Abstract
Toxin-based identification procedures are useful for differentiating Pseudomonas syringae pathovars. A biological test on peptone-glucose-NaCl agar in which the yeast Rhodotorula pilimanae was used proved to be more reliable for detecting lipodepsipeptide-producing strains of P. syringae than the more usual test on potato dextrose agar in which Geotrichum candidum is used. A PCR test performed with primers designed to amplify a 1,040-bp fragment in the coding sequence of the syrD gene, which was assumed to be involved in syringomycin and syringopeptin secretion, efficiently detected the gene in pathovars that produce the lipodepsipeptides. Comparable results were obtained in both tests performed with strains of the syringomycin-producing organisms P. syringae pv. syringae, P. syringae pv. atrofaciens, and P. syringae pv. aptata, but the PCR test failed with a syringotoxin-producing Pseudomonas fuscovaginae strain. The specificity of the test was verified by obtaining negative PCR test results for related pathovars or species that do not produce the toxic lipodepsipeptides. P. syringae pv. syringae was detected repeatedly in liquid medium inoculated with diseased vegetative tissue and assayed by the PCR test. Our procedure was also adapted to detect P. syringae pv. morsprunorum with a cfl gene-based PCR test.
The species Pseudomonas syringae is heterogeneous and is divided into 57 pathovars (12). Rapid identification procedures are needed to avoid the physiological, biochemical, and pathological tests used to characterize P. syringae pathovars. One of the methods investigated, production of different phytotoxins by members of this species (14), has led to the development of identification tests based on detection of genes involved in the production of toxins (4, 24, 32, 34, 37, 40, 42). Such tests are particularly necessary when different pathogens induce similar symptoms on the same crop. In the case of bacterial canker of cherry trees, two pathogens, P. syringae pv. morsprunorum and P. syringae pv. syringae, can be responsible for the disease (6, 7, 24, 47); P. syringae pv. syringae is a pathogen of numerous monocot and dicot plants (47). These two P. syringae pathovars are frequently encountered in Belgium as fruit tree pathogens.
Syringomycin, syringotoxin, and syringostatin are produced by strains of P. syringae pv. syringae (3, 10, 21, 41). These molecules are lipodepsipeptides that are toxic to a wide range of organisms, including plants; they mainly differ in the amino acids found in the peptide parts of the molecules (11). Syringopeptins are more phytotoxic and more complex lipodepsipeptides produced by the same pathovar (1, 20). Both classes of lipodepsipeptides are pore-forming cytotoxins that act by promoting passive transmembrane ion flux (19). Additive effects and possible interactions in channel formation (19), as well as synergism (20), have been reported for the two classes of toxins. The sequences of four genes putatively involved in syringomycin regulation (syrP), synthesis (syrB and syrC), and secretion (syrD) are known (36, 48, 49). The syrD gene is also necessary to produce syringopeptin (13). Two DNA probes constructed from syrB and syrD genes verified that these genes are conserved in toxin-producing strains as single copies in the bacterial genome (35). PCR tests for detection of the syrB and syrD genes in P. syringae pv. syringae strains have been described recently (42). The test for detection of the syrB gene is specific, but additional amplified products were obtained by amplifying the predicted syrD gene fragment (42).
Syringomycin production and syringopeptin production are not limited to P. syringae pv. syringae. These compounds have been found in P. syringae pv. atrofaciens (44), a monocot pathogen that is considered the main bacterial disease of cereal crops in central and eastern Europe (43). Syringomycin production and hybridization to syrB and syrD probes have been reported for a strain of P. syringae pv. aptata (16, 35), a sugar beet pathogen. Strains of both pathovars inhibit the growth of the fungus Geotrichum candidum on potato dextrose agar (PDA) (a biotest used to visualize syringomycin production) (15, 26). Surprisingly, it has been reported that syringotoxin is produced by a distantly related species, Pseudomonas fuscovaginae, a rice pathogen in cold and humid areas (9).
In this paper, we describe a rapid test that can be used to determine the production of toxic lipodepsipeptides by strains of P. syringae, as well as an original PCR test that can be used to specifically detect the presence of the syrD gene. In addition, a PCR method for detecting P. syringae pv. syringae in diseased cherry twigs is described. The ability of this method to detect P. syringae pv. morsprunorum, another pathogen of cherry trees, was demonstrated by using primers specific for the cfl gene, which is involved in production of the phytotoxin coronatine (4).
MATERIALS AND METHODS
Bacterial strains and culture.
The bacterial strains used in this study are described in Table 1. P. syringae pv. aptata UPB110 and UPB165 are nonfluorescent variant strains (26). P. syringae pv. morsprunorum LMG 2222 is a coronatine-producing strain; strains LMG 5075t1, LMG 5467, and LMG 5698 are considered coronatine nonproducers (27). All precultures were incubated for 24 h at 28°C on medium 2 agar, a modified nutrient agar which contained (per liter) 2 g of yeast extract, 5 g of peptone, 5 g of NaCl, 0.45 g of KH2PO4, 0.96 g of Na2HPO4, and 8 g of agar; the pH was adjusted to 7 with NaOH.
TABLE 1.
