This randomized clinical trial investigates the effect that limiting recreational screen media use has on the physical activity of children.
Key Points
Question
Does limiting recreational screen media use increase habitual physical activity in children?
Findings
In this cluster randomized clinical trial that included 181 children and 164 adults from 89 families, an intervention designed to limit recreational screen use resulted in a mean (SD) increase in children’s nonsedentary time of 44.8 (63.5) minutes per day with a corresponding mean (SD) change of 1.0 (55.1) minute per day in children from the control group. The between-group difference was statistically significant.
Meaning
Balancing children’s recreational screen media use should be a public health priority as it increases their physical activity substantially.
Abstract
Importance
Children and adults spend large amounts of their leisure time using screen media, which may affect their health and behavior.
Objective
To investigate the effect of reducing household recreational screen media use on physical activity and sleep in children and adults.
Design, Setting, and Participants
This was a cluster randomized clinical trial with a 2-week follow-up. Enrollment began on June 6, 2019, and ended on March 30, 2021. This study included a population-based sample from 10 Danish municipalities. A total of 89 families (181 children and 164 adults) were recruited based on a population-based survey on screen media habits in families with children. To be eligible, the responding parent had to list self-reported recreational screen use greater than the 40th percentile of recreational screen time use in the source population (>2.4 hours per day). In addition, the parent had to be full-time employed (with no regular night shifts) or enrolled in full-time education.
Interventions
Families were randomly assigned to the screen media reduction intervention (45 families, 86 children, 82 adults) designed to ensure participant compliance to a maximum use of screen media (≤3 hours per week) for a 2-week period. Families randomly assigned to the control group (44 families, 95 children, 82 adults) were instructed to carry on as usual.
Main Outcomes and Measures
The primary outcome was between-group difference in leisure nonsedentary activity (in minutes per day) measured by combined thigh and waist accelerometry. Secondary outcomes included other physical activity and sleep parameters measured by single-channel electroencephalography.
Results
Among the 89 randomized families (intervention group [45 families]: 86 children; mean [SD] age, 8.6 [2.7] years; 44 boys [51%]; 42 girls [49%]; control group [44 families]: 95 children, mean [SD] age, 9.5 [2.5] years; 38 boys [40%]; 57 girls [60%]), 157 children (87%) had complete data on the primary outcome. Eighty-three children (97%) in the intervention group were compliant to the screen use reduction during the intervention. The mean (SD) change in leisure nonsedentary activity in the intervention group was 44.8 (63.5) minutes per day and in the control group was 1.0 (55.1) minute per day (intention-to-treat between-group mean difference, 45.8 minutes per day; 95% CI, 27.9-63.6 minutes per day; P < .001). No significant between-group mean differences were observed between intervention and control for the electroencephalography-based sleep outcomes.
Conclusions and Relevance
In this cluster randomized clinical trial, a recreational screen media reduction intervention resulted in a substantial increase in children’s engagement in physical activity. The large effect size suggests that the high levels of recreational screen media use seen in many children should be a public health concern.
Trial Registration
ClinicalTrials.gov Identifier: NCT04098913
Introduction
Children spend much of their leisure time engaging with screen media, which continues to raise concerns among health authorities, health professionals, and parents.1,2,3,4 Recent evidence synthesis suggests that high screen media use is associated with a variety of health outcomes, including adiposity and poorer mental health.5 However, the evidence for causal associations between screen use and health outcomes is considered weak, giving rise to controversy and debate over whether high frequency of recreational screen media use is harmful.
Recreational screen use may influence the health and well-being of children by reducing engagement in physical activity and delaying bedtime and disturbing sleep. Screen media devices are often used while being sedentary; thus, time displacement is thought to be the primary mechanism through which screen media use may affect physical activity. Yet, recreational screen media use may also simply replace time otherwise spent being sedentary. Also, children or adults who need less sleep or who have difficulty sleeping may be more likely to have bedtime routines that include screen use. These suggested associations could, therefore, be an artifact of reverse causation or confounding. Trials have investigated the effect of screen media reduction interventions on physical activity in children, with most studies reporting no effect.6,7,8,9,10,11,12,13 However, owing to nonadherence, partial adherence, or unknown adherence to the interventions, it is possible that the estimates of causal effects of previous trials may have been biased. A number of observational studies and randomized trials suggest a relationship between screen media use with sleep duration and earlier bedtime, but there are methodological concerns (eg, use of self-reported sleep duration and nonadherence to intervention) that lower confidence in the results of prior studies.14,15,16
In the present trial, we investigate the outcome of reducing recreational screen media use in families on several physical activity and sleep parameters in children and adults. Our trial design addresses important shortcomings of previous studies by using a screen media reduction intervention that optimizes participant compliance and includes rigorous objective assessments of compliance with the intervention and outcome measures.
Methods
Trial Design
The Short-term Efficacy of Reducing Screen-Based Media Use (SCREENS) trial was a parallel cluster randomized clinical trial.17 The trial was reported in compliance with the Consolidated Standards of Reporting Trials (CONSORT) statement for cluster randomized trials.18 Families (ie, cluster units) were randomly assigned to a screen media reduction intervention or usual screen media use (control) using a 1:1 allocation ratio. A cluster design was used for 2 reasons: (1) family members can encourage each other to maintain adherence and (2) randomly assigning individuals within 1 family to the intervention or control group would increase risk of contamination. Participant enrollment began on June 6, 2019, and follow-up assessments ended March 30, 2021. More information on the trial protocol and analysis plan is available in Supplements 1 and 2, respectively. Ethical approval was obtained from the Ethical Committee of Southern Denmark. Written informed consent was provided by parents on behalf of their children before baseline.
