Abstract
Objective
The ongoing COVID-19 pandemic has caused an indefinite delay to cancer screening programs worldwide. This study aims to explore the impact on breast cancer screening outcomes such as mammography and diagnosis rates.
Methods
We searched Ovid MEDLINE, Ovid Embase, medRxiv and bioRxiv between January 2020 to October 2021 to identify studies that reported on the rates of screening mammography and breast cancer diagnosis before and during the pandemic. The effects of ‘lockdown’ measures, age and ethnicity on outcomes were also examined. All studies were assessed for risk of bias using the Newcastle-Ottawa Scale (NOS). Rate ratios were calculated for all outcomes and pooled using standard inverse-variance random effects meta-analysis.
Results
We identified 994 articles, of which 7 registry-based and 24 non-registry-based retrospective cohort studies, including data on 4,860,786 and 629,823 patients respectively across 18 different countries, were identified. Overall, breast cancer screening and diagnosis rates dropped by an estimated 41–53% and 18–29% respectively between 2019 and 2020. No differences in mammogram screening rates depending on patient age or ethnicity were observed. However, countries that implemented lockdown measures were associated with a significantly greater reduction in mammogram and diagnosis rates between 2019 and 2020 in comparison to those that did not.
Conclusion
The pandemic has caused a substantial reduction in the screening and diagnosis of breast cancer, with reductions more pronounced in countries under lockdown restrictions. It is early yet to know if delayed screening during the pandemic translates into higher breast cancer mortality.
Keywords: Breast neoplasms, COVID-19, screening, systematic review, meta-analysis
Introduction
Mass screening programs for colorectal, cervical, and breast neoplasms are available in many developed countries.1–3 These programs have been widely successful in reducing cancer deaths in multiple settings by identifying premalignant conditions and early-stage malignancies to permit early treatment.1–3 For instance, it is estimated that the BreastScreen Program in Australia has prevented approximately 8 deaths for every 1000 women who participate in mammographic screening, even at a 56% turnout rate.4,5 Similarly, in Europe it has been reported that screening mammography prevented 21,680 deaths attributed to breast cancer each year.6
The ongoing COVID-19 pandemic has affected the delivery of cancer screening services worldwide.7 In many nations, healthcare resources were redirected to combat the COVID-19 pandemic; hence, cancer prevention and control services were categorised as non-urgent medical procedures.8 A survey among 66 participating centres across 35 countries from the International Cancer Screening Network reported that 65 settings (97%) experienced a disruption to cancer screening activities, while 60 settings (91%) from 31 countries suspended their cancer screening programs during the pandemic.9 In particular, the national screening program for breast cancer was suspended entirely by the Italian government between March and April 2020 in line with the nationwide full lockdown to slow the spread of coronavirus.10 Despite the gradual resumption of the screening program from May 2020, 980,994 fewer invitations were sent out for mammography screening in Italy from January to December 2020 compared with 2021.10
Currently, little information is available about the effect of the COVID-19 pandemic on breast cancer screening and diagnosis rates worldwide. Therefore, a rapid review was carried out to investigate the change in rates of mammographic screening and breast cancer diagnosis during the COVID-19 pandemic in comparison to the same time period prior to the beginning of the pandemic. The effects of ‘lockdown’ policies, age and ethnicity on cancer screening rates were studied, as well as the effects of the pandemic on the diagnosis of different stages of breast cancer. We anticipate that the findings of this review will inform policymaking with respect to the impact of the postponement of breast cancer screening programs, as well as raising attention on the impact of the pandemic on at-risk populations outside of the developed world.
Methods
Data sources and search strategy
This rapid review is reported in accordance with the PRISMA 2020 statement.11 In the interest of time, the authors did not pre-register the protocol for this review. An extensive electronic search of MEDLINE and Embase (both via Ovid) was conducted on October 1st, 2021 to identify relevant articles using free-text terms and controlled vocabulary relating to “breast cancer”, “cancer screening” and “cancer diagnosis” and the built-in COVID-19 filter in the Ovid search engine. A manual search of the pre-print servers medRxiv and bioRxiv was also performed. Given the aim of the review was to explore COVID-19 related impacts on breast cancer screening and diagnosis rates, reports published before January 1st, 2020 were excluded. No language restrictions were placed on any of the searches. The full electronic search strategy is reported in Supplementary Table S1.
Eligibility criteria
Retrospective cohort studies that examined the impact of COVID-19 on mammographic screening and breast cancer diagnosis rates were eligible for this review. Specifically, studies that reported the number of women eligible for mammographic screening and/or who were diagnosed with breast cancer during the pandemic were considered. January 1st, 2020 was designated as the start of the pandemic, in line with the announcement of a mysterious pneumonia caused by the novel coronavirus in Wuhan, China by the World Health Organisation (WHO).12 Studies that did not compare breast cancer screening and/or diagnosis rates during the pandemic with the same period of time in 2019 were excluded. Reports on breast cancer screening through clinical breast examination and/or self-breast examination were also omitted. Only studies that reported on real-world data were considered; thus, studies using simulated data were also excluded.
Selection procedures
All references returned from the search were imported into Covidence (Covidence systematic review software, Veritas Health Innovation, Melbourne, Australia), a database management system for systematic reviews, and deduplicated.13 Titles and abstracts, then full-text articles (when available), were then screened against the selection criteria outlined above. Articles that were found to be eligible after both stages were included for further analysis. If the same data were reported by more than one article, articles were merged into a single study for the extraction process. The entire selection process was completed by a single reviewer (JSN) in view of restricted time and resources.
Data extraction
A data extraction template was developed in Covidence based on a pre-prepared checklist.13 A test run was conducted on several studies before commencing the formal data extraction process. Details including the surname of first author, year of publication, location of study, number of participants, implementation of lockdown measures, duration of the study, and count data for the key outcomes of interest (screening and diagnosis rates) were extracted. A single reviewer completed the data collection process from each report in view of limited time and resources. When required outcome data was unavailable, the authors of the studies were contacted.
