Abstract
Background
Digital health interventions could help to prevent age-related diseases, but little is known about how older adults engage with such interventions, especially in the long term, or whether engagement is associated with changes in clinical, behavioral, or biological outcomes in this population. Disparities in engagement levels with digital health interventions may exist among older people and be associated with health inequalities.
Objective
This study aimed to describe older adults’ engagement with an eHealth intervention, identify factors associated with engagement, and examine associations between engagement and changes in cardiovascular and dementia risk factors (blood pressure, cholesterol, BMI, physical activity, diet, and cardiovascular and dementia risk scores).
Methods
This was a secondary analysis of the 18-month randomized controlled Healthy Ageing Through Internet Counselling in the Elderly trial of a tailored internet-based intervention encouraging behavior changes, with remote support from a lifestyle coach, to reduce cardiovascular and cognitive decline risk in 2724 individuals aged ≥65 years, recruited offline in the Netherlands, Finland, and France. Engagement was assessed via log-in frequency, number of lifestyle goals set, measurements entered and messages sent to coaches, and percentage of education materials read. Clinical and biological data were collected during in-person visits at baseline and 18 months. Lifestyle data were self-reported on a web-based platform.
Results
Of the 1389 intervention group participants, 1194 (85.96%) sent at least one message. They logged in a median of 29 times, and set a median of 1 goal. Higher engagement was associated with significantly greater improvement in biological and behavioral risk factors, with evidence of a dose-response effect. Compared with the control group, the adjusted mean difference (95% CI) in 18-month change in the primary outcome, a composite z-score comprising blood pressure, BMI, and cholesterol, was −0.08 (−0.12 to −0.03), −0.04 (−0.08 to 0.00), and 0.00 (−0.08 to 0.08) in the high, moderate, and low engagement groups, respectively. Low engagers showed no improvement in any outcome measures compared with the control group. Participants not using a computer regularly before the study engaged much less with the intervention than those using a computer up to 7 (adjusted odds ratio 5.39, 95% CI 2.66-10.95) or ≥7 hours per week (adjusted odds ratio 6.58, 95% CI 3.21-13.49). Those already working on or with short-term plans for lifestyle improvement at baseline, and with better cognition, engaged more.
Conclusions
Greater engagement with an eHealth lifestyle intervention was associated with greater improvement in risk factors in older adults. However, those with limited computer experience, who tended to have a lower level of education, or who had poorer cognition engaged less. Additional support or forms of intervention delivery for such individuals could help minimize potential health inequalities associated with the use of digital health interventions in older people.
Keywords: aging, eHealth, disparities, engagement, prevention, cardiovascular, lifestyle, risk factors
Introduction
Background
The number of people aged ≥60 years increased from 382 million worldwide in 1980 to 962 million in 2017 and is expected to reach nearly 2.1 billion by 2050 [1]. In parallel, there are an increasing number of cases of age-related diseases, including cardiovascular disease (CVD) and dementia, placing an ever-increasing burden on health and social care systems [2]. For example, the worldwide cost of dementia increased by 35% between 2010 and 2015 to reach US $818 billion and was estimated to exceed US $1 trillion in 2018 [3]. CVD and dementia share many potentially modifiable lifestyle-based risk factors, including physical inactivity, unhealthy diet, obesity, and hypertension [4,5], offering opportunities for prevention that could bring about huge public health gains. There is a need to establish, using rigorously conducted research studies, the extent to which interventions might influence behavior in the short, medium, and longer term, providing evidence for the best policies to reduce noncommunicable disease risk.
Digital health tools are a possible approach for delivering preventive interventions for CVD and dementia [6,7], which, if effective and efficient, can be rolled out at scale. However, nonuse of digital health interventions is a fundamental problem, with persistent reports of high discontinuation rates, even in research studies involving atypically motivated individuals [8,9]. Specifying the dose of nonpharmacological interventions, in terms of engagement, is inherently more difficult than for drug treatments, even more so for digital interventions where use is often at participants’ discretion [8] and requires more investment and motivation than simply taking a daily medication [10]. Although increased engagement with digital interventions is associated with greater improvements in health outcomes, including behavior change, in young and middle-aged adults [10-12], very little is known about engagement with digital interventions [13], or its association with health outcomes, in older people, especially in the long term.
A concern about using digital health interventions in older populations is that they may further widen existing health inequalities [14]. Although the use of digital technologies is increasing in this age group, digital exclusion is still common, particularly in individuals aged >75 years, and older adults who do not use the internet have poorer health and lower socioeconomic status than those who do [15]. Even among older internet users, the levels of engagement with digital technologies may vary and be associated with individuals’ characteristics. It is vital to understand better how older people use and interact with such tools and to identify potential disparities in use.
Objectives
To explore this, we drew on data from a large international trial of an eHealth intervention designed to encourage behavior changes for the prevention of CVD and cognitive decline in older individuals to (1) describe engagement with the different components of the eHealth intervention, (2) identify factors associated with engagement, and (3) examine associations between engagement and changes in cardiovascular and dementia risk factors.
Methods
Setting and Participants
We analyzed data from the previously described 18-month Healthy Ageing Through Internet Counselling in the Elderly (HATICE) parallel group randomized controlled trial (ISRCTN48151589) [16-18]. Between March 2015 and August 2016, a total of 2724 dementia-free community dwellers aged ≥65 years with at least basic computer literacy and either 2 or more CVD risk factors (hypertension, dyslipidemia, overweight, smoking, or physical inactivity) or a history of CVD or diabetes were enrolled in Finland, France, and the Netherlands. Participants were randomized in a 1:1 ratio to either (1) the intervention, a multicomponent internet-based platform designed to encourage lifestyle changes, with remote support from a lifestyle coach (see the Intervention section), or (2) a control group receiving access to a simple static internet platform containing only basic health information and no coach support. Participants were recruited offline, primarily through a population registry (Finland), commercial mailing lists and a prevention center (France), and general practitioners (Netherlands) [17,19]. The intervention had a modest but significant beneficial effect on the trial’s primary outcome, a composite cardiovascular risk score [17]. Clinical, demographic, and biological data were collected during face-to-face study visits at baseline and 18 months. Data concerning lifestyle, mood, and health self-management were self-reported via the study’s web-based platform at baseline and 12 and 24 months. Adverse events were self-reported on the web-based platform every 3 months.
Ethics Approval
The local ethical committees in each country approved the protocol (Academic Medical Centre, the Netherlands: METC 2014_126; Northern Savonia Hospital District Research Ethics Committee, Finland: 35/2014; Comité de Protection des Personnes Sud Ouest et Outre Mer, France: 2014-A01287–40), and all participants provided written informed consent.
Intervention
Intervention group participants had access to a secure internet-based platform (Figure 1), with remote support from a lifestyle coach trained in motivational interviewing and healthy lifestyle advice. Full details of the development and content of the platform have been previously published [18]. The intervention aimed to facilitate the self-management of cardiovascular risk factors, including hypertension, obesity, physical inactivity, diet, smoking, diabetes, and hypercholesterolemia, to improve the overall risk profile. It was designed using national and European guidelines for primary and secondary CVD prevention [20] and input from members of the target population, health professionals, and patient organizations [16,21,22].
Figure 1.
