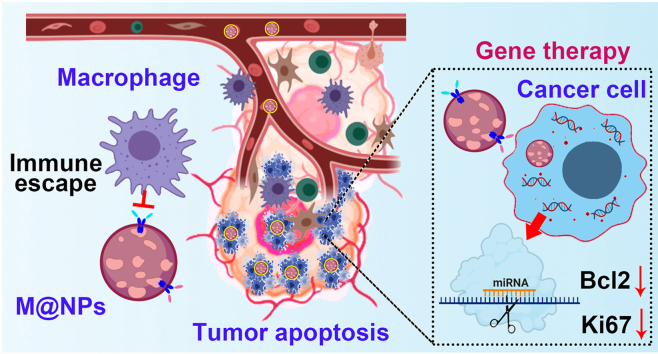
Abstract
Colon cancer is one of the most common gastrointestinal tumors in the world. Currently, the commonly used methods such as radiotherapy, chemotherapy and drug treatments are often ineffective and have significant side effects. Here we developed a safe and efficient biomaterials based anti-tumor nanoplatform (M@NPs/miR365), which was formed with poly (citrate-peptide) (PCP), miRNA365 mimic and MC38 cancer cell membrane (M). PCP could efficiently deliver miR365 mimic into MC38 cancer cells, promote the apoptosis of MC38 tumor cells and regulate the expression of Bcl2 and Ki67 in vitro. Tumor cell membranes were prepared by a fast and convenient sonication method. This tumor cell membrane-coated drug delivery system M@NPs can effectively reduce macrophage uptake and increase the stability of NPs. And the MC38 tumor model mice experiment showed that M@NPs/miR365 via caudal vein injection effectively inhibit tumor development. Based on the immune escape and homologous targeting of cancer cells and efficient gene transfection ability of NPs, this “Trojan horse” like “Pseudotumor cell” carries the target gene miR365 mimic to the tumor site and realizes cancer therapy. Noteworthy, the drug delivery system has good biocompatibility. Thus, this safe drug delivery strategy mediated by cancer cell membrane and gene therapy may have a certain significance for reducing the gap between nanoplatform and tumor clinical treatment.
Keywords: Bioactive biomaterials, Polycitrate, Gene delivery, Pseudotumor cell, Colon cancer therapy
Graphical abstract
1. Introduction
Colon cancer is one of the most common gastrointestinal tumors in the world. Although colon cancer incidence ranks the fourth highest after lung cancer, breast cancer and prostate cancer [1], its mortality rate is second behind lung cancer in America [2]. Early tumors can be removed by surgery, but it is almost impossible to completely remove tumor cells by surgery. However, with the further development of tumors, a combination of radiotherapy, chemotherapy, and drug therapy is used in order to alleviate or achieve the purpose of tumor treatment. Due to the indiscriminate mode of action of radiation between cancer tissue and normal tissue, such therapy will cause serious side effects and often lead to low survival rate [3]. Therefore, it is urgent to develop a safe and effective new strategy for the treatment of colon cancer.
As one of the branches of gene drugs, large numbers of studies have shown that microRNA (miRNA) plays an important role in tumor tissues [4,5]. Unlike gene editing and gene knockout, miRNA mediated gene interference does not cause genomic changes [6,7]. Meanwhile, miRNAs bind to target genes through base pairing, which makes them have the advantages of strong targeting and low side effects in regulating abnormal gene expression [8,9]. And regulate miRNA expression showed great value in tumor therapy [10,11]. A large number of studies have shown that miR365 worked as a tumor suppressor gene in a variety of tumors, such as lung cancer, liver cancer, breast cancer and colon cancer. It can suppress the metastasis and proliferation of tumor cells, or promote the apoptosis of tumor cells [[12], [13], [14], [15]]. Therefore, we speculate that regulating miR365 expression may be a potential breakthrough in the treatment of colon cancer.
However, due to the complex microenvironment, how to achieve miRNA delivery in vivo has become an important obstacle to its clinical application. Although some viral vectors application has made great progress in the field of gene therapy strategies, these methods still need to be further optimized due to their safety [16]. With the development of nanomaterials, nanoscale gene vectors have brought important changes to tumor treatment [17]. By optimizing the size, shape and surface charge, nanoparticles are endowed with higher miRNA delivery efficiency [18,19]. Although nanoscale gene vectors have made great progress in cancer diagnosis and treatment in recent decades years, a variety of biological barriers such as biocompatibility, low targeting, systemic blood circulation and immune cell removal still limit their efficient application in vivo cancer therapy [20].
To overcome these challenges and reduce the gap between nanoplatform and clinical application, the natural biological cell membranes coated nanoparticles that not only has the physical and chemical properties of nanomaterials, but also possesses the advantages of homologous cells have attracted more and more attention [[21], [22], [23], [24]]. Studies have showed that cancer cells have immune escape ability due to cancer associated antigens and immune adjuvants on the surface of the membrane [[25], [26], [27]], and homologous targeting ability [28,29]. During the progression of cancer, cancer cells may escape from the immune system through various mechanisms. One of the mechanisms is that some antigens on the surface of the cancer cell membrane can escape the recognition of immune cells. Such as Cluster of Differentiation 47 (CD47) protein, which is located in the membrane of both healthy and cancer cell, delivering a “don't eat me” signal to the Signal-regulatory protein alpha (SIRPα) receptor on macrophages, monocytes, neutrophils and dendritic cells [30,31]. Drugs with immune escape that designed against the CD47-SIRPα immune checkpoint have been used in clinical practice [[32], [33], [34]]. And previous studies have demonstrated that biomimetic nanoparticles coated with cancer cell membranes can inherit the homologous targeting properties [28] of cancer cells due to the homologous targeting related protein galectin-3 on the cancer cell membrane [35,36]. This allowed the nanoparticles that modified by cancer cell membranes to maximize their anti-tumor effects in vivo.
On the other hand, polycitrate biomedical polymer has shown special advantages in biomedical applications including facile synthesis, bioactive components, high biocompatibility and good biodegradation, which has been approved by FDA in 2021. To extend the biomedical applications, our group developed various functionalized polycitrate and demonstrated their applications in tissue regeneration and cancer therapy [37,38]. In recent years, we found that the ε-poly-L-lysine polypeptide-functionalized poly (citrate-peptide) (PCP) could efficiently load miRNA and show the high intracellular delivery, while presenting good biocompatibility [8]. It is very promising to use PCP as the bioactive vector for targeted cancer gene therapy.
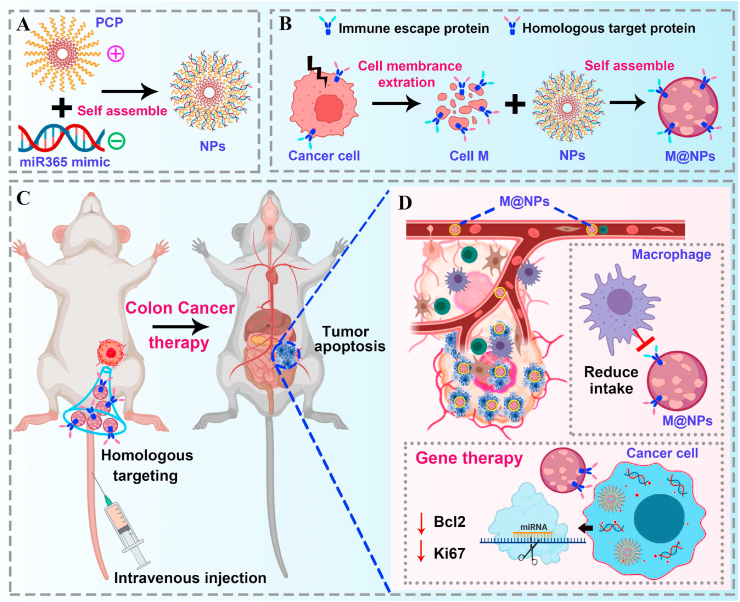
In this paper, we report a PCP/miRNA365 delivery nanoplatform (NPs) camouflaged by MC38 colon cancer cell membrane for targeted colon cancer therapy. This “Trojan horse” like “Pseudotumor cell” carries the target gene miR365 mimic to the tumor site and realizes targeted delivery based on the homologous targeting of tumor cells and immune escape. As showed in Scheme 1, NPs/miR365 (NPs) was self-assembled by miR365 mimic and PCP, which have been previously proven to have efficient gene delivery capabilities and biosafety [8]. Then, the cancer cell membrane prepared by ultrasonic crushing method was encapsulated onto NPs to form M@NPs. Based on the bionic characteristics of the cancer cell membrane, M@NPs could reach the tumor site after caudal vein injection to realize target gene therapy in tumor tissue.
