Abstract
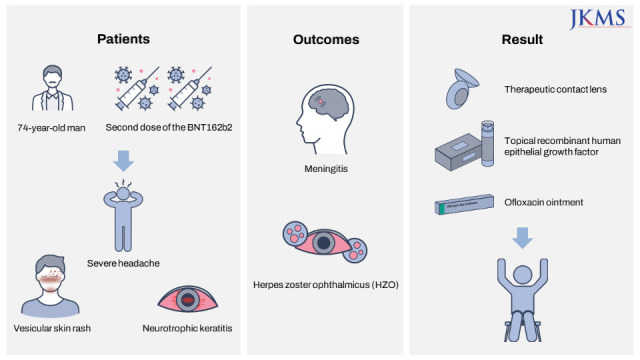
There are several reports that herpes zoster characterized by reactivation of varicella zoster virus (VZV) following coronavirus disease 2019 (COVID-19) vaccines can occur. Herein, we report VZV meningitis, herpes zoster ophthalmicus (HZO), and late neurotrophic keratitis after receiving a second dose of messenger RNA (mRNA) COVID-19 vaccine. A 74-year-old man developed a vesicular skin rash on the forehead, scalp, nose, and left upper eyelid with a severe headache. Five days earlier, he received a second dose of the BNT162b2 mRNA vaccine on his left arm. Ocular examination revealed conjunctival hyperemia and pseudodendrite in the peripheral cornea. VZV was detected in the cerebrospinal fluid using polymerase chain reaction. The patient was diagnosed with HZO and meningitis. The patient was treated with intravenous acyclovir and topical acyclovir ointment and levofloxacin 1.5% eye drops. One month later, he developed a central epithelial defect with a rolled margin, typical of a neurotrophic ulcer. Treatment with a therapeutic contact lens and a combination of topical recombinant human epithelial growth factor and ofloxacin ointment was initiated. At six months after vaccination, the slit-lamp examination findings were stable with a mild corneal superficial stromal haze.
Keywords: COVID-19 Vaccination, Herpes Zoster Ophthalmicus, Meningitis, Neurotropic Keratitis
Graphical Abstract
INTRODUCTION
Globally, several types of vaccines against coronavirus disease 2019 (COVID-19), caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), are being administered with overall effectiveness against severe infection to suppress the spread of the pandemic.1,2,3 Among them, the messenger RNA (mRNA)-based COVID-19 vaccine has shown a good safety profile.4 However, BNT162b2 mRNA COVID-19 vaccination was strongly associated with an elevated risk of myocarditis, lymphadenopathy, appendicitis, and herpes zoster (HZ) infection in Israel.4 Recently, HZ or meningitis characterized by reactivation of varicella zoster virus (VZV) following COVID-19 vaccines have been reported worldwide in the case reports and systematic review.1,2,3,5 We report a case of herpes zoster ophthalmicus (HZO) and meningitis following BNT162b2 mRNA COVID-19 vaccination. To the best of our knowledge, no study to date has described HZO and meningitis simultaneously after mRNA vaccination.
CASE DESCRIPTION
A 74-year-old man presented to the emergency department with a severe headache and forehead pain. The patient also complained of eyelid swelling on the left side of the face and photophobia of the left eye. He had regular visits to the hospital for hypertension, diabetes mellitus, and left hemiplegia due to a cerebrovascular accident 17 years previously. Five days earlier, he received a second dose of the BNT162b2 mRNA vaccine on his left arm. On the day of vaccination, the patient had mild injection site tenderness, but developed a tingling and itching sensation on the left side of the face after 2 days, followed by a vesicular skin rash on the forehead. The lesions progressed rapidly, involving the left side of the scalp, nose, and left upper eyelid (Fig. 1). He had a history of chickenpox in childhood but no history of herpes zoster or vaccination for herpes zoster virus.
Fig. 1. Photographs of herpes zoster ophthalmicus. Initial photograph showing erythematous grouped vesicle and crust on the left side of the face in 74-year-old man.
