Abstract
Alzheimer’s disease is a progressive and deadly neurodegenerative disorder and one of the most common causes of dementia globally. Current, insufficiently sensitive and specific methods of early diagnosing and monitoring this disease prompt a search for new tools. Numerous literature data have indicated that the pathogenesis of Alzheimer’s disease (AD) is not limited to the neuronal compartment but involves various immunological mechanisms. Neuroinflammation has been recognized as a very important process in AD pathology. It seems to play pleiotropic roles, both neuroprotective and neurodegenerative, in the development of cognitive impairment depending on the stage of the disease. Mounting evidence demonstrates that inflammatory proteins could be considered biomarkers of disease progression. Therefore, the present review summarizes the role of some inflammatory molecules and their potential utility in detecting and monitoring dementia severity. This paper also provides a valuable insight into new mechanisms leading to the development of dementia, which might be useful in discovering possible anti-inflammatory treatment.
Keywords: Neuroinflammation, Alzheimer’s disease, dementia disorders, biomarkers, neurodegeneration, chemokines, interleukins
1. INTRODUCTION
Alzheimer’s disease (AD) is the most common cause of dementia in the world. It is an untreatable and progressive neurodegenerative disease. AD develops mainly in individuals over the age of 65. Every year, we observe an increasing number of new cases correlated with the aging society [1]. It is estimated that 50 million people worldwide suffer from dementia, and this number will triple by 2050 [2]. The highest number of cases are reported in North America and Western Europe. In the USA, AD is the sixth leading cause of death [3]. The disorder imposes an enormous burden not only on affected individuals but also on their relatives, caregivers, and healthcare systems. Despite significant advances in the diagnosis of neurodegenerative disorders, there are still no specific biomarkers that reflect the molecular mechanism underlying AD. Therefore, it is crucial to find new biomarkers that can help detect AD earlier in the course of the disease and potentially contribute to the discovery of treatment.
AD is a chronic disorder in which pathological processes begin at least 20 years prior to disease onset [3]. The etiology of AD is heterogeneous and not fully understood. It is assumed that genetic and environmental factors may play a role in the pathogenesis of the disease. However, genetic cases constitute less than 5% of AD cases overall, with several gene mutations associated with the disease. In early-onset
Alzheimer's disease (EOAD), we can identify mutations in three genes: presenilin 1, presenilin 2, and amyloid precursor protein (APP) [4], whereas sporadic, late-onset AD (LOAD) is associated with the APOE ε4 gene [5]. The histopathological hallmarks of AD include the gradual formation of extracellular plaques composed of amyloid β and neurofibrillary tangles (NFTs) formed by hyperphosphorylated tau. They cause neuronal and synaptic loss [6]. The most toxic molecules involved in AD pathology are insoluble peptides of amyloid-beta 1-42 (Aβ-42), which can be recognized as unfamiliar by the immune system, triggering the inflammatory response. They are formed by proteolytic processing of amyloid precursor protein by several secretases [7]. The accumulation and aggregation of Aβ-42 initiate a cascade of pathological processes, including neuroinflammation, which leads to CNS (Central Nervous System) cell damage [8, 9]. However, several mechanisms are involved in Aβ removal from the brain, such as transport through the blood-brain barrier (BBB), microglia, enzymatic degradation, and retention by CSF into the circulatory and lymphatic system [10]. Disturbances in these processes can result in increased amyloid accumulation. Amyloid-beta may also be implicated in tau hyperphosphorylation by modulating the activity of kinases or phosphatases.
Another hallmark of AD is neurofibrillary tangles connected with hyperphosphorylated tau [11]. Tau is a protein responsible for stabilizing microtubules in neurons. A decline in tau attachment to microtubules results in synaptic dysfunction. Moreover, tau is a subject of many post-translational modifications, such as acetylation, glycation, nitration, and phosphorylation, particularly important in AD pathology [12]. The imbalance between tau kinase and phosphatase activity results in increased tau phosphorylation and intracellular tau aggregation, leading to the creation of NFTs in AD patients. Finally, formed NFTs cause the weakening of synaptic plasticity [13, 14], leading to the damage of neuronal cells.
In the current review, we summarize recent studies investigating the role of various inflammatory molecules in AD pathology. Furthermore, advances in understanding potentially novel mechanisms leading to AD and key genetic polymorphisms of inflammatory molecules associated with enhanced AD risk are described.
2. MECHANISMS OF NEUROINFLAMMATION
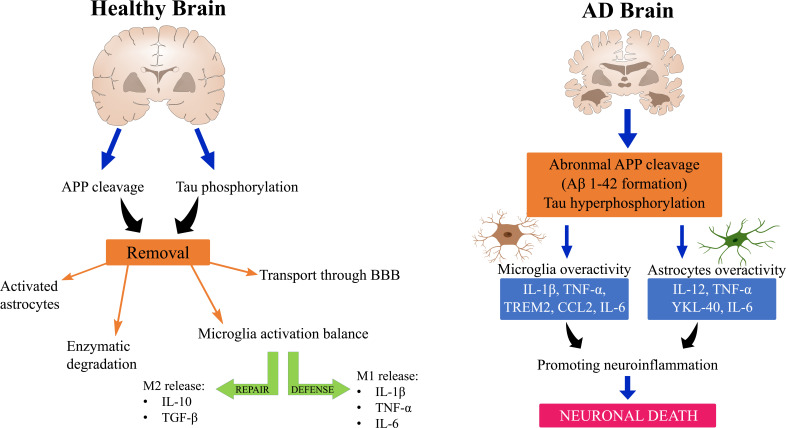
Mechanisms underlying AD neuroinflammation are still not fully understood, although they have been investigated for over 20 years. Numerous genetic and immunological analyses have shown a significant connection between inflammation and AD pathology (Fig. 1) [15].
Fig. (1).
The mechanism of neuroinflammation in healthy and AD brains. (A higher resolution / colour version of this figure is available in the electronic copy of the article).
Krstic et al. presented a new perspective on inflammation related to late-onset AD, which illustrates the evolution of AD pathology. According to Krstic, a healthy brain reacts with elevated APP synthesis in normal conditions following exposure to inflammation. This results in the aggregation of its products. Moreover, in LOAD, tau hyperphosphorylation and mislocalization are observed. Typically, microglia and astrocytes are responsible for eliminating unnecessary aggregates [16, 17]. Pathological processes in the brain may lead to microglia overactivity and enhanced synthesis of pro-inflammatory cytokines without protecting normal microglia. Consequently, neurons are more prone to neurotoxic degradation and formation of plaques, thus increasing the pro-inflammatory response [17]. It is suggested that one of the mechanisms of neuroinflammation may be related to the presence of antigen CD14 on microglia and LPS (Lipopolysaccharide), which coordinate with toll-like receptor 4 (TLR-4). This receptor is an important signaling protein capable of inducing microglial activation, which initiates the production of proinflammatory cytokines [17]. Furthermore, LPS may be associated with enhanced β- and γ-secretase activity, resulting in increased accumulation of Aβ1-42. These relationships indicate a link between amyloidogenesis and neuroinflammation, although exact mechanisms behind this process are still not fully known [18, 19]. As mentioned above, the key contributors of the neuroinflammatory reaction are microglia and astrocytes, which, when activated, release inflammatory molecules, such as cytokines and chemokines, and regulate the intensity and duration of the immune response.
Microglia are macrophages of brain tissue that play a primarily protective function [20]. During brain development, they are responsible for eliminating the surplus of synaptic connections, while in a fully-developed, adult brain, they engage in synaptic plasticity regulation and remodeling of neuronal circuits [21]. Microglia can be transformed into their activated form by a number of triggers, such as injury, pro-inflammatory cytokines, or loss of ion homeostasis. In this state, microglia are capable of releasing cytotoxic factors, free radicals, and proinflammatory cytokines, such as TNF-α [22]. Two activation states are generally distinguished: M1: classical activation state and M2: alternative phenotype. Lipopolysaccharide (LPS), IFN-γ, or TNF-α result in classical activation, which is involved in defense mechanisms against pathogens by secreting pro-inflammatory factors, such as IL-1β, TNF-α, IL-6, and reactive oxygen species [23]. On the contrary, the M2 phenotype is induced by IL-4 and IL-13, resulting in the release of neuroprotective factors, e.g., TGF-β, IL-10, and IGF-1 [23]. Through balancing inflammation, M2 microglia are capable of improving the remodeling and repairing of brain tissue. The M1-to-M2 switch can be very rapid [24, 25]. On the other hand, microglia are also implicated in the development of some pathological states. They are involved in binding to Aβ plaques via several receptors, such as CD14, CD36, and toll-like receptors, TLR4 and TLR2, which activate microglia and stimulate the enhanced release of proinflammatory factors by microglia [15, 26]. Moreover, the activated microglia around senile plaques, which generate the proinflammatory environment, promote senile plaque formation [27, 28].
Similar to microglia, astrocytes can also be activated, which are expressed by increased release of glial fibrillary acidic protein (GFAP). Despite their neuroprotective function, they can also create an inflammatory environment by releasing a number of proinflammatory cytokines, such as TNF-α, IL-6, or IL-12 [29, 30]. It is suggested that astrocytes may accumulate in close proximity to plaques and amyloid β, thus promoting astrocytes activation [31]. Studies have confirmed the presence of activated astrocytes in the brains of animal models and AD patients [29, 32]. There are possibly a few mechanisms of astrocyte involvement in neuroinflammation. One of them may be astrocyte activation by NFκB releasing complement protein C3, which binds neuronal C3aR. NFκB controls neuronal function and durability, thus proving the role of astroglia in neuronal damage [33, 34].
Neutrophils are white blood cells that act at the first line of early innate immunity throughout phagocytosis, releasing neutrophil extracellular traps (NETs) and producing reactive oxygen species [35]. Studies have revealed abnormal reactions of neutrophils in AD [36]. Research conducted on mouse models of AD has demonstrated the presence of neutrophils near amyloid plaques and the overactivity of neutrophils caused by amyloid. Moreover, inhibiting neutrophils through blocking LFA-1 integrin in the early stages of dementia improved memory in mice [37]. These discoveries may suggest that the overactivity of neutrophils has a detrimental effect on the brain in AD. Stock et al. revealed that neutrophil granulate proteins might act as either neuroprotective or neurotoxic molecules. Considering their protective functions, CAP37, cathepsin G, and neutrophil elastase may help in Aβ cleavage, resulting in plaque removal [38].
3. RELATIONSHIP BETWEEN THE GUT MICROBIOTA AND AD
A new perspective on neuroinflammation is offered by exploring the connection between gut microbiota and the nervous system. The so-called “brain-gut axis” is discussed in a large number of new studies exploring its contribution to AD pathology [39]. Research on rodent AD models and AD patients has demonstrated different compositions of the gut microbiota in AD patients in comparison to healthy controls, which is associated with the loss of epithelial barrier integrity and chronic systemic and intestinal inflammation [40, 41]. Moreover, the gut microbiota synthesizes LPS, bacterial amyloid, and other toxins, which can alter physiological barriers and can be associated with systemic inflammation. Despite differences in structure between CNS and gut amyloid, the latter can also cause enhanced immune responses, resulting in neuronal amyloid aggregation [39, 42, 43]. Furthermore, LPS release from the gut microbiota, in particular, can result in microglial activation, thus disturbing amyloid removal and neurotoxicity [39]. A study by Kim et al. demonstrated that transplanting healthy mice microbiota into AD models alleviated glial activation and formation of Aβ plaques and NFTs [40]. Furthermore, the abundant presence of several bacterial, pro-inflammatory taxa, belonging to Escherichia/Shigella, positively correlated with elevated blood levels of pro-inflammatory cytokines, such as cytokines IL-1β, NLRP3, and CXCL2 in amyloid-positive patients, and negatively correlated with anti-inflammatory E.rectale [44]. Interestingly, probiotic treatment resulted in a reduction in the levels of IL1α, IL1β, IL2, IL12, IFNγ, and TNFα, and an increase in the levels of IL4 and IL6, which are anti-inflammatory cytokines [45].
4. POTENTIAL DIAGNOSTIC USEFULNESS OF INFLAMMATORY PROTEINS IN AD PATHOLOGY
Inflammation plays a pivotal role in the development and progression of AD. Comprehensive brain imaging studies have supported the relationship between potentiated neuroinflammation and progression of MCI (Mild Cognitive Impairment) and AD [46]. However, little is known about inflammation during the early stages of cognitive decline or whether it differs in different neurodegenerative diseases. Identifying inflammatory profiles in the cerebrospinal fluid (CSF) and blood of patients may help find novel biomarkers reflecting AD pathology and explore possible therapeutic targets.
Many proteomic studies have analyzed the direction of changes, significance, and diagnostic usefulness of specific inflammatory biomarkers in the pathology and progression of AD [47]. CSF levels of selected inflammatory proteins allow for the discrimination of patients with dementia (MCI and AD) from subjects without cognitive decline. Furthermore, CSF levels can be associated with amyloid and/or tau pathology. A recent study has revealed that although the correlations between neuropsychological performance and inflammatory markers were weak, they were most apparent in AD and cognitive tests [48]. It is indicated that a panel of specific proteins reflecting different pathological mechanisms could be a valuable and potent tool for a more definitive diagnosis of AD and a more precise classification of patients suffering from the disorder.
Current data have suggested the utility of machine learning tools to investigate novel biomarkers for AD. It is an artificial intelligence algorithm that chooses the best model to decode available data [49]. It has been revealed that the specificity and sensitivity of diagnosis may be significantly enhanced by combining machine learning, numerous variables, and novel biomarkers [50]. A recent comprehensive meta-analysis has demonstrated that naive Bayes and random forest models seem to be the best models for determining susceptibility to MCI and AD [50]. Abate et al. revealed that machine learning models use plasma levels of unfolded p53 (U-p532D3A8+), MMSE scale, and apolipoprotein E epsilon-4 (APOEε4), allowing AD prediction with nearly 87% agreement with clinical diagnosis. Moreover, plasma levels of unfolded p53 (U-p532D3A8+) could be a promising potential blood biomarker for AD [51]. Another group of researchers found that combining different methods of deep-learning convolutional neural networks (CNNs) with an automatic image interpretation system of FDG and florbetapir tau PETcould accurately predict future cognitive decline in patients with MCI with significantly higher prediction accuracy (84.2%) for conversion to AD in comparison to conventional methods [52]. Studies have demonstrated encouraging results concerning machine learning techniques and novel biomarkers as a promising approach for AD prediction [50].