Scheme 1.
Schematically describe the synthesis of M@NPs and the application of M@NPs/miR365 in colon cancer therapy. A) Positively charged PCP and negatively charged miRNA mimic self assembled into NPs. B) The cancer cell membrane fragments prepared by ultrasonic crushing method was encapsulated onto NPs to form M@NPs. C) M@NPs reach the tumor site after caudal vein injection to realize target gene therapy in tumor tissue. D) The molecular mechanism of tumor therapy of M@NPs.
2. Results
2.1. MiRNA365 associated with colon cancer cells proliferation and apoptosis
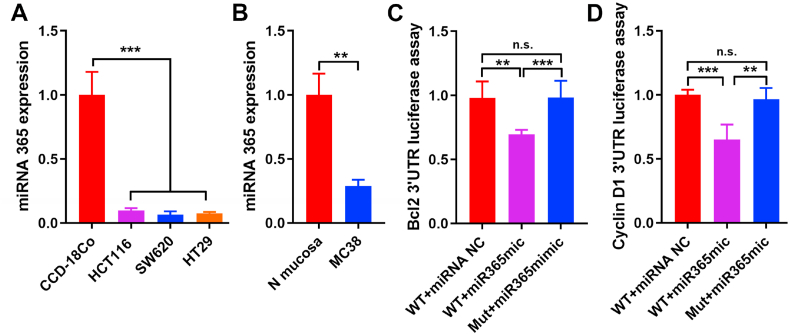
In order to confirm the miRNA365 expression level in different cell lines, we separately tested the miR365 expression in human (HCT116, SW620 and HT29) and murine (MC38) colon cancer cell lines. The results showed that, compared with normal colon tissue cells, the expression of miRNA365 in colon cancer cell lines was significantly reduced (Fig. 1A and B), regardless of whether it was in human (CCD-18Co) or murine (Normal mucosa) cells. These results suggested that the low expression of miRNA365 may be related to the occurrence of colon cancer. One of the main reasons for the lethality of malignant tumors is the high proliferation and anti-apoptosis ability of cancer cells, which is significantly different from healthy cells. Therefore, we speculated that the expression of miRNA365 is related to cell proliferation gene Cyclin D1 and apoptosis gene Bcl2. And our results of dual-luciferase reporter assay showed that both Bcl2 (Fig. 1C) and Cyclin D1 (Fig. 1D) were the target genes of miRNA365. These results suggested that miRNA365 is a potential target for the treatment of colon cancer.
Fig. 1.
MiRNA365 associated with colon cancer cell proliferation and apoptosis. A) The expression of miR365 in human normal colon tissue cell (CCD-18Co) and human colon cancer cell lines (HCT116, SW620 and HT29). B) MiR365 expression in murine normal colon tissue cell (N-mucosa) and murine colon cancer cell line (MC38). C-D) Dual-luciferase reporter assay of the role of miRNA365 on Bcl2 and cyclin D1. n. s. (no significance). ∗∗P < 0.01, ∗∗∗P < 0.001 (n = 3).
2.2. Biological function evaluation of PCP/miR365 nanocomposites
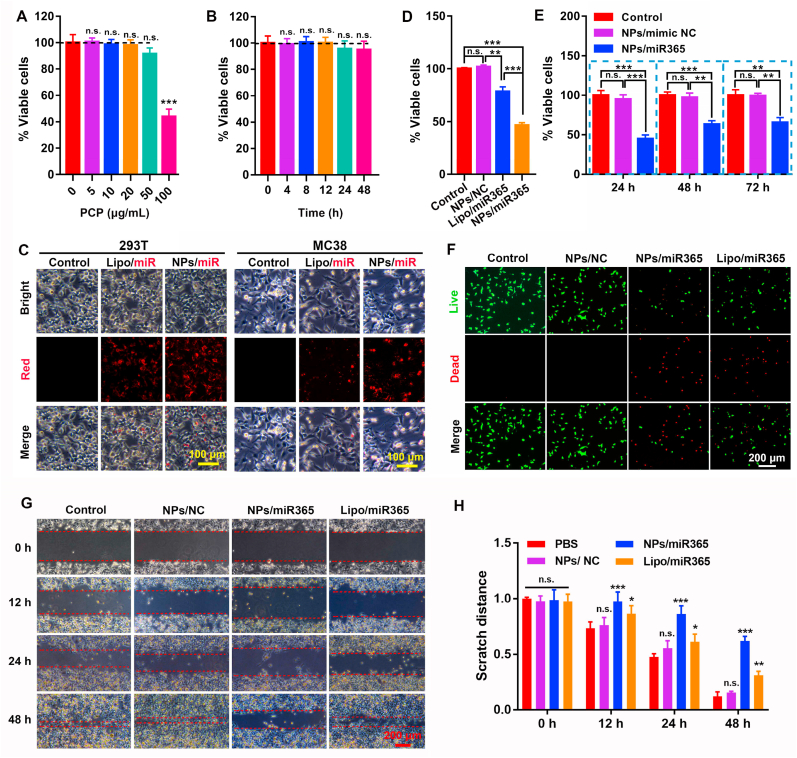
Our previous study showed that PCP has the good biocompatibility in mammalian cells [8]. In order to detect the cytocompatibility of PCP in cancer cells (MC38), the cytotoxicity of different concentrations of PCP (0–100 μg mL−1) and different cell treatment time at the same concentration (50 μg mL−1) were measured. The results showed that PCP showed good cytocompatibility when the concentration was lower than 50 μg mL−1 (Fig. 2A and B). In addition, our results also demonstrated that different concentrations of PCP (0–100 μg mL−1) was safe and non-toxic to L929 cells and intestinal epithelial cells NCM460 (Figure S1).
Fig. 2.
The cytotoxicity and biological function of PCP and NPs/miR365 nanocomplex. A) Cytotoxicity of PCP on MC38 cells at different concentrations (all data was compared with PCP at 0 μg mL−1 concentration). B) Cytotoxicity of 50 μg mL−1 of PCP on MC38 cells at different time points (compared with 0 h treatment). C) Cells uptake of NPs/miRNA in 293 T and MC38 cells. D) Inhibitory effect of NPs/miR365 on MC38 cells proliferation after 24 h treatment. PCP mediated miRNA mimic control (NPs/NC) and commercial lipofectamine 2000 mediated miR365 delivery (Lipo/miR365) were used as controls. E) The effects of NPs/miR365 on the proliferation of MC38 cells after 24 h, 48 h and 72 h treatment respectively. F) Live/dead staining of MC38 cells after 24 h treatment of NPs/miR365. G) Cell scratch images at 0, 12, 24 and 48 h were recorded using a microscope. H) Statistical analysis of scratch distance. n. s. (no significance). ∗P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.001 (n = 3).
