Abstract
Phytase improves the bioavailability of phytate phosphorus in plant foods to humans and animals and reduces phosphorus pollution of animal waste. Our objectives were to express an Aspergillus niger phytase gene (phyA) in Saccharomyces cerevisiae and to determine the effects of glycosylation on the phytase’s activity and thermostability. A 1.4-kb DNA fragment containing the coding region of the phyA gene was inserted into the expression vector pYES2 and was expressed in S. cerevisiae as an active, extracellular phytase. The yield of total extracellular phytase activity was affected by the signal peptide and the medium composition. The expressed phytase had two pH optima (2 to 2.5 and 5 to 5.5) and a temperature optimum between 55 and 60°C, and it cross-reacted with a rabbit polyclonal antibody against the wild-type enzyme. Due to the heavy glycosylation, the expressed phytase had a molecular size of approximately 120 kDa and appeared to be more thermostable than the commercial enzyme. Deglycosylation of the phytase resulted in losses of 9% of its activity and 40% of its thermostability. The recombinant phytase was effective in hydrolyzing phytate phosphorus from corn or soybean meal in vitro. In conclusion, the phyA gene was expressed as an active, extracellular phytase in S. cerevisiae, and its thermostability was affected by glycosylation.
Phytate, myo-inositol hexophosphate, is the major storage form of phosphorus in food or feeds of plant origin (23). Phytases, a specific group of phosphatases, are required to initiate the release of phosphorus from phytate. Simple-stomached animals such as swine and poultry, as well as humans, have little phytase activity in their gastrointestinal tracts. Thus, nearly all of the dietary phytate phosphorus ingested by these species is excreted into the environment, resulting in phosphorus pollution in areas of intensive animal production (3). Supplemental microbial phytase in corn-soybean meal diets for swine and poultry effectively improves phytate phosphorus utilization by these animals and reduces their fecal phosphorus excretion by up to 50% (15, 16).
Aspergillus niger phytase (EC 3.1.3.8) has been well characterized: it is an extracellular glycoprotein, and the mature enzyme has a mass of approximately 80 kDa (26). The gene, phyA, has been cloned from A. niger and sequenced (20, 26a). There are 10 potential N glycosylation sites in the primary structure (26a). The gene was first expressed in A. niger (26a), producing a commercially available phytase called Natuphos (BASF, Mt. Olive, N.J.). However, the thermostability constraint and the cost of the enzyme limit its use in animal production (24). Later, the gene was also expressed in tobacco seeds (21) or leaves (27) and in soybean cells (17). Although there are differences in glycosylation among these hosts, the expressed phytase enzymes are all active, and the sizes of the deglycosylated proteins are similar. In contrast, Escherichia coli was unable to express an active phytase enzyme from the phyA gene, producing a nonglycosylated, intracellular inclusion protein (56 kDa) (22). The in vitro solubilized and refolded protein showed only 1% of the activity of the purified phytase (26). Seemingly, a lack of glycosylation and/or secretion of phytase in E. coli precluded the expression of active phyA phytase. The yeast Saccharomyces cerevisiae has been widely used as a host organism to produce heterologous proteins, and the expressed proteins are generally hyperglycosylated (4, 6). Our objectives were to determine if S. cerevisiae was able to express functional phyA phytase and to study the effects of glycosylation on the phytase’s activity and thermostability.
MATERIALS AND METHODS
Plasmid cassettes.
Three plasmids with different signal peptides were constructed (Table 1). The phyA gene (GenBank accession no. M94550) was kindly provided by E. J. Mullaney of the U.S. Department of Agriculture (USDA). To facilitate cloning, we initially amplified a 1.4-kb DNA fragment containing the coding region of phyA from pMD4.21 (22) by PCR and inserted it into pET25b(+) (Novagen, Madison, Wis.). The resulting construct, pEP1 (6,893 bp), was used as a template to amplify the phyA gene by PCR in the following experiments.
TABLE 1.
