Abstract
Background
Vaccination of children to prevent coronavirus disease 2019 (Covid-19) is an urgent public health need. The safety, immunogenicity, and efficacy of the mRNA-1273 vaccine in children 6 to 11 years of age are unknown.
Methods
Part 1 of this ongoing phase 2–3 trial was open label for dose selection; part 2 was an observer-blinded, placebo-controlled expansion evaluation of the selected dose. In part 2, we randomly assigned children (6 to 11 years of age) in a 3:1 ratio to receive two injections of mRNA-1273 (50 μg each) or placebo, administered 28 days apart. The primary objectives were evaluation of the safety of the vaccine in children and the noninferiority of the immune response in these children to that in young adults (18 to 25 years of age) in a related phase 3 trial. Secondary objectives included determination of the incidences of confirmed Covid-19 and severe acute respiratory syndrome coronavirus 2 infection, regardless of symptoms. Interim analysis results are reported.
Results
In part 1 of the trial, 751 children received 50-μg or 100-μg injections of the mRNA-1273 vaccine, and on the basis of safety and immunogenicity results, the 50-μg dose level was selected for part 2. In part 2 of the trial, 4016 children were randomly assigned to receive two injections of mRNA-1273 (50 μg each) or placebo and were followed for a median of 82 days (interquartile range, 14 to 94) after the first injection. This dose level was associated with mainly low-grade, transient adverse events, most commonly injection-site pain, headache, and fatigue. No vaccine-related serious adverse events, multisystem inflammatory syndrome in children, myocarditis, or pericarditis were reported as of the data-cutoff date. One month after the second injection (day 57), the neutralizing antibody titer in children who received mRNA-1273 at a 50-μg level was 1610 (95% confidence interval [CI], 1457 to 1780), as compared with 1300 (95% CI, 1171 to 1443) at the 100-μg level in young adults, with serologic responses in at least 99.0% of the participants in both age groups, findings that met the prespecified noninferiority success criterion. Estimated vaccine efficacy was 88.0% (95% CI, 70.0 to 95.8) against Covid-19 occurring 14 days or more after the first injection, at a time when B.1.617.2 (delta) was the dominant circulating variant.
Conclusions
Two 50-μg doses of the mRNA-1273 vaccine were found to be safe and effective in inducing immune responses and preventing Covid-19 in children 6 to 11 years of age; these responses were noninferior to those in young adults. (Funded by the Biomedical Advanced Research and Development Authority and the National Institute of Allergy and Infectious Diseases; KidCOVE ClinicalTrials.gov number, NCT04796896.)
The Coronavirus Efficacy (COVE) and Teen COVE trials1-3 showed that the mRNA-1273 vaccine (Moderna) had mainly low-grade transient adverse effects and high efficacy in preventing symptomatic coronavirus disease 2019 (Covid-19) in persons who were 12 years of age or older, and mRNA-1273 is approved for vaccination of adults in the United States. Although the highest risk of illness and death from Covid-19 occurs among older adults and populations with underlying coexisting conditions,4 children are at risk for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection that can lead to severe Covid-19–related outcomes, including hospitalization, the use of life-supporting interventions, and death.5,6 The burden of Covid-19 in children extends to social issues such as school interruptions and other life disruptions that may result in long-term consequences for academic development and well-being.7 Complications of SARS-CoV-2 infection in children and adolescents can include the development of multisystem inflammatory syndrome in children (MIS-C) and sequelae such as long Covid-198-10; these outcomes indicate a compelling need to protect children through vaccination.
Previous studies have shown that vaccination of 12-to-17-year-old adolescents reduced the risks of MIS-C and hospitalization.5,11,12 The Covid-19 BNT162b2 vaccine (Pfizer–BioNTech) received emergency use authorization (EUA) from the Food and Drug Administration (FDA) for immunization of adolescents and children 5 to 11 years of age.13 Recently, the mRNA-1273 vaccine received provisional approval in some countries outside the United States for use in children who are 6 to 11 years of age.14-16 Here, we report the interim results of the ongoing phase 2–3 KidCOVE trial, which evaluated the safety, immunogenicity, and efficacy of two 50-μg doses of the mRNA-1273 vaccine, as compared with placebo, administered 28 days apart in children who were 6 to 11 years of age.
Methods
Trial Oversight and Participants
The trial participants in three age cohorts (6 to 11 years, 2 to 5 years, and 6 months to 23 months) were enrolled at 79 sites in the United States and 8 sites in Canada. The trial was conducted in two parts, with an open-label dose-selection phase in part 1 and an observer-blinded, randomized, placebo-controlled expansion phase in part 2, which assessed safety, immunobridging (an approach in which the immune response in the test population is compared with that in a population in which efficacy has been shown), and efficacy. Here, we report the results of the interim analysis of part 1 and part 2 of the trial in the cohort of 6-to-11-year-old children. After the EUA for the BNT162b2 vaccine was updated on October 29, 2021, to include children 5 to 11 years of age in the United States, participants became eligible to have their data unblinded and placebo recipients became eligible to cross over to receive the mRNA-1273 vaccine. The cutoff date for blinded data was November 10, 2021.
Eligible children were generally healthy, but children with stable chronic conditions were also included. Children with a known recent history of SARS-CoV-2 infection, those who had received an investigational or approved SARS-CoV-2–related vaccine, and those with known hypersensitivity to vaccine components or excipients were excluded. Additional inclusion and exclusion criteria and information on the trial oversight, conduct, and design are provided in the Supplementary Appendix, available with the full text of this article at NEJM.org.
Trial Procedures
In both parts of the trial, the vaccination regimen involved two doses of the mRNA-1273 vaccine or saline placebo administered by intramuscular (deltoid) injection 28 days apart. Part 1 of the trial was designed to select a dose of mRNA-1273 vaccine for evaluation in part 2 (Fig. S1 in the Supplementary Appendix). The safety and immunogenicity of the mRNA-1273 vaccine at a dose level of 50 μg were evaluated in the 6-to-11-year-old age group before escalation to a 100-μg dose level after review by the internal safety team. When a prespecified subgroup of participants completed day 57, an analysis was performed, and the 50-μg dose level was selected by the sponsor and endorsed by the data and safety monitoring board.
