Abstract
Background
Annually, close to 5000 children under age 6 years are treated in emergency departments or admitted for care due to opioid exposures. Naloxone is effectively used to treat opioid overdose in both children and adults. Non‐cardiogenic pulmonary edema is a rare but serious adverse effect of naloxone administration that has been reported in adults.
Case Report
We present the case of a 3‐year‐old male with suspected opioid overdose who developed acute hypoxia due to pulmonary edema after administration of naloxone following a likely prolonged downtime.
Why Should an Emergency Physician Be Aware of This?
The copious fluid in the airway made for difficult intubation at a pediatric tertiary care center. Given the incidence of opioid exposures in children, clinicians should be aware of this rare, but dangerous adverse effect of naloxone and consider airway precautions and pediatric critical care availability early in the presentation.
Keywords: fentanyl, intubation, opiate, opiate overdose, pediatric emergencytoxicology, pulmonary edema
1. INTRODUCTION
The opioid epidemic is a growing public health concern that has become increasingly important in pediatric medicine. The incidence of opioid exposures has been noted to be 22.6 per 100,000 children and was highest among children less than 5 years old, with 73% of exposures being unintentional. 1 Hydrocodone has been shown to be the most common source of opioid poisoning in children, but children with methadone or buprenorphine ingestions have been shown to have higher morbidity. 2 Classic findings of opioid toxicity include miosis, central nervous system depression or altered mental status, and respiratory depression. Hyporeflexia, bradycardia, and hypotension are also possible. However, when young children present with opioid overdose, the medication and the dose ingested are often unknown, which can lead to delayed diagnosis.
Naloxone is an opioid antagonist that is effective at reversing respiratory complications in cases of opioid overdose. It is a synthetic derivative of oxymorphone that is a competitive inhibitor of mu opioid receptors. Due to its lipophilic nature, naloxone rapidly reaches the central nervous system and has an onset of action of within minutes, with a half‐life of 30–120 minutes. 3 Most studies supporting the use of naloxone for opioid overdose have been in adult patients, however, some smaller studies have shown naloxone effectively reduces opioid intoxication in children as well. The recommended dose for naloxone for patients weighing less than 20 kg is 0.1 mg/kg intravenously, to be repeated every 3 minutes until there is improvement in respiratory depression. 4
The most common adverse effect of naloxone is opioid withdrawal syndrome, which presents with agitation, nausea, vomiting, or anxiety in opioid‐dependent patients. It should be noted that opioid dependence is uncommon in young children, so this is less likely to be observed after administration of naloxone. Although naloxone is otherwise considered safe, there have been rare adverse effects reported in adults, one of which is pulmonary edema. Thought to be caused by catecholamine increase, this adverse effect has been well‐documented in adult patients, both with and without pre‐existing heart disease, at an incidence of 0.2%–3.6%. 5 Although it has been reported infrequently in adolescent patients, to our knowledge, it has not been reported in young children. 6 We report a case of naloxone‐induced non‐cardiogenic pulmonary edema in an otherwise healthy 3‐year‐old male with suspected opioid overdose.
2. CASE REPORT
A 3‐year‐old male presented to the emergency department (ED) by ambulance after being found unresponsive. He was last seen in his normal state of health around 7 PM on the night before presentation when his mother dropped him off at a friend's hotel room. When she returned around 5 AM the next morning, he was found unresponsive in the hotel bed and emergency medical services (EMS) were called. Per EMS, the patient had poor respiratory effort, strong femoral pulses, and was unresponsive. Bag mask ventilation (BVM) was initiated, and the patient was brought emergently to the pediatric ED. The patient had no previous medical problems, and mother was unaware if any drugs or toxic substances were present in the hotel room.
On arrival to the ED, the patient was responsive only to painful stimuli. He had strong central and peripheral pulses with a heart rate of 120 bpm. He had slow, shallow respirations with oxygen saturations of 95%–100% on room air. He would pause respirations intermittently and required BVM. He had a normal temperature, clear lungs, normal heart sounds, and no obvious signs of trauma. His pupils were noted to be pinpoint.
An intravenous line was placed and a venous blood gas was drawn, showing a pH of 6.93 and pCO2 of 106 mm Hg. The initial chest x‐ray was normal (Figure 1). Given his respiratory depression, altered mental status, and pinpoint pupils, there was clinical concern for opioid overdose, and 0.1 mg/kg of naloxone was administered intravenously. Within 60 seconds of administration of naloxone, the patient began spontaneously moving all extremities and resumed spontaneous respirations at a rate >12. Pupils after initial naloxone were 4 mm and reactive bilaterally.