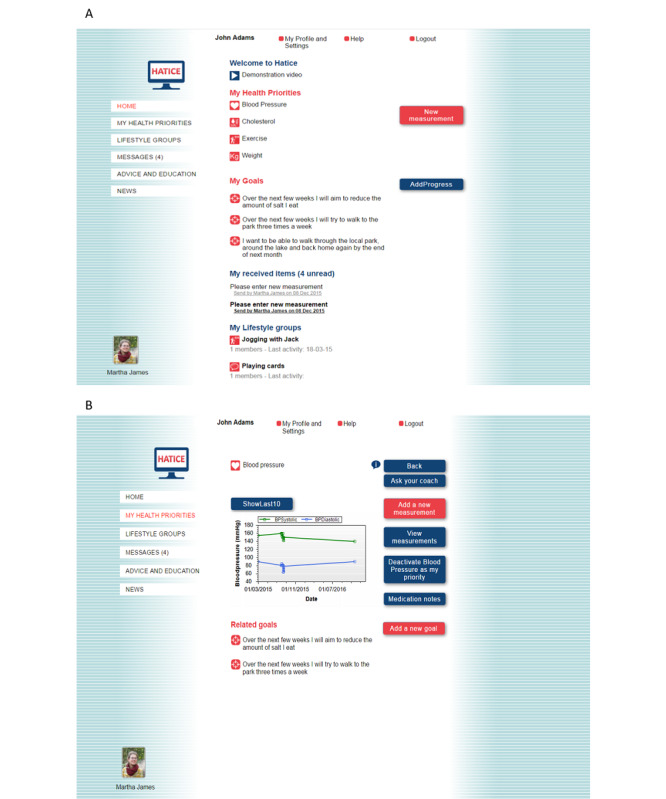
Screenshots of the HATICE intervention platform: (A) home page and (B) measurements page. HATICE: Healthy Ageing Through Internet Counselling in the Elderly.
After secure login, participants were able to (1) view their individual cardiovascular risk profile (based on baseline measurements), (2) set personal goals for lifestyle change and make corresponding action plans, (3) monitor goals by entering data (eg, blood pressure measurements or food diaries) and receive graphical or automated feedback, and (4) obtain health information from education modules (including text, videos, and quizzes) and peer-to-peer videos. News items related to CVD, healthy aging, or eHealth were added regularly to the platform. All content was provided in the local language, and advice was adapted to local guidelines where necessary. Owing to the older age of the study’s participants and their expected level of computer experience, the navigation structure and layout of the intervention platform were kept as simple as possible (Figure 1).
Coaches met with participants face-to-face at baseline and thereafter communicated with them via a computer messaging system. There was also a booster telephone call at 12 months. Using motivational interviewing techniques, they supported participants in making lifestyle changes by encouraging them to prioritize up to 3 health factors (the home page layout then reflected the chosen health priorities) and set at least one goal at baseline, interact with the platform, and set additional goals over time. Coaches also provided motivational feedback. Participants could see a photograph and the name of their coach when they logged in to the intervention platform (Figure 1).
The intervention was designed by the academic researchers involved in the project. Technical development was performed by Vital Health Software.
Outcomes
Engagement Outcomes
Engagement with digital health interventions, similar to engagement with serious games [23], is thought to encompass behavioral, emotional, and cognitive factors [24-26], but there is no consensus on how it should be measured [10]. Similar to previous studies [10,11,27,28], we assessed engagement (only in the intervention group) with our eHealth intervention through system use metrics, including number and dates of logins, number of goals set, messages sent to coaches, monitoring measurements or goal diary entries, and percentage of advice and education materials read.
Different studies have found different components of eHealth intervention use, including number of logins, number of activities completed per login, percentage of study modules completed, amount of goals set, and number of self-monitoring measures, to be associated with outcomes [26,29,30], and it has been suggested that composite measures may be the best way to measure engagement or adherence with such interventions [10]. Therefore, we developed a composite indicator of overall engagement throughout the study, defined as the sum of the points obtained for logins (0, 1, and 2 points for the first, second, and third tertiles of total logins, respectively), number of goals set (0: 0 points; 1: 1 point; ≥2: 2 points), sending of at least one message to the coach (no: 0 points; yes: 1 point), entering at least one measurement (no: 0 points; yes: 1 point), and reading some of the advice and education materials (no: 0 points; yes: 1 point). The composite engagement score ranged from 0 to 7 points, with higher scores indicating greater engagement. It was categorized into low (0-2 points), moderate (3-5 points), and high (6-7 points) engagement, thus avoiding potential difficulties with nonlinear relationships with outcome measures [26,29]. This categorization was decided before the analysis and was intended to capture meaningful differences in engagement with the platform use among the 3 groups (ie, low, moderate, and high engagers). In sensitivity analyses, we categorized the engagement scores using tertiles.
In addition, login data were used to study platform use over time. As participants were asked to log in every 3 months to complete an adverse event questionnaire, and at 12 and 18 months to complete study evaluations, and we could not distinguish these logins from other types, rather than calculating the time to last login, we calculated the time to the first occurrence of nonuse attrition, that is, no login during the previous month [8]. Because they only logged in once every 3 months, at most, participants who only logged in to complete adverse event questionnaires or study evaluations (and did not otherwise use the intervention platform) were considered to display nonuse attrition.
Risk Factor Outcomes
The trial’s primary outcome was a composite cardiovascular risk z score based on systolic blood pressure, low-density lipoprotein cholesterol, and BMI [17]. Secondary outcomes included individual components of the composite z score, physical activity [31], dietary intake (Mediterranean Diet Adherence Screener score) [32], and estimated cardiovascular (Systematic Coronary Risk Estimation–Older People) [33] and dementia (Cardiovascular Risk Factors, Aging, and Incidence of Dementia score) [34] risk.
Predictor Variables
The following baseline variables were assessed as predictors of engagement and nonuse attrition: age, sex, level of education, country of residence, living status, history of CVD or diabetes, current smoking, physical activity, hypertension, dyslipidemia, obesity, intention to make lifestyle changes, cognition, depressive symptoms, anxiety, chronic condition self-management, physical performance, computer use during the 4 weeks before baseline, and diet. Table S1 in Multimedia Appendix 1 provides further details.
Statistical Analyses
Baseline characteristics were described using means (SD), medians (IQR), or numbers (%) and were compared among low, moderate, and high engagers using 1-way ANOVA or Kruskal-Wallis tests for continuous variables and chi-square tests for categorical variables.
Baseline predictors of overall engagement (categorized as low, moderate, or high) were assessed using a multivariate generalized ordered logit (partial proportional odds) model using the Stata gologit2 command (StataCorp LP) [35]. In this model, the proportional odds assumption (ie, that the relationship between each pair of outcome categories was the same) was assessed using Wald tests for each predictor variable. If the assumption held, only 1 coefficient was calculated for the predictor variable, as in standard ordinal logistic regression. If it was violated, separate coefficients were calculated for the comparison of low versus moderate and high engagement categories and for low and moderate versus high engagement categories. The initial multivariate model included all variables associated with engagement in bivariate models at the .2 significance level, and the final model was determined using a manual backward stepwise selection procedure (sequentially eliminating variables with a P>.05).
Baseline factors associated with the first occurrence of nonuse attrition were examined using multivariate Cox proportional hazards models. As the proportional hazards assumption (verified using Schoenfeld residuals) was not met in the initial analysis, the follow-up period was split, following visual inspection of Kaplan-Meier survival curves, into early (ie, months 0-2) and late (ie, month 3 onward) periods, and analyses were run separately for each period. Variable selection was performed as for the generalized ordered logit model.
Finally, 18-month changes in the HATICE primary and secondary outcome variables were compared between the control group and the 3 engagement categories in the intervention group using linear regression models, adjusted for baseline variables associated with engagement, age, education, physical status, and smoking. Models were further adjusted for the baseline score of the outcome of interest if it differed significantly among engagement groups.
All analyses were exploratory and performed using Stata (version 14.1).
Results
Description of Engagement
The median number of logins per participant in the intervention group (N=1389) during the 18-month follow-up period was 29 (IQR 16-48; range 0-700; Figure S1A in Multimedia Appendix 1). In comparison, the median number of logins to the static platform in the control group (N=1335) was 12 (IQR 9-16).