In part 2 of the trial, children were randomly assigned with the use of a centralized interactive response technology system, in a 3:1 ratio, to receive two injections of the selected mRNA-1273 vaccine dose or placebo. The trial participants and personnel were unaware of the trial-group assignments until the initiation of unblinding (on the date of the EUA); however, personnel who prepared and administered injections were aware of these assignments.
The primary objectives were to determine the safety and reactogenicity of two injections of the selected dose of mRNA-1273 vaccine, administered 28 days apart, and to infer efficacy on the basis of the noninferiority of serum antibody levels and serologic response as compared with those among young adults (18 to 25 years of age) from the randomly selected immunogenicity subgroup of the phase 3 COVE trial.1 Key secondary objectives were to determine the incidences of confirmed Covid-19 and SARS-CoV-2 infection (regardless of symptoms) after administration of the mRNA-1273 vaccine or placebo (Table S1).
Safety
Evaluations of reactogenicity included assessment of solicited local and systemic adverse reactions that occurred within 7 days after each injection, as recorded in electronic diaries by the parents or guardians of the participants. Safety assessments included the following: unsolicited adverse events that occurred within 28 days after each injection and adverse events leading to nonreceipt of the second injection, discontinuation from the trial, or both; medically attended adverse events, serious adverse events, and adverse events of special interest, including MIS-C, myocarditis, and pericarditis; and SARS-CoV-2 infection assessed from day 1 through trial completion. Enhanced surveillance of myocarditis (in part 2 of the trial) included solicitation of cardiac symptoms (safety telephone calls) and medical follow-up and cardiac assessment if indicated by the evaluating provider.
Immunogenicity
The geometric mean titers of neutralizing antibodies were measured with the use of a validated lentivirus pseudovirus (D614G) assay with a 50% inhibitory dilution (ID50) and an 80% inhibitory dilution (ID80).1 Anti-spike geometric mean levels of binding antibodies were measured with the use of a Meso Scale Discovery assay (see the Supplementary Appendix). Serologic responses in participants were defined as an increase in antibody titers from below the lower limit of quantitation to titers that were at least 4 times the lower limit of quantitation, or at least 4 times as high as the baseline value if the baseline titers were equal to or above the lower limit of quantitation. In part 1 of the trial, we also assessed the geometric mean titers of neutralizing antibodies against the B.1.617.2 (delta) variant.
Efficacy
In both parts 1 and 2 of the trial, the incidence of confirmed symptomatic Covid-19 was defined in accordance with the guidelines of the Centers for Disease Control and Prevention (CDC). This definition included one systemic or respiratory symptom and a positive reverse-transcriptase–polymerase-chain-reaction (RT-PCR) assay for SARS-CoV-2. We also used the definition from the phase 3 COVE trial involving adults; that definition included at least two prespecified systemic symptoms or at least one respiratory symptom and a positive RT-PCR assay.1 SARS-CoV-2 infection (regardless of symptoms) was defined by a negative SARS-CoV-2 status at baseline in participants who later had a positive RT-PCR assay at a scheduled nasal-swab test or at a visit prompted by SARS-CoV-2 symptoms or exposure or by the detection of SARS-CoV-2–binding antibody levels against SARS-CoV-2 nucleocapsid protein measured by means of the Elecsys (Roche) serologic assay at a scheduled postbaseline visit (described in the protocol, available at NEJM.org).
Statistical Analysis
We planned to enroll up to 1275 participants in the dose-selection cohorts in part 1 of the trial and up to 12,000 participants (approximately 4000 per age group) in part 2. Details regarding statistical methods are provided in the Supplementary Methods section in the Supplementary Appendix, and details regarding the analysis populations are provided in Table S2.
The safety population consisted of all the participants who had received at least one trial injection, and the solicited safety population consisted of those who had received at least one injection and had at least one assessment for solicited adverse events. Solicited adverse events were evaluated according to trial and age group. We summarized the numbers and percentages of participants who had solicited adverse events within 7 days after each injection according to toxicity grade. We also summarized unsolicited adverse events (presented according to the Medical Dictionary for Regulatory Activities preferred terms and system organ class categories), serious adverse events, medically attended adverse events, severe adverse events, and adverse events leading to nonreceipt of the second injection or discontinuation from the trial. Descriptive summary data are provided for all other safety measures.
For the primary objective of inference of mRNA-1273 vaccine efficacy, we assessed the immunogenicity subgroups of children (6 to 11 years of age) in this trial and young adults (18 to 25 years of age) in a related phase 3 trial. The noninferiority of geometric mean titers of neutralizing antibodies at day 57 in children as compared with young adults was indicated if the lower boundary of the 95% confidence interval for the geometric mean titer ratio was at least 0.67 and if the lower boundary of the 95% confidence interval for the difference in serologic response was −10 percentage points or more (see the statistical analysis plan, which is included with the protocol). We provide the geometric mean titers and geometric mean ratios (with 95% confidence intervals calculated according to the analysis of covariance model), the number and percentage of participants with a serologic response (with 95% confidence intervals calculated with the Clopper–Pearson method), and the between-group difference in serologic response (with 95% confidence intervals calculated with the Miettinen–Nurminen method) at day 57. The same analysis was performed for binding-antibody values.
In part 2 of the trial, for the secondary efficacy objective of determining the incidences of Covid-19 and SARS-CoV-2 infection, regardless of symptoms, we evaluated all randomly assigned participants who did not have serologic or virologic evidence of SARS-CoV-2 infection at baseline and who received at least one planned injection, excluding those who received an incorrect injection (the modified-intention-to-treat-1 population, hereafter called the mITT1 population) and those who received both injections of trial vaccine according to the schedule, without a major protocol deviation (the per-protocol population). Incidence rates, which were calculated as the number of cases divided by the number of person-years (defined as the total years from the first day of the analysis to the date of the event, to the last date of trial participation, to censoring time, or to the efficacy data-cutoff date, whichever was earliest) accrued during the blinded phase before unblinding, and 95% confidence intervals were calculated with the exact method (Poisson distribution).
Results
Trial Population
Between March and August 2021, a total of 751 participants who were 6 to 11 years of age were enrolled in part 1 of the trial and 4016 participants were enrolled in part 2. In part 1, all 380 participants who were enrolled in the 50-μg dose-level mRNA-1273 group and 371 participants who were enrolled in the 100-μg dose-level mRNA-1273 group received one injection, and 379 participants who were enrolled in the 50-μg dose-level group and 371 participants who were enrolled in the 100-μg dose-level group received two injections (Fig. S2).