Methodological quality and reporting biases
The Newcastle-Ottawa Scale (NOS) for the assessment of cohort studies was used to evaluate the methodological quality of eligible studies. The three main domains addressed by the scale are: selection, comparability and outcomes.14 Studies were graded by a single reviewer as high (8-9), moderate (6-7) or low (≤5) quality based on the number of stars awarded in the assessment scale. The risk of publication bias was assessed by looking for asymmetry in the funnel plots, as well as discussed narratively.
Statistical analysis
The results of the studies were expressed as rate ratios; defined as the ratio of the mammographic screening or breast cancer diagnosis rates during the pandemic to the mammographic screening or breast cancer diagnosis rates recorded in the period prior to the pandemic.15 For the purposes of the study we divided rate ratios into those derived from registry-based studies and those that weren't. We also assumed that the population of women eligible for screening mammography and/or diagnosed with breast cancer remained stable between 2019 and 2020 and thus defined the rate ratio as the number of women who received a screening mammogram or were diagnosed with breast cancer during the pandemic divided by the number prior to the pandemic. Rate ratios from eligible studies were log-transformed then pooled in Review Manager 5.4 using standard inverse-variance random effects meta-analysis according to the method proposed by Der Simonian and Laird.16 The standard error (SE) of the log rate ratio was estimated as outlined in the Cochrane Handbook for Systematic Reviews of Interventions.15 Statistical heterogeneity was examined by inspecting the distribution of effects within forest plots and the magnitude of corresponding I2 statistics and their 95% confidence intervals.
Subgroup and sensitivity analyses
Where data were available, subgroup analyses were performed to investigate changes in study outcomes according to whether participants were: 1) ≥65 years of age or not, 2) identified as Caucasian or not, 3) diagnosed with early- or late-stage breast cancer; and 4) whether the location of interested had implemented lockdown measures during the period of interest We used ≥65 years old as the cut-off age for the sub-analysis of a screening population because this group has a higher risk of death from COVID-19; 62 times higher than the 54 years or younger population.17 Hence, these elderly individuals were generally advised to stay at home and limit public gatherings during the pandemic.17 Sensitivity analyses were also performed to assess the robustness of pooled estimates based on the observed risk of bias.
Results
Search results
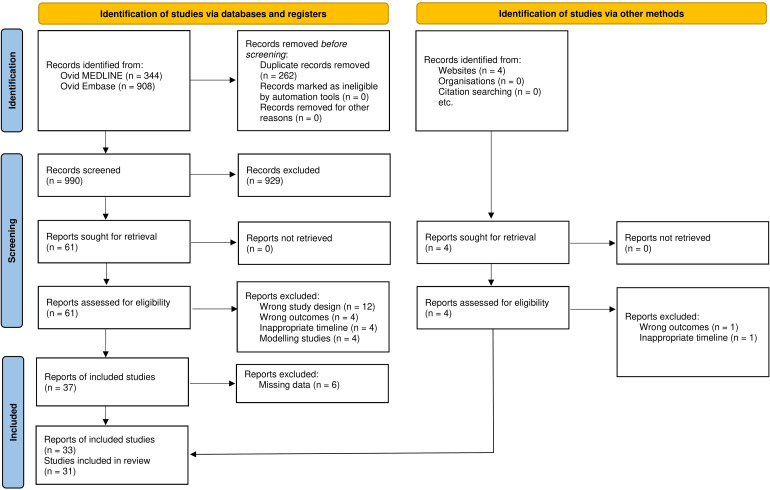
The initial electronic database search identified 1252 records, of which 990 were available for screening following deduplication of 262 duplicate records. Of these, 929 reports were excluded following title and abstract screening, leaving 61 articles for full-text review. Following full-text review, 24 further reports were eliminated due to study design (n = 12), incorrect study outcomes (n = 4), inappropriate timelines (n = 4) and the use of simulated data (n = 4). Authors of eight reports which did not report the necessary count data were contacted to retrieve the data; however only two responded, resulting in further elimination of six reports. Four reports were identified through manual searches of medRxiv and bioRxiv of which two were excluded due to incorrect study outcomes and inappropriate timelines. Therefore, 33 reports consisting of 31 studies formed the basis of this review.18–50 A PRISMA flow diagram outlining the screening process is presented in Figure 1. The list of excluded studies with reasons of exclusion is reported in Supplementary Table S2.
Figure 1.
PRISMA flowchart.
Description of included studies
Table 1 provides an overview of the characteristics of the included studies. From the 31 studies, 7 analysed data from national registries while the rest reported at an institutional level. Eighteen different countries were represented with the United States being the most studied country (n = 8), followed by Italy (n = 3), United Kingdom (n = 3), Austria (n = 2) and Taiwan (n = 2). Based on the World Bank country classification,51 Fourteen of the countries are classified as developed nations and four as low-to-middle-income nations. Four countries did not have a national screening program for breast cancer of which two were non-developed nations (Brazil and South Africa). Table 2 reports on the breast cancer screening protocol for each country, whether screening was suspended and the highest grade of COVID-19 lockdown implemented during the pandemic. All countries reported some form of lockdown during COVID-19 except for Chile, Hong Kong, South Korea and Taiwan.19,23,31,48 Interestingly, Denmark was the only country under lockdown with normal operation of its breast cancer screening program.40
Table 1.