Characteristics of strains used in this study
| Straina | Host | State or Country | Size of inhibition zone (mm) onb:
|
PCR results with syrD primers | Lipodepsipeptide producedc | Sourced | |
|---|---|---|---|---|---|---|---|
| PGNaClA | PDA | ||||||
| P. syringae pv. syringae strains | |||||||
| B3A | Peach | California | 13.7 | 12.6 | +/+e | SR, SPf | D. C. Gross |
| B301D | Pear | England | 24.2 | 18.4 | +/+ | SR, SPf | D. C. Gross |
| HS191 | Millet | Australia | 15 | 6.2 | +/+ | SRg | D. C. Gross |
| B457 | Orange | California | 32.4 | 19.4 | +/+ | STh | H. Maraite |
| Ps268 | Lemon | California | 30.7 | 19.4 | +/+ | STg | H. Maraite |
| CFBP 2117 | Sweet cherry | France | 14.2 | 2.5 | +/+ | L. Gardan | |
| CFBP 2118 | Sweet cherry | France | 6 | 6.4 | +/+ | L. Gardan | |
| 6186-M85-7 | Sweet cherry | France | 21.2 | 12.2 | +/+ | L. Gardan | |
| LMG 1247T | Lilac | England | 20 | 12.9 | +/+ | LMG | |
| LMG 5084 | Pear | England | 23.4 | 17.7 | +/+ | LMG | |
| LMG 5494 | Sweet cherry | Hungary | 7.5 | 0 | +/+ | LMG | |
| LMG 5191 | Sour cherry | Switzerland | 0 | 0 | −/− | LMG | |
| LMG 6104 | Plum | South Africa | 2 | 0 | +/+ | LMG | |
| LMG 5141 | Pear | England | 0 | 0 | +/+ | LMG | |
| PsP1 | Pear | Belgium | 19.5 | 15.2 | +/+ | This study | |
| PsP2 | Pear | Belgium | 21.9 | 8.5 | +/+ | This study | |
| PsP4 | Pear | Belgium | 20.7 | 10.9 | +/+ | This study | |
| Rom1 | Apple | Bulgaria | 22 | 12.2 | +/+ | R. Penev | |
| P. syringae pv. aptata strains | |||||||
| LMG 5059T | Sugar beet | United States | 17 | 13.1 | +/+ | LMG | |
| LMG 5646 | Sugar beet | New Zealand | 5.4 | 4.2 | +/NT | LMG | |
| LMG 5143 | Sugar beet | Unknown | 10.5 | 14.2 | +/NT | LMG | |
| UPB 165 | Sugar beet | France | 15.5 | 16.1 | +/NT | H. Maraite | |
| UPB 110 | Sugar beet | Belgium | 18.4 | 11.5 | +/NT | H. Maraite | |
| P. syringae pv. atrofaciens strains | |||||||
| LMG 5095T | Wheat | New Zealand | 23 | 16 | +/+ | SR, SPi | LMG |
| LMG 5000 | Wheat | Unknown | 5 | 5.2 | +/+ | LMG | |
| P. syringae pv. morsprunorum strains | |||||||
| LMG 5075t1T | Plum | Unknown | NT/− | LMG | |||
| LMG 2222 | Sweet cherry | England | 0 | 0 | −/− | LMG | |
| LMG 5467 | Prunus sp. | South Africa | NT/− | LMG | |||
| LMG 5468 | Sweet cherry | England | NT/− | LMG | |||
| LMG 5698 | Plum | France | NT/− | LMG | |||
| PmC14 | Sweet cherry | Belgium | NT/− | This study | |||
| PmC36 | Sweet cherry | Belgium | NT/− | This study | |||
| P. syringae pv. tomato LMG 5093T | Tomato | England | 0 | −/− | LMG | ||
| P. syringae pv. garcae strains | |||||||
| LMG 5064T | Coffee | Brazil | 0 | −/NT | LMG | ||
| LMG 5065 | Coffee | Kenya | 0 | −/NT | LMG | ||
| P. syringae pv. oryzae LMG 10912T | Rice | Japan | 0 | −/NT | LMG | ||
| P. syringae pv. tabaci LMG 5393T | Tobacco | Hungary | 1 | −/NT | LMG | ||
| P. syringae pv. papulans LMG 5076T | Common apple | Canada | 0 | −/NT | LMG | ||
| P. syringae pv. pisi LMG 5079T | Pea | New Zealand | 0 | −/NT | LMG | ||
| P. viridiflava LMG 2352T | Bean | Switzerland | 0 | 0 | −/NT | LMG | |
| P. fuscovaginae strains | |||||||
| LMG 2158T | Rice | Japan | 12 | 15.7 | −/− | LMG | |
| UPB 264b | Rice | Burundi | 25.9 | 20.5 | −/− | ST, FPj | H. Maraite |
T = pathotype strain.
Each plate was sprayed with an R. pilimanae cell suspension after incubation for 48 h at 28°C and was incubated at 20°C after spraying. The values are averages of the values from two trials; two plates were used for each trial.
SR, syringomycin; ST, syringotoxin; SP, syringopeptin; FP, fuscopeptin.
LMG, Laboratorium voor Microbiologie, Gent, Belgian Coordinated Collections of Microorganisms, Ghent, Belgium.
PCR results obtained with purified DNA/PCR results obtained with lysed cells. +, positive; −, negative; NT, not tested.
Data from reference 17.
Data from reference 27.
Data from reference 43.
Data from reference 7.
Estimation of lipodepsipeptide production on agar media.
Lipodepsipeptide production on agar media was estimated by using PDA and peptone-glucose-NaCl agar (PGNaClA). PGNaClA contained (per liter) 5 g of peptone, 10 g of glucose, 5 g of NaCl, and 8 g of agar. Petri dishes containing 30 ml of medium were incubated for 48 h at 37°C before they were used. A pellet of cells taken from a preculture was placed (a 3-cm-long and 0.8-cm-wide line) in the center of each petri dish. The cultures were incubated for 24 or 48 h at 28°C or for 4 days at 25°C, sprayed with a cell suspension of the yeast Rhodotorula pilimanae MUCL 3039 or a cell suspension of the fungus G. candidum MUCL 31566, and incubated at 20 or 25°C for 48 h. The maximum inhibition zones between the two organisms were measured.
