Abstract
Aims
Guidelines recommend the use of potent P2Y12 inhibitors over clopidogrel for the reduction of ischaemic events in patients with acute coronary syndrome (ACS). However, this comes at the expense of increased bleeding. A guided selection of P2Y12 inhibiting therapy has the potential to overcome this limitation. We aimed at evaluating the comparative safety and efficacy of guided vs. routine selection of potent P2Y12 inhibiting therapy in patients with ACS.
Methods and results
We performed a network meta-analysis of randomized controlled trials (RCTs) comparing different oral P2Y12 inhibitors currently recommended for the treatment of patients with ACS (clopidogrel, prasugrel, and ticagrelor). RCTs including a guided approach (i.e. platelet function or genetic testing) vs. standard selection of P2Y12 inhibitors among patients with ACS were also included. Incidence rate ratios (IRR) and associated 95% confidence intervals (CIs) were estimated. P-scores were used to estimate hierarchies of efficacy and safety. The primary efficacy endpoint was major adverse cardiovascular events (MACE) and the primary safety endpoint was all bleeding. A total of 61 898 patients from 15 RCTs were included. Clopidogrel was used as reference treatment. A guided approach was the only strategy associated with reduced MACE (IRR: 0.80, 95% CI: 0.65–0.98) without any significant trade-off in all bleeding (IRR: 1.22, 95% CI: 0.96–1.55). A guided approach and prasugrel were associated with reduced myocardial infarction. A guided approach, prasugrel, and ticagrelor were associated with reduced stent thrombosis. Ticagrelor was also associated with reduced total and cardiovascular mortality. Prasugrel was associated with increased major bleeding. Prasugrel and ticagrelor were associated with increased minor bleeding. The incidence of stroke did not differ between treatments.
Conclusion
In patients with an ACS, compared with routine selection of potent P2Y12 inhibiting therapy (prasugrel or ticagrelor), a guided selection of P2Y12 inhibiting therapy is associated with the most favourable balance between safety and efficacy. These findings support a broader adoption of guided approach for the selection of P2Y12 inhibiting therapy in patients with ACS.
Study registration number
This study is registered in PROSPERO (CRD42021258603).
Key Question
A guided selection of P2Y12 inhibiting therapy using platelet function or genetic testing improves outcomes among patients undergoing percutaneous coronary intervention. Nevertheless, the comparative safety and efficacy of a guided versus routine selection of potent P2Y12-inhibiting therapy in acute coronary syndrome has not been explored.
Key Finding
In a comprehensive network meta-analysis including the totality of available evidence and using clopidogrel as treatment reference, a guided approach was the only strategy associated with reduced major adverse cardiovascular events without any significant trade-off in bleeding. Prasugrel and ticagrelor increased bleeding and only ticagrelor reduced mortality.
Take Home Message
A guided selection of P2Y12-inhibiting therapy represents the strategy associated with the most favourable balance between safety and efficacy. These findings support a broader adoption of guided P2Y12 inhibiting therapy in patients with acute coronary syndrome.
Keywords: Antiplatelet therapy, Acute coronary syndrome, Clopidogrel, Prasugrel, Ticagrelor, Platelet function, Genetic testing, P2Y12 inhibitors
Graphical Abstract
Structured Graphical Abstract.
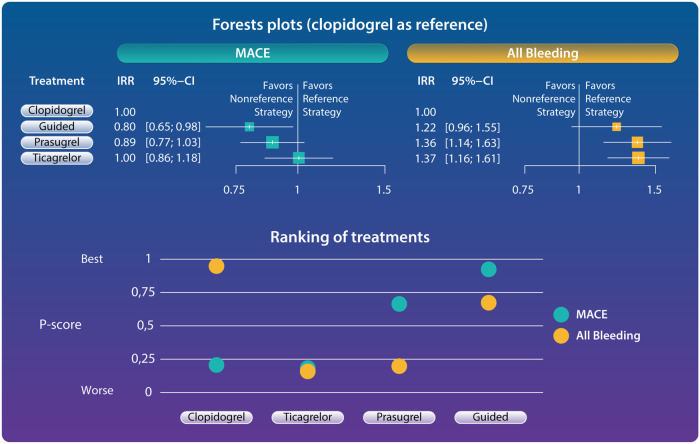
Forest plots and ranking of treatments for primary outcomes. Guided selection of P2Y12 inhibiting was the only strategy associated with reduced major adverse cardiovascular events without any increase of bleeding (forest plots). Ranking of treatments according to P-scores showed guided therapy to be the strategy with the best balance between safety and efficacy. P-scores range between 0 and 1: the higher the P-score value, the higher the likelihood that a therapy is more effective or safe. Guided approach showed the best P-score for MACE (0.931) and the second-best P-score for all bleeding (0.569). Clopidogrel showed the best P-score for all bleeding (0.983) but the poorest P-score for MACE (0.199). Prasugrel showed the second-best P-score for MACE (0.673) but a poor P-score for all bleeding (0.233). Ticagrelor showed a poor P-score for both MACE and all bleeding (0.198 and 0.214, respectively). CI, confidence interval; IRR, incidence rate ratio; MACE, major adverse cardiovascular events.