Characteristics of included studies (N = 31).
| First author (year) | Study location | Sample size | Data source | Reporting period (2019 & 2020) | Outcomes examined | Risk of bias |
|---|---|---|---|---|---|---|
| Amram, 202118 | USA | 83,200 | Multi-institutional | Apr-Dec | Screening | Low |
| Barriga, 202119 | Chile | 156 | Single-institution | Apr-Jul | Diagnosis | Moderate |
| Bessa, 202120 | Brazil | 2,848,181 | Registry | Jan-Dec | Screening | Moderate |
| Borsky, 202121 | UK | 238 | Single-institution | Aug-Oct | Diagnosis | Moderate |
| Chang, 202122 | USA | 1069 | Multi-institutional | Mar-May | Diagnosis | Moderate |
| Chou, 202125 | Taiwan | 243 | Single-institution | Jan-Jul | Diagnosis | Low |
| de Degani, 202126 | Argentina | 12,016 | Multi-institutional | Mar-Sep | Screening | Moderate |
| DeGroff, 202127 | USA | 75,610 | Multi-institutional | Apr-Jun* | Screening | Moderate |
| Eijkelboom, 20219,28 | Netherlands | 9430 | Registry | Week 9-35 | Diagnosis | Low |
| Ferrara, 202129 | Italy | 1003 | Multi-institutional | Week 11-20** | Diagnosis | Moderate |
| Hamilton, 202130 | UK | 1444 | Multi-institutional | Mar-Sep | Diagnosis | Moderate |
| Kaltofen, 202131 | Germany | 320 | Single-institution | Jan-Jun | Diagnosis | Moderate |
| Kang, 202132 | South Korea | 35,943 | Multi-institutional | Feb-Jul | Both | Moderate |
| Kempf, 202133 | France | 1145 | Single-institution | Mar-Sep | Diagnosis | Moderate |
| Knoll, 202134 | Austria | 475 | Single-institution | Mar-Dec | Diagnosis | Low |
| Lloyd, 202135 | USA | 11,011 | Single-institution | Jan-Dec*** | Both | Moderate |
| Mantellini, 202010 | Italy | 1,283,703 | Registry | Jan-May | Screening | Moderate |
| Miller, 202136 | USA | 29,180 | Single-institution | Mar-Oct | Screening | Low |
| Morais, 202137 | Portugal | 597 | Single-institution | Mar-Jul | Diagnosis | Moderate |
| O’Brien, 202138 | UK | 396 | Single-institution | Feb-Jul | Diagnosis | Moderate |
| Sacoto, 202139 | Spain | 21,775 | Single-institution | Jan-Dec | Both | Moderate |
| Skovlund, 202140 | Denmark | 823 | Registry | Feb-May* | Diagnosis | Moderate |
| Sprague, 202141 | USA | 316,494 | Multi-institutional | Jan-Jul | Screening | Moderate |
| Tonneson, 202142 | USA | 571 | Single-institution | Mar-Aug | Diagnosis | Moderate |
| Toss, 202143 | Italy | 25,389 | Single-institution | May-Jul | Both | Moderate |
| Tsai, 202044 | Taiwan | 704,834 | Registry | Jan-Apr | Screening | Low |
| Tsibulak, 202045,46 | Austria | 552 | Multi-institutional | Mar-May | Diagnosis | Moderate |
| van Wyk, 202147 | South Africa | 386 | Single-institution | Apr-Jun | Diagnosis | Moderate |
| Vardhanabhuti, 202148 | Hong Kong | 8092 | Registry | Jan-Dec | Diagnosis | Moderate |
| Velazquez, 202149 | USA | 9047 | Single-institution | Jan-Dec | Screening | Low |
| Vrdoljak, 202150 | Croatia | 5723 | Registry | Jan-Dec | Diagnosis | Moderate |
*Pre-COVID period included 2015-2019 [average].
**Pre-COVID period included 2018-2019 [average].
***Pre-COVID period included 2016-2019 [average].
Table 2.
List of countries covered and their associated breast cancer screening protocol, whether screening was suspended, and the highest grade of COVID-19 lockdown reported.
| Country | Screening protocol | Suspension of screening program | Highest grade of lockdown |
|---|---|---|---|
| Argentina | Mammography biennially for women aged 50-69 years old | Yes | 3 |
| Austria | Mammography biennially for women aged 45-69 years old | Yes | 2 |
| Brazil | Mammography biennially for women aged 50-69 years old | NA* | 3 |
| Chile | Mammography triennially for women aged 50-69 years old | No | No lockdown |
| Croatia | Mammography biennially for women aged 50-69 years old | Yes | 2 |
| Denmark | Mammography biennially for women aged 50-69 years old | No | 1 |
| France | Mammography biennially for women aged 50-74 years old | Yes | 2 |
| Germany | Mammography biennially for women aged 50-69 years old | Yes | 2 |
| Hong Kong | Insufficient evidence for population-based mammography. Self-referral for women at increased risk | NA* | No lockdown |
| Italy | Mammography biennially for women aged 50-69 years old | Yes | 2 |
| Netherlands | Mammography biennially for women aged 50-75 years old | Yes | 2 |
| Portugal | Mammography biennially for women aged 45-69 years old | Yes | 2 |
| South Africa | Mammography annually for women aged 40-54 years old, biennially for women aged 55 years and older | NA* | 3 |
| South Korea | Mammography biennially for women aged 40-69 years old | No | No lockdown |
| Spain | Mammography biennially for women aged 50-69 years old | Yes | 2 |
| Taiwan | Mammography biennially for women aged 45-69 years old | No | No lockdown |
| UK | Mammography triennially for women aged 50-69 years old | Yes | 2 |
| USA | Multiple guidelines; American Cancer Society proposed mammography annually for women aged 50-54 years old, biennially for women aged 55-74 years old | NA* | 2 |
*Not applicable as national screening programs are not available.
Grade of household lockdowns.52
1- Recommended not to leave the house.
2- Required to stay at home with exceptions (daily exercise, grocery shopping).
3- Required to stay at home with minimal exceptions (e.g. allowed to leave once weekly, or only one person per household to leave at a time, etc.).
Methodological quality appraisal and risk of bias
All studies used a retrospective cohort study design which is considered Level III-2 evidence by the NHMRC evidence hierarchy.53 Seven studies were classified as low risk of bias and 24 as moderate risk of bias according to the NOS. Most studies lost one or two points in the comparability domain for not accounting for the effects of confounders in the studies (see Supplementary Table S3 for the full results). The risk of publication bias was also considered to be low given the visual symmetry in the funnel plots (see Supplementary Figures S1 and S2) and the inclusion of preprints and abstracts from conference proceedings in this review.