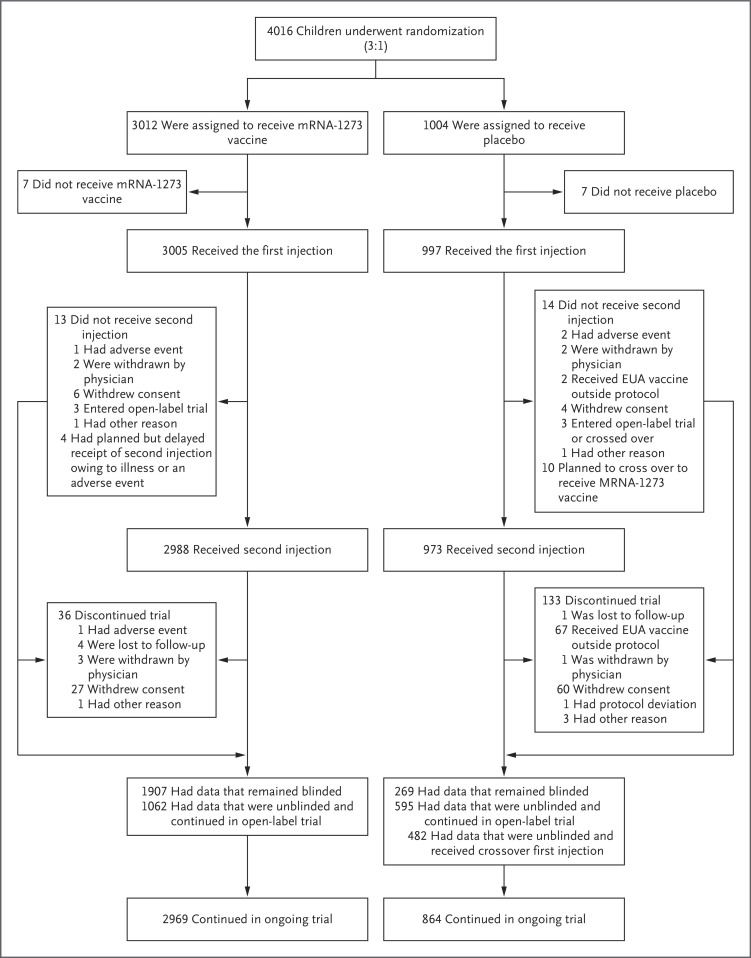
In part 2 of the trial, 4016 participants were randomly assigned to receive two 50-μg injections of the mRNA-1273 vaccine or two injections of placebo; 3005 participants in the vaccine group and 997 participants in the placebo group received the first injection, and 2988 participants (99.2%) in the vaccine group and 973 participants (96.9%) in the placebo group received both injections (Figure 1). A total of 13 participants (0.4%) in the vaccine group and 14 participants (1.4%) in the placebo group did not receive the second injection, most commonly because of withdrawal of consent in both groups; 2 participants (0.2%) in the placebo group received a vaccine that was available under an EUA, outside the protocol. In the vaccine group, 36 participants (1.2% of those who received the first injection) discontinued the trial, and these discontinuations were attributed mainly to withdrawal of consent. In the placebo group, 133 participants (13.3% of those who received the first injection) discontinued the trial; these discontinuations were attributed mainly to receipt of an EUA vaccine outside the protocol.
Figure 1. Randomization and Analysis Populations in Part 2 of the Trial.
The populations of trial participants (6 to 11 years of age) who received the mRNA-1273 vaccine at a dose level of 50 μg or placebo are shown. The reasons for not receiving a first injection included withdrawal of consent (in 8 participants), screening failure because of error in randomization (in 5), and physician decision owing to a medication change 1 month before consent (in 1). Two participants who were randomly assigned to receive placebo received the mRNA-1273 vaccine. In the placebo group, the two adverse events were related to coronavirus disease 2019 (Covid-19). In the mRNA-1273 vaccine group, of the 36 participants who discontinued the trial, 9 had received a first injection and 27 had received a second injection. In the placebo group, of the 133 participants who discontinued the trial, 10 had received a first injection and 123 had received a second injection. The number of trial discontinuations includes 9 participants in the vaccine group and 67 participants in the placebo group who had data that were unblinded and discontinued the trial. After October 29, 2021, the date of emergency use authorization (EUA) of the BNT162b2 vaccine for children 5 to 11 years of age, participants became eligible to have their data unblinded. The cutoff date for blinded data was November 10, 2021.
The demographic characteristics of the participants at baseline were generally balanced between the trial groups, similar in parts 1 and 2 of the trial, and representative of a diverse population (Table 1 and Tables S3 and S4). In part 2, the mean age of the participants in the safety population was 8.5 years (approximately 50% of the participants were 6 to 8 years of age), 49.2% were female, 51.9% were White non-Hispanic, and 47.9% were from communities of color. The distribution of race groups included 65.6% White, 10.0% Black, 9.9% Asian, and 10.6% multiracial participants, and 18.5% of the participants were Hispanic or Latinx. Characteristics of the per-protocol immunogenicity subgroup in part 2 of the trial, including representativeness of communities of color, were generally similar to those in the safety population in part 2 and those in the per-protocol immunogenicity subgroup in the COVE trial involving young adults (18 to 25 years of age).