4.1. Changes in the Concentrations of Proinflammatory Proteins in Dementia
Growing evidence from comprehensive proteomic studies has suggested that increased concentrations of inflammatory proteins could be a useful biological marker of cognitive decline severity. Measuring changes in the levels of selected inflammatory proteins in CSF and blood may allow for monitoring of disease progression (Table 1).
Table 1.
Direction of changes in the concentrations of proinflammatory proteins in patients with dementia.
| Protein | Patients | Controls | CSF | Serum | Plasma | Whole Blood | Refs. |
|---|---|---|---|---|---|---|---|
| IL-1β | AD=60 | 94 | - | ↑ | - | - | [53] |
| IL-1β | AD=58 | 31 | - | ↑ | - | - | [54] |
| IL-1β | AD=11 | 12 | ↑ | - | - | - | [55] |
| IL-6 | AD=2295 | 2498 | - | - | - | ↑ | [56] |
| TNF-α | AD=2433 | 1984 | - | - | - | ↑ | [56] |
| TNF-α | EOAD=22 | 50 | - | ↑ | - | - | [57] |
| TNF-α | LOAD=54 | 50 | - | ↑ | - | - | [57] |
| TNF-α | AD=32 | 17 | ↑ | - | - | - | [58] |
| CCL2 | AD=310 | 120 | - | - | ↑ | - | [59] |
| CCL2 | MCI=66 | 120 | - | - | ↑ | - | [59] |
| CX3CL1 | AD=42 | 20 | ↑ | - | - | ↑ | [60] |
| CX3CL1 | MCI=18 | 20 | ↑ | - | - | ↑ | [60] |
| IL-15 | AD=17 | 32 | ↑ | - | - | - | [61] |
| IL-15 | AD=57 | 508 | ↑ | - | - | - | [62] |
| IL-15 | MCI=256 | 508 | ↑ | - | - | - | [62] |
| IL-4 | MCI=21 | 20 | - | - | ↑ | - | [63] |
| IL-10 | AD=27 | 18 | - | ↑ | - | - | [64] |
| IL-10 | MCI=21 | 20 | - | - | ↑ | - | [63] |
| CXCL-12 | AD=30 | 30 | - | - | ↓ | - | [65] |
| sTREM2 | AD=66 | 100 | ↑ | - | - | - | [66] |
| sTREM2 | MCI=184 | 100 | ↑ | - | - | - | [66] |
| YKL-40 | AD=11 | 35 | ↑ | - | - | - | [67] |
| HMGB1 | MCI=12 | 12 | - | ↑ | - | - | [68] |
| GPNMB | AD=10 | 10 | ↑ | - | - | - | [69] |
Cytokines are a large family of proteins, including interleukins and chemokines, which are released following activation by infection, inflammation, or damage of brain cells. They are a heterogeneous group of proteins that can act as pro-and anti-inflammatory factors [70]. One of the largest groups of proinflammatory proteins modulating inflammatory response in the CNS is interleukins. A growing body of evidence indicates that assessing cytokine profiles in the CSF and serum of patients with dementia could be a useful diagnostic tool for monitoring disease progression. Studies on mice and humans have identified several interleukins, including IL-1, IL-6, TNF-α, which contribute to the progression of AD.
The contribution of IL-1, particularly IL-1β isoform, to the pathogenesis of AD is well described in the literature [18, 23, 58]. During inflammation in the CNS, interleukin 1 is released by activated microglial cells. This is confirmed by a study conducted by Griffin et al., in which increased levels of IL-1 in the brain tissue, CSF, and serum of patients with AD were found [71]. Studies by Griffin et al. revealed that elevated expression of IL-1 is associated with tau phosphorylation and NFT formation through kinase MAPK-P38 activation and IL-1-driven cascades [71, 72]. A comparable tendency in IL-1β has been described in patients with MCI [54, 73]. However, CSF IL-1β levels are decreased in amnestic MCI patients [74].
Mounting evidence indicates the significance of IL-6 in the development of inflammation in the brains of AD patients [75-77]. IL-6 is a major proinflammatory cytokine, the expression of which is increased in the brains of AD patients [76]. This interleukin is released during microglia activation caused by APP amino acids and is found near amyloid plaques [78]. Various cellular models have demonstrated that IL-6 has a pleiotropic effect. By way of illustration, IL-6 contributed to the activation of APP expression in primary rat cortical neurons, whereas no effect was observed on glial cells [79, 80]. It is suggested that higher levels of IL-6 and IL-1β mRNA are associated with different expressions. The involvement of IL-6 in neuroinflammation was confirmed by treating hippocampal neurons with IL-6, which resulted in the elevation of hyperphosphorylated tau [81]. In the Mayo Clinic Study of Aging, a greater likelihood of diagnosing MCI was connected with enhanced IL-6 levels [82]. Moreover, it seems that soluble receptor IL-6Rα may also be a marker for early pathology in AD. Wang et al. assessed a panel of 43 inflammatory proteins in the CSF of patients with AD and subjective cognitive decline (SCI). The authors reported that elevated levels of IL-6Rα in the SCI of patients may be related to an undiagnosed psychological stress reaction and may lead to the development of future cognitive decline [83].
IL-15 is a pleiotropic cytokine expressed in astrocytes and activated microglia. In a pro-inflammatory state, this cytokine is responsible for T and B cell proliferation. Nevertheless, IL-15 may also act as a neuroprotective protein. It promotes T cell recruitment in an injured brain, which is associated with neuroprotection [84, 85], but its specific role in neurodegeneration is still not fully understood. However, studies have demonstrated a close connection between serum IL-15 levels and dementia [84]. Research on the brains of AD patients has revealed markedly elevated IL-15 levels in the advanced stages of the disease [86]. Moreover, analyses of the CSF of AD patients also revealed elevated levels of IL-15 [58, 62, 83], which were correlated with age of onset [61].
Anti-inflammatory cytokines are responsible for neutralizing and preventing inflammation. IL-4 has been described as a cytokine, which inhibits the release of TNF-α, IL-1β, and IL-6 by activated monocytes [87]. In AD, IL-4 induces M2 microglia activation, in which microglia release anti-inflammatory cytokines, such as IL-10. Higher IL-4 levels were observed in MCI and AD patients, although they were negatively correlated with disease severity [63]. However, Leung et al. discovered that in patients with AD showing rapid cognitive decline, IL-4 levels were significantly elevated compared to patients with slow cognitive decline [88]. As acetylcholinesterase is linked to amyloid deposits, studies involving treatment with Acetylcholinesterase inhibitors (AChEI) were conducted. It was found that the therapy caused upregulation of IL-4 in AD patients [89, 90]. Moreover, treating mice with IL-4 was associated with both a decline in Aβ aggregation and a reduction in tau phosphorylation [91, 92].
Il-10 is another anti-inflammatory cytokine found in the M2 microglia activation state [23]. This cytokine is predominantly regarded as an inhibitor of the expression of several inflammatory cytokines, such as IL-1β and TNF-α, as well as an inhibitor of antigen presentation through inhibiting MHC II and CD80 on cells [93]. In mouse models of AD, improvement in neurogenesis was linked with the upregulation of IL-10 [94]. Research on gene polymorphism in the IL-10 gene, which relates it to AD pathology, is currently underway [95]. Studies examining the CSF and blood of AD patients have demonstrated elevated levels of this cytokine in comparison to non-demented controls [58, 63]. Moreover, IL-10 plasma levels were negatively correlated with ventricular and brain volume obtained using neuroimaging MRI tests [88]. Additionally, CSF levels of Aβ42 and Aβ42/p/tau ratio were negatively correlated with IL-10 serum levels [64].
The physiological role of TNF-α (Tumor Necrosis Factor-α) in the CNS is extensive, which includes promoting cell migration and proliferation, impacting synaptic plasticity, and maintaining ion homeostasis. However, TNF-α is also involved in neuropathology as it stimulates the activation of microglia and astrocytes and causes synaptic loss [96]. Activated microglia and astrocytes are the main structures releasing this protein [22]. Increased expression of TNF-α in the brains of AD patients [97] and elevated levels of TNF-α in body fluids of AD patients compared to healthy controls have been evaluated in recent articles [56, 58, 98]. It has been suggested that TNF-α contributes to AD pathology through modulation of Aβ plaque formation and regulation of the glial response by recruiting peripheral monocytes into the brain [99, 100]. Furthermore, some studies have indicated a relationship between elevated CSF and serum concentrations of TNF-α in EOAD and LOAD compared to non-demented controls [57, 58].
Physiologically, chemokines expressed within the CNS may play a role in initiating progenitor cell and neuronal migration during brain development and may function as trophic factors for neurons. A close relationship between the expression of selected chemokines (e.g., CX3CL1, CXCL12, CCL2) and the influx of inflammatory cells into the CNS has been demonstrated. Chemokines expressed in the CNS, i.e., CX3CL1 and CXCL12, seem to be key players in the development of inflammation present in the brains of AD patients. Fractalkine (CX3CL1) seems to be a pivotal factor in the development of dementing disorders, particularly in the early stages of the disease [60]. Fractalkine and its CX3CR1 receptor are particularly interesting in the context of an association between AD and inflammation. CX3CL1 is engaged in the communication between neurons and microglia cells expressing CX3CR1. It has been demonstrated that the CX3CR1/CX3CL1 complex modulates neuronal survival, plaque load, and cognition [15]. Therefore, dysregulation of the CXC3CL1 pathway exerts harmful effects on neurogenesis, synaptic plasticity as well as cognition [101, 102]. Disturbance in the CX3CL1 signaling axis has been described in several neurodegenerative diseases, including AD, although its specific role has not been fully elucidated to date [103-106]. Findings from investigations involving animal models of AD confirmed that CX3CR1/CX3CL1 interaction participates in the clearance of amyloid plaques and may play a role in the intraneuronal accumulation of hyperphosphorylated tau [60]. CX3CL1 may be useful as an early biomarker in the diagnosis of cognitive disorders, particularly MCI and AD. Furthermore, it is suggested that increased levels of CX3CL1 may predict the risk of future dementia in individuals who are cognitively normal. The inclusion of CX3CL1 in the biomarker panel may improve the diagnostic performance of established biomarkers (Aβ1-42, tau, pTau), particularly in patients with mild cognitive decline [60].
A very important chemokine in the process of neuroregeneration seems to be CXCL12, also known as stromal cell-derived factor 1 (SDF1). Studies have revealed that mice without CXCL12 died during the embryonic period due to serious defects in nervous and hematopoietic systems [107]. Furthermore, Trousse et al. demonstrated that CXCL12 knockdown mice models have disturbed memory and impaired ability to learn [108]. This chemokine binds to the CXCR4 receptor, being the only chemokine ligand that binds to this receptor [109]. It is not only expressed in peripheral blood but also in microglia, astrocytes, and neurons [110]. Studies on transgenic mice have demonstrated altered expression of CXCR4 near NFT [111]. Moreover, it has been found that the brains of both AD patients and mice expressed decreased levels of CXCL12 in comparison to controls [109, 112]. Furthermore, Janssens et al. reported that injection of CXCL12 into brain ventricles of mice was associated with a decreased number of amyloid aggregates [109]. This may contribute to reduced plasma and CSF levels of CXCL12 in patients with mild AD [65]. Interestingly, treating mouse models of AD with SDF1 resulted in a decrease in the number and range of AB plaques [113], indicating a protective role of this cytokine.
As CCL2, also known as monocyte chemoattractant protein-1 (MCP-1), is responsible for macrophage and monocyte migration [114] and microglia activation during inflammation, it is linked to aggregation of Aβ plaques during AD pathology [115]. Research on AD brains has demonstrated the overexpression of CCL2, particularly in microglia, astrocytes, and near Aβ plaques [59, 115, 116]. Interestingly, MCP-1 expression is also regulated by amyloid 1-42, as proven in a study on cell cultures [117] and AD brains. The expression could be mediated by the JNK-AP1 signaling pathway [118]. The axis between CCL2 and its receptor, CCR2, is linked to AD development through provoking the inflammatory cascade, facilitating the migration of macrophages to the brain, and regulating other cytokines linked to inflammation [119]. Recent data have shown that plasma and CSF levels of CCL2 are elevated in AD and MCI compared to controls, higher in AD patients than individuals with MCI, and correlated with disease progression [59, 120, 121]. Furthermore, rapidity of cognitive decline was described in prodromal AD patients in combination with CCL2 levels [122].
CXCR3 is a chemokine receptor expressed not only in astrocytes and microglia, both in the activated and resting states, but also in neurons [123, 124]. Studies have demonstrated that lowering the expression of CXCR3 in transgenic mice reduced the number of amyloid plaques and decreased Aβ protein levels [125]. As CXCR3 binds to a group of chemokines, including CXCL9, CXCL10, and CXCL11, scientists have also decided to examine them. Elevated levels of CXCL10 were found in MCI and AD patients compared to controls. Furthermore, the chemokine was positively correlated with MMSE [126, 127]. In a study by Corrêa et al., the same observations were made between CXCL10 and Aβ in AD patients, although the levels of this chemokine did not differ between the groups [128].
4.2. Genetic Polymorphisms of Inflammatory Proteins and Risk of Dementia
Studies have demonstrated that not only monitoring of changes in the concentrations of proteins associated with inflammation but also polymorphism in inflammation-related genes may be important in the assessment of cognitive impairment development. Advanced technologies for the analysis of genetic polymorphism have identified numerous genetic loci that affect AD. Moreover, it has been observed that both pro- and anti-inflammatory molecules, particularly IL-1A, TNF-α, VEGF, IL-4, IL-10, and TGF-β1, are associated with a higher risk of AD [129]. Interestingly, the analysis of numerous polymorphisms in different ethnic groups from different regions showed that the same genotype might either increase or exert no impact on AD risk, depending on the population. By way of illustration, IL-1A 889 C/T polymorphism seems to be a risk factor for Caucasians but not Asians [130, 131]. It is suggested that polymorphism may influence the expression of cytokines in AD, leading to increased gene transcriptional activity and abundant proinflammatory cytokine release by microglia after stimulation [132, 133]. Investigations of the relationship between polymorphisms in inflammatory molecules and AD risk could provide a deeper insight into the biological mechanisms of the disease and may contribute to finding effective therapies.