Mammographic screening outcomes
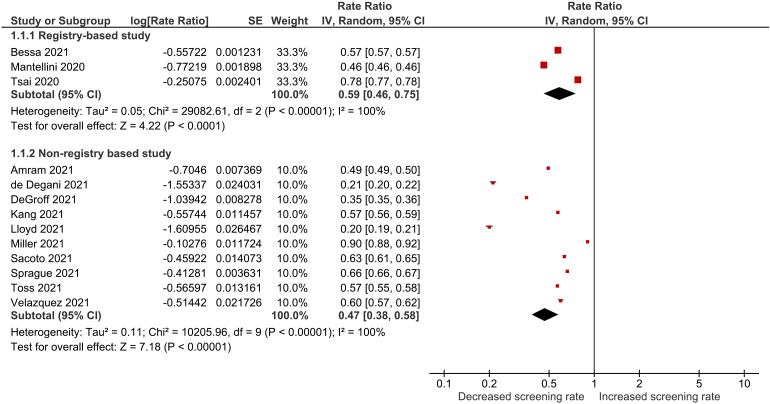
Table 3 compares the number of mammograms performed during the pandemic vs. the same time period before the pandemic. Reductions in screening mammography rates were reported in all studies and ranged from 53.8% to 22.2% among registry-based studies20,35,44 and 80.0% to 9.8% among non-registry-based studies.18,24,25,31,34,36,39,41,43,49Figure 2 displays the results of the meta-analysis of mammographic screening rate ratios. Overall, we noted that mammogram rates declined between 2019 and 2020 by 41% (RR: 0.59, 95% CI: 0.46–0.75) and 53% (RR: 0.47, 95% CI: 0.38–0.58) according to data derived from 3 registry-based and 10 non-registry-based studies respectively. Considerable statistical heterogeneity was observed among the registry-based (I2 = 100%) and non-registry-based studies (I2 = 100%); however this was difficult to interpret due to the high precision (i.e. low standard error) of individual summary estimates. Consequently, outliers and other clinical aspects of included studies were examined and the authors were satisfied that included studies were sufficiently clinically homogeneous.
Table 3.
Number of screening mammograms performed and/or new female breast cancer cases detected before and during the COVID-19 pandemic.
| First author (year) | Data source | Follow-up period (months) | Number of screening mammograms | Number of new breast cancer cases | ||||
|---|---|---|---|---|---|---|---|---|
| Pre-COVID | COVID | Change from baseline (%) | Pre-COVID | COVID | Change from baseline (%) | |||
| Bessa, 202122 | Registry | 12 | 1,810,901 | 1,037,280 | −42.7% | NA | NA | NA |
| Eijkelboom, 20219,28 | Registry | 7 | NA | NA | NA | 5749 | 3681 | −36.0% |
| Mantellini, 202010 | Registry | 5 | 878,046 | 405,657 | −53.8% | NA | NA | NA |
| Skovlund, 202140 | Registry | 4 | NA | NA | NA | 484 | 339 | −30.0% |
| Tsai, 202044 | Registry | 4 | 396,371 | 308,463 | −22.2% | NA | NA | NA |
| Vardhanabhuti, 202148 | Registry | 12 | NA | NA | NA | 4035 | 4057 | 0.5% |
| Vrdoljak, 202150 | Registry | 12 | NA | NA | NA | 2875 | 2848 | −0.9% |
| Amram, 202120 | Non-registry | 9 | 55,678 | 27,522 | −50.6% | NA | NA | NA |
| Barriga, 202121 | Non-registry | 4 | NA | NA | NA | 86 | 70 | −18.6% |
| Borsky, 202123 | Non-registry | 3 | NA | NA | NA | 136 | 102 | −25.0% |
| Chang, 202124 | Non-registry | 2 | NA | NA | NA | 790 | 279 | −64.7% |
| Chou, 202125 | Non-registry | 7 | NA | NA | NA | 128 | 115 | −10.2% |
| de Degani, 202126 | Non-registry | 7 | 9918 | 2098 | −78.8% | NA | NA | NA |
| DeGroff, 202127 | Non-registry | 3 | 55,856 | 19,754 | −64.6% | NA | NA | NA |
| Ferrara, 202129 | Non-registry | 2.5 | NA | NA | NA | 620 | 383 | −38.2% |
| Hamilton, 202130 | Non-registry | 7 | NA | NA | NA | 769 | 642 | −16.5% |
| Kaltofen, 202131 | Non-registry | 6 | NA | NA | NA | 170 | 150 | −11.8% |
| Kang, 202132 | Non-registry | 6 | 20,923 | 11,982 | −42.7% | 1669 | 1369 | −18.0% |
| Kempf, 202133 | Non-registry | 7 | NA | NA | NA | 1482 | 1259 | −15.0% |
| Knoll, 202134 | Non-registry | 10 | NA | NA | NA | 263 | 212 | −19.4% |
| Lloyd, 202135 | Non-registry | 4 | 8566 | 1713 | −80.0% | 399 | 333 | −16.6% |
| Miller, 202136 | Non-registry | 8 | 15,339 | 13,841 | −9.8% | NA | NA | NA |
| Morais, 202137 | Non-registry | 5 | NA | NA | NA | 370 | 227 | −38.6% |
| O’Brien, 202138 | Non-registry | 6 | NA | NA | NA | 201 | 195 | −3.0% |
| Sacoto, 202139 | Non-registry | 12 | 13,041 | 8239 | −36.8% | 285 | 210 | −26.3% |
| Sprague, 202141 | Non-registry | 7 | 190,454 | 126,040 | −33.8% | NA | NA | NA |
| Tonneson, 202142 | Non-registry | 5 | NA | NA | NA | 390 | 181 | −53.6% |
| Toss, 202143 | Non-registry | 3 | 15,942 | 9052 | −43.2% | 221 | 174 | −21.3% |
| Tsibulak, 202045,46 | Non-registry | 3 | NA | NA | NA | 351 | 201 | −42.7% |
| van Wyk, 202147 | Non-registry | 3 | NA | NA | NA | 247 | 139 | −43.7% |
| Velazquez, 202149 | Non-registry | 12 | 5662 | 3385 | −40.2% | NA | NA | NA |
NA - Not available.
