Abstract
Objective
Coronavirus disease 19 (COVID-19) has an increased risk of coagulopathy with high frequency of antiphospholipid antibodies (aPL). Recent reports of thrombosis associated with adenovirus-based vaccines raised concern that SARS-CoV-2 immunization in primary antiphospholipid syndrome (PAPS) patients may trigger clotting complications. Our objectives were to assess immunogenicity, safety, and aPL production in PAPS patients, after vaccinating with Sinovac-CoronaVac, an inactivated virus vaccine against COVID-19.
Methods
This prospective controlled phase-4 study of PAPS patients and a control group (CG) consisted of a two-dose Sinovac-CoronaVac (D0/D28) and blood collection before vaccination (D0), at D28 and 6 weeks after second dose (D69) for immunogenicity/aPL levels. Outcomes were seroconversion (SC) rates of anti-SARS-CoV-2 S1/S2 IgG and/or neutralizing antibodies (NAb) at D28/D69 in naïve participants. Safety and aPL production were also assessed.
Results
We included 44 PAPS patients (31 naïve) and 132 CG (108 naïve) with comparable age (p=0.982) and sex (p>0.999). At D69, both groups had high and comparable SC (83.9% vs. 93.5%, p=0.092), as well as NAb positivity (77.4% vs. 78.7%, p=0.440), and NAb-activity (64.3% vs. 60.9%, p=0.689). Thrombotic events up to 6 months or other moderate/severe side effects were not observed. PAPS patients remained with stable aPL levels throughout the study at D0 vs. D28 vs. D69: anticardiolipin (aCL) IgG (p=0.058) and IgM (p=0.091); anti-beta-2 glycoprotein I (aβ2GPI) IgG (p=0.513) and IgM (p=0.468).
Conclusion
We provided novel evidence that Sinovac-CoronaVac has high immunogenicity and safety profile in PAPS. Furthermore, Sinovac-CoronaVac did not trigger thrombosis nor induced changes in aPL production.
Keywords: COVID-19, vaccine immunogenicity, SARS-CoV-2 vaccine, antiphospholipid syndrome, antiphospholipid antibodies
Background
Coronavirus disease 19 (COVID-19) has an increased risk of coagulopathy, especially the occurrence of thromboembolic events. The intense inflammatory response evoked by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) replication may induce a dysregulation of coagulation toward a hypercoagulable state,1-3 and both large vessels and microcirculation may be affected.4-6
Antiphospholipid syndrome (APS) is the most frequent acquired thrombophilia.7 Half of the cases, known as primary APS (PAPS), occur without the concomitance of other autoimmune rheumatic diseases (ARD).8 APS is characterized by the persistent presence of antiphospholipid antibodies (aPL), namely, lupus anticoagulant (LA), IgG, and/or IgM aCL and IgG and/or IgM aβ2GPI, which play an important role in the pathogenesis of thrombosis in those patients.9
Interestingly, both diseases share common mechanisms of thrombosis: activation of endothelial cells, resulting in inhibition of endothelial nitric oxide synthase production, and consequently, decreasing nitric oxide production; complement activation; and unchecked inflammatory signals responsible for the formation of neutrophil extracellular traps (NETosis).10
Recent studies reported the presence of aPL in patients infected with SARS-CoV-2.11-14 Furthermore, infectious etiologies may act as the “second hit,” crucial for the thrombogenesis in APS and/or aPL-positive patients.15-16 Therefore, vaccinating these patients to prevent COVID-19 is of utmost importance.
Paradoxically, two of the vaccines against SARS-CoV-2 using adenovirus platforms developed by AstraZeneca and Janssen have been associated with the occurrence of rare and atypical thromboembolic events, especially in women under 50 years of age, a condition that has been called vaccine-induced immune thrombotic thrombocytopenia (VITT).17,18 As a consequence, vaccinating patients with thrombophilia using other platforms, such as inactivated virus or mRNA, may be preferable in this subset of patients. However, studies on the efficacy and safety of those vaccines in APS are still lacking.
CoronaVac (Sinovac Life Sciences, Beijing, China) is an inactivated vaccine against COVID-19, which is supporting vaccination campaigns in more than 40 countries, including Brazil, and has shown good tolerance and efficacy in inducing humoral responses against SARS-CoV-2 in the general population.19-21 Jara et al.22 demonstrated that Sinovac-CoronaVac reduced rates of infection, hospitalization, ICU admission, and death by 65.9%, 87.5%, 90.3%, and 86.3%, respectively, in the overall population of 10.2 million people in Chile.
The aims of the present prospective study were to evaluate immunogenicity of Sinovac-CoronaVac vaccine in naïve PAPS patients compared to a balanced age- and sex-control group (CG). We further assessed safety, including thrombotic events, and the possible vaccine-induced aPL production throughout the study period.
Methods
Study design
This study is a subgroup analysis of patients with PAPS from a large phase four prospective controlled trial with ARD patients performed at a single tertiary center in Brazil.23
Patients and controls
All consecutive PAPS patients who fulfilled the current classification criteria for PAPS (Sidney) 9 and were regularly followed in our Outpatient Rheumatology Clinics and were ≥18 years old were invited to participate. Subsequently, a CG of hospital maintenance, administrative personal, or their relatives balanced by sex and age (±5 years differences) using an Excel program (ratio 1PAPS: 3CG) were also invited to participate. Exclusion criteria for both groups were the following: ARD (other than APS, for the patient’s group), use of immunosuppressive drugs, HIV infection, history of anaphylactic response to vaccine components, acute febrile illness or symptoms compatible to COVID-19 at vaccination, previous demyelinating disease (including Guillain-Barré syndrome), symptomatic heart failure (class III or IV), previous vaccination with any SARS-CoV-2 vaccine, history of vaccination with live virus vaccine in the previous 4 weeks or with virus vaccine inactivated in the previous 2 weeks, history of having received blood products in the previous 6 months, individuals who refused to participate in the study, and hospitalized patients.
Participants who developed RT-PCR-confirmed COVID-19 after receiving the first vaccine dose (incident cases) and with positive COVID-19 serology and/or NAb at baseline (collected on the day of vaccination) were excluded from the immunogenicity and aPL analysis; however, they were included in the safety evaluation.