Knowing that cytokines play an important role in neuroinflammation, researchers have started to investigate the influence of gene polymorphisms on overall AD risk [129]. It was found that the IL-1 beta -511 C/T genotype was linked to the risk of developing AD in the Caucasian population independent of the presence or absence of APOE4 allele [134], similar to IL1B +3953 TT polymorphism, which was also associated with a higher risk of AD [135]. TNF-α -308 A/G gene alterations, similar to IL-1, were found to be associated with an increased risk of AD but only in the East Asian population, with no such association found in European or Middle Eastern subjects [136]. IL-4 polymorphism was also studied, and it was revealed that −590 C/T and −1098 T/G alterations were associated with altered IL-4 transcription, causing an increased risk of AD [137, 138].
Although gene alterations are commonly negatively correlated with disease, some of them act as protective agents. A meta-analysis by Hua et al. demonstrated that −174 G/C polymorphism occurring in IL-6 could display a protective ability in the Asian population [130]. Studies on IL-10 -1082A genotype in the Brazilian population revealed a decreased risk of AD compared to other examined genotypes [139].
Recent genome-wide association studies (GWAS) have established that TREM2 triggering receptors expressed in myeloid cells-2 and CD33, which have been proven to participate in innate immune activation, are genes related to the risk of AD. Recent investigations have linked TREM2 and antigenCD33 dysfunction to the promotion of inflammation, which leads to Aβ production, although further research is needed. To confirm GWAS findings, the expression of CD33 mRNA was tested, and the results showed its elevated expression in microglia [140]. Furthermore, it was found to be positively correlated with the number of plaques and Aβ1-42 levels [141]. In turn, TREM2 variants were linked to accelerated brain atrophy compared to non-carriers [140]. Furthermore, these variants may alter TREM2 receptors, probably affecting Aβ clearance and, as a result, promoting neurodegeneration [140, 142]. Mouse models of AD with TREM2 and CD33 deletions confirmed this hypothesis by associating them with a microglial impairment that causes Aβ production [143, 144].
4.3. Biomarkers of Glial Activation
Glial cells are non-neuronal cells located within the whole nervous system (central and peripheral NS), providing structural and metabolic support for the neurons, maintaining synaptic homeostasis, involving in neuronal repair, and eliminating pathogens and dead CNS cells. Glial activation occurs as part of an altered immune response characterized by an enhanced inflammatory response during AD progression. Notably, a longitudinal TSPO-PET study revealed diminished microglial activation over time in patients at the MCI stage and increased activation in patients at the AD stage of dementia [145]. These results suggest that at the early onset of dementia, microglial activation has beneficial effects, whereas it is rather detrimental in the later stages of the disease. Well-known markers of glial activation seem to be TREM2 and chitinase-3-like protein 1 (CHI3L1), also known as YKL-40.
TREM2, when expressed in microglia, is thought to positively regulate neuroinflammation through plaque phagocytosis. On the other hand, it can exert a negative effect in the later stages of the disease by inducing inflammatory responses. It has been established that TREM2 is overexpressed in the brains of AD patients [146]. Moreover, significantly higher concentrations of soluble TREM2 have been observed in the CSF of patients with AD. sTREM releases into CSF through ADAM10 and ADAM17 cleavage. Additionally, significantly elevated levels of TREM2 have been observed in the CSF of early symptomatic AD patients [147-149]. A recent study has found that CSF levels of sTREM2 are positively correlated with T-tau and P-tau concentrations, suggesting a role of this protein in the neurodegenerative process of AD [147-150].
A crucial marker of glial activation is YKL-40, a glycoprotein found in the astrocytes of healthy and AD patients' brain tissues [151]. The overall role of YKL-40 in the physiological state remains unknown. However, based on a number of studies, we can assume that in AD, pro-inflammatory cytokines, such as TNF-α and IL-1β, may trigger YKL-40 expression from astrocytes [152]. Furthermore, based on studies using knockout mice, we can hypothesize the neuroprotective role of this cytokine. These studies have revealed more severe neuropathology and noticeable gliosis in knockout mice than wild-type controls [153, 154]. YKL-40 has a few homologs, such as chitinase-3 like 3 (CHI3L3) and chitinase-3 like 2 (CHI3L2), which are shown to increase mRNA expression, and are found in the brains of AD mouse models as well as the human AD brain [155]. Increased concentrations of YKL-40 have been observed in fully developed AD and, importantly, in the very early stages of the disease (preclinical AD, very mild, and mild dementia) [67, 156]. The correlation with elevated levels of total and phosphorylated tau may indicate a pivotal role of this protein in AD pathology. Notably, it appears that YKL-40 may also be a prognostic marker for monitoring disease progression in individuals with and without cognitive impairment symptoms. Interestingly, a recent study has demonstrated that higher levels of YKL-40 are present only in AD with ongoing dementia and correlated with increased astrogliosis [157]. Furthermore, elevated concentrations of YKL-40 predicted progression from MCI to symptomatic AD and other types of dementia, as measured by an annual assessment of MMSE during the follow-up. Elevated YKL-40 concentrations in patients without dementia are found to be associated with a higher risk of developing AD dementia [62]. These findings indicate the prognostic value of YKL-40 as a potential biomarker for AD.
4.4. Novel Biomarkers
High mobility group box-1 (HMGB1), a novel protein, is considered a potential biomarker for AD. It is a nonhistone, nuclear protein, part of the damage-associated molecular pattern (DAMP) expressed in many types of cells, including the brain, microglia, and neurons [158]. It has been proven that HMGB1 induces inflammation through binding to RAGE and TLRs, which results in the production of proinflammatory cytokines [159]. Research on AD has revealed that HMGB1 is involved in maintaining amyloid β 1-42 oligomer stability. Studies on brain tissue have demonstrated that AD patients have elevated levels of this protein than controls. Moreover, a study used immunostaining to detect HMGB1 in Aβ plaques, suggesting a tendency for this protein to accumulate in plaques [160]. Elevated serum levels of HMGB1 in AD patients in comparison to controls were found. Moreover, subjects with MCI showed even higher concentrations in comparison to patients with AD [68], which may indicate its crucial role in the early onset of the disease.
Another factor of potential significance in AD pathology, which is also considered a possible biomarker, may be glycoprotein non-metastatic melanoma protein B (GPNMB). It is a type I transmembrane protein, firstly isolated from a poorly metastatic melanoma cell line [161]. GPNMB is involved in osteoclast differentiation and deterioration, and T-cell activation. It acts as a negative regulator of macrophage inflammatory responses [162, 163]. Studies using transgenic mice have demonstrated GPNMB expression in neurons and astrocytes. Nonetheless, its role in the brain is still not fully known. Overexpression of GPNMB has been detected in some neurodegenerative disorders, such as Amyotrophic Lateral Sclerosis (ALS) [164]. Moreover, Murata et al. showed its potential neuroprotective function by improving memory in overexpressed mice [165]. Satoh et al. demonstrated that activated microglia accumulating around senile plaques caused elevated levels of this protein in AD brains [166]. Recent studies have associated GPNMB only with activated microglia occurring during AD. Furthermore, analysis of immortalized microglia cell lines produced data, associating soluble Aβ with GPNMB expression. The same study confirmed these results as elevated levels of this protein were not only found in the brains but also in the CSF of AD patients in comparison to controls [69]. However, a study conducted by Aichholzer et al. did not confirm these findings [167].
The proteins described in this paper play an important role not only in the pathogenesis of neurodegenerative diseases but may also be useful for diagnostic purposes. Determination of their concentrations in CSF requires a lumbar puncture, which has certain medical and legal limitations. Since it is more invasive than drawing blood, it requires additional consent from the patient, which is regulated differently in every country in which the patient is treated [168]. Various branches of law generally contain provisions that regulate specific areas of social relations and protect the rights of the parties to these relations. This also applies to the norms of medical law, which, among other things, defines the relationship between medical professionals and their patients. The regulation of these relations is manifested, inter alia, in the indication of the rights of the patient and the corresponding duties of the doctor or another individual performing medical procedures. Therefore, it seems that the possibility of determining some of the proteins described in this work in the blood is very important not only for medical reasons but is also associated with fewer legal restrictions. Considering the fact that the person's welfare can be endangered in various medical activities, they are often subjected to detailed regulation not only by medical but also by criminal law. This is particularly evident in the case of AD patients. They are not always able to consent to additional medical procedures concerning them, including a lumbar puncture. Therefore, the issues addressed in this paper regarding the significance of inflammatory markers in the pathogenesis of AD, and the possibility of their determination in blood, which is less complicated to obtain than a lumbar puncture, encompasses medical, economic as well as legal issues.
4.5. Other Molecules Associated with AD
Recent studies have suggested that it is not only proteins that can be used in diagnosing and monitoring the disease. Iron, copper, and zinc are important metals participating in healthy brain processes. However, their accumulation and homeostatic imbalance may act as a trigger of the disease and facilitate its progression. A recent analysis of the brains of AD patients has revealed significant aggregation of these metal ions [169]. The aforementioned molecules tend to accumulate near senile plaques and NFTs [170]. Interestingly, they also have a significant association with the molecular basis of AD. An excessive amount of copper is known to enhance APP expression and production of Aβ [171]. Moreover, iron and copper modulate APP levels by controlling transcription and translation of APP genes [172]. Therefore, a recent study has analyzed metal levels in the CSF of AD patients. It was demonstrated that ferritin concentrations are correlated with lower levels of Aβ 1-42. The discovery demonstrated that iron might be linked to amyloid deposition and, as a result, stimulate AD progression [173]. Another study revealed a correlation between higher iron and copper levels, and elevated concentrations of the established markers of the disease, such as Aβ 1-42 and p-Tau [174]. These findings confirmed the potential utility of metal ions as novel biomarkers for AD or even as a therapeutic strategy [172].
5. AVAILABLE AND POTENTIAL TREATMENT FOR AD
At the beginning of June 2021, the US Food and Drug Administration (FDA) approved the first drug, Aduhelm (aducanumab), for the treatment of AD. It is a monoclonal antibody targeted amyloid β plaques immunization [175]. Unfortunately, Aduhelm (aducanumab) is not a cure for the disease. Until this year, established drugs are only symptomatic, not capable of halting or slowing neurodegeneration. Donepezil, galantamine, and rivastigmine work as cholinesterase inhibitors, while memantine is an antagonist of the N-methyl-D-aspartate receptor [176]. Knowing how important neuroinflammation is, researchers have started to consider new strategies regarding this issue, particularly using anti-inflammatory drugs [177]. Epidemiological studies conducted in the last decades have demonstrated that the use of non-steroidal anti-inflammatory drugs (NSAIDs) could protect individuals against AD. However, findings from clinical trials concerning the effectiveness of anti-inflammatory drugs in AD therapy are controversial and divergent [178]. However, research on a possible treatment for AD in various fields is still being pursued with a particular focus on non-pharmacological approaches. Therefore, plant extracts and nutritional approaches to the treatment of the disease are being investigated. Garlic has an established role in the treatment of different diseases, such as cardiovascular and gastrointestinal disorders and cancer [185-187]. Therefore, researchers have started to examine the impact of “Aged Garlic Extract” (AGE) and S-Allyl-L-Cysteine (SAC) in vitro on AD models with positive outcomes [179, 180]. A recent study on neuronal cells treated with AGE and SAC stimulated by reactive oxygen species (ROS) has demonstrated that both substances may exert neuroprotective and neurorescue effects, protecting neurons from ROS insults and maintaining pre-synaptic proteins [182]. The same study showed the benefits of pre-treatment with AGE, protecting around 80% of neuronal cells from ROS [179]. Furthermore, another in vitro study indicated that both SAC and AGE may affect Aβ, resulting in a decrease in the number of plaques in AD model brains, although the specific mechanism has not yet been fully known [181, 182]. A different substance worth mentioning is curcumin. It is a polyphenol found in turmeric, a spice with a distinct yellow color that is widely used in cooking [183]. Curcumin is known for its anti-inflammatory, antitumor, and antioxidant properties [184]. In Alzheimer’s disease, research regarding curcumin in AD has demonstrated that the compound has the ability to inhibit Aβ and tau aggregation in vivo [188]. Moreover, a study by Khanna et al. revealed that curcumin might have anti-inflammatory and neuroprotective effects [189]. The main pathway by which curcumin work is believed to be through NFkB inhibition by preventing phosphorylation of IkB and consecutive activation of NFkB [190]. Interestingly, this substance is capable of inhibiting enzymes, such as β-secretase (BACE-1), which are responsible for APP cleavage [191]. The limitation of using curcumin is its poor bioavailability due to its solubility in water. Thus, a new form of the substance, a polymeric nanoparticle encapsulated curcumin (NanoCurc™), has been developed [192]. A study by Ray et al. demonstrated that the use of NanoCurc™ protected neuronally differentiated human cells from oxidative stress. Furthermore, cell cultures treated with NanoCurc™ displayed elevated levels of Neuron Specific Enolase (NSE), which may suggest the protective role of the molecule on the neuronal phenotype. On the other hand, animal models treated with this molecule showed decreased levels of ROS, but also attenuated activity of caspase-3 and caspase-7 in the brain. Interestingly, it also exerted a positive effect on already ROS insulted cells. These findings suggested the therapeutic application of NanoCurc™ in AD patients, particularly considering its ability to cross the BBB [193].
Another interesting theory concerns music therapy as a potential AD agent. Increasing evidence indicates that listening to music may improve aspects of memory in patients with dementia [194]. Although the exact mechanism has not been fully elucidated, it is known that music interacts with brain regions responsible for emotions and decision-making. This has led to the establishment of some possible mechanisms underlying this phenomenon, such as dopaminergic pathway activation or sympathetic arousal [194]. A systematic review conducted by Garcia-Casares et al. confirmed the beneficial influence of music therapy on cognition, emotion, and behavior in AD patients [195]. Interestingly, this type of therapy resulted in a marked improvement in memory and orientation and alleviated depression and anxiety in AD patients after only four sessions [196]. However, further research is needed in this regard.