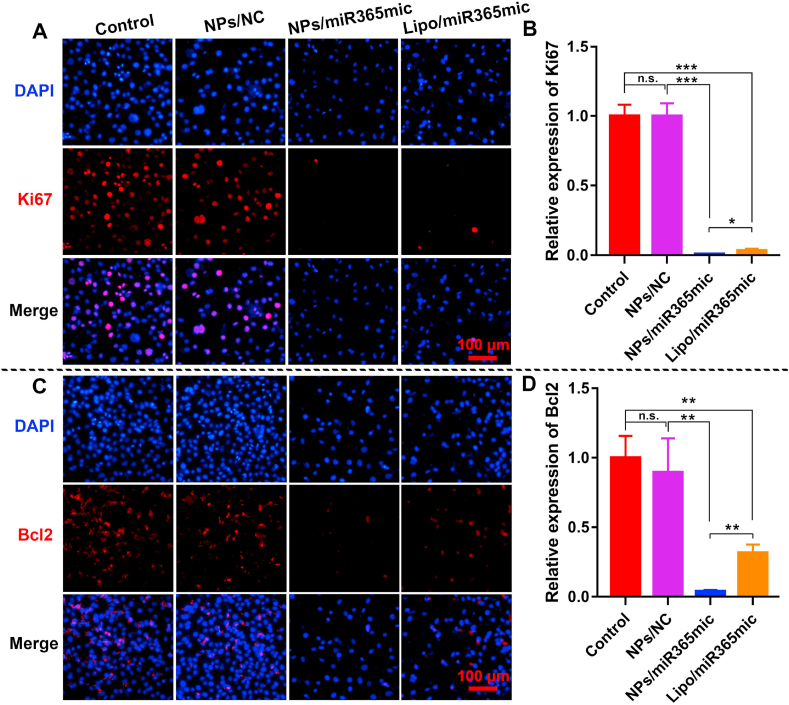
In order to detect the optimal binding ratio between PCP (10 μg μL−1) and miRNA (20 μM)), we assembled PCP with miRNA in different volume ratios. The results of agarose gel electrophoresis showed that when the volume ratio of PCP to miRNA was 4:1, they could be perfectly combined (Figure S2). NPs/cy3miR365 that self-assembled by Cy3 labeled miRNA365 and PCP was used to test the miRNA delivery ability (cells uptake ability). Compared with negative control and commercial lipofectamine 2000, PCP showed better miRNA delivery ability both in 293 T and MC38 cells (Fig. 2C). Subsequently, the inhibitory effect of NPs/miR365 on MC38 cells proliferation after 24 h treatment was performed. Compared with the PCP mediated miRNA mimic control (NPs/NC) and/or commercial lipofectamine 2000 mediated miR365 (Lipo/miR365) groups, NPs/miR365 showed excellent ability to inhibit cell proliferation (Fig. 2D and E). The result of Live/Dead staining showed that NPs/miR365 could promote the cell death of MC38 cells (Fig. 2F). The number of MC38 cells in the NPs/miR365 treatment group was significantly reduced compared to the control groups (Figure S3). The results of flow cytometry also showed that NPs/miR365 significantly promoted tumor cells apoptosis (Figure S4). Compared with the control groups, the cell scratch test showed that NPs/miR365 could effectively inhibit the migration of tumor cells in vitro (Fig. 2G and H). Moreover, the immunofluorescence results showed that PCP mediated miR365 delivery could effectively inhibit the expression of Ki67 and Bcl2 proteins (Fig. 3). In addition, the Live/Dead staining results also showed that NPs/miR365 was safe to intestinal epithelial cells NCM460 (Figure S5). These results indicated that regulating the expression of miR365 has potential application value for tumor therapy.
Fig. 3.
NPs/miR365 efficiently inhibited the expression of Ki67 and Bcl2 in MC38 tumor cells. A) Immunofluorescence staining of Ki67 after NPs/miR365 treatment in MC38 cells. B) Quantitative analysis of Ki67 fluorescence. C) Immunofluorescence staining of Bcl2 in MC38 cells. D) Quantitative analysis of Bcl2 fluorescence. Data was expressed as mean ± SD. n. s. (no significance). ∗P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.001 (n = 3).
2.3. Characterization of tumor cell membrane fragments and M@NPs
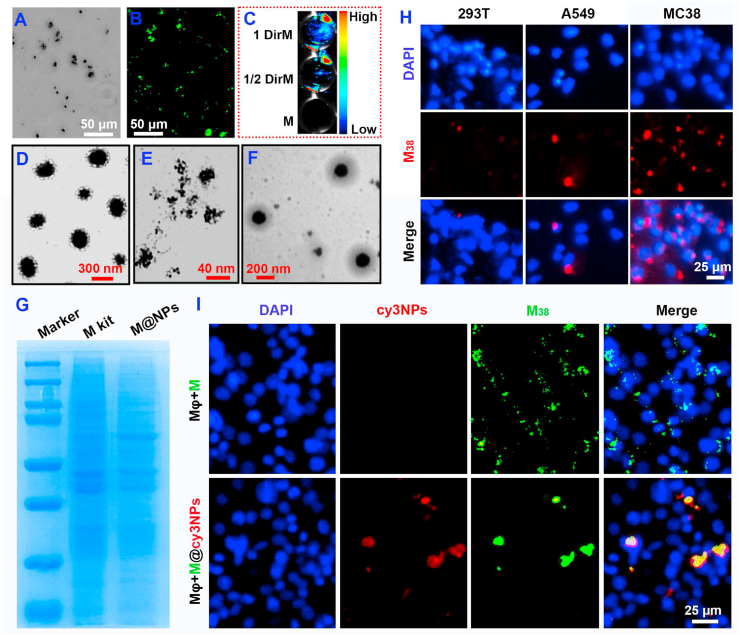
In order to visually demonstrate the formation of cancer cell membrane fragments (M), fluorescent dyes DiO (green dye, 484/501) and Dir (near-infrared fluorescent dye, 748/780) were used to label the cell membrane, respectively. Cell membrane fragments (M) (Fig. 4A), DiO labeled DiOM (Fig. 4B) and the Dir labeled DirM (Fig. 4C) significant fluorescence signal compared with the unlabeled M. The TEM results of NPs/miR365 (Fig. 4D), M (Fig. 4E) and M@NPs/miR365 (Fig. 4F) showed that the cell membrane coated NPs was successfully prepared. The average size of PCP/miR365 (NPs) and M@NPs/miR365 (M@NPs) were ∼255 nm and ∼295 nm, respectively (Figure S6). And the UV spectra results showed that NPs and M@NPs have the similar absorption peaks (Figure S7). The stability of NPs and M@NPs were analyzed by the 10% FBS buffer. The prepared NPs and M@NPs were treated with 10% FBS buffer at 37 °C for 2 h, 4 h and 6 h, respectively. Their stability was checked by agarose gel electrophoresis (Figure S8). The results showed that both NPs and M@NPs began to release miRNA slowly after 2 h of FBS treatment. After 4 h of FBS treatment, miRNAs in NPs were released in large quantities. And after 6 h, the miRNAs in NPs were almost completely released and degraded. However, a large number of miRNAs were still detected in M@NPs at 6 h. This indicated that the stability of M@NPs is greatly improved compared with NPs, and it can effectively release miRNAs.
Fig. 4.
Characterization of M38 tumor cell membrane fragments (M) and M@NPs. A) Morphology of M38 under microscope. B) The morphology of DiOM under fluorescence microscope. C) Dir near-infrared fluorescent labeled DirM. D-F) TEM of NPs, M and M@NPs. G) Coomassie Blue Staining of cell mmbrance (kit method) and M@NPs. H) Uptake of red labeled M38 by different cells. I) Immune escape ability of M38 coated nanoparticles to macrophages.
To further prove the presence and function of M@NPs, a membrane protein extraction kit (P0033, Beyotime) was used as a control. The results of Coomassie Blue Staining showed that M@NPs was successfully coated with a functionalized cell membrane (Fig. 4G). To detect the homologous targeting of cancer cells, the DiI labeled MC38 cell membrane fragments (M38) were separately added into 293 T, A549 and MC38 cells. The results showed that MC38 cells were more likely to absorb more M38 cell membrane fragments than the control groups (Fig. 4H and S9). We also tested the immune escape ability of cancer cell membrane coated nanoparticles to macrophages. M38@cy3NPs prepared from cy3 labeled NPs and DiO labeled M38 membrane was added into macrophages. Non-cellular structure M38 fragments with very small size were removed by macrophages as foreign matters, but “Trojan horse” like “pseudotumor cell” are more likely to escape the phagocytosis of macrophages (Fig. 4I).