DNA purification.
A liquid medium 2 culture was started from a preculture and incubated for 24 to 48 h at 28°C. DNA was extracted as described by Pitcher et al. (33), except that Eppendorf tubes were centrifuged for 30 min at 20,000 × g after the chloroform–2-pentanol solution was added and the washing steps were as follows: two washes with ethanol and three washes with a solution containing ethanol and H2O (70:30, vol/vol). The DNA was dried at 37°C, resolubilized overnight at 20°C in a 10 mM Tris–0.1 mM EDTA buffer (pH 8), and stored at −20°C. The PCR tests were carried out with 1 ng of purified DNA unless otherwise specified.
Preparation of samples for direct PCR with lysed cells.
A liquid culture was started in 4 ml of medium 2 and incubated for 24 h at 28°C. After homogenization, the optical density was measured at 610 nm. The culture was centrifuged at 11,068 × g for 5 min, and the culture medium was removed. The cells were washed twice with 1 ml of sterile water and resuspended in 500 μl of sterile water. The cells were lysed by incubating them for 15 min at 100°C and then transferring them four times from −20°C (15 min) to 70°C (5 min). Lysed cells were stored at −20°C in sterile water. Suspension volumes containing approximately 5 × 106, 5 × 105, and 5 × 104 lysed cells were estimated on the basis of the initial cell suspension optical density measurements and were used in PCR.
Primers and PCR.
The following primers were used to detect the syrD gene: the 21-mer oligonucleotide 5′-CAGCGGCGTTGCGTCCATTGC-3′ (primer syD1) and the 23-mer oligonucleotide 5′-TGCCGCCGACGATGTAGACCAGC-3′ (primer syD2). These primers amplify a 1,040-bp product located in the coding sequence of the syrD gene at positions 566 to 1606 of the open reading frame (36). The primers used by Bereswill et al. (4) to detect the cfl gene were used to amplify a 655-bp fragment. Each PCR was carried out in a 50-μl (final volume) mixture containing 25 pmol of each primer and 0.5 U of Taq DNA polymerase (Boehringer Mannheim). The concentration of the buffer used with the enzyme was the concentration recommended by the manufacturer, and each deoxynucleoside triphosphate (dATP, dCTP, dGTP, and dTTP [Boehringer Mannheim]) was added at a final concentration of 0.2 mM. Two thermocycler programs (Techne Cyclogene) were used. Program 1 was used with a relatively low annealing temperature in order to detect the syrD gene in purified DNA or lysed cells of strains of P. syringae pv. syringae and other pathovars or species. It consisted of 37 cycles, as follows: denaturation at 93°C for 3 min in cycle 1 and for 1 min in cycles 2 to 37; annealing at 60°C for 1 min in all cycles; and polymerization at 72°C for 1 min in cycles 1 to 36 and for 6 min in cycle 37. The same program was used with an annealing temperature of 64°C. The purpose of program 2 was to allow the simultaneous detection of the two pathovars responsible for bacterial canker of cherry trees. This program was used in order to verify the absence of the syrD gene in strains of P. syringae pv. morsprunorum, as well as to perform both PCR tests in studies of diseased cherry twigs. Program 2 has been described by Bereswill et al. (4, 5) but was modified as follows: denaturation was performed for 3 min in cycle 1 and annealing was carried out for 2 min at 57°C. PCR products were analyzed on a 0.8% agarose gel, stained with ethidium bromide, and visualized under UV light.
Plant inoculation.
One-year-old dormant shoots of the Napolean variety taken from a cherry orchard in the morning were cut to the required length and washed. Cell suspensions of P. syringae were inoculated onto cut ends in the case of P. syringae pv. syringae (6 × 105 CFU) and onto exposed cortical tissues in the case of P. syringae pv. morsprunorum (1.2 × 106 CFU). Sterile deionized water was used as a negative control. The preparations were incubated at 15°C in glass tubes in the dark for 1 month; in some instances tubes were incubated at −10°C between days 6 and 9. Then the twigs were stored at 4°C until they were used in PCR tests.
Recovery of bacteria from diseased twigs and PCR analysis.
Deep-seated pieces of damaged tissues were carefully excised with a flame-sterilized scalpel slightly away from disease lesions and put in 4 ml of liquid medium 2 in glass tubes. Control samples were excised from healthy tissues of water-inoculated shoots. The tubes were incubated for 3 to 4 days at 28°C, and the bacterial suspensions were collected. The protocols described above for cell lysis and PCR analysis of lysed cells were used. Several samples were excised from the same or different diseased shoots 36, 39, and 50 days after inoculation. The smallest populations necessary to induce bacterial growth in medium 2 were estimated by inoculating liquid medium with diluted cell suspensions of both pathogens.
RESULTS
Biological test for lipodepsipeptide production.
The results obtained after the yeast R. pilimanae was sprayed onto 2-day cultures are summarized in Table 1. Among the organisms tested, inhibition zones were observed only for strains of P. fuscovaginae and P. syringae pv. syringae, P. syringae pv. atrofaciens, P. syringae pv. aptata, and P. syringae pv. tabaci. The largest inhibition zones were obtained with syringotoxin-producing strains B457, Ps268, and UPB 264b. The zones of inhibition were generally larger on PGNaClA than on PDA, but the sizes of the inhibition zones varied by strain. In addition, strain CFBP 2118 of P. syringae pv. syringae, strains LMG 5143 and UPB 110 of P. syringae pv. aptata, strain LMG 5000 of P. syringae pv. atrofaciens, and strain LMG 2158 of P. fuscovaginae produced slightly larger zones on PDA than on PGNaClA. The inhibition zones obtained with P. syringae pv. tabaci LMG 5393 were very small. Two P. syringae pv. syringae strains, LMG 5494 and LMG 6104, produced zones of inhibition of R. pilimanae on PGNaClA but not on PDA. Incubation times of more than 2 days before the plates were sprayed with R. pilimanae did not result in the formation of zones of inhibition for negative strains regardless of the medium used (Table 2).