CONCLUSION
A growing number of scientific reports have demonstrated that neuroinflammation seems to be an important factor in Alzheimer’s pathology, which influences amyloid deposition, NFT formation, and consequently leads to neuronal death. During this process, activated microglia and astrocytes release pro- and anti-inflammatory cytokines that modulate inflammation in the CNS. In a healthy brain, they are capable of eliminating abnormalities, whereas, in AD, their over-activation cause elevated expression of cytokines, thus inducing neuroinflammation. Investigation of these cytokines released by activated microglia might be helpful in diagnosing the disease in the early stages due to anti-inflammatory M2 type microglia, which are involved in Aβ clearance before they switch to a more damaging M1 phenotype. Studies in recent years have highlighted the relationship between inflammation in the gut and brain and its association to neuroinflammation in AD. Comprehension and recognition of the mechanisms of inflammatory molecule release might establish a panel of proteins that may potentially be used as new biomarkers. Considering that AD is a heterogenic disorder, it seems that a combination of multiple markers reflecting various pathomechanisms and different stages of disease progression could be the most effective strategy. These discoveries would improve the detectability of the disease and enable the discovery of possible anti-inflammatory treatment. In this review, we described pivotal inflammatory molecules with the inclusion of new and potential ones and hypothesized their use as novel biomarkers for AD. However, further studies are needed to fully understand the contribution of neuroinflammation to AD pathology and determine the clinical utility of these inflammatory markers in the diagnosis, prognosis, and management of AD.
ACKNOWLEDGEMENTS
AKP and PM received consultation and/or lecture honoraria from the Roche company.
LIST OF ABBREVIATIONS
- AChEI
Acetylcholinesterase Inhibitors
- AD
Alzheimer’s Disease
- AGE
“Aged Garlic Extract”
- ALS
Amyotrophic lateral sclerosis
- APOEε4
Apolipoprotein E epsilon-4
- APP
Amyloid precursor protein
- Aβ
Amyloid Beta
- BACE-1
β-secretase
- BBB
Blood Brain Barrier
- CNNs
Convolutional Neural Networks
- CNS
Central Nervous System
- CSF
Cerebrospinal Fluid
- DAMP
Damage-associated Molecular Pattern
- EOAD
Early-onset Alzheimer's Disease
- FDA
Food and Drug Administration
- GFAP
Glial Fibrillary Acidic Protein
- GPNMB
Glycoprotein Non-metastatic Melanoma Protein B
- GWAS
Genome-wide Association Studies
- HMGB1
High Mobility Group Box-1
- LOAD
Late-onset Alzheimer's Disease
- LPS
Lipopolysaccharide
- MCI
Mild Cognitive Impairment
- MCP-1
Monocyte Chemoattractant Protein-1
- NETs
Neutrophil Extracellular Traps
- NFT
Neurofibrillary Tangle
- NSAIDs
Non-Steroidal Anti-Inflammatory Drugs
- NSE
Neuron Specific Enolase
- ROS
Reactive Oxygen Species
- SAC
S-Allyl-L-Cysteine
- SCI
Subjective Cognitive Decline
- SDF-1
Stromal cell-Derived Factor 1
- TLR-4
Toll-Like Receptor 4
- TNF
Tumor Necrosis Factor
CONSENT FOR PUBLICATION
Not applicable.
FUNDING
None.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
REFERENCES
- 1.Lane C.A., Hardy J., Schott J.M. Alzheimer’s disease. Eur. J. Neurol. 2018;25(1):59–70. doi: 10.1111/ene.13439. [DOI] [PubMed] [Google Scholar]
- 2.WHO. Risk Reduction of cognitive decline and dementia. World Health Organization; 2019. p. 96. Available from: https://www.who.int/publications/i/item/risk-reduction-of-cognitive-decline-and-dementia. [PubMed] [Google Scholar]
- 3.Alzheimer’s Association. Alzheimer’s Facts and Figures Report. Available from: https://www.alz.org/alzheimers-dementia/facts-figures. [Google Scholar]
- 4.Clarimón J., Djaldetti R., Lleó A., Guerreiro R.J., Molinuevo J.L., Paisán-Ruiz C., Gómez-Isla T., Blesa R., Singleton A., Hardy J. Whole genome analysis in a consanguineous family with early onset Alzheimer’s disease. Neurobiol. Aging. 2009;30(12):1986–1991. doi: 10.1016/j.neurobiolaging.2008.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Muñoz S.S., Garner B., Ooi L. Understanding the role of apoe fragments in alzheimer’s disease. Neurochem. Res. 2019;44(6):1297–1305. doi: 10.1007/s11064-018-2629-1. [DOI] [PubMed] [Google Scholar]
- 6.Crews L., Masliah E. Molecular mechanisms of neurodegeneration in Alzheimer’s disease. Hum. Mol. Genet. 2010;19(R1):R12–R20. doi: 10.1093/hmg/ddq160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Weiner M.W., Veitch D.P., Aisen P.S., Beckett L.A., Cairns N.J., Cedarbaum J., Green R.C., Harvey D., Jack C.R., Jagust W., Luthman J., Morris J.C., Petersen R.C., Saykin A.J., Shaw L., Shen L., Schwarz A., Toga A.W., Trojanowski J.Q. 2014 Update of the Alzheimer’s Disease Neuroimaging Initiative: A review of papers published since its inception. Alzheimers Dement. 2015;11(6):e1–e120. doi: 10.1016/j.jalz.2014.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen G.F., Xu T.H., Yan Y., Zhou Y.R., Jiang Y., Melcher K., Xu H.E. Amyloid beta: structure, biology and structure-based therapeutic development. Acta Pharmacol. Sin. 2017;38(9):1205–1235. doi: 10.1038/aps.2017.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kojro E., Fahrenholz F. Alzheimer’s Disease. Vol. 38. Springer US; 2005. The Non-Amyloidogenic Pathway: Structure and Function of α-Secretases. ; pp. 105–127. [DOI] [PubMed] [Google Scholar]
- 10.Tarasoff-Conway J.M., Carare R.O., Osorio R.S., Glodzik L., Butler T., Fieremans E., Axel L., Rusinek H., Nicholson C., Zlokovic B.V., Frangione B., Blennow K., Ménard J., Zetterberg H., Wisniewski T., de Leon M.J. Clearance systems in the brain-implications for Alzheimer disease. Nat. Rev. Neurol. 2015;11(8):457–470. doi: 10.1038/nrneurol.2015.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Iba M., Guo J.L., McBride J.D., Zhang B., Trojanowski J.Q., Lee V.M.Y. Synthetic tau fibrils mediate transmission of neurofibrillary tangles in a transgenic mouse model of Alzheimer’s-like tauopathy. J. Neurosci. 2013;33(3):1024–1037. doi: 10.1523/JNEUROSCI.2642-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hoskin J.L., Sabbagh M.N., Al-Hasan Y., Decourt B. Tau immunotherapies for Alzheimer’s disease. Expert Opin. Investig. Drugs. 2019;28(6):545–554. doi: 10.1080/13543784.2019.1619694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Martin L., Latypova X., Wilson C.M., Magnaudeix A., Perrin M-L., Yardin C., Terro F. Tau protein kinases: involvement in Alzheimer’s disease. Ageing Res. Rev. 2013;12(1):289–309. doi: 10.1016/j.arr.2012.06.003. [DOI] [PubMed] [Google Scholar]
- 14.Naseri N.N., Wang H., Guo J., Sharma M., Luo W. The complexity of tau in Alzheimer’s disease. Neurosci. Lett. 2019;705:183–194. doi: 10.1016/j.neulet.2019.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Heneka M.T., Carson M.J., El Khoury J., Landreth G.E., Brosseron F., Feinstein D.L., Jacobs A.H., Wyss-Coray T., Vitorica J., Ransohoff R.M., Herrup K., Frautschy S.A., Finsen B., Brown G.C., Verkhratsky A., Yamanaka K., Koistinaho J., Latz E., Halle A., Petzold G.C., Town T., Morgan D., Shinohara M.L., Perry V.H., Holmes C., Bazan N.G., Brooks D.J., Hunot S., Joseph B., Deigendesch N., Garaschuk O., Boddeke E., Dinarello C.A., Breitner J.C., Cole G.M., Golenbock D.T., Kummer M.P. NeuroInflammation in Alzheimer’s disease. Lancet Neurol. 2015;14(4):388–405. doi: 10.1016/S1474-4422(15)70016-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Krstic D., Knuesel I. Deciphering the mechanism underlying late-onset Alzheimer disease. Nat. Rev. Neurol. 2013;9(1):25–34. doi: 10.1038/nrneurol.2012.236. [DOI] [PubMed] [Google Scholar]
- 17.Nazem A., Sankowski R., Bacher M., Al-Abed Y. Rodent models of neuroinflammation for Alzheimer’s disease. J. Neuroinflammation. 2015;12:74. doi: 10.1186/s12974-015-0291-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Webers A., Heneka M.T., Gleeson P.A. The role of innate immune responses and neuroinflammation in amyloid accumulation and progression of Alzheimer’s disease. Immunol. Cell Biol. 2020;98(1):28–41. doi: 10.1111/imcb.12301. [DOI] [PubMed] [Google Scholar]
- 19.Lee J.W., Lee Y.K., Yuk D.Y., Choi D.Y., Ban S.B., Oh K.W., Hong J.T. Neuro-inflammation induced by lipopolysaccharide causes cognitive impairment through enhancement of beta-amyloid generation. J. Neuroinflammation. 2008;5:37. doi: 10.1186/1742-2094-5-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ransohoff R.M., Perry V.H. Microglial physiology: unique stimuli, specialized responses. Annu. Rev. Immunol. 2009;27:119–145. doi: 10.1146/annurev.immunol.021908.132528. [DOI] [PubMed] [Google Scholar]
- 21.Schafer D.P., Stevens B. Microglia function in central nervous system development and plasticity. Cold Spring Harb. Perspect. Biol. 2015;7(10):a020545. doi: 10.1101/cshperspect.a020545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cai Z., Hussain M.D., Yan L-J. Microglia, neuroinflammation, and beta-amyloid protein in Alzheimer’s disease. Int. J. Neurosci. 2014;124(5):307–321. doi: 10.3109/00207454.2013.833510. [DOI] [PubMed] [Google Scholar]
- 23.Tang Y., Le W. Differential roles of M1 and M2 microglia in neurodegenerative diseases. Mol. Neurobiol. 2016;53(2):1181–1194. doi: 10.1007/s12035-014-9070-5. [DOI] [PubMed] [Google Scholar]
- 24.Jin X., Liu M-Y.Y., Zhang D-F.F., Zhong X., Du K., Qian P., Gao H., Wei M-J.J. Natural products as a potential modulator of microglial polarization in neurodegenerative diseases. Pharmacol. Res. 2019;145:104253. doi: 10.1016/j.phrs.2019.104253. [DOI] [PubMed] [Google Scholar]
- 25.Zhang Z., Zhang Z., Lu H., Yang Q., Wu H., Wang J. Microglial polarization and inflammatory mediators after intracerebral hemorrhage. Mol. Neurobiol. 2017;54(3):1874–1886. doi: 10.1007/s12035-016-9785-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chee S.E.J., Solito E. The impact of ageing on the CNS immune response in Alzheimer’s disease. Front. Immunol. 2021;12:738511. doi: 10.3389/fimmu.2021.738511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Frank S., Burbach G.J., Bonin M., Walter M., Streit W., Bechmann I., Deller T. TREM2 is upregulated in amyloid plaque-associated microglia in aged APP23 transgenic mice. Glia. 2008;56(13):1438–1447. doi: 10.1002/glia.20710. [DOI] [PubMed] [Google Scholar]
- 28.Hickman S.E., El Khoury J. TREM2 and the neuroimmunology of Alzheimer’s disease. Biochem. Pharmacol. 2014;88(4):495–498. doi: 10.1016/j.bcp.2013.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Medeiros R., LaFerla F.M. Astrocytes: conductors of the Alzheimer disease neuroinflammatory symphony. Exp. Neurol. 2013;239:133–138. doi: 10.1016/j.expneurol.2012.10.007. [DOI] [PubMed] [Google Scholar]
- 30.Fakhoury M. Microglia and astrocytes in Alzheimer’s disease: implications for therapy. Curr. Neuropharmacol. 2018;16(5):508–518. doi: 10.2174/1570159X15666170720095240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wyss-Coray T., Rogers J. Inflammation in Alzheimer disease-a brief review of the basic science and clinical literature. Cold Spring Harb. Perspect. Med. 2012;2(1):a006346–a006346. doi: 10.1101/cshperspect.a006346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Olabarria M., Noristani H.N., Verkhratsky A., Rodríguez J.J. Concomitant astroglial atrophy and astrogliosis in a triple transgenic animal model of Alzheimer’s disease. Glia. 2010;58(7):831–838. doi: 10.1002/glia.20967. [DOI] [PubMed] [Google Scholar]
- 33.Van Eldik L.J., Carrillo M.C., Cole P.E., Feuerbach D., Greenberg B.D., Hendrix J.A., Kennedy M., Kozauer N., Margolin R.A., Molinuevo J.L., Mueller R., Ransohoff R.M., Wilcock D.M., Bain L., Bales K. The roles of inflammation and immune mechanisms in Alzheimer’s disease. Alzheimers Dement. (N. Y.) 2016;2(2):99–109. doi: 10.1016/j.trci.2016.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lian H., Yang L., Cole A., Sun L., Chiang A.C-A., Fowler S.W., Shim D.J., Rodriguez-Rivera J., Taglialatela G., Jankowsky J.L., Lu H.C., Zheng H. NFκB-activated astroglial release of complement C3 compromises neuronal morphology and function associated with Alzheimer’s disease. Neuron. 2015;85(1):101–115. doi: 10.1016/j.neuron.2014.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dong Y., Lagarde J., Xicota L., Corne H., Chantran Y., Chaigneau T., Crestani B., Bottlaender M., Potier M.C., Aucouturier P., Dorothée G., Sarazin M., Elbim C. Neutrophil hyperactivation correlates with Alzheimer’s disease progression. Ann. Neurol. 2018;83(2):387–405. doi: 10.1002/ana.25159. [DOI] [PubMed] [Google Scholar]
