Abstract
Trichophyton indotineae, a new species of dermatophytes, has become a significant concern in treating dermatophytosis due to the high level of terbinafine resistance reported in this organism. This is the first report of Trichophyton indotineae infection in Central Vietnam. Antifungal susceptibility testing showed that this isolate was susceptible to itraconazole, voriconazole, and terbinafine. Therefore, the patient was successfully treated with oral itraconazole and ketoconazole topical cream.
Keywords: Dermatophytes, Trichophyton indotineae, Trichophyton interdigitale, Tinea corporis
1. Introduction
Trichophyton spp. is a significant cause of dermatophytosis worldwide [1]. Most common species are Trichophyton rubrum, Trichophyton mentagrophytes, Trichophyton interdigitale, and Trichophyton tonsurans [2]. This pathogenic fungal genus could produce a broad spectrum of clinical dermatophytosis, including tinea corporis, tinea capitis, tinea manuum, tinea pedis, tinea cruris, and onychomycosis [3]. Trichophyton interdigitale (T. interdigitale) is the common etiologies isolated from humans' skin and nails [1], and terbinafine-resistance of this species is one of the major concerns in treatment. Nowadays, some new species are identified, and with the help of molecular methods, it is possible to have a more accurate understanding the taxonomic status of dermatophytes [2]. Trichophyton indotineae (T. indotineae), a species newly reported, has been determined as Trichophyton interdigitale-like species [4]. Although they share similarities in morphology and genetic characteristics [4,5], the result of urease test seems to differ between the two species, with T. interdigitale is mostly positive, while T. indotineae is weakly positive or negative [4,6]. A high level of terbinafine resistance has been reported of T. indotineae in India [4].
2. Case
A 27-year-old Vietnamese man who lived alone in the urban area of Hue city developed a skin lesion on his right leg for two months. He was working as a carpenter, with no contact to animals and no recent travel history. When the lesion appeared, the man initially tried to use some traditional herbal medicines, however, the lesion progressed in size requiring a visit to Hue University of Medicine and Pharmacy Hospital, Hue City, Vietnam in May 2020. No disease was noted in his medical history.
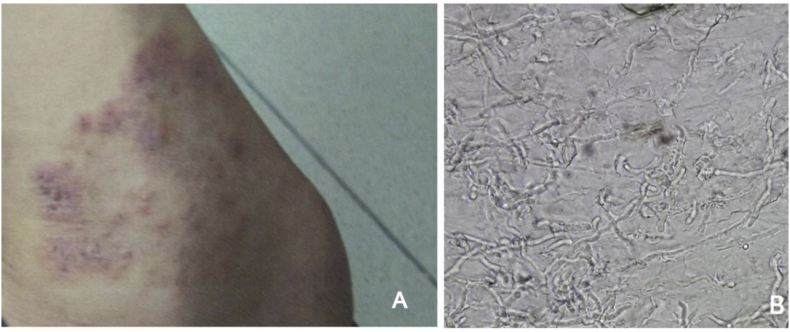
The dermatological examination revealed a large red skin rash on his right leg with a scaly and itchy presentation (Fig. 1A), and dermatophytes were considered the causative agent. His skin samples were collected at the Department of Parasitology for fungal examination. The direct microscopic examination of the skin scraping sample showed filamentous structure and arthrospores, which was consistent with fungal infection (Fig. 1B).
Fig. 1.