Of the 1389, intervention group participants, 1194 (85.96%) sent at least one message to their coach during the 18-month study period (Figure S1B in Multimedia Appendix 1), and the median (IQR) number of messages sent was 6 (2-10). The median (IQR) number of goals set was 1 (1-2), and of the 1389 participants, 151 (10.87%) did not set any goals, and 560 (40.32%) set 2 or more goals (Figure S1C in Multimedia Appendix 1). Weight was the health factor most commonly targeted by goals, followed by physical activity, and nutrition (Figure S1D in Multimedia Appendix 1). Participants were most likely to read advice and education pages for cholesterol, blood pressure, and diabetes (Figure S1E in Multimedia Appendix 1). Physical activity measurements (min/week, subjectively reported) were the most frequent type of monitoring data entered, followed by weight and blood pressure measurements (Figure S1F in Multimedia Appendix 1). Additional descriptive engagement data are provided in Table S2 in Multimedia Appendix 1.
The median (IQR) composite engagement score was 5 (3-6), and of the 1389 participants, 208 (14.97%) were classified as having low engagement with the platform, 681 (49.03%) moderate engagement, and 500 (36%) high engagement (Table S3 in Multimedia Appendix 1). All components of platform use significantly increased across the three categories (and tertiles; Table S4 in Multimedia Appendix 1).
Predictors of Engagement
At baseline, participants in the low engagement category were younger, had a lower level of education, had poorer cognitive and physical performance, and had more depressive symptoms than those who engaged more. They were also more often from the Netherlands, more likely to be smokers, and less likely to have used a computer in the preceding 4 weeks or be planning or already acting on lifestyle change (Table 1).
Table 1.
HATICEa participants’ (intervention group) baseline characteristics by overall engagement during the trial.
|
|
Low engagement (N=208) | Moderate engagement (N=681) | High engagement (N=500) | P value | ||
| Age (years), median (IQR) | 69.6 (67.5-73.9) | 69.5 (67.3-72.8) | 69.0 (67.0-72.4) | .05 | ||
| Men, n (%) | 102 (49) | 385 (56.5) | 244 (48.8) | .02 | ||
| Educationb, n (%) | .02 | |||||
|
|
Low | 82 (39.4) | 201 (29.5) | 134 (26.8) |
|
|
|
|
Medium | 58 (27.9) | 206 (30.3) | 159 (31.8) |
|
|
|
|
High | 68 (32.7) | 274 (40.2) | 207 (41.4) |
|
|
| Country, n (%) | .006 | |||||
|
|
Netherlands | 121 (58.2) | 402 (59.0) | 244 (48.8) |
|
|
|
|
France | 22 (10.6) | 86 (12.6) | 69 (13.8) |
|
|
|
|
Finland | 65 (31.3) | 193 (28.3) | 187 (37.4) |
|
|
| Living with partner, n (%) | 146 (70.2) | 500 (73.4) | 361 (72.2) | .65 | ||
| Cognitive z scorec, mean (SD) | −0.14 (0.69) | 0.03 (0.58) | 0.00 (0.08) | .003 | ||
| SPPBd,e<10, n (%) | 47 (22.6) | 93 (13.7) | 78 (15.6) | .008 | ||
| Depressive symptomsf, n (%) | 27 (13) | 54 (7.9) | 39 (7.8) | .05 | ||
| HADSg anxiety scoreh, median (IQR) | 4 (2-6) | 4 (2-6) | 4 (2-6) | .16 | ||
| History of CVDi, n (%) | 60 (28.9) | 210 (31) | 154 (30.9) | .83 | ||
| Diabetes, n (%) | 41 (19.7) | 154 (22.7) | 101 (20.2) | .50 | ||
| Hypertension, n (%) | 170 (82.9) | 557 (83.5) | 409 (83.3) | .98 | ||
| Dyslipidemia, n (%) | 201 (97.1) | 653 (96) | 480 (96.6) | .74 | ||
| Currently smoking, n (%) | 20 (12.1) | 47 (7.5) | 29 (5.9) | .03 | ||
| Physically activej, n (%) | 128 (61.5) | 452 (66.5) | 334 (66.8) | .36 | ||
| Obese, n (%) | 82 (39.4) | 262 (38.5) | 185 (37) | .79 | ||
| MEDASk scorel, mean (SD) | 5.8 (2.0) | 6.2 (2.0) | 6.0 (1.9) | .10 | ||
| PIHm scoren, median (IQR) | 87 (79-91) | 87 (81-91) | 86 (81-91) | .53 | ||
| Trying to change lifestyle? n (%) | .001 | |||||
|
|
No plans | 25 (12) | 45 (6.6) | 30 (6) |
|
|
|
|
Long-term plans | 27 (13) | 78 (11.5) | 40 (8) |
|
|
|
|
Short-term plans | 35 (16.8) | 82 (12) | 88 (17.6) |
|
|
|
|
Short-term acting | 39 (18.8) | 120 (17.6) | 92 (18.4) |
|
|
|
|
Long-term acting | 82 (39.4) | 356 (52.3) | 250 (50) |
|
|
| Computer use in the last 4 weeks, n (%) | <.001 | |||||
|
|
No | 22 (11) | 18 (3) | 3 (1) |
|
|
|
|
Yes, <7 hours/week | 113 (55) | 395 (58) | 276 (55) |
|
|
|
|
Yes, ≥7 hours/week | 72 (35) | 267 (39) | 221 (44) |
|
|
aHATICE: Healthy Ageing Through Internet Counselling in the Elderly.
bLow, medium, and high education levels correspond to basic, postsecondary nontertiary, and tertiary levels, respectively.
cCognitive z score indicates average z scores of the Mini Mental Status Examination, Category Fluency, Stroop Color-Word Test, and Rey Auditory Verbal Learning Test.
dSPPB: Short Physical Performance Battery.
eRange 0-12 points, where higher scores indicate better performance.
fGeriatric Depression Scale–15 score ≤5.
gHADS: Hospital Anxiety and Depression Scale.
hRange 0-21, where higher scores indicate increasing symptoms of anxiety.
iCVD: cardiovascular disease.
jDefined as meeting the World Health Organization guidelines of ≥150 minutes’ moderate-intensity or ≥75 minutes’ vigorous-intensity physical activity per week.
kMEDAS: Mediterranean Diet Adherence Screener.
lRange 0-14, where higher scores indicate higher adherence to Mediterranean diet.
mPIH: Partners in Health.
nRange 0-96, where higher scores indicate better chronic disease self-management.
In the multivariate analysis (Table 2), increasing engagement was independently predicted by country of residence, having short-term (ie, within the next month) plans for lifestyle change or acting on it for more than 6 months, and regular computer use at baseline. Furthermore, compared with those in the low engagement category, participants in the moderate and high engagement categories had better baseline cognitive performance, and compared with those in the low and moderate categories, those in the high engagement category were more likely to be women.
Table 2.