- 36.Vitte J., Michel B.F., Bongrand P., Gastaut J.L. Oxidative stress level in circulating neutrophils is linked to neurodegenerative diseases. J. Clin. Immunol. 2004;24(6):683–692. doi: 10.1007/s10875-004-6243-4. [DOI] [PubMed] [Google Scholar]
- 37.Zenaro E., Pietronigro E., Della Bianca V., Piacentino G., Marongiu L., Budui S., Turano E., Rossi B., Angiari S., Dusi S., Montresor A., Carlucci T., Nanì S., Tosadori G., Calciano L., Catalucci D., Berton G., Bonetti B., Constantin G. Neutrophils promote Alzheimer’s disease-like pathology and cognitive decline via LFA-1 integrin. Nat. Med. 2015;21(8):880–886. doi: 10.1038/nm.3913. [DOI] [PubMed] [Google Scholar]
- 38.Stock A.J., Kasus-Jacobi A., Pereira H.A. The role of neutrophil granule proteins in neuroinflammation and Alzheimer’s disease. J. Neuroinflammation. 2018;15(1):240. doi: 10.1186/s12974-018-1284-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kowalski K., Mulak A. Brain-gut-microbiota axis in Alzheimer’s disease. J. Neurogastroenterol. Motil. 2019;25(1):48–60. doi: 10.5056/jnm18087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kim M-S., Kim Y., Choi H., Kim W., Park S., Lee D., Kim D.K., Kim H.J., Choi H., Hyun D-W., Lee J.Y., Choi E.Y., Lee D.S., Bae J.W., Mook-Jung I. Transfer of a healthy microbiota reduces amyloid and tau pathology in an Alzheimer’s disease animal model. Gut. 2020;69(2):283–294. doi: 10.1136/gutjnl-2018-317431. [DOI] [PubMed] [Google Scholar]
- 41.Park J-C., Han S-H., Mook-Jung I. Peripheral inflammatory biomarkers in Alzheimer’s disease: A brief review. BMB Rep. 2020;53(1):10–19. doi: 10.5483/BMBRep.2020.53.1.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Megur A., Baltriukienė D., Bukelskienė V., Burokas A. The microbiota-gut-brain axis and alzheimer’s disease: neuroinflammation is to blame? Nutrients. 2020;13(1):37. doi: 10.3390/nu13010037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Friedland R.P., Chapman M.R. The role of microbial amyloid in neurodegeneration. PLoS Pathog. 2017;13(12):e1006654. doi: 10.1371/journal.ppat.1006654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cattaneo A., Cattane N., Galluzzi S., Provasi S., Lopizzo N., Festari C., Ferrari C., Guerra U.P., Paghera B., Muscio C., Bianchetti A., Volta G.D., Turla M., Cotelli M.S., Gennuso M., Prelle A., Zanetti O., Lussignoli G., Mirabile D., Bellandi D., Gentile S., Belotti G., Villani D., Harach T., Bolmont T., Padovani A., Boccardi M., Frisoni G.B., INDIA-FBP Group Association of brain amyloidosis with pro-inflammatory gut bacterial taxa and peripheral inflammation markers in cognitively impaired elderly. Neurobiol. Aging. 2017;49:60–68. doi: 10.1016/j.neurobiolaging.2016.08.019. [DOI] [PubMed] [Google Scholar]
- 45.Bonfili L., Cecarini V., Berardi S., Scarpona S., Suchodolski J.S., Nasuti C., Fiorini D., Boarelli M.C., Rossi G., Eleuteri A.M. Microbiota modulation counteracts Alzheimer’s disease progression influencing neuronal proteolysis and gut hormones plasma levels. Sci. Rep. 2017;7(1):2426. doi: 10.1038/s41598-017-02587-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bradburn S., Murgatroyd C., Ray N. Neuroinflammation in mild cognitive impairment and Alzheimer’s disease: A meta-analysis. Ageing Res. Rev. 2019;50:1–8. doi: 10.1016/j.arr.2019.01.002. [DOI] [PubMed] [Google Scholar]
- 47.Pedrero-Prieto C.M., García-Carpintero S., Frontiñán-Rubio J., Llanos-González E., Aguilera García C., Alcaín F.J., Lindberg I., Durán-Prado M., Peinado J.R., Rabanal-Ruiz Y. A comprehensive systematic review of CSF proteins and peptides that define Alzheimer’s disease. Clin. Proteomics. 2020;17:21. doi: 10.1186/s12014-020-09276-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Brosseron F., Traschütz A., Widmann C.N., Kummer M.P., Tacik P., Santarelli F., Jessen F., Heneka M.T. Characterization and clinical use of inflammatory cerebrospinal fluid protein markers in Alzheimer’s disease. Alzheimers Res. Ther. 2018;10(1):25. doi: 10.1186/s13195-018-0353-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.El Naqa I., Murphy M.J. Machine Learning in Radiation Oncology. Cham: Springer International Publishing; 2015. What Is Machine Learning? pp. 3–11. [DOI] [Google Scholar]
- 50.Chang C-H., Lin C-H., Lane H-Y. Machine learning and novel biomarkers for the diagnosis of Alzheimer’s disease. Int. J. Mol. Sci. 2021;22(5):2761. doi: 10.3390/ijms22052761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Abate G., Vezzoli M., Polito L., Guaita A., Albani D., Marizzoni M., Garrafa E., Marengoni A., Forloni G., Frisoni G.B., Cummings J.L., Memo M., Uberti D. A conformation variant of p53 combined with machine learning identifies Alzheimer disease in preclinical and prodromal stages. J. Pers. Med. 2020;11(1):14. doi: 10.3390/jpm11010014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Choi H., Jin K.H., Alzheimer’s Disease Neuroimaging Initiative Predicting cognitive decline with deep learning of brain metabolism and amyloid imaging. Behav. Brain Res. 2018;344:103–109. doi: 10.1016/j.bbr.2018.02.017. [DOI] [PubMed] [Google Scholar]
- 53.Italiani P., Puxeddu I., Napoletano S., Scala E., Melillo D., Manocchio S., Angiolillo A., Migliorini P., Boraschi D., Vitale E., Di Costanzo A. Circulating levels of IL-1 family cytokines and receptors in Alzheimer’s disease: new markers of disease progression? J. Neuroinflammation. 2018;15(1):342. doi: 10.1186/s12974-018-1376-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Forlenza O.V., Diniz B.S., Talib L.L., Mendonça V.A., Ojopi E.B., Gattaz W.F., Teixeira A.L. Increased serum IL-1β level in Alzheimer’s disease and mild cognitive impairment. Dement. Geriatr. Cogn. Disord. 2009;28(6):507–512. doi: 10.1159/000255051. [DOI] [PubMed] [Google Scholar]
- 55.Blum-Degen D., Müller T., Kuhn W., Gerlach M., Przuntek H., Riederer P. Interleukin-1 β and interleukin-6 are elevated in the cerebrospinal fluid of Alzheimer’s and de novo Parkinson’s disease patients. Neurosci. Lett. 1995;202(1-2):17–20. doi: 10.1016/0304-3940(95)12192-7. [DOI] [PubMed] [Google Scholar]
- 56.Lai K.S.P., Liu C.S., Rau A., Lanctôt K.L., Köhler C.A., Pakosh M., Carvalho A.F., Herrmann N. Peripheral inflammatory markers in Alzheimer’s disease: A systematic review and meta-analysis of 175 studies. J. Neurol. Neurosurg. Psychiatry. 2017;88(10):876–882. doi: 10.1136/jnnp-2017-316201. [DOI] [PubMed] [Google Scholar]
- 57.Gezen-Ak D., Dursun E., Hanağası H., Bilgiç B., Lohman E., Araz Ö.S., Atasoy I.L., Alaylıoğlu M., Önal B., Gürvit H., Yılmazer S. BDNF, TNFα, HSP90, CFH, and IL-10 serum levels in patients with early or late onset Alzheimer’s disease or mild cognitive impairment. J. Alzheimers Dis. 2013;37(1):185–195. doi: 10.3233/JAD-130497. [DOI] [PubMed] [Google Scholar]
- 58.Taipa R., das Neves S.P., Sousa A.L., Fernandes J., Pinto C., Correia A.P., Santos E., Pinto P.S., Carneiro P., Costa P., Santos D., Alonso I., Palha J., Marques F., Cavaco S., Sousa N. Proinflammatory and anti-inflammatory cytokines in the CSF of patients with Alzheimer’s disease and their correlation with cognitive decline. Neurobiol. Aging. 2019;76:125–132. doi: 10.1016/j.neurobiolaging.2018.12.019. [DOI] [PubMed] [Google Scholar]
- 59.Lee W.J., Liao Y.C., Wang Y.F., Lin I.F., Wang S.J., Fuh J.L. Plasma MCP-1 and cognitive decline in patients with Alzheimer’s disease and mild cognitive impairment: A two-year follow-up study. Sci. Rep. 2018;8(1):1280. doi: 10.1038/s41598-018-19807-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kulczyńska-Przybik A., Słowik A., Mroczko P., Borawski B., Groblewska M., Borawska R., Mroczko B. Cerebrospinal fluid and blood CX3Cl1 as a potential biomarker in early diagnosis and prognosis of dementia. Curr. Alzheimer Res. 2020;17(8):709–721. doi: 10.2174/1567205017666201109095657. [DOI] [PubMed] [Google Scholar]
- 61.Rentzos M., Zoga M., Paraskevas G.P., Kapaki E., Rombos A., Nikolaou C., Tsoutsou A., Vassilopoulos D. IL-15 is elevated in cerebrospinal fluid of patients with Alzheimer’s disease and frontotemporal dementia. J. Geriatr. Psychiatry Neurol. 2006;19(2):114–117. doi: 10.1177/0891988706286226. [DOI] [PubMed] [Google Scholar]
- 62.Janelidze S., Mattsson N., Stomrud E., Lindberg O., Palmqvist S., Zetterberg H., Blennow K., Hansson O. CSF biomarkers of neuroinflammation and cerebrovascular dysfunction in early Alzheimer disease. Neurology. 2018;91(9):e867–e877. doi: 10.1212/WNL.0000000000006082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.King E., O’Brien J.T., Donaghy P., Morris C., Barnett N., Olsen K., Martin-Ruiz C., Taylor J-P., Thomas A.J. Peripheral inflammation in prodromal Alzheimer’s and Lewy body dementias. J. Neurol. Neurosurg. Psychiatry. 2018;89(4):339–345. doi: 10.1136/jnnp-2017-317134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.D’Anna L., Abu-Rumeileh S., Fabris M., Pistis C., Baldi A., Sanvilli N., Curcio F., Gigli G.L., D’Anna S., Valente M. Serum interleukin-10 levels correlate with cerebrospinal fluid amyloid beta deposition in alzheimer disease patients. Neurodegener. Dis. 2017;17(4-5):227–234. doi: 10.1159/000474940. [DOI] [PubMed] [Google Scholar]
- 65.Laske C., Stellos K., Eschweiler G.W., Leyhe T., Gawaz M. Decreased CXCL12 (SDF-1) plasma levels in early Alzheimer’s disease: A contribution to a deficient hematopoietic brain support? J. Alzheimers Dis. 2008;15(1):83–95. doi: 10.3233/JAD-2008-15107. [DOI] [PubMed] [Google Scholar]
- 66.Ewers M., Franzmeier N., Suárez-Calvet M., Morenas-Rodriguez E., Caballero M.A.A., Kleinberger G., Piccio L., Cruchaga C., Deming Y., Dichgans M., Trojanowski J.Q., Shaw L.M., Weiner M.W., Haass C., Alzheimer’s Disease Neuroimaging Initiative Increased soluble TREM2 in cerebrospinal fluid is associated with reduced cognitive and clinical decline in Alzheimer’s disease. Sci. Transl. Med. 2019;11(507):eaav6221. doi: 10.1126/scitranslmed.aav6221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wang L., Gao T., Cai T., Li K., Zheng P., Liu J., Alzheimer’s Disease Neuroimaging Initiative Cerebrospinal fluid levels of YKL-40 in prodromal Alzheimer’s disease. Neurosci. Lett. 2020;715:134658. doi: 10.1016/j.neulet.2019.134658. [DOI] [PubMed] [Google Scholar]
- 68.Festoff B.W., Sajja R.K., van Dreden P., Cucullo L. HMGB1 and thrombin mediate the blood-brain barrier dysfunction acting as biomarkers of neuroinflammation and progression to neurodegeneration in Alzheimer’s disease. J. Neuroinflammation. 2016;13(1):194. doi: 10.1186/s12974-016-0670-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hüttenrauch M., Ogorek I., Klafki H., Otto M., Stadelmann C., Weggen S., Wiltfang J., Wirths O. Glycoprotein NMB: A novel Alzheimer’s disease associated marker expressed in a subset of activated microglia. Acta Neuropathol. Commun. 2018;6(1):108. doi: 10.1186/s40478-018-0612-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zheng C., Zhou X-W., Wang J-Z. The dual roles of cytokines in Alzheimer’s disease: update on interleukins, TNF-α, TGF-β and IFN-γ. Transl. Neurodegener. 2016;5:7. doi: 10.1186/s40035-016-0054-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Griffin W.S.T. Alzheimer’s - Looking beyond plaques. F1000 Med. Rep. 2011;3:24. doi: 10.3410/M3-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sheng J.G., Jones R.A., Zhou X.Q., McGinness J.M., Van Eldik L.J., Mrak R.E., Griffin W.S.T. Interleukin-1 promotion of MAPK-p38 overexpression in experimental animals and in Alzheimer’s disease: potential significance for tau protein phosphorylation. Neurochem. Int. 2001;39(5-6):341–348. doi: 10.1016/S0197-0186(01)00041-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gorska-Ciebiada M., Saryusz-Wolska M., Borkowska A., Ciebiada M., Loba J. Adiponectin, leptin and IL-1 β in elderly diabetic patients with mild cognitive impairment. Metab. Brain Dis. 2016;31(2):257–266. doi: 10.1007/s11011-015-9739-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Rizzi L., Roriz-Cruz M. Cerebrospinal fluid inflammatory markers in amnestic mild cognitive impairment. Geriatr. Gerontol. Int. 2017;17(2):239–245. doi: 10.1111/ggi.12704. [DOI] [PubMed] [Google Scholar]