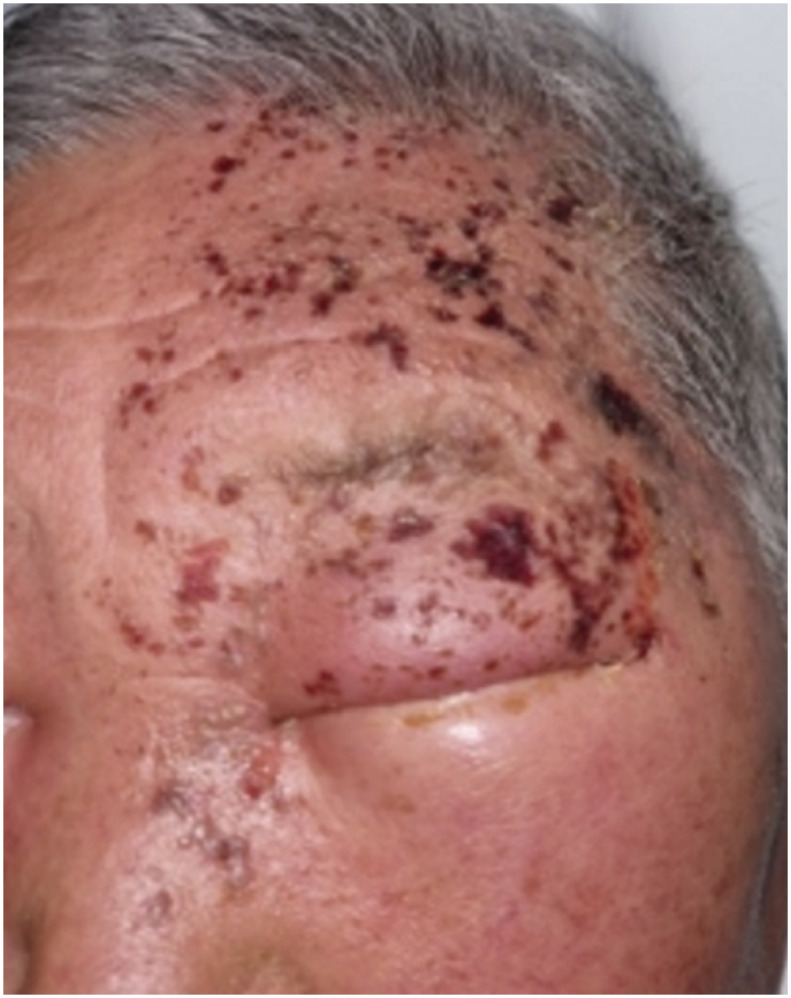
The figures are published under agreement of the patient.
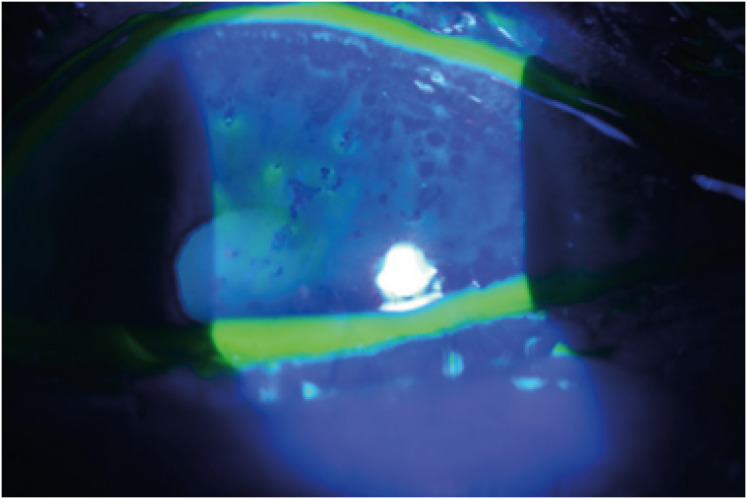
Ocular examination revealed a visual acuity of 20/32 in the left eye, and slit-lamp examination revealed conjunctival chemosis, hyperemia, and pseudodendrite in the peripheral cornea (Fig. 2). The anterior chamber was quiet, and the posterior segment examination findings were normal. Blood test results showed an elevated C-reactive protein (18.97 mg/L) and normal white blood cell count (4,710 cells/μL) with no lymphopenia (35%). The patient had 1.0 IU/mL of varicella-zoster immunoglobulin M antibody. The patient tested negative for human immunodeficiency virus (HIV) infection. Cerebrospinal fluid examination revealed a high leukocyte count (47 cells/μL) with lymphocyte predominance (100%), and high protein (74.5 mg/dL) and glucose (86 mg/dL) levels. Magnetic resonance imaging of brain did not reveal any significant findings. Varicella zoster virus was detected in the cerebrospinal fluid using polymerase chain reaction. The patient was diagnosed with HZO and meningitis. The patient was treated with intravenous acyclovir and topical acyclovir ointment four times daily and levofloxacin 1.5% eye drops four times daily. On hospital day 3, he showed decreased pseudodendrite and eyelid swelling, and improvement in his headache. The patient was discharged on hospital day 10.
Fig. 2. Anterior segment photography of the patient at first day. Slit-lamp examination revealed multiple pseudodendrite with rarely staining with fluorescein in the peripheral cornea.