Vaccine protocol
PAPS patients and CG were scheduled to receive a two-dose vaccine. The first dose was given on February 9–18th 2021 (D0, with baseline blood collection immediately before it); the second dose was given 28 days later (D28, with blood collection immediately before it). A third blood sample was obtained 6 weeks after the second dose at day 69 (D69). This protocol was delayed 4 weeks for participants with incident COVID-19 infection during the study. Ready-to-use syringes loaded with CoronaVac (Sinovac Life Sciences, Beijing, China, batch #20200412), that consists of 3 μg in 0.5 mL of β-propiolactone inactivated SARS-CoV-2 (derived from the CN02 strain of SARS-CoV-2 grown in African green monkey kidney cells—Vero 25 cells) with aluminum hydroxide as an adjuvant, were administered intramuscularly in the deltoid area. The sera of each blood sample (20 mL) from all participants obtained at days D0, D28, and D69 were stored in a −70°C freezer.
Anti-SARS-CoV-2 S1/S2 IgG antibodies
A chemiluminescent immunoassay was used to measure human IgG antibodies against the S1 and S2 proteins in the receptor binding domain (RBD) (Indirect ELISA, LIAISON® SARS-CoV-2 S1/S2 IgG, DiaSorin, Italy). Seroconversion rate (SC) was defined as positive serology (>15.0 UA/mL) post vaccination, since only patients with pre-vaccination negative serology were included. Geometric mean titers (GMT) of these antibodies and 95% confidence intervals were also calculated at all time points, attributing the value of 1.9 UA/mL (half of the lower limit of quantification 3.8 UA/mL) to undetectable levels (<3.8 UA/mL). The factor increase in GMT (FI-GMT) is the ratio of the GMT after vaccination to the GMT before vaccination, used to demonstrate growth in IgG titers. They are also presented and compared as geometric means and 95% confidence intervals (CI).
SARS-CoV-2 cPass virus-neutralization antibodies
The SARS-CoV-2 sVNT Kit (GenScript, Piscataway, NJ, USA) was performed according to manufacturer instructions. This analysis detects circulating neutralizing antibodies against SARS-CoV-2 that block the interaction between the RBD of the viral spike glycoprotein with the ACE2 cell surface receptor. The tests were performed on the ETI-MAX-3000 equipment (DiaSorin, Italy). The samples were classified as either “positive” (inhibition ≥30%) or “negative” (inhibition <30%), as suggested by the manufacturer.24 The frequency of positive samples was calculated at all time points. Medians (interquartile range) of the percentage of neutralizing activity only for positive samples were calculated.
Outcomes
Immunogenicity outcome was assessed by two criteria SC rates of total anti-SARS-Cov-2 S1/S2 IgG and presence of NAb at D69. Other endpoints were the following: anti-S1/S2 IgG SC and presence of NAb at D28 (after vaccine first dose); geometric mean titers of anti-S1/S2 IgG and their FI-GMT at D28 and D69; and median (interquartile range) neutralizing activity of NAb at D28 and D69.
Vaccine adverse events and incident cases of COVID-19
Patients and CG were advised to report any side effects of the vaccine. They received on D0 (first dose) and on D28 (second dose) a standardized diary for local and systemic manifestations. The standardized diary of adverse events (AE) was carefully reviewed with each participant on the day of the second dose (D28) and at the last visit (D69). COVID-19 incident cases were followed for 40 days (from D0 to 10 days after the second dose [D39]) and thereafter for the following 40 days (from D40 to D79).
Vaccine AE severity was defined according to WHO definitions.25 A rigorous surveillance for any kind of thrombotic event was performed during a period of 6 months after full-vaccination.
Additionally, all participants were instructed to communicate any manifestation associated or not with COVID-19 through telephone, smartphone instant messaging, or email. Suspicious cases of COVID-19 were instructed to seek medical care near the residence and, if recommended, to come to our tertiary hospital to have the RT-PCR exam or in-person visit. Patients were clinically followed for 6 months (August 18, 2021).
Study data were collected and managed using REDCap electronic data capture tools hosted at our Institution.26-27
RT-PCR for SARS-CoV-2
Clinical samples for SARS-CoV-2 RT-PCR consisted of nasopharyngeal and oropharyngeal swabs, using a laboratory developed test.28
Antiphospholipid antibodies
We assessed the criteria antiphospholipid antibodies IgG/IgM aCL and IgG/IgM anti-β2GPI in PAPS patients. Peripheral blood samples were collected in dry tubes (2 tubes), respecting the time between collection and centrifugation of at most 1 hour. Samples were centrifuged at 3200 r/min for 15 min and aliquoted in a volume of 500 μL. The aCL antibodies were detected by commercial fluoro immunoenzymatic assay (EliA) Thermo Scientific™/Phadia™ 250 Immunoassay Analyzers and they were considered positive if present in medium or high titers (≥40 GPL or MPL). The aβ2GPI antibodies were measured through the enzyme-linked immunosorbent assay (ELISA) QUANTALite®, InovaDiagnostics and their positivity was defined if titers were > 20UI/mL. Antiphospholipid antibodies at D28 and D69 were compared to baseline (D0) to verify if there was any increase in titers after vaccination. The thrombosis score risk aGAPSS (adjusted Global AntiPhospholipid Syndrome Score) that includes the three criteria aPL,29-30 besides arterial hypertension and dyslipidemia, was calculated at baseline and at D69 using LA previously registered in our electronic database. LA detection was performed according to updated guidelines.31
Statistical analysis
A convenience sample of PAPS patients was selected with a CG in a 1:3 ratio. Continuous variables are presented as medians (interquartile ranges) with intergroup comparison using Mann–Whitney test. Categorical variables are presented as number (percentage) and compared using chi-square or Fisher’s exact tests, as appropriate. Continuous data regarding anti-S1/S2 serology titers are presented as geometric means (95% CI) and compared with the same tests, but in Napierian logarithm (ln) transformed data. Longitudinal comparisons of ln-transformed anti-S1/S2 IgG titers between PAPS and CG were performed using generalized estimating equations (GEE) with normal marginal distribution and gamma distribution, respectively. Results were followed by Bonferroni multiple comparisons to identify differences between groups and time points. Multivariate logistic regression analyses were performed using as dependent variables SC or presence of NAb, and as independent variables those with p <0.2 in univariate analysis. The isotypes of each aPL were analyzed categorically (according to aPL cutoff positivity definitions) using Chi-square test and continuously by Friedman Repeated Measures Analysis of Variance on Ranks at D0, D28, and D69. aGAPSS score of APS patients was also compared between the three time points using Friedman Repeated Measures Analysis of Variance on Ranks.