Participants
We recruited families from 10 municipalities in the region of Southern Denmark by sending a digital survey invitation via a Danish electronic secure mail system. The invitation was sent to a randomly selected parent in each household where at least 1 child aged 6 to 10 years resided full time. This sampling frame was obtained from the Danish Civil Registration System via The Danish Health Data Authority. The survey included questions on family screen media habits,19,20 and respondents indicated yes or no if they were interested in taking part in the SCREENS trial at the end of the survey. We assessed eligibility of families among those who answered yes. Eligible families included parents (the responding parent) with self-reported recreational screen use greater than the 40th percentile (>2.4 hours per day) of recreational screen use in the source population, those with no residents in the household younger than 4 years, and those where the parent had full-time employment (with no regular night shifts) or was enrolled in full-time education. After assessment of family eligibility, the responding parent was telephoned to confirm that at least 1 adult and 1 child were willing to participate, and at least 1 participating adult and all participating children were able to hand over their smartphone(s) and tablets if allocated to the intervention group. Participants were excluded if they were unable to engage in regular physical activities during everyday life, diagnosed with a current sleep disorder or any neuropsychiatric or developmental disorders according to self-report, or had been on sick leave owing to stress within the last 3 months. Race and ethnicity information was available for only some of the included children: those who were randomly selected to participate in the SCREENS survey through which we recruited participants. This information was provided by the Danish Health Data Authority. Race and ethnicity were used for descriptive statistics of survey data, not to describe participants in the trial. Ethnicity provided by the Danish Health Data Authority was coded to Statistics Denmark; categories included Danish, non-Western background, and other Western background.
Randomization and Masking
The randomization sequence was generated by a statistical third-party contributor at Odense Patient Data Explorative Network (OPEN) not involved in intervention delivery or data collection. A block randomization (permuted blocks of 2-4 families) was performed using an online randomization platform managed by OPEN. A member of the research team logged in to the randomization platform and performed the randomization in the family’s home. Thus, members of the research team had no knowledge of to which group (intervention or control) the families would be allocated before the randomization took place. The study was open label as it was not possible to blind participants owing to the nature of the intervention.
Interventions
The trial was designed to investigate the efficacy of limiting recreational screen media use on physical activity and sleep, rather than the pragmatic effectiveness of the intervention. Thus, the aim of the intervention was to ensure the family members’ compliance with the intervention. Based on our pilot work,21 a 2-week intervention assured that families had time to adapt to a lifestyle with limited screen media use while still being able to be compliant with the intervention, which is a requirement to obtain unbiased estimates of causal effects.22,23 Families allocated to the control group were instructed to carry on with their lives as usual. All participants allocated to the intervention group had to hand over their portable devices (ie, smartphones, tablets) for the full duration of the intervention. Some adults were not able to do so owing to their jobs, but at least 1 adult had to relinquish their smartphone. Participants received a simple, non–smart phone with their SIM card inserted, allowing them to call and send text messages during the intervention. All participants were instructed to reduce their recreational screen use to 3 hours or less per week during the intervention. Adults were allowed up to 30 minutes per day of necessary screen use for activities such as coordinating appointments. All participants randomly assigned to the intervention group registered their recreational and necessary screen media use during the intervention period in simple diaries. Three to 5 intervention reminder signs were placed in household locations where family members usually gather or engage with screen devices. Finally, families who completed the study received a reimbursement of €70 ($75.50).17
Intervention Compliance
Participants were considered compliant if they had 7 hours or less per week of screen media use during the experiment period. Screen media use was objectively assessed using noncommercial DeviceTracker apps and a television monitor developed in-house and installed by a research team member.24 The apps collected second-to-second screen media use on smartphones, tablets, and computers, whereas the television monitor tracked minute-to-minute television usage by assessing the power cord current (eAppendix and eTable 1 in Supplement 3). The objectively measured screen use and self-registered screen use (only for intervention families) were used to calculate intervention compliance (eAppendix in Supplement 3).
Outcome Assessments
Children and adults wore 2 Axivity AX3 (Axivity Ltd) triaxial accelerometers using elastic belts 24 hours per day through 7 consecutive days at baseline (days −7 to 0 from randomization) and follow-up (days 8-15). One accelerometer was placed at the right side of the waist above the right hip, and the other was placed on the front of the thigh midway between the hip and the knee. Children’s nonsedentary activity (all waking activities other than sitting and lying down) was determined using algorithms by Brønd et al.25 We classified the body postures of sitting, lying down, standing still, and standing with minor movement, and the activity types, including walking, running, and cycling, using a 1-second time resolution. In adults, nonsedentary activity was derived using the method developed by Skotte et al.26 The algorithms classify body postures and activity types with high accuracy in children and adults.25,26 Moderate to vigorous physical activity (MVPA) was derived from ActiGraph counts generated from the waist accelerometer (in 10-second epochs).27,28,29 Details of the physical activity data processing are described in the study protocol (Supplement 1).17
Sleep was assessed at home using single-channel electroencephalography (Zmachine Insight+ model DT-200 [General Sleep Corporation]) during 3 nights at baseline (days −4 to −1 from randomization) and follow-up (days 12-14). At baseline, an additional test night (day −7) was included to familiarize the participants with the equipment. The Zmachine automatically scores sleep data into awake, light sleep (stages N1 and N2), deep sleep (stage N3), and rapid eye movement (REM) sleep, and it has demonstrated high validity when compared with polysomnography.30,31 Participants younger than 6 years did not sleep with the Zmachine because the equipment has not been tested in preschool children. Participants’ instructions are described elsewhere.32 Details on sleep data processing are available in the eAppendix in Supplement 3.