TABLE 2.
Biological tests performed on two agar media with various incubation times and test organisms
| Strain | Sizes of inhibition zones (mm)a
|
||||||
|---|---|---|---|---|---|---|---|
| PGNaClA
|
PDA
|
||||||
| Expt Ab | Expt Bc | Expt Cd | Expt Ab | Expt Bc | Expt Cd | Expt De | |
| P. syringae pv. syringae strains | |||||||
| B301D | 17.7 | 24.2 | 28.7 | 12.5 | 18.4 | 25.5 | 18.7 |
| LMG 5494 | 6.5 | 7.5 | 12.9 | 0 | 0 | 0.5f | 0 |
| LMG 5191 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| LMG 6104 | 0 | 2 | 2.7 | 0 | 0 | 0 | 0 |
| LMG 5141 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| P. syringae pv. morsprunorum LMG 2222 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
Sizes of inhibition zones between test organisms and bacteria. The values are the averages of the values from the two trials; two plates were used for each trial.
Each plate was sprayed with an R. pilimanae cell suspension after incubation for 24 h at 28°C and was incubated at 20°C after spraying.
Each plate was sprayed with an R. pilimanae cell suspension after incubation for 48 h at 28°C and was incubated at 20°C after spraying.
Each plate was sprayed with an R. pilimanae cell suspension after incubation for 4 days at 25°C and was incubated at 25°C after spraying.
Each plate was sprayed with a G. candidum spore suspension after incubation for 4 days at 25°C and was incubated at 25°C after spraying.
Irregular result (a small inhibition zone was observed on only one plate).
PCR detection of the syrD gene by using purified DNA.
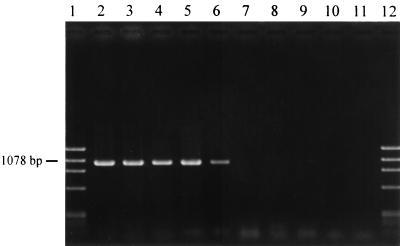
The specificity of the PCR test performed with an annealing temperature of 64°C is shown in Fig. 1. The results of the systematic search for the syrD gene in P. syringae pathovars and other species are shown in Table 1. In this study, the tests were carried out by using purified DNA and a relatively low annealing temperature, 60°C. All P. syringae pv. syringae strains in which toxins have been found (B301D, B3A, B457, Ps268, and HS191) produced an approximately 1,040-bp DNA fragment in PCR performed with primers syD1 and syD2. The same results were obtained with almost all of the strains of this pathovar, including field strains isolated in Belgium; the only exception was strain LMG 5191. The latter strain, which is not able to inhibit growth in biological tests, produced negative responses in PCR assays regardless of the DNA concentration used. Strains LMG 6104 and LMG 5494, which produced no or very weak and poorly reproducible inhibition of R. pilimanae on PDA but greater inhibition on PGNaClA, gave positive responses in PCR assays. Strain LMG 5141 was the only strain which was positive in PCR assays and negative in biological tests.
FIG. 1.
PCR products amplified with syrD primers from 1-ng portions of DNA from various phytobacteria. Program 1 was used with an annealing temperature of 64°C. Lanes 1 and 12, HaeIII-digested φX174 replicative-form DNA; lane 2, P. syringae pv. syringae B301D; lane 3, P. syringae pv. syringae P268; lane 4, P. syringae pv. syringae HS191; lane 5, P. syringae pv. atrofaciens LMG 5095; lane 6, P. syringae pv. aptata LMG 5059; lane 7, P. syringae pv. tabaci LMG 5393; lane 8, P. syringae pv. pisi LMG 5079; lane 9, P. syringae pv. morsprunorum LMG 2222; lane 10, P. syringae pv. papulans LMG 5076; lane 11, P. viridiflava LMG 2352. Products were separated on a 0.8% agarose gel.
P. syringae pv. atrofaciens LMG 5095, which is known to be syringomycin and syringopeptin producer (44), and LMG 5000 reacted like the strains of P. syringae pv. syringae in PCR assays. The same results were obtained with all of the strains of P. syringae pv. aptata tested. Despite the inhibition zones observed in the biological tests and the changes in the quantities of DNA used in the reaction mixtures, negative results were always obtained with the two P. fuscovaginae strains used in PCR tests. The Pseudomonas viridiflava pathotype strain and strains that were representatives of a series of P. syringae pathovars were negative in PCR tests, which confirmed the bioassay results obtained for toxin production except for the P. syringae pv. tabaci LMG 5393 results.
PCR detection of the syrD gene by using lysed cells.
In PCR studies of lysed cells, the use of approximately 5 × 106 and 5 × 105 lysed cells in PCR mixtures confirmed the results obtained when the syrD primers and purified DNA were used (Table 1). In the case of strains of P. syringae pv. morsprunorum that produce coronatine, the search for the syrD gene with lysed cells was carried out with the less specific PCR program, program 2, but all of the strains tested gave negative responses. A weak nonspecific amplification reaction was sometimes noticed, but there was no possible confusion with the expected 1,040-bp fragment.
PCR tests performed with diseased plant material.
Inoculation of P. syringae pv. syringae PsP2 onto cut ends of cherry twigs could cause damage in the cambium and xylem up to 70 mm from the inoculation point after 1 month. Inoculation of P. syringae pv. morsprunorum PmC36 onto cortical tissues of cherry twigs resulted in darkening of the cortical tissues in and around the inoculated area. Inoculation of liquid medium 2 with a deep-seated piece of diseased plant material obtained a small distance away from a disease lesion always gave enough bacterial growth after 3 to 4 days to carry out cell lysis.