Figure 2.
Forest plot for combined effects of mammographic screening rate.
Breast cancer diagnosis rates
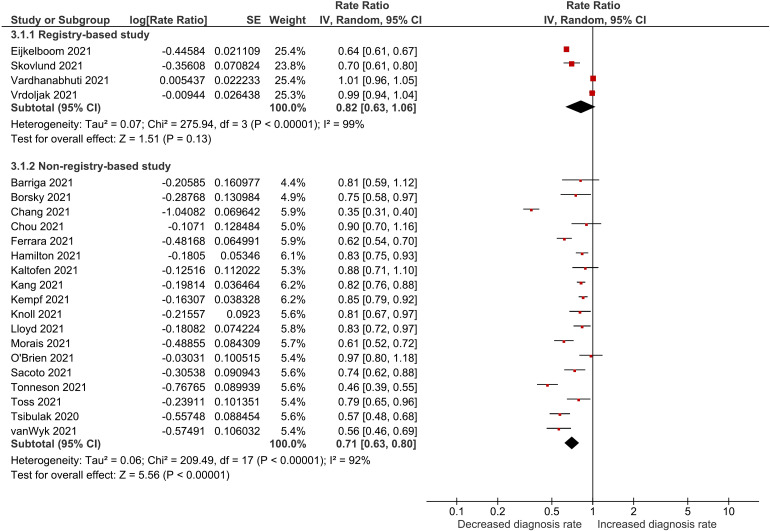
Table 3 compares the number of new female breast cancer cases detected during the pandemic versus the same time period before the pandemic. Reductions in the diagnosis rate of breast cancer were observed in all but one study, by Vardhanabhuti et al.,48 with reported rates ranging from 36.0% reduction to a 0.5% increase among registry-based studies26,27,40,48,50 and 64.7% to 3.0% reduction in non-registry-based studies.19,21–23,28–34,37–39,42,43,45–47Figure 3 displays the results of the meta-analysis of the diagnosis rate ratios. Similar to the changes in mammogram screening rates, we noted an estimated 18% (RR: 0.82, 95% CI: 0.63–1.06) and 29% (RR: 0.71, 95% CI: 0.63–0.80) reduction in diagnosis rates between 2019 and 2020 based on data derived from 4 registry-based and 18 non-registry-based studies respectively. Again, despite considerable statistical heterogeneity among the registry-based (I2 = 99%) and non-registry-based studies (I2 = 92%) the results of the individual studies were pooled for the same reason outlined previously.
Figure 3.
Forest plot for combined effects of breast cancer diagnosis rate.
Subgroup and sensitivity analyses
We did not observe any differences in mammogram screening rates depending on patient age or ethnicity (see Supplementary Figures S3 and S4); however, we note that countries that implemented lockdown measures were associated with a significantly higher reduction in mammogram screening rates between 2019 and 2020 (RR: 0.47 vs. 0.67, Chi2 = 4.54, p = 0.03) (see Supplementary Figure S5). Similarly, greater reduction in breast cancer diagnosis rates between 2019 and 2020 were observed among countries that implemented lockdown measures in comparison to those that did not (RR: 0.70 vs. 0.89, Chi2 = 6.08, p = 0.01) (see Supplementary Figure S6). We also noted greater reductions in the number of early-stage breast cancers diagnosed compared to late-stage cancers between 2019 and 2020 (RR: 0.58 vs. 0.79, Chi2 = 5.85, p = 0.02) (see Supplementary Figure S7). Performing meta-analysis after excluding the moderate to high risk of bias studies did not result in any clinically significant changes to pooled estimates.
Discussion
The aim of this rapid review was to estimate the impact of the COVID-19 pandemic on both breast cancer screening and diagnosis rates. Our results suggest that almost half of the women eligible for breast cancer screening did not get their mammograms during the pandemic in comparison to 2019. This finding can likely be largely attributed to the suspension of most breast cancer screening activities in areas with high COVID-19 cases18,20,24 as a result of the inability to maintain ‘safe distancing’ during breast imaging studies, given the short distance between the radiographer's face and the face of the patient, a distance that can be as little as 20–30 cm during the radiographic procedure.54,55 In addition to the suspension of screening programs, we also note that the use of lockdown measures likely also significantly impacted screening and diagnosis rates.
However, the effects of the pandemic also appear to extend beyond countries with a high COVID-19 incidence.24,25,34 For example, modest declines in mammographic screening rates were also observed in low COVID-19 incidence countries like Taiwan despite the usual operation of breast cancer screening acitvities.44 Studies that examined factors influencing mammography attendance rates under these circumstances highlighted that fear of using public transport and of visiting hospitals was associated with reduction in the number of outpatient visits in breast centres.56,57
Reduced uptake of mammographic screening, which can detect breast cancer in asymptomatic women, also likely largely explains the observed reductions in diagnosis rates. However, interestingly, our findings suggest that diagnosis rates did not fall as much as the screening rates, which is likely due to the preservation of breast services for symptomatic patients compared to the breast screening units, as evidenced by the stable flow of patients presenting with symptomatic breast cancer during the pandemic.58 This finding is also consistent with the results of our subgroup analysis, which suggested smaller declines in diagnosis rates for late-stage cancers than for early-stage cancers, as well as mathematical modelling from Yong et al. and Sharpless.59,60 However, any reductions in the number of new cases of breast cancer diagnosed, particularly the modest decline in the number of early-stage cases detected, is a cause for concern.