Participants recorded time of awakening and bedtime, as well as time going to and leaving school and/or work in diaries throughout the baseline and follow-up periods. Information from the diaries was subsequently used to time annotate accelerometry and screen media use time-series data into awake vs sleep time, as well as leisure and school or work periods as described in the data management and statistical analysis plan available in Supplement 2.
Outcomes
The primary outcome was the between-group difference in change in children’s leisure nonsedentary activity (in minutes per day) from baseline to 2-week follow-up. Individual mean leisure nonsedentary activity (in minutes per day) was calculated by dividing the total leisure nonsedentary activity by the number of valid days (days with <10% nonwear time during leisure). Participants with at least 3 valid weekdays and 1 valid weekend day were considered compliant with the monitoring protocol.
Secondary outcome measures included a combination of physical activity and sleep parameters in children and adults. Secondary physical activity measures were change in leisure nonsedentary activity (only adults), leisure MVPA, and leisure nonsedentary activity on weekdays and weekend days separately. Sleep measures were change in total sleep duration, sleep onset latency, wake after sleep onset, and sleep stages (ie, light sleep, deep sleep, and REM sleep).
We added post hoc analyses to strengthen the interpretation of our results. These included total nonsedentary activity during awake time, total MVPA, leisure MVPA on weekdays and weekend days separately, accelerometry-derived total sleep duration using a machine learning algorithm (eAppendix in Supplement 3), and self-reported total sleep duration.
Statistical Analysis
As described in the study protocol, assuming an intraclass correlation coefficient of 0.3 for sibling nonsedentary activity and a cluster size of 1.96 children per family, we can detect a minimum difference of 24 minutes per day of leisure nonsedentary activity by including 174 children from 88 families with 80% power and α = .05 (2-sided).17 No interim analyses were performed. We used a linear mixed-effects model to estimate the between-group mean difference at follow-up. We accounted for the correlation owing to clustering of children and parents within families by including family-level random intercepts. Analyses were carried out on the principle of intention-to-treat with adjustment for the baseline value of the respective outcome and age (we judged age of children and adults to be slightly imbalanced between the 2 groups at baseline). Missing values were imputed on summary level of the outcomes using chained equations (eAppendix in Supplement 3).
We conducted a per protocol analysis for the primary outcome where noncompliant children from the intervention group were omitted from the analysis. We conducted prespecified subgroup analyses for the primary outcome by adding an interaction term between group allocation and the subgroup indicator (age group, sex, having a sibling of similar age, season of the intervention, and baseline amounts of nonsedentary activity, amount of leisure time, and amount of shared leisure time in the family) with main outcomes included (statistical analysis plan in Supplement 2). Statistical analyses were conducted using Stata17, version 17 (StataCorp). A 2-sided P value < .05 was considered significant.
Results
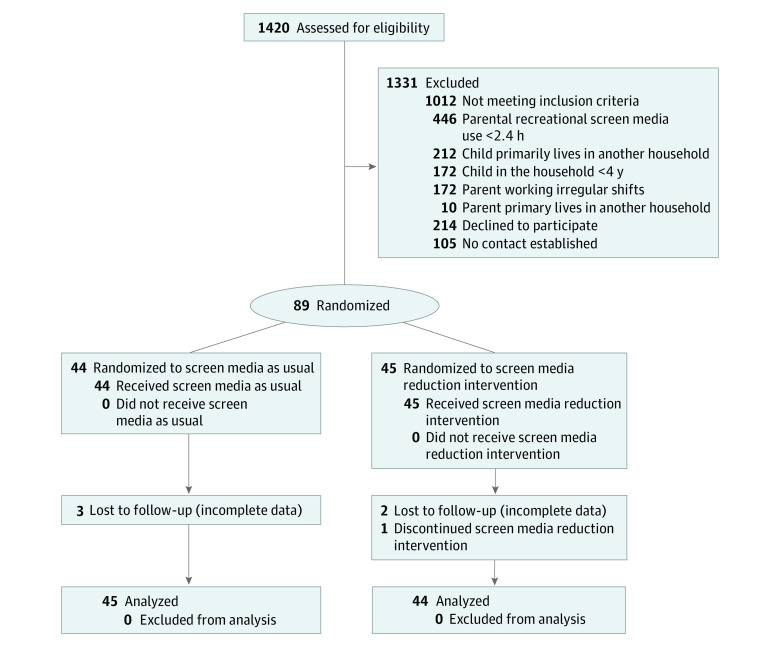
A total of 1420 participants from the population-based survey sample agreed to be contacted about the SCREENS trial. A total of 89 families were randomly assigned: 45 families were allocated to the intervention group (86 children; mean [SD] age, 8.6 [2.7] years; 44 boys [51%]; 42 girls [49%]) and 44 families were allocated to the control group (95 children, mean [SD] age, 9.5 [2.5] years; 38 boys [40%]; 57 girls [60%]) (Figure 1; eFigure 1 in Supplement 3). Most children with information on ethnicity had a Danish background. One family had a child categorized as other Western, and 1 family had a child categorized as other non-Western.
Figure 1. Consolidated Standards of Reporting Trials Flow Diagram.
Flow of families from eligibility assessment invitation to analysis. For more details see eFigure 1 in the Supplement.
Baseline characteristics were generally similar between groups (Table). However, children and adults in the intervention group appeared slightly younger. Characteristics of study participants were similar to those of eligible nonparticipants (eTable 2 in Supplement 3), and baseline characteristics for children with complete data vs incomplete data for the primary outcome were similar (eTable 3 in Supplement 3).