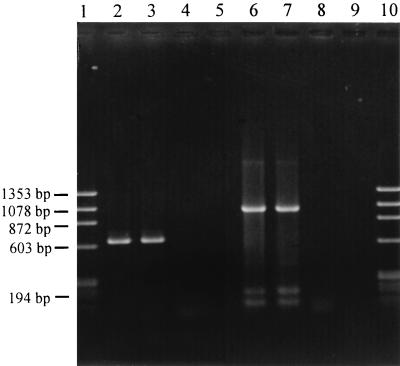
Despite a low annealing temperature and prolongation of annealing, the syrD PCR test was reliable when PCR program 2 was used, although weak nonspecific amplification was observed (Fig. 2). The adaptability of the method for detecting other phytopathogenic bacteria was demonstrated by detecting P. syringae pv. morsprunorum with the cfl gene-based PCR test. This test was specific for coronatine-producing strains under the PCR conditions described above, and conservation of the cfl gene in P. syringae pv. morsprunorum strains isolated from diseased cherry trees in Belgium was verified (unpublished results). Using PCR program 2 with both sets of primers allowed simultaneous detection of the two pathogens (Fig. 2). Multiple samples from the same twig and samples from several twigs always gave the expected results; twigs inoculated with strain PsP2 were positive in the syrD PCR test, and twigs inoculated with strain PmC36 were positive in the cfl PCR test. In some cases, bacterial growth was obtained in liquid medium inoculated with healthy tissues from control twigs; however, PCR assays of lysed cells were always negative with the syrD or cfl primers. Positive results that correctly identified the pathovars inoculated were obtained with diseased tissues for the three inoculation times investigated.
FIG. 2.
PCR products amplified with cfl primers (lanes 2 to 5) and syrD primers (lanes 6 to 9) from lysed cells grown in culture from diseased cherry twigs. Program 2 was used with an annealing temperature of 57°C. Lanes 1 and 10, HaeIII-digested φX174 replicative-form DNA; lanes 2, 3, and 9, P. syringae pv. morsprunorum PmC36-inoculated cherry twigs; lanes 4 and 8, water-treated cherry twigs; lanes 5 to 7, P. syringae pv. syringae PsP2-inoculated cherry twigs. Products were separated on a 0.8% agarose gel.
The limits of detection for both PCR procedures, which corresponded to the populations of pathogens necessary to induce bacterial growth in the liquid medium, were estimated to be less than 5 CFU.
DISCUSSION
The objective of this study was to develop rapid phytotoxin-specific tests which identified phytopathogenic strains of P. syringae pv. syringae. The virulence of P. syringae pv. syringae is largely determined by production of syringomycin and syringopeptin (18), but mutants that are not able to produce syringomycin exhibit only a 35% reduction in virulence (13, 36). Consequently, the possibility that some phytopathogenic strains could produce only one class of lipodepsipeptide was taken into account when the identification tests were developed. Thus, the choice of a syrD gene-based PCR test was justified because this gene should be conserved in all P. syringae strains that produce a lipodepsipeptide since it is necessary to produce both syringomycins and syringopeptins (13). In addition, using R. pilimanae was preferable to using G. candidum for detecting lipodepsipeptide production because the former species allowed detection of lower concentrations of each of the essential syringomycins and syringopeptins (23). These toxins are simultaneously produced by the field strains that have been investigated so far (13, 20, 44). Thus, the zones of inhibition observed in the biological tests in this study should most likely be attributed to a mixture of lipodepsipeptides, and syringomycins could play an essential role because of their higher level of toxicity for R. pilimanae than that of syringopeptins (23). Some strains which are able to produce lipodepsipeptides do not produce toxins on PDA (35). PGNaClA seems to be better suited as it allows detection of toxin-producing strains which repeatedly fail to produce toxins on PDA (Table 2). The variation observed among strains of a given pathovar in a medium favorable for toxin production (Table 1) confirms that regulation of toxin production is somewhat variable in a pathovar (15).
Amplification of a unique DNA product in the syrD-based PCR test (Fig. 1) revealed the high level of specificity of the primers used for the syrD gene. The use of different primers and PCR conditions probably explains the differences between our results and the results of a previous study (42). Similar results were obtained with P. syringae pv. syringae strains classified in three different clusters of this pathovar (B3A and B301D; Ps268 and B457; HS191) (35) and with strains of P. syringae pv. atrofaciens and P. syringae pv. aptata, including nonfluorescent variant strains UPB110 and UPB165 (Table 1). These results seem to indicate the specific and general presence of the syrD gene in lipodepsipeptide-producing strains of these pathovars, and they further validate the use of the primers to detect this gene. This is the first report of PCR identification of toxigenic strains of P. syringae pv. atrofaciens and P. syringae pv. aptata. Due to the putative function of the syrD gene (36), the results suggest that toxin secretion could be facilitated by the same protein in the three pathovars. Some heterogeneity is observed within each of the pathovars (45), and the separation of the pathovars is also still disputed (26, 30, 31, 35, 46). Maraite and Weyns (26) found differences in pathogenicity among P. syringae strains isolated from woody plants, monocots, and sugar beet, but there is still some confusion with respect to P. syringae strains from monocytes (26, 30, 43, 44, 46). However, the three pathovars are genetically closely related (25), and conservation of a gene involved in toxin production is thus not as remarkable as conservation in the case of coronatine-producing pathovars is (4, 25).