See the editorial comment for this article ‘Time for a paradigm shift? Making the case for tailored selection of antiplatelet therapy’, by Michelle L. O'Donoghue and Nicholas A. Marston, https://doi.org/10.1093/eurheartj/ehac046.
Introduction
Oral P2Y12 inhibitors (clopidogrel, prasugrel, and ticagrelor) used in conjunction with aspirin constitute a key strategy for the prevention of ischaemic recurrences in patients with acute coronary syndrome (ACS).1 Prasugrel and ticagrelor are characterized by more potent P2Y12 inhibitory effects and have shown to reduce major adverse cardiovascular events (MACE) compared with clopidogrel in large randomized controlled trials (RCTs).2–4 Accordingly, in the absence of contraindications, guidelines recommend their use over clopidogrel for the treatment of patients with ACS.1,5–7 However, such ischaemic benefit is counterbalanced by an increased risk of bleeding.2–4 Importantly, bleeding carries significant prognostic implications, including increased mortality, similar or worse than a recurrent ischaemic event.8 There is growing evidence that, compared with more potent P2Y12 inhibitors, the reduced efficacy of clopidogrel largely depends on its interindividual variability in pharmacokinetic and pharmacodynamic effects resulting in high platelet reactivity (HPR), a modifiable marker of thrombotic risk, in up to 30% of treated patients (‘clopidogrel poor responders’).9,10 Indeed, clopidogrel is a pro-drug that requires a two-step oxidation process by the hepatic cytochrome P450 (CYP) system to be activated, and carriers of genetic variants affecting the function of this enzyme significantly impact clopidogrel response.10,11 On the other hand, enhanced platelet inhibition induced by prasugrel and ticagrelor is associated with increased risk of bleeding without any reduction of ischaemic events among patients responding to clopidogrel.9,12,13
Against this background, a number of RCTs have tested the safety and efficacy of platelet function and genetic testing as tools to guide P2Y12 inhibiting therapy by selectively administering the more potent and predictable ticagrelor or prasugrel to clopidogrel poor responders.10,13 Although results of individual RCTs have not yielded consistent results, largely driven by their limited sample sizes, a recent meta-analysis overcoming such limitation found that a strategy of a guided selection of antiplatelet therapy is associated with improved outcomes as compared with standard selection of antiplatelet therapy among patients undergoing percutaneous coronary interventions (PCI).14 Nevertheless, pooled analysis specifically focused on the acute setting and comparing a guided selection of P2Y12 inhibitors to the standard of care potent P2Y12 inhibitors are lacking. Therefore, the default use of potent P2Y12 inhibitors may still be considered the preferred treatment option for patients with ACS in light of the higher level of evidence derived from large-scale pivotal RCTs.5,6,15 Comprehensive analyses providing comparative safety and efficacy of currently recommended oral P2Y12 inhibitor strategies, including a guided selection approach by means of platelet function or genetic testing, may provide important insights on the best treatment to be used in the specific setting of ACS.
Methods
Search strategy and selection criteria
This network meta-analysis was conducted according to the Cochrane Handbook for Systematic Reviews of Interventions, reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2020 guidelines16 (Supplementary material online, Table S1 for PRISMA-NMA checklist) and registered in PROSPERO (CRD42021258603). We included RCTs comparing different oral P2Y12 inhibitors currently recommended for the treatment of patients with ACS (clopidogrel, prasugrel, ticagrelor). RCTs including a guided approach (i.e. platelet function or genetic testing) vs. standard selection of P2Y12 inhibitors among patients with ACS were also included. We excluded studies in which patients without ACS accounted for more than 30% of the total population, as well as those with small sample size (<200 per arm), limited follow-up (<3 months), or using non-approved dose regimens and non-randomized studies.
From database inception to 25 June 2021, we carried out a systematic digital search using the MEDLINE with PubMed interface, the Cochrane Central Register of Controlled Trials, Embase, and the Web of Science databases. Moreover, we screened abstracts and presentations from the following cardiovascular (CV) societies: European Society of Cardiology, European Association of Percutaneous Cardiovascular Interventions, American Heart Association, American College of Cardiology, Transcatheter Cardiovascular Therapeutics, and Society of Cardiovascular Angiography and Interventions. Search terms included: ‘clopidogrel’, ‘prasugrel’, ‘ticagrelor’, ‘acute coronary syndromes’, ‘randomized controlled trials’, ‘percutaneous coronary intervention’, ‘P2Y12 inhibitor’, ‘antiplatelet’, ‘guided’, ‘platelet function’, ‘genotype’, as well as combinations of these terms (Supplementary material online, Table S2 for the full search strategy). No restrictions were applied for publication date. Previous meta-analyses on similar topics were examined. References of the included articles were inspected with a snowball approach. Literature search terms were reviewed by an experienced medical librarian.