Cloning and expression plasmids
| Plasmid | Description | Use | Source or reference |
|---|---|---|---|
| pET25b(+) | Expression vector | Cloning | Novagen |
| pEP1 | pET25b(+) + phyA | Cloning | This study |
| pSES1 | Expression vector | Cloning | 10 |
| pSPP1 | pSES1 + Spe2a + phyA | Cloning | This study |
| pYES2 | Expression vector | Expression | Invitrogen |
| pYEP1 | pYES2 + Spe2 + phyA | Expression | This study |
| pYXP1 | pYES2 + Spxyb + phyA | Expression | This study |
| pYPP1 | pYES2 + Sphyc + phyA | Expression | This study |
The first plasmid, pYEP1 (7,783 bp), was the pYES2 expression vector (Invitrogen, San Diego, Calif.) with the signal peptide of endoglucanase E2 from Thermomonospora fusca, Spe2 (28), and the coding region of phyA isolated from a shuttle plasmid, pSPP1, which was made from pSES1 (10) to express phyA in Streptomyces lividans in our preliminary experiment. Initially, the pLT1 promoter (14) and the signal peptide of Spe2 were amplified from pBW2 (10) by PCR. An upstream primer, 5′-CAG CTA TGA CCA TGA TTA CGC C-3′, and a downstream primer, 5′-CGT AGA ACG GGA ATT CAT TGG CCG CC-3′, were used. The PCR fragment was digested with the restriction enzymes PstI and EcoRI, ligated with the coding region of the phyA gene that was isolated from pEP1 by PCR, and digested at the EcoRI and HindIII sites. To add a KpnI site at the 3′ end, this ligated fragment was then inserted into pBluescript SK+ (Stratagene, La Jolla, Calif.) at the PstI and HindIII sites. Subsequently, a PstI-KpnI fragment was inserted into pSES1 to obtain the shuttle plasmid pSPP1, and a HindIII fragment digested from pSPP1 was inserted into pYES2 to produce pYEP1.
The second plasmid, pYXP1 (7,219 bp), was pYES2 ligated with the coding region of the phyA gene joined to Spxy, the signal peptide of a xylanase gene from Aureobasidium pullulans (18). Spxy was joined with phyA by overlap extension PCR (8) with four different primers. Primer 1 (5′-CCC AAG CTT GAT CAC ATC CAT TCA-3′) and primer 2 (5′-CGG GGA CTG CTA GCG CAC GTT CGA T-3′) were used to amplify the Spxy sequence from pCE4 (18). Primer 3 (5′-ATC GAA CGT GCG CTA GCA GTC CCC G-3′) and primer 4 (5′-GCT CTA GAC TAA GCA AAA CAC TCC-3′) were used to amplify the coding region of phyA from pEP1. The two amplified fragments were then mixed as templates and joined by PCR with primers 1 and 4. The final product was inserted into pYES2 at the HindIII and XbaI sites.
The third plasmid, pYPP1 (7,176 bp), was identical to pYXP1 except that it contained Sphy, the signal peptide of phyA phytase. The lead sequence and the coding region of the phyA gene, excluding the intron between them, were joined by PCR. The template was pEP1, with a long upstream primer that consisted of a Sphy sequence plus a KpnI restriction site at the 5′ end (GG GGT ACC ATG GGC GTC TCT GCT GTT CTA CTT CCT TTG TAT CTC CTG TCT GGA GTC ACC TCC GGA CTG GCA) and primer 4 (see above) as the downstream primer. The PCR fragment was inserted into pYES2 at the KpnI and XbaI sites to produce pYPP1.
Transformation, induction, and sample preparation.
The three plasmids were transformed into S. cerevisiae INVSc1 (Invitrogen) by the lithium chloride method (9). Transformants were initially grown at 30°C in Sabouraud-raffinose (4%) medium (18), Sabouraud-glycerol medium (4% raffinose was replaced by 5% glycerol and 0.1% dextrose), or yeast extract-peptone-dextrose (YEPD) medium. When the culture optical density at 600 nm (OD600) reached 2, 2% galactose was added to induce phytase expression. Extracellular and intracellular samples were prepared for various assays (18). The total protein concentration in the samples was determined by the method of Lowry et al. (19).
Northern blotting.