Final multivariatea generalized ordered logistic regression model showing factors significantly associated with increasing overall engagement during follow-up (categorized as low, moderate, or high platform engagement; N=1238).
|
|
ORb (95% CI) | P valuec | ||||
| Variables meeting the proportional odds assumptiond | ||||||
|
|
Country | .02 | ||||
|
|
|
Netherlands (refe) | 1 | N/Af | ||
|
|
|
France | 1.41 (0.98-2.02) | .07 | ||
|
|
|
Finland | 1.55 (1.16-2.06) | .003 | ||
|
|
Trying to change lifestyle? | .002 | ||||
|
|
|
No plans (ref) | 1 | N/A | ||
|
|
|
Long-term plans | 1.20 (0.70-2.07) | .51 | ||
|
|
|
Short-term plans | 2.25 (1.33-3.80) | .002 | ||
|
|
|
Short-term acting | 1.51 (0.92-2.50) | .11 | ||
|
|
|
Long-term acting | 2.02 (1.26-3.25) | .004 | ||
|
|
Computer use in last 4 weeks before baseline visit | <.001 | ||||
|
|
|
None (ref) | 1 | N/A | ||
|
|
|
<7 hours/week | 5.39 (2.66-10.95) | <.001 | ||
|
|
|
≥7 hours/week | 6.58 (3.21-13.49) | <.001 | ||
| Variables not meeting the proportional odds assumptiong | ||||||
|
|
Low engagement (ref) vs moderate and high engagement | |||||
|
|
|
Sex (male) | 1.20 (0.84-1.72) | .31 | ||
|
|
|
Cognitive z score | 1.67 (1.26-2.21) | <.001 | ||
|
|
Low and moderate engagement (ref) vs high engagement | |||||
|
|
|
Sex (male) | 0.77 (0.60-0.98) | .03 | ||
|
|
|
Cognitive z score | 0.99 (0.81-1.22) | .95 | ||
aThe following baseline variables were included in the initial multivariate model but did not remain significantly associated with engagement following a backward stepwise selection procedure: age, education, current smoking, physical status (Short Physical Performance Battery), depressive symptoms (Geriatric Depression Scale), anxiety (Hospital Anxiety and Depression Scale), and nutrition score.
bOR: odds ratio.
cP values in italics are overall Wald tests for categorical variables.
dFor independent variables meeting the proportional odds assumption, the relationship between each pair of outcome categories (ie, moderate and high engagement vs low engagement and high engagement vs low and moderate engagement) is the same; therefore, only 1 OR is calculated per variable.
eref: reference.
fN/A: not applicable.
gFor independent variables not meeting the proportional odds assumption, separate ORs are calculated between each pair of outcome categories.
Changes Over Time in Engagement and Its Associated Factors
As shown in Figure 2A, intervention use (measured using logins) declined over time. The roughly (reverse) sigmoidal Kaplan-Meier nonuse attrition curve (Figure 2B) shows a sharp decrease in the proportion of participants logging in at least once during the previous month from the end of month 2 onward. In a sensitivity analysis using a 6-week nonuse attrition definition, the curve was similar, although slightly elongated (Figure S2 in Multimedia Appendix 1). The median time to the first occurrence of nonuse attrition was 3 months. Of the 1389 participants in the intervention group, 465 (33.48%) demonstrated early nonuse attrition (during months 1-2), 747 (53.78%) demonstrated late nonuse attrition (between 2 and 18 months), and 145 (10.44%) were highly consistent platform users, logging in at least once every month for the entire follow-up period (32/1389, 2.30% logged in every month, during which they participated in the trial but dropped out before the end of follow-up). The highly consistent users demonstrated significantly higher engagement with all parts of the platform than the other 2 groups, but even in the early nonuse attrition group, 24.30% (113/465) logged in to the platform at least once a month for at least 12 of the 18 months of follow-up (Table S5 in Multimedia Appendix 1).
Figure 2.
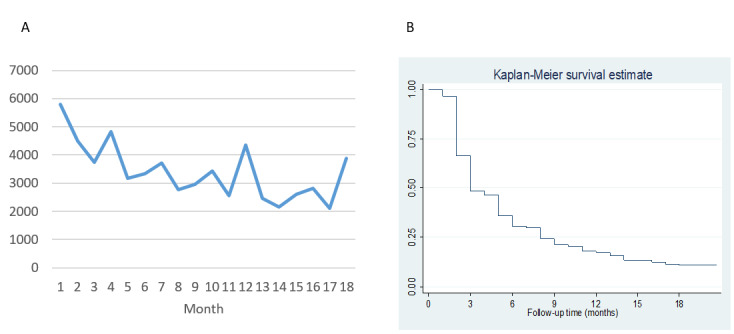
Changes in engagement over time in the intervention group: (A) total number of logins per month in the intervention group and (B) time to nonuse attrition (ie, no login during the previous month).
Not using a computer regularly before baseline and a lower baseline chronic condition self-management score predicted early nonuse attrition, whereas living in the Netherlands and acting on lifestyle change for <6 months at baseline predicted late nonuse attrition (Table 3).
Table 3.
Baseline factors associated with early (model 1) and late (model 2) nonuse attrition.
|
|
HRa | 95% CI | P value | |||||
| Model 1: early nonuse attritionb (N=1351; 448 events) | ||||||||
|
|
Computer use in the last 4 weeks | |||||||
|
|
|
None (refc) | 1 | N/Ad | N/A | |||
|
|
|
<7 hours/week | 0.46 | 0.31-0.69 | <.001 | |||
|
|
|
≥7 hours/week | 0.44 | 0.29-0.66 | <.001 | |||
|
|
Partners in Health score (points)e | 0.99 | 0.98-1.00 | .03 | ||||
| Model 2: late nonuse attritionf (N=848; 693 events) | ||||||||
|
|
Country | |||||||
|
|
|
Netherlands (ref) | 1 | N/A | N/A | |||
|
|
|
France | 0.66 | 0.51-0.84 | .001 | |||
|
|
|
Finland | 0.57 | 0.47-0.69 | <.001 | |||
|
|
Trying to change lifestyle? | |||||||
|
|
|
No plans to change lifestyle (ref) | 1 | N/A | N/A | |||
|
|
|
Long-term plans to change lifestyle | 0.96 | 0.66-1.40 | .83 | |||
|
|
|
Short-term plans to change lifestyle | 1.31 | 0.96-1.78 | .09 | |||
|
|
|
Short-term acting on lifestyle change | 1.49 | 1.15-1.93 | .002 | |||
|
|
|
Long-term acting on lifestyle change | 1.15 | 0.91-1.45 | .23 | |||
aHR: hazard ratio.
bThe first instance of nonuse attrition during months 1 to 2. The following baseline variables were included in the initial multivariate model but did not remain significantly associated with early nonuse attrition following a backward stepwise selection procedure: education, history of cardiovascular disease, history of diabetes, history of hypertension, Geriatric Depression Scale score, and verbal fluency score.
cref: reference.
dN/A: not applicable.
eHigher scores indicate better chronic disease self-management.
fThe first instance of nonuse attrition from month 3 onward. The analysis included individuals who had not already undergone an episode of nonuse attrition during the first 2 months. The following variables were included in the initial multivariate model but did not remain significantly associated with late nonuse attrition following a backward stepwise selection procedure: education, current smoking, obesity, age, Mini Mental Status Examination score, verbal fluency score, Stroop score, Rey Auditory Verbal Learning Test recall score, Short Physical Performance Battery score, Partners in Health score, and Mediterranean Diet Adherence Screener nutrition score.
Association Between Engagement and Intervention Outcomes
There was a significantly greater improvement in the HATICE primary outcome measure, comprising systolic blood pressure, BMI, and low-density lipoprotein cholesterol, over 18 months in the high engagement category than in the control group (adjusted mean difference −0.08, 95% CI −0.12 to −0.03; P=.001), with an indication of a dose-response effect (Figure 3 and Table S6 in Multimedia Appendix 1; overall P value across the 3 adherence groups=.005). Similarly, compared with those in the control group, there was a significantly greater decrease in systolic blood pressure and BMI and significantly less decline in physical activity and Mediterranean Diet Adherence Screener score (all indicating improvement of cardiovascular or dementia risk) in the high engagement category over 18 months (Figure 3 and Table S6 in Multimedia Appendix 1). The results were also numerically, if not significantly, in favor of greater improvement in the other outcome measures, except for Systematic Coronary Risk Estimation–Older People (SCORE-OP), in the high and moderate engagement groups. The results were comparable when the engagement scores were categorized into tertiles (Table S7 in Multimedia Appendix 1).