Table. Baseline Characteristics.
| Variable | Median (IQR) | |
|---|---|---|
| Control (n = 44 families) | Intervention (n = 45 families) | |
| Children | ||
| No. | 95 | 86 |
| Age, mean (SD), y | 9.5 (2.5) | 8.6 (2.7) |
| Sex, No. (%) | ||
| Female | 57 (60) | 42 (49) |
| Male | 38 (40) | 44 (51) |
| Height, mean (SD), cm | 144.6 (16.6) | 137.8 (17.4) |
| Body weight, mean (SD), kg | 37.0 (12.6) | 33.3 (12.5) |
| BMI,a mean (SD), z score | 0.1 (1.2) | 0.2 (1.0) |
| Recreational smartphone/tablet use, h/wk | 9.6 (3.1-15.9) | 10.4 (3.6-19.7) |
| Recreational computer use, h/wk | 10.1 (6.0-19.7) | 9.4 (2.1-27.0) |
| Recreational television use, h/wk | 4.7 (1.5-10.2) | 6.2 (2.4-9.6) |
| Adults | ||
| No. | 82 | 82 |
| Age, mean (SD), y | 42.4 (4.8) | 40.2 (5.5) |
| Sex, No. (%) | ||
| Female | 44 (54) | 44 (54) |
| Male | 38 (46) | 38 (46) |
| Height, mean (SD), cm | 176.0 (9.6) | 175.0 (8.5) |
| Weight, mean (SD), kg | 80.0 (14.4) | 80.4 (13.5) |
| BMI, mean (SD) | 25.8 (4.0) | 26.2 (3.5) |
| Educational attainment, ISCED, No. (%) | ||
| 0-3 | 16 (19) | 14 (17) |
| 4-6 | 40 (49) | 48 (59) |
| 7-8 | 26 (32) | 20 (24) |
| Recreational smartphone/tablet use, h/wk | 13.8 (8.0-19.8) | 14.6 (8.8-20.1) |
| Recreational computer use, h/wk | 2.5 (0.7-9.5) | 1.2 (0.2-6.3) |
| Recreational television use, h/wk | 8.8 (4.8-14.5) | 8.0 (2.8-13.4) |
| Family environment | ||
| Children in the household, No. of children | 2.0 (2.0-3.0) | 2.0 (2.0-2.0) |
| Adults in the household, No. of adults | 2.0 (2.0-2.0) | 2.0 (2.0-2.0) |
| Residual recreational television use, h/wkb | 2.5 (0.5-7.7) | 0.9 (0.0-5.5) |
| Family recreational screen media use, h/wk | 96.0 (69.6-129.1) | 80.3 (56.7-104.9) |
| Household screen media devices, No. of devices | 11.0 (8.0-12.0) | 9.0 (7.0-11.0) |
Abbreviations: BMI, body mass index; ISCED, International Standard Classification of Education.
Calculated as weight in kilograms divided by height in meters squared.
Residual recreational television use is all television use that could not be assigned to any family members.
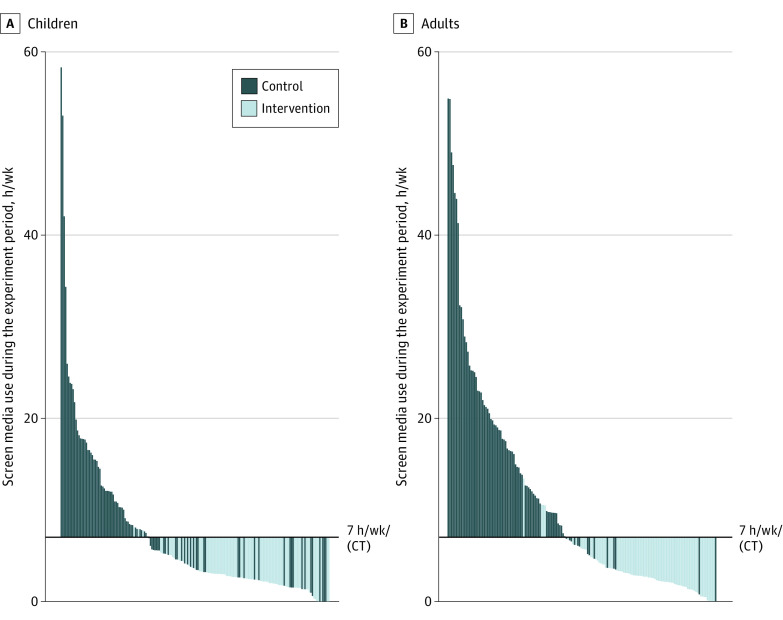
Family-level compliance with the intervention was 96% (43 of 45 families). On an individual level, 83 of 86 children (97%) and 78 of 82 adults (95%) in the intervention group did not exceed the compliance threshold of 7 or less hours per week per person of recreational screen media use (Figure 2). All adults handed over their smartphones in 24 of 45 of the intervention families (53%). Details about compliance and screen media availability during the intervention are provided in eTable 4 in Supplement 3.
Figure 2. Individual-Level Compliance With the Intervention.
Mean recreational screen media use (television, tablet, smartphone, computer) per child (A) and adult (B) during the experiment plotted against the compliance threshold (CT) of 7 hours per week per person. Baseline television watching was used for control group participants because they did not complete screen media diaries during the experiment, which permitted individual assignment of television use during the experiment.