Data extraction and risk of bias evaluation
Two investigators (M.G., S.B.) independently screened titles and abstracts for eligibility as well as the full-text, supplementary material, online appendices, and reference lists of each eligible study, to confirm the inclusion criteria and to identify further published studies. The same two investigators independently performed data extraction. There were no restrictions with respect to the language used or publication status. Disagreements were solved by consensus, and if unresolved, discussed with the senior author. Among the RCTs that met the eligibility criteria, risk of bias was independently assessed by two investigators (M.G., S.B.) according to the Cochrane Collaboration risk-of-bias-tool 2.17 Five domains of bias were evaluated: (i) randomization process, (ii) deviations from intended interventions, (iii) missing outcome data, (iv) measurement of the outcome, and (v) selection of the reported results.
Outcome measure
The primary efficacy outcome was MACE as defined in each individual included trial (Supplementary material online, Table S3). Secondary efficacy endpoints included all-cause death, CV death, myocardial infarction (MI), stroke, and definite or probable stent thrombosis (ST). The primary safety endpoint was all bleeding. Secondary safety endpoints included major bleeding and minor bleeding. Major, minor, or all bleeding were defined according to trial definitions. We prioritized the Bleeding Academic Research Consortium definition when available. If not reported, we chose the Thrombolysis in Myocardial Infarction criteria or Global Utilization of Streptokinase and t-PA for Occluded Coronary Arteries criteria.
Statistical analysis
We performed a frequentist network meta-analysis of all available treatments combining direct and indirect estimates by accounting for the correlation among multi-arm trials.18 Clopidogrel represented the reference treatment because RCTs testing the potent P2Y12 inhibitors considered clopidogrel in the control arm.2,3 A patient/year approach was adopted to address different follow-up times. Statistical heterogeneity was assessed using the Cochran Q test, Higgins and Thompson’s I2 statistic and heterogeneity variance τ2, and standard deviation τ.19 DerSimonian and Laird random-effects model was used in case of high heterogeneity, otherwise Mantel–Haenszel fixed-effects model were preferred. We estimated incidence rate ratios (IRR) and associated 95% confidence intervals (CIs), considering results as significant when the latter did not include the null value. Consistency between direct and indirect sources was examined by the node-splitting technique and net heat plots.20 Ranking of treatments according to P-scores were calculated as previously described.21 Ranks range between 0 and 1: the higher the P-score value for a given endpoint, the higher the likelihood that a therapy is more effective or safe. We carried out a pre-specified sensitivity analysis by removing studies in which an invasive approach was not performed. Three post-hoc sensitivity analyses were also included: (i) since indirect comparisons from network meta-analysis provide observational evidence across RCTs and may suffer the biases of observational studies, we run a sensitivity analysis of direct comparisons; (ii) because the use of platelet function tests results in a delay in treatment allocation based on drug response in the setting of ACS, we run a sensitivity analysis excluding the studies adopting this approach; and (iii) although we only included studies in which the population consisted in large part of patients with ACS, we performed a sensitivity analysis excluding trials not enrolling only ACS patients. Finally, pre-specified meta-regressions with a Bayesian framework and simple adjustment were carried out to assess the impact of age, proportion of patients with ST-elevation MI (STEMI) and year of publication on outcomes, considering the trial-level value obtained by averaging those of the study arms as the covariate. The goodness-of-fit of the regression models was compared with that of the original model by means of the Deviance Information Criterion (DIC), considering a five-unit DIC reduction suggestive for a goodness-of-fit improvement. All analyses were performed using R 3.6 (The R Project for Statistical Computing, Vienna, Austria), packages ‘netmeta’ and ‘gemtc’.
Results
Study selection and baseline characteristics
After removal of duplicates, a total of 9083 potentially relevant articles were screened. The PRISMA flow diagram is illustrated in Supplementary material online, Table S4. Fifteen RCTs with a total of 61 898 patients and a mean follow-up of 11.9 months met the eligibility criteria and were included in the analysis. The network of treatment regimens used in the present study is represented in Figure 1. Direct effects estimate included direct comparisons for all treatments and the contribution of direct vs. indirect comparisons for each outcome is reported in Supplementary material online, Table S5. Among RCTs testing a guided selection of P2Y12 inhibitor, two used platelet function testing and three used genetic testing.22–26 The use of an invasive strategy was high among included studies except for TRILOGY-ACS,27 which was for patients with ACS undergoing medical therapy. A description of the main features of each trial is reported in Supplementary material online, Table S3 and baseline clinical features are shown in Supplementary material online, Table S6. The risk of bias for each study and the estimate of overall risk of bias are reported in Supplementary material online, Table S7. Eight RCTs were at low risk for bias, three raised some concerns and four were at high risk of bias. There was no significant inconsistency between direct and indirect estimates for all included outcomes (Supplementary material online, Tables S8, S10, and S11). The definition of the composite outcomes of MACE and all bleeding varied slightly between studies leading to moderate/high statistical heterogeneity (Supplementary material online, Table S12). Therefore, the more conservative random effect model was used to assess these outcomes.
Figure 1.