Statistical significance was defined as p <0.05. All statistical analyses were performed using IBM-SPSS for Windows software version 22.0.
Ethics statement
The protocol was approved by the National and Institutional Ethical Committee of Hospital das Clínicas da Faculdade de Medicina da Universidade de São Paulo (HCFMUSP), Brazil (CAAE: 42566621.0.0000.0068). It was in accordance with the Declaration of Helsinki and local regulations, and all participants signed a written informed consent before enrollment.
Results
Participants
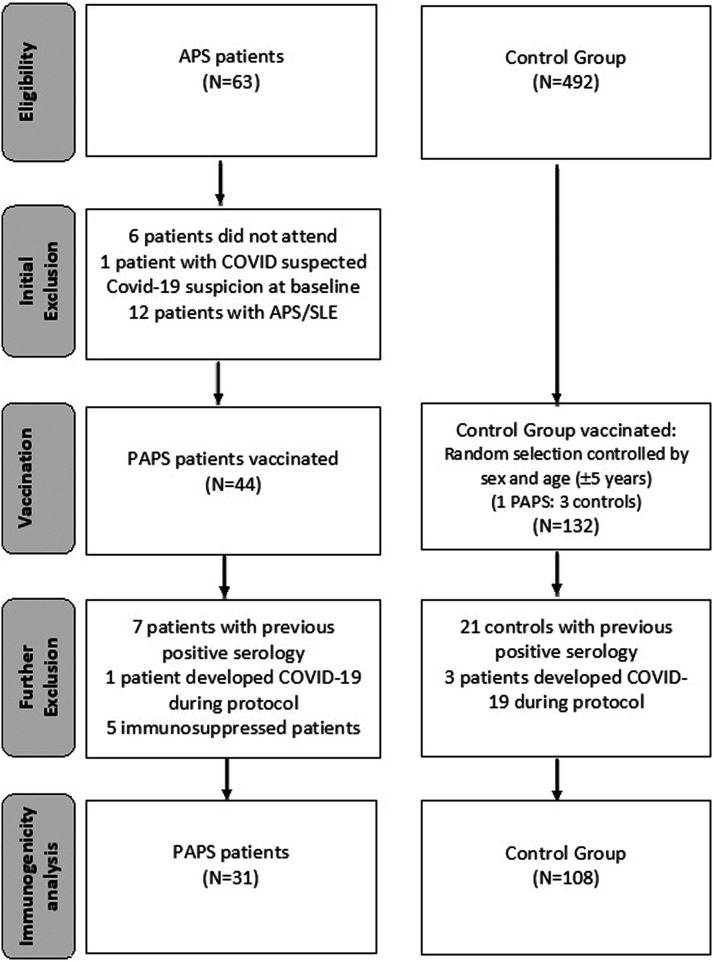
We initially selected 63 patients, but six patients did not attend the vaccine appointment, one patient had symptoms compatible with COVID-19 at the day of vaccination and 12 patients had associated systemic lupus erythematosus (SLE) and were excluded. The remaining 44 PAPS patients and 132 controls were included in the study. Forty-three patients had thrombotic criteria (97.7%) and 18 (40.9%) had obstetric criteria. Only one patient was classified as exclusively obstetric. Triple positivity was present in 45.4% of cases (Table 1). The number of triple positives was even higher (54.8%) considering only the 31 naïve-PAPS.
Table 1.
Baseline characteristics of primary antiphospholipid syndrome patients and controls.
| PAPS (n=44) | Controls (n=132) | p-Value | |
|---|---|---|---|
| Demographics | |||
| Current age, years | 46 (31–73) | 46 (31–78) | 0.982 |
| Age at diagnosis, years | 29 (17–67) | - | - |
| Disease duration, years | 16.7 ± 8.4 | - | - |
| Female sex | 44 (86.4) | 114 (86.4) | >0.999 |
| Caucasian race | 27 (61.4) | 64 (48.5) | 0.139 |
| Comorbidities | |||
| Systemic arterial hypertension | 18 (40.9) | 39 (29.5) | 0.163 |
| Diabetes mellitus | 3 (6.8) | 16 (12.1) | 0.411 |
| Dyslipidemia | 26 (59.1) | 11 (8.3) | <0.001 |
| Obesity | 21 (47.7) | 42 (32.3) | 0.066 |
| Current smoking | 17 (38.6) | 11 (8.3) | <0.001 |
| APS criteria manifestations | |||
| Thrombotic | 43 (97.7) | - | - |
| Arterial | 21 (47.7) | - | - |
| Stroke | 13 (29.5) | 0 (0) | <0.001 |
| Venous | 25 (56.8) | - | - |
| Obstetric | 18 (40.9) | - | - |
| aPL profile | |||
| Single positivity | 11 (25.0) | - | - |
| Double positivity | 13 (29.5) | - | - |
| Triple positivity | 20 (45.5) | ||
| APS treatment | |||
| VKA | 39 (88.6) | - | - |
| LMWH | 3 (6.8) | - | - |
| LDA | 8 (18.2) | - | |
| Hydroxychloroquine | 17 (38.6) | - | |
Results are expressed in mean ± standard deviation, median (minimum and maximum values), and n (%).
PAPS—primary antiphospholipid syndrome; aPL—antiphospholipid antibody; VKA—vitamin K antagonist; LMWH—low-molecular-weight heparin; LDA—low dose aspirin.
PAPS patients and CG had comparable median ages (46 [31–73] vs. 46 [31–78] years, p=0.982) and female sex (86.4% in both groups, p=1.0) at study entry. The mean duration of disease in PAPS patients was 16.7 ± 8.4 years. Of note, the PAPS group had more stroke than CG (29.5% vs. 0%, p<0.001), besides dyslipidemia (59.1% vs. 8.3%, p<0.001) and smoking (38.6% vs. 8.3%, p<0.001). These characteristics are shown in Table 1.