Table 1. Demographic and Clinical Characteristics in the Safety Population at Baseline (Part 2 of the Trial).*.
| Characteristic | mRNA-1273, 50 μg (N=3007) |
Placebo (N=995) |
Total (N=4002) |
|---|---|---|---|
| Age — yr | 8.5±1.7 | 8.5±1.6 | 8.5±1.7 |
| Age category — no. (%) | |||
| 6–8 yr | 1514 (50.3) | 484 (48.6) | 1998 (49.9) |
| 9–11 yr | 1493 (49.6) | 511 (51.4) | 2004 (50.1) |
| Sex — no. (%) | |||
| Male | 1554 (51.7) | 481 (48.3) | 2035 (50.8) |
| Female | 1453 (48.3) | 514 (51.7) | 1967 (49.2) |
| Race or ethnic group — no. (%)† | |||
| White | 1957 (65.1) | 668 (67.1) | 2625 (65.6) |
| Black | 309 (10.3) | 93 (9.3) | 402 (10.0) |
| Asian | 298 (9.9) | 100 (10.1) | 398 (9.9) |
| American Indian or Alaska Native | 14 (0.5) | 3 (0.3) | 17 (0.4) |
| Native Hawaiian or Other Pacific Islander | 4 (0.1) | 0 | 4 (<0.1) |
| Multiracial | 327 (10.9) | 97 (9.7) | 424 (10.6) |
| Other race | 62 (2.1) | 22 (2.2) | 84 (2.1) |
| Not reported | 23 (0.8) | 10 (1.0) | 33 (0.8) |
| Unknown | 9 (0.3) | 1 (0.1) | 10 (0.2) |
| Missing data | 4 (0.1) | 1 (0.1) | 5 (0.1) |
| Hispanic or Latinx — no. (%)† | |||
| Yes | 561 (18.7) | 181 (18.2) | 742 (18.5) |
| No | 2417 (80.4) | 805 (80.9) | 3222 (80.5) |
| Not reported | 22 (0.7) | 5 (0.5) | 27 (0.7) |
| Unknown | 7 (0.2) | 4 (0.4) | 11 (0.3) |
| Race and ethnic group — no. (%)† | |||
| White non-Hispanic | 1542 (51.3) | 536 (53.9) | 2078 (51.9) |
| Communities of color | 1459 (48.5) | 456 (45.8) | 1915 (47.9) |
| Missing data | 6 (0.2) | 3 (0.3) | 9 (0.2) |
| Weight — kg | |||
| Mean | 33.3±11.3 | 33.5±11.4 | 33.4±11.3 |
| Median (range) | 30.6 (14.0–112.0) | 30.9 (14.2–99.8) | 30.7 (14.0–112.0) |
| Underlying conditions — no. (%) | |||
| Obesity‡ | 608 (20.2) | 195 (19.6) | 803 (20.1) |
| Chronic lung disease | 279 (9.3) | 90 (9.0) | 369 (9.2) |
| Asthma | 250 (8.3) | 83 (8.3) | 333 (8.3) |
| Cardiac disease | 19 (0.6) | 7 (0.7) | 26 (0.6) |
| Diabetes mellitus | 9 (0.3) | 5 (0.5) | 14 (0.3) |
| HIV infection | 4 (0.1) | 0 | 4 (<0.1) |
| SARS-CoV-2 status — no. (%)§ | |||
| Negative | 2703 (89.9) | 880 (88.4) | 3583 (89.5) |
| Positive | 257 (8.5) | 87 (8.7) | 344 (8.6) |
| Missing data | 47 (1.6) | 28 (2.8) | 75 (1.9) |
Plus–minus values are means ±SD. Percentages may not total 100 because of rounding. HIV denotes human immunodeficiency virus, and SARS-CoV-2 severe acute respiratory syndrome coronavirus 2.
Race and ethnic group were reported by the participants or by their parents or guardians. Participants could be included in more than one category. Race and ethnic group categories shown are White (non-Hispanic) and communities of color (i.e., White Hispanic participants, non-White participants, all others whose race or ethnic group was reported as not known, not reported, or missing).
Obesity was defined as a body-mass index (BMI; the weight in kilograms divided by the square of the height in meters) at or above the 95th percentile according to the revised World Health Organization Child Growth Standards.
Baseline SARS-CoV-2 status was positive if there was immunologic or virologic evidence of previous Covid-19, as defined by a positive reverse transcriptase–polymerase-chain-reaction (RT-PCR) test or a positive immunoassay result (i.e., detection of binding antibodies against the SARS-CoV-2 nucleocapsid [Elecsys, Roche] above the limit of detection or the lower limit of quantification at day 1). Baseline SARS-CoV-2 status was negative if there was a negative RT-PCR test and a negative immunoassay result (i.e., no detection of binding antibodies against the SARS-CoV-2 nucleocapsid [Elecsys, Roche] below the limit of detection and the lower limit of quantitation at day 1). The data-cutoff date was November 10, 2021.
Safety
On the basis of the combined safety, reactogenicity, and immunogenicity results in part 1 of the trial, the 50-μg dose level was selected for evaluation in the 6-to-11-year-old age group in part 2 (Part 1 Results section in the Supplementary Appendix). Data on safety are provided in Tables S5 through S10, and data on immunogenicity are provided in Figures S3 and S4 and Tables S11 through S14.
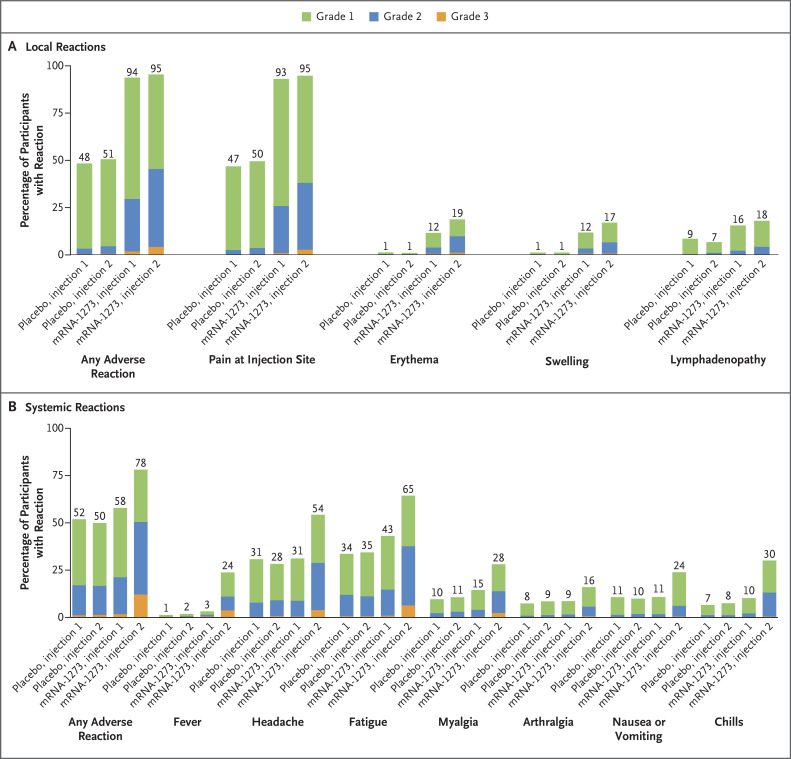
In part 2 of the trial, the median duration of follow-up was 82 days (interquartile range, 14 to 94) after the first injection and 51 days (interquartile range, 45 to 57) after the second injection. Solicited local adverse events were more common in the mRNA-1273 vaccine group than in the placebo group after the first injection (94% vs. 48%) and after the second injection (95% vs. 51%); the most common adverse event was injection-site pain (Figure 2A and Table S15). Most solicited local adverse events after any injection were grades 1 or 2. In the mRNA-1273 group, the incidence of local grade 3 adverse events was higher after the second injection (4%) than after the first injection (2%).