A. Patient's leg skin lesions, B. Numerous hyphae in skin sample checking with 20% KOH solution.
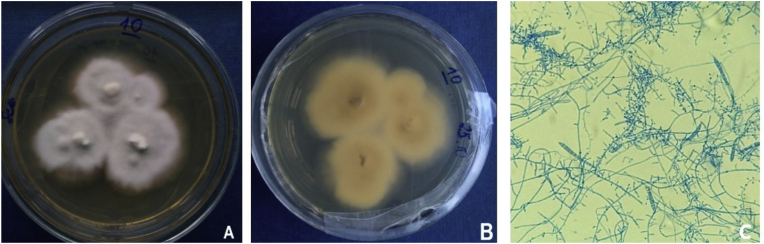
Skin scrapings were initially cultured on Sabouraud - Chloramphenicol - Cycloheximide dish medium, incubated at 28 °C, and observed every two days to follow the fungal growth. After ten days, the fungal colonies had a distinct velvety white color, a flat appearance, and elevated slightly raised in the center; the reverse showed a light yellow pigment. Microscopy revealed numerous microconidia with the pyriform and clavate form; macroconidia with 4–8 septa (Fig. 2A, B, C). Morphologically, this isolate was similar to T. interdigitale.
Fig. 2.
A and B. Macroscopic colonies morphology, C. Microscopic morphology in Lactophenol cotton blue solution. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
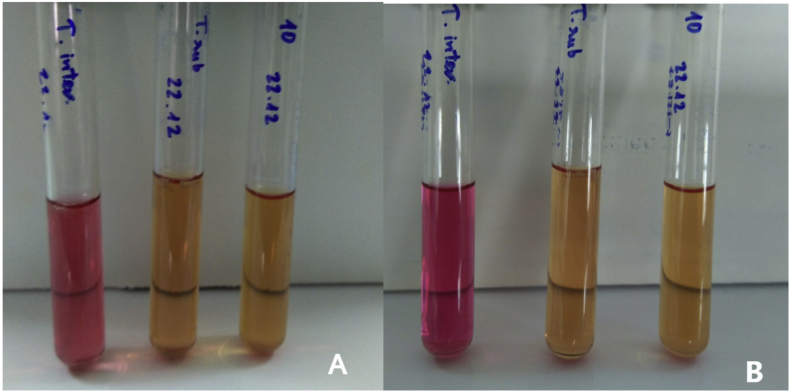
The urease test was also performed using urea broth medium (HiMedia, India) and the negative result was recorded after seven days and fourteen days. Trichophyton interdigitale ATCC 9533 and Trichophyton rubrum ATCC 28188 were used as positive and negative controls, respectively (Fig. 3). This isolate was then cultured on Tween agar medium to evaluate lipolytic activity [7]. The result confirmed a positive lipase reaction (Fig. 4).
Fig. 3.
Urease test results of T. interdigitale ATCC 9533, T. rubrum ATCC 28188, and isolate No.10 after 7 days incubation (A), 14 days incubation (B).
Fig. 4.
Tween agar positive result after three weeks.
For molecular identification, fungal DNA was extracted according to the instruction of the MasterPure™ Yeast DNA Purification kit. Polymerase chain reaction (PCR) was then performed using universal fungal primers ITS1 (5′-TCCGTAGGTGAACCTGCGG-3′) and ITS4 (5′-TCCTCCGCTTATTGATATGC-3′) (Integrated DNA Technologies) to amplify the ITS rDNA region [8]. The final volume for PCR amplification was 50 μL, which contained two μL of template DNA, 25 μL of PCR 2X Master Mix (Invitrogen, USA), two μL of each 10 μM oligo primer, and 19 μL water. PCR reactions were performed using SureCycler 8800 Thermal Cycler with cycling conditions previously published [8]. PCR products were visualized by running electrophoresis on a 1% agarose gel staining with GelRed™ (Biotium). The result was observed under a UV transilluminator. Both T. rubrum ATCC 28188 and T. interdigitale ATCC 9533 were used as positive controls. The presence of a specific band of around 700 bp was considered as a demonstration DNA target (Fig. 5).
Fig. 5.
Electrophoretic patterns PCR of T. rubrum ATCC 28188 (T.rub), T. interdigitale ATCC 9533 (T.inte), the control negative (C.neg), and isolate No.10.