Vaccine immunogenicity
For this analysis, we excluded 37 (21.0%) participants (13 PAPS patients and 24 CG) due to pre-vaccination positive COVID-19 serology (6 PAPS and 18 CG) and/or NAb (1 PAPS and 3 CG) and the incidents confirmed cases of COVID-19 during the study (1 PAPS and 3 CG). Further exclusions for the immunogenicity analyses were related to continuous immunosuppression (not related to APS): two patients were using azathioprine and prednisone (one due to autoimmune hepatitis and the other due to idiopathic interstitial pulmonary disease); one patient with renal transplant was on mycophenolate mofetil, tacrolimus, and prednisone; one patient with cardiac transplant was on mycophenolate mofetil and cyclosporine; and one patient was using prednisone to treat livedoid vasculopathy.
The final immunogenicity analysis included 31 naïve-PAPS patients and 108 controls. Flow chart of the study is illustrated in Figure 1.
Figure 1.
Flowchart of patients and controls submitted to Sinovac-CoronaVac vaccination.
Anti-SARS-CoV-2 IgG antibodies
There was a modest initial response of anti-SARS-CoV-2 IgG in both groups after the first dose with comparable SC in naïve-PAPS patients a CG at D28 (25.8% vs. 30.6%, p=0.609). The SC rates at D69 increased approximately 3-fold after the second dose with similar immunogenicity for naïve-PAPS and GC groups: SC rates (83.9% vs. 93.5%, p=0.092) and geometric mean titers (GMT) (50.2 [95%CI 34.5–73.2] in PAPS vs. 61.7 [95%CI 52.8–72.3] in CG, p=0.249). The factor increase in GMT (FI-GMT) at D69 was also elevated in naïve-PAPS and CG (21.4 [95%CI 14.5–31.6] vs. 26.5 [95%CI 22.3–31.4], p=0.586) and at D69, respectively (Table 2).
Table 2.
Seroconversion rates and anti-SARS-CoV-2 S1/S2 IgG titers before and after CoronaVac in näive-PAPS and controls.
| Seroconversion (SC) | Geometric mean titer (GMT) | Factor increase in GMT | |||||
|---|---|---|---|---|---|---|---|
| D28 | D69 | D0 | D28 | D69 | D28 | D69 | |
| PAPS, n=31 | 8 (25.8) | 26 (83.9) | 2.4 (2.0–2.7) | 7.7 (5.1–11.6)a | 50.2 (34.5–73.2)a,b | 3.3 (2.2–4.9) | 21.4 (14.5–31.6) |
| Controls, n=108 | 33 (30.6) | 101 (93.5) | 2.3 (2.1–2.6) | 9.8 (7.6–12.6)c | 61.7 (52.8–72.3)c,d | 4.2 (3.4–5.1) | 26.5 (22.3–31.4) |
| p-Value (PAPS vs CG) | 0.609 | 0.092 | 0.936 | 0.359 | 0.249 | 0.600 | 0.586 |
PAPS—Primary antiphospholipid syndrome; CG—control group; SC—Seroconversion (defined as post-vaccination titer >15 AU/mL—Indirect ELISA, LIAISON® SARS-CoV-2 S1/S2 IgG, DiaSorin, Italy); GMT—Geometric mean titers (AU/mL).
Frequencies of SC are presented as number (%) and they were compared using chi-square between PAPS patients and CG at pre-specified time points (D28 and D69). IgG antibody titers and FI-GMT are expressed as geometric means with 95% confidence interval (95%CI). Comparisons of ln-transformed anti-S1/S2 IgG titers between PAPS and CG were performed using generalized estimating equations (GEE) with normal marginal distribution and gamma distribution, respectively. Results were followed by Bonferroni multiple comparisons to identify differences between groups and time points.
ap<0.001 for longitudinal comparisons of GMT in PAPS patients at D28 and D69 vs. baseline.
bp<0.001 for longitudinal comparison of GMT in PAPS patients at D69 vs. D28.
cp<0.001 for longitudinal comparison of GMT in control at D28 and D69 vs. baseline.
dp<0.001 for longitudinal comparison of GMT in control at D69 vs. D28.
According to Bonferroni’s multiple comparison, there was a significant GMT increase when we performed longitudinal comparisons of GMT in naïve-PAPS patients at baseline versus D28 and D69 (p<0.001, for both) and at D28 vs. D69 (p<0.001). Likewise, the results of longitudinal GMT comparisons in CG at D28 and D69 vs. baseline and between D69 vs. D28 also showed a significant increase (p<0.001, for all comparisons) (Table 2).
SARS-CoV-2 cPass virus-neutralization antibodies (NAb)
The frequency of NAb at D28 was lower in naïve-PAPS patients than CG (16.1% vs. 35.2%, p=0.043), with a robust rise at D69 and comparable NAb positivity rates among both groups (77.4% vs. 78.7%, p=0.440). NAb-activity was comparable in naïve-PAPS patients and CG at D28 (38.1% [32.0–55.5] vs. 43.7% [34.2–66.4], p=0.275) and D69 (64.3 [49.0–77.0%] vs. 60.9 [45.6–81.3%], p=0.689) (Table 3).
Table 3.
Frequency of neutralizing antibodies and neutralizing activity (%) after CoronaVac in näive-PAPS compared to controls.
| After vaccine 1st dose | After vaccine 2nd dose | |||
|---|---|---|---|---|
| Subjects with positive NAb, n (%) | Neutralizing activity (%) median (interquartile range) | Subjects with positive NAb, n (%) | Neutralizing activity (%) median (interquartile range) | |
| PAPS, n=31 | 5 (16.1)a | 38.1 (32–55.5) | 24 (77.4) | 64.3 (49.0–77.0) |
| Controls, n=108 | 38 (35.2) | 43.7 (34.2–66.4) | 85 (78.7) | 60.9 (45.6–81.3) |
| p-Value (PAPS vs CG) | p =0.043 | p=0.275 | p=0.440 | p=0.689 |
Results are expressed in median (interquartile range) and n (%).
Nab—neutralizing antibodies; PAPS—primary antiphospholipid syndrome; CG—control group.
Positivity for Nab defined as a neutralizing activity ≥30% (cPass sVNT Kit, GenScript, Piscataway, USA).
ap <0.05 in comparison to controls.
