Abstract
Objective
To examine the relative effectiveness of a fourth dose of the Pfizer-BioNTech mRNA (BNT162b2) vaccine compared with three vaccine doses over the span of 10 weeks.
Design
Retrospective, test negative, case-control study, with a matched analysis and an unmatched multiple tests analysis.
Setting
Nationally centralised database of Maccabi Healthcare Services, an Israeli national health fund for 2.5 million people; from 10 January 2022 (seven days after the fourth dose was first given to eligible individuals) to 13 March 2022, an omicron dominant period in Israel.
Participants
97 499 Maccabi Healthcare Services members aged 60 years and older, who were eligible to receive a fourth vaccine dose and obtained at least one polymerase chain reaction (PCR) test during the study.
Main outcome measures
Breakthrough SARS-CoV-2 infection, defined as a positive PCR test performed seven or more days after inoculation with the BNT162b2 vaccine; and breakthrough SARS-CoV-2 infection resulting in severe covid-19 disease, defined as hospital admission or death related to covid-19.
Results
27 876 participants received the fourth BNT162b2 vaccine dose and 69 623 received three doses only. Of 106 participants who died during the follow-up period, 77 had had their third doses only and 23 had had their fourth doses during the first three weeks after inoculation. In the first three weeks, a fourth dose provided additional protection against both SARS-CoV-2 infection and severe disease relative to three doses of the vaccine. However, relative vaccine effectiveness against infection quickly decreased over time, peaking during the third week at 65.1% (95% confidence interval 63.0% to 67.1%) and falling to 22.0% (4.9% to 36.1%) by the end of the 10 week follow-up period. Unlike relative effectiveness against SARS-CoV-2 infection, the relative effectiveness of a fourth dose against severe covid-19 was maintained at a high level (>72%) throughout follow-up. However, severe disease was a relatively rare event, occurring in <1% of study participants who received four doses or three doses only.
Conclusions
A fourth dose of the BNT162b2 vaccine appears to have provided additional protection against both SARS-CoV-2 infection and severe covid-19 disease relative to three vaccine doses. However, relative effectiveness of the fourth dose against infection appears to wane sooner than that of the third dose.
Introduction
In August 2021, Israel launched a national third dose (booster) vaccination campaign against covid-19, in response to increased SARS-CoV-2 infections with the delta (B.1.617.2) variant during the summer of 20211 and growing evidence indicating that the immunity induced by the Pfizer-BioNTech mRNA vaccine (BNT162b2) had waned.2 3 4 5 The booster was shown to largely restore short term effectiveness,6 7 8 and by 31 December 2021, over 4.2 million individuals (~45% of individuals of all ages in Israel) received a booster shot.1 However, the rapid spread of the omicron variant (B.1.1.529) from late December to March 20229 caused a sharp increase in infection rates and covid-19 related hospital admission.1 The rising incidence of omicron infections alongside evidence of a relatively rapid decrease in protection against infection conferred by the third dose10 11 prompted Israeli authorities to administer a fourth dose (a second booster). Recommendations were first given for immunosuppressed individuals on 30 December 2021,12 and three days later for all individuals aged 60 years or older and those at high risk of exposure (eg, healthcare workers). Eligible individuals must have received the third dose at least four months before the fourth dose.
Sufficient real world evidence indicating the effectiveness of a fourth dose against infection with SARS-CoV-2 and severe covid-19 has been lacking so far. By using the centralised database of Maccabi Healthcare Services (MHS), an Israeli national health fund that covers 2.5 million people, we examined the short term effectiveness of a fourth BNT162b2 dose relative to three doses on covid-19 breakthrough infection and severe disease over the span of 10 weeks.
Methods
Data sources and data extraction
MHS, which covers 26.7% of the population and provides a representative sample of the Israeli population, has maintained a centralised database of electronic medical records for three decades, with a disengagement rate of less than 1% among its members, allowing for comprehensive longitudinal medical follow-up. The database includes demographic data, measurements, procedures and diagnoses for inpatients and outpatients, drug treatments, imaging records, and laboratory data from a single central laboratory.
Anonymised electronic medical records were retrieved from MHS’s centralised computer database. Individual level data for the study population included age, biological sex, socioeconomic status index, city of residence (including over 1300 cities), and whether a person currently resided at a nursing home or an assisted living residence. The socioeconomic status index was measured on a scale of 1 (lowest) to 10 based on several parameters including household income, educational qualifications, household crowding, and car ownership. Data collected also covered the most recently documented body mass index (where obesity was defined as body mass index ≥30) and information on chronic diseases from automated MHS registries, including cardiovascular diseases,13 diabetes,14 chronic kidney disease,15 chronic obstructive pulmonary disease, and immunocompromised conditions. SARS-CoV-2 related information included dates of vaccinations and results of any PCR tests for SARS-CoV-2 (including tests performed within and outside of MHS) and dates of hospital admission related to covid-19.
Study population
The study population included all MHS members aged 60 years and older who were eligible to receive a fourth vaccine dose from 3 January 2022, the first day of eligibility according to the Israeli Ministry of Health. Vaccination status was assessed on the test day, and eligible individuals were those who received at least three vaccine doses and at least four months had passed since the third (booster) dose. We excluded individuals who ever had a positive SARS-CoV-2 polymerase chain reaction (PCR) test before the start of the study period (that is, between March 2020 and 10 January 2022) and people with a possibly incomplete medical history related to covid-19 during the pandemic (that is, those who joined MHS after March 2020).
Design and statistical analysis
Analyses focused on the period from 10 January 2022 (seven days after the fourth dose was first given to eligible individuals) to 13 March 2022, an omicron dominant period in Israel.9 We evaluated two outcomes related to SARS-CoV-2 infection. Firstly, we looked at breakthrough infection, defined as a positive PCR test performed seven or more days after inoculation with the BNT162b2 vaccine, where the seven day cut-off period was based on previous breakthrough infection definitions in vaccine effectiveness studies.6 16 Secondly, we investigated breakthrough infection resulting in severe covid-19 disease, defined as hospital admission (≤21 days from the first positive PCR test) or death related to covid-19.
Previous SARS-CoV-2 studies5 10 11 have shown a time dependent increase followed by a decrease in the level of protection conferred by the BNT162b2 vaccine. Therefore, we stratified the analysis by time since vaccination, in equal day intervals (7-13, 14-20, 21-27, 28-34, 35-41, 42-48, 49-55, 56-62, 63-69 days), aiming to estimate the reduction in the odds of a positive outcome at these different time points after inoculation with a fourth dose. Vaccination status was determined at the time of the PCR test, or the time of covid-19 related hospital admission or death if either event occurred before testing positive. For the outcome of severe disease, we stratified time since vaccination by three week intervals, owing to insufficient numbers of severe outcomes.
For our primary analysis, we used a test negative, case-control design, which has been strongly argued for in covid-19 studies because of its ability to better control for bias stemming from healthcare seeking and testing behaviour.6 17 18 We defined cases as individuals with a positive PCR test for SARS-CoV-2 infection during the study, or individuals who had a diagnosis of severe covid-19 during the study; cases were defined separately for each outcome. Eligible controls were individuals with a negative PCR test result who had not tested positive previously.