Total RNA was isolated with TRIzol reagent (GIBCO BRL, Gaithersburg, Md.) from the phyA transformants before and after induction. The RNA samples (20 μg per lane) were subjected to Northern blotting (2). A 1.4-kb EcoRI-HindIII DNA fragment containing the phyA coding region was used as a probe.
SDS-PAGE and Western blotting.
To detect phytase protein expression, the medium supernatant cultured with the phyA transformants was subjected to sodium dodecyl sulfate–12.5% polyacrylamide gel electrophoresis (SDS-PAGE) with the use of a Mini-Protein I cell (Bio-Rad Laboratories, Hercules, Calif.) (13). Prior to gel electrophoresis, the supernatants were concentrated (∼10-fold) with the use of a stirred ultrafiltration cell (M8050; Amicon, Beverly, Mass.) with a membrane (YM10) having an apparent molecular weight cutoff of 10,000. Protein was stained with Coomassie bright blue and quantified by an IS-1000 digital imaging system (Alpha Innotech Co., San Leandro, Calif.), using a 10-kDa protein ladder as the standard (Promega, Madison, Wis.). For Western blot analysis, the separated proteins were transferred onto a Protran nitrocellulose membrane (Schleicher & Schuell, Keene, N.H.) with a Mini Trans-Blot cell (Bio-Rad Laboratories). A rabbit polyclonal immunoglobulin G (provided by A. H. J. Ullah of the USDA) raised against purified native A. niger phytase was used as the primary antibody and was diluted 1:5,000 prior to application. A goat anti-rabbit immunoglobulin G–horseradish peroxidase system (Bio-Rad Laboratories) was used for the final colorimetric detection.
Enzyme activity and properties.
Phytase activity was determined in the culture supernatants and the total soluble and insoluble fractions of the transformed cells as previously described (7). Briefly, 0.5 ml of sample (containing 0.02 to 0.2 U of phytase, diluted with 0.2 M citric acid [pH 5.5]) was transferred to a tube and incubated in a water bath (37°C) for 5 min. Then, 0.5 ml of 1% sodium phytate (diluted with 0.2 M citric acid [pH 5.5]; Sigma, St. Louis, Mo.) was added to start the reaction, which was carried out at 37°C for 15 min and stopped by adding 1 ml of 15% trichloroacetic acid. Blanks were run by incubating the enzyme samples with trichloroacetic acid for 15 min before adding the substrate. The mixture was centrifuged (2,000 × g, 10 min) and 0.2 ml of supernatant was mixed with sulfuric acid, ammonium molybdate, and ascorbic acid for the determination of free-phosphorus concentration. One phytase unit was defined as the activity that releases 1 μmol of inorganic phosphorus from sodium phytate per min at pH 5.5 and 37°C. The optimal pH of the expressed phytase was determined (at 37°C; n = 3) by using buffers of 0.2 M glycine-HCl (pH 1.0, 2.0, and 2.5), 0.2 M sodium citrate (pH 3.0, 4.0, 5.0, and 5.5), and 0.2 M Tris-HCl (pH 6.0 and 8.0). The optimal temperature was determined (at pH 5.5; n = 3) at 25, 37, 45, 50, 55, 60, 75, and 80°C. For a direct comparison of thermostability with the commercial phytase, the expressed phytase was partially purified by ammonium sulfate precipitation at 75% saturation and DEAE-Sepharose chromatography (5). Before the heat treatment, both phytases were adjusted to the same protein concentration (1 mg/ml) with 0.2 M citric acid, pH 5.5. Immediately after the enzymes (400 μl) were heated at 55 and 80°C for 15 min, the samples were chilled on ice and their remaining phytase activities were measured at 37°C as described above.
Phytate phosphorus hydrolysis by the expressed phytase.
To confirm the effectiveness of the expressed phytase on hydrolysis of phytate in natural food, 5 g of either corn or soybean meal was incubated with phytase (0, 100, 250, 500, and 1,000 U/kg of sample) in 0.2 M sodium citrate, pH 5.5, at 37°C for 1 or 4 h (n = 4). The phosphorus released in the supernatant was assayed as described by Chen et al. (1). A direct comparison between the expressed phytase, in the partially purified form as described above, and the commercial phytase for phytate phosphorus release from soybean meal was made at the level of 250 U/kg of feed (with similar protein concentrations).