Antiphospholipid antibodies and vaccination
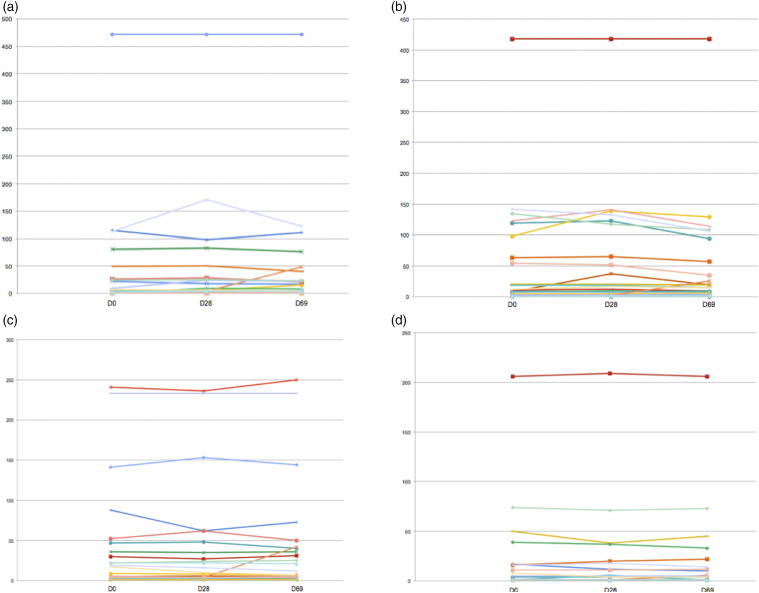
High titers of aCL at baseline were identified in 13/31 (41.9%) of the naïve-APS patients (seven of IgG isotype, four of IgM isotype, and 1 with both isotypes). Fourteen (45.2%) patients had high titers of aβ2GPI at baseline (four with IgG isotype, eight of IgM isotype, and two with both isotypes). All patients remained positive for aCL and/or aβ2GPI without significant changes in titers, but one patient with negative IgM aCL (5 MPL) and IgM aβ2GPI (5 UI/mL) at baseline and at D28 (IgM aCL: four MPL and IgM aβ2GPI:4 UI/mL) had an increment to 48 MPL and 42 UI/mL, respectively, at day 69.
No significant difference was found between samples collected before and after vaccination for all four autoantibodies (Figure 2). In the quantitative analysis, titers remained stable over time. In the qualitative assessment, frequencies of positivity also did not change for all aPL: IgG aCL positivity rates were 25.8% (n=8/31) vs. 25.8% (n=8/31) vs. 22.6% (n=7/31), p=0.944, at D0, D28, and D69; IgM aCL positivity rates were 16.1% (n=5/31) vs. 16.1% (n=5/31) vs. 19.4% (n=6/31), p=0.927, at D0, D28, and D69; IgG aβ2GPI positivity rates were 12.9% (n=4/31) vs. 12.9% (n=4/31) vs. 16.1% (n=5/31), p=0.914, at D0, D28, and D69; and IgM aβ2GPI positivity rates were 16.1% (n=5/31) vs. 16.1% (n=5/31) vs. 19.4% (n=6/31), p=0.927, at D0, D28, and D69.
Figure 2.
Antiphospholipid antibody titers evaluation in näive primary antiphospholipid patients before (baseline—D0) and after Sinovac-CoronaVac vaccination (first dose—D28 and second dose—D69). (a) Anticardiolipin antibody IgM (aCL, titers in MPL), (b) anticardiolipin antibody IgG (aCL, titers in GPL), (c) anti-beta-2 glycoprotein I IgM (aβ2GPI, titers in UI/mL), and (d) anti-beta-2 glycoprotein I IgG (aβ2GPI, titers in UI/mL).
The median (interquartile range) aGAPSS of the 31 naïve-APS patients did not modify after completing vaccination (D0 vs D28 vs D69: 13 [4–17] vs. 13 [4–17] vs. 13 [4–17], p=0.717).
Vaccine safety and tolerance
We did not observe any moderate/severe AE in any group. Local and systemic reactions were more common in the PAPS group after the first dose compared to controls, but not after the second dose. The overall description of AE in PAPS patients and controls is summarized in Table 4.
Table 4.
Adverse events of CoronaVac vaccination in primary antiphospholipid syndrome patients and controls.
| After vaccine 1st dose | After vaccine 2nd dose | |||||
|---|---|---|---|---|---|---|
| PAPS (n=44) | Controls (n=132) | p-Value | PAPS (n=44) | Controls (n=132) | p-Value | |
| No symptoms | 26 (59.1) | 81 (61.4) | 0.789 | 25 (59.5) | 86 (66.7) | 0.400 |
| Local reactions (at the injection site) | 14 (31.8) | 27 (20.5) | 0.123 | 8 (19.0) | 26 (20.2) | 0.876 |
| Pain | 12 (27.3) | 22 (16.7) | 0.123 | 6 (14.3) | 25 (19.4) | 0.457 |
| Erythema | 5 (11.4) | 1 (0.8) | 0.004 | 2 (4.8) | 1 (0.8) | 0.150 |
| Swelling | 2 (4.5) | 5 (3.8) | >0.999 | 3 (7.1) | 8 (6.2) | 0.732 |
| Bruise | 6 (13.6) | 4 (3.0) | 0.017 | 0 (0) | 1 (0.8) | >0.999 |
| Pruritus | 2 (4.5) | 1 (0.8) | 0.155 | 2 (4.8) | 6 (4.7) | >0.999 |
| Induration | 4 (9.1) | 6 (4.5) | 0.270 | 2 (4.8) | 9 (7.0) | >0.999 |
| Systemic reactions | 16 (36.4) | 39 (29.5) | 0.398 | 15 (35.7) | 37 (28.7) | 0.390 |
| Fever | 2 (4.5) | 2 (1.5) | 0.260 | 2 (4.8) | 3 (2.3) | 0.597 |
| Malaise | 6 (13.6) | 5 (3.8) | 0.019 | 3 (7.1) | 13 (10.1) | 0.764 |
| Somnolence | 6 (13.6) | 10 (7.6) | 0.226 | 1 (2.4) | 9 (7.0) | 0.454 |
| Lack of appetite | 2 (4.5) | 4 (3.0) | 0.641 | 1 (2.4) | 6 (4.7) | >0.999 |
| Nausea | 6 (13.6) | 1 (0.8) | 0.001 | 3 (7.1) | 9 (7.0) | >0.999 |
| Vomit | 1 (2.3) | 1 (0.8) | 0.439 | 0 (0) | 1 (0.8) | >0.999 |
| Diarrhea | 4 (9.1) | 7 (5.3) | 0.471 | 3 (7.1) | 7 (5.4) | 0.709 |
| Abdominal pain | 3 (6.8) | 6 (4.5) | 0.693 | 3 (7.1) | 7 (5.4) | 0.709 |
| Vertigo | 5 (11.4) | 3 (2.3) | 0.024 | 2 (4.8) | 6 (4.7) | >0.999 |
| Tremor | 3 (6.8) | 0 (0) | 0.015 | 1 (2.4) | 2 (1.6) | 0.573 |
| Headache | 6 (13.6) | 16 (12.1) | 0.792 | 8 (19.0) | 23 (17.8) | 0.859 |
| Fatigue | 8 (18.2) | 5 (3.8) | 0.002 | 5 (11.9) | 14 (10.9) | 0.851 |
| Sweating | 4 (9.1) | 2 (1.5) | 0.035 | 3 (7.1) | 3 (2.3) | 0.159 |
| Myalgia | 4 (9.1) | 3 (2.3) | 0.067 | 6 (14.3) | 14 (10.9) | 0.548 |
| Muscle weakness | 5 (11.4) | 4 (3.0) | 0.044 | 5 (11.9) | 10 (7.8) | 0.409 |
| Arthralgia | 6 (13.6) | 5 (3.8) | 0.019 | 4 (9.5) | 9 (7.0) | 0.737 |
| Back pain | 7 (15.9) | 7 (5.3) | 0.024 | 3 (7.1) | 16 (12.4) | 0.413 |
| Cough | 4 (9.1) | 4 (3.0) | 0.109 | 0 (0) | 6 (4.7) | 0.338 |
| Sneezing | 4 (9.1) | 6 (4.5) | 0.270 | 5 (11.9) | 11 (8.5) | 0.514 |
| Coryza | 3 (6.8) | 11 (8.3) | >0.999 | 5 (11.9) | 16 (12.4) | 0.932 |