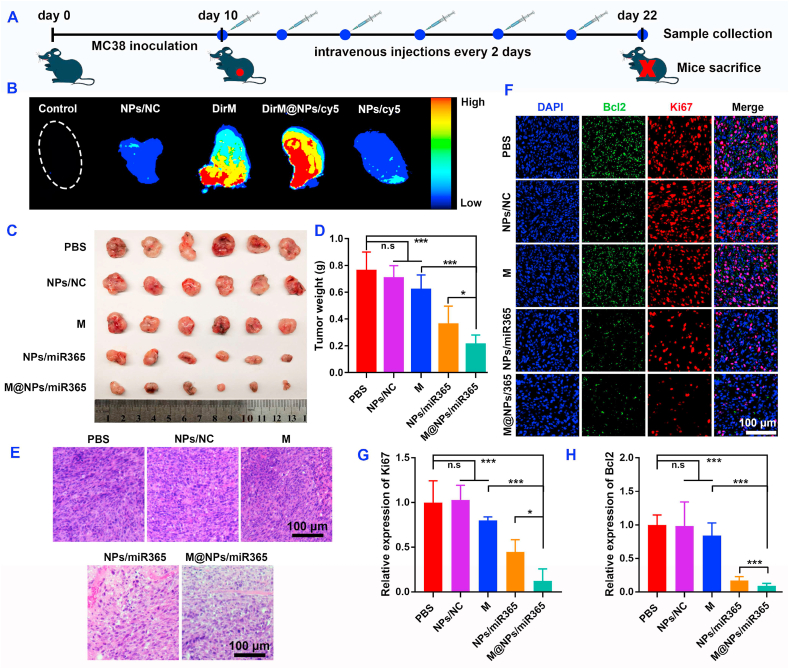
Fig. 5A showed the timeline of animal experiments. After two consecutive intravenous injections (10 h per time), the tumor tissues (Fig. 5B) and main organs (Figure S10) of mice were collected and the fluorescence signal was measured by Odyssey CLX imaging system. Compared with the control groups, fluorescence signal intensity in the DirM@NPs/cy5miR group was the highest. Compared with the DirM and NPs/cy5 group, the stronger fluorescence signal in DirM@NPs/cy5miR indicated that the cancer cell membrane fragments improved the tumor targeting of the NPs. Since the material will eventually be excreted from the body through renal or hepatic metabolism, a large number of fluorescent signals are also detected in the kidney and liver (Figure S10).
Fig. 5.
M@NPs/miR365 effectively improved the therapeutic effect of tumor. A) Timeline of animal experiment. B) Representative ex vivo fluorescence image of tumor after intravenous injection of NPs/NC (PCP with negative control miRNA), DirM (Dir near-infrared fluorescent labeled cell membrane), NPs/cy5 (PCP with Cy5 fluorescent labeled miR365) and DirM@NPs/cy5 (DirM encapsulated NPs/cy5) (n = 4). C) Tumor size. D) Weight statistics of tumor tissue (n = 6). E) Representative photos of H&E staining of tumor tissue. F) Immunofluorescence staining of Bcl2 and Ki67 in tumor tissues. G-H) Quantitative analysis of Ki67 and Bcl2 fluorescence (n = 4). n. s (no significance). ∗P < 0.05, ∗∗∗P < 0.001.
In order to evaluate the effect of M@NPs/miR365 on tumor therapy in vivo, mice with subcutaneous tumors prepared from MC38 cells were randomly divided into PBS, NPs/NC, M, NPs/miR365 and M@NPs/miR365 groups. The timeline of animal experiment was showed in Fig. 5A. After intravenous injection of M@NPs every two days for 6 times, mice blood and tumor were collected. M@NPs could effectively inhibit the tumor growth (Fig. 5C–D and S11). The result of H&E staining (Fig. 5E) showed that the cancer cell density in M@NPs/miR365 group decreased significantly compared with the control groups (PBS and NPs/NC group). And less expression of Ki67 and Bcl2 in the immunofluorescence staining (Fig. 5F) suggested that M@NPs/miR365 inhibit the occurrence and development of tumors. The quantitative analysis of Ki67 and Bcl2 was shown in Fig. 5G and H. Compared with NPs/miR365 group, M@NPs/miR365 that mediated by cancer cell membrane fragments showed a better effect in inhibiting the expression of Ki67 and Bcl2. The above results indicated that PCP mediated miR365 can effectively resist tumor progress. And the tumor cell membrane fragments-coated M@NPs/miR365 showed a better effect.
2.4. Biocompatibility evaluation of M@NPs in vivo
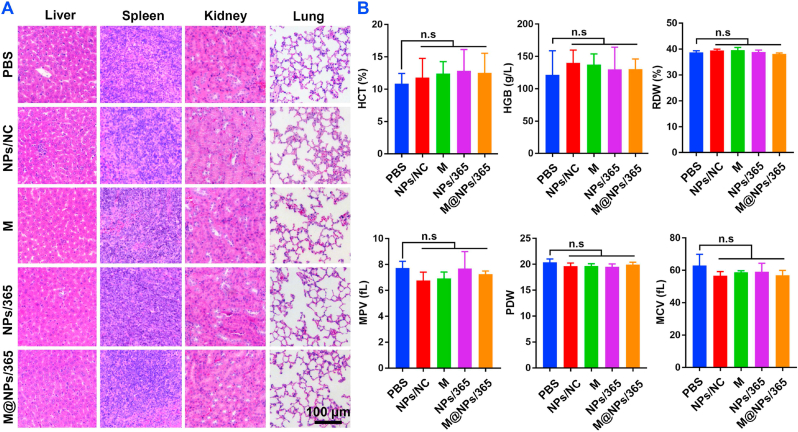
Although PCP showed excellent cytocompatibility in vitro, we still need to test the safety of NPs or M@NPs in tumor treatment in vivo. After intravenous injections of NPs or M@NPs/miR365 every two days for 6 times, the blood and main organ such as lung, liver, spleen and kidney were collected for blood chemistry test and H&E staining to evaluate the toxicity of M@NPs in vivo. Compared with the control group, no obvious difference in tissue integrity and cell structure was observed in the experimental groups (Fig. 6A). Besides, some blood routine indexes, such as HCT (Hematocrit), HGB (Hemoglobin), RDW (Red cell volume distribution width), MPV (Mean platelet volume), PDW (Platelet distribution width) and MCV (Mean red blood cell volume) in all groups showed no significant difference (Fig. 6B). These results suggested that NPs/miR365 or M@NPs/miR365 was safe in tumor treatment.
Fig. 6.
Evaluated the biocompatibility of M@NPs/miR365 in vivo. After intravenous injections of M@NPs/miR365 every two days for 6 times, blood and main organ such as lung, liver, spleen and kidney were collected for blood chemistry test and H&E staining to evaluate the toxicity of M@NPs in vivo. A) H&E staining of liver, spleen, kidney and lung (n = 5). B) Hematological toxicity test. HCT (Hematocrit), HGB (Hemoglobin), RDW (Red cell volume distribution width), MPV (Mean platelet volume), PDW (Platelet distribution width) and MCV (Mean red blood cell volume). n. s (no significance) (n = 4).
3. Discussion
Numbers of studies showed that miR365 worked as a tumor suppressor gene in a variety of tumors, such as lung cancer, liver cancer, breast cancer and colon cancer [[12], [13], [14], [15]]. It can suppress the metastasis and proliferation of tumor cells, or promote the apoptosis of tumor cells. In order to examine the relationship between miRNA365 and colon cancer, we separately detected the expression of miR365 in human colon cancer cell lines and murine colon cancer cell lines. The results showed that miR365 in cancer cells was significantly lower than that in normal colon tissue cells (Fig. 1A and B). And the results of dual-luciferase reporter assay showed that Bcl2 and Cyclin D1 was one of the target genes of miR365 (Fig. 1C). This result was consistent with previous study [15]. After transfected miR365 mimic into MC38 colon cancer cells, the results of CCK8, Live/Dead staining and Bcl2 and Ki67 immunofluorescence showed that miR365 can inhibit the proliferation of tumor cells and promote their apoptosis in vitro (Fig. 2, Fig. 3 and S3–S4). Therefore, regulating the expression of miR365 by gene therapy may be a potential breakthrough in the treatment of colon cancer.
The critical issue needs to be solved in the application of gene therapy in vivo is how to realize gene delivery and reduce the clearance of biological barrier such as enzyme degradation, systemic blood circulation and immune cell removal [20]. Although some virus mediated gene delivery strategies in vivo has made some progress, their application is limited due to the safety [16]. With the development of nanobiomaterials, nanomedicine has brought important changes to tumor treatment [17]. By optimizing the size, shape and surface charge, nanoparticles are endowed with higher miRNA delivery efficiency [18,19]. We previously proven that PCP have efficient gene delivery capabilities and biosafety both in vitro and in vivo [8]. Moreover, miR365 mimic mediated by PCP showed higher tumor proliferation inhibition and cell apoptosis efficiency than commercial lipofectamine 2000 in vitro (Fig. 2, Fig. 3).