Figure 3.
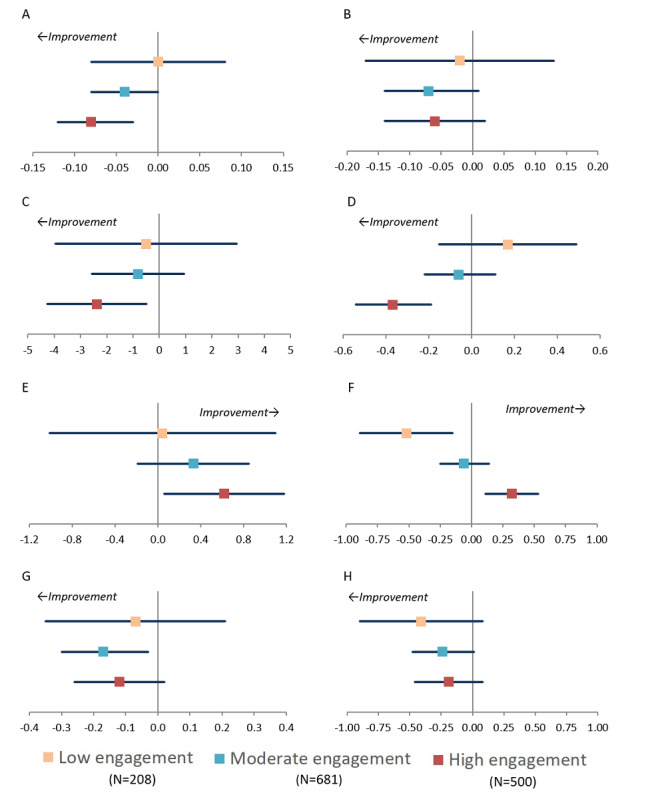
Adjusted mean difference in 18-month changes in outcome measures in low, moderate, and high engagement categories in the HATICE intervention group compared with control group: (A) HATICE composite z-score (BMI, LDL, and SBP), (B) LDL cholesterol (mmol/L), (C) SBP (mm Hg), (D) BMI (kg/m2), (E) moderate-intense physical activity (hours/week), (F) MEDAS score (range 0-14 points), (G) CAIDE dementia risk score (range 0-15 points), and (H) SCORE-OP (10-year CVD mortality risk). Point estimates are the mean difference in 18-month change compared with the control group. Bars are 95% CIs. Each model was adjusted for baseline age, sex, education, country, physical function, smoking, plans to make lifestyle changes, computer use, and cognition and for baseline score of the outcome of interest if it differed across engagement groups. The HATICE primary outcome measure was a composite score based on the average of 18-month changes in SBP, LDL cholesterol, and BMI z-scores. CAIDE: Cardiovascular Risk Factors, Aging, and Incidence of Dementia; CVD: cardiovascular disease; HATICE: Healthy Ageing Through Internet Counselling in the Elderly; LDL: low-density lipoprotein; MEDAS: Mediterranean Diet Adherence Screener; SCORE-OP: Systematic Coronary Risk Estimation–Older People; SBP: systolic blood pressure.
Discussion
Principal Findings
In an 18-month randomized trial in older adults, compared with those in the control group, those in the intervention group who engaged most with the eHealth intervention designed to encourage lifestyle changes showed significantly greater improvement in objectively and subjectively measured cardiovascular and dementia risk factors. Those with low engagement showed no difference compared with the control group. Participants who reported that they were already working on improving their lifestyle at baseline, or had short-term plans to do so, were more engaged with the intervention. Those who reported not using a computer in the month before baseline were extremely unlikely to engage, irrespective of their intentions regarding lifestyle change.
Although most intervention group participants engaged with the HATICE platform to some extent (eg, 1238/1389, 89.13% set at least one goal), some intervention components, notably the advice and education sections, were used less frequently than others. Interestingly, lifestyle factors (ie, weight loss and physical activity) were the most frequent targets for goal setting, but participants tended to read more advice and education materials when they set a goal relating to a clinical risk factor (ie, cholesterol, blood pressure, or diabetes), suggesting potential differences in engagement depending on underlying motivations.
Strengths and Limitations
Our study provides comprehensive data concerning the engagement of older adults with a tailored digital health intervention over a relatively long period. To date, this population has received little attention in this field. We used data from a large international randomized controlled trial and used multiple objective measures of engagement combined into a composite indicator and a large range of validated predictor and health outcome variables. Our results can be interpreted alongside the qualitative research conducted within the same trial [19,21,36]. A limitation is that, given the difficulty in defining a suitable dose of eHealth interventions and a lack of consensus in the literature on how to measure engagement [10], our engagement indicator was arbitrarily defined after trial completion based on the distributions of the different metrics. However, the definition was chosen before conducting any of the comparative analyses presented here, and it adequately captured differences in engagement. Nonetheless, although some participants demonstrated very high levels of engagement with the eHealth intervention, this subsample was very small. Therefore, we could not specifically study the associations between this very high level of engagement and study outcomes. In addition, our participants, who had at least basic computer literacy (due to study eligibility criteria) and had consented to participate in an eHealth behavior change intervention trial, are not representative of the general older population, in which disparities in engagement would likely be greater. However, the multinational context and use of various recruitment methods have increased the diversity of our population.
Comparison With Previous Work
As in younger populations, engagement declined over time in our trial, with a typical sigmoidal pattern of nonuse attrition indicating a curiosity plateau followed by a rejection phase [8]. Similarly, although older adults were less likely to participate in a web-based chronic disease self-management intervention trial than were younger adults, those who did participate engaged with the intervention in a manner similar to the younger participants [13]. Sustaining engagement may be vital for the long-term effectiveness of digital health interventions, as efficacy declines over time [6]. Automated reminders, and in particular human support, may increase engagement. Adherence to supervised lifestyle interventions appears greater than that to unsupervised interventions in older populations [37], and qualitative research with the HATICE participants underlined the motivational role of the coaches in this trial [36]. Participants also reported that user-friendliness, notably in terms of the attractiveness of the platform, and technical difficulties (eg, login problems) also influenced their engagement with the platform [36]. Integrating digital health interventions into primary care might enhance sustainability and provide additional motivation to older individuals not yet considering lifestyle changes or with reticence regarding such programs [22,36].
Similar to a Finnish computerized cognitive training intervention for older adults [38], and as mentioned in our qualitative work [36], previous level of computer use was the strongest predictor of engagement with our web-based intervention, even in our more contemporary population, in which basic computer literacy was an inclusion criterion. The notion of computer use may reflect both computer literacy and computer access (or quality of access) and is likely to be an indicator of inequalities in access to the eHealth intervention. Indeed, compared with HATICE participants who reported regular use at baseline (N=1344), those who did not regularly use a computer (N=43) were significantly more likely to be older and women and to have a lower level of cognition and education (data not shown). Moreover, Dutch participants were less likely to be regular computer users at baseline and engaged less with the platform than those in France and Finland. This could reflect cultural differences in attitudes toward behavior change, prevention, and research participation [19,21]. Furthermore, self-selection due to recruitment methods may have led the French and Finnish samples to be biased toward more motivated and health-focused individuals. Dutch participants, who were recruited via their general practitioners and were likely influenced by medical authority [19], appeared to be more representative of the general population, notably in terms of education level [17]. Participants’ intentions to make lifestyle changes were also associated with platform engagement, and better self-management of chronic conditions was associated with a lower risk of early nonuse attrition. Both relate to self-efficacy, an established predictor of adherence to lifestyle interventions [39].