- 75.Swardfager W., Lanctôt K., Rothenburg L., Wong A., Cappell J., Herrmann N., Lanctt K., Rothenburg L., Wong A., Cappell J. A meta-analysis of cytokines in Alzheimer’s disease. Biol. Psychiatry. 2010;68(10):930–941. doi: 10.1016/j.biopsych.2010.06.012. [DOI] [PubMed] [Google Scholar]
- 76.Hampel H., Haslinger A., Scheloske M., Padberg F., Fischer P., Unger J., Teipel S.J., Neumann M., Rosenberg C., Oshida R., Hulette C., Pongratz D., Ewers M., Kretzschmar H.A., Möller H.J. Pattern of interleukin-6 receptor complex immunoreactivity between cortical regions of rapid autopsy normal and Alzheimer’s disease brain. Eur. Arch. Psychiatry Clin. Neurosci. 2005;255(4):269–278. doi: 10.1007/s00406-004-0558-2. [DOI] [PubMed] [Google Scholar]
- 77.Keegan A.P., Paris D., Luis C.A., Abdullah L., Ait-Ghezala G., Beaulieu-Abdelahad D., Pryor M., Chaykin J., Crynen G., Crawford F., Mullan M. Plasma cytokine IL-6 levels and subjective cognitive decline: preliminary findings. Int. J. Geriatr. Psychiatry. 2018;33(2):358–363. doi: 10.1002/gps.4752. [DOI] [PubMed] [Google Scholar]
- 78.Erta M., Quintana A., Hidalgo J. Interleukin-6, a major cytokine in the central nervous system. Int. J. Biol. Sci. 2012;8(9):1254–1266. doi: 10.7150/ijbs.4679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Del Bo R., Angeretti N., Lucca E., De Simoni M.G., Forloni G. Reciprocal control of inflammatory cytokines, IL-1 and IL-6, and β-amyloid production in cultures. Neurosci. Lett. 1995;188(1):70–74. doi: 10.1016/0304-3940(95)11384-9. [DOI] [PubMed] [Google Scholar]
- 80.Domingues C., da Cruz E Silva O.A.B., Henriques A.G. Impact of cytokines and chemokines on Alzheimer’s disease neuropathological hallmarks. Curr. Alzheimer Res. 2017;14(8):870–882. doi: 10.2174/1567205014666170317113606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Quintanilla R.A., Orellana D.I., González-Billault C., Maccioni R.B. Interleukin-6 induces Alzheimer-type phosphorylation of tau protein by deregulating the cdk5/p35 pathway. Exp. Cell Res. 2004;295(1):245–257. doi: 10.1016/j.yexcr.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 82.Wennberg A.M.V., Hagen C.E., Machulda M.M., Knopman D.S., Petersen R.C., Mielke M.M. The cross-sectional and longitudinal associations between IL-6, IL-10, and TNFα and cognitive outcomes in the mayo clinic study of aging. J Gerontol Ser A. 2019;74(8):1289–1295. doi: 10.1093/gerona/gly217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wang Y., Emre C., Gyllenhammar-Schill H., Fjellman K., Eyjolfsdottir H., Eriksdotter M., Schultzberg M., Hjorth E. Cerebrospinal fluid inflammatory markers in alzheimer’s disease: influence of comorbidities. Curr. Alzheimer Res. 2021;18(2):157–170. doi: 10.2174/1567205018666210330162207. [DOI] [PubMed] [Google Scholar]
- 84.Bishnoi R.J., Palmer R.F., Royall D.R. Serum interleukin (IL)-15 as a biomarker of Alzheimer’s disease. PLoS One. 2015;10(2):e0117282. doi: 10.1371/journal.pone.0117282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Takano M., Nishimura H., Kimura Y., Mokuno Y., Washizu J., Itohara S., Nimura Y., Yoshikai Y. Protective roles of γ δ T cells and interleukin-15 in Escherichia coli infection in mice. Infect. Immun. 1998;66(7):3270–3278. doi: 10.1128/IAI.66.7.3270-3278.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Asby D., Boche D., Allan S., Love S., Miners J.S. Systemic infection exacerbates cerebrovascular dysfunction in Alzheimer’s disease. Brain. 2021;144(6):1869–1883. doi: 10.1093/brain/awab094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.te Velde A.A., Huijbens R.J., Heije K., de Vries J.E., Figdor C.G. Interleukin-4 (IL-4) inhibits secretion of IL-1 beta, tumor necrosis factor alpha, and IL-6 by human monocytes. Blood. 1990;76(7):1392–1397. doi: 10.1182/blood.V76.7.1392.1392. [DOI] [PubMed] [Google Scholar]
- 88.Leung R., Proitsi P., Simmons A., Lunnon K., Güntert A., Kronenberg D., Pritchard M., Tsolaki M., Mecocci P., Kloszewska I., Vellas B., Soininen H., Wahlund L.O., Lovestone S. Inflammatory proteins in plasma are associated with severity of Alzheimer’s disease. PLoS One. 2013;8(6):e64971. doi: 10.1371/journal.pone.0064971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lugaresi A., Di Iorio A., Iarlori C., Reale M., De Luca G., Sparvieri E., Michetti A., Conti P., Gambi D., Abate G. IL-4 in vitro production is upregulated in Alzheimer's disease patients treated with acetylcholinesterase inhibitors. Exp Gerontol. 2004;39(4):653–657. doi: 10.1016/j.exger.2003.08.012. [DOI] [PubMed] [Google Scholar]
- 90.Han S.H., Park J.C., Byun M.S., Yi D., Lee J.H., Lee D.Y., Mook-Jung I., KBASE Research Group Blood acetylcholinesterase level is a potential biomarker for the early detection of cerebral amyloid deposition in cognitively normal individuals. Neurobiol. Aging. 2019;73:21–29. doi: 10.1016/j.neurobiolaging.2018.09.001. [DOI] [PubMed] [Google Scholar]
- 91.Kawahara K., Suenobu M., Yoshida A., Koga K., Hyodo A., Ohtsuka H., Kuniyasu A., Tamamaki N., Sugimoto Y., Nakayama H. Intracerebral microinjection of interleukin-4/interleukin-13 reduces β-amyloid accumulation in the ipsilateral side and improves cognitive deficits in young amyloid precursor protein 23 mice. Neuroscience. 2012;207:243–260. doi: 10.1016/j.neuroscience.2012.01.049. [DOI] [PubMed] [Google Scholar]
- 92.Dionisio-Santos D.A., Behrouzi A., Olschowka J.A., O’Banion M.K. Evaluating the effect of interleukin-4 in the 3xtg mouse model of Alzheimer’s disease. Front. Neurosci. 2020;14:441. doi: 10.3389/fnins.2020.00441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Porro C., Cianciulli A., Panaro M.A. The regulatory role of IL-10 in neurodegenerative diseases. Biomolecules. 2020;10(7):1–15. doi: 10.3390/biom10071017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kiyota T., Ingraham K.L., Swan R.J., Jacobsen M.T., Andrews S.J., Ikezu T. AAV serotype 2/1-mediated gene delivery of anti-inflammatory interleukin-10 enhances neurogenesis and cognitive function in APP+PS1 mice. Gene Ther. 2012;19(7):724–733. doi: 10.1038/gt.2011.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zhang Y., Zhang J., Tian C., Xiao Y., Li X., He C., Huang J., Fan H. The -1082G/A polymorphism in IL-10 gene is associated with risk of Alzheimer’s disease: A meta- analysis. J. Neurol. Sci. 2011;303(1-2):133–138. doi: 10.1016/j.jns.2010.12.005. [DOI] [PubMed] [Google Scholar]
- 96.Decourt B., Lahiri D.K., Sabbagh M.N. Targeting tumor necrosis factor alpha for alzheimer’s disease. Curr. Alzheimer Res. 2017;14(4):412–425. doi: 10.2174/1567205013666160930110551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zhao M., Cribbs D.H., Anderson A.J., Cummings B.J., Su J.H., Wasserman A.J., Cotman C.W. The induction of the TNFalpha death domain signaling pathway in Alzheimer’s disease brain. Neurochem. Res. 2003;28(2):307–318. doi: 10.1023/A:1022337519035. [DOI] [PubMed] [Google Scholar]
- 98.Brosseron F., Krauthausen M., Kummer M., Heneka M.T. Body fluid cytokine levels in mild cognitive impairment and Alzheimer’s disease: A comparative overview. Mol. Neurobiol. 2014;50(2):534–544. doi: 10.1007/s12035-014-8657-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Paouri E., Tzara O., Kartalou G-I.I., Zenelak S., Georgopoulos S. Peripheral tumor necrosis factor-alpha (tnf-α) modulates amyloid pathology by regulating blood-derived immune cells and glial response in the brain of AD/TNF transgenic mice. J. Neurosci. 2017;37(20):5155–5171. doi: 10.1523/JNEUROSCI.2484-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Paouri E., Tzara O., Zenelak S., Georgopoulos S. Genetic deletion of tumor necrosis factor-α attenuates amyloid-β production and decreases amyloid plaque formation and glial response in the 5xfad model of Alzheimer’s disease. J. Alzheimers Dis. 2017;60(1):165–181. doi: 10.3233/JAD-170065. [DOI] [PubMed] [Google Scholar]
- 101.Bachstetter A.D., Morganti J.M., Jernberg J., Schlunk A., Mitchell S.H., Brewster K.W., Hudson C.E., Cole M.J., Harrison J.K., Bickford P.C., Gemma C. Fractalkine and CX 3 CR1 regulate hippocampal neurogenesis in adult and aged rats. Neurobiol. Aging. 2011;32(11):2030–2044. doi: 10.1016/j.neurobiolaging.2009.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Rogers J.T., Morganti J.M., Bachstetter A.D., Hudson C.E., Peters M.M., Grimmig B.A., Weeber E.J., Bickford P.C., Gemma C. CX3CR1 deficiency leads to impairment of hippocampal cognitive function and synaptic plasticity. J. Neurosci. 2011;31(45):16241–16250. doi: 10.1523/JNEUROSCI.3667-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Xu Y., Zeng K., Han Y., Wang L., Chen D., Xi Z., Wang H., Wang X., Chen G. Altered expression of CX3CL1 in patients with epilepsy and in a rat model. Am. J. Pathol. 2012;180(5):1950–1962. doi: 10.1016/j.ajpath.2012.01.024. [DOI] [PubMed] [Google Scholar]
- 104.Donohue M.M., Cain K., Zierath D., Shibata D., Tanzi P.M., Becker K.J. Higher plasma fractalkine is associated with better 6-month outcome from ischemic stroke. Stroke. 2012;43(9):2300–2306. doi: 10.1161/STROKEAHA.112.657411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Bhaskar K., Konerth M., Kokiko-Cochran O.N., Cardona A., Ransohoff R.M., Lamb B.T. Regulation of tau pathology by the microglial fractalkine receptor. Neuron. 2010;68(1):19–31. doi: 10.1016/j.neuron.2010.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Cho S.H., Sun B., Zhou Y., Kauppinen T.M., Halabisky B., Wes P., Ransohoff R.M., Gan L. CX3CR1 protein signaling modulates microglial activation and protects against plaque-independent cognitive deficits in a mouse model of Alzheimer disease. J. Biol. Chem. 2011;286(37):32713–32722. doi: 10.1074/jbc.M111.254268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ma Q., Jones D., Borghesani P.R., Segal R.A., Nagasawa T., Kishimoto T., Bronson R.T., Springer T.A. Impaired B-lymphopoiesis, myelopoiesis, and derailed cerebellar neuron migration in CXCR4- and SDF-1-deficient mice. Proc. Natl. Acad. Sci. USA. 1998;95(16):9448–9453. doi: 10.1073/pnas.95.16.9448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Trousse F., Jemli A., Silhol M., Garrido E., Crouzier L., Naert G., Maurice T., Rossel M. Knockdown of the CXCL12/CXCR7 chemokine pathway results in learning deficits and neural progenitor maturation impairment in mice. Brain Behav. Immun. 2019;80:697–710. doi: 10.1016/j.bbi.2019.05.019. [DOI] [PubMed] [Google Scholar]
- 109.Janssens R., Struyf S., Proost P. Pathological roles of the homeostatic chemokine CXCL12. Cytokine Growth Factor Rev. 2018;44:51–68. doi: 10.1016/j.cytogfr.2018.10.004. [DOI] [PubMed] [Google Scholar]
- 110.Tanabe S., Heesen M., Yoshizawa I., Berman M.A., Luo Y., Bleul C.C., Springer T.A., Okuda K., Gerard N., Dorf M.E. Functional expression of the CXC-chemokine receptor-4/fusin on mouse microglial cells and astrocytes. J. Immunol. 1997;159(2):905–911. [PubMed] [Google Scholar]
- 111.Bonham L.W., Karch C.M., Fan C.C., Tan C., Geier E.G., Wang Y., Wen N., Broce I.J., Li Y., Barkovich M.J., Ferrari R., Hardy J., Momeni P., Höglinger G., Müller U., Hess C.P., Sugrue L.P., Dillon W.P., Schellenberg G.D., Miller B.L., Andreassen O.A., Dale A.M., Barkovich A.J., Yokoyama J.S., Desikan R.S., International FTD-Genomics Consortium (IFGC) International Parkinson’s Disease Genetics Consortium (IPDGC) International Genomics of Alzheimer’s Project (IGAP) CXCR4 involvement in neurodegenerative diseases. Transl. Psychiatry. 2018;8(1):73. doi: 10.1038/s41398-017-0049-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Sanfilippo C., Castrogiovanni P., Imbesi R., Nunnari G., Di Rosa M. Postsynaptic damage and microglial activation in AD patients could be linked CXCR4/CXCL12 expression levels. Brain Res. 2020;1749:147127. doi: 10.1016/j.brainres.2020.147127. [DOI] [PubMed] [Google Scholar]
- 113.Wang Q., Xu Y., Chen J-C.C., Qin Y-Y.Y., Liu M., Liu Y., Xie M-J.J., Yu Z-Y.Y., Zhu Z., Wang W. Stromal cell-derived factor 1α decreases β-amyloid deposition in Alzheimer’s disease mouse model. Brain Res. 2012;1459:15–26. doi: 10.1016/j.brainres.2012.04.011. [DOI] [PubMed] [Google Scholar]