Primary End Points
A total of 157 children (87%) had complete data on the primary outcome. The mean (SD) within-group changes for children’s nonsedentary activity in the control group were 1.0 (55.1) minutes per day and in the intervention group were 44.8 (63.5) minutes per day. The intention-to-treat analysis for between-group change in children’s leisure nonsedentary activity (n = 181) showed a significant between-group difference of 45.8 minutes per day (95% CI, 27.9-63.6 minutes per day; P < .001) in favor of the intervention (Figure 3; eFigure 2A, eTable 5 in Supplement 3). The complete case analysis (n = 157) also showed a significant between-group difference of 46.5 minutes per day (95% CI, 29.5-63.6 minutes per day) in children’s leisure nonsedentary time in favor of intervention. This corresponded to a 30% (95% CI, 20-39 minutes per day) mean difference in nonsedentary activity during leisure. The per-protocol analysis (n = 155) of children’s leisure nonsedentary time yielded similar results (group mean difference, 44.6 minutes per day; 95% CI, 27.1-62.1 minutes per day).
Figure 3. Physical Activity Outcomes at 2-Week Follow-up.

Between-group mean differences in physical activity outcomes (β coefficients with 95% CI) for children (A) and adults (B). Analyses were adjusted for age, the baseline value of the outcome, and included family-level random intercepts. The primary outcome of the SCREENS trial is the total leisure time spent nonsedentary. Full information is available in eTable 5 in the Supplement. MVPA indicates moderate to vigorous physical activity.
The subgroup analyses for the primary outcome are presented in eFigure 3 in Supplement 3. The subgroup analyses revealed significant interactions between the group allocation and baseline amount of nonsedentary time (below and above median) and having close siblings (within 3 years of age). The size of the intervention effect was larger in children with lower levels of baseline nonsedentary activity and in children with no close siblings. No correction for false discovery rate was made and findings from subgroup analyses should be interpreted as exploratory.
Secondary End Points
The intervention significantly increased children’s leisure MVPA in favor of the screen reduction group. Likewise, significant between-group differences were found for children’s weekday and weekend day leisure nonsedentary time in favor of the intervention with larger between-group differences in change on weekend days compared with weekdays (weekday nonsedentary time during leisure, 33.8 minutes per day; 95% CI, 16.2-51.5; weekend day nonsedentary time, 73.4 minutes per day; 95% CI, 40.8-106.1) (Figure 3; eTable 5 in Supplement 3). We observed no significant effects on leisure nonsedentary time, leisure MVPA per day, or leisure nonsedentary time per weekday or weekend day in adults (Figure 3; eFigure 2B, eTable 5 in Supplement 3).
The intervention had no significant effect on total sleep duration, sleep onset latency, and wake after sleep onset in children and adults (Figure 4; eTable 6 in Supplement 3). Similarly, no significant between-group differences were observed for changes in proportions of light (stages N1 and N2), deep (stage N3), and REM sleep in children or adults (eTable 6 and eFigure 4 in Supplement 3).
Figure 4. Sleep Duration, Sleep Onset Latency, and Wake After Sleep Onset at 2-Week Follow-up.
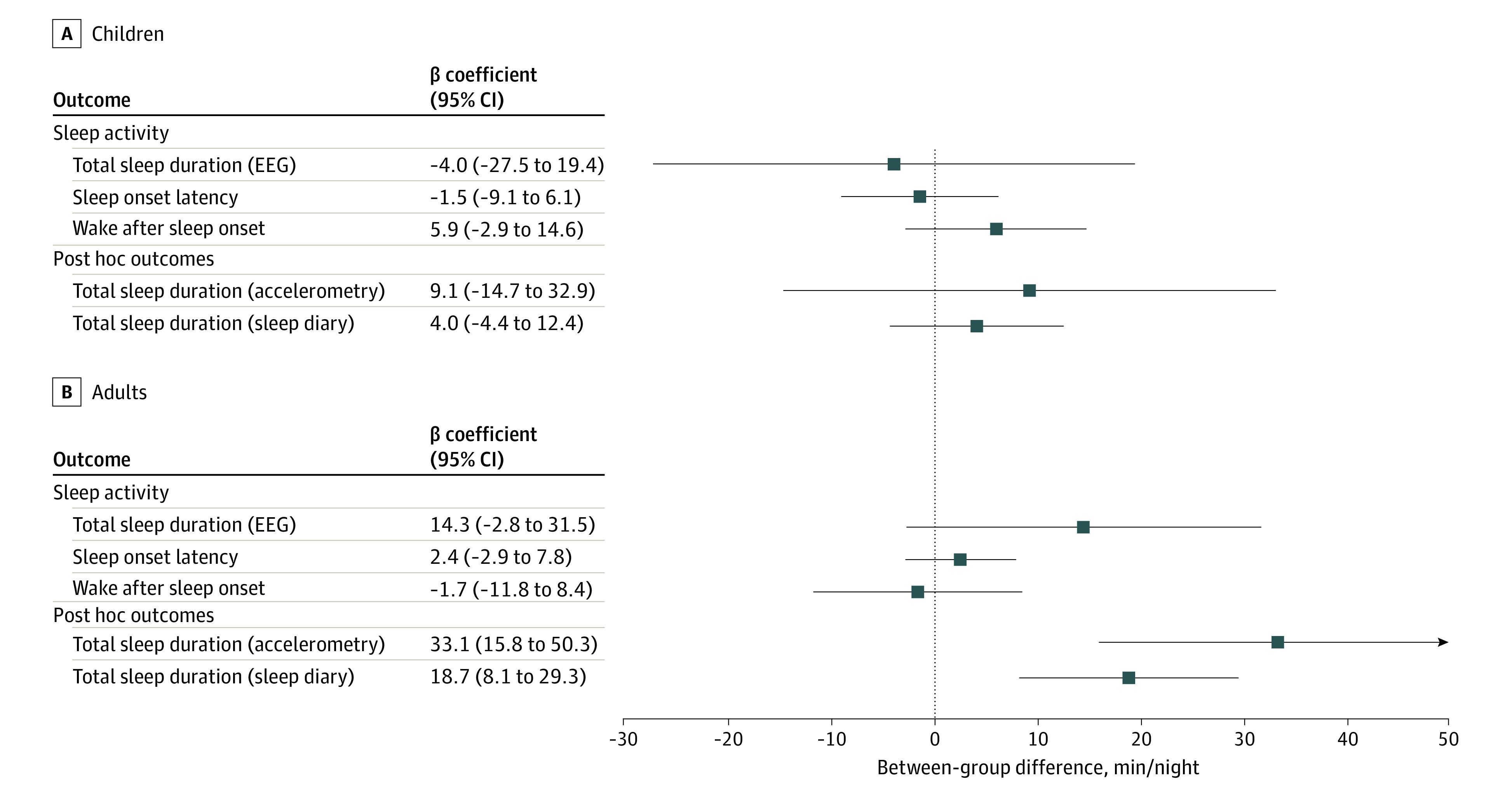
Between-group mean differences in sleep outcomes (β coefficients with 95% CI) for children (A) and adults (B). All analyses were adjusted for age, the baseline value of the outcome, and included family-level random intercepts. Full information is available in eTable 6 in the Supplement.