Undoubtedly, although nanomedicine has made great progress in cancer diagnosis and treatment, a variety of biological barriers such as low targeting and immune cell removal are still a challenge in vivo [39,40]. More and more studies have shown that nanoparticles encapsulated with natural biological cell membranes can have the advantages of homologous cells, which has attracted more and more attention [22,23,41,42]. Cancer cells may escape from the immune system due to some antigens on the surface of the cancer cell membrane. Such as Cluster of Differentiation 47 (CD47) protein, which is located in the membrane of both healthy and cancer cell, delivering a “don't eat me” signal to the Signal-regulatory protein alpha (SIRPα) receptor on macrophages, monocytes, neutrophils and dendritic cells [30,31]. And previous studies have demonstrated that biomimetic nanoparticles coated with cancer cell membranes can inherit the homologous targeting properties [28] of cancer cells due to the homologous targeting related protein galectin-3 on the cancer cell membrane [35,36].
In this study, ultrasonication was used to prepare cell membrane fragments. Compared with the kit method, this method has the advantages of simple operation, time saving and low cost. And the results of Coomassie Blue Staining showed that the cell membrane prepared by ultrasonic method had the same protein bands as the cell membrane prepared by kit (Fig. 4G). Here, a bioactive nanoplatform camouflaged by MC38 colon cancer cell membrane with immune escape and tumor tissue targeting for colon cancer gene therapy was constructed. This “Trojan horse " like “pseudotumor cell” M@NPs could carry the target gene miR365 mimic to the tumor site and realizes targeted delivery based on the homologous targeting of tumor cells [28,29]. DiI labeled MC38 cell membrane fragments (M38) were added to 293 T cells, A549 and MC38 cells respectively to detect the uptake of M38 fragments by different type cells. The results showed that M38 fragments prepared by MC38 cell membrane were easier to fuse with homologous MC38 cells (Fig. 4H). Meanwhile, M38 fragments and M38 fragments coated M@NPs could escape the phagocytosis from macrophages. Non-cellular structure M38 fragments with very small size were removed by macrophages as foreign matters, but “Trojan horse” like “pseudotumor cell” are more likely to escape the phagocytosis of macrophages. This may be because cells size of " pseudotumor cells” have immune escape ability due to cancer associated antigens and immune adjuvants on the surface of the membrane [[25], [26], [27]]. The further in vivo cancer gene therapy showed that M@NPs could efficiently deliver miRNA365 to the tumor tissue after intravenous injection. After continuous intravenous administration, tumors tissue size in NPs/miR365 and M@NPs/miR365 groups were significantly inhibited. The H&E staining of lung, liver, spleen and kidney and blood chemistry test results also showed that M@NPs/miR365 mediated gene therapy was safe in tumor treatment.
Although the M@NPs/miR365 has not achieved complete removal of tumor tissue, it has shown excellent effect only by relying on a single tumor treatment strategy (gene therapy). The possible reasons were as follows: 1) Considering the ethical factors of control mice, the administration times were relatively short. Longer administration may demonstrate better tumor clearance; 2) RNA interference technology is only an artificial compensatory supplement to miRNA, but does not completely modify the abnormal gene expression. This compensatory method of administration may require longer treatment; 3) As RNA interference technology is relatively mild and safe for individuals, it is worth considering giving a relatively long time of administration for complete removal of tumor tissues in the future research or clinical applications. Several limitations in this study can be done better in the future. First of all, whether this gene therapy can be used for long-term anti-tumor therapy and its safety needs to be verified by more comprehensive tests. Second, although miRNA mimics have been used in many preclinical studies [[43], [44], [45], [46]], the control of drug concentration and dosing frequency deserves further study.
4. Conclusion
In conclusion, as a gene delivery system for cancer therapy, colon cancer cell membrane coated PCP/miRNA365 nanocomplex could efficiently enter the cancer cells, and enhance the tumor cell apoptosis by inhibiting the Ki67/Bcl2 expression, target the tumor cells and tumor tissue in vitro/in vivo. The M@NPs/miR365 drug delivery strategy could effectively inhibit the progression of tumor tissue and exhibits non-toxic side effects in mice. This drug delivery strategy may have a certain significance for reducing the gap between nanoparticles platform and tumor clinical treatment.
5. Materials and methods
5.1. miRNA365 expression in colon cancer cell lines
Human (HCT116, SW620 and HT29) and murine (MC38) colon cancer cell lines (ATCC) were used for miRNA extraction. And the human colon tissue cells CCD-18Co and mice normal mucosa (N-mucosa) were used as control. The expression of miRNA365 was measured by Bulge-loopTM miRNA qRT-PCR Primer Set (Business secrecy) that bought from RiboBio (RiboBio, Guangzhou, China). The U6 gene was used as an internal gene. QPCR reaction was performed by using SYBR green mix (NovoStart, E096) and the 2−ΔΔCt method was used for data analysis.
5.2. Dual-luciferase reporter assay
The 293 T cells (ATCC) were used for dual-luciferase reporter assay. 104 cells were seeded into 96 well plates, and luciferase reporter gene was transfected into 293 T cells when the cell confluence was 70%. Then 50 ng of wild-type plasmid pmirGLO-WT-Vec-3′UTR (or mutant plasmid Mut-Vec-3′UTR) and 50 ng of control plasmid was transfected into 293 T cells. Then 100 pmol mL−1 of miRNA365 mimic (miR365mic) or miR365 negative control mimic (miRNA NC) was transfected into 293 T cells, respectively. After 48 h transfection, cell culture medium was discarded and washed one time with 100 μl PBS. Then cells were lysed and the fluorescence activity was measured by Thermo Fisher Varioskan Luminometer. The fluorescence activity of luciferase was determined by calibrating with the activity of Renilla luciferase.
5.3. Fabrication and cytotoxicity of PCP/miRNA complex
PCP were synthesized according to the method we described previously [8]. To detect the cytotoxicity of PCP, about 7000 MC38 cells per well were seeded into 96 well plates and cultured in RPMI-1640 medium with 10% FBS (GIBCO, New Zealand) under a humid atmosphere containing 5% CO2 at 37 °C. When the confluence of cells was 70%, RPMI-1640 medium containing 10% FBS and different concentrations (0, 5, 10, 20, 50, 100 μg mL−1) of PCP were added into the plate. After 24 h, 10 μl of CCK8 (TargetMol, C0005) was added to each well. Then cell viability was measured by using Thermo Fisher Varioskan (Thermo) according to the manufacturer's instructions. And the cytotoxicity of PCP (50 μg mL−1) at different time points (0, 4, 8, 12, 24, 48 h) was also measured. For miRNA delivery ability test, NPs/cy3miRNA nanocomplexes self-assembled with PCP (80 μg mL−1) and cy3 fluorescently labeled miRNA365 (40 nM) were added into 293 T cells and MC38 cells culture medium, respectively. After 24 h of transfection, photos were collected by fluorescence microscope.
5.4. Cell proliferation, apoptosis and scratch assay
About 7000 MC38 cells/well were seeded into 96 well plates and cultured in RPMI-1640 medium with 10% FBS. When the confluence of cells was 70%, NPs/miR365 or NPs/miR NC (miR365 negative control mimic) nanocomposites (50 μg mL−1 of PCP with 25 nM of miR365mimic or mimic control) were added to the cell culture medium respectively. Commercial lipofectamine 2000 was used as control. After 24 h of treatment, CCK8 (TargetMol, C0005) and live/dead staining (Thermo, L3224) were separately used to detect the cell viability according to the instructions. Meanwhile, cells that were treated with NPs/miR365 or NPs/miR NC at 24 h, 48 h and 72 h were also tested by CCK8. And NPs/miR365 or NPs/miR NC treated MC38 cells were used for cell apoptosis test by cell flow cytometry. A sterile 10 μL pipette tip was used to make a cell scratch when MC38 cells that seeded in the 6-well plate reached the appropriate confluence. Then NPs/miR365 or NPs/miR NC was added into the RPMI-1640 medium with 1% FBS. Cell scratch images at 0, 12, 24 and 48 h were recorded using a microscope.