FIGURE 1.
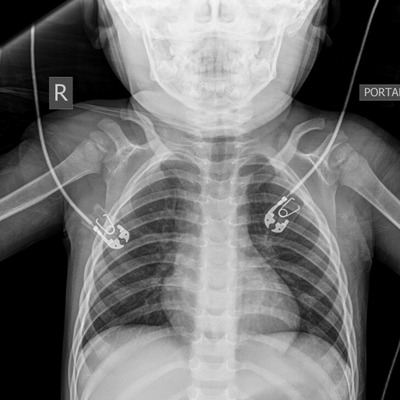
Chest radiograph prior to administration of naloxone shows clear lung fields without any acute cardiopulmonary abnormality
After ∼30 minutes, the patient again had intermittent apnea and a second dose of naloxone 0.1 mg/kg was given with resultant improvement in respiratory depression. Approximately 5 minutes after the second dose of naloxone, the patient became severely hypoxic with gasping respirations. BVM was being performed as the physician team set up for rapid sequence intubation. BVM was becoming increasingly difficult and frothy pink fluid began draining from the nose and mouth. Within 30 seconds, this fluid changed to copious clear fluid. Upon direct laryngoscopy, copious fluid was visualized pouring from his airway. Aggressive suctioning was unable to clear the view and the intubation was performed without any view of the airway. Tube placement was verified by capnography, auscultation of breath sounds, and later by the second chest x‐ray (Figure 2), which also showed new bilateral interstitial infiltrates. The patient was then transferred to the pediatric intensive care unit. Although he initially required 100% FiO2 and positive end expiratory pressure (PEEP) of 12 to maintain O2 saturations over 80%, he was able to be weaned down to room air and PEEP of 8 within 24 hours with improvement in pulmonary edema on chest x‐ray (Figure 3). Of note, the patient developed a pneumothorax after central line placement requiring a chest tube (Figure 3). He was noted to have hypoxic ischemic brain injury on brain magnetic resonance imaging (MRI) and after a 10‐day hospital course, was discharged to an inpatient rehabilitation facility. Ultimately, extended drug screening returned positive for fentanyl. Department of family services was notified of the incident and an investigation of child abuse/neglect was initiated.
FIGURE 2.
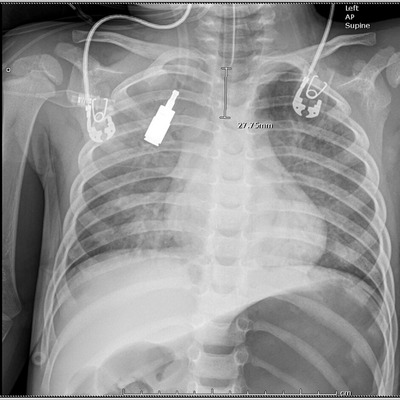
Chest radiograph after administration of naloxone and intubation showing endotracheal tube in place and new bilateral interstitial infiltrates
FIGURE 3.
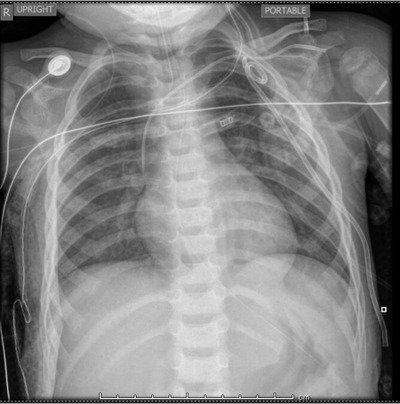
Chest radiograph 24 hours after administration of naloxone showing interval improvement of pulmonary edema as well as interval placement of central line and chest tube
3. DISCUSSION
Pulmonary edema is a rare but well‐reported side effect of naloxone administration in adult patients. 7 , 8 , 9 Naloxone is thought to cause pulmonary edema due to increased catecholamine‐induced vasoconstriction resulting in significant fluid shifts. 10 Some studies have shown that higher doses of naloxone are associated with higher risk of pulmonary complications. 11 “Flash” pulmonary edema is characterized by acute, significant hypoxia, which if untreated, results in cardiac compromise. Copious frothy, pink secretions can also be seen on intubation. It is important to note that opioid overdose itself can also cause pulmonary edema through a multifactorial mechanism including histamine release and prolonged hypoxia and acidosis in the setting of respiratory depression that leads to increased pulmonary vasopermeability. 12 It is clinically difficult to differentiate between naloxone associated pulmonary edema from opioid overdose‐associated pulmonary edema. Our patient had a likely prolonged downtime leading to significant acidosis at presentation, but an initially clear chest radiograph indicating no pre‐existing pulmonary edema; however, there likely is some baseline level of increased vascular permeability that predisposed our patient to subsequent naloxone‐associated pulmonary edema.