Matched analysis
For our primary analysis, cases were matched to controls using 1:m matching, with up to five controls per case, based on seven factors: sex, age group (using a binary cut-off age of ≥70 years, because studies19 have indicated that older age groups are at higher risk, especially for severe disease), city of residence, socioeconomic status, calendar week of first test (to account for potential time-varying risk within the outcome period), the month of receipt of the third dose (to mitigate possible bias related to waning of the third dose), and a categorical variable for the living environment (a medical nursing home, an assisted living facility, or a private residence). The last factor was included because of early pandemic increases in SARS-CoV-2 infection in nursing homes and assisted living facilities, which led to differential regulations in these institutions, mandating staff and residents to be vaccinated and limiting visits from non-residents. Therefore, exposure was substantially different in these facilities compared with the rest of the population.
The first positive PCR test (or first hospital admission or death related to covid-19 in the severe disease analysis) and the first negative PCR test during the study period were the only tests included for each case and control, respectively.20 Negative tests for cases were excluded, rendering participants as either cases or controls, but not both. The rationale was to avoid a potential bias stemming from repeated tests, indicating different healthcare seeking behaviour and potentially a lower pre-test risk of infection.20
A conditional logistic regression model that accounted for the matching was fit to the data. The relative vaccine effectiveness6 21 of the fourth dose compared with having received three doses only was calculated as 100%×(1−odds ratio) for each week since vaccination. The odds ratio estimated the multiplicative effect of the fourth dose compared with the third dose (that is, the proportional change in the odds ratio),22 rather than the absolute effectiveness compared with a person being unvaccinated; comparing the absolute effectiveness was not possible given the rapid rollout and high vaccination coverage among older individuals in Israel.
To deal with potential confounders, we adjusted for underlying comorbidities, including obesity, cardiovascular diseases, diabetes, hypertension, chronic kidney disease, chronic obstructive pulmonary disease, and immunosuppression conditions. We also adjusted for a categorical variable consisting of the number of PCR tests each person undertook from the beginning of the pandemic (March 2020) to the start of the outcome period, as a proxy for healthcare seeking behaviour related to SARS-CoV-2. The covariate included the entire pandemic period apart from the outcome period in order to differentiate this behavioural variable from the dependent variable.5 23
Sensitivity analysis: unmatched multiple tests analysis
As a sensitivity analysis, we allowed people to contribute multiple negative tests, but excluded them once they tested positive or were admitted to hospital with covid-19.6 11 We used an unmatched logistic regression analysis, adjusting for all of the covariates included in the matched approach (that is, comorbidities and the number of previous PCR tests) as well as the previously matched parameters (biological sex, age group (<70 v ≥70 years), calendar week of testing, socioeconomic status, month of receipt of the third dose, and residence in a nursing home or assisted living facility). We did not include the city of residence, which included over 1300 cities. In the unmatched approach, a logistic regression model using generalised estimating equations was fit to the data to account for the possibility of repeated samples from the same participant,24 assuming an unstructured correlation matrix. The relative vaccine effectiveness was estimated in the same way as the primary matched analysis.
Additional sensitivity analyses
To examine how the time-varying risk of exposure might influence effectiveness, we conducted an additional sensitivity analysis in which we created an accumulative attack rate variable, which we later adjusted for in the unmatched multiple test analysis. The attack rate was calculated for every day in which a PCR test was performed and represented the average proportion of infected inhabitants in a city within the past 14 days divided by the overall number of dwellers in that city.
In another additional analysis, the measured outcome for severe covid-19 included only people who met the US National Institutes of Health’s definition of severe disease (oxygen saturation <94% on room air, respiratory rate >30 breaths per minute, or lung infiltrates >50%), critical disease,25 or death due to covid-19. The rationale was to ensure that designating covid-19 related hospital admission and death in the main analysis as severe disease was not prone to misclassification when comparing with internationally recognised criteria for severe covid-19. We applied both the matched and unmatched approaches to this analysis.
Finally, we also examined the interval between the second and third vaccine doses, because the dosing interval was possibly associated with both the administration and effectiveness of later doses, namely, the fourth dose. To this end, we devised three temporal categories framing the interval between the second and third dose: less than six months, six to nine months, and more than nine months. The second category (the most common by far) was the reference. This covariate was then adjusted for in the unmatched multiple test analysis. All analyses were performed using R Studio version 3.6 with the MatchIt, gee, geepack, and survival packages.
Patient and public involvement
Owing to use of retrospective data sources and lack of funding, no members of the public or patients were formally involved. Patients were informally involved in devising the research question through the team’s clinical practice, which included three practicing physicians and a nurse (SG, AP, TP, and GP). The manuscript was also internally reviewed by the leadership of MHS and by key policy officials in Israel.
Results
A total of 97 499 MHS members aged 60 years and older were eligible for the study and obtained at least one PCR test during the outcome period of 10 January to 13 March 2022. During that time period, 233 582 PCR tests were performed on 27 876 participants who received a fourth dose of the BNT162b2 vaccine and on 69 623 people who were eligible for a fourth dose but had not (yet) received one. Of those who had PCR tests, 572 (0.25%) participants were either admitted to hospital or died as a result of covid-19. Of 106 participants who died during the follow-up period, 77 had had their third doses only and 23 had had their fourth doses during the first three weeks after inoculation. Table 1 describes characteristics of the study population. The recipients of the fourth dose were more chronically ill overall than recipients of the third dose only, possibly related to targeted vaccination campaigns and stronger compliance to recommendations. These differences stress the need for the performed adjustment.
Table 1.