Deglycosylation of the expressed phytase.
Endo Hf (New England Biolabs, Beverly, Mass.) was used to deglycosylate the expressed phytase. The reaction was carried out by incubating phytase samples (supernatants of the yeast culture, with 100 μg of total protein) with 3,000 New England Biolab units (0.3 IU) of Endo Hf in 0.5 M sodium citrate [pH 5.5]–1 mM phenylmethylsulfonyl fluoride for 5 h at 37°C (no further change after overnight digestion). The reaction mixture was subjected to SDS-PAGE and Western blot analyses as described above. Phytase activity and the thermostability of the enzyme before and after deglycosylation (with similar protein concentrations) were compared as described above (n = 4).
RESULTS
Functional expression of phyA in S. cerevisiae.
Both the pYEP1 and the pYPP1 transformants showed moderate levels of extracellular phytase activity at 15 h after induction in Sabouraud-raffinose medium, while the pYXP1 or the pYES2 (vector control) transformants had no detectable activity (Table 2). There were no significant differences among these transformants in cell densities (OD600). The pYPP1 transformants produced quite different phytase activities in three different media, with considerably higher activity and cell density in YEPD medium. In contrast, the pYEP1 transformants expressed similar phytase activities in Sabouraud-raffinose and Sabouraud-glycerol media but had no activity in YEPD medium (data not shown).
TABLE 2.
Extracellular phytase activity in medium supernatant 15 h after induction
| Plasmid | Medium | Activity (u/liter) | Cell density (OD600) |
|---|---|---|---|
| pYES2 | Sabouraud-raffinose | Not detectable | 6.83 |
| pYXP1 | Sabouraud-raffinose | Not detectable | 6.89 |
| pYEP1 | Sabouraud-raffinose | 80 | 6.49 |
| pYPP1 | Sabouraud-raffinose | 146 | 6.75 |
| pYPP1 | Sabouraud-glycerol | 375 | 5.79 |
| pYPP1 | YEPD | 2,797 | 51.1 |
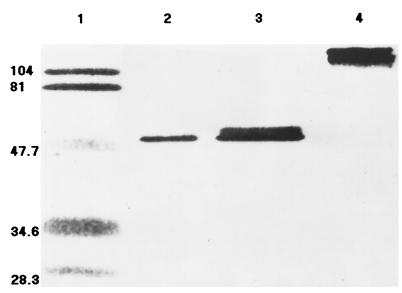
Because of the relatively high phytase activity yield, the pYPP1 transformants were grown in YEPD medium for further characterization of the enzyme. Nearly all the phytase produced in the system was secreted into the medium, because no intracellular phytase activity was detected. The phyA mRNA was detected in the pYPP1 transformants 20 h after induction, but not in the pYES2 control (data not shown). In the medium supernatant, there was a significant protein band of approximately 120 kDa shown by the SDS-PAGE that was recognized by the phytase polyclonal antibody. The deglycosylated phytase formed a sharp band (50 kDa) (Fig. 1).
FIG. 1.
Western blot analysis of the extracellular phytase protein expressed in pYPP1 transformants of S. cerevisiae (20 h after induction) before and after deglycosylation, using a primary antibody raised against purified A. niger phytase. Lane 1, prestained SDS-PAGE marker; lanes 2 and 3, deglycosylated phytase (10 and 20 μg of protein, respectively); lane 4, glycosylated phytase (20 μg of protein; the band was approximately 120 kDa as estimated by SDS-PAGE). Numbers on the left are molecular masses, in kilodaltons.
Properties of the expressed phytase.