Figure 2. Solicited Local and Systemic Adverse Reactions in Part 2 of the Trial.
Shown is the percentage of participants in the solicited safety population who had a solicited local or systemic adverse reaction within 7 days after the first or second 50-μg injection of the mRNA-1273 vaccine or placebo. The numbers above the bars are the percentage of participants in each group with the specified reaction. Lymphadenopathy was defined as axillary or groin swelling or tenderness. The data-cutoff date was November 10, 2021.
The incidence of solicited systemic adverse events after the first injection was similar in the mRNA-1273 vaccine group (58%) and the placebo group (52%) and higher after the second injection in the mRNA-1273 vaccine group than in the placebo group (in 78% vs. 50%) (Figure 2B). In both groups, the most common solicited systemic adverse events were headache and fatigue. In the mRNA-1273 vaccine group, the incidences of chills and fever were higher after the second injection than after the first injection; these increases in incidence were greater than the increases observed with other adverse events. Most systemic adverse events were grade 1 or 2. After the second injection, the incidence of systemic grade 3 adverse events — most commonly fatigue, headache, and fever — was higher in the mRNA-1273 group (12%) than in the placebo group (1%).
The majority of solicited adverse events in the vaccine group occurred within 1 or 2 days after either injection and persisted for medians of 2 or 3 days. The median duration of fever was 1 day (Table S16).
The incidences of local adverse events were similar in children who received the mRNA-1273 vaccine at the 50-μg dose level and in young adults (18 to 25 years of age) who received the mRNA-1273 vaccine at the 100-μg dose level in the COVE trial; the incidences of systemic adverse events were lower among the children than among the young adults. The incidence of grade 3 adverse events was also lower in children than in young adults, with the exception of fever, which occurred more often in children than in young adults after either injection, and in particular, after the second injection (in 4% vs. 1%) (Tables S17 and S18).
In the current trial, the incidence of unsolicited adverse events that occurred up to 28 days after either injection was similar in the mRNA-1273 vaccine group (29.6%) and the placebo group (25.1%) (Tables S19 through S21). The incidence of unsolicited adverse events that were considered by the investigator to be related to the trial vaccine or placebo was higher in the mRNA-1273 group (10.6%) than in the placebo group (5.0%). These events were mostly reactogenicity events; injection-site erythema was the most common. Serious unsolicited adverse events that occurred up to 28 days after any injection were reported for three participants (<0.1%) in the mRNA-1273 group and two participants (0.2%) in the placebo group.
All serious adverse events in the mRNA-1273 vaccine group (appendicitis, cellulitis, and orbital cellulitis) and in the placebo group (affective disorder and Covid-19) were considered by the investigators to be unrelated to the trial vaccine or placebo. The incidence of medically attended adverse events was similar in the mRNA-1273 group (13.4%) and the placebo group (14.2%). No vaccination-related adverse events led to nonreceipt of the second injection, discontinuation from the trial, or both. As of the data-cutoff date, the investigators had not attributed any serious adverse events to the trial vaccine or placebo, and no deaths or cases of anaphylaxis, MIS-C, myocarditis, or pericarditis were reported.
Efficacy
At day 57, the geometric mean titer of neutralizing antibodies was 1610 (95% CI, 1457 to 1780) in 320 children who had received the mRNA-1273 vaccine at the 50-μg dose level as compared with 1300 (95% CI, 1171 to 1443) in 295 young adults who had received the mRNA-1273 vaccine at the 100-μg dose level, with a serologic response in at least 99% of the participants in both groups (Table 2 and S22 and Fig. S5). The geometric mean titer ratio of neutralizing antibodies in children as compared with young adults was 1.2 (95% CI, 1.1 to 1.4), and the between-group difference in the serologic response was 0.1 percentage points (95% CI, −1.9 to 2.1), findings that met the noninferiority criterion for the coprimary immunogenicity objective. These findings were further supported by similar results with respect to the distribution of binding antibodies (Tables S23 and S24 and Fig. S6).
Table 2. Immunogenicity of the mRNA-1273 Vaccine in Part 2 of the Trial.*.
| Variable | Children, 6–11 Yr mRNA-1273, 50 μg (N=320) |
Young Adults, 18–25 Yr mRNA-1273, 100 μg (N=295) |
Children vs. Young Adults |
|---|---|---|---|
| Baseline | |||
| No. of participants with nonmissing data | 317 | 295 | |
| Geometric mean pseudovirus neutralizing antibody titer (95% CI)† | 9.3 (NE to NE) | 9.3 (9.2 to 9.4) | |
| Day 57 | |||
| No. of participants with nonmissing data | 319 | 295 | |
| Geometric mean pseudovirus neutralizing antibody titer (95% CI)‡ | 1610 (1457 to 1780) | 1300 (1171 to 1443) | Geometric mean titer ratio, 1.2 (95% CI, 1.1 to 1.4)‡ |
| Serologic response | |||
| No. of participants/total no. (%)§ | 313/316 (99.1) | 292/295 (99.0) | 0.1 percentage points (95% CI, −1.9 to 2.1)¶ |
| 95% CI‖ | 97.3 to 99.8 | 97.1 to 99.8 |
The 50% inhibitory dilution titer of neutralizing antibodies was determined for participants in the immunogenicity population. For neutralizing antibody values that were assessed by pseudovirus neutralizing antibody assay and reported as being below the lower limit of quantitation (18.5), 0.5 times the lower limit of quantitation was used in the analysis. For values greater than the upper limit of quantitation (45,118), the upper limit of quantitation was used in the analysis if actual values were not available. The young adults group includes participants who were 18 to 25 years of age in the mRNA-1273 group in the COVE trial. The upper limit of quantitation was different for selected COVE trial participants who had undergone testing previously. The data-cutoff date was November 10, 2021. To convert the values for geometric mean pseudovirus neutralizing antibody titers to international units, multiply by 0.242.