Characteristics of study participants with at least three BNT162b2 vaccine doses who were tested between 10 January and 13 March 2022. Data are number (%) of participants unless stated otherwise
| Characteristic | Vaccination status | Overall (n=97 499) | |
|---|---|---|---|
| Three doses (n=69 623) | Four doses (n=27 876) | ||
| Sex | |||
| Female | 37 777 (54.3%) | 15 583 (55.9%) | 53 360 (54.7%) |
| Male | 31 846 (45.7%) | 12 293 (44.1%) | 44 139 (45.3%) |
| Age (years) | |||
| Mean (standard deviation) | 70.1 (7.63) | 72.6 (8.66) | 70.8 (8.02) |
| Median (range) | 68.8 (60.0-104) | 71.2 (60.0-108) | 69.4 (60.0-108) |
| Socioeconomic status index (scale 1-10) | |||
| High (7-10) | 29 759 (42.7) | 10 723 (38.5) | 40 482 (41.5) |
| Middle (4-6) | 31 814 (45.7) | 13 243 (47.5) | 45 057 (46.2) |
| Low (1-3) | 8050 (11.6) | 3910 (14.0) | 11 960 (12.3) |
| Cardiovascular disease | |||
| No | 51 897 (74.5) | 19 807 (71.1) | 71 704 (73.5) |
| Yes | 17 726 (25.5) | 8069 (28.9) | 25 795 (26.5) |
| Diabetes mellitus | |||
| No | 53 004 (76.1) | 20 151 (72.3) | 73 155 (75.0) |
| Yes | 16 619 (23.9) | 7725 (27.7) | 24 344 (25.0) |
| Hypertension | |||
| No | 31 704 (45.5) | 11 034 (39.6) | 42 738 (43.8) |
| Yes | 37 919 (54.5) | 16 842 (60.4) | 54 761 (56.2) |
| Chronic kidney disease | |||
| No | 46 043 (66.1) | 16 366 (58.7) | 62 409 (64.0) |
| Yes | 23 580 (33.9) | 11 510 (41.3) | 35 090 (36.0) |
| Chronic obstructive pulmonary disease | |||
| No | 65 301 (93.8) | 25 929 (93.0) | 91 230 (93.6) |
| Yes | 4322 (6.2) | 1947 (7.0) | 6269 (6.4) |
| Obesity (body mass index ≥30) | |||
| No | 53 598 (77.0) | 20 904 (75.0) | 74 502 (76.4) |
| Yes | 16 025 (23.0) | 6972 (25.0) | 22 997 (23.6) |
| Immunosuppression | |||
| No | 60 690 (87.2) | 23 740 (85.2) | 84 430 (86.6) |
| Yes | 8933 (12.8) | 4136 (14.8) | 13 069 (13.4) |
| Assisted living | |||
| No | 68 892 (99.0) | 27 318 (98.0) | 96 210 (98.7) |
| Yes | 731 (1.0) | 558 (2.0) | 1289 (1.3) |
| Nursing home | |||
| No | 68 078 (97.8) | 26 182 (93.9) | 94 260 (96.7) |
| Yes | 1545 (2.2) | 1694 (6.1) | 3239 (3.3) |
| Test-taking behaviours (No of previous PCR tests) | |||
| 0 | 12 507 (18.0) | 4502 (16.2) | 17 009 (17.4) |
| 1-2 | 21 295 (30.6) | 7569 (27.2) | 28 864 (29.6) |
| ≥3 | 35 821 (51.4) | 15 805 (56.7) | 51 626 (53.0) |
| Third dose month | |||
| August 2021 | 64 172 (92.2) | 26 924 (96.6) | 91 096 (93.4) |
| September 2021 | 4407 (6.3) | 849 (3.0) | 5256 (5.4) |
| October 2021 | 995 (1.4) | 103 (0.4) | 1098 (1.1) |
| November 2021 | 49 (0.1) | 0 (0) | 49 (0.1) |
PCR=polymerase chain reaction.
Relative vaccine effectiveness against breakthrough SARS-CoV-2 infections
The matched analysis included 30 731 cases and 60 054 matched controls (table 2); 47% (n=14 303) of cases were matched to one control, 29% (n=8824) of cases had two controls, and 24% (n=7604) had at least three controls. Estimates of relative effectiveness by week since receipt of the fourth vaccine dose are detailed in table 2; table S1 shows the adjusted odds ratios from which these estimates were derived. Relative effectiveness of the fourth dose against SARS-CoV-2 infection peaked in the second week of the outcome period (three weeks after inoculation) at 65.1% (95% confidence interval 63.0% to 67.1%) compared with those individuals vaccinated with only three doses (table 2, fig 1). However, the relative effectiveness began to decline in the fourth week, and by week 5 it dropped back to levels roughly similar to those observed during the first week, before reaching 22.0% (4.9% to 36.1%) by week 9.
Table 2.
PCR test results among study participants with at least three BNT162b2 vaccine doses at different time points, between 10 January and 13 March 2022, and adjusted relative vaccine effectiveness against SARS-CoV-2 infection for participants receiving a fourth dose
| Time after receiving fourth dose | Matched analysis* | Multiple tests analysis† | |||||
|---|---|---|---|---|---|---|---|
| Positive PCR | Negative PCR | Adjusted rVE (%; 95% CI) | Positive PCR | Negative PCR | Adjusted rVE (%; 95% CI) | ||
| Received only three doses (reference) | 19 211 | 25 861 | — | 22 046 | 74 268 | — | |
| 7-13 days | 3263 | 11 376 | 57.7 (55.6 to 59.7) | 3555 | 23 423 | 46 (43.7 to 48.3) | |
| 14-20 days | 2131 | 6264 | 65.1 (63 to 67.1) | 2439 | 19 478 | 61.7 (59.8 to 63.6) | |
| 21-27 days | 1785 | 4972 | 64 (61.6 to 66.3) | 2033 | 17 484 | 63.9 (62 to 65.8) | |
| 28-34 days | 1416 | 3716 | 58.1 (54.8 to 61.1) | 1613 | 15 274 | 61 (58.6 to 63.2) | |
| 35-41 days | 930 | 2569 | 55 (50.6 to 58.9) | 1084 | 12 664 | 58.8 (55.7 to 61.6) | |
| 42-48 days | 684 | 1940 | 50.2 (44.5 to 55.3) | 796 | 11 146 | 57.1 (53.4 to 60.5) | |
| 49-55 days | 556 | 1501 | 42.5 (35.1 to 49.1) | 654 | 9719 | 52.8 (48.2 to 57) | |
| 56-62 days | 542 | 1364 | 33.4 (23.8 to 41.8) | 625 | 8214 | 42.6 (36.6 to 48.1) | |
| 63-69 days | 213 | 491 | 22 (4.9 to 36.1) | 256 | 2662 | 29.5 (18.1 to 39.2) | |
| Overall | 30 731 | 60 054 | — | 35 101 | 194 332 | — | |
PCR=polymerase chain reaction; rVE=relative vaccine effectiveness (calculated as 100%×(1−odds ratio) for each week since vaccination).
Matched analysis used 1:m matching, with up to five controls per case, based on seven factors: sex, age group, city of residence, socioeconomic status, calendar week of first test, month of receipt of the third dose, and a categorical variable for the living environment (a medical nursing home, assisted living facility, or private residence). Analysis also adjusted for underlying comorbidities and previous test-taking behaviour.
Odds ratios were adjusted for comorbidities, age group, nursing home or assisted living residence, previous test-taking behaviour, biological sex, calendar week of testing, residential socioeconomic status, and month of receipt of the third dose
Fig 1.
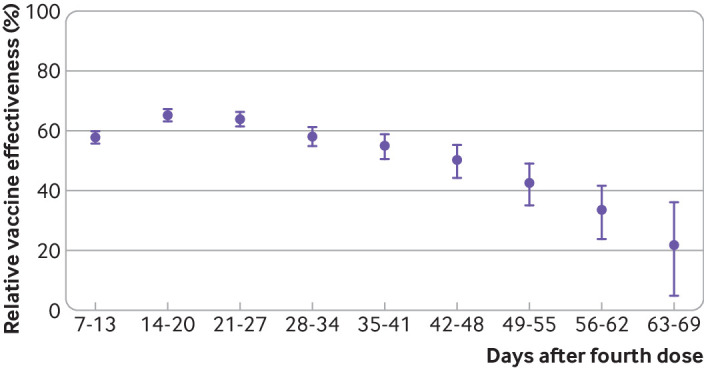
Adjusted fourth dose effectiveness of BNT162b2 vaccine against SARS-CoV-2 infection, relative to receipt of only three doses. Data based on results from primary matched analysis. Relative vaccine effectiveness=100%×(1−odds ratio) for each week since vaccination; error bars=95% confidence intervals
Relative vaccine effectiveness against severe covid-19 disease
A total of 494 hospital admissions and deaths associated with covid-19 were matched to 2184 controls, where 80% of cases had five controls and 12.4% had two to four controls. Table 3 presents estimates of relative vaccine effectiveness per three week interval since receipt of the fourth dose; table S3 shows the adjusted odds ratios from which these estimates were derived. Unlike relative effectiveness against SARS-CoV-2 infection, relative effectiveness of a fourth dose against severe covid-19 did not vary significantly as a function of time since vaccination, reaching 86.5% (95% confidence interval 63.4% to 95%) compared with a third dose during weeks 6-9 of the outcome period (table 3, fig 2). Owing to the relative paucity of observations in the matched analysis, we also estimated relative effectiveness by dividing the outcome period into early and late intervals after inoculation, with similar estimates (tables S4-S5).