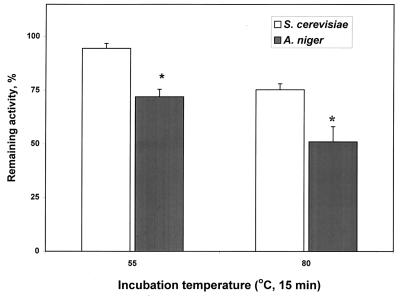
The relative activities of the expressed phytase were 0.53, 62.8, 68.2, 50.6, 85.3, 105.3, 100, 69.8, and 0.84% at pH 1, 2, 2.5, 3, 4, 5, 5.5, 6, and 8, respectively. The differences between pH 2 and 2.5 or 5 and 5.5 were not significant. Thus, the enzyme had two pH optima: 2 to 2.5 and 5 to 5.5, with less activity (P < 0.05) at the lower pH than at the higher pH. The relative activities were 24.2, 44.6, 63.9, 83.6, 89.8, 100, 0.6, and 0.9% at 25, 37, 45, 50, 55, 60, 75, and 80°C, respectively. The optimal temperature of the enzyme was between 55 and 60°C, because the difference in activity between these two temperatures was not significant. The partially purified phytase (4 U/mg) expressed in S. cerevisiae retained 23 and 24% more (P < 0.01) of the initial activity after it was heated for 15 min at 55 and 80°C, respectively, than did the commercial phytase (Fig. 2).
FIG. 2.
Comparison of the thermostabilities of the phyA phytase expressed by pYPP1 transformants (of S. cerevisiae) and of the commercial phytase (from A. niger; BASF). The sources of phytase were adjusted to the same protein concentration (1 mg/ml) with 0.2 M citric acid, pH 5.5, before the test. Samples (400 μl) were heated for 15 min at 55 and 80°C and chilled on ice immediately thereafter. The remaining phytase activity was determined at 37°C and expressed as a percentage of the initial activity. Results are means ± standard errors of the means from five independent tests; an asterisk indicates a significant difference (P < 0.01) between these two enzymes.
Phytate phosphorus hydrolysis by the expressed phytase.
The total amounts of phosphorus released from soybean meal within 1 h were 0.68, 1.18, 1.62, 2.48, and 3.31 g/kg of food for 0, 100, 250, 500, and 1,000 U/kg of food, respectively. The differences between any two levels of phytase activity were significant. At any given level of phytase activity, longer incubation (4 versus 1 h) also resulted in additional phosphorus release from soybean meal. But the hydrolysis reached a plateau at 500 U/kg of soybean meal for the 4-h incubation. There were no differences in the amount of phosphorus released from corn between the 1- and 4-h incubations when phytase activity levels were greater than 250 U/kg of food. The amount of phosphorus released from corn at any given activity level was lower than that from soybean meal. At the same activity (250 U/kg of food), the expressed phytase and the commercial phytase released similar amounts of phosphorus from soybean meal within 1 h (1.41 versus 1.51 g/kg; the blank was 0.73 g/kg).
Effects of deglycosylation on the expressed phytase activity and thermostability.
After deglycosylation by Endo Hf, the expressed phytase had 91% (standard error [SE] = 2.1%, P = 0.22) of the original activity. However, deglycosylation caused a substantial reduction (P < 0.05) in enzyme thermostability. While 62.6% (SE = 2.2%) of the activity remained after the nondeglycosylated enzyme was heated for 15 min at 80°C, only 37.3% (SE = 1.8) of its activity remained in the deglycosylated enzyme treated under the same condition.