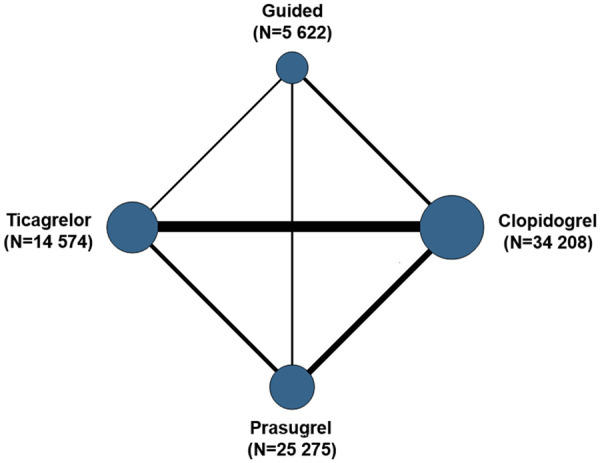
Treatment network. Numbers refer to patient/year.
Network meta-analysis results
Primary endpoints
Compared with clopidogrel, there was a statistically significant reduction in the primary efficacy endpoint of MACE with a guided approach (IRR: 0.80, 95% CI: 0.65–0.98) but not with prasugrel (IRR: 0.89, 95% CI: 0.77–1.03) and ticagrelor (IRR: 1.00, 95% CI; 0.86–1.18) (Figure 2). Guided selection of P2Y12 inhibiting therapy was associated with a marginally significant reduction of MACE compared with ticagrelor (IRR: 0.79, 95% CI: 0.63–1.00) but the reduction was not statistically significant when this was compared with prasugrel (IRR: 0.90, 95% CI: 0.72–1.12) (Supplementary material online, Table S8).
Figure 2.
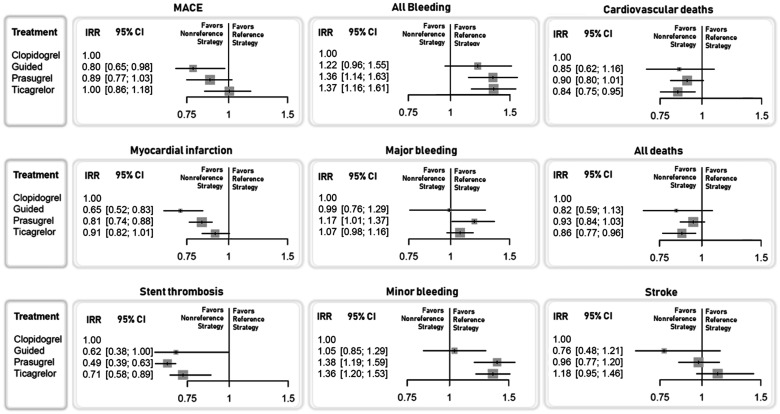
Forest plots for the included outcomes. Incidence rate ratio and 95% confidence intervals compared with clopidogrel (reference strategy) are plotted. The outcomes of major adverse cardiovascular events, all bleeding, and myocardial infarction were analysed with a random-effect model, while in the other outcomes, a fixed-effect model was used.
The primary safety endpoint of all bleeding was significantly increased with prasugrel (IRR: 1.36, 95% CI: 1.14–1.63) and ticagrelor (IRR: 1.37, 95% CI: 1.16–1.61) but not with a guided approach (IRR: 1.22, 95% CI: 0.96–1.55), compared with clopidogrel (Figure 2). There was a non-significant reduction of all bleeding favouring a guided selection of P2Y12 inhibiting therapy compared with both prasugrel and ticagrelor (Supplementary material online, Table S8).
Secondary efficacy endpoints
Compared with clopidogrel, a guided selection of P2Y12 inhibiting therapy was associated with a marginally significant reduction and prasugrel and ticagrelor with a significant reduction of ST, while only a guided approach and prasugrel significantly reduced the incidence of MI (Figure 2). A guided selection of P2Y12 inhibiting therapy was associated with a numerical reduction and ticagrelor with a numerical increase of stroke, but none of the treatments significantly impacted this risk compared with clopidogrel (Figure 2). There was a significant reduction of MI favouring a guided selection of P2Y12 inhibiting therapy compared with ticagrelor and a borderline significant reduction of ST with prasugrel compared with ticagrelor (Supplementary material online, Table S8).
Secondary safety endpoints
Prasugrel and ticagrelor, but not a guided selection of P2Y12 inhibiting therapy, were associated with increased the risk of minor bleeding while only prasugrel significantly increased the risk of major bleeding, compared with clopidogrel (Figure 2). There was a significant reduction of minor bleeding favouring a guided selection of P2Y12 inhibiting therapy compared with both prasugrel and ticagrelor (Supplementary material online, Table S8).
Mortality endpoints
Guided selection of P2Y12 inhibiting therapy as well as prasugrel and ticagrelor were associated with similar numerical reduction of all-cause and CV mortality, albeit reaching statistical significance only with ticagrelor, compared with clopidogrel (Figure 2). There were no significant differences between other treatment comparisons (Supplementary material online, Table S8).
Sensitivity analysis and meta-regression
Pre-specified analysis
Results were consistent at sensitivity analyses conducted only in patients undergoing invasive treatment, except for a significant reduction of MI with ticagrelor compared with clopidogrel and a borderline significant increase of major bleeding with prasugrel compared with clopidogrel (Supplementary material online, Table S13). Meta-regression analyses did not show any significant impact of age, percentage patients with STEMI, and year of publication on outcomes, except for a significant interaction between year of publication and all bleeding (Supplementary material online, Table S14).