5.5. Immunofluorescence staining
MC38 cells were seeded into 48 well plates. NPs/miR365 or NPs/miR NC (miR365 negative control mimic) nanocomposites (50 μg mL−1 of PCP with 25 nM of miR365mimic or mimic control) were added to the cell culture medium respectively. Commercial lipofectamine 2000 mediated miRNA delivery was used as control. After 48 h of treatment, primary antibody Ki67 (Abcam, ab15580) or Bcl2 (Abcam, ab194583) and secondary antibody CoraLite594-conjugated Goat Anti-Rabbit IgG (H + L) (Proteintech, SA00013-4) or CoraLite488-conjugated Goat Anti-Rabbit IgG (H + L) (Proteintech, SA00013-2) were used for immunofluorescence staining respectively. The immunofluorescence staining of tissue sections was performed according to our previously reported [19].
5.6. Preparation and characterizations of cancer cell membrane fragments and M@NPs
To prepare fluorescently labeled MC38 tumor cell membrane fragments, 10 μL of DiO dye (25 mg mL−1) (AAT Bioquest, 22066), DiI (AAT Bioquest, 22044) or DiR (AAT Bioquest, 22070) was added to 10 mL of cell suspension containing 2 × 107 MC38 cells. The cells were stained in the dark for 20 min at room temperature. Then fluorescently labeled cells were centrifuged at 1000 g for 5 min and washed with equal volume of pre-cooled PBS for twice. Cells were resuspended with 20 mL pre-cooled PBS containg 1 mM PMSF and sonicated at 100 amplitude for 3 min (Q700, Qsonica, USA) for twice. After centrifugation at 4000 rpm for 5 min at 4 °C, the supernatant was collected and transferred to a new centrifuge tube to continue centrifugation at 20,000g for 10 min at 4 °C. Washed the precipitate with pre-cooled deionized sterilized water once. Cell membrane fragments were resuspended with 4 mL pre-cooled deionized sterilized water and sonicated at 100 amplitude for 2 min. Then the cell membrane fragments suspension was stored at −20 °C for further use. For PCP/miR365 (NPs) preparation, we used the method previously described [8]. Briefly, 12 μL of PCP (10 μg μL−1) and 3 μL of miRNA (20 μM) were mixed (v/v ratio = 4:1), and then sonicated at 100 amplitude for 90 s (Q700, Qsonica, USA). After standing at room temperature for 5 min, add 50 μL of cancer cell membrane, mix well and continue to stand for 30 min to obtain M@NPs nanoparticles.
In order to detect the homologous targeting of tumor cells, 50 μL of DiI labeled MC38 cell membrane fragments (M38) were separately added into 293 T, A549 and MC38 cells culture medium. After 4 h co-culture, the uptake of M38 fragments by 293 T, A549 and MC38 cells was detected with fluorescence microscope. Subsequently, we also tested the immune escape ability of cancer cell membrane coated nanoparticles to macrophages. M38@cy3NPs prepared from cy3 labeled NPs and DiO labeled M38 membrane was added into macrophages. After 4 h co-culture, the fluorescence signal was detected.
5.7. Animal experiment
Aged six weeks female C57BL/6 mice were purchased from the Animal Center of Xi'an Jiaotong University. They are placed under standard conditions of room temperature and dark-light cycle, with sufficient food and water. All mice were treated in accordance with the policies and regulations of the Institutional Animal Care and Use Committee (Number: 2021–1592). C57BL/6 mice bearing MC38 tumor were obtained by subcutaneous injection of 100 μL of 107 MC38 cells/mL for further study.
5.8. Targeted miRNA delivery and biotoxicity of M@NPs in vivo
In order to test whether the M@NPs can realize the targeted delivery of miRNA365 to the tumor tissues, PBS (control), NPs/NC (PCP/miRNA control), DirM (near-infrared fluorescent dye DiR labeled cancer cell membrane fragments), DirM@NPs/cy5 (self-assembled by DiR labeled cell membrane fragments and Cy5 fluorescent labeled NPs) and NPs/cy5 (Cy5 fluorescent labeled NPs) was injected into MC38 induced cancer mice through caudal vein, respectively. M@NPs/cy5 containing 0.5 nmol Cy5 fluorescent labeled miRNA365 were prepared using the method described above. After 10 h intravenous injections, the tumor tissue was collected, and the fluorescence signal was detected by the near-infrared fluorescence imaging system (Odyssey CLX, USA).
The biological safety of M@NPs in vivo was detected. Mice were injected with NPs/NC, NPs/miR365 or M@NPs/miR365 (0.6 mg PCP with 0.3 nmol miR365 or miRNA NC per time) through the caudal vein every two days for 6 times. The main organs such as liver, spleen, kidney and lung were collected for Hematoxylin-eosin (H&E) staining and blood was used for blood routine examination by animal automatic blood cell analyzer (Mindray, BC-2800vet).
5.9. Antitumor efficiency in vivo
Then the MC38 tumor-bearing mice were randomly divided into five groups, PBS, NPs/NC (PCP/miR control), M (M38 cancer cell membrane fragments), NPs (PCP/miR365) and M@NPs (self-assembled with M38 cell membrane fragments and PCP/miR365). Mice were respectively injected with PBS (100 μL PBS), NPs/NC (0.6 mg PCP with 0.3 nmol miR NC), M (15 μL of membrane), NPs/miR365 (0.6 mg PCP and 0.3 nmol miR365) or M@NPs/miR365 (15 μL of membrane, 0.6 mg PCP and 0.3 nmol miR365) through caudal vein every two days for 6 times. Then tumors in each group were collected for hematoxylin-eosin (HE) and immunofluorescence staining.
Author contributions
L. Zhang, W. Zhang, H. Peng, T L. Shen and F Y. Qu performed the in vivo experiments. L. Zhang, M. Wang, M. Luo, X Y. Qu, W G. Liu and S Y. Yang performed the part of the experiments in vitro. L. Zhang prepared the figures and wrote the manuscript. L. Zhang and B. Lei designed and drafted the work. All authors discussed the data and final approval of the version published.
Conceptualization: Long Zhang; Data curation: Long Zhang, Wan Zhang, Hang Peng, Tianli Shen, Fengyi Qu, Min Wang, Meng Luo, Xiaoyan Qu, Wenguang Liu and Shuanying Yang; Formal analysis: All authors; Funding acquisition: Long Zhang; Investigation: Wan Zhang and Hang Peng; Methodology: Tianli Shen, Min Wang, Xiaoyan Qu, Wenguang Liu; Project administration: Long Zhang, Bo Lei and Shuanying Yang; Resources: All authors; Software: Long Zhang, Min Wang, Tianli Shen, Xiaoyan Qu and Hang Peng; Supervision: Long Zhang, Bo Lei and Shuanying Yang; Validation: All authors; Visualization: Long Zhang and Tianli Shen; Roles/Writing – original draft: Long Zhang and Bo Lei; Writing – review & editing: All authors.
Statistical analysis
Means ± SD was used for all statistical results. Results were analyzed using GraphPad Prism version 7 (GraphPad) for Windows. An unpaired two-tailed Student's t-test was used for comparisons between two groups. A P value of <0.05 was considered statistically significant.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This work was supported by "Project funded by China Postdoctoral Science Foundation (2021M692578)". The picture materials in the schematic diagram are provided by “BioRender.com".
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.mtbio.2022.100294.
Contributor Information
Long Zhang, Email: longzhang@xjtu.edu.cn.
Bo Lei, Email: rayboo@xjtu.edu.cn.
Shuanying Yang, Email: yangshuanying@xjtu.edu.cn.