DISCUSSION
Up to now, the phyA gene has been expressed as an active enzyme in A. niger (26a), tobacco seeds (21) or leaves (27), and soybean cells (17). In the present study, we have demonstrated that S. cerevisiae is also able to express the phyA gene. The expressed extracellular phytase has been verified, by Northern and Western blotting analyses, to be a specific product of the phyA gene and a functional enzyme. Its molecular mass is approximately 120 kDa, and the enzyme is the largest among the isolated or overexpressed phyA proteins. Deglycosylation of the enzyme produced a protein with a mass (50 kDa) similar to those from other hosts (17, 21, 26a, 27), indicating that the increased mass is due to the sugar moieties at the N glycosylation sites. Probably because of heavy glycosylation, this expressed phytase seems to have a slightly wider temperature optimum (55 to 60°C) and to be more thermostable than the commercial phytase or the ones expressed in other hosts. After being heated at 55 and 80°C for 15 min, our partially purified enzyme retained 95 and 75% of its phytase activity, whereas the commercial phytase retained 72 and 51% of its activity, respectively. Wild-type phytase retained only 40% of its activity after it was heated at 68°C for 10 min (26). Activity of the phyA phytase expressed in soybean cells declined rapidly at temperatures above 63°C (17). Because phytases from these sources are less glycosylated than the one expressed in S. cerevisiae, their apparent inferior thermostabilities are probably related to their lower levels of glycosylation. More directly, deglycosylation of the phytase expressed in S. cerevisiae resulted in a 40% loss of its thermostability at 80°C. Kanai et al. (11) have demonstrated that N glycosylation of cycloinulo-oligosaccharide fructanotransferase improves the thermostability of the enzyme expressed in S. cerevisiae. Others have shown a similar role of glycosylation in protein thermostability (12, 25). Because the main constraint of the commercial phytase is the activity loss due to heat from feed pelleting (24), our results suggest a promising direction for tackling the problem.
The catalytic and immunological properties of the phyA phytase expressed in S. cerevisiae are not significantly affected by glycosylation. Deglycosylation of the enzyme reduced its specific activity by only 9%. Both the heavy glycosylated and the deglycosylated proteins cross-reacted with the polyclonal antibody raised against the wild-type enzyme. The hyperglycosylation did not change the pH activity profile of the expressed phytase, which was similar to that of the commercial or the wild-type A. niger phytase (26). Because the gastric pH of simple-stomached animals is around 2.5 to 3.5, this recombinant phytase is expected to be effective, as is the commercial phytase (24), in releasing phytate phosphorus from digesta. This idea is supported by this recombinant phytase’s in vitro effectiveness in releasing phytate phosphorus from corn and soybean meal. Its ability to hydrolyze not only chemically pure, soluble sodium phytate but also natural phytate in foods is practically important, because phytate in plant foods exists as insoluble mixed salts of phytic acid (23). It is also interesting that the expressed and the commercial phytases have similar capacities to hydrolyze the natural phytate in soybean meal at the same activity level as that of sodium phytate. In addition, the amounts of phytate phosphorus released from corn or soybean meal at a given level of phytase activity were affected by the source or amount of phytate and the incubation time. Because corn has a lower phytate concentration than soybean meal, less phytase activity and less time are needed to produce maximal hydrolysis of its phytate phosphorus.
The total yield of extracellular phyA phytase activity was affected by the signal peptides and the media. The signal peptide (Spxy) of xylanase A from A. pullulans (18) did not give a functional expression of phytase. This might be due to the lack of proper splicing or instability of the phyA transcript containing Spxy. Spe2 of endoglucanase E2 from T. fusca (28) resulted in a lower extracellular phytase activity than the phyA signal peptide (Sphy [26a]). Because there were similar levels of cell growth (OD600) without detectable intracellular phytase activity among transformants containing different signal peptides, the differences in their total yields of extracellular phytase activity were probably due to differences in the specific activity of the produced enzyme. However, further purification of these phytases is necessary to accurately compare their specific activities and to determine whether the expression rate of the gene causes the differences in yield. Phytase activity was higher when the pYPP1 transformants were grown in YEPD medium than in the other two complex media, largely due to a higher total cell mass in the YEPD medium. Because of the relatively low cost, YEPD medium would potentially be a good choice for phytase production.
ACKNOWLEDGMENTS
This research was supported by a project of the Cornell Biotechnology Program, New York State Science and Technology.
We are grateful to E. J. Mullaney and A. H. J. Ullah of the USDA, X. L. Li of the University of Georgia, and S. Zhang, J. Porres, E. Rodriguez, D. Irwin, and T. Xiang of Cornell University for providing experimental materials and technical help.