Post-hoc analysis
The sensitivity analysis of direct comparisons showed the results were consistent with that of the main analysis (Supplementary material online, Table S9). At sensitivity analysis excluding the two studies adopting platelet function tests (ANTARCTIC and TROPICAL-ACS), results were consistent with the main analysis excepting for a borderline non-significant reduction of MACE and a non-significant reduction of ST with guided approach, compared with clopidogrel (Supplementary material online, Table S15). Finally, at sensitivity analysis excluding the only trial not enrolling only ACS (TAILOR-PCI), results were consistent with the main analysis excepting for a non-significant reduction of ST with guided approach, compared with clopidogrel (Supplementary material online, Table S16). The results of these two latter sensitivity analyses should be interpreted in the light of some statistical limitations (Supplementary material online, Tables S15 and S16).
Ranking of treatment strategies
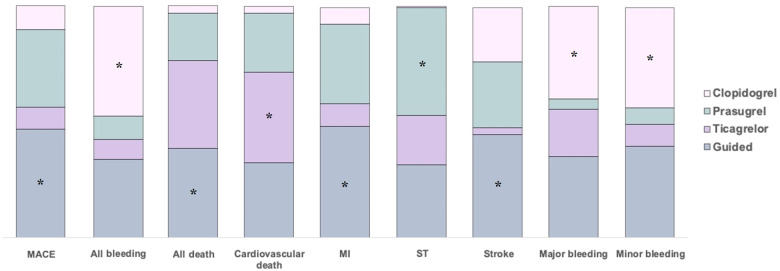
Ranking of treatments according to P-scores for each outcome are displayed in Figure 3. A guided selection of P2Y12 inhibiting therapy ranked as best treatment with respect to the outcomes of MACE, MI, stroke, and all-cause death. Prasugrel ranked as the best treatment for the outcome of ST but showed the poorest performance for bleeding outcomes. Ticagrelor ranked as best treatment for the outcome of CV death but showed a poor performance, similar to that of clopidogrel, for ischaemic outcomes and a poor performance for bleeding outcomes. Clopidogrel ranked as the best treatment for safety outcomes but showed the poorest performance on ischaemic and mortality outcomes as compared with other P2Y12 inhibitor regimens. A guided selection of P2Y12 inhibiting therapy ranked second in terms of bleeding outcomes and was only slightly inferior to clopidogrel for the outcome of major bleeding (P-score = 0.701 vs. 0.779). Overall, a guided strategy showed the most favourable safety and efficacy profile (Graphical Abstract).
Figure 3.
Ranking of treatments according to P-scores for each outcome. Coloured bars show the surface under the cumulative ranking curve values for each endpoint. Higher bars correspond to higher surface under the cumulative ranking curve and indicate better performing treatments. P-scores range between 0 and 1: the higher the P-score value, the higher the likelihood that a therapy is more effective or safe. major adverse cardiovascular events: 1° = guided (0.931), 2° = prasugrel (0.673), 3° = clopidogrel (0.199), and 4° = ticagrelor (0.198); all bleeding: 1° = clopidogrel (0.983), 2° = guided (0.569), 3° = prasugrel (0.233), and 4° = ticagrelor (0.214); all death: 1° = guided (0.764), 2° = ticagrelor (0.756), 3° = prasugrel (0.411), and 4° = clopidogrel (0.069); cardiovascular death: 1° = ticagrelor (0.771), 2° = guided (0.650), 3° = prasugrel (0.512), and 4° = clopidogrel (0.066); myocardial infarction: 1° = guided (0.983), 2° = prasugrel (0.669), 3° = ticagrelor (0.337), and 4° = clopidogrel (0.011); stent thrombosis: 1° = prasugrel (0.930), 2° = guided (0.633), 3° = ticagrelor (0.428), and 4° = clopidogrel (0.009); stroke: 1° = guided (0.891), 2° = prasugrel (0.574), 3° = clopidogrel (0.472), and 4° = ticagrelor (0.063); major bleeding: 1° = clopidogrel (0.779), 2° = guided (0.701), 3° = ticagrelor (0.413), and 4° = prasugrel (0.088); minor bleeding: 1° = clopidogrel (0.889), 2° = guided (0.776), 3° = ticagrelor (0.200), and 4° = prasugrel (0.136). *, best treatment.
Discussion
The results of this comprehensive network meta-analysis embracing 61 898 patients from 15 RCTs and comparing different P2Y12 inhibiting strategies among patients with ACS showed that, compared with clopidogrel, a guided selection of P2Y12 inhibitor is the only strategy associated with a reduction of ischaemic events without a significant trade-off in bleeding. The superior performance of a guided approach, using either platelet function or genetic testing, compared with potent P2Y12 inhibitor regimens, was consistent at ranking of treatments according to P-scores. In particular, a guided selection of P2Y12 inhibiting therapy was associated with an efficacy profile similar or superior to prasugrel and ticagrelor and was only slightly inferior to clopidogrel in terms of safety (Graphical Abstract).