Table 3.
PCR test results and severe covid-19 outcomes among study participants with at least three BNT162b2 vaccine doses at different time points, between 10 January and 13 March 2022, and adjusted relative vaccine effectiveness against severe covid-19 for participants receiving a fourth dose
| Time after receiving fourth dose | Matched analysis* | Multiple tests analysis† | |||||
|---|---|---|---|---|---|---|---|
| Severe disease | Negative PCR | Adjusted rVE (%; 95% CI) | Severe disease | Negative PCR | Adjusted rVE (%; 95% CI) | ||
| Received only three doses (reference) | 331 | 860 | — | 380 | 74 268 | — | |
| 7-27 days | 98 | 886 | 77.5 (69.7 to 83.2) | 117 | 60 385 | 73.3 (66.3 to 78.9) | |
| 28-48 days | 57 | 346 | 72.8 (58.8 to 82.1) | 64 | 39 084 | 73.9 (64.3 to 80.9) | |
| 49-69 days | 8 | 92 | 86.5 (63.4 to 95) | 11 | 20 595 | 86.1 (73.4 to 92.8) | |
| Overall | 494 | 2184 | — | 572 | 194 332 | — | |
PCR=polymerase chain reaction; rVE=relative vaccine effectiveness (calculated as 100%×(1−odds ratio) for each week since vaccination).
Matched analysis used 1:m matching, with up to five controls per case, based on seven factors: sex, age group, city of residence, socioeconomic status, calendar week of first test, month of receipt of the third dose, and a categorical variable for the living environment (a medical nursing home, assisted living facility, or private residence). Analysis also adjusted for underlying comorbidities and previous test-taking behaviour.
Odds ratios were adjusted for comorbidities, age group, nursing home or assisted living residence, previous test-taking behaviour, biological sex, calendar week of testing, residential socioeconomic status, and month of receipt of the third dose
Fig 2.
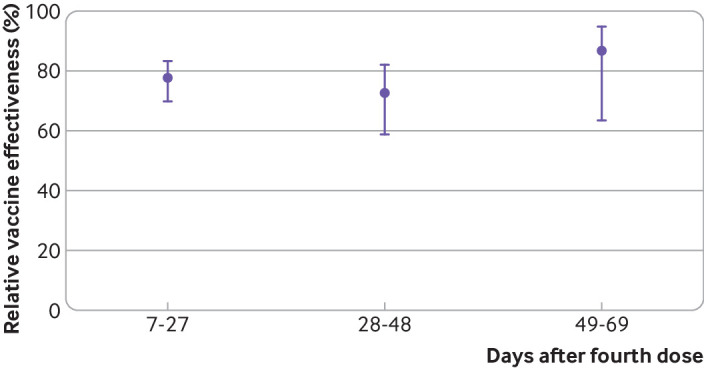
Adjusted fourth dose effectiveness of BNT162b2 vaccine against severe covid-19, relative to receipt of only three doses. Severe disease was defined as hospital admission or death related to covid-19. Relative vaccine effectiveness=100%×(1−odds ratio) for each week since vaccination; error bars=95% confidence intervals
Unmatched multiple tests analysis
The unmatched multiple test analysis included 229 433 PCR tests, of which 35 101 (15.3%) were positive and 572 were admitted to hospital or died due to covid-19 (table 2 and table 3). Relative vaccine effectiveness was similar to the primary analysis for both SARS-CoV-2 infection and severe covid-19, although the decline in these estimates against infection with time since inoculation was slightly attenuated (table 2, table 3, and figs S1-S2).
Most comorbidities were not significantly associated with the odds of infection, although chronic obstructive pulmonary disease and chronic kidney disease were associated with slightly lower odds of infection, possibly because of risk averse behaviour (tables S1-S2). However, comorbidities were mostly associated with an increased odds of severe disease (table S6). Likewise, individuals aged 70 years or older had a lower odds of infection than those younger than 70 years, but a significantly higher odds of severe disease (odds ratio 2.34, 95% confidence interval 1.87 to 2.93). Participants receiving three or more PCR tests before the study period were associated with a significantly reduced odds of infection and severe disease. Importantly, the unmatched multiple test analysis highlighted the importance of controlling for test week, where the odds of infection were highest between late January and early February 2022. Additionally, residence in a nursing home or an assisted living facility was associated with a significantly lower odds of infection, as hypothesised, but was not associated with severe disease. Having received the third dose after August 2021 was associated with a slightly lower odds of infection, but was not significantly associated with the odds of hospital admission or death associated with covid-19; figure S5 shows the distribution of months between the third and fourth dose.
Additional sensitivity analyses
The attack rate analysis pointed to the importance of exposure, where each 1% increase in the attack rate in each city of residence was associated with a 10.12-fold (95% confidence interval 4.17 to 24.58) increase in the odds of SARS-CoV-2 infection. However, adjusting for the attack rate did not change estimates of relative effectiveness of the fourth vaccine dose (table S7). Estimates of the relative effectiveness of a fourth dose against criteria-defined severe covid-19 (that is, cases that met the National Institutes of Health’s definition of severe disease) were similar to those estimates for fourth doses against any hospital admission or death related to covid-19, without significant differences in relative effectiveness during the 10 week follow-up period (tables S8-S10, figs S3-S4).
Finally, when examining the interval between the second and third vaccine dose, we found that having received the second dose more than nine months before the third one was associated with a significantly decreased odds of infection with SARS-CoV-2 (odds ratio 0.14, 95% confidence interval 0.02 to 0.95), compared with having received the second dose between six and nine months before the third dose (or first booster shot; table S11). However, only 52 individuals in our analysis had an interval of more than nine months between the second and third dose (fig S5). Again, estimates of relative vaccine effectiveness of the fourth dose were largely unchanged.
Discussion
Principal findings
This study investigated the relative vaccine effectiveness of a fourth dose of the BioNTech-Pfizer mRNA BNT162b2 vaccine compared with receiving three doses only, against both infection with the SARS-CoV-2 omicron variant and infection resulting in severe covid-19, assessed by hospital admissions and death, among individuals aged 60 years and older in Israel. We found that, relative to three vaccine doses, a fourth dose initially provided additional protection against both SARS-CoV-2 infection and severe disease (assessed by hospital admissions and deaths). However, relative vaccine effectiveness against infection quickly decreased over time, peaking during the third week after inoculation at 65.1% (95% confidence interval 63.0% to 67.1%) and declining to 22.0% (4.9% to 36.1%) by the end of the 10 week follow-up period. Similar results were obtained with different analytical approaches.