The results of the syrD-based PCR test suggest that the syrD gene is not present in P. fuscovaginae and confirm that there are differences in secretion between P. syringae and P. fuscovaginae (8). On the other hand, the similar results obtained in the biological test for the two species (Table 1) indicate the adequacy of this test for detecting lipodepsipeptide-producing strains of the two species. The two other differences observed in the two tests described above remain unexplained (Table 1). Despite detection of the syrD gene, the ability of P. syringae pv. syringae LMG 5141 to actually produce lipodepsipeptides is not known. On the other hand, the weak inhibition zones observed in the biological tests with P. syringae pv. tabaci LMG 5393 may not be attributed to lipodepsipeptide production. Strains comparable to P. syringae LMG 5191 (Table 1) should not be detected by the tests described here. However, this strain is not able to infect cherry twigs (unpublished results) and apparently is an epiphytic strain that is not able to produce syringomycin and is similar to epiphytic strains described by Quigley and Gross (35). The need for rapid tests to identify different pathogens on the same crop was mentioned in the case of cherry trees (6, 24, 35), peas (35), and rice (22). The tests described here could help discriminate P. syringae pv. syringae from P. syringae pv. morsprunorum on cherry trees, P. syringae pv. pisi on peas, and P. syringae pv. oryzae and P. fuscovaginae on rice (Table 1).
Pure cultures can generally be obtained directly from natural infections of bacterial canker occurring in cortical tissues of cherry trees. These observations led to the development of the method used to identify the two pathovars responsible for this disease with diseased cherry twigs. This method is similar to the combined biological and enzymatic amplification technique called BIO-PCR, which was able to detect the presence of the seedborne pathogen P. syringae pv. phaseolicola in bean seed water extracts (29, 38, 39). A different procedure involves inoculation of a liquid medium with a carefully selected piece of diseased vegetative tissue. The biological amplification that occurred prior to the standard PCR test avoided the presence of PCR inhibitors from plant extracts (unpublished results) and considerably improved the sensitivity of detection. Consequently, the limit of detection corresponds to the size of the population of the pathogen necessary to induce bacterial growth in the liquid medium. Attempts to directly detect resident populations in the diseased twigs by PCR were unsuccessful (unpublished results). The procedure described here allows for identification of two pathovars of P. syringae by using two different PCR tests, suggesting that such a procedure could probably be applied to other plants and pathogens.
ACKNOWLEDGMENTS
We thank D. C. Gross (Washington State University, Pullman), H. Maraite (Université Catholique de Louvain, Louvain-la-Neuve, Belgium), L. Gardan (Institut National de Recherches Agronomiques, Beaucouzé, France), and R. Penev (Fruit Growing Institute, Plovdiv, Bulgaria) for providing the strains used in this study, P. Boxus for supporting and encouraging this project, and J. M. Jacquemain and B. Watillon for helpful discussions.
This work was supported by the Ministère des Classes Moyennes et de l’Agriculture de Belgique.
REFERENCES
- 1.Ballio A, Barra D, Bossa F, Collina A, Grgurina I, Marino G, Moneti G, Paci M, Pucci P, Segre A, Simmaco M. Syringopeptins, new phytotoxic lipodepsipeptides of Pseudomonas syringae pv. syringae. FEBS Lett. 1991;291:109–112. doi: 10.1016/0014-5793(91)81115-o. [DOI] [PubMed] [Google Scholar]
- 2.Ballio A, Barra D, Bossa F, DeVay J E, Grgurina I, Iacobellis N S, Marino G, Pucci P, Simmaco M, Surico G. Multiple forms of syringomycin. Physiol Mol Plant Pathol. 1988;33:493–496. [Google Scholar]
- 3.Ballio A, Bossa F, Collina A, Gallo M, Iacobellis N S, Paci M, Pucci P, Scaloni A, Segre A, Simmaco M. Structure of syringotoxin, a bioactive metabolite of Pseudomonas syringae pv. syringae. FEBS Lett. 1990;269:377–380. doi: 10.1016/0014-5793(90)81197-v. [DOI] [PubMed] [Google Scholar]
- 4.Bereswill S, Bugert P, Völksch B, Ullrich M, Bender C L, Geider K. Identification and relatedness of coronatine-producing Pseudomonas syringae pathovars by PCR analysis and sequence determination of the amplification products. Appl Environ Microbiol. 1994;60:2924–2930. doi: 10.1128/aem.60.8.2924-2930.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bereswill S, Pahl A, Belleman P, Zeller W, Geider K. Sensitive and species-specific detection of Erwinia amylovora by polymerase chain reaction analysis. Appl Environ Microbiol. 1992;58:3522–3526. doi: 10.1128/aem.58.11.3522-3526.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burkowicz A, Rudolph K. Evaluation of pathogenicity and of cultural and biochemical tests for identification of Pseudomonas syringae pathovars syringae, morsprunorum and persicae from fruit trees. J Phytopathol. 1994;141:59–76. [Google Scholar]