Post Hoc Analyses
Post hoc analyses on total nonsedentary activity, total MVPA, and weekday and weekend day MVPA showed significant increases in children (total nonsedentary activity, 46.3 minutes per day; 95% CI, 22.3-70.4; total MVPA, 8.8 minutes per day; 95% CI, 3.6-13.9; weekday MVPA, 4.4 minutes per day; 95% CI, 0-8.7; weekend day MVPA, 16.9 minutes per day; 95% CI, 9.2-24.7), but not in adults (Figure 3; eTable 5 in Supplement 3). Finally, post hoc analyses on accelerometry-derived total sleep duration and self-reported sleep duration showed no significant effects of the intervention in children, whereas significant increases in sleep duration were observed in adults (Figure 4; eTable 6 in Supplement 3).
Discussion
In this population-based randomized clinical trial of families with children, an intervention limiting recreational screen media use resulted in a substantial increase in children’s daily time spent being nonsedentary during leisure as measured by combined thigh and waist accelerometry. The intervention also increased children’s objectively measured MVPA, whereas no differences were found on sleep parameters. The intervention did not improve any physical activity or electroencephalography-based sleep parameters in adults.
Children who took part in this study had a mean of 35.9 hours per week (just over 5 hours per day) of screen media use at baseline, which is somewhat less of that recently reported among US children aged 12 to 13 years.33 With an overall between-group mean difference of 46 minutes per day (73 minutes per day on weekend days) of nonsedentary activity, the apparent causal effects of recreational screen media use on physical activity in children appear large enough to warrant high levels of screen time as a public health problem causing physical inactivity in children.
The absence of an effect in adults suggests that adults predominantly replaced time usually spend on screen media with other types of nonscreen-based sedentary activities. We observed that all physical activity outcomes were consistent in direction of a positive effect in adults; therefore, it is possible that effects are smaller in adults and that our study was underpowered to detect these.
The results for sleep parameters in children are not consistent with evidence from a systematic review of trials targeting screen media use (often in combination with co-interventions),16 but all studies were deemed at risk of bias in multiple domains.16 We are unaware of any trials investigating screen media restriction in the home environment on adult’s sleep. A few laboratory-based trials have investigated light exposure from screens before bedtime and reported that it suppressed sleepiness and prolonged sleep latency.34,35 A study also reported that short-wavelength (blue) light affected sleep architecture and shortened sleep duration.36 The effects that we found on sleep duration in adults based on self-report and accelerometry in adults warrant further investigation.
Strengths and Limitations
The strengths of this trial include the use of combined waist and thigh accelerometry to assess the primary outcome (which allows for accurate classification of sedentary and nonsedentary activity), minimal dropout rate, and objective verification of compliance to the intervention. In addition, families recruited into our study were from a well-defined source population, with a similar distribution of sociodemographic factors (eTable 2 in Supplement 3), which suggests wider generalizability.
The results should be interpreted with the following limitations in mind. First, blinding of participants was not possible owing to the nature of the intervention. Although we documented successful participant compliance in the intervention group, the participants allocated to the control group appeared to have reduced their screen use to some extent during the 2-week experiment period, which is likely explained by some children and parents in the control group being motivated to change screen use and which may have caused an underestimation of the effect estimates. Second, the sleep assessment protocol may have been inadequate to achieve sufficient reliability of sleep parameters, and analyses may, therefore, have been underpowered.32 Finally, the findings in adults may not generalize to adults living without children and those being of older age.
Conclusions
In conclusion, results of this randomized clinical trial showed that limiting recreational screen media use substantially increased children’s physical activity. The large effect suggests that the high levels of recreational screen media use seen in many children should be a public health concern and emphasizes the importance of developing and implementing measures to balance recreational screen media use to prevent physical inactivity in children.