Comparison with other studies
The waning of vaccine effectiveness against SARS-CoV-2 infection is consistent with previous observations of the second and third doses of the BNT162b2b vaccine.10 11 Nonetheless, compared with the previously demonstrated waning pattern of the relative vaccine effectiveness of three doses versus two doses in real world settings (which begins around three months after inoculation), the relative effectiveness of the fourth dose against infection appears to wane sooner, just as waning after the third dose was sooner than after the second dose.11 26 This more rapid decline could be explained by a reduced effectiveness of the BNT162b2b vaccine against the omicron variant.27 28 However, the effect of waning protection of sequential vaccine doses is difficult to differentiate from the real world circulation of variants.
Additionally, we need to consider that frequent stimulation with the same mRNA vaccine triggers an immunological response that is yet to be fully understood in terms of duration, effectiveness, and patterns of waning when exposed to different variants. In this respect, the interval between doses could have an impact on the duration of immunity. In Israel, the intervals between doses have been short compared with other countries. The second dose of BNT162b2b was issued between three and four weeks after the first, and the third dose was initially given to those who received the second dose at least five months before; however, a few months later, authorities shortened the required interval to three months in the light of a new increase of SARS-CoV-2 infections.29 The fourth dose, as mentioned previously, required a minimal interval of four months. Nonetheless, a growing number of studies suggest that an extended dosing interval amplifies both the humoral immune response30 and perhaps the cellular response,31 and is an effective global strategy given the discrepancies between regions with high and low vaccination coverage.32 But even recent studies have so far only investigated the second dose and have not evaluated responses to the omicron variant.30 33 Our present analysis showed that receiving the third dose later (that is, after August 2021) was associated with slight protection against infection, but was not significantly associated with the odds of hospital admission or death related to covid-19. Although our findings point to a protective effect of a longer interval between the second and third doses, owing to Israeli regulations, the distribution in the population is narrow overall, and the number of people with longer intervals is small; thus, these questions should be further researched in other countries.
Unlike the relative vaccine effectiveness against SARS-CoV-2 infection, the relative effectiveness of a fourth dose against severe covid-19 was maintained at high level (>72%) throughout the 10 week follow-up period. Sustained vaccine effectiveness against severe disease has been shown for previous doses also.20 34 However, severe disease was a relatively rare event, occurring in <1% of participants receiving fourth doses or third doses only. The difference between waning of protection against infection and sustained protection against severe disease could imply a different underlying immunological mechanism. A recent clinical study by Terreri et al33 suggested that breakthrough infections could be explained by a decrease in the concentration of specific antibodies, which are probably not generated by a parenteral vaccine and are slow to reach nasopharyngeal mucosal sites of viral entry. By contrast, immunological memory (memory B and T cells) does not wane, and might be important for protection against severe disease.33 However, this hypothesis does not explain why a third vaccine dose protects against severe disease compared with a second one,6 and why a fourth improves the protection of a third dose. A stabilisation effect of additional doses on immune memory should be further investigated, as well as studies on mucosal vaccines.35
Strengths and limitations of this study
Our analysis had several limitations. Firstly, to provide timely evidence of the relative vaccine effectiveness of a fourth dose of BNT162b2b vaccine, we were only able to include 10 weeks of data. Although the pattern of a short term increase in protection against SARS-CoV-2 infection followed by waning is already present, long term vaccine effectiveness needs to be evaluated and is particularly important for estimates of relative effectiveness of the fourth dose against severe covid-19. Protection from previous doses against severe disease has been shown to wane more slowly than protection against infection.20 34 Nonetheless, our study suggests a more rapid waning of protection against infection from a fourth dose than from previous doses; therefore, waning of relative vaccine effectiveness against hospital admission and death related to covid-19 needs to be further examined over a longer period.
A second limitation stems from the varying dominance of different SARS-CoV-2 variants over time. The period after fourth dose vaccination in Israel has been dominated by the omicron variant, which makes assessing the relative effectiveness of the fourth dose against other covid-19 variants difficult—a well recognised limitation of real world analyses during this pandemic.6 36 37 Furthermore, because the eligible population for a fourth dose comprised individuals aged 60 years or older, we cannot infer similar relative vaccine effectiveness and potential waning in younger people. Additionally, fourth dose recipients were more unwell overall, possibly stemming from targeted vaccination campaigns and previous rollout policies. Adjusting for comorbidities by virtue of an available comprehensive medical history, as well as adjustment and matching by other factors including timing of the third vaccine dose, residential and social factors, and previous testing renders residual confounding less likely.
The lack of pre-defined PCR testing protocols implemented in the study population also presented a challenge. This limitation has been discussed extensively in previous covid-19 observational studies and could lead to potential biases relating to healthcare seeking behaviour.6 11 38 39 The test negative design attempts to mitigate this potential bias. Firstly, those participants not tested are not eligible to be considered uninfected controls, thus reducing potential misclassification of SARS-CoV-2 infection status (which is more likely to occur in a cohort study), especially when the omicron variant was spreading rapidly and patients with no or mild symptoms might not be tested. Secondly, previous studies37 have shown that improved healthcare seeking behaviour might be related both to improved vaccine uptake as well as behaviours that could influence the risk of SARS-CoV-2 infection (eg, mask wearing, social distancing, and handwashing) or to more severe outcomes if infected (such as chronic disease management). Thus, healthcare seeking behaviour could confound the association between timely receipt of a fourth vaccine dose and SARS-CoV-2 related outcomes.
These potential confounders are illustrated by a directed acyclic graph (fig 3), following the conceptual scheme presented by Sullivan et al.40 When restricting the analysis only to those individuals with measurable and reasonable healthcare seeking behaviour (fig 3C)—in a similar concept yet different execution to the adjustment of other confounders (shown in fig 3B)—this potential confounding does not bias the relation between exposure and outcome. Because healthcare seeking behaviour is not a binary variable, this solution is insufficient when applied to real world data analysis. Nevertheless, a more comprehensive discussion should resolve any potential collider bias. The criterion for minimal eligibility in a test negative design is the fact that a patient was tested, so this selection bias could create a scenario where we condition on the collider (testing is a common effect of both healthcare seeking behaviour and potentially of the severity of the SARS-CoV-2 manifestation, which prompts the patient to be tested, as seen in fig 3C).41 However, controlling for both healthcare seeking behaviour and the propensity to be tested (inherent to the design) blocks the biasing path, albeit incompletely, under the limitation of measuring healthcare seeking behaviour adequately (fig 3E).42
Fig 3.