- 7.Crosse J E. Bacterial canker of stone-fruits. 1. Field observations on the avenues of autumnal infection of cherry. J Hortic Sci. 1954;30:131–142. [Google Scholar]
- 8.Flamand M C, Ewbank E, Goret B, Maraite H. Production of phytotoxic lipodepsipeptides by Pseudomonas fuscovaginae. In: Rudolph K, Burr T J, Mansfield J W, Stead D, Vivian A, Von Kietzell J, editors. Pseudomonas syringae pathovars and related pathogens. London, United Kingdom: Kluwer Academic Publishers; 1997. pp. 221–226. [Google Scholar]
- 9.Flamand M C, Pelsser S, Ewbank E, Maraite H. Production of syringotoxin and other bioactive peptides by Pseudomonas fuscovaginae. Physiol Mol Plant Pathol. 1996;48:217–231. [Google Scholar]
- 10.Fukuchi N, Isogai A, Nakayama J, Suzuki A. Structure of syringotoxin B, a phytotoxin produced by citrus isolates of Pseudomonas syringae pv. syringae. Agric Biol Chem. 1990;54:3377–3379. [PubMed] [Google Scholar]
- 11.Fukuchi N, Isogai A, Nakayama J, Takayama S, Yamashita S, Suyama K, Takemoto J Y, Suzuki A. Structure and stereochemistry of three phytotoxins, syringomycin, syringotoxin and syringostatin, produced by Pseudomonas syringae pv. syringae. J Chem Soc Perkin Trans 1. 1992;1992:1149–1157. [Google Scholar]
- 12.Gardan L, Shafif H, Grimont P A D. DNA relatedness among pathovars of P. syringae and related bacteria. In: Rudolph K, Burr T J, Mansfield J W, Stead D, Vivian A, Von Kietzell J, editors. Pseudomonas syringae pathovars and related pathogens. London, United Kingdom: Kluwer Academic Publishers; 1997. pp. 445–448. [Google Scholar]
- 13.Grgurina I, Gross D C, Iacobellis N S, Lavermicocca P, Takemoto J Y. Phytotoxin production by Pseudomonas syringae pv. syringae—syringopeptin production by syr mutants defective in biosynthesis or secretion of syringomycin. FEMS Microbiol Lett. 1996;138:35–39. [Google Scholar]
- 14.Gross D C. Molecular and genetic analysis of toxin production by pathovars of Pseudomonas syringae. Annu Rev Phytopathol. 1991;29:247–278. [Google Scholar]
- 15.Gross D C, DeVay J E. Population dynamics and pathogenesis of Pseudomonas syringae in maize and cowpea in relation to the in vitro production of syringomycin. Phytopathology. 1976;67:475–483. [Google Scholar]
- 16.Gross D C, DeVay J E. Production and purification of syringomycin, a phytotoxin produced by Pseudomonas syringae. Physiol Plant Pathol. 1977;11:13–28. [Google Scholar]
- 17.Gross D C, DeVay J E, Stadtman F H. Chemical properties of syringomycin and syringotoxin: toxigenic peptides produced by Pseudomonas syringae. J Appl Bacteriol. 1977;43:453–463. [Google Scholar]
- 18.Gross D C, Hutchison M L, Scholz B K, Zhang J-H. Genetic analysis of the role of toxin production by Pseudomonas syringae pv. syringae in plant pathogenesis. In: Rudolph K, Burr T J, Mansfield J W, Stead D, Vivian A, Von Kietzell J, editors. Pseudomonas syringae pathovars and related pathogens. London, United Kingdom: Kluwer Academic Publishers; 1997. pp. 163–169. [Google Scholar]
- 19.Hutchison M L, Gross D C. Lipopeptide phytotoxins produced by Pseudomonas syringae pv. syringae: comparison of the biosurfactant and ion channel-forming activities of syringopeptin and syringomycin. Mol Plant Microbe Interact. 1997;10:347–354. doi: 10.1094/MPMI.1997.10.3.347. [DOI] [PubMed] [Google Scholar]
- 20.Iacobellis N S, Lavermicocca P, Grgurina I, Simmaco M, Ballio A. Phytotoxic properties of Pseudomonas syringae pv. syringae toxins. Physiol Mol Plant Pathol. 1992;40:107–116. [Google Scholar]
- 21.Isogai A, Fukuchi N, Yamashita S, Suyama K, Suzuki A. Structures of syringostatins A and B, novel phytotoxins produced by Pseudomonas syringae pv. syringae isolated from lilac blights. Tetrahedron Lett. 1990;31:695–698. [Google Scholar]
- 22.Kim H M, Song W Y. Characterization of ribosomal RNA intergenic spacer region of several seedborne bacterial pathogens of rice. Seed Sci Technol. 1996;24:571–580. [Google Scholar]
- 23.Lavermicocca P, Iacobellis N S, Simmaco M, Graniti A. Biological properties and spectrum of activity of Pseudomonas syringae pv. syringae toxins. Physiol Mol Plant Pathol. 1997;50:129–140. [Google Scholar]
- 24.Liang L Z, Sobiczewski P, Paterson J M, Jones A L. Variation in virulence, plasmid content, and genes for coronatine synthesis between Pseudomonas syringae pv. morsprunorum and P. s. syringae from Prunus. Plant Dis. 1994;78:389–392. [Google Scholar]
- 25.Manceau C, Horvais A. Assessment of genetic diversity among strains of Pseudomonas syringae by PCR-restriction fragment length polymorphism analysis of rRNA operons with special emphasis on P. syringae pv. tomato. Appl Environ Microbiol. 1997;63:498–505. doi: 10.1128/aem.63.2.498-505.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maraite H, Weyns J. Pseudomonas syringae pv. aptata and pv. atrofaciens, specific pathovars or members of pv. syringae. In: Rudolph K, Burr T J, Mansfield J W, Stead D, Vivian A, Von Kietzell J, editors. Pseudomonas syringae pathovars and related pathogens. London, United Kingdom: Kluwer Academic Publishers; 1997. pp. 515–520. [Google Scholar]
- 27.Mitchell R E. Coronatine production by some phytopathogenic pseudomonads. Physiol Plant Pathol. 1981;20:83–89. [Google Scholar]