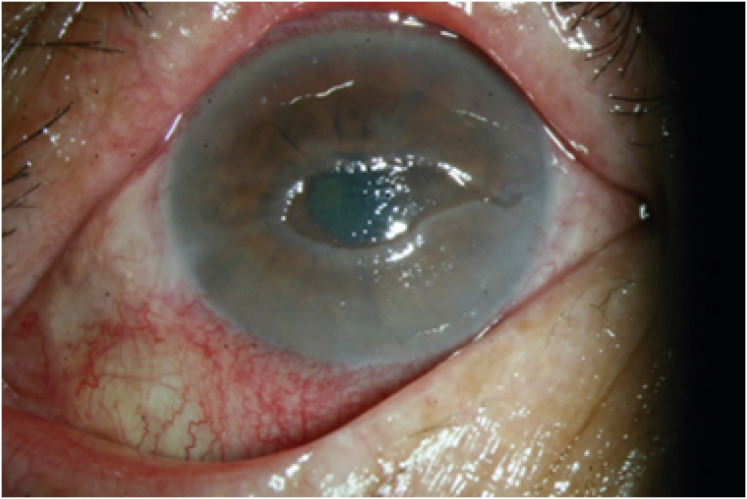
Twenty days later, after discharge, he developed an oval central epithelial defect with a rolled margin, typical of a neurotrophic ulcer (Fig. 3). Corneal sensation was markedly reduced compared with that in the contralateral eye. Treatment with a therapeutic contact lens and a mixed combination of topical recombinant human epithelial growth factor (0.005% solution) and ofloxacin ointment was initiated. Over the following two months, the corneal epithelium resolved, and the topical epithelial growth factor and antibiotics were gradually tapered. At his last visit 6 months after vaccination, his visual acuity was 20/63, and the slit-lamp examination findings were stable with a mild corneal superficial stromal haze.
Fig. 3. Anterior segment photography of the patient at one month. Neurotrophic ulcer showing central corneal smooth, oval with heaped-up epithelium at border. The figures are published under agreement of the patient.
DISCUSSION
VZV is a DNA virus from the human alpha-herpes virus that causes chickenpox as the primary infection.6
Following primary infection, this virus becomes latent in neurons of dorsal root ganglia, cranial nerve ganglia, and autonomic ganglia.1 Up to decades later, reactivation of latent VZV may arise and the secondary infection causes HZ. Typically, HZ presents as a painful or pruritic cutaneous vesicular eruption that is usually confined to the ipsilateral dermatome.6 VZV reactivation occurs when down-regulation of toll-like receptor results in inhibition of interferon response, thus triggering the viral replication.7 Several triggering factors that are associated with this down-regulation of the immune response are advanced age, stress, physical trauma, malignancy, HIV infection, and immunosuppressive drugs.8 Of particular interest, viral replication appears more frequently in older individuals because of their diminished cell-mediated immunity, a phenomenon known as immunosenescence.9 Our patient was of older age suffering from diabetes mellitus and hemiplegia.
The newly developed COVID-19 vaccines have established an acceptable safety profile; however, some individuals experience a wide range of adverse events. Recently, HZO, meningitis, and acute retinal necrosis after receiving a COVID-19 vaccine have been reported in immunocompetent patients.1,2,3,5,10 Vaccination with COVID-19 mRNA may cause an immunomodulatory response that leads to the reactivation of dormant VZV. Stimulation of the immune system following vaccination induces a strong T-cell response. Vaccination with BNT162b2 produces a cellular response with increased spike-specific CD8+ T cells and T helper type 1 CD4+ T cells after booster dosage.11 A possible hypothesis suggests that VZV-specific CD8+ cells are temporarily incapable of regulating VZV after the massive shift of naive CD8+ cells in the setting of SARS-CoV-2 mRNA vaccination.1,3
HZO most commonly manifests as eyelid lesions, corneal diseases, and uveitis, which can result in long-term sequelae. Jastrzebski et al.12 reported reactivation of HZ keratitis with corneal melt after live attenuated zoster vaccination. Jabbour et al.13 reported a case of HZO reactivation after recombinant zoster vaccination. Rehman et al.14 described two cases of HZO after receiving a live COVID-19 vaccine. We encountered a patient who developed VZV meningitis and late neurotrophic keratitis after receiving a second dose of the BNT162b2 mRNA COVID-19 vaccine.
Although the treatment of refractory neurotrophic keratitis remains challenging, the emergence of new therapies, such as nerve growth factor, autologous serum, thymosin beta-4, and insulin-like-growth factor, has enabled the clinician to promote ocular surface repair.15 We successfully managed the neurotrophic keratitis with mixed use of topical recombinant human epithelial growth factor (off-label use) and ofloxacin ointment.
Therefore, we believe that it is important to inform patients about this potential risk and recommend careful monitoring after vaccination given the possibility of VZV reactivation. Early diagnosis and antiviral treatment can halt disease progression and prevent debilitating complications.
Footnotes
Disclosure: The authors have no potential conflicts of interest to disclose.