Supplemental information
Supplemental information can be found in the supplemental materials.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Bray F., Ferlay J., Soerjomataram I., Siegel R.L., Torre L.A., Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Siegel R.L., Miller K.D., Jemal A. Cancer statistics, 2020, CA. Cancer J. Clin. 2020;70:7–30. doi: 10.3322/caac.21590. [DOI] [PubMed] [Google Scholar]
- 3.Ngwa W., Irabor O.C., Schoenfeld J.D., Hesser J., Demaria S., Formenti S.C. Using immunotherapy to boost the abscopal effect. Nat. Rev. Cancer. 2018;18:313–322. doi: 10.1038/nrc.2018.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Valihrach L., Androvic P., Kubista M. Circulating miRNA analysis for cancer diagnostics and therapy. Mol. Aspect. Med. 2020;72:100825. doi: 10.1016/j.mam.2019.10.002. [DOI] [PubMed] [Google Scholar]
- 5.Orso F., Quirico L., Dettori D., Coppo R., Virga F., Ferreira L.C., Paoletti C., Baruffaldi D., Penna E., Taverna D. Role of miRNAs in tumor and endothelial cell interactions during tumor progression. Semin. Cancer Biol. 2020;60:214–224. doi: 10.1016/j.semcancer.2019.07.024. [DOI] [PubMed] [Google Scholar]
- 6.Liu R., Liang L., Freed E.F., Gill R.T. Directed evolution of CRISPR/Cas systems for precise gene editing. Trends Biotechnol. 2021;39:262–273. doi: 10.1016/j.tibtech.2020.07.005. [DOI] [PubMed] [Google Scholar]
- 7.Wilson P.C., Humphreys B.D. Single-cell genomics and gene editing: implications for nephrology. Nat. Rev. Nephrol. 2019;15:63–64. doi: 10.1038/s41581-018-0094-3. [DOI] [PubMed] [Google Scholar]
- 8.Zhang L., Wang M., Chen M., Niu W., Liu W., Leng T., Ji W., Lei B. A safe and efficient bioactive citrate-lysine/miRNA33 agonist nanosystem for high fat diet-induced obesity therapy. Chem. Eng. J. 2021;408:127304. [Google Scholar]
- 9.Lee Y.S., Dutta A. MicroRNAs in cancer. Annu. Rev. Pathol. 2009;4:199–227. doi: 10.1146/annurev.pathol.4.110807.092222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pastor F., Berraondo P., Etxeberria I., Frederick J., Sahin U., Gilboa E., Melero I. An RNA toolbox for cancer immunotherapy. Nat. Rev. Drug Discov. 2018;17:751–767. doi: 10.1038/nrd.2018.132. [DOI] [PubMed] [Google Scholar]
- 11.Biagioni A., Skalamera I., Peri S., Schiavone N., Cianchi F., Giommoni E., Magnelli L., Papucci L. Update on gastric cancer treatments and gene therapies. Cancer Metastasis Rev. 2019;38(3):537–548. doi: 10.1007/s10555-019-09803-7. [DOI] [PubMed] [Google Scholar]
- 12.Kang S.M., Lee H.J., Cho J.Y. MicroRNA-365 regulates NKX2-1, a key mediator of lung cancer. Cancer Lett. 2013;335:487–494. doi: 10.1016/j.canlet.2013.03.006. [DOI] [PubMed] [Google Scholar]
- 13.Jiang Z.B., Ma B.Q., Liu S.G., Li J., Yang G.M., Hou Y.B., Si R.H., Gao P., Yan H.T. miR-365 regulates liver cancer stem cells via RAC1 pathway. Mol. Carcinog. 2019;58:55–65. doi: 10.1002/mc.22906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu F., Zhuang L., Wu R., Li D. miR-365 inhibits cell invasion and migration of triple negative breast cancer through ADAM10. J. buon. 2019;24:1905–1912. [PubMed] [Google Scholar]
- 15.Nie J., Liu L., Zheng W., Chen L., Wu X., Xu Y., Du X., Han W. microRNA-365, down-regulated in colon cancer, inhibits cell cycle progression and promotes apoptosis of colon cancer cells by probably targeting Cyclin D1 and Bcl-2. Carcinogenesis. 2012;33:220–225. doi: 10.1093/carcin/bgr245. [DOI] [PubMed] [Google Scholar]
- 16.Zaimy M.A., Saffarzadeh N., Mohammadi A., Pourghadamyari H., Izadi P., Sarli A., Moghaddam L.K., Paschepari S.R., Azizi H., Torkamandi S., Tavakkoly-Bazzaz J. New methods in the diagnosis of cancer and gene therapy of cancer based on nanoparticles. Cancer Gene Ther. 2017;24:233–243. doi: 10.1038/cgt.2017.16. [DOI] [PubMed] [Google Scholar]
- 17.Vaughan H.J., Green J.J., Tzeng S.Y. Cancer-targeting nanoparticles for combinatorial nucleic acid delivery. Adv. Mater. 2020;32 doi: 10.1002/adma.201901081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xie Y., Hang Y., Wang Y., Sleightholm R., Prajapati D.R., Bader J., Yu A., Tang W., Jaramillo L., Li J., Singh R.K., Oupický D. Stromal modulation and treatment of metastatic pancreatic cancer with local intraperitoneal triple miRNA/siRNA nanotherapy. ACS Nano. 2020;14:255–271. doi: 10.1021/acsnano.9b03978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang L., Peng H., Zhang W., Li Y., Liu L., Leng T. Yeast cell wall particle mediated nanotube-RNA delivery system loaded with miR365 antagomir for post-traumatic osteoarthritis therapy via oral route. Theranostics. 2020;10:8479–8493. doi: 10.7150/thno.46761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen Z., Zhao P., Luo Z., Zheng M., Tian H., Gong P., Gao G., Pan H., Liu L., Ma A., Cui H., Ma Y., Cai L. Cancer Cell Membrane–biomimetic nanoparticles for homologous-targeting dual-modal imaging and photothermal therapy. ACS Nano. 2016;10:10049–10057. doi: 10.1021/acsnano.6b04695. [DOI] [PubMed] [Google Scholar]
- 21.Jiang Q., Liu Y., Guo R., Yao X., Sung S., Pang Z., Yang W. Erythrocyte-cancer hybrid membrane-camouflaged melanin nanoparticles for enhancing photothermal therapy efficacy in tumors. Biomaterials. 2019;192:292–308. doi: 10.1016/j.biomaterials.2018.11.021. [DOI] [PubMed] [Google Scholar]
- 22.Wang D., Wang S., Zhou Z., Bai D., Zhang Q., Ai X., Gao W., Zhang L. White blood cell membrane-coated nanoparticles: recent development and medical applications. Adv. Healthc. Mater. 2022;11:2101349. doi: 10.1002/adhm.202101349. [DOI] [PubMed] [Google Scholar]
- 23.Park J.H., Jiang Y., Zhou J., Gong H., Mohapatra A., Heo J., Gao W., Fang R.H., Zhang L. Genetically engineered cell membrane coated nanoparticles for targeted delivery of dexamethasone to inflamed lungs. Sci. Adv. 2021;7 doi: 10.1126/sciadv.abf7820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang S., Duan Y., Zhang Q., Komarla A., Gong H., Gao W., Zhang L. Drug targeting via platelet membrane–coated nanoparticles. Small Struc. 2020;1:2000018. doi: 10.1002/sstr.202000018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li S.Y., Cheng H., Xie B.R., Qiu W.X., Zeng J.Y., Li C.X., Wan S.S., Zhang L., Liu W.L., Zhang X.Z. Cancer cell membrane camouflaged cascade bioreactor for cancer targeted starvation and photodynamic therapy. ACS Nano. 2017;11:7006–7018. doi: 10.1021/acsnano.7b02533. [DOI] [PubMed] [Google Scholar]
- 26.Rabinovich G.A., Gabrilovich D., Sotomayor E.M. Immunosuppressive strategies that are mediated by tumor cells. Annu. Rev. Immunol. 2007;25:267–296. doi: 10.1146/annurev.immunol.25.022106.141609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yue X.S., Murakami Y., Tamai T., Nagaoka M., Cho C.S., Ito Y., Akaike T. A fusion protein N-cadherin-Fc as an artificial extracellular matrix surface for maintenance of stem cell features. Biomaterials. 2010;31(20):5287–5296. doi: 10.1016/j.biomaterials.2010.03.035. [DOI] [PubMed] [Google Scholar]