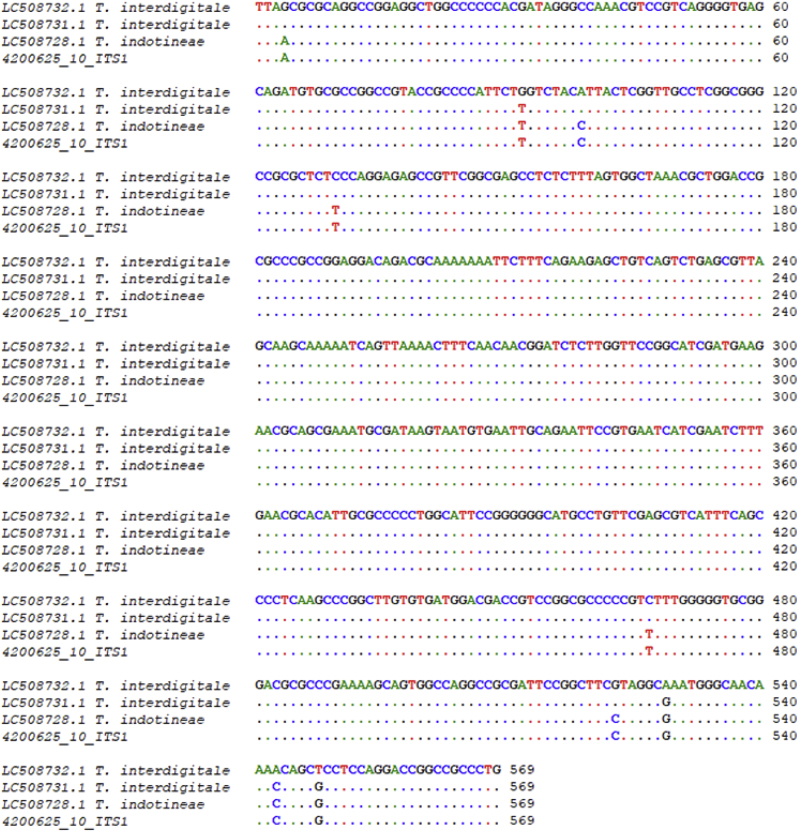
The amplicons were sent for purification and Sanger sequencing to Malaysia's 1st Base DNA Sequencing Service (https://www.base-asia.com/dna-sequencing-services). Newly generated sequences were analyzed using BLAST in GenBank. Furthermore, sequences were aligned by Bioedit 7.2.5 to check the similarity with reference isolates, including T. interdigitale LC508732 and LC508731, T. indotineae LC508728 [4]. The result showed some nucleotide differences between the new sequence and T. interdigitale, but 100% was found identical to T. indotineae LC508728 (Fig. 6). Our ITS sequence has been deposited in the GenBank database under accession number OM108103.
Fig. 6.
ITS rDNA region aligned results of our isolate (No.10: 4200625) and reference strains: T. interdigitale LC508732.1 and LC508731.1, T. indotineae LC508728.
The Minimum inhibitory concentrations (MICs) values of itraconazole, voriconazole, and terbinafine for this fungal isolate were measured following the EUCAST recommendations (E.Def.11.0) for Trichophyton [9]. Itraconazole (Sigma - Aldrich), voriconazole (AK Scientific, Inc, USA), and terbinafine (Sigma - Aldrich) were provided by the manufacturers as standard powder. The experiment was performed in 96-well microdilution plates with flat-bottom, incubated at 28 °C, and MICs were read at day +5 [10]. A. fumigatus ATCC 204305 was used as a quality control strain. The result showed that MIC values were 0.125 mg/L to itraconazole, and 0.25mg/L to both voriconazole and terbinafine. The patient was treated with oral itraconazole in a dose of 200mg/day for one week and topical ketoconazole for two weeks. His skin lesion and symptoms almost disappeared after the treatment period.
