Oncol. 2021;164:275–281. doi: 10.1016/j.radonc.2021.09.023. [DOI] [PubMed] [Google Scholar]
- 19.Acharya S., Fischer-Valuck B.W., Mazur T.R., et al. Magnetic resonance image guided radiation therapy for external beam accelerated partial-breast irradiation: evaluation of delivered dose and intrafractional cavity motion. Int J Radiat Oncol Biol Phys. 2016;96:785–792. doi: 10.1016/j.radonc.2021.09.023. [DOI] [PubMed] [Google Scholar]
- 20.Lee H., Shin K.H., Kim K., Kim J.H., Chang J.H. The Acute and Late Toxicities of MRI-Guided External Beam Partial Breast Irradiation Delivered Using a Once-per-Day Regimen. Int J Radiat Oncol Biol Phys. 2021;11:201. doi: 10.1016/j.ijrobp.2021.07.719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vinnicombes S. How I report breast magnetic resonance imaging studies for breast cancer staging and screening. Cancer Imaging. 2016;16:17. doi: 10.1186/s40644-016-0078-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.van Heijst T., van Asselen B., Pijnappel R.M., Cloos-van Balen M., Lagendijk J.J.W., van den Bongard D., et al. MRI sequences for the detection of individual lymph nodes in regional breast radiotherapy planning. Br J Radiol. 2016;89:20160072. doi: 10.1259/bjr.20160072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang W., Li J.B., Hu H.G., Li F.X., Xu M., Sun T., et al. Correlation between target motion and the dosimetric variance of breast and organ at risk during whole breast radiotherapy using 4DCT. Radiat Oncol. 2013;8:111. doi: 10.1186/1748-717x-8-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stemkens B, Paulson ES, Tijssen RHN. Nuts and bolts of 4D-MRI for radiotherapy. Phys Med Biol 2018;63:21TR01. https://doi:10.1088/1361-6560/aae56d. [DOI] [PubMed]
- 25.Groot Koerkamp M.L., de Hond Y.J.M., Maspero M., Kontaxis C., Mandija S., Vasmel J.E. Synthetic CT for single-fraction neoadjuvant partial breast irradiation on an MRI-linac. Phys Med Biol. 2021;66 doi: 10.1088/1361-6560/abf1ba. 10.1088/1361-6560/abf1ba. [DOI] [PubMed] [Google Scholar]