- 28.Morgan M K, Chatterjee A K. Genetic organization and regulation of proteins associated with production of syringotoxin by Pseudomonas syringae pv. syringae. J Bacteriol. 1988;170:5689–5697. doi: 10.1128/jb.170.12.5689-5697.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mosquedacano G, Herreaestrella L. A simple and efficient PCR method for the specific detection of Pseudomonas syringae pv. phaseolicola in bean seeds. World J Microbiol Biotechnol. 1997;13:463–467. [Google Scholar]
- 30.Otta J D. Pseudomonas syringae incites a leaf necrosis on spring and winter wheats in South Dakota. Plant Dis Rep. 1974;58:1061–1064. [Google Scholar]
- 31.Otta J D, English H. Serology and pathology of Pseudomonas syringae. Phytopathology. 1971;61:443–452. [Google Scholar]
- 32.Paterson J M, Jones A L. Detection of Pseudomonas syringae pv. morsprunorum on cherries in Michigan with a DNA hybridization probe. Plant Dis. 1991;75:893–896. [Google Scholar]
- 33.Pitcher D G, Saunders N A, Owen R J. Rapid extraction of bacterial genomic DNA with guanidium thiocyanate. Letters Appl Microbiol. 1989;8:151–156. [Google Scholar]
- 34.Prossen D, Hatziloukas E, Schaad N W, Panopoulos N J. Specific detection of Pseudomonas syringae pv. phaseolicola DNA in bean seed by polymerase chain reaction-based amplification of a phaseolotoxin gene region. Phytopathology. 1993;83:965–970. [Google Scholar]
- 35.Quigley N B, Gross D C. Syringomycin production among strains of Pseudomonas syringae pv. syringae: conservation of the syrB and syrD genes and activation of phytotoxin production by plant signal molecules. Mol Plant Microbe Interact. 1994;7:78–90. doi: 10.1094/mpmi-7-0078. [DOI] [PubMed] [Google Scholar]
- 36.Quigley N B, Mo Y Y, Gross D C. syrD is required for syringomycin production by Pseudomonas syringae pathovar syringae and is related to a family of ATP-binding secretion proteins. Mol Microbiol. 1993;9:787–801. doi: 10.1111/j.1365-2958.1993.tb01738.x. [DOI] [PubMed] [Google Scholar]
- 37.Schaad N W, Azad H, Peet R C, Panopoulos N J. Identification of Pseudomonas syringae pv. phaseolicola by a DNA hybridization probe. Phytopathology. 1989;79:903–907. [Google Scholar]
- 38.Schaad N W, Cheong S S, Tamaki S, Hatziloukas E, Panopoulos N J. A combined biological and enzymatic amplification (BIO-PCR) technique to detect Pseudomonas syringae pv. phaseolicola in bean seed extracts. Phytopathology. 1995;85:243–248. [Google Scholar]
- 39.Schaad N W, Hatziloukas E, Panopoulos N J. Detection of Pseudomonas syringae pv. phaseolicola in agroecosystems using BIO-PCR. In: Rudolph K, Burr T J, Mansfield J W, Stead D, Vivian A, Von Kietzell J, editors. Pseudomonas syringae pathovars and related pathogens. London, United Kingdom: Kluwer Academic Publishers; 1997. pp. 449–452. [Google Scholar]
- 40.Scheck H J, Canfield M L, Pscheidt J W, Moore L W. Rapid evaluation of pathogenicity in Pseudomonas syringae pv. syringae with a lilac tissue culture bioassay and syringomycin DNA probes. Plant Dis. 1997;81:905–910. doi: 10.1094/PDIS.1997.81.8.905. [DOI] [PubMed] [Google Scholar]
- 41.Segre A, Bachmann R C, Ballio A, Bossa F, Grgurina I, Iacobellis N S, Marino G, Pucci P, Simmaco M, Takemoto J Y. The structure of syringomycins A1, E and G. FEBS Lett. 1989;255:27–31. doi: 10.1016/0014-5793(89)81054-3. [DOI] [PubMed] [Google Scholar]
- 42.Sorensen K N, Kim K-H, Takemoto J Y. PCR detection of cyclic lipodepsinonapeptide-producing Pseudomonas syringae pv. syringae and similarity of strains. Appl Environ Microbiol. 1998;64:226–230. doi: 10.1128/aem.64.1.226-230.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Toben H, Mavridis A, Rudolph K W E. Basal glume rot (Pseudomonas syringae pv. atrofaciens) on wheat and barley in FRG and resistance screening of wheat. EPPO (Eur Mediterr Plant Prot Org) Bull. 1989;19:119–125. [Google Scholar]
- 44.Vassilev V, Lavermicocca P, Di Giorgio D, Iacobellis N S. Production of syringomycins and syringopeptins by Pseudomonas syringae pv. atrofaciens. Plant Pathol. 1996;45:316–322. [Google Scholar]
- 45.Weingart H, Völksch B. Genetic fingerprinting of Pseudomonas syringae pathovars using ERIC-, REP-, and IS50-PCR. J Phytopathol. 1997;145:339–345. [Google Scholar]
- 46.Wilkie J P. Basal glume rot of wheat in New Zealand. N Z J Agric Res. 1973;16:155–160. [Google Scholar]
- 47.Young J M. Pathogenicity and identification of the lilac pathogen, Pseudomonas syringae pv. syringae van Hall 1902. Ann Appl Biol. 1991;118:283–298. [Google Scholar]
- 48.Zhang J H, Quigley N B, Gross D C. Analysis of the syrB and syrC genes of Pseudomonas syringae pv. syringae indicates that syringomycin is synthesized by a thiotemplate mechanism. J Bacteriol. 1995;177:4009–4020. doi: 10.1128/jb.177.14.4009-4020.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang J H, Quigley N B, Gross D C. Analysis of the syrP gene, which regulates syringomycin synthesis by Pseudomonas syringae pv. syringae. Appl Environ Microbiol. 1997;63:2771–2778. doi: 10.1128/aem.63.7.2771-2778.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]