The 95% confidence intervals were calculated with the use of t-distribution of the log-transformed values for geometric mean titer (observed or model-based, which is estimated by geometric least-squares means), respectively, then back-transformed to the original scale for presentation.
The log-transformed antibody levels were analyzed with the use of an analysis of covariance model (primary approach) with the group variable (children and young adults in the COVE trial) as a fixed effect. The resulting least-squares means, the difference of least-squares means, and 95% confidence intervals were back-transformed to the original scale for presentation.
The serologic response in participants was defined as an increase in antibody titers from below the lower limit of quantitation to titers that were at least 4 times the lower limit of quantitation, or at least 4 times as high as the baseline value if the baseline titers were equal to or above the lower limit of quantitation. Percentages were based on the number of participants with nonmissing data at baseline and the corresponding time point.
The 95% confidence interval was calculated with the use of the confidence limits in the Miettinen–Nurminen method.
The 95% confidence interval was calculated with the use of the Clopper–Pearson method.
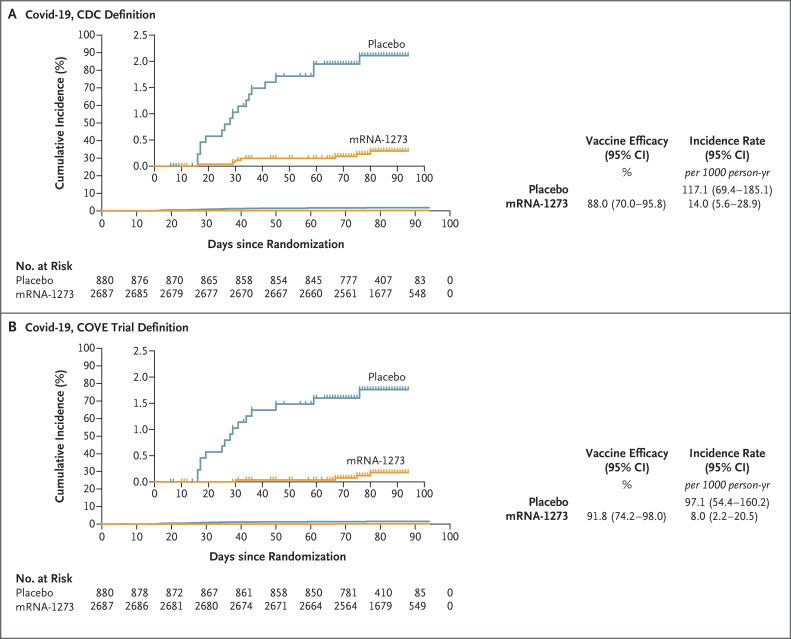
In the mITT1 population, among children who did not have evidence of SARS-CoV-2 infection at baseline, the estimates of vaccine efficacy at least 14 days after the first injection were 88.0% (95% CI, 70.0 to 95.8) according to the CDC definition, with 7 cases (0.3%) in the mRNA-1273 group and 18 cases (2.1%) in the placebo group and 91.8% (95% CI, 74.2 to 98.0) according to the definition used in the phase 3 COVE trial involving adults, with 4 cases (0.1%) in the mRNA-1273 group and 15 cases (1.7%) in the placebo group (Figure 3 and Table S25). The estimated vaccine efficacy against SARS-CoV-2 infection was 74.0% (95% CI. 57.9 to 84.1), regardless of symptoms that occurred at least 14 days after the first injection, with 34 cases (1.3%) in the vaccine group and 40 cases (4.6%) in the placebo group. The estimated vaccine efficacy against asymptomatic SARS-CoV-2 infection was 62.5% (95% CI, 30.9 to 79.4), with 22 cases (2.5%) in the mRNA-1273 group and 27 cases (1.0%) in the placebo group. The analysis of vaccine efficacy at least 14 days after two injections (in the per-protocol population) was limited by the small number of Covid-19 cases and the shortened period of blinded follow-up (Table S26).
Figure 3. Vaccine Efficacy after the First Injection in Part 2 of the Trial.
The cumulative incidence of Covid-19 was based on the Centers for Disease Control and Prevention (CDC) definition (Panel A) and the primary case definition in the COVE trial (Panel B) in the modified-intention-to-treat-1 population, 14 days after the first injection. Covid-19 cases are based on one symptom according to the CDC definition and two symptoms in the primary case definition used in the COVE trial.1 The number of person-years was defined as the total years from the first day of the analysis to the date of the event, to the last date of trial participation, to censoring time, or to the efficacy data-cutoff date, whichever was earliest. Incidence was defined as the number of participants with an event divided by the number of participants at risk, with adjustment for person-years (total time at risk) in each trial group. The 95% confidence interval (CI) was calculated with the use of the exact method (Poisson distribution) with adjustment for person-years. Vaccine efficacy was defined as 1 minus the ratio of the incidence rate (mRNA-1273 vaccine vs. placebo), and the 95% confidence interval of the ratio was calculated with the use of the exact method conditional on the total number of cases, with adjustment for person-years. The data-cutoff date was November 10, 2021. The insets show the same data on an expanded y axis. Tick marks in both panels indicate censored data.
In part 1 of the trial, a preliminary analysis showed an increase by a factor of 81.8 (95% CI, 70.4 to 95.0) in the geometric mean titer of neutralizing antibodies (ID50) against the delta variant from baseline to day 57. A total of 99.3% of the children had a serologic response, findings that are similar to those indicated by increased geometric mean titers measured in adults who had received a booster against this variant (Table S27).
Discussion
The ongoing KidCOVE trial to evaluate vaccination of children (6 to 11 years of age) with two 50-μg doses of the mRNA-1273 vaccine administered 28 days apart showed safety, immunogenicity, and vaccine efficacy that were consistent with those previously observed in adolescents and adults.1,2 These results extend the evidence of the safety and efficacy of the mRNA-1273 vaccine seen in adults and adolescents and provide support for the use of this vaccine to prevent Covid-19 in children.