Trial Protocol
Statistical Analysis Plan
eAppendix.
eTable 1. Individual Assignment of TV Monitor Data
eTable 2. Participants vs Nonparticipants
eTable 3. Dropouts/Missing Data in the Primary Analysis
eTable 4. Available Screen Media Devices and Tracking
eTable 5. Physical Activity Outcomes at 2-Week Follow-up
eTable 6. Sleep Outcomes at 2-Week Follow-up
eFigure 1. Detailed Flow Chart
eFigure 2. Individual Change in Leisure Nonsendentary Time at 2-Week Follow-up
eFigure 3. Subgroup Analyses: Children’s Leisure Nonsendentary Time at 2-Week Follow-up
eFigure 4. Sleep Stages at 2-Week Follow-up
eReferences
Data Sharing Statement
References
- 1.Ashton JJ, Beattie RM. Screen time in children and adolescents: is there evidence to guide parents and policy? Lancet Child Adolesc Health. 2019;3(5):292-294. doi: 10.1016/S2352-4642(19)30062-8 [DOI] [PubMed] [Google Scholar]
- 2.Social media, screen time, and young people’s mental health. Lancet. 2019;393(10172):611. doi: 10.1016/S0140-6736(19)30358-7 [DOI] [PubMed] [Google Scholar]
- 3.Royal College of Paediatrics and Child Health. The health impacts of screen time—a guide for clinicians and parents. Accessed December 10, 2021. https://www.rcpch.ac.uk/resources/health-impacts-screen-time-guide-clinicians-parents
- 4.World Health Organization. WHO guidelines on physical activity and sedentary behaviour. November 25, 2020. Accessed December 10, 2021. https://apps.who.int/iris/rest/bitstreams/1315866/retrieve [PubMed]
- 5.Stiglic N, Viner RM. Effects of screen time on the health and well-being of children and adolescents: a systematic review of reviews. BMJ Open. 2019;9(1):e023191. doi: 10.1136/bmjopen-2018-023191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ni Mhurchu C, Roberts V, Maddison R, et al. Effect of electronic time monitors on children’s television watching: pilot trial of a home-based intervention. Prev Med. 2009;49(5):413-417. doi: 10.1016/j.ypmed.2009.09.003 [DOI] [PubMed] [Google Scholar]
- 7.Epstein LH, Roemmich JN, Robinson JL, et al. A randomized trial of the effects of reducing television viewing and computer use on body mass index in young children. Arch Pediatr Adolesc Med. 2008;162(3):239-245. doi: 10.1001/archpediatrics.2007.45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Todd MK, Reis-Bergan MJ, Sidman CL, et al. Effect of a family-based intervention on electronic media use and body composition among boys aged 8–11 years: a pilot study. J Child Health Care. 2008;12(4):344-358. doi: 10.1177/1367493508097404 [DOI] [PubMed] [Google Scholar]
- 9.Ford BS, McDonald TE, Owens AS, Robinson TN. Primary care interventions to reduce television viewing in African American children. Am J Prev Med. 2002;22(2):106-109. doi: 10.1016/S0749-3797(01)00410-X [DOI] [PubMed] [Google Scholar]
- 10.Hinkley T, Cliff DP, Okely AD. Reducing electronic media use in 2- to 3-year-old children: feasibility and efficacy of the Family@play pilot randomised controlled trial. BMC Public Health. 2015;15(1):779. doi: 10.1186/s12889-015-2126-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mendoza JA, Baranowski T, Jaramillo S, et al. Fit 5 kids TV reduction program for Latino preschoolers: a cluster randomized controlled trial. Am J Prev Med. 2016;50(5):584-592. doi: 10.1016/j.amepre.2015.09.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Maddison R, Marsh S, Foley L, et al. Screen Time Weight-Loss Intervention Targeting Children at Home (SWITCH): a randomized controlled trial. Int J Behav Nutr Phys Act. 2014;11(1):111. doi: 10.1186/s12966-014-0111-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Babic MJ, Smith JJ, Morgan PJ, et al. Intervention to reduce recreational screen time in adolescents: outcomes and mediators from the Switch-Off 4 Healthy Minds (S4HM) cluster randomized controlled trial. Prev Med. 2016;91:50-57. doi: 10.1016/j.ypmed.2016.07.014 [DOI] [PubMed] [Google Scholar]
- 14.Hale L, Guan S. Screen time and sleep among school-aged children and adolescents: a systematic literature review. Sleep Med Rev. 2015;21:50-58. doi: 10.1016/j.smrv.2014.07.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carter B, Rees P, Hale L, Bhattacharjee D, Paradkar MS. Association between portable screen-based media device access or use and sleep outcomes: a systematic review and meta-analysis. JAMA Pediatr. 2016;170(12):1202-1208. doi: 10.1001/jamapediatrics.2016.2341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Martin KB, Bednarz JM, Aromataris EC. Interventions to control children’s screen use and their effect on sleep: a systematic review and meta-analysis. J Sleep Res. 2021;30(3):e13130. doi: 10.1111/jsr.13130 [DOI] [PubMed] [Google Scholar]
- 17.Rasmussen MGB, Pedersen J, Olesen LG, et al. Short-term efficacy of reducing screen media use on physical activity, sleep, and physiological stress in families with children aged 4-14: study protocol for the SCREENS randomized controlled trial. BMC Public Health. 2020;20(1):380. doi: 10.1186/s12889-020-8458-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Campbell MK, Piaggio G, Elbourne DR, Altman DG; CONSORT Group . Consort 2010 statement: extension to cluster randomised trials. BMJ. 2012;345:e5661. doi: 10.1136/bmj.e5661 [DOI] [PubMed] [Google Scholar]