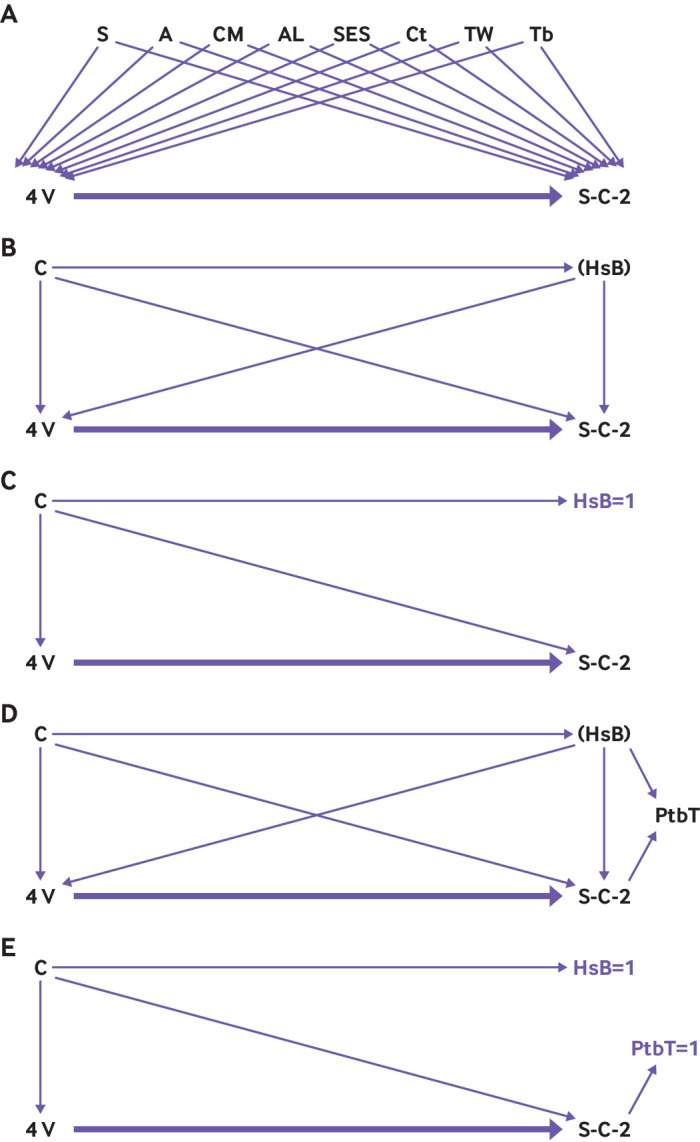
Directed acyclic graph illustrating biases and their attempted mitigation in this test negative design study. S=sex; A=older age groups; CM=comorbidities; AL=assisted living or nursing home; SES=socioeconomic status; Ct=city of residence; TW=week of testing; Tb=time passed since third dose or first booster. (A) Known confounders by a priori knowledge of previous studies possibly confounding the association between time from the fourth dose (4V) and SARS-CoV-2 related outcomes (S-C-2). (B) For simplicity, all confounders in panel A were combined as C; healthcare seeking behaviour (HsB) possibly confounds the association between a fourth dose (4V) and S-C-2; additionally, some confounders in panel A could be causes of HsB, such as age or comorbidities. (C) In this test negative design, only study participants with a measurable and reasonable healthcare seeking behaviour (that is, HsB=1) were included, thus removing this biasing path. (D) Healthcare seeking behaviour potentially influences the propensity to be tested (PtbT); hence, when a test negative design includes only patients tested for SARS-CoV-2 (or conditioning on the possible collider, PtbT), this inclusion could create a collider bias. (E) When healthcare seeking behaviour is controlled, as attempted by the test negative design, the path is blocked
Healthcare in Israel is free and universal to all residents, as are PCR tests for the study population. Therefore, implementing this study design in Israel increases the likelihood of engagement in two groups of people who have already chosen to be at least thrice vaccinated. But alongside its advantages, generalisability of the test negative design is limited, because of the association in other populations who did not choose to be tested.42 Nonetheless, supporting this design were previous covid-19 studies implementing a test negative design that yielded comparable results to cohort studies on this population,6 11 while presenting diminished bias in the short term outcomes of vaccinee effectiveness.
Some studies have included rapid antigen tests in their analysis, treating them equally to PCR tests, whereas this study did not. Although our study had fewer observations by excluding rapid antigen tests, such tests are generally considered less reliable, and negative at-home tests are not reported. Furthermore, a policy was in place to use PCR tests for the entire examined age group (≥60 years, eligible for a fourth dose) during the follow-up period, making testing accessible to the study population.
Lastly, the relative metric of our main analysis warrants discussion. We compared the effectiveness of the fourth dose to that of a third one, thereby estimating the relative vaccine effectiveness rather than the absolute vaccine effectiveness comparing fourth dose recipients with unvaccinated individuals.21 Admittedly, the nature of relative vaccine effectiveness requires contextuality in its interpretation.21 Nonetheless, two overarching principles guided our choice. Firstly, owing to the rapid rollout and high compliance rates, most of the SARS-CoV-2 naive population older than 60 years had received at least two vaccine doses.1 Therefore, apart from potential scarcity of data in comparing to unvaccinated individuals, this eligible yet unvaccinated population in a massively campaigned environment such as Israel is plausibly different in terms health related behaviour, which could introduce a bias in rendering it the reference group,43 as has been pointed out in previous vaccination studies of Israeli populations measuring the effectiveness of three doses relative to two doses.6 11 44 Secondly, the recent global spread of the omicron variant obliges policy makers worldwide to issue acute recommendations, equipped with limited information, as has been the case throughout the pandemic. The immediate question facing many countries now is whether, in the light of the rapid spread and recent studies pointing to waning of the third vaccine dose,10 11 45 a second booster should be recommended. This question is specific to those individuals who are eligible for the fourth dose—that is, those who have already received three vaccine doses. Therefore, the relative nature between the fourth dose and three doses is inherent to this healthcare policy question. Focusing efforts on additional doses, of course, has important implications on resource allocation.
Conclusions and policy implications
The implications of our finding should be considered in the light of local and global vaccine resource allocation, as the fourth vaccine dose is deliberated in high risk older populations. This study has shown additional protection of the fourth dose against both SARS-CoV-2 infection and severe covid-19 relative to three doses. However, the relative vaccine effectiveness against infection varied over time and waned sooner than that of the third dose, by peaking at 65.1% three weeks after inoculation and falling to 22.0% by the end of the 10 week follow-up period. Relative effectiveness of a fourth dose against severe covid-19 stayed at a high level (>72%) throughout follow-up, although severe disease was a rare event, occurring in <1% of study participants receiving fourth doses or third doses only.
What is already known on this topic
Waning protection of the second and third doses of the BioNTech-Pfizer (BNT162b2) vaccine against SARS-CoV-2 infection has been shown, although protection against severe covid-19 remains high
Administration of a fourth vaccine dose is currently being considered globally, although its effectiveness after a month is unknown, as well as the effectiveness of populations at risk (eg, residents of long care facilities) or effect of specific comorbidities
The rapid spread of the omicron variant (B.1.1.529) alongside evidence of waning effectiveness of the third dose of the BioNTech-Pfizer (BNT162b2) vaccine against SARS-CoV-2 infection prompted Israeli authorities to administer a fourth dose in January 2022
What this study adds
Relative effectiveness of the fourth dose of the BNT162b2 vaccine compared with a third dose against SARS-CoV-2 infection wanes sooner than the relative effectiveness of previous doses
Relative effectiveness of the fourth dose against severe covid-19 stayed at a high level throughout the 10 week follow-up, although severe disease was a rare event, occurring in <1% of study participants receiving four doses or three doses only
By the fifth week after vaccination, relative effectiveness of the fourth dose against SARS-CoV-2 infection dropped back to levels similar to those observed during the first week
Web extra.