- 28.Rao L., Yu G.T., Meng Q.F., Bu L.L., Tian R., Lin L.S., Deng H., Yang W., Zan M., Ding J., Li A., Xiao H., Sun Z.J., Liu W., Chen X. Cancer cell membrane-coated nanoparticles for personalized therapy in patient-derived xenograft models. Adv. Funct. Mater. 2019;29:1905671. [Google Scholar]
- 29.Xie W., Deng W.W., Zan M., Rao L., Yu G.T., Zhu D.M., Wu W.T., Chen B., Ji L.W., Chen L., Liu K., Guo S.S., Huang H.M., Zhang W.F., Zhao X., Yuan Y., Dong W., Sun Z.J., Liu W. Cancer cell membrane camouflaged nanoparticles to realize starvation therapy together with checkpoint blockades for enhancing cancer therapy. ACS Nano. 2019;13:2849–2857. doi: 10.1021/acsnano.8b03788. [DOI] [PubMed] [Google Scholar]
- 30.Logtenberg M.E.W., Scheeren F.A., Schumacher T.N. The CD47-SIRPα immune checkpoint. Immunity. 2020;52:742–752. doi: 10.1016/j.immuni.2020.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang Y., Zhang Z., Li S., Zhao L., Li D., Cao Z., Xu X., Yang X. A siRNA-assisted assembly strategy to simultaneously suppress "self" and upregulate "eat-me" signals for nanoenabled chemo-immunotherapy. ACS Nano. 2021;15:16030–16042. doi: 10.1021/acsnano.1c04458. [DOI] [PubMed] [Google Scholar]
- 32.Jiang N., Xie B., Xiao W., Fan M., Xu S., Duan Y., Hamsafar Y., Evans A.C., Huang J., Zhou W., Lin X., Ye N., Wanggou S., Chen W., Jing D., Fragoso R.C., Dugger B.N., Wilson P.F., Coleman M.A., Xia S., Li X., Sun L.Q., Monjazeb A.M., Wang A., Murphy W.J., Kung H.J., Lam K.S., Chen H.W., Li J.J. Fatty acid oxidation fuels glioblastoma radioresistance with CD47-mediated immune evasion. Nat. Commun. 2022;13:1511. doi: 10.1038/s41467-022-29137-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Willingham S.B., Volkmer J.P., Gentles A.J., Sahoo D., Dalerba P., Mitra S.S., Wang J., Contreras-Trujillo H., Martin R., Cohen J.D., Lovelace P., Scheeren F.A., Chao M.P., Weiskopf K., Tang C., Volkmer A.K., Naik T.J., Storm T.A., Mosley A.R., Edris B., Schmid S.M., Sun C.K., Chua M.S., Murillo O., Rajendran P., Cha A.C., Chin R.K., Kim D., Adorno M., Raveh T., Tseng D., Jaiswal S., Enger P., Steinberg G.K., Li G., So S.K., Majeti R., Harsh G.R., van de Rijn M., Teng N.N., Sunwoo J.B., Alizadeh A.A., Clarke M.F., Weissman I.L. The CD47-signal regulatory protein alpha (SIRPa) interaction is a therapeutic target for human solid tumors. Proc. Natl. Acad. Sci. U.S.A. 2012;109:6662–6667. doi: 10.1073/pnas.1121623109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chao M.P., Alizadeh A.A., Tang C., Myklebust J.H., Varghese B., Gill S., Jan M., Cha A.C., Chan C.K., Tan B.T., Park C.Y., Zhao F., Kohrt H.E., Malumbres R., Briones J., Gascoyne R.D., Lossos I.S., Levy R., Weissman I.L., Majeti R. Anti-CD47 antibody synergizes with rituximab to promote phagocytosis and eradicate non-Hodgkin lymphoma. Cell. 2010;142:699–713. doi: 10.1016/j.cell.2010.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rao L., Bu L.L., Cai B., Xu J.H., Li A., Zhang W.F., Sun Z.J., Guo S.S., Liu W., Wang T.H., Zhao X.Z. Cancer cell membrane-coated upconversion nanoprobes for highly specific tumor imaging. Adv. Mater. 2016;28:3460–3466. doi: 10.1002/adma.201506086. [DOI] [PubMed] [Google Scholar]
- 36.Fang R.H., Hu C.M., Luk B.T., Gao W., Copp J.A., Tai Y., O'Connor D.E., Zhang L. Cancer cell membrane-coated nanoparticles for anticancer vaccination and drug delivery. Nano Lett. 2014;14:2181–2188. doi: 10.1021/nl500618u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Du Y., Ge J., Li Y., Ma P.X., Lei B. Biomimetic elastomeric, conductive and biodegradable polycitrate-based nanocomposites for guiding myogenic differentiation and skeletal muscle regeneration. Biomaterials. 2018;157:40–50. doi: 10.1016/j.biomaterials.2017.12.005. [DOI] [PubMed] [Google Scholar]
- 38.Xi Y., Ge J., Wang M., Chen M., Niu W., Cheng W., Xue Y., Lin C., Lei B. Bioactive anti-inflammatory, antibacterial, antioxidative silicon-based nanofibrous dressing enables cutaneous tumor photothermo-chemo therapy and infection-induced wound healing. ACS Nano. 2020;14:2904–2916. doi: 10.1021/acsnano.9b07173. [DOI] [PubMed] [Google Scholar]
- 39.Feng Q., Yang X., Hao Y., Wang N., Feng X., Hou L., Zhang Z. Cancer cell membrane-biomimetic nanoplatform for enhanced sonodynamic therapy on breast cancer via autophagy regulation strategy. ACS Appl. Mater. Interfaces. 2019;11:32729–32738. doi: 10.1021/acsami.9b10948. [DOI] [PubMed] [Google Scholar]
- 40.Xuan M., Shao J., Dai L., He Q., Li J. Macrophage cell membrane camouflaged mesoporous silica nanocapsules for in vivo cancer therapy. Adv. Healthc. Mater. 2015;4:1645–1652. doi: 10.1002/adhm.201500129. [DOI] [PubMed] [Google Scholar]
- 41.Jiang Y., Krishnan N., Zhou J., Chekuri S., Wei X., Kroll A.V., Yu C.L., Duan Y., Gao W., Fang R.H., Zhang L. Engineered cell-membrane-coated nanoparticles directly present tumor antigens to promote anticancer immunity. Adv. Mater. 2020;32:2001808. doi: 10.1002/adma.202001808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kroll A.V., Fang R.H., Jiang Y., Zhou J., Wei X., Yu C.L., Gao J., Luk B.T., Dehaini D., Gao W., Zhang L. Nanoparticulate delivery of cancer cell membrane elicits multiantigenic antitumor immunity. Adv. Mater. 2017;29:1703969. doi: 10.1002/adma.201703969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rupaimoole R., Slack F.J. MicroRNA therapeutics: towards a new era for the management of cancer and other diseases. Nat. Rev. Drug Discov. 2017;16:203–222. doi: 10.1038/nrd.2016.246. [DOI] [PubMed] [Google Scholar]
- 44.Liu G., Yang D., Rupaimoole R., Pecot C.V., Sun Y., Mangala L.S., Li X., Ji P., Cogdell D., Hu L., Wang Y., Rodriguez-Aguayo C., Lopez-Berestein G., Shmulevich I., De Cecco L., Chen K., Mezzanzanica D., Xue F., Sood A.K., Zhang W. Augmentation of response to chemotherapy by microRNA-506 through regulation of RAD51 in serous ovarian cancers. JNCI-J. Natl. Cancer. 2015;107 doi: 10.1093/jnci/djv108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wiggins J.F., Ruffino L., Kelnar K., Omotola M., Patrawala L., Brown D., Bader A.G. Development of a lung cancer therapeutic based on the tumor suppressor microRNA-34. Cancer Res. 2010;70:5923–5930. doi: 10.1158/0008-5472.CAN-10-0655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Joshi H.P., Subramanian I.V., Schnettler E.K., Ghosh G., Rupaimoole R., Evans C., Saluja M., Jing Y., Cristina I., Roy S., Zeng Y., Shah V.H., Sood A.K., Ramakrishnan S. Dynamin 2 along with microRNA-199a reciprocally regulate hypoxia-inducible factors and ovarian cancer metastasis. P. Natl. A. Sci. 2014;111:5331–5336. doi: 10.1073/pnas.1317242111. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.