- 114.Deshmane S.L., Kremlev S., Amini S., Sawaya B.E. Monocyte chemoattractant protein-1 (MCP-1): An overview. J. Interferon Cytokine Res. 2009;29(6):313–326. doi: 10.1089/jir.2008.0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Kiyota T., Gendelman H.E., Weir R.A., Higgins E.E., Zhang G., Jain M. CCL2 affects β-amyloidosis and progressive neurocognitive dysfunction in a mouse model of Alzheimer’s disease. Neurobiol. Aging. 2013;34(4):1060–1068. doi: 10.1016/j.neurobiolaging.2012.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Sokolova A., Hill M.D., Rahimi F., Warden L.A., Halliday G.M., Shepherd C.E. Monocyte chemoattractant protein-1 plays a dominant role in the chronic inflammation observed in Alzheimer’s disease. Brain Pathol. 2009;19(3):392–398. doi: 10.1111/j.1750-3639.2008.00188.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Smits H.A., Rijsmus A., van Loon J.H., Wat J.W.Y., Verhoef J., Boven L.A., Nottet H.S.L.M. Amyloid-β-induced chemokine production in primary human macrophages and astrocytes. J. Neuroimmunol. 2002;127(1-2):160–168. doi: 10.1016/S0165-5728(02)00112-1. [DOI] [PubMed] [Google Scholar]
- 118.Vukic V., Callaghan D., Walker D., Lue L.F., Liu Q.Y., Couraud P.O., Romero I.A., Weksler B., Stanimirovic D.B., Zhang W. Expression of inflammatory genes induced by beta-amyloid peptides in human brain endothelial cells and in Alzheimer’s brain is mediated by the JNK-AP1 signaling pathway. Neurobiol. Dis. 2009;34(1):95–106. doi: 10.1016/j.nbd.2008.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Liu C., Cui G., Zhu M., Kang X., Guo H. NeuroInflammation in Alzheimer’s disease: chemokines produced by astrocytes and chemokine receptors. Int. J. Clin. Exp. Pathol. 2014;7(12):8342–8355. [PMC free article] [PubMed] [Google Scholar]
- 120.Kimura A., Yoshikura N., Hayashi Y., Inuzuka T. Cerebrospinal fluid c-c motif chemokine ligand 2 correlates with brain atrophy and cognitive impairment in Alzheimer’s disease. J. Alzheimers Dis. 2018;61(2):581–588. doi: 10.3233/JAD-170519. [DOI] [PubMed] [Google Scholar]
- 121.Shen X.N., Niu L.D., Wang Y.J., Cao X.P., Liu Q., Tan L., Zhang C., Yu J.T. Inflammatory markers in Alzheimer’s disease and mild cognitive impairment: A meta-analysis and systematic review of 170 studies. J. Neurol. Neurosurg. Psychiatry. 2019;90(5):590–598. doi: 10.1136/jnnp-2018-319148. [DOI] [PubMed] [Google Scholar]
- 122.Westin K., Buchhave P., Nielsen H., Minthon L., Janciauskiene S., Hansson O. CCL2 is associated with a faster rate of cognitive decline during early stages of Alzheimer’s disease. PLoS One. 2012;7(1):e30525. doi: 10.1371/journal.pone.0030525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Flynn G., Maru S., Loughlin J., Romero I.A., Male D. Regulation of chemokine receptor expression in human microglia and astrocytes. J. Neuroimmunol. 2003;136(1-2):84–93. doi: 10.1016/S0165-5728(03)00009-2. [DOI] [PubMed] [Google Scholar]
- 124.Xia M.Q., Bacskai B.J., Knowles R.B., Qin S.X., Hyman B.T. Expression of the chemokine receptor CXCR3 on neurons and the elevated expression of its ligand IP-10 in reactive astrocytes: In vitro ERK1/2 activation and role in Alzheimer’s disease. J. Neuroimmunol. 2000;108(1-2):227–235. doi: 10.1016/S0165-5728(00)00285-X. [DOI] [PubMed] [Google Scholar]
- 125.Krauthausen M., Kummer M.P., Zimmermann J., Reyes-Irisarri E., Terwel D., Bulic B., Heneka M.T., Müller M. CXCR3 promotes plaque formation and behavioral deficits in an Alzheimer’s disease model. J. Clin. Invest. 2015;125(1):365–378. doi: 10.1172/JCI66771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Galimberti D., Schoonenboom N., Scheltens P., Fenoglio C., Venturelli E., Pijnenburg Y.A.L., Bresolin N., Scarpini E. Intrathecal chemokine levels in Alzheimer disease and frontotemporal lobar degeneration. Neurology. 2006;66(1):146–147. doi: 10.1212/01.wnl.0000191324.08289.9d. [DOI] [PubMed] [Google Scholar]
- 127.Koper O.M., Kamińska J., Sawicki K., Kemona H. CXCL9, CXCL10, CXCL11, and their receptor (CXCR3) in neuroinflammation and neurodegeneration. Adv. Clin. Exp. Med. 2018;27(6):849–856. doi: 10.17219/acem/68846. [DOI] [PubMed] [Google Scholar]
- 128.Corrêa J.D., Starling D., Teixeira A.L., Caramelli P., Silva T.A. Chemokines in CSF of Alzheimer’s disease patients. Arq. Neuropsiquiatr. 2011;69(3):455–459. doi: 10.1590/S0004-282X2011000400009. [DOI] [PubMed] [Google Scholar]
- 129.Su F., Bai F., Zhang Z. Inflammatory cytokines and Alzheimer’s disease: a review from the perspective of genetic polymorphisms. Neurosci. Bull. 2016;32(5):469–480. doi: 10.1007/s12264-016-0055-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Hua Y., Guo X., Huang Q., Kong Y., Lu X. Association between interleukin-6 -174G/C polymorphism and the risk of Alzheimer’s disease: A meta-analysis. Int. J. Neurosci. 2013;123(9):626–635. doi: 10.3109/00207454.2013.784286. [DOI] [PubMed] [Google Scholar]
- 131.Qin X., Peng Q., Zeng Z., Chen Z., Lin L., Deng Y., Huang X., Xu J., Wu H., Huang S., Li S., Zhao J. Interleukin-1A -889C/T polymorphism and risk of Alzheimer’s disease: A meta-analysis based on 32 case-control studies. J. Neurol. 2012;259(8):1519–1529. doi: 10.1007/s00415-011-6381-6. [DOI] [PubMed] [Google Scholar]
- 132.Singhal G., Jaehne E.J., Corrigan F., Toben C., Baune B.T. Inflammasomes in neuroinflammation and changes in brain function: A focused review. Front. Neurosci. 2014;8:315. doi: 10.3389/fnins.2014.00315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Mrak R.E., Griffin W.S.T. Glia and their cytokines in progression of neurodegeneration. Neurobiol. Aging. 2005;26(3):349–354. doi: 10.1016/j.neurobiolaging.2004.05.010. [DOI] [PubMed] [Google Scholar]
- 134.Déniz-Naranjo M.C., Muñoz-Fernandez C., Alemany-Rodríguez M.J., Pérez-Vieitez M.C., Aladro-Benito Y., Irurita-Latasa J., Sánchez-García F. Cytokine IL-1 beta but not IL-1 alpha promoter polymorphism is associated with Alzheimer disease in a population from the Canary Islands, Spain. Eur. J. Neurol. 2008;15(10):1080–1084. doi: 10.1111/j.1468-1331.2008.02252.x. [DOI] [PubMed] [Google Scholar]
- 135.Di Bona D., Plaia A., Vasto S., Cavallone L., Lescai F., Franceschi C., Licastro F., Colonna-Romano G., Lio D., Candore G., Caruso C. Association between the interleukin-1β polymorphisms and Alzheimer’s disease: A systematic review and meta-analysis. Brain Res. Brain Res. Rev. 2008;59(1):155–163. doi: 10.1016/j.brainresrev.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 136.Lee Y.H., Choi S.J., Ji J.D., Song G.G. Association between TNF-α promoter -308 A/G polymorphism and Alzheimer’s disease: A meta-analysis. Neurol. Sci. 2015;36(6):825–832. doi: 10.1007/s10072-015-2102-8. [DOI] [PubMed] [Google Scholar]
- 137.Ribizzi G., Fiordoro S., Barocci S., Ferrari E., Megna M. Cytokine polymorphisms and Alzheimer disease: possible associations. Neurol. Sci. 2010;31(3):321–325. doi: 10.1007/s10072-010-0221-9. [DOI] [PubMed] [Google Scholar]
- 138.Li W., Qian X., Teng H., Ding Y., Zhang L. Association of interleukin-4 genetic polymorphisms with sporadic Alzheimer’s disease in Chinese Han population. Neurosci. Lett. 2014;563:17–21. doi: 10.1016/j.neulet.2014.01.019. [DOI] [PubMed] [Google Scholar]
- 139.Moraes C.F., Benedet A.L., Souza V.C., Lins T.C., Camargos E.F., Naves J.O.S., Brito C.J., Córdova C., Pereira R.W., Nóbrega O.T. Cytokine gene polymorphisms and Alzheimer’s disease in Brazil. Neuroimmunomodulation. 2013;20(5):239–246. doi: 10.1159/000350368. [DOI] [PubMed] [Google Scholar]
- 140.Karch C.M., Goate A.M. Alzheimer’s disease risk genes and mechanisms of disease pathogenesis. Biol. Psychiatry. 2015;77(1):43–51. doi: 10.1016/j.biopsych.2014.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Griciuc A., Serrano-Pozo A., Parrado A.R., Lesinski A.N., Asselin C.N., Mullin K., Hooli B., Choi S.H., Hyman B.T., Tanzi R.E. Alzheimer’s disease risk gene CD33 inhibits microglial uptake of amyloid beta. Neuron. 2013;78(4):631–643. doi: 10.1016/j.neuron.2013.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Reitz C., Mayeux R., Alzheimer’s Disease Genetics Consortium TREM2 and neurodegenerative disease. N. Engl. J. Med. 2013;369(16):1564–1565. doi: 10.1056/NEJMc1306509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Tejera D., Heneka M.T. Methods in Molecular Biology. Vol. 2034. Humana Press Inc.; 2019. Microglia in Neurodegenerative Disorders. pp. 57–67. [DOI] [PubMed] [Google Scholar]
- 144.Wang Y., Cella M., Mallinson K., Ulrich J.D., Young K.L., Robinette M.L., Gilfillan S., Krishnan G.M., Sudhakar S., Zinselmeyer B.H., Holtzman D.M., Cirrito J.R., Colonna M. TREM2 lipid sensing sustains the microglial response in an Alzheimer’s disease model. Cell. 2015;160(6):1061–1071. doi: 10.1016/j.cell.2015.01.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Fan Z., Brooks D.J., Okello A., Edison P. An early and late peak in microglial activation in Alzheimer’s disease trajectory. Brain. 2017;140(3):792–803. doi: 10.1093/brain/aww349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Yeh F.L., Hansen D.V., Sheng M. TREM2, Microglia, and Neurodegenerative Diseases. Trends Mol. Med. 2017;23(6):512–533. doi: 10.1016/j.molmed.2017.03.008. [DOI] [PubMed] [Google Scholar]
- 147.Lashley T., Schott J.M., Weston P., Murray C.E., Wellington H., Keshavan A., Foti S.C., Foiani M., Toombs J., Rohrer J.D., Heslegrave A., Zetterberg H. Molecular biomarkers of Alzheimer’s disease: progress and prospects. Dis. Model. Mech. 2018;11(5):dmm031781. doi: 10.1242/dmm.031781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Suárez-Calvet M., Kleinberger G., Araque Caballero M.Á., Brendel M., Rominger A., Alcolea D., Fortea J., Lleó A., Blesa R., Gispert J.D., Sánchez-Valle R., Antonell A., Rami L., Molinuevo J.L., Brosseron F., Traschütz A., Heneka M.T., Struyfs H., Engelborghs S., Sleegers K., Van Broeckhoven C., Zetterberg H., Nellgård B., Blennow K., Crispin A., Ewers M., Haass C. sTREM2 cerebrospinal fluid levels are a potential biomarker for microglia activity in early-stage Alzheimer’s disease and associate with neuronal injury markers. EMBO Mol. Med. 2016;8(5):466–476. doi: 10.15252/emmm.201506123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Piccio L., Deming Y., Del-Águila J.L., Ghezzi L., Holtzman D.M., Fagan A.M., Fenoglio C., Galimberti D., Borroni B., Cruchaga C. Cerebrospinal fluid soluble TREM2 is higher in Alzheimer disease and associated with mutation status. Acta Neuropathol. 2016;131(6):925–933. doi: 10.1007/s00401-016-1533-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Yang J., Fu Z., Zhang X., Xiong M., Meng L., Zhang Z. TREM2 ectodomain and its soluble form in Alzheimer’s disease. J. Neuroinflammation. 2020;17(1):204. doi: 10.1186/s12974-020-01878-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Querol-Vilaseca M., Colom-Cadena M., Pegueroles J., San Martín-Paniello C., Clarimon J., Belbin O., Fortea J., Lleó A. YKL-40 (Chitinase 3-like I) is expressed in a subset of astrocytes in Alzheimer’s disease and other tauopathies. J. Neuroinflammation. 2017;14(1):118. doi: 10.1186/s12974-017-0893-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Craig-Schapiro R., Perrin R.J., Roe C.M., Xiong C., Carter D., Cairns N.J., Mintun M.A., Peskind E.R., Li G., Galasko D.R., Clark C.M., Quinn J.F., D’Angelo G., Malone J.P., Townsend R.R., Morris J.C., Fagan A.M., Holtzman D.M. YKL-40: A novel prognostic fluid biomarker for preclinical Alzheimer’s disease. Biol. Psychiatry. 2010;68(10):903–912. doi: 10.1016/j.biopsych.2010.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Wiley C.A., Bonneh-Barkay D., Dixon C.E., Lesniak A., Wang G., Bissel S.J., Kochanek P.M. Role for mammalian chitinase 3-like protein 1 in traumatic brain injury. Neuropathology. 2015;35(2):95–106. doi: 10.1111/neup.12158. [DOI] [PubMed] [Google Scholar]