The need for less aggressive antithrombotic regimens to prevent ischaemic complications with newer generation drug-eluting stents, together with the increased understanding of the prognostic relevance of bleeding events in patients undergoing PCI, have prompted investigations aimed at identifying antiplatelet treatment regimens associated with a more individualized and potentially favourable balance between ischaemic and bleeding risk.8,28 The cohort of ACS patients represent a further challenge for the optimization of antithrombotic therapy, since they are not only at higher ischaemic but are also at a higher bleeding risk.29 Patients treated with clopidogrel, but not those treated with prasugrel or ticagrelor, are characterized by interindividual variability in pharmacokinetic and pharmacodynamic response with up to 30% of patients persisting with HPR, a modifiable marker of thrombotic risk, also known as clopidogrel poor responders.10 These findings contribute to the reduced efficacy of clopidogrel compared with more potent P2Y12 inhibitors and are largely attributable to genetic polymorphisms of the CYP2C19 enzyme that lead to reduced clopidogrel bioactivation and reduced effectiveness at reducing platelet inhibition among patients carrying at least one loss-of-function allele.10,13 On the other hand, enhanced platelet inhibition induced by prasugrel and ticagrelor is associated with increased risk of bleeding without any reduction of ischaemic events among patients responding to clopidogrel.9,12,13 Against this background, the use of platelet function and genetic testing have been implemented to guide the selection of P2Y12 inhibiting therapy to the individual patient, favouring the use of clopidogrel for patients with adequate platelet inhibitory effects (‘clopidogrel responders’) and reserving alternative agents (i.e. prasugrel or ticagrelor) to those who do not (‘clopidogrel poor responders’).10,13
Early RCTs investigating a guided selection of P2Y12 inhibiting therapy failed to provide results supporting this strategy.13,14,26 An understanding of some of the limitations of these earlier studies as well as the recognition that a guided strategy may result in different outcomes depending on whether this results in escalation or de-escalation of therapy according to the clinical setting (i.e. ACS or non-ACS) in which it is adopted, led to the design of investigations better suited to define the potential benefits of guided selection of antiplatelet therapy.13,14,26 TROPICAL-ACS, POPular Genetics, and TAILOR-PCI were three major RCTs that have recently provided important insights on the use of a guided strategy in the setting of ACS.24–26 In particular, TAILOR-PCI was the largest RCT (n = 5302) that was expected to better define the effectiveness of a genotype-guided vs. a standard antiplatelet selection among PCI patients.25 Although a 34% reduction of MACE favoured the guided strategy at 12 months, this was not statistically significant given to the choice of a very ambitious 85% power to show a 50% reduction of the primary endpoint with guided therapy.25 Collectively, the inconclusive results of RCTs in this setting were largely driven by their limited sample sizes. A recent comprehensive pooled analysis overcoming low statistical power of individual RCTs found a guided approach to improve outcomes by reducing bleeding without any trade-off in efficacy when de-escalation occurred and by reducing ischaemic events without any trade-off in safety with escalation.14 Nevertheless, the lack of data on the comparative safety and efficacy of guided vs. potent P2Y12 inhibiting therapy in the specific setting of ACS may still favour the default use of prasugrel or ticagrelor in these patients, given the evidence in support of their use stems from large-scale pivotal RCTs.5,6,15 Furthermore, while broadly available, many centres are still not familiar with the use of tools to guide antiplatelet selection, which together with the lack of data on the comparative safety and efficacy of guided selection of P2Y12 inhibitors vs. the currently reference standards in ACS prasugrel or ticagrelor, might limit this strategy in clinical practice.
In this network meta-analysis embracing the totality of available evidence on the topic, we found that a guided selection of P2Y12 inhibiting therapy represents the strategy with the best balance between safety and efficacy. Indeed, prasugrel was effective in reducing individual ischaemic events compared with clopidogrel, and more effective than ticagrelor in reducing ST, but was associated with a significant increase of bleeding, including major bleeding. These findings are consistent with prior trials of prasugrel and indeed attributed to its potent and irreversible P2Y12 inhibitory effects.2,30 Notably, prasugrel ranked second for the outcome of MACE but the benefit it yielded compared with clopidogrel was not statistically significant in our analysis. This may be due to use of the more conservative random-effects model, as heterogeneity for this outcome was high, and to the fact MACE was the composite of CV death, MI, and stroke in the majority of trials. Indeed, this did not include the outcome of ST that was strongly reduced by prasugrel, but included stroke, an outcome in which prasugrel showed neutral results. Moreover, the effect size prasugrel yielded for the outcome of CV death and MI was lower compared with that of a guided approach. On the other hand, compared with clopidogrel, ticagrelor was the only strategy associated with a significant reduction of the risk of mortality, but increased minor and all bleeding, and yielded a less pronounced reduction of ischaemic events compared with a guided approach and prasugrel. Of note, we found the guided approach to reduce MACE and MI compared with ticagrelor. The superiority of prasugrel vs. ticagrelor and non-inferiority of clopidogrel vs. ticagrelor on ischaemic events have been previously described in RCTs and observational studies.31–34 Similarly, other reports have shown ticagrelor to be associated with reduced mortality in patients with ACS.3,35 This finding can potentially be attributed to the pleiotropic mechanisms of ticagrelor, including increased plasma levels of adenosine, and support the concept that there may be a mortality benefit independent of a reduction of ischaemic events.36 However, such observations have not been observed in other studies and warrants further investigation.32,34 Finally, as expected, clopidogrel represented the safest treatment (i.e. lowest risk of bleeding) but showed a poor performance on ischaemic and mortality outcomes as compared with others P2Y12 inhibitor regimens.