Extra material supplied by authors
Web appendix: Supplementary materials
Contributors: SG and TP conceived the study idea and designed the study. SG performed the literature search. SG, YS, and TP contributed to the methodology. GP, YS, and SG contributed to the data extraction. YS performed the statistical analysis. SG, YS, and TP interpreted the analysis. SG and TP drafted the manuscript. GP, AP, and VEP critically revised the manuscript. AP provided administrative support. TP supervised the project. All authors gave final approval for the version to be submitted. SG is the guarantor. The corresponding author attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted.
Funding: Research reported in this publication was supported by the National Institute of Allergy and Infectious Diseases of the US National Institutes of Health (award R01AI137093 (VEP)). The content is solely the responsibility of the authors and does not necessarily represent the official views of the US National Institutes of Health.
Competing interests: All authors have completed the ICMJE uniform disclosure form at www.icmje.org/disclosure-of-interest/ and declare: support for VEP from the National Institutes of Health for the submitted work; VEP has received reimbursement from Merck and Pfizer for travel to scientific input engagements unrelated to the topic of this manuscript and is a member of the WHO Immunization and Vaccine-related Implementation Research Advisory Committee; all other authors declare they have no conflict of interest.
The lead author SG affirms that the manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned (and, if relevant, registered) have been explained.
Dissemination to participants and related patient and public communities: Dissemination of this study to patients, clinicians, researchers, and the public will be done through the Maccabi Research and Innovation website (https://www.ksminnovation.com), through the researchers’ private social media accounts and the Maccabi Healthcare Services’ social media platforms, including LinkedIn and Twitter, and potentially through a dedicated press release with a plain language summary, including direct engagements and interview with the media, locally and globally. We will share the study’s information and conclusions with clinicians and patients through national and international conferences (in epidemiology, infectious diseases, and public health).
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics statements
Ethical approval
This study was approved by the Maccabi Healthcare Services’ institutional review board. Owing to the retrospective design of the study, informed consent was waived by the institutional review board, because all identifying details of the participants were removed before computational analysis.
Data availability statement
According to the Israel Ministry of Health regulations, individual level data cannot be shared openly. Specific requests for remote access to de-identified community level data should be referred to Kahn Sagol Maccabi Research and Innovation Centre, Maccabi Healthcare Services. Specific requests for remote access to the code used for data analysis should be referred to Kahn Sagol Maccabi Research and Innovation Centre, Maccabi Healthcare Services.
References
- 1.COVID-19 in Israel dashboard. Isr. Minist. Heal. https://datadashboard.health.gov.il/COVID-19/general (accessed 15 Apr 2022).
- 2. Seow J, Graham C, Merrick B, et al. Longitudinal observation and decline of neutralizing antibody responses in the three months following SARS-CoV-2 infection in humans. Nat Microbiol 2020;5:1598-607. 10.1038/s41564-020-00813-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ruopp MD, Strymish J, Dryjowicz-Burek J, Creedon K, Gupta K. Durability of SARS-CoV-2 IgG Antibody Among Residents in a Long-Term Care Community. J Am Med Dir Assoc 2021;22:510-1. 10.1016/j.jamda.2021.01.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Shrotri M, Navaratnam AMD, Nguyen V, et al. Virus Watch Collaborative . Spike-antibody waning after second dose of BNT162b2 or ChAdOx1. Lancet 2021;398:385-7. 10.1016/S0140-6736(21)01642-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mizrahi B, Lotan R, Kalkstein N, et al. Correlation of SARS-CoV-2-breakthrough infections to time-from-vaccine. Nat Commun 2021;12:6379. 10.1038/s41467-021-26672-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Patalon T, Gazit S, Pitzer VE, Prunas O, Warren JL, Weinberger DM. Odds of Testing Positive for SARS-CoV-2 Following Receipt of 3 vs 2 Doses of the BNT162b2 mRNA Vaccine. JAMA Intern Med 2022;182:179-84. 10.1001/jamainternmed.2021.7382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bar-On YM, Goldberg Y, Mandel M, et al. Protection of BNT162b2 Vaccine Booster against Covid-19 in Israel. N Engl J Med 2021;385:1393-400. 10.1056/NEJMoa2114255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Levine-Tiefenbrun M, Yelin I, Alapi H, et al. Viral loads of Delta-variant SARS-CoV-2 breakthrough infections after vaccination and booster with BNT162b2. Nat Med 2021;27:2108-10. 10.1038/s41591-021-01575-4. [DOI] [PubMed] [Google Scholar]
- 9.SARS-CoV-2 variants in analyzed sequences, Israel. https://ourworldindata.org/grapher/covid-variants-area?country=~ISR (accessed 30 Dec 2021).
- 10. Levine-Tiefenbrun M, Yelin I, Alapi H, et al. Waning of SARS-CoV-2 booster viral-load reduction effectiveness. Nat Commun 2022;13:1237. 10.1038/s41467-022-28936-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Patalon T, Saciuk Y, Ma M, et al. Waning Effectiveness of the Third Dose of the BNT162b2 mRNA COVID-19 Vaccine. medRxiv 2022;2022.02.25.22271494. 10.1101/2022.02.25.22271494 [DOI] [PMC free article] [PubMed]
- 12.Israeli Ministry of Health. Fourth Dose of the Vaccine Approved for People with a Weakened Immune System. 2021.https://www.gov.il/en/departments/news/30122021-05
- 13. Weitzman D, Chodick G, Shalev V, Grossman C, Grossman E. Prevalence and factors associated with resistant hypertension in a large health maintenance organization in Israel. Hypertension 2014;64:501-7. 10.1161/HYPERTENSIONAHA.114.03718. [DOI] [PubMed] [Google Scholar]
- 14. Chodick G, Heymann AD, Shalev V, Kookia E. The epidemiology of diabetes in a large Israeli HMO. Eur J Epidemiol 2003;18:1143-6. 10.1023/B:EJEP.0000006635.36802.c8. [DOI] [PubMed] [Google Scholar]