- 154.Llorens F., Thüne K., Tahir W., Kanata E., Diaz-Lucena D., Xanthopoulos K., Kovatsi E., Pleschka C., Garcia-Esparcia P., Schmitz M., Ozbay D., Correia S., Correia Â., Milosevic I., Andréoletti O., Fernández-Borges N., Vorberg I.M., Glatzel M., Sklaviadis T., Torres J.M., Krasemann S., Sánchez-Valle R., Ferrer I., Zerr I. YKL-40 in the brain and cerebrospinal fluid of neurodegenerative dementias. Mol. Neurodegener. 2017;12(1):83. doi: 10.1186/s13024-017-0226-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Colton C.A., Mott R.T., Sharpe H., Xu Q., Van Nostrand W.E., Vitek M.P. Expression profiles for macrophage alternative activation genes in AD and in mouse models of AD. J. Neuroinflammation. 2006;3:27. doi: 10.1186/1742-2094-3-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Rosén C., Andersson C-H., Andreasson U., Molinuevo J.L., Bjerke M., Rami L., Lladó A., Blennow K., Zetterberg H. Increased levels of chitotriosidase and ykl-40 in cerebrospinal fluid from patients with Alzheimer’s disease. Dement. Geriatr. Cogn. Disord. Extra. 2014;4(2):297–304. doi: 10.1159/000362164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Blanco-Palmero V.A., Rubio-Fernández M., Antequera D., Villarejo-Galende A., Molina J.A., Ferrer I., Bartolome F., Carro E. Increased YKL-40 but not C-reactive protein levels in patients with Alzheimer’s disease. Biomedicines. 2021;9(9):1094. doi: 10.3390/biomedicines9091094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Nishibori M., Wang D., Ousaka D., Wake H. High mobility group box-1 and blood-brain barrier disruption. Cells. 2020;9(12):2650. doi: 10.3390/cells9122650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Venegas C., Heneka M.T. Danger-associated molecular patterns in Alzheimer’s disease. J. Leukoc. Biol. 2017;101(1):87–98. doi: 10.1189/jlb.3MR0416-204R. [DOI] [PubMed] [Google Scholar]
- 160.Takata K., Kitamura Y., Kakimura J., Shibagaki K., Tsuchiya D., Taniguchi T., Smith M.A., Perry G., Shimohama S. Role of high mobility group protein-1 (HMG1) in amyloid-β homeostasis. Biochem. Biophys. Res. Commun. 2003;301(3):699–703. doi: 10.1016/S0006-291X(03)00024-X. [DOI] [PubMed] [Google Scholar]
- 161.Tsui K-H., Chang Y-L., Feng T-H., Chang P-L., Juang H-H. Glycoprotein transmembrane nmb: An androgen-downregulated gene attenuates cell invasion and tumorigenesis in prostate carcinoma cells. Prostate. 2012;72(13):1431–1442. doi: 10.1002/pros.22494. [DOI] [PubMed] [Google Scholar]
- 162.Tanaka H., Shimazawa M., Kimura M., Takata M., Tsuruma K., Yamada M., Takahashi H., Hozumi I., Niwa J., Iguchi Y., Nikawa T., Sobue G., Inuzuka T., Hara H. The potential of GPNMB as novel neuroprotective factor in amyotrophic lateral sclerosis. Sci. Rep. 2012;2:573. doi: 10.1038/srep00573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Ripoll V.M., Irvine K.M., Ravasi T., Sweet M.J., Hume D.A. Gpnmb is induced in macrophages by IFN-γ and lipopolysaccharide and acts as a feedback regulator of proinflammatory responses. J. Immunol. 2007;178(10):6557–6566. doi: 10.4049/jimmunol.178.10.6557. [DOI] [PubMed] [Google Scholar]
- 164.Nagahara Y., Shimazawa M., Tanaka H., Ono Y., Noda Y., Ohuchi K., Tsuruma K., Katsuno M., Sobue G., Hara H. Glycoprotein nonmetastatic melanoma protein B ameliorates skeletal muscle lesions in a SOD1G93A mouse model of amyotrophic lateral sclerosis. J. Neurosci. Res. 2015;93(10):1552–1566. doi: 10.1002/jnr.23619. [DOI] [PubMed] [Google Scholar]
- 165.Murata K., Yoshino Y., Tsuruma K., Moriguchi S., Oyagi A., Tanaka H., Ishisaka M., Shimazawa M., Fukunaga K., Hara H. The extracellular fragment of GPNMB (Glycoprotein nonmelanosoma protein B, osteoactivin) improves memory and increases hippocampal GluA1 levels in mice. J. Neurochem. 2015;132(5):583–594. doi: 10.1111/jnc.13010. [DOI] [PubMed] [Google Scholar]
- 166.Satoh J.I., Kino Y., Yanaizu M., Ishida T., Saito Y. Microglia express GPNMB in the brains of Alzheimer’s disease and Nasu-Hakola disease. Intractable Rare Dis. Res. 2019;8(2):120–128. doi: 10.5582/irdr.2019.01049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Aichholzer F., Klafki H-W., Ogorek I., Vogelgsang J., Wiltfang J., Scherbaum N., Weggen S., Wirths O. Evaluation of cerebrospinal fluid glycoprotein NMB (GPNMB) as a potential biomarker for Alzheimer’s disease. Alzheimers Res. Ther. 2021;13(1):94. doi: 10.1186/s13195-021-00828-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Guzik-Makaruk E.M., Pływaczewski E.W., Laskowska K., Filipkowski W., Jurgielewicz-Delegacz E., Mroczko P. A comparative analysis of the treatment of decision-making by or for patients with neurodegenerative diseases in four legal jurisdictions. J. Alzheimers Dis. 2019;70(1):1–10. doi: 10.3233/JAD-190259. [DOI] [PubMed] [Google Scholar]
- 169.Liu Y., Nguyen M., Robert A., Meunier B. Metal ions in Alzheimer’s disease: a key role or not? Acc. Chem. Res. 2019;52(7):2026–2035. doi: 10.1021/acs.accounts.9b00248. [DOI] [PubMed] [Google Scholar]
- 170.Guo T., Zhang D., Zeng Y., Huang T.Y., Xu H., Zhao Y. Molecular and cellular mechanisms underlying the pathogenesis of Alzheimer’s disease. Mol. Neurodegener. 2020;15(1):40. doi: 10.1186/s13024-020-00391-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Zhao Y., Wang Y., Hu J., Zhang X., Zhang Y.W., Cut A. CutA divalent cation tolerance homolog (Escherichia coli) (CUTA) regulates β-cleavage of β-amyloid precursor protein (APP) through interacting with β-site APP cleaving protein 1 (BACE1). J. Biol. Chem. 2012;287(14):11141–11150. doi: 10.1074/jbc.M111.330209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Cahill C.M., Lahiri D.K., Huang X., Rogers J.T. Amyloid precursor protein and alpha synuclein translation, implications for iron and inflammation in neurodegenerative diseases. Biochim. Biophys. Acta. 2009;1790(7):615–628. doi: 10.1016/j.bbagen.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Ayton S., Diouf I., Bush A.I., Alzheimer’s disease Neuroimaging Initiative Evidence that iron accelerates Alzheimer’s pathology: A CSF biomarker study. J. Neurol. Neurosurg. Psychiatry. 2018;89(5):456–460. doi: 10.1136/jnnp-2017-316551. [DOI] [PubMed] [Google Scholar]
- 174.Shams M., Martola J., Charidimou A., Granberg T., Ferreira D., Westman E., Wintermark M., Iv M., Larvie M., Kristoffersen Wiberg M., Kaijser M., Forsgard N., Zetterberg H., Wahlund L.O., Shams S. Cerebrospinal fluid metals and the association with cerebral small vessel disease. J. Alzheimers Dis. 2020;78(3):1229–1236. doi: 10.3233/JAD-200656. [DOI] [PubMed] [Google Scholar]
- 175.Cummings J., Aisen P., Apostolova L.G., Atri A., Salloway S., Weiner M. Aducanumab: appropriate use recommendations. J. Prev. Alzheimers Dis. 2021;8(4):398–410. doi: 10.14283/jpad.2021.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Cummings J.L., Tong G., Ballard C. Treatment combinations for alzheimer’s disease: current and future pharmacotherapy options. J. Alzheimers Dis. 2019;67(3):779–794. doi: 10.3233/JAD-180766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Fakhoury M. Inflammation in Alzheimer’s disease. Curr. Alzheimer Res. 2020;17(11):959–961. doi: 10.2174/156720501711210101110513. [DOI] [PubMed] [Google Scholar]
- 178.Ozben T., Ozben S. Neuro-inflammation and anti-inflammatory treatment options for Alzheimer’s disease. Clin. Biochem. 2019;72:87–89. doi: 10.1016/j.clinbiochem.2019.04.001. [DOI] [PubMed] [Google Scholar]
- 179.Ray B., Chauhan N.B., Lahiri D.K. Oxidative insults to neurons and synapse are prevented by aged garlic extract and S-allyl-L-cysteine treatment in the neuronal culture and APP-Tg mouse model. J. Neurochem. 2011;117(3):388–402. doi: 10.1111/j.1471-4159.2010.07145.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Gupta V.B., Rao K.S.J. Anti-amyloidogenic activity of S-allyl-L-cysteine and its activity to destabilize Alzheimer’s β-amyloid fibrils in vitro. Neurosci. Lett. 2007;429(2-3):75–80. doi: 10.1016/j.neulet.2007.09.042. [DOI] [PubMed] [Google Scholar]
- 181.Chauhan N.B. Effect of aged garlic extract on APP processing and tau phosphorylation in Alzheimer’s transgenic model Tg2576. J. Ethnopharmacol. 2006;108(3):385–394. doi: 10.1016/j.jep.2006.05.030. [DOI] [PubMed] [Google Scholar]
- 182.Ray B., Chauhan N.B., Lahiri D.K. The “aged garlic extract:” (AGE) and one of its active ingredients S-allyl-L-cysteine (SAC) as potential preventive and therapeutic agents for Alzheimer’s disease (AD). Curr. Med. Chem. 2011;18(22):3306–3313. doi: 10.2174/092986711796504664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Chainoglou E., Hadjipavlou-Litina D. Curcumin in health and diseases: Alzheimer’s disease and curcumin analogues, derivatives, and hybrids. Int. J. Mol. Sci. 2020;21(6):1975. doi: 10.3390/ijms21061975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Fang L., Gou S., Liu X., Cao F., Cheng L. Design, synthesis and anti-Alzheimer properties of dimethylaminomethyl-substituted curcumin derivatives. Bioorg. Med. Chem. Lett. 2014;24(1):40–43. doi: 10.1016/j.bmcl.2013.12.011. [DOI] [PubMed] [Google Scholar]
- 185.Sobenin I.A., Pryanishnikov V.V., Kunnova L.M., Rabinovich Y.A., Martirosyan D.M., Orekhov A.N. The effects of time-released garlic powder tablets on multifunctional cardiovascular risk in patients with coronary artery disease. Lipids Health Dis. 2010;9:119. doi: 10.1186/1476-511X-9-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Millen A.E., Subar A.F., Graubard B.I., Peters U., Hayes R.B., Weissfeld J.L., Yokochi L.A., Ziegler R.G., PLCO Cancer Screening Trial Project Team Fruit and vegetable intake and prevalence of colorectal adenoma in a cancer screening trial. Am. J. Clin. Nutr. 2007;86(6):1754–1764. doi: 10.1093/ajcn/86.5.1754. [DOI] [PubMed] [Google Scholar]
- 187.Gullett N.P., Ruhul Amin A.R.M., Bayraktar S., Pezzuto J.M., Shin D.M., Khuri F.R., Aggarwal B.B., Surh Y-J., Kucuk O. Cancer prevention with natural compounds. Semin. Oncol. 2010;37(3):258–281. doi: 10.1053/j.seminoncol.2010.06.014. [DOI] [PubMed] [Google Scholar]
- 188.Okuda M., Fujita Y., Hijikuro I., Wada M., Uemura T., Kobayashi Y., Waku T., Tanaka N., Nishimoto T., Izumi Y., Kume T., Akaike A., Takahashi T., Sugimoto H. PE859, A novel curcumin derivative, inhibits amyloid-β and tau aggregation, and ameliorates cognitive dysfunction in senescence-accelerated mouse prone 8. J. Alzheimers Dis. 2017;59(1):313–328. doi: 10.3233/JAD-161017. [DOI] [PubMed] [Google Scholar]
- 189.Khanna S., Park H-A., Sen C.K., Golakoti T., Sengupta K., Venkateswarlu S., Roy S. Neuroprotective and antiinflammatory properties of a novel demethylated curcuminoid. Antioxid. Redox Signal. 2009;11(3):449–468. doi: 10.1089/ars.2008.2230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Ray B., Lahiri D.K. NeuroInflammation in Alzheimer’s disease: different molecular targets and potential therapeutic agents including curcumin. Curr. Opin. Pharmacol. 2009;9(4):434–444. doi: 10.1016/j.coph.2009.06.012. [DOI] [PubMed] [Google Scholar]
- 191.Konno H., Endo H., Ise S., Miyazaki K., Aoki H., Sanjoh A., Kobayashi K., Hattori Y., Akaji K. Synthesis and evaluation of curcumin derivatives toward an inhibitor of beta-site amyloid precursor protein cleaving enzyme 1. Bioorg. Med. Chem. Lett. 2014;24(2):685–690. doi: 10.1016/j.bmcl.2013.11.039. [DOI] [PubMed] [Google Scholar]
- 192.Bisht S., Khan M.A., Bekhit M., Bai H., Cornish T., Mizuma M., Rudek M.A., Zhao M., Maitra A., Ray B., Lahiri D., Maitra A., Anders R.A. A polymeric nanoparticle formulation of curcumin (NanoCurc™) ameliorates CCl4-induced hepatic injury and fibrosis through reduction of pro-inflammatory cytokines and stellate cell activation. Lab. Invest. 2011;91(9):1383–1395. doi: 10.1038/labinvest.2011.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Ray B., Bisht S., Maitra A., Maitra A., Lahiri D.K. Neuroprotective and neurorescue effects of a novel polymeric nanoparticle formulation of curcumin (NanoCurc™) in the neuronal cell culture and animal model: implications for Alzheimer’s disease. J. Alzheimers Dis. 2011;23(1):61–77. doi: 10.3233/JAD-2010-101374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Peck K.J., Girard T.A., Russo F.A., Fiocco A.J. Music and memory in alzheimer’s disease and the potential underlying mechanisms. J. Alzheimers Dis. 2016;51(4):949–959. doi: 10.3233/JAD-150998. [DOI] [PubMed] [Google Scholar]
- 195.García-Casares N., Moreno-Leiva R.M., García-Arnés J.A. Music therapy as a non-pharmacological treatment in Alzheimer’s disease. A systematic review. Rev. Neurol. 2017;65(12):529–538. doi: 10.33588/rn.6512.2017181. [DOI] [PubMed] [Google Scholar]
- 196.Gómez Gallego M., Gómez García J. Musicoterapia en la enfermedad de Alzheimer: efectos cognitivos, psicológicos y conductuales. Neurologia. 2017;32(5):300–308. doi: 10.1016/j.nrl.2015.12.003. [DOI] [PubMed] [Google Scholar]