Higher engagement with the HATICE intervention platform was associated with more favorable changes in the trial’s main cardiovascular and dementia risk outcome measures, with evidence of a dose-response effect. Similar results have been reported for eHealth interventions targeting various health conditions or behaviors in younger populations [10], but the mechanisms (or mediators, eg, increased knowledge, motivation, self-efficacy, or affect management) underlying such relationships are not well understood [24,25]. Furthermore, we assessed engagement using an aggregate indicator, suggested to be the most useful measure of engagement [10], but it is important to understand whether measures of frequency (eg, logins) and intensity (eg, number of messages sent or amount of advice and education read) of engagement and passive (eg, reading advice and education) and active (eg, entering measurements and sending messages) platform use all influenced outcomes similarly. Not all forms of engagement with eHealth interventions are necessarily associated with outcomes [29], and the frequency of engagement may be more associated with physical health outcomes [25], whereas intensity is more associated with psychological health outcomes [10]. In addition, it is important to understand whether intervention use is a valid indicator of engagement in behavior change [24] and, if so, through which mechanisms. Finally, engagement with eHealth interventions may encompass more than just objective measures of use, and factors such as interest, enjoyment, and attention may also play a role [24,40].
Conclusions
Engaging older people in an eHealth lifestyle self-management intervention is feasible, and greater engagement is associated with greater improvement in biological and behavioral dementia and cardiovascular risk factors. Further work is required to determine more specifically the strength or type of engagement with such interventions required to obtain a meaningful impact on health outcomes and how best to sustain engagement over time. Our results also suggest disparities in engagement, which, given biases in trial participation, are likely to be accentuated in real-world settings. Older adults with limited computer experience, poorer cognition, and no concrete plans for lifestyle change may require extra support to reach a level of engagement with digital lifestyle interventions that is sufficient to bring about health benefits or require access to alternative methods of intervention delivery to mitigate potential health inequalities that could be associated with the widespread roll-out of digital health interventions in older populations.
Acknowledgments
The research leading to these results received funding from the European Union’s Horizon 2020 research and innovation program under grant agreement 779238 and the National Key R&D Programmes of China (2017YFE0118800).
Healthy Ageing Through Internet Counselling in the Elderly is a collaborative project cofunded by the European Union’s Seventh Framework Program (2007-2013), under grant agreement 305374.
The members of the Prevention of Dementia using Mobile Phone Applications (PRODEMOS) group are as follows: Edo Richard, Pim van Gool, Eric Moll van Charante, Marieke Hoevenaar-Blom, Esmé Eggink, Melanie Hafdi, and Patrick Witvliet (Academic Medical Center, University of Amsterdam, Amsterdam, The Netherlands); Carol Brayne, Linda Barnes, and Rachael Brooks (University of Cambridge, Cambridge, United Kingdom); Wei Wang, Wenzhi Wang, Youxin Wang, and Manshu Song (Capital Medical University, Beijing, China; Edith Cowan University, Perth, Australia); Anders Wimo and Ron Handels (Karolinska Institutet, Stockholm, Sweden); Sandrine Andrieu and Nicola Coley (INSERM UMR1295, Toulouse France); Jean Georges and Cindy Birck (Alzheimer Europe, Luxembourg); Bram van de Groep and Mark van der Meijden (Vital Health Software, Ede, the Netherlands)
The members of the Healthy Ageing Through Internet Counselling in the Elderly group are as follows: Edo Richard, Pim van Gool, Eric Moll van Charante, Cathrien Beishuizen, Susan Jongstra, Tessa van Middelaar, Lennard van Wanrooij, and Marieke Hoevenaar-Blom (Academic Medical Center, University of Amsterdam, Amsterdam, The Netherlands); Hilkka Soininen, Tiia Ngandu, and Mariagnese Barbera (University of Eastern Finland, Kuopio, Finland); Miia Kivipelto and Francesca Mangiasche (Karolinska Institutet, Stockholm, Sweden); Sandrine Andrieu, Nicola Coley, and Juliette Guillemont (INSERM–Toulouse University UMR1027, Toulouse, France); Yannick Meiller (Novapten, Paris, France); Bram van de Groep (Vital Health Software, Ede, the Netherlands); and Carol Brayne (University of Cambridge, Cambridge, United Kingdom).
The funders played no role in the study design; collection, analysis, and interpretation of data; writing of the report; or the decision to submit the paper for publication. The researchers were independent from the funders. All authors had full access to the data and take responsibility for the integrity of the data and the accuracy of the data analysis, and all gave approval for publication of the manuscript.
Abbreviations
- aOR
adjusted odds ratio
- CVD
cardiovascular disease
- HATICE
Healthy Ageing Through Internet Counselling in the Elderly
Supplementary tables and figures.
Data Availability
All individual participant data collected during the trial, after deidentification, and study documents, including the study protocol, will be available to academic researchers who provide a methodologically sound proposal, approved by the Healthy Ageing Through Internet Counselling in the Elderly steering group, and agree to collaborate on the analysis with at least one member of the Healthy Ageing Through Internet Counselling in the Elderly study team. Enquiries or proposals should be directed to MHB.
Footnotes
Authors' Contributions: NC, TN, C Beishuizen, MB, MK, HS, WvG, C Brayne, EMVC, ER, and SA made a substantial contribution to the study concept and design. NC, MHB, C Beishuizen, MB, LvW, EMVC, ER, HS, and SA contributed to data collection. MHB, C Beishuizen, and LvW contributed to data management. NC performed the data analysis. NC, LA, MHB, TN, C Beishuizen, MB, LvW, MK, HS, WvG, C Brayne, EMVC, ER, and SA contributed to data interpretation. NC drafted the manuscript, tables, and figures. All authors contributed to the revision of the manuscript.
Conflicts of Interest: None declared.
References
- 1.United Nations World Population Ageing 2017: Highlights. UN iLibrary. 2018. Oct, [2022-04-27]. https://www.un-ilibrary.org/content/books/9789213627457 .