A 50-μg dose level of the mRNA-1273 vaccine was selected for evaluation in part 2 of the trial on the basis of the lower reactogenicity than the 100-μg dose level and supportive immunogenicity results in part 1. Dose levels of less than 50 μg were not evaluated because of the risk of inferior immunogenicity among children as compared with that among young adults. In more than 3000 children who received at least one 50-μg dose of the mRNA-1273 vaccine, no new safety concerns were identified. Reactogenicity events — most commonly injection-site pain, fatigue, and headache — were generally mild to moderate. These events started within 1 or 2 days after vaccination and resolved after 1 to 3 days. The incidence of local adverse events was similar, and the incidence of systemic adverse events was lower in children 6 to 11 years of age than in young adults (18 to 25 years of age) in the COVE trial. The incidence of grade 3 adverse events, except for fever, was lower in children. The incidence of fever, including grade 3 fever, was higher among children than among young adults after any injection, with a median duration of 1 day; no febrile seizures were reported. Analysis of all safety data, including unsolicited adverse events, identified no short-term safety issues. No deaths, anaphylaxis events, MIS-C, myocarditis, pericarditis, or vaccine-related serious adverse events were reported through the data-cutoff date.
The efficacy of the 50-μg dose level of the mRNA-1273 vaccine in children in this trial was inferred by successful immunobridging to data in young adults who had received the 100-μg dose level of the vaccine in the COVE trial, which had shown high efficacy.1 Moreover, the magnitude of the geometric mean titer of neutralizing antibodies ID50 (1610, or 390 IU per milliliter) at day 57 was comparable to that of titers (high tertile; >363 IU per milliliter) that correlated with a 69% Covid-19 risk reduction in the COVE trial.17 These results indicate that vaccinated children who are 6 to 11 years of age may be protected from Covid-19 and subsequent sequelae.
The low incidences of Covid-19 and SARS-CoV-2 infections were expected given the limited blinded follow-up period and 3:1 (mRNA-1273:placebo) randomization. Nevertheless, the mRNA-1273 vaccine at a dose level of 50 μg in children was protective against Covid-19 beginning 14 days after the first injection (in the mITT1 population), as assessed either by the less-stringent CDC criteria (reflecting the milder cases of Covid-19 among children) or the COVE trial definitions. This vaccine was also protective against SARS-CoV-2 infection regardless of symptoms or asymptomatic infection. These findings were consistent with the vaccine efficacy observed in the COVE trial involving adults and the Teen COVE trial involving adolescents.1,2
Our trial showed the efficacy of mRNA-1273 vaccine when delta was the predominant circulating variant and provided preliminary results indicating that in children mRNA-1273 vaccine elicits neutralizing antibody responses that are similar to those observed against the delta variant after booster vaccination in adults. These findings suggest that this vaccine provides a protective benefit for children against variants of concern. Our findings are also consistent with those reported in other studies showing the effectiveness of mRNA-1273 vaccine in reducing Covid-19–related hospital admissions during the circulation of the delta variant5 and the neutralization of variants 18-20, including the B.1.1.529 (omicron) variant, after vaccination in children and adolescents.21-23
The diversity of enrolled participants in this large trial, which was similar to that in the COVE trial,1 reflects the generalizability of the results to various populations of children. The limitations of the trial data include a lack of long-term results. Data from the 1-year follow-up after the second injection are being evaluated to assess the safety of the mRNA-1273 vaccine and the durability of its protection in children.24,25 Owing to the few cases of Covid-19 that occurred during the short, blinded phase of the trial, evaluation of the efficacy of mRNA-1273 after two injections was limited when the number of cases would be expected to be higher; nonetheless, the efficacy observed after one injection is reassuringly similar to that seen in adolescents and adults who have received mRNA-1273.1,2 Although the trial was powered to assess the safety of rare events (0.1%), even rarer events were identified during the global distribution of Covid-19 vaccines; thus, continued vigilance in monitoring safety is warranted.26
The effectiveness of the mRNA-1273 vaccine in the trial shown during the delta variant outbreak in the United States and preliminary results that show neutralization of the delta variant suggest that the vaccine can provide a protective benefit in children against variants, findings that are consistent with those of other studies.18-22 However, the trial was conducted before the surge of the omicron variant, and assessment of the benefit of the vaccine against this variant is ongoing in the trial.
The trial results indicate that a 50-μg dose level of the mRNA-1273 vaccine had an acceptable safety profile and was efficacious in children 6 to 11 years of age. The trial is ongoing to provide additional safety and efficacy data in this age group as well as in younger children (6 months to 5 years of age). Vaccination of children may help to protect them from Covid-19 and may reduce community circulation of SARS-CoV-2 variants and lead to a return toward normal routines.
Acknowledgments
We thank the trial participants and their families; the staff at the trial sites for their dedication and devotion in implementing the protocol; members of the data and safety monitoring board (David Bernstein, M.D., [chair] Cincinnati Children’s Hospital Medical Center; Michael Polis, M.D., M.P.H., Medical Consulting; and Anne Chang, Ph.D., M.P.H.T.M., M.B., B.S., Children’s Health Queensland Hospital, Australia); David C. Montefiori, Ph.D., and the Immune Assay Team at Duke University Medical Center for performing the neutralization assays; Adrian McDermont, Ph.D., and the team at the Vaccine Research Center, National Institute of Allergy and Infectious Diseases, National Institutes of Health, for performing the Meso Scale Discovery multiplex assays; and Frank Dutko, Ph.D., (a Moderna contractor) for editorial support with an earlier version of the manuscript.
Protocol
Supplementary Appendix
Disclosure Forms
Data Sharing Statement
This article was published on May 11, 2022, at NEJM.org.
A data sharing statement provided by the authors is available with the full text of this article at NEJM.org.
Footnotes
Supported by the Office of the Assistant Secretary for Preparedness and Response, Biomedical Advanced Research and Development Authority (contract 75A50120C00034), and by the National Institute of Allergy and Infectious Diseases, part of the National Institutes of Health (grants UM1AI148576, UM1AI148452, UM1AI148689, UM1AI148450, UM1AI148372, and UM1AI148575).