| Stuffy nose | 6 (13.6) | 6 (4.5) | 0.038 | 2 (4.8) | 12 (9.3) | 0.522 |
| Sore throat | 0 (0) | 5 (3.8) | 0.333 | 2 (4.8) | 7 (5.4) | >0.999 |
| Shortness of breath | 2 (4.5) | 4 (3.0) | 0.641 | 0 (0) | 4 (3.1) | 0.573 |
| Conjunctivitis | 0 (0) | 0 (0) | - | 0 (0) | 1 (0.8) | >0.999 |
| Pruritus | 2 (4.5) | 0 (0) | 0.061 | 2 (4.8) | 2 (1.6) | 0.253 |
| Skin rash | 1 (2.3) | 2 (1.5) | >0.999 | 0 (0) | 1 (0.8) | >0.999 |
Results are presented in n (%). PAPS—primary antiphospholipid syndrome.
COVID-19 incident cases
During the study, four participants (one PAPS patient and three CG) had incident symptomatic cases of COVID-19, all confirmed by RT-PCR. All cases occurred from D0 to D32 and none of them was hospitalized.
Discussion
To the best of our knowledge, this is the first study to demonstrate that the Sinovac-CoronaVac vaccine is highly immunogenic and safe in PAPS patients and did not trigger short- and medium-term thrombosis or increase of aPL-related antibodies production.
Recent studies focusing on an overall evaluation of mRNA COVID-19 immunized ARD patients have shown a good safety profile, with no severe AE or underlying disease flare.30,31 However, lower antibody titers compared to controls were observed, which may impact protection against the virus.32-33 In line with these findings, our recent study revealed a moderate, but reduced SC rate with Sinovac-CoronaVac in 910 adults with naïve ARD (vs. 182 naïve volunteers in CG). Immunosuppressive drugs and prednisone were identified as factors associated with diminished immunogenicity evaluating the entire group of ARD patients.23
However, PAPS patients may have some distinct clinical and immunological features 34 compared to other ARDs. A previous study published by our group evaluating the response to the H1N1 vaccine in 1668 ARD patients demonstrated that PAPS patients presented higher rates of SC than several other ARDs.35 The present study with Sinovac-CoronaVac vaccine showed that PAPS patients had a high SC and high NAb positivity, comparable to the CG. The most likely explanation is the fact that the cornerstone of treatment in this syndrome is lifelong anticoagulation and not immunosuppressive therapy.36 The accuracy of this data was improved by the fact that both groups were balanced by age and sex, one of the most important parameters to influence vaccine response.37 In addition, the impact of previous exposure in vaccine response was excluded, since only naïve-PAPS patients were evaluated for immunogenicity. In fact, previous studies have demonstrated that vaccine-induced antibody response is greatly enhanced in pre-exposed individuals.38-39
The safety profile of inactivated COVID-19 vaccines has been tested and confirmed by mass immunization programs; those vaccines are highly relevant for the population evaluated in the present study.40 Our PAPS patients had more minor adverse effects compared to controls. Perhaps the awareness of having a thrombophilia might have alerted them to report any symptom after the first dose. The occurrence of more bruises was expected because of anticoagulation.
Even though the thrombotic risk assessed with aGAPSS in our PAPS patients was very high, no thrombotic event was recorded during our study.41 In addition, these patients have a high frequency of comorbidities associated with endothelial dysfunction, such as hypertension, obesity, and dyslipidemia, which may also favor clot events.42-44 Despite the very small sample size, it is reassuring that no cases of venous and arterial thromboses were observed in this high-risk population, after 6 months of follow-up.
Supporting this notion, aPL titers were comparable before and after complete vaccination, an encouraging finding since aPL has an important role in the PAPS thrombogenesis.10 Consistent with this observation, we have not detected a significant production of aPL-related antibodies nor thrombotic events after the pandemic influenza immunization in PAPS patients.45 Furthermore, a larger Chinese study with 406 healthy-workers immunized with inactivated SARS-CoV-2 vaccine (BBIBPCorV, Sinopharm, Beijing, China) also found no significant difference in aPL measurement in serial blood samples before and 4 weeks after the second dose.46
Our study has some limitations. The routine blood collection used to perform immunogenicity assays of SARS-CoV-2 could not be extrapolated to LA functional assays, a known high-risk parameter for thrombosis in PAPS and perhaps also for COVID-19 infection.47 Another flaw in our study was the small convenience sample size but very much related to the general prevalence of this disease in the population, which is approximately 50 per 100,000 population,48 with numbers being even lower when considering only PAPS.