- 2.GBD 2019 Diseases and Injuries Collaborators Global burden of 369 diseases and injuries in 204 countries and territories, 1990-2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet. 2020 Oct 17;396(10258):1204–22. doi: 10.1016/S0140-6736(20)30925-9. https://linkinghub.elsevier.com/retrieve/pii/S0140-6736(20)30925-9 .S0140-6736(20)30925-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wimo A, Guerchet M, Ali G, Wu Y, Prina AM, Winblad B, Jönsson L, Liu Z, Prince M. The worldwide costs of dementia 2015 and comparisons with 2010. Alzheimers Dement. 2017 Jan;13(1):1–7. doi: 10.1016/j.jalz.2016.07.150. https://linkinghub.elsevier.com/retrieve/pii/S1552-5260(16)30043-7 .S1552-5260(16)30043-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.D'Agostino RB, Pencina MJ, Massaro JM, Coady S. Cardiovascular disease risk assessment: insights from Framingham. Glob Heart. 2013 Mar;8(1):11–23. doi: 10.1016/j.gheart.2013.01.001. https://linkinghub.elsevier.com/retrieve/pii/S2211-8160(13)00005-7 .S2211-8160(13)00005-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Livingston G, Huntley J, Sommerlad A, Ames D, Ballard C, Banerjee S, Brayne C, Burns A, Cohen-Mansfield J, Cooper C, Costafreda SG, Dias A, Fox N, Gitlin LN, Howard R, Kales HC, Kivimäki M, Larson EB, Ogunniyi A, Orgeta V, Ritchie K, Rockwood K, Sampson EL, Samus Q, Schneider LS, Selbæk G, Teri L, Mukadam N. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet. 2020 Aug 08;396(10248):413–46. doi: 10.1016/S0140-6736(20)30367-6. http://europepmc.org/abstract/MED/32738937 .S0140-6736(20)30367-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beishuizen CR, Stephan BC, van Gool WA, Brayne C, Peters RJ, Andrieu S, Kivipelto M, Soininen H, Busschers WB, Moll van Charante EP, Richard E. Web-based interventions targeting cardiovascular risk factors in middle-aged and older people: a systematic review and meta-analysis. J Med Internet Res. 2016 Mar 11;18(3):e55. doi: 10.2196/jmir.5218. https://www.jmir.org/2016/3/e55/ v18i3e55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wesselman LM, Hooghiemstra AM, Schoonmade LJ, de Wit MC, van der Flier WM, Sikkes SA. Web-based multidomain lifestyle programs for brain health: comprehensive overview and meta-analysis. JMIR Ment Health. 2019 Apr 09;6(4):e12104. doi: 10.2196/12104. https://mental.jmir.org/2019/4/e12104/ v6i4e12104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eysenbach G. The law of attrition. J Med Internet Res. 2005 Mar 31;7(1):e11. doi: 10.2196/jmir.7.1.e11. https://www.jmir.org/2005/1/e11/ v7e11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kelders SM, Kok RN, Ossebaard HC, Van Gemert-Pijnen JE. Persuasive system design does matter: a systematic review of adherence to web-based interventions. J Med Internet Res. 2012 Nov 14;14(6):e152. doi: 10.2196/jmir.2104. https://www.jmir.org/2012/6/e152/ v14i6e152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Donkin L, Christensen H, Naismith SL, Neal B, Hickie IB, Glozier N. A systematic review of the impact of adherence on the effectiveness of e-therapies. J Med Internet Res. 2011 Aug 05;13(3):e52. doi: 10.2196/jmir.1772. https://www.jmir.org/2011/3/e52/ v13i3e52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fuhr K, Schröder J, Berger T, Moritz S, Meyer B, Lutz W, Hohagen F, Hautzinger M, Klein JP. The association between adherence and outcome in an Internet intervention for depression. J Affect Disord. 2018 Mar 15;229:443–9. doi: 10.1016/j.jad.2017.12.028.S0165-0327(17)32480-1 [DOI] [PubMed] [Google Scholar]
- 12.Mclaughlin M, Delaney T, Hall A, Byaruhanga J, Mackie P, Grady A, Reilly K, Campbell E, Sutherland R, Wiggers J, Wolfenden L. Associations between digital health intervention engagement, physical activity, and sedentary behavior: systematic review and meta-analysis. J Med Internet Res. 2021 Feb 19;23(2):e23180. doi: 10.2196/23180. https://www.jmir.org/2021/2/e23180/ v23i2e23180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Portz JD, LaMendola WF. Participation, retention, and utilization of a Web-based chronic disease self-management intervention among older adults. Telemed J E Health. 2019 Feb;25(2):126–31. doi: 10.1089/tmj.2017.0208. http://europepmc.org/abstract/MED/29782228 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rueda S, Artazcoz L, Navarro V. Health inequalities among the elderly in western Europe. J Epidemiol Community Health. 2008 Jun;62(6):492–8. doi: 10.1136/jech.2006.059279.62/6/492 [DOI] [PubMed] [Google Scholar]
- 15.Davidson S. Digital Inclusion Evidence Review 2018. Age UK. 2018. Nov, [2022-04-27]. https://www.ageuk.org.uk/globalassets/age-uk/documents/reports-and-publications/age_uk_digital_inclusion_evidence_review_2018.pdf .
- 16.Richard E, Jongstra S, Soininen H, Brayne C, Moll van Charante EP, Meiller Y, van der Groep B, Beishuizen CR, Mangialasche F, Barbera M, Ngandu T, Coley N, Guillemont J, Savy S, Dijkgraaf MG, Peters RJ, van Gool WA, Kivipelto M, Andrieu S. Healthy Ageing Through Internet Counselling in the Elderly: the HATICE randomised controlled trial for the prevention of cardiovascular disease and cognitive impairment. BMJ Open. 2016 Jun 10;6(6):e010806. doi: 10.1136/bmjopen-2015-010806. https://bmjopen.bmj.com/lookup/pmidlookup?view=long&pmid=27288376 .bmjopen-2015-010806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Richard E, Moll van Charante EP, Hoevenaar-Blom MP, Coley N, Barbera M, van der Groep A, Meiller Y, Mangialasche F, Beishuizen CB, Jongstra S, van Middelaar T, Van Wanrooij LL, Ngandu T, Guillemont J, Andrieu S, Brayne C, Kivipelto M, Soininen H, Van Gool WA. Healthy ageing through internet counselling in the elderly (HATICE): a multinational, randomised controlled trial. Lancet Digit Health. 2019 Dec;1(8):e424–34. doi: 10.1016/S2589-7500(19)30153-0. https://linkinghub.elsevier.com/retrieve/pii/S2589-7500(19)30153-0 .S2589-7500(19)30153-0 [DOI] [PubMed] [Google Scholar]
- 18.Jongstra S, Beishuizen C, Andrieu S, Barbera M, van Dorp M, van de Groep B, Guillemont J, Mangialasche F, van Middelaar T, Moll van Charante E, Soininen H, Kivipelto M, Richard E. Development and validation of an interactive internet platform for older people: the healthy ageing through internet counselling in the elderly study. Telemed J E Health. 2017 Feb;23(2):96–104. doi: 10.1089/tmj.2016.0066. [DOI] [PubMed] [Google Scholar]
- 19.Coley N, Rosenberg A, van Middelaar T, Soulier A, Barbera M, Guillemont J, Steensma J, Igier V, Eskelinen M, Soininen H, Moll van Charante E, Richard E, Kivipelto M, Andrieu S, MIND-AD. HATICE groups Older adults' reasons for participating in an eHealth prevention trial: a cross-country, mixed-methods comparison. J Am Med Dir Assoc. 2019 Jul;20(7):843–9.e5. doi: 10.1016/j.jamda.2018.10.019.S1525-8610(18)30599-1 [DOI] [PubMed] [Google Scholar]
- 20.Barbera M, Mangialasche F, Jongstra S, Guillemont J, Ngandu T, Beishuizen C, Coley N, Brayne C, Andrieu S, Richard E, Soininen H, Kivipelto M, HATICE study group Designing an Internet-based multidomain intervention for the prevention of cardiovascular disease and cognitive impairment in older adults: the HATICE trial. J Alzheimers Dis. 2018;62(2):649–63. doi: 10.3233/JAD-170858.JAD170858 [DOI] [PubMed] [Google Scholar]