While, to the authors’ knowledge, this is the first systematic review to present pooled estimates on the impact of the pandemic on breast cancer screening and diagnosis rates, we note some limitations of the study. First, the quality of evidence from this review was limited by the design of the studies included. As each of the retrospective studies was unblinded, the inherent risk of biases in evaluating and reporting the outcomes was difficult to avoid. Nevertheless, the risk of selection bias across studies in this review was considered low given that the outcome measures were highly objective. Second, most of the studies were non-registry-based studies and did not include a method to avoid double counting of patients in the clinical databases. Hence, we analysed the seven registry-based studies separately from the non-registry-based studies. Third, restricting the pre-COVID-19 period to before 1st January 2020 also meant the exclusion of three studies with a one-year long assessment period.61–63 Lastly, the validity of rate measures in this review was limited by the assumption of stability of population between the pre-pandemic and pandemic periods. The effects of the COVID-19 pandemic on life expectancy are built upon different assumptions about the age-specific mortality rates and COVID-19 prevalence rates which differ widely across different countries; therefore, it is complex and not straightforward to predict the trends in demographic stability during the COVID-19 pandemic.64,65
Recommendations
Implications for practice
The postponement of breast cancer screening throughout the pandemic to preserve healthcare resources and protect patients from COVID-19 infection is not feasible and will likely delay the diagnosis and treatment of breast cancer. Accessibility is one of the important factors that promotes screening participation.66 Decentralisation of national breast cancer screening programs into multiple community-based programs is encouraged to overcome transportation barriers during the pandemic. In fact, the community breast cancer screening program was proven safe and reliable during the pandemic in Taiwan.44 In the long run, patient hesitancy to leave their home has to be surmounted along with the gradual reopening of breast screening centres. A Polish survey found that the availability of transparent medical information is effective in restoring patient confidence in healthcare services during the pandemic.67 Thus, future subjects invited for breast cancer screening should be, at minimum, informed about safety precautions practiced by the facilities, i.e. mandatory masking and temperature screening, and, if possible, supplied with the latest information about COVID-19 in a timely manner.
Implications for research
Future investigations into breast cancer mortality after the pandemic are of significant interest. This review also highlights the ongoing need for the development of innovative, cost-effective, less invasive and independent ways of detecting breast cancer. We also strongly recommend that future studies investigating the impact of the pandemic on breast cancer outcomes, where possible, use longitudinal designs to assess the long-term outcomes, and follow best practice reporting guidelines to ensure key outcomes of interest are clearly reported.
Conclusion
This systematic review has demonstrated that breast cancer screening has been severely affected by the COVID-19 pandemic as evidenced by the reduced rates of screening mammography and breast cancer diagnosis in 30 out of 31 studies. The difference in magnitude of reduction in breast cancer screening and diagnosis between studies reflects the variation in the reactions of different countries and health institutions to COVID-19. While the postponement of breast cancer screening programs and services during the COVID-19 pandemic was necessary and reasonable at one time, given that the future trajectory of COVID-19 is difficult to predict, delays in the resumption of screening programs may create another public health crisis in the near future. These findings support the immediate restoration of breast cancer screening as well as our patients’ confidence in the healthcare system amid the pandemic-stricken era.
Supplemental Material
Supplemental material, sj-docx-1-msc-10.1177_09691413221101807 for Assessing the impact of the COVID-19 pandemic on breast cancer screening and diagnosis rates: A rapid review and meta-analysis by Jay Shen Ng and Daniel G. Hamilton in Journal of Medical Screening
Acknowledgements
The authors thank A/Prof Sue Finch for her assistance with the design and execution of the statistical analysis.
Footnotes
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Data availability statement: All data and materials from the review are publicly available on the Open Science Framework (DOI: 10.17605/OSF.IO/W9FGX).
Ethical approval statement: Ethics approval was not acquired because data will be obtained and analysed from previous published studies. Informed consent was not required for this systematic review.
ORCID iD: Jay Shen Ng https://orcid.org/0000-0002-8491-4066
Supplemental material: Supplemental material for this article is available online.
References
- 1.Gini A, Jansen EEL, Zielonke N, et al. Impact of colorectal cancer screening on cancer-specific mortality in Europe: a systematic review. Eur J Cancer 2020; 127: 224–235. [DOI] [PubMed] [Google Scholar]
- 2.Jansen EEL, Zielonke N, Gini A, et al. Effect of organised cervical cancer screening on cervical cancer mortality in Europe: a systematic review. Eur J Cancer 2020; 127: 207–223. [DOI] [PubMed] [Google Scholar]
- 3.Zielonke N, Gini A, Jansen EEL, et al. Evidence for reducing cancer-specific mortality due to screening for breast cancer in Europe: a systematic review. Eur J Cancer 2020; 127: 191–206. [DOI] [PubMed] [Google Scholar]
- 4.Nickson C, Mason KE, English DR, et al. Mammographic screening and breast cancer mortality: a case-control study and meta-analysis. Cancer Epidemiol Biomarkers Prev 2012; 21: 1479–1488. [DOI] [PubMed] [Google Scholar]
- 5.Roder D, Houssami N, Farshid G, et al. Population screening and intensity of screening are associated with reduced breast cancer mortality: evidence of efficacy of mammography screening in Australia. Breast Cancer Res Treat 2008; 108: 409–416. [DOI] [PubMed] [Google Scholar]
- 6.Zielonke N, Kregting LM, Heijnsdijk EAM, et al. The potential of breast cancer screening in Europe. Int J Cancer 2021; 148: 406–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Richards M, Anderson M, Carter P, et al. The impact of the COVID-19 pandemic on cancer care. Nature Cancer 2020; 1: 565–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marron JM, Charlot M, Gaddy J, et al. The ethical imperative of equity in oncology: lessons learned from 2020 and a path forward. Am Soc Clin Oncol Educ Book 2021; 41: e13–e19. [DOI] [PubMed] [Google Scholar]
- 9.Puricelli Perin DM, Elfström KM, Bulliard J-L, et al. Early assessment of the first wave of the COVID-19 pandemic on cancer screening services: the international cancer screening network COVID-19 survey. Prev Med 2021; 151: 106642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Battisti F, Falini P, Gorini G, et al. Cancer screening programmes in Italy during the COVID-19 pandemic: an update of a nationwide survey on activity volumes and delayed diagnoses. Ann Ist Super Sanita 2022; 58: 16–24. [DOI] [PubMed] [Google Scholar]
- 11.Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Syst Rev 2021; 10: 89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.A Timeline of COVID-19 Developments in 2020, https://www.ajmc.com/view/a-timeline-of-covid19-developments-in-2020(2021, accessed 2021 Nov 22).