The implementation of a guided selection of P2Y12 inhibiting therapy may have an important economic impact not only by reducing costs related to the use of less expensive and broadly available treatments (i.e. clopidogrel), but also because of its associated improved outcomes.37,38 Indeed, these considerations largely offset any concerns surrounding the added costs of platelet function or genetic tests, which are now broadly available as rapid and easy to use bedside assays.13 Platelet function and genetic testing each have advantages and disadvantages.10,13 While platelet function testing provides a direct measure of individual response to P2Y12 inhibitors, it is characterized by an assay-dependent variability in results and requires the patient to be on treatment with clopidogrel to assess clopidogrel responsiveness, a feature that may represent a limitation in the setting of ACS. Genetic testing performed to identify functional alleles of the hepatic cytochrome CYP2C19 enzyme does not require the patient to be on clopidogrel and results remain unchanged over time. Nevertheless, CYP2C19 genotypes only contribute in part to clopidogrel response, and integrating results of genetic testing with clinical variables has shown to increase the accuracy in identifying patients with HPR.39 Of note, awareness of how an individual responds to clopidogrel may represent important information in the light of the emerging evidence supporting the use of P2Y12 inhibitor monotherapy for long-term secondary prevention.11,40,41
Study limitations
Our meta-analysis has limitations. First, the absence of patient-level data prevents assessment of baseline clinical and procedural characteristics that may potentially impact safety and efficacy outcomes as well as of outcomes not previously reported. In particular, despite we only included studies in which the population consisted in large part of ACS, a small percentage of non-ACS patients was enrolled in one of the included studies. Nevertheless, we have run a post-hoc sensitivity analysis excluding this study. Second, amongst studies testing a guided vs. standard selection of P2Y12 inhibitors, both platelet function and genetic testing were used as tools to guide the drug selection in the guided therapy arm. Nevertheless, a previous meta-analysis on the topic found no differences in the use of one or the other strategy.14 Third, there was some statistical and baseline heterogeneity among studies for the outcomes of MACE and all bleeding. To at least partially overcome this limitation, we used the more conservative random-effects model for these outcomes. Fourth, it may be argued that the ranking of treatments may be imprecise, could include minor absolute difference between comparisons, and could hide risk of bias/limitations within each comparison. Nevertheless, we used ranking of treatments as a graphical tool to facilitate the appraisal of the main results as recommended by Cochrane Handbook, and the results of ranking were consistent with the results of the main analysis. Fifth, there was an imbalance in the number of patients included in some comparisons. Nevertheless, even the less represented treatment strategy of guided selection accounts for a total of 5622 patients/year from 5 RCTs, and direct effects estimate included direct comparisons based on RCTs for all treatments. Finally, as the overall use of invasive strategy among included RCTs was very high and only one RCT (which was excluded in a sensitivity analysis) included conservatively treatment patients, the results of our analysis apply to ACS patients treated with PCI.
Conclusions
Among patients with ACS, a guided selection of P2Y12 inhibiting therapy represents the strategy associated with the most favourable balance between safety and efficacy providing significant protection against ischaemic recurrences without enhancing the risk of bleeding. Whether a guided approach represents the best strategy for reducing mortality warrants further investigations. These findings support a broader adoption of tools, including platelet function and genetic testing, to enable a more personalized selection of antiplatelet therapy among patients with ACS.
Supplementary material
Supplementary material is available at European Heart Journal online.
Funding
This work was not funded. M.G. is supported by a grant from Fondazione Enrico ed Enrica Sovena (Rome, Italy). L.H.C., F.F., and D.J.A. are supported by NIH/NCATS UL1 TR001427 and NIH/NHLBI R01 HL149752.