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
References
- 1.El Sahly HM, Baden LR, Essink B, et al. Efficacy of the mRNA-1273 SARS-CoV-2 vaccine at completion of blinded phase. N Engl J Med 2021;385:1774-1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ali K, Berman G, Zhou H, et al. Evaluation of mRNA-1273 SARS-CoV-2 vaccine in adolescents. N Engl J Med 2021;385:2241-2251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Food and Drug Administration. Coronavirus (COVID-19) update: FDA takes key action by approving second covid-19 vaccine. Press release, January 31, 2022. (https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-takes-key-action-approving-second-covid-19-vaccine).
- 4.Kim L, Garg S, O’Halloran A, et al. Risk factors for intensive care unit admission and in-hospital mortality among hospitalized adults identified through the US Coronavirus Disease 2019 (COVID-19)–Associated Hospitalization Surveillance Network (COVID-NET). Clin Infect Dis 2021;72(9):e206-e214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Delahoy MJ, Ujamaa D, Whitaker M, et al. Hospitalizations associated with COVID-19 among children and adolescents — COVID-NET, 14 states, March 1, 2020–August 14, 2021. MMWR Morb Mortal Wkly Rep 2021;70:1255-1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Woodruff RC, Campbell AP, Taylor CA, et al. Risk factors for severe covid-19 in children. Pediatrics 2022;149(1):e2021053418-e2021053418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim L, Whitaker M, O’Halloran A, et al. Hospitalization rates and characteristics of children aged <18 years hospitalized with laboratory-confirmed COVID-19 — COVID-NET, 14 states, March 1–July 25, 2020. MMWR Morb Mortal Wkly Rep 2020;69:1081-1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dembiński Ł, Vieira Martins M, Huss G, et al. SARS-CoV-2 Vaccination in children and adolescents — a joint statement of the European Academy of Paediatrics and the European Confederation for Primary Care Paediatricians. Front Pediatr 2021;9:721257-721257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.National Center for Immunization and Respiratory Diseases. Health department-reported cases of multisystem inflammatory syndrome in children (MIS-C) in the United States. Centers for Disease Control and Prevention, May 11, 2021. (https://stacks.cdc.gov/view/cdc/106439#).
- 10.Stephenson T, Pinto Pereira SM, Shafran R, et al. Physical and mental health 3 months after SARS-CoV-2 infection (long COVID) among adolescents in England (CLoCk): a national matched cohort study. Lancet Child Adolesc Health 2022;6:230-239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marks KJ, Whitaker M, Anglin O, et al. Hospitalizations of children and adolescents with laboratory-confirmed COVID-19 — COVID-NET, 14 states, July 2021–January 2022. MMWR Morb Mortal Wkly Rep 2022;71:271-278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zambrano LD, Newhams MM, Olson SM, et al. Effectiveness of BNT162b2 (Pfizer-BioNTech) mRNA vaccination against multisystem inflammatory syndrome in children among persons aged 12-18 years — United States, July–December 2021. MMWR Morb Mortal Wkly Rep 2022;71:52-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Walter EB, Talaat KR, Sabharwal C, et al. Evaluation of the BNT162b2 Covid-19 vaccine in children 5 to 11 years of age. N Engl J Med 2021;386:35-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Australian Technical Advisory Group on Immunisation. ATAGI recommendations on the use of Spikevax (Moderna) COVID-19 vaccine in children aged 6 to 11 years. Australian Government Department of Health, February 23, 2022. (https://www.health.gov.au/news/atagi-recommendations-on-the-use-of-spikevax-moderna-covid-19-vaccine-in-children-aged-6-to-11-years).
- 15.Health Canada. Health Canada authorizes use of the Moderna Spikevax (50 mcg) COVID-19 vaccine in children 6 to 11 years of age. March 17, 2022. (https://www.canada.ca/en/health-canada/news/2022/03/health-canada-authorizes-use-of-the-moderna-spikevax-50-mcg-covid-19-vaccine-in-children-6-to-11-years-of-age.html).
- 16.European Medicines Agency. EMA recommends approval of Spikevax for children aged 6 to 11. February 24, 2022. (https://www.ema.europa.eu/en/news/ema-recommends-approval-spikevax-children-aged-6-11).
- 17.Gilbert PB, Montefiori DC, McDermott AB, et al. Immune correlates analysis of the mRNA-1273 COVID-19 vaccine efficacy clinical trial. Science 2022;375:43-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bruxvoort KJ, Sy LS, Qian L, et al. Effectiveness of mRNA-1273 against delta, mu, and other emerging variants of SARS-CoV-2: test negative case-control study. BMJ 2021;375:e068848-e068848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pajon R, Doria-Rose NA, Shen X, et al. SARS-CoV-2 Omicron variant neutralization after mRNA-1273 booster vaccination. N Engl J Med 2022;386:1088-1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Baden LR, El Sahly HM, Essink B, et al. Phase 3 trial of mRNA-1273 during the Delta-variant surge. N Engl J Med 2021;385:2485-2487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Girard B, Tomassini JE, Deng W, et al. mRNA-1273 vaccine-elicited neutralization of SARS-CoV-2 Omicron in adolescents and children. January 25, 2022. (https://www.medrxiv.org/content/10.1101/2022.01.24.22269666v1). preprint.
- 22.Bartsch YC, St Denis KJ, Kaplonek P, et al. Comprehensive antibody profiling of mRNA vaccination in children. October 9, 2021. (https://www.biorxiv.org/content/10.1101/2021.10.07.463592v1). preprint.
- 23.Burns MD, Bartsch YC, Boribong BP, et al. Durability and cross-reactivity of SARS-CoV-2 mRNA vaccine in adolescent children. January 10, 2022. (https://www.medrxiv.org/content/10.1101/2022.01.05.22268617v1). preprint. [DOI] [PMC free article] [PubMed]
- 24.Chu VT, Yousaf AR, Chang K, et al. Household transmission of SARS-CoV-2 from children and adolescents. N Engl J Med 2021;385:954-956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McLean HQ, Grijalva CG, Hanson KE, et al. Household transmission and clinical features of SARS-CoV-2 infections by age in 2 US communities. August 20, 2021. (https://www.medrxiv.org/content/10.1101/2021.08.16.21262121v2). preprint. [DOI] [PMC free article] [PubMed]
- 26.Straus W, Urdaneta V, Esposito DB, et al. Myocarditis after mRNA-1273 vaccination: a population-based analysis of 151 million vaccine recipients worldwide. November 12, 2021. (https://www.medrxiv.org/content/10.1101/2021.11.11.21265536v1). preprint.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.