- 19.Klakk H, Wester CT, Olesen LG, et al. The development of a questionnaire to assess leisure time screen-based media use and its proximal correlates in children (SCREENS-Q). BMC Public Health. 2020;20(1):664. doi: 10.1186/s12889-020-08810-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pedersen J, Rasmussen MG, Olesen LG, Klakk H, Kristensen PL, Grøntved A. Recreational screen media use in Danish school-aged children and the role of parental education, family structures, and household screen media rules. Prev Med. 2022;155:106908. doi: 10.1016/j.ypmed.2021.106908 [DOI] [PubMed] [Google Scholar]
- 21.Rasmussen MGB, Pedersen J, Olesen LG, Kristensen PL, Brønd JC, Grøntved A. Feasibility of 2 screen media reduction interventions: results from the SCREENS pilot trial. PLoS One. 2021;16(11):e0259657. doi: 10.1371/journal.pone.0259657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.DeMets DL, Cook T. Challenges of nonintention-to-treat analyses. JAMA. 2019;321(2):145-146. doi: 10.1001/jama.2018.19192 [DOI] [PubMed] [Google Scholar]
- 23.Hernán MA, Robins JM. Causal Inference: What If. Chapman & Hall/CRC; 2020. [Google Scholar]
- 24.Kristensen PL, Olesen LG, Egebæk HK, Pedersen J, Rasmussen MG, Grøntved A. Criterion validity of a research-based application for tracking screen time on android and iOS smartphones and tablets. Comput Hum Behav. 2021;5:100164. doi: 10.1016/j.chbr.2021.100164 [DOI] [Google Scholar]
- 25.Brønd JC, Grøntved A, Andersen LB, Arvidsson D, Olesen LG. Simple method for the objective activity type assessment with preschoolers, children, and adolescents. Children (Basel). 2020;7(7):72. doi: 10.3390/children7070072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Skotte J, Korshøj M, Kristiansen J, Hanisch C, Holtermann A. Detection of physical activity types using triaxial accelerometers. J Phys Act Health. 2014;11(1):76-84. doi: 10.1123/jpah.2011-0347 [DOI] [PubMed] [Google Scholar]
- 27.Brønd JC, Andersen LB, Arvidsson D. Generating ActiGraph counts from raw acceleration recorded by an alternative monitor. Med Sci Sports Exerc. 2017;49(11):2351-2360. doi: 10.1249/MSS.0000000000001344 [DOI] [PubMed] [Google Scholar]
- 28.Petersen TL, Brønd JC, Kristensen PL, Aadland E, Grøntved A, Jepsen R. Resemblance in accelerometer-assessed physical activity in families with children: the Lolland-Falster Health Study. Int J Behav Nutr Phys Act. 2020;17(1):161. doi: 10.1186/s12966-020-01067-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brønd JC, Aadland E, Andersen LB, Resaland GK, Andersen SA, Arvidsson D. The ActiGraph counts processing and the assessment of vigorous activity. Clin Physiol Funct Imaging. 2019;39(4):276-283. doi: 10.1111/cpf.12571 [DOI] [PubMed] [Google Scholar]
- 30.Wang Y, Loparo KA, Kelly MR, Kaplan RF. Evaluation of an automated single-channel sleep staging algorithm. Nat Sci Sleep. 2015;7:101-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kaplan RF, Wang Y, Loparo KA, Kelly MR, Bootzin RR. Performance evaluation of an automated single-channel sleep-wake detection algorithm. Nat Sci Sleep. 2014;6:113-122. doi: 10.2147/NSS.S71159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pedersen J, Rasmussen MGB, Olesen LG, Kristensen PL, Grøntved A. Self-administered electroencephalography-based sleep assessment: compliance and perceived feasibility in children and adults. Sleep Sci Pract. 2021;5(1):8. doi: 10.1186/s41606-021-00059-1 [DOI] [Google Scholar]
- 33.Nagata JM, Cortez CA, Cattle CJ, et al. Screen time use among US adolescents during the COVID-19 pandemic: findings from the Adolescent Brain Cognitive Development (ABCD) study. JAMA Pediatr. 2022;176(1):94-96. doi: 10.1001/jamapediatrics.2021.4334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chang A-M, Aeschbach D, Duffy JF, Czeisler CA. Evening use of light-emitting eReaders negatively affects sleep, circadian timing, and next-morning alertness. Proc Natl Acad Sci U S A. 2015;112(4):1232-1237. doi: 10.1073/pnas.1418490112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Grønli J, Byrkjedal IK, Bjorvatn B, Nødtvedt Ø, Hamre B, Pallesen S. Reading from an iPad or from a book in bed—the impact on human sleep: a randomized controlled crossover trial. Sleep Med. 2016;21:86-92. doi: 10.1016/j.sleep.2016.02.006 [DOI] [PubMed] [Google Scholar]
- 36.Green A, Cohen-Zion M, Haim A, Dagan Y. Evening light exposure to computer screens disrupts human sleep, biological rhythms, and attention abilities. Chronobiol Int. 2017;34(7):855-865. doi: 10.1080/07420528.2017.1324878 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
Statistical Analysis Plan
eAppendix.
eTable 1. Individual Assignment of TV Monitor Data
eTable 2. Participants vs Nonparticipants
eTable 3. Dropouts/Missing Data in the Primary Analysis
eTable 4. Available Screen Media Devices and Tracking
eTable 5. Physical Activity Outcomes at 2-Week Follow-up
eTable 6. Sleep Outcomes at 2-Week Follow-up
eFigure 1. Detailed Flow Chart
eFigure 2. Individual Change in Leisure Nonsendentary Time at 2-Week Follow-up
eFigure 3. Subgroup Analyses: Children’s Leisure Nonsendentary Time at 2-Week Follow-up
eFigure 4. Sleep Stages at 2-Week Follow-up
eReferences
Data Sharing Statement