- 21.Akenine U, Barbera M, Beishuizen CR, Fallah Pour M, Guillemont J, Rosenberg A, Coley N, Mangialasche F, Salo L, Savy S, Pols AJ, Andrieu S, Richard E, Soininen H, Moll van Charante E, Kivipelto M, HATICE study group Attitudes of at-risk older adults about prevention of cardiovascular disease and dementia using eHealth: a qualitative study in a European context. BMJ Open. 2020 Aug 06;10(8):e037050. doi: 10.1136/bmjopen-2020-037050. https://bmjopen.bmj.com/lookup/pmidlookup?view=long&pmid=32764085 .bmjopen-2020-037050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Beishuizen CR, Akenine U, Barbera M, Rosenberg A, Fallah Pour M, Richard E, Soininen H, Mangialasche F, Kivipelto M, Pols AJ, Moll van Charante E. Integrating nurses' experiences with supporting behaviour change for cardiovascular prevention into a self-management internet platform in Finland and the Netherlands: a qualitative study. BMJ Open. 2019 Jun 06;9(6):e023480. doi: 10.1136/bmjopen-2018-023480. https://bmjopen.bmj.com/lookup/pmidlookup?view=long&pmid=31175194 .bmjopen-2018-023480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hookham G, Nesbitt K. A systematic review of the definition and measurement of engagement in serious games. Proceedings of the Australasian Computer Science Week Multiconference; ACSW '19; January 29-31, 2019; Sydney, Australia. 2019. pp. 1–10. [DOI] [Google Scholar]
- 24.Perski O, Blandford A, West R, Michie S. Conceptualising engagement with digital behaviour change interventions: a systematic review using principles from critical interpretive synthesis. Transl Behav Med. 2017 Jun;7(2):254–67. doi: 10.1007/s13142-016-0453-1. http://europepmc.org/abstract/MED/27966189 .10.1007/s13142-016-0453-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Short CE, DeSmet A, Woods C, Williams SL, Maher C, Middelweerd A, Müller AM, Wark PA, Vandelanotte C, Poppe L, Hingle MD, Crutzen R. Measuring engagement in eHealth and mHealth behavior change interventions: viewpoint of methodologies. J Med Internet Res. 2018 Nov 16;20(11):e292. doi: 10.2196/jmir.9397. https://www.jmir.org/2018/11/e292/ v20i11e292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang R, Nicholas J, Knapp AA, Graham AK, Gray E, Kwasny MJ, Reddy M, Mohr DC. Clinically meaningful use of mental health apps and its effects on depression: mixed methods study. J Med Internet Res. 2019 Dec 20;21(12):e15644. doi: 10.2196/15644. https://www.jmir.org/2019/12/e15644/ v21i12e15644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Antypas K, Wangberg SC. An Internet- and mobile-based tailored intervention to enhance maintenance of physical activity after cardiac rehabilitation: short-term results of a randomized controlled trial. J Med Internet Res. 2014 Mar 11;16(3):e77. doi: 10.2196/jmir.3132. https://www.jmir.org/2014/3/e77/ v16i3e77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kelders SM, Bohlmeijer ET, Van Gemert-Pijnen JE. Participants, usage, and use patterns of a Web-based intervention for the prevention of depression within a randomized controlled trial. J Med Internet Res. 2013 Aug 20;15(8):e172. doi: 10.2196/jmir.2258. https://www.jmir.org/2013/8/e172/ v15i8e172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Donkin L, Hickie IB, Christensen H, Naismith SL, Neal B, Cockayne NL, Glozier N. Rethinking the dose-response relationship between usage and outcome in an online intervention for depression: randomized controlled trial. J Med Internet Res. 2013 Oct 17;15(10):e231. doi: 10.2196/jmir.2771. https://www.jmir.org/2013/10/e231/ v15i10e231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Power JM, Phelan S, Hatley K, Brannen A, Muñoz-Christian K, Legato M, Tate DF. Engagement and weight loss in a web and mobile program for low-income postpartum women: fit moms/ Mamás Activas. Health Educ Behav. 2019 Dec;46(2_suppl):114–23. doi: 10.1177/1090198119873915. [DOI] [PubMed] [Google Scholar]
- 31.Stewart AL, Mills KM, King AC, Haskell WL, Gillis D, Ritter PL. CHAMPS physical activity questionnaire for older adults: outcomes for interventions. Med Sci Sports Exerc. 2001 Jul;33(7):1126–41. doi: 10.1097/00005768-200107000-00010. [DOI] [PubMed] [Google Scholar]
- 32.Schröder H, Fitó M, Estruch R, Martínez-González MA, Corella D, Salas-Salvadó J, Lamuela-Raventós R, Ros E, Salaverría I, Fiol M, Lapetra J, Vinyoles E, Gómez-Gracia E, Lahoz C, Serra-Majem L, Pintó X, Ruiz-Gutierrez V, Covas M. A short screener is valid for assessing Mediterranean diet adherence among older Spanish men and women. J Nutr. 2011 Jun;141(6):1140–5. doi: 10.3945/jn.110.135566.jn.110.135566 [DOI] [PubMed] [Google Scholar]
- 33.Cooney MT, Selmer R, Lindman A, Tverdal A, Menotti A, Thomsen T, DeBacker G, De Bacquer D, Tell GS, Njolstad I, Graham IM, SCORE and CONOR investigators Cardiovascular risk estimation in older persons: SCORE O.P. Eur J Prev Cardiol. 2016 Jul;23(10):1093–103. doi: 10.1177/2047487315588390.2047487315588390 [DOI] [PubMed] [Google Scholar]
- 34.Kivipelto M, Ngandu T, Laatikainen T, Winblad B, Soininen H, Tuomilehto J. Risk score for the prediction of dementia risk in 20 years among middle aged people: a longitudinal, population-based study. Lancet Neurol. 2006 Sep;5(9):735–41. doi: 10.1016/S1474-4422(06)70537-3.S1474-4422(06)70537-3 [DOI] [PubMed] [Google Scholar]
- 35.Williams R. Generalized ordered logit/partial proportional odds models for ordinal dependent variables. Stata J. 2006 Feb 01;6(1):58–82. doi: 10.1177/1536867x0600600104. [DOI] [Google Scholar]
- 36.van Middelaar T, Beishuizen CR, Guillemont J, Barbera M, Richard E, Moll van Charante EP, HATICE consortium Engaging older people in an Internet platform for cardiovascular risk self-management: a qualitative study among Dutch HATICE participants. BMJ Open. 2018 Jan 21;8(1):e019683. doi: 10.1136/bmjopen-2017-019683. https://bmjopen.bmj.com/lookup/pmidlookup?view=long&pmid=29358447 .bmjopen-2017-019683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Coley N, Ngandu T, Lehtisalo J, Soininen H, Vellas B, Richard E, Kivipelto M, Andrieu S, HATICE‚ FINGER‚ and MAPT/DSA groups Adherence to multidomain interventions for dementia prevention: data from the FINGER and MAPT trials. Alzheimers Dement. 2019 Jun;15(6):729–41. doi: 10.1016/j.jalz.2019.03.005.S1552-5260(19)30076-7 [DOI] [PubMed] [Google Scholar]
- 38.Turunen M, Hokkanen L, Bäckman L, Stigsdotter-Neely A, Hänninen T, Paajanen T, Soininen H, Kivipelto M, Ngandu T. Computer-based cognitive training for older adults: determinants of adherence. PLoS One. 2019 Jul 10;14(7):e0219541. doi: 10.1371/journal.pone.0219541. https://dx.plos.org/10.1371/journal.pone.0219541 .PONE-D-18-36201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Collado-Mateo D, Lavín-Pérez AM, Peñacoba C, Del Coso J, Leyton-Román M, Luque-Casado A, Gasque P, Fernández-Del-Olmo MÁ, Amado-Alonso D. Key factors associated with adherence to physical exercise in patients with chronic diseases and older adults: an umbrella review. Int J Environ Res Public Health. 2021 Feb 19;18(4):2023. doi: 10.3390/ijerph18042023. https://www.mdpi.com/resolver?pii=ijerph18042023 .ijerph18042023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kelders SM, van Zyl LE, Ludden GD. The concept and components of engagement in different domains applied to eHealth: a systematic scoping review. Front Psychol. 2020 May 27;11:926. doi: 10.3389/fpsyg.2020.00926. doi: 10.3389/fpsyg.2020.00926. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary tables and figures.
Data Availability Statement
All individual participant data collected during the trial, after deidentification, and study documents, including the study protocol, will be available to academic researchers who provide a methodologically sound proposal, approved by the Healthy Ageing Through Internet Counselling in the Elderly steering group, and agree to collaborate on the analysis with at least one member of the Healthy Ageing Through Internet Counselling in the Elderly study team. Enquiries or proposals should be directed to MHB.