- Conceptualization: You IC, Cho NC.
- Data curation: You IC, Ahn M.
- Supervision: You IC.
- Visualization: You IC.
- Writing - original draft: You IC.
- Writing - review & editing: You IC, Ahn M, Cho NC.
References
- 1.Katsikas Triantafyllidis K, Giannos P, Mian IT, Kyrtsonis G, Kechagias KS. Varicella zoster virus reactivation following COVID-19 vaccination: a systematic review of case reports. Vaccines (Basel) 2021;9(9):1013. doi: 10.3390/vaccines9091013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Maruki T, Ishikane M, Suzuki T, Ujiie M, Katano H, Ohmagari N. A case of varicella zoster virus meningitis following BNT162b2 mRNA COVID-19 vaccination in an immunocompetent patient. Int J Infect Dis. 2021;113:55–57. doi: 10.1016/j.ijid.2021.09.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Desai HD, Sharma K, Shah A, Patoliya J, Patil A, Hooshanginezhad Z, et al. Can SARS-CoV-2 vaccine increase the risk of reactivation of varicella zoster? A systematic review. J Cosmet Dermatol. 2021;20(11):3350–3361. doi: 10.1111/jocd.14521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barda N, Dagan N, Ben-Shlomo Y, Kepten E, Waxman J, Ohana R, et al. Safety of the BNT162b2 mRNA Covid-19 vaccine in a nationwide setting. N Engl J Med. 2021;385(12):1078–1090. doi: 10.1056/NEJMoa2110475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Iwanaga J, Fukuoka H, Fukuoka N, Yutori H, Ibaragi S, Tubbs RS. A narrative review and clinical anatomy of herpes zoster infection following COVID-19 vaccination. Clin Anat. 2022;35(1):45–51. doi: 10.1002/ca.23790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Psichogiou M, Samarkos M, Mikos N, Hatzakis A. Reactivation of varicella zoster virus after vaccination for SARS-CoV-2. Vaccines (Basel) 2021;9(6):572. doi: 10.3390/vaccines9060572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ku CC, Besser J, Abendroth A, Grose C, Arvin AM. Varicella-Zoster virus pathogenesis and immunobiology: new concepts emerging from investigations with the SCIDhu mouse model. J Virol. 2005;79(5):2651–2658. doi: 10.1128/JVI.79.5.2651-2658.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thimmanagari K, Veeraballi S, Roach D, Al Omour B, Slim J. Ipsilateral zoster ophthalmicus post COVID-19 vaccine in healthy young adults. Cureus. 2021;13(7):e16725. doi: 10.7759/cureus.16725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Crooke SN, Ovsyannikova IG, Poland GA, Kennedy RB. Immunosenescence and human vaccine immune responses. Immun Ageing. 2019;16(1):25. doi: 10.1186/s12979-019-0164-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zheng F, Willis A, Kunjukunju N. Acute retinal necrosis from reactivation of varicella zoster virus following BNT162b2 mRNA COVID-19 vaccination. Ocul Immunol Inflamm. 2021 doi: 10.1080/09273948.2021.2001540. Forthcoming. [DOI] [PubMed] [Google Scholar]
- 11.Sahin U, Muik A, Vogler I, Derhovanessian E, Kranz LM, Vormehr M, et al. BNT162b2 vaccine induces neutralizing antibodies and poly-specific T cells in humans. Nature. 2021;595(7868):572–577. doi: 10.1038/s41586-021-03653-6. [DOI] [PubMed] [Google Scholar]
- 12.Jastrzebski A, Brownstein S, Ziai S, Saleh S, Lam K, Jackson WB. Reactivation of herpes zoster keratitis with corneal perforation after zoster vaccination. Cornea. 2017;36(6):740–742. doi: 10.1097/ICO.0000000000001203. [DOI] [PubMed] [Google Scholar]
- 13.Jabbour S, Shekhawat NS, Chen A, Woreta FA. Presumed herpes zoster ophthalmicus reactivation following recombinant zoster vaccination. Cornea. 2021;40(2):248–250. doi: 10.1097/ICO.0000000000002537. [DOI] [PubMed] [Google Scholar]
- 14.Rehman O, Arya SK, Jha UP, Nayyar S, Goel I. Herpes zoster ophthalmicus after COVID-19 vaccination: chance occurrence or more than that? Cornea. 2022;41(2):254–256. doi: 10.1097/ICO.0000000000002881. [DOI] [PubMed] [Google Scholar]
- 15.Jabbour S, Ashton C, Balal S, Kaye A, Ahmad S. The management of neurotrophic keratitis. Curr Opin Ophthalmol. 2021;32(4):362–368. doi: 10.1097/ICU.0000000000000766. [DOI] [PubMed] [Google Scholar]