- 13.Covidence systematic review software. Melbourne, Australia: Veritas Health Innovation, 2020.
- 14.Wells GA, Shea B, O’Connell D, et al. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses. In: 2014.
- 15.Higgins JPT, Li T, Deeks JJ. Chapter 6: Choosing effect measures and computing estimates of effect, www.training.cochrane.org/handbook(accessed 2021 Nov 3).
- 16.Review Manager (RevMan) [Computer program]. The Cochrane Collaboration, 2020.
- 17.Yanez ND, Weiss NS, Romand J-A, et al. COVID-19 mortality risk for older men and women. BMC Public Health 2020; 20: 1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Amram O, Robison J, Amiri S, et al. Socioeconomic and racial inequities in breast cancer screening during the COVID-19 pandemic in Washington state. JAMA Netw Open 2021; 4: e2110946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Barriga S, Camacho N, Roman L, et al. Evaluation of the impact of the COVID-19 pandemic in the diagnosis and treatment of breast cancer patients treated at clinica alemana de Santiago. Rev Cir (Mex) 2021; 73: 301–306. [Google Scholar]
- 20.Bessa JdF. Breast imaging hindered during COVID-19 pandemic, in Brazil. Rev Saude Publica 2021; 55: 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Borsky K, Shah K, Cunnick G, et al. P088. Pattern of breast cancer presentation during the COVID-19 pandemic. Eur J Surg Oncol 2021; 47: e319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chang SB, Savitz AC, Miller AM, et al. Characterization of breast cancer management during the COVID 19 pandemic in a large integrated healthcare delivery system: stage at diagnosis and timing/modality of first treatment. Cancer Res 2021; 81: SS2-06. DOI: 10.1158/1538-7445.SABCS20-SS2-06 [DOI] [Google Scholar]
- 23.Chou C-P, Lin H-S. Delayed breast cancer detection in an Asian country (Taiwan) with low COVID-19 incidence. Cancer Manag Res 2021; 13: 5899–5906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.de Degani Lopez G, Duarte L, Ismael J, et al. The impact of the COVID-19 pandemic on cancer care in the public health subsector, province of Santa Fe, Argentina. ecancermedicalscience 2021; 15: 1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.DeGroff A, Miller J, Melillo S, et al. COVID-19 impact on screening test volume through the National Breast and Cervical Cancer early detection program, January-June 2020, in the United States. Prev Med 2021; 151: 106559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Eijkelboom AH, de Munck L, Lobbes MBI, et al. Impact of the suspension and restart of the Dutch breast cancer screening program on breast cancer incidence and stage during the COVID-19 pandemic. Prev Med 2021; 151: 106602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Eijkelboom AH, de Munck L, Verloop J, et al. Impact of the COVID-19 pandemic on diagnosis, stage, and initial treatment of breast cancer in the Netherlands: a population-based study. Journal of Hematology and Oncology 2021; 14: 64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ferrara G, De Vincentiis L, Ambrosini-Spaltro A, et al. Cancer diagnostic delay in northern and central Italy during the 2020 lockdown due to the coronavirus disease 2019 pandemic. Am J Clin Pathol 2021; 155: 64–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hamilton AC, Donnelly DW, Loughrey MB, et al. Inequalities in the decline and recovery of pathological cancer diagnoses during the first six months of the COVID-19 pandemic: a population-based study. Br J Cancer 2021; 125: 798–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kaltofen T, Hagemann F, Harbeck N, et al. Changes in gynecologic and breast cancer diagnoses during the first wave of the COVID-19 pandemic: analysis from a tertiary academic gyneco-oncological center in Germany. Arch Gynecol Obstet 2022; 305: 713–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kang Y-J, Oh SJ, Baek JM, et al. Impact of COVID-19 pandemic in 2020 on the diagnosis and management of breast cancer in Korea: a multi-institutional study. J Clin Oncol 2021; 39: 10566. DOI: 10.1200/JCO.2021.39.15-suppl.10566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kempf E, Lame G, Layese R, et al. New cancer cases at the time of SARS-Cov2 pandemic and related public health policies: a persistent and concerning decrease long after the end of the national lockdown. Eur J Cancer 2021; 150: 260–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Knoll K, Leitner K, Kogl J, et al. The impact of COVID-19 pandemic on the rate of newly diagnosed gynecological and breast cancers: a tertiary center perspective. Arch Gynecol Obstet 2022; 305 :945–953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lloyd MR, Stephens SJ, Hong JC, et al. The impact of COVID-19 on breast cancer stage at diagnosis. J Clin Oncol 2021; 39: 528. DOI: 10.1200/JCO.2021.39.15-suppl.528 [DOI] [Google Scholar]
- 35.Mantellini P, Battisti F, Armaroli P, et al. Oncological organized screening programmes in the COVID-19 era: an Italian survey on accrued delays, reboot velocity, and diagnostic delay estimates. Epidemiol Prev 2020; 44: 344–352. [DOI] [PubMed] [Google Scholar]
- 36.Miller MM, Meneveau MO, Rochman CM, et al. Impact of the COVID-19 pandemic on breast cancer screening volumes and patient screening behaviors. Breast Cancer Res Treat 2021; 189: 237–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Morais S, Antunes L, Rodrigues J, et al. The impact of the COVID-19 pandemic on the short-term survival of patients with cancer in Northern Portugal. Int J Cancer 2021; 149: 287–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.O’Brien AC, O’Doherty A, McNally S, et al. The COVID-19 impact on symptomatic breast cancer referrals and diagnosis. Ir Med J 2021; 114: P335. [Google Scholar]
- 39.Sacoto MC, Moreno R, Rodriguez A, et al. COVID-19 pandemic impact in newly diagnosed breast cancer patients (BCP) at a third level hospital. Ann Oncol 2021; 32: 95. [Google Scholar]
- 40.Skovlund CW, Friis S, Morch LS, et al. Hidden morbidities: drop in cancer diagnoses during the COVID-19 pandemic in Denmark. Acta Oncol (Madr) 2021; 60: 20–23. [DOI] [PubMed] [Google Scholar]