REFERENCES
- 1.Chen P S, Toribara T Y, Warner H. Microdetermination of phosphorus. Anal Chem. 1956;28:1756–1758. [Google Scholar]
- 2.Cheng W, Ho Y, Ross D A, Han Y, Combs G F, Jr, Lei X G. Overexpression of cellular glutathione peroxidase does not affect expression of plasma glutathione peroxidase or phospholipid hydroperoxide glutathione peroxidase in mice offered diets adequate or deficient in selenium. J Nutr. 1997;127:675–680. doi: 10.1093/jn/127.5.675. [DOI] [PubMed] [Google Scholar]
- 3.Cromwell G L, Coffey R D. P—a key essential nutrient, yet a possible major pollutant—its central role in animal nutrition. In: Lyons T P, editor. Biotechnology in the feed industry. Proceedings, Alltech 7th Annual Symposium. Nicholasville, Ky: Alltech Technical Publications; 1991. p. 133. [Google Scholar]
- 4.Curry C, Gilkes N, O’Neill G, Miller R C, Jr, Skipper N. Expression and secretion of a Cellulomonas fimi exoglucanase in Saccharomyces cerevisiae. Appl Environ Microbiol. 1988;54:476–484. doi: 10.1128/aem.54.2.476-484.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Greiner R, Konietzny U, Jany K-D. Purification and characterization of two phytases from Escherichia coli. Arch Biochem Biophys. 1993;303:107–113. doi: 10.1006/abbi.1993.1261. [DOI] [PubMed] [Google Scholar]
- 6.Grinna L S, Tschopp J F. Size distribution and general structural features of N-linked oligosaccharides from the methylotrophic yeast, Pichia pastoris. Yeast. 1989;5:107–116. doi: 10.1002/yea.320050206. [DOI] [PubMed] [Google Scholar]
- 7.Han Y M, Yang F, Zhou A G, Miller E R, Ku P K, Hogberg M G, Lei X G. Supplemental phytases of microbial and cereal sources improve dietary phytate phosphorus utilization by pigs from weaning through finishing. J Anim Sci. 1997;75:1017–1025. doi: 10.2527/1997.7541017x. [DOI] [PubMed] [Google Scholar]
- 8.Horton R M. In vitro recombination and mutagenesis of DNA: SOEing together tailor-made genes. In: White B A, editor. PCR protocols: current methods and applications. Totowa, N.J: Humana Press Inc.; 1993. pp. 251–261. [DOI] [PubMed] [Google Scholar]
- 9.Ito H, Fukuda Y, Murata K, Kimura A. Transformation of intact yeast cells treated with alkali cations. J Bacteriol. 1983;153:163–168. doi: 10.1128/jb.153.1.163-168.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jung E D, Lao G, Irwin D, Barr B K, Benjamin A, Wilson D B. DNA sequences and expression in Streptomyces lividans of an exoglucanase gene and an endoglucanase gene from Thermomonospora fusca. Appl Environ Microbiol. 1993;59:3032–3043. doi: 10.1128/aem.59.9.3032-3043.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kanai T, Ueki N, Kawaguchi T, Teranishi Y, Atomi H, Tomorbaatar C, Ueda M, Tanaka A. Recombinant thermostable cycloinulo-oligosaccharide fructanotransferase produced by Saccharomyces cerevisiae. Appl Environ Microbiol. 1997;63:4956–4960. doi: 10.1128/aem.63.12.4956-4960.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kodama S, Tsujimoto M, Tsuruoka N, Sugo T, Endo T, Kobata A. Role of sugar chains in the in vitro activity of recombinant human interleukin 5. Eur J Biochem. 1993;211:903–908. doi: 10.1111/j.1432-1033.1993.tb17624.x. [DOI] [PubMed] [Google Scholar]
- 13.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature (London) 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 14.Lao G, Ghangas G S, Jung E D, Wilson D B. DNA sequences of three β-1,4-endoglucanase genes from Thermomonospora fusca. J Bacteriol. 1991;173:3397–3407. doi: 10.1128/jb.173.11.3397-3407.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lei X G, Ku P K, Miller E R, Yokoyama M T. Supplementing corn-soybean meal diets with microbial phytase linearly improves phytate P utilization by weanling pigs. J Anim Sci. 1993;71:3359–3367. doi: 10.2527/1993.71123359x. [DOI] [PubMed] [Google Scholar]