Susceptibility to terbinafine was also evaluated molecularly by checking the specific hotspots mutation on Squalene Monooxygenase gene SQLE (Leu393Ser/Phe or Phe397Leu) associated with resistance previously described by Kano et al. [5]. Generated sequences were compared with the reference strain of T. mentagrophytes (KU242352) [5]. We detected three mutations (A3360G; G3606T; A3734G), none associated with terbinafine resistance. The SQLE sequence has been deposited in the GenBank database under accession number ONO54179.
3. Discussion
This is the first case report of tinea corporis caused by T. indotineae in Vietnam. Physicochemical properties were checked including urease test and lipolytic activity. Antifungal susceptibility testing showed that our isolate was susceptible to itraconazole, voriconazole, and terbinafine with MICs of 0.125mg/L, 0.25mg/L, and 0.25mg/L, respectively. Treatment was successful with antifungal therapy combined with oral itraconazole and topical ketoconazole.
T. indotineae, a newly described species of Trichophyton, was first reported in 2020 from North India, and showed a high level of terbinafine resistance [4]. This species, which mainly reported in India [6], has been reported until now from in other parts of the world [[11], [12], [13]]. In France, T. indotineae were isolated from patients returning from countries such as India, Bangladesh, Myanmar, suggesting the presence of T. indotineae in Asian countries [13]. Fungal identification is usually based on macroscopy and microscopy, but it is not appropriate in this case since T. indotineae is similar to T. interdigitale and even to other species of the T. mentagrophytes complex [6]. Urease production is one of the physical characteristics of fungi that seems to be useful to discriminate between T. indotineae and T. interdigitale. While the former has a negative result, the latter shows a positive. According to a study by Tang et al., almost all strains of T. interdigitale were positive for urease, while most strains of T. indotineae were negative [6]. The isolate in the present study was negative for urease after 7 days and 14 days. In addition, our isolate was able to produce the lipolytic enzyme, indicating it is probable belonging to the T. indotineae species as previously indicated by Tang et al. [6].
Slight differences between T. indotineae and T. interdigitale have been described in the previous studies by sequencing ITS region [4]. The result showed that our isolate had 100% identity to T. indotineae LC508728 [4] (Fig. 6).
Although T. indotineae was known as a high terbinafine-resistant strain [4,6,14], the NUBS20020 from Japan was susceptible to terbinafine showing a MIC of <0.03mg/L [5]. Kano et al. correlated a specific missense mutation (Phe397Leu) in the squalene epoxidase-(SQLE) gene of T. indotineae resistance to terbinafine, recommending a PCR method to identify hotspot mutation [5]. According to this study, our terbinafine-sensitive isolate did not show this hotspot mutation.
In conclusion, this was the first detection of T. indotineae in Vietnam, further studies will be conducted, to better understand Trichophyton species’ phenotypic and genotypic characteristics.
Declaration of competing interest
The authors have no conflict of interest.
Acknowledgments
This work was supported by Hue University, Vietnam (Grant code: DHH-2020-04-124). We would like to acknowledge the staffs of the Parasitology Department, Hue University of Medicine and Pharmacy Hospital who are not listed as authors in this work. We thank Dr. Antonella Santona, Department of Biomedical Science, Sassari University-Italy, who supported us in analyzing the SQLE gene.