In conclusion, Sinovac-CoronaVac vaccine was highly immunogenic, demonstrated a good safety profile, and did not trigger short- and medium-term thrombosis or production of aPL in naïve-PAPS patients. Our findings support the recommendation of SARS-CoV-2 vaccination for PAPS patients.
Acknowledgments
We thank the contribution of the Central Laboratory Division, Registry Division, Security Division, IT Division, Superintendency, Pharmacy Division, and Vaccination Center (CRIE) for their technical support. We also thank the volunteers for participating in the three in-person visits of the protocol, handling the biological material and those responsible for the follow-up of all participants.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP) (#2015/03756-4 to SGP, CAS, NEA and EB, #2019/17272-0 to LVKK); Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq #304984/2020-5 to CAS, #305242/2019-9 to EB, #306879/2018-2 to EFB), B3 - Bolsa de Valores do Brasil to EB, and Instituto Butantan supplied the study product and had no other role in the trial.
Trial registration: ClinicalTrials.gov- NCT04754698 first registered on February 8th 2021. https://clinicaltrials.gov/ct2/show/NCT04754698?term=NCT04754698&draw=2&rank=1
ORCID iDs
Flavio Signorelli https://orcid.org/0000-0002-5565-1017
Gustavo Guimarães Moreira Balbi https://orcid.org/0000-0003-0235-8834
Nadia E Aikawa https://orcid.org/0000-0002-7585-4348
Clovis A Silva https://orcid.org/0000-0001-9250-6508
Sandra G Pasoto https://orcid.org/0000-0002-7343-6804
Eduardo F Borba https://orcid.org/0000-0001-6194-5129
Danieli Castro Oliveira Andrade https://orcid.org/0000-0002-0381-1808
Eloisa Bonfá https://orcid.org/0000-0002-0520-4681
References
- 1.Chan NC, Weitz JI. COVID-19 coagulopathy, thrombosis, and bleeding. Blood 2020; 136: 381–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Levi M, van der Poll T. Inflammation and coagulation. Crit Care Med 2010; 38(2 Suppl): S26–S34. [DOI] [PubMed] [Google Scholar]
- 3.Gustine JN, Jones D. Immunopathology of hyperinflammation in COVID-19. Am J Pathol 2021; 191: 4–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Price LC, McCabe C, Garfield B, et al. Thrombosis and COVID-19 pneumonia: the clot thickens! Eur Respir J 2020; 56: 2001608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Suh YJ, Hong H, Ohana M, et al. Pulmonary embolism and deep vein thrombosis in COVID-19: A systematic review and meta-analysis. Radiology 2021; 298: E70–E80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tan BK, Mainbourg S, Friggeri A, et al. Arterial and venous thromboembolism in COVID-19: a study-level meta-analysis. Thorax 2021; 76: 970–979. [DOI] [PubMed] [Google Scholar]
- 7.Radin M, Cecchi I, Rubini E, et al. Treatment of antiphospholipid syndrome. Clin Immunol 2020; 221: 108597. [DOI] [PubMed] [Google Scholar]
- 8.Cervera R. Lessons from the "Euro-Phospholipid" project. Autoimmun Rev 2008; 7: 174–178. [DOI] [PubMed] [Google Scholar]
- 9.Miyakis S, Lockshin MD, Atsumi T, et al. International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS). J Thromb Haemost 2006; 4: 295–306. [DOI] [PubMed] [Google Scholar]
- 10.Wang X, Krouzman E, Andrade DCO, et al. COVID-19 and antiphospholipid antibodies: A position statement and management guidance from AntiPhospholipid Syndrome Alliance for Clinical Trials and InternatiOnal Networking (APS ACTION). Lupus 2021; 31: 143–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tung ML, Tan B, Cherian R, et al. Anti-phospholipid syndrome and COVID-19 thrombosis: connecting the dots. Rheumatol Advances Practice 2021; 5: rkaa081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kipshidze N, Dangas G, White CJ, et al. Viral coagulopathy in patients with COVID-19: Treatment and care. Clin Applied Thrombosis/hemostasis : Official Journal Int Acad Clin Appl Thrombosis/Hemostasis 2020; 26: 1076029620936776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zuo Y, Estes SK, Ali RA, et al. Prothrombotic autoantibodies in serum from patients hospitalized with COVID-19. Sci Translational Medicine 2020; 12: eabd3876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Taha M, Samavati L. Antiphospholipid antibodies in COVID-19: a meta-analysis and systematic review. RMD Open 2021; 7: e001580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Willis R, Harris E, Pierangeli S. Pathogenesis of the antiphospholipid syndrome. Semin Thromb Hemost 2012; 38: 305–321. [DOI] [PubMed] [Google Scholar]
- 16.Giannakopoulos B, Krilis SA. The pathogenesis of the antiphospholipid syndrome. New Engl J Med 2013; 368: 1033–1044. [DOI] [PubMed] [Google Scholar]
- 17.El Hasbani G, Taher AT, Jawad A, et al. COVID-19, Antiphospholipid Antibodies, and Catastrophic Antiphospholipid Syndrome: A Possible Association? Clin Medicine Insights. Arthritis Musculoskeletal Disorders 2020; 13: 1179544120978667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Makris M, Pavord S, Lester W, et al. Vaccine-induced immune thrombocytopenia and thrombosis (VITT). Res Practice Thrombosis Haemostasis 2021; 5: e12529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang Y, Zeng G, Pan H, et al. Safety, tolerability, and immunogenicity of an inactivated SARS-CoV-2 vaccine in healthy adults aged 18-59 years: a randomised, double-blind, placebo-controlled, phase 1/2 clinical trial. Lancet Infect Dis 2021; 21: 181–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mallapaty S. WHO approval of Chinese CoronaVac COVID vaccine will be crucial to curbing pandemic. Nature 2021; 594: 161–162. [DOI] [PubMed] [Google Scholar]
- 21.Palacios R, Batista AP, Albuquerque CSN, et al. Efficacy and safety of a COVID-19 inactivated vaccine in healthcare professionals in Brazil: The PROFISCOV study. SSRN Electron J Published 2021; 21(1):853. [Google Scholar]