- 41.Sprague BL, Lowry KP, Miglioretti DL, et al. Changes in mammography utilization by women’s characteristics during the first 5 months of the COVID-19 pandemic. J Natl Cancer Inst 2021; 113: 1161–1167. DOI: 10.1093/jnci/djab045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tonneson JE, Hoskin TL, Durgan DM, et al. Impact of COVID-19 pandemic on breast cancer stage at diagnosis, presentation, and patient management. Ann Surg Oncol 2021; 28: S375–S376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Toss A, Isca C, Venturelli M, et al. Two-month stop in mammographic screening significantly impacts on breast cancer stage at diagnosis and upfront treatment in the COVID era. ESMO Open 2021; 6: 100055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tsai H-Y, Chang Y-L, Shen C-T, et al. Effects of the COVID-19 pandemic on breast cancer screening in Taiwan. The Breast 2020; 54: 52–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tsibulak I, Reiser E, Ebner C, et al. Strong decline of newly diagnosedgynecologic and breast cancers during the COVID-19pandemic: a retrospective analysis from Innsbruck, Austria. Clin Cancer Res 2020; 26: 18. DOI: 10.1158/1557-3265.COVID-19-PO-038 [DOI] [Google Scholar]
- 46.Tsibulak I, Reiser E, Bogner G, et al. Decrease in gynecological cancer diagnoses during the COVID-19 pandemic: an Austrian perspective. Int J Gynecol Cancer 2020; 30: 1667–1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.van Wyk AC, de Jager LJ, Razack R, et al. The initial impact of the COVID-19 pandemic on the diagnosis of new cancers at a large pathology laboratory in the public health sector, Western Cape Province, South Africa. S Afr Med J 2021; 111: 570–574. [PubMed] [Google Scholar]
- 48.Vardhanabhuti V, Ng KS. Differential impact of COVID-19 on cancer diagnostic services based on body regions: a public facility-based study in Hong Kong. Int J Radiat Oncol Biol Phys 2021; 111: 331–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Velazquez AI, Hayward JH, Gregory B, et al. Trends in breast cancer screening in a safety-net hospital during the COVID-19 pandemic. JAMA Netw Open 2021; 4: e2119929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vrdoljak E, Balja MP, Marušić Z, et al. COVID-19 Pandemic effects on breast cancer diagnosis in Croatia: a population- and registry-based study. Oncologist 2021; 26: e1156–e1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.World Bank Country and Lending Groups, https://datahelpdesk.worldbank.org/knowledgebase/articles/906519-world-bank-country-and-lending-groups(2021, accessed 2021 Nov 23).
- 52.Ritchie H, Mathieu E, Rodés-Guirao L, et al. Coronavirus pandemic (COVID-19). Our World in Data 2020; 2: 1–5. [Google Scholar]
- 53.Merlin T, Weston A, Tooher R. Extending an evidence hierarchy to include topics other than treatment: revising the Australian ‘levels of evidence’. BMC Med Res Methodol 2009; 9; 34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lu M. The Front Line: Visualizing the Occupations with the Highest COVID-19 Risk, https://www.visualcapitalistcom/the-front-line-visualizing-the-occupations-with-the-highest-covid-19-risk/(2020, accessed 2021 Nov 25).
- 55.Granata V, Fusco R, Izzo F, et al. COVID-19 infection in cancer patients: the management in a diagnostic unit. Radiol Oncol 2021; 55: 121–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Liu YA, Hsu YC, Lin MH, et al. Hospital visiting policies in the time of coronavirus disease 2019: a nationwide website survey in Taiwan. J Chin Med Assoc 2020; 83: 566–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tiikkaja H, Viri R. The effects of COVID-19 epidemic on public transport ridership and frequencies. A case study from Tampere, Finland. Transp Res Interdiscip Perspect 2021; 10: 100348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dave RV, Kim B, Courtney A, et al. Breast cancer management pathways during the COVID-19 pandemic: outcomes from the UK ‘alert level 4’ phase of the B-MaP-C study. Br J Cancer 2021; 124: 1785–1794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yong JH, Mainprize JG, Yaffe MJ, et al. The impact of episodic screening interruption: COVID-19 and population-based cancer screening in Canada. J Med Screen 2021; 28: 100–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sharpless NE. COVID-19 and cancer. Science 2020; 368: 1290. [DOI] [PubMed] [Google Scholar]
- 61.Koca B, Yildirim M. Delay in breast cancer diagnosis and its clinical consequences during the coronavirus disease pandemic. J Surg Oncol 2021; 124: 261–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ruiz-Medina S, Gil S, Jimenez B, et al. Significant decrease in annual cancer diagnoses in Spain during the COVID-19 pandemic: a real-data study. Cancers (Basel) 2021; 13: 3215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Vilaca M, Silva D, Magalhaes D, et al. 1628P Impact of COVID-19 pandemia on the diagnosis of breast cancer in one region of north of Portugal: one year experience. Ann Oncol 2021; 32: S1155–S1156. [Google Scholar]
- 64.Harper S. The impact of the COVID-19 pandemic on global population ageing. J Popul Ageing 2021; 14: 137–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Marois G, Muttarak R, Scherbov S. Assessing the potential impact of COVID-19 on life expectancy. PloS one 2020; 15: e0238678–e0238678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hsieh H-M. Effect of accessibility improvement in a national population-based breast cancer screening policy on mammography utilization among women with comorbid conditions in Taiwan. Soc Sci Med 2021; 284: 114245. [DOI] [PubMed] [Google Scholar]
- 67.Lewandowski R, Goncharuk AG, Cirella GT. Restoring patient trust in healthcare: medical information impact case study in Poland. BMC Health Serv Res 2021; 21: 65. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-msc-10.1177_09691413221101807 for Assessing the impact of the COVID-19 pandemic on breast cancer screening and diagnosis rates: A rapid review and meta-analysis by Jay Shen Ng and Daniel G. Hamilton in Journal of Medical Screening