References
- 1.Brescini L., Fioriti S., Morroni G., Barchiesi F. Antifungal combinations in dermatophytes. J. Fungi. 2021;7(9):727. doi: 10.3390/jof7090727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhan P., Liu W. The changing face of dermatophytic infections worldwide. Mycopathologia. 2017;182(1–2):77–86. doi: 10.1007/s11046-016-0082-8. [DOI] [PubMed] [Google Scholar]
- 3.Nenoff P., Kruger C., Ginter-Hanselmayer G., Tietz H.J. Mycology - an update. Part 1: dermatomycoses: causative agents, epidemiology and pathogenesis. Journal der Deutschen Dermatologischen Gesellschaft = J. German Soc. Dermatol.: JDDG. 2014;12(3):188–210. doi: 10.1111/ddg.12245. [DOI] [PubMed] [Google Scholar]
- 4.Kano R., Kimura U., Kakurai M., Hiruma J., Kamata H., Suga Y., et al. Trichophyton indotineae sp. nov.: a new highly terbinafine-resistant anthropophilic dermatophyte species. Mycopathologia. 2020;185(6):947–958. doi: 10.1007/s11046-020-00455-8. [DOI] [PubMed] [Google Scholar]
- 5.Kano R., Noguchi H., Harada K., Hiruma M. Rapid molecular detection of terbinafine-resistant dermatophytes. Med. Mycol. J. 2021;62(2):41–44. doi: 10.3314/mmj.21-00001. [DOI] [PubMed] [Google Scholar]
- 6.Tang C., Kong X., Ahmed S.A., Thakur R., Chowdhary A., Nenoff P., et al. Taxonomy of the Trichophyton mentagrophytes/T. Interdigitale species complex harboring the highly virulent, Multiresistant Genotype T. indotineae. Mycopathologia. 2021;186(3):315–326. doi: 10.1007/s11046-021-00544-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Elavarashi E., Kindo A.J., Rangarajan S. Enzymatic and non-enzymatic virulence activities of dermatophytes on solid media. J. Clin. Diagn. Res. : J. Clin. Diagn. Res. 2017;11(2):DC23–D25. doi: 10.7860/JCDR/2017/23147.9410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Do N.A., Nguyen T.D., Nguyen K.L., Le T.A. Distribution of species of dermatophyte among patients at a dermatology centre of Nghean Province, Vietnam, 2015-2016. Mycopathologia. 2017;182(11–12):1061–1067. doi: 10.1007/s11046-017-0193-x. [DOI] [PubMed] [Google Scholar]
- 9.Arendrup M.C., Kahlmeter G., Guinea J., Meletiadis J. How to: perform antifungal susceptibility testing of microconidia-forming dermatophytes following the new reference EUCAST method E.Def 11.0, exemplified by Trichophyton. Clin. Microbiol. Infect. 2021;27(1):55–60. doi: 10.1016/j.cmi.2020.08.042. [DOI] [PubMed] [Google Scholar]
- 10.Dogra S., Shaw D., Rudramurthy S. Antifungal drug susceptibility testing of dermatophytes: laboratory findings to clinical implications. Indian Dermatol. Online J. 2019;10(3):225–233. doi: 10.4103/idoj.IDOJ_146_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Astvad K.M.T., Hare R.K., Jørgensen K.M., Saunte D.M.L., Thomsen P.K., Arendrup M.C. Increasing terbinafine resistance in Danish Trichophyton isolates 2019–2020. J. Fungi. 2022;8(2):150. doi: 10.3390/jof8020150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Posso-De Los Rios C.J., Tadros E., Summerbell R.C., Scott J.A. Terbinafine resistant Trichophyton indotineae isolated in patients with superficial dermatophyte infection in Canadian patients. J. Cutan. Med. Surg. 2022 doi: 10.1177/12034754221077891. 12034754221077891. [DOI] [PubMed] [Google Scholar]
- 13.Jabet A., Brun S., Normand A.C., Imbert S., Akhoundi M., Dannaoui E., et al. Extensive dermatophytosis caused by terbinafine-resistant Trichophyton indotineae, France. Emerg. Infect. Dis. J. 2022;28(1):229–233. doi: 10.3201/eid2801.210883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kong X., Tang C., Singh A., Ahmed S.A., Al-Hatmi A.M.S., Chowdhary A., et al. Antifungal susceptibility and mutations in the squalene epoxidase gene in dermatophytes of the Trichophyton mentagrophytes species complex. Antimicrob. Agents Chemother. 2021;65(8) doi: 10.1128/AAC.00056-21. e00056-21. [DOI] [PMC free article] [PubMed] [Google Scholar]