- 22.Jara A, Undurraga EA, González C, et al. Effectiveness of an inactivated SARS-CoV-2 vaccine in Chile. New Engl J Med 2021; 385: 875–884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Medeiros-Ribeiro AC, Aikawa NE, Saad CGS, et al. Immunogenicity and safety of the CoronaVac inactivated vaccine in patients with autoimmune rheumatic diseases: a phase 4 trial. Nat Med 2021; 27: 1744–1751. [DOI] [PubMed] [Google Scholar]
- 24.Taylor SC, Hurst B, Charlton CL, et al. A new SARS-CoV-2 dual-purpose serology test: Highly accurate infection tracing and neutralizing antibody response detection. J Clinical Microbiology 2021; 59: e02438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.World Health Organization . World alliance for patient safety: WHO draft guidelines for adverse event reporting and learning systems: from information to action. World Health Organization, 2005. [Google Scholar]
- 26.Harris PA, Taylor R, Minor BL, et al. The REDCap consortium: Building an international community of software platform partners. J Biomed Inform 2019; 95: 103208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Harris PA, Taylor R, Thielke R, et al. Research electronic data capture (REDCap)-A metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009; 42: 377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Corman VM, Landt O, Kaiser M, et al. Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. Euro Surveill: Eur Commun Dis Bull 2020; 25: 2000045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sciascia S, Sanna G, Murru V, et al. GAPSS: the Global Anti-Phospholipid Syndrome Score. Rheumatology 2013; 52: 1397–1403. [DOI] [PubMed] [Google Scholar]
- 30.Radin M, Schreiber K, Costanzo P, et al. The adjusted Global AntiphosPholipid Syndrome Score (aGAPSS) for risk stratification in young APS patients with acute myocardial infarction. Int J Cardiol 2017; 240: 72–77. [DOI] [PubMed] [Google Scholar]
- 31.Pengo V, Tripodi A, Reber G, et al. Update of the guidelines for lupus anticoagulant detection. J Thromb Haemost 2009; 7: 1737–1740. [DOI] [PubMed] [Google Scholar]
- 32.Geisen UM, Berner DK, Tran F, et al. Immunogenicity and safety of anti-SARS-CoV-2 mRNA vaccines in patients with chronic inflammatory conditions and immunosuppressive therapy in a monocentric cohort. Ann Rheum Dis 2021; 80: 1306–1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Simon D, Tascilar K, Fagni F, et al. SARS-CoV-2 vaccination responses in untreated, conventionally treated and anticytokine-treated patients with immune-mediated inflammatory diseases. Ann Rheum Dis 2021; 80: 1312–1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cervera R, Piette J-C, Font J, et al. Antiphospholipid syndrome: Clinical and immunologic manifestations and patterns of disease expression in a cohort of 1,000 patients. Arthritis Rheum 2002; 46: 1019–1027. [DOI] [PubMed] [Google Scholar]
- 35.Saad CGS, Borba EF, Aikawa NE, et al. Immunogenicity and safety of the 2009 non-adjuvanted influenza A/H1N1 vaccine in a large cohort of autoimmune rheumatic diseases. Ann Rheum Dis 2011; 70: 1068–1073. [DOI] [PubMed] [Google Scholar]
- 36.Signorelli F, Balbi GGM, Domingues V, et al. New and upcoming treatments in antiphospholipid syndrome: A comprehensive review. Pharmacol Res 2018; 133: 108–120. [DOI] [PubMed] [Google Scholar]
- 37.Sadarangani M, Abu Raya B, Conway JM, et al. Importance of COVID-19 vaccine efficacy in older age groups. Vaccine 2021; 39: 2020–2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sadarangani M, Marchant A, Kollmann TR. Immunological mechanisms of vaccine-induced protection against COVID-19 in humans. Nat Rev Immunol 2021; 21: 475–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ebinger JE, Fert-Bober J, Printsev I, et al. Antibody responses to the BNT162b2 mRNA vaccine in individuals previously infected with SARS-CoV-2. Nat Med 2021; 27: 981–984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.He Q, Mao Q, Zhang J, et al. COVID-19 Vaccines: Current Understanding on Immunogenicity, Safety, and Further Considerations. Front Immunol 2021; 12: 669339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Radin M, Sciascia S, Erkan D, et al. The adjusted global antiphospholipid syndrome score (aGAPSS) and the risk of recurrent thrombosis: Results from the APS ACTION cohort. Semin Arthritis Rheum 2019; 49: 464–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lowe GDO. Common risk factors for both arterial and venous thrombosis. Br J Haematol 2008; 140: 488–495. [DOI] [PubMed] [Google Scholar]
- 43.Greinacher A, Thiele T, Warkentin TE, et al. Thrombotic Thrombocytopenia after ChAdOx1 nCov-19 Vaccination. New Engl J Med 2021; 384: 2092–2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hunter PR. Thrombosis after covid-19 vaccination. BMJ 2021; 373: n9. [DOI] [PubMed] [Google Scholar]
- 45.de Medeiros DM, Silva CA, Bueno C, et al. Pandemic influenza immunization in primary antiphospholipid syndrome (PAPS): a trigger to thrombosis and autoantibody production? Lupus 2014; 23: 1412–1416. [DOI] [PubMed] [Google Scholar]
- 46.Liu T, Dai J, Yang Z, et al. Inactivated SARS-CoV-2 vaccine does not influence the profile of prothrombotic antibody nor increase the risk of thrombosis in a prospective Chinese cohort. Sci Bull 2021; 66: 2312–2319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Devreese KMJ, Linskens EA, Benoit D, et al. Antiphospholipid antibodies in patients with COVID‐19: A relevant observation? J Thromb Haemost 2020; 18: 2191–2201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Duarte-García A, Pham MM, Crowson CS, et al. The epidemiology of antiphospholipid syndrome: A population-based study. Arthritis Rheumatol 2019; 71: 1545–1552. [DOI] [PMC free article] [PubMed] [Google Scholar]