- 15. Coresh J, Turin TC, Matsushita K, et al. Decline in estimated glomerular filtration rate and subsequent risk of end-stage renal disease and mortality. JAMA 2014;311:2518-31. 10.1001/jama.2014.6634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Dagan N, Barda N, Kepten E, et al. BNT162b2 mRNA Covid-19 Vaccine in a Nationwide Mass Vaccination Setting. N Engl J Med 2021;384:1412-23. 10.1056/NEJMoa2101765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Dean NE, Hogan JW, Schnitzer ME. Covid-19 Vaccine Effectiveness and the Test-Negative Design. 2021;385:1431-3. 10.1056/NEJMe2113151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Abu-Raddad LJ, Chemaitelly H, Butt AA, National Study Group for COVID-19 Vaccination . Effectiveness of the BNT162b2 Covid-19 Vaccine against the B.1.1.7 and B.1.351 Variants. N Engl J Med 2021;385:187-9. 10.1056/NEJMc2104974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gazit S, Shlezinger R, Perez G, et al. SARS-CoV-2 Naturally Acquired Immunity vs. Vaccine-induced Immunity, Reinfections versus Breakthrough Infections: a Retrospective Cohort Study. Clin Infect Dis 2022;ciac262. 10.1093/cid/ciac262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chemaitelly H, Tang P, Hasan MR, et al. Waning of BNT162b2 Vaccine Protection against SARS-CoV-2 Infection in Qatar. N Engl J Med 2021;385:e83. 10.1056/NEJMoa2114114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lewis NM, Chung JR, Uyeki TM, Grohskopf L, Ferdinands JM, Patel MM. Interpretation of Relative Efficacy and Effectiveness for Influenza Vaccines. Clin Infect Dis 2021;7:ciab1016. 10.1093/cid/ciab1016. [DOI] [PubMed] [Google Scholar]
- 22. Boslaugh S. Encyclopedia of epidemiology. Sage Publications, 2007. [Google Scholar]
- 23. Goldberg Y, Mandel M, Bar-On YM, et al. Waning Immunity after the BNT162b2 Vaccine in Israel. N Engl J Med 2021;385:e85. 10.1056/NEJMoa2114228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ballinger GA. Using Generalized Estimating Equations for Longitudinal Data Analysis. 2016;7:127-50. 10.1177/1094428104263672 [DOI] [Google Scholar]
- 25.Clinical Spectrum of SARS-CoV-2 Infection. National Institutes of Health. 2021. https://www.covid19treatmentguidelines.nih.gov/overview/clinical-spectrum/
- 26. Chemaitelly H, Ayoub HH, Almukdad S, et al. Duration of protection of BNT162b2 and mRNA-1273 COVID-19 vaccines against symptomatic SARS-CoV-2 Omicron infection in Qatar. medRxiv 2022;2022.02.07.22270568. 10.1101/2022.02.07.22270568 [DOI]
- 27. Girard B, Tomassini JE, Deng W, et al. mRNA-1273 Vaccine-elicited Neutralization of SARS-CoV-2 Omicron in Adolescents and Children. medRxiv 2022. 10.1101/2022.01.24.22269666 [DOI]
- 28. Abu-Raddad LJ, Chemaitelly H, Ayoub HH, et al. Effect of mRNA Vaccine Boosters against SARS-CoV-2 Omicron Infection in Qatar. N Engl J Med 2022;9. 10.1056/NEJMoa2200797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Israel to offer COVID boosters 3 months after second vaccine dose. Times Isr. https://www.timesofisrael.com/israel-to-offer-covid-boosters-3-months-after-second-vaccine-dose/ (accessed 20 Apr 2022).
- 30. Hall VG, Ferreira VH, Wood H, et al. Delayed-interval BNT162b2 mRNA COVID-19 vaccination enhances humoral immunity and induces robust T cell responses. Nat Immunol 2022;23:380-5. 10.1038/s41590-021-01126-6 [DOI] [PubMed] [Google Scholar]
- 31. Payne RP, Longet S, Austin JA, et al. PITCH Consortium . Immunogenicity of standard and extended dosing intervals of BNT162b2 mRNA vaccine. Cell 2021;184:5699-5714.e11. 10.1016/j.cell.2021.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Skowronski DM, Febriani Y, Ouakki M, et al. Two-dose SARS-CoV-2 vaccine effectiveness with mixed schedules and extended dosing intervals: test-negative design studies from British Columbia and Quebec, Canada. Clin Infect Dis 2022;19:ciac290. 10.1093/cid/ciac290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Terreri S, Piano Mortari E, Vinci MR, et al. Persistent B cell memory after SARS-CoV-2 vaccination is functional during breakthrough infections. Cell Host Microbe 2022;30:400-408.e4. 10.1016/j.chom.2022.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Tenforde MW, Self WH, Naioti EA, et al. IVY Network Investigators. IVY Network . Sustained Effectiveness of Pfizer-BioNTech and Moderna Vaccines Against COVID-19 Associated Hospitalizations Among Adults - United States, March-July 2021. MMWR Morb Mortal Wkly Rep 2021;70:1156-62. 10.15585/mmwr.mm7034e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lycke N. Recent progress in mucosal vaccine development: potential and limitations. Nat Rev Immunol 2012;12:592-605. 10.1038/nri3251 [DOI] [PubMed] [Google Scholar]
- 36. Levine-Tiefenbrun M, Yelin I, Alapi H, et al. Viral loads of Delta-variant SARS-CoV2 breakthrough infections following vaccination and booster with the BNT162b2 vaccine. medRxiv 2021;2021.08.29.21262798. 10.1101/2021.08.29.21262798 [DOI] [PubMed]
- 37. Gazit S, Mizrahi B, Kalkstein N, et al. BNT162b2 mRNA Vaccine Effectiveness Given Confirmed Exposure: Analysis of Household Members of COVID-19 Patients. Clin Infect Dis 2021;24:ciab973. 10.1093/cid/ciab973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Bar-On YM, Goldberg Y, Mandel M, et al. Protection of BNT162b2 Vaccine Booster against Covid-19 in Israel. N Engl J Med 2021;385:1393-400. 10.1056/NEJMoa2114255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Mizrahi B, Lotan R, Kalkstein N, et al. Correlation of SARS-CoV-2-breakthrough infections to time-from-vaccine. Nat Commun 2021;12:6379. 10.1038/s41467-021-26672-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Sullivan SG, Tchetgen Tchetgen EJ, Cowling BJ. Theoretical Basis of the Test-Negative Study Design for Assessment of Influenza Vaccine Effectiveness. Am J Epidemiol 2016;184:345-53. 10.1093/aje/kww064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Holmberg MJ, Andersen LW. Collider Bias. JAMA 2022;327:1282-3. 10.1001/jama.2022.1820. [DOI] [PubMed] [Google Scholar]
- 42. Westreich D, Hudgens MG. Invited Commentary: Beware the Test-Negative Design. Am J Epidemiol 2016;184:354-6. 10.1093/aje/kww063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Andrews N, Stowe J, Kirsebom F, et al. Effectiveness of COVID-19 booster vaccines against COVID-19-related symptoms, hospitalization and death in England. Nat Med 2022;28:831-7. 10.1038/s41591-022-01699-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Patel MK. Booster Doses and Prioritizing Lives Saved. N Engl J Med 2021;385:2476-7. 10.1056/NEJMe2117592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Prunas O, Weinberger DM, Pitzer VE, et al. Waning Effectiveness of the BNT162b2 Vaccine Against Infection in Adolescents. medRxiv 2022;2022.01.04.22268776. 10.1101/2022.01.04.22268776 [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Web appendix: Supplementary materials
Data Availability Statement
According to the Israel Ministry of Health regulations, individual level data cannot be shared openly. Specific requests for remote access to de-identified community level data should be referred to Kahn Sagol Maccabi Research and Innovation Centre, Maccabi Healthcare Services. Specific requests for remote access to the code used for data analysis should be referred to Kahn Sagol Maccabi Research and Innovation Centre, Maccabi Healthcare Services.