- 16.Lei X G, Ku P K, Miller E R, Yokoyama M T, Ullrey D E. Supplementing corn-soybean meal diets with microbial phytase maximizes phytate P utilization by weanling pigs. J Anim Sci. 1993;71:3368–3375. doi: 10.2527/1993.71123368x. [DOI] [PubMed] [Google Scholar]
- 17.Li J, Hegeman C E, Hanlon R W, Hanlon G H, Lacy G H, Denbow M D, Grabau E A. Secretion of active recombinant phytase from soybean cell-suspension cultures. Plant Physiol (Rockville) 1997;114:1103–1111. doi: 10.1104/pp.114.3.1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li X-L, Ljungdahl L G. Expression of Aureobasidium pullulans xynA in, and secretion of the xylanase from, Saccharomyces cerevisiae. Appl Environ Microbiol. 1996;62:209–213. doi: 10.1128/aem.62.1.209-213.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lowry O H, Rosebrough N J, Farr A L, Randall R J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 20.Mullaney E J, Gibson D M, Ullah A H J. Positive identification of a lambda gt11 clone containing a region of fungal phytase gene by immunoprobe and sequence verification. Appl Microbiol Biotechnol. 1991;55:611–614. doi: 10.1007/BF00169625. [DOI] [PubMed] [Google Scholar]
- 21.Pen J, Verwoerd T C, van Paridon P A, Beudeker R F, van den Elzen P J M, Geerse K, van der Klis J D, Versteegh H A J, van Ooyen A J J, Hoekema A. Phytase-containing transgenic seeds as a novel feed additive for improved phosphorus utilization. Bio/Technology. 1993;11:811–814. [Google Scholar]
- 22.Phillippy B Q, Mullaney E J. Expression of an Aspergillus niger phytase (phyA) in Escherichia coli. J Agric Food Chem. 1997;45:3337–3342. [Google Scholar]
- 23.Reddy N R, Sathe S K, Salunkhe D K. Phytates in legumes and cereals. Adv Food Res. 1982;28:1–92. doi: 10.1016/s0065-2628(08)60110-x. [DOI] [PubMed] [Google Scholar]
- 24.Simons P C M, Versteegh H A, Jongbloed A W, Kemme P A, Slump P, Bos K D, Wolters M G E, Beudeker R F, Verschoor G J. Improvement of phosphorus availability by microbial phytase in broilers and pigs. Br J Nutr. 1990;64:525–540. doi: 10.1079/bjn19900052. [DOI] [PubMed] [Google Scholar]
- 25.Terashima M, Kubo A, Suzawa M, Itoh Y, Katoh S. The role of the N-linked carbohydrate chain of rice α-amylase in thermostability and enzyme kinetics. Eur J Biochem. 1994;226:249–254. doi: 10.1111/j.1432-1033.1994.tb20048.x. [DOI] [PubMed] [Google Scholar]
- 26.Ullah A H J, Gibson D M. Extracellular phytase (E. C. 3. 1. 3. 8) from Aspergillus ficuum NRRL 3135: purification and characterization. Prep Biochem. 1987;17:63–91. doi: 10.1080/00327488708062477. [DOI] [PubMed] [Google Scholar]
- 26a.van Hartingsveldt W, van Zeijl C M J, Harteveld G M, Gouka R J, Suukerbuyk M E G, Luiten R G M, van Paridon P A, Selten G C M, Veenstra A E, van Gorcom R F M, van den Hondel A M J J. Cloning, characterization and overexpression of the phytase-encoding gene (phyA) of Aspergillus niger. Gene. 1993;127:87–94. doi: 10.1016/0378-1119(93)90620-i. [DOI] [PubMed] [Google Scholar]
- 27.Verwoerd T C, van Paridon P A, van Ooyen A J J, van Lent J W M, Hoekema A, Pen J. Stable accumulation of Aspergillus niger phytase in transgenic tobacco leaves. Plant Physiol (Rockville) 1995;109:1199–1205. doi: 10.1104/pp.109.4.1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wilson D B. Biochemistry and genetics of actinomycete cellulases. Crit Rev Biotechnol. 1992;12:45–63. doi: 10.3109/07388559209069187. [DOI] [PubMed] [Google Scholar]