Some case reports have shown furosemide to be an effective treatment for naloxone‐associated pulmonary edema with resultant quick progression to extubation in most adult patients but has not been formally studied. 13 Our patient was also able to wean down quickly on respiratory support without the use of furosemide.
3.1. Why should an emergency physician be aware of this?
As opioid use continues to be highly prevalent in the community, children are at increased risk for accidental ingestion and overdose. Naloxone can be a life‐saving medication for opioid overdose. Although the rare adverse event of pulmonary edema had not previously been reported in children, it should be considered in all patients when giving naloxone. In pediatric patients in particular, clinicians should be aware of the potential need for intubation when giving naloxone. Clinicians should also consider timely transfer to a pediatric critical care unit as these patients may be difficult to manage when mechanically ventilated and may require pediatric cardiac or pulmonary specialists. Social workers familiar with pediatric care can also help evaluate for abuse or neglect in these situations.
CONFLICTS OF INTEREST
The authors declare no conflicts of interest.
Grout S, Dave M, Lefort R. Naloxone‐associated pulmonary edema in a 3‐year‐old with opioid overdose. JACEP Open. 2022;3:e12740. 10.1002/emp2.12740
Funding and support: By JACEP Open policy, all authors are required to disclose any and all commercial, financial, and other relationships in any way related to the subject of this article as per ICMJE conflict of interest guidelines (see www.icmje.org). The authors have stated that no such relationships exist.
Supervising Editor: Karl Sporer, MD.
REFERENCES
- 1. Patel MA, Wheeler DC, Rose SR, et al. Prevalence and characteristics of pediatric opioid exposures and poisonings in the United States. J Pediatrics. 2019;206(4):148‐155. [DOI] [PubMed] [Google Scholar]
- 2. Boyer EW. Management of opioid analgesic overdose. N Engl J Med. 2012;367(2):146‐155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Allen JD, Casavant MJ, Spiller HA, et al. Prescription opioid exposures among children and adolescents in the United States: 2001–2015. Pediatrics. 2017;139(4) e20163382. [DOI] [PubMed] [Google Scholar]
- 4. Committee on Drugs . Naloxone dosage and route of administration for infants and children: addendum to emergency drug doses for infants and children. Pediatrics. 1990;86(3):484‐485. [PubMed] [Google Scholar]
- 5. Sporer KA, Firestone J, Isaacs SM. Out‐of‐hospital treatment of opioid overdoses in an urban setting. Acad Emerg Med. 1996;3(7):660‐667. [DOI] [PubMed] [Google Scholar]
- 6. Gurji HA, Ham AA. Postoperative pulmonary edema in an adolescent. Consultant. 2021;61(10):e33‐e36. [Google Scholar]
- 7. Bansal S, Khan R, Tietjen PA. Naloxone‐induced pulmonary edema. Chest. 2007;132:692A. [Google Scholar]
- 8. Schwartz JA, Koenigsberg MD. Naloxone‐induced pulmonary edema. Ann Emerg Med. 1987;16(11):1294‐1296. [DOI] [PubMed] [Google Scholar]
- 9. Patti R, Ponnusamy V, Somal N, et al. Naloxone‐induced noncardiogenic pulmonary edema. Am J Ther. 2020;27(6):e672‐e673. [DOI] [PubMed] [Google Scholar]
- 10. Silverstein JH, Gintautas J, Tadoori P, et al. Effects of naloxone on pulmonary capillary permeability. Prog Clin Biol Res. 1990;328:389‐392. [PubMed] [Google Scholar]
- 11. Farkas A, Lynch MJ, Westover R, et al. Pulmonary complications of opioid overdose treated with naloxone. Ann Emerg Med. 2020;75(1):39‐48. [DOI] [PubMed] [Google Scholar]
- 12. Sporer KA, Dorn E. Heroin‐related noncardiogenic pulmonary edema: a case series. Chest. 2001;120(5):1628‐1632. [DOI] [PubMed] [Google Scholar]
- 13. Jiwa N, Sheth H, Silverman R. Naloxone‐induced non‐cardiogenic pulmonary edema: a case report. Drug Saf Case Rep. 2018;5(1):20. [DOI] [PMC free article] [PubMed] [Google Scholar]