Conflict of interest: F.F. declares that he has received consulting fees or honoraria from AstraZeneca, Bayer, and Sanofi, outside the present work. F.R. declares that he has received honoraria from Chiesi, outside the present work. D.C. declares that he has received consulting and speaker’s fee from Amgen, Boehringer Ingelheim, Daiichi Sankyo, and Sanofi, outside the present work. G.B.Z. has consulted for Cardionovum, CrannMed, InnovHeart, Meditrial, Opsens Medical, and Replycare, outside the present work. L.H.C. receives support from Accriva Diagnostics and Inc, outside the present work. B.B. reports that he is a consulting expert, on behalf of the plaintiff, for litigation related to two specific brand models of IVC filters. J.t.B. reports lecture or consultancy fees from AstraZeneca, Eli Lilly, Daiichi Sankyo, The Medicines Company, Accu-Metrics, Boehringer Ingelheim, Bristol-Myers Squibb, Pfizer, Bayer, Ferrer, and Idorsia. He received institutional research grants from ZonMw and AstraZeneca, outside the present work. R.M. reports institutional research grants from Abbott, Abiomed, Applied Therapeutics, Arena, AstraZeneca, Bayer, Biosensors, Boston Scientific, Bristol-Myers Squibb, CardiaWave, CellAegis, CERC, Chiesi, Concept Medical, CSL Behring, DSI, Insel Gruppe AG, Medtronic, Novartis Pharmaceuticals, OrbusNeich, Philips, Transverse Medical, and Zoll; personal fees from ACC, Boston Scientific, California Institute for Regenerative Medicine (CIRM), Cine-Med Research, Janssen, WebMD, and SCAI; consulting fees paid to the institution from Abbott, Abiomed, AM-Pharma, Alleviant Medical, Bayer, Beth Israel Deaconess, CardiaWave, CeloNova, Chiesi, CSL Behring, Concept Medical, DSI, Duke University, Idorsia Pharmaceuticals, Medtronic, Novartis, Philips; Equity <1% in Applied Therapeutics, Elixir Medical, STEL, CONTROLRAD (spouse); Scientific Advisory Board for AMA, Biosensors (spouse); and Faculty CRF (no fee). C.M.G. receives research support from Johnson & Johnson. He receives consulting support from Astra-Zenca, Johnson & Johnson, and Janssen & Bayer, outside the present work. F.C. declares that he has received consulting and speaker fees from Biotronic, Amgen, Astra Zeneca, Servier, Menarini, and BMS, outside the present work. D.S. reports having received speaker fees and fees for advisory board activities in the area of antithrombotic treatment from Bayer, Sanofi Aventis, Pfizer, Daiichi Sankyo, Astra Zeneca, and Roche Diagnostics, outside the present work. D.J.A. declares that he has received consulting fees or honoraria from Abbott, Amgen, Aralez, AstraZeneca, Bayer, Biosensors, Boehringer Ingelheim, Bristol-Myers Squibb, Chiesi, Daiichi-Sankyo, Eli Lilly, Haemonetics, Janssen, Merck, PhaseBio, PLx Pharma, Pfizer, Sanofi, and The Medicines Company and has received payments for participation in review activities from CeloNova and St Jude Medical, outside the present work. D.J.A. also declares that his institution has received research grants from Amgen, AstraZeneca, Bayer, Biosensors, CeloNova, CSL Behring, Daiichi-Sankyo, Eisai, Eli Lilly, Gilead, Janssen, Matsutani Chemical Industry Co., Merck, Novartis, Osprey Medical, Renal Guard Solutions, and Scott R. MacKenzie Foundation. The remaining authors report no disclosures.
Data availability
The data underlying this article are available in the individual trials included in the analysis and have been fully reported in the article and in its online supplementary material.
Supplementary Material
References
- 1.Capodanno D, Alfonso F, Levine GN, Valgimigli M, Angiolillo DJ.. ACC/AHA versus ESC guidelines on dual antiplatelet therapy: JACC Guideline comparison. J Am Coll Cardiol 2018;72:2915–2931. [DOI] [PubMed] [Google Scholar]
- 2.Wiviott SD, Braunwald E, McCabe CH. et al. ; TRITON-TIMI 38 Investigators. Prasugrel versus clopidogrel in patients with acute coronary syndromes. N Engl J Med 2007;357:2001–2015. [DOI] [PubMed] [Google Scholar]
- 3.Wallentin L, Becker RC, Budaj A. et al. ; PLATO Investigators. Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N Engl J Med 2009;361:1045–1057. [DOI] [PubMed] [Google Scholar]
- 4.Franchi F, Angiolillo DJ.. Novel antiplatelet agents in acute coronary syndrome. Nat Rev Cardiol 2015;12:30–47. [DOI] [PubMed] [Google Scholar]
- 5.Collet JP, Thiele H, Barbato E. et al. ; ESC Scientific Document Group. 2020 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur Heart J 2021;42:1289–1367. [DOI] [PubMed] [Google Scholar]
- 6.Ibanez B, James S, Agewall S. et al. ; ESC Scientific Document Group. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: the Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J 2018;39:119–177. [DOI] [PubMed] [Google Scholar]
- 7.Levine GN, Bates ER, Bittl JA. et al. 2016 ACC/AHA Guideline Focused Update on Duration of Dual Antiplatelet Therapy in Patients With Coronary Artery Disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines: an Update of the 2011 ACCF/AHA/SCAI Guideline for Percutaneous Coronary Intervention, 2011 ACCF/AHA Guideline for Coronary Artery Bypass Graft Surgery, 2012 ACC/AHA/ACP/AATS/PCNA/SCAI/STS Guideline for the Diagnosis and Management of Patients With Stable Ischemic Heart Disease, 2013 ACCF/AHA Guideline for the Management of ST-Elevation Myocardial Infarction, 2014 AHA/ACC Guideline for the Management of Patients With Non-ST-Elevation Acute Coronary Syndromes, and 2014 ACC/AHA Guideline on Perioperative Cardiovascular Evaluation and Management of Patients Undergoing Noncardiac Surgery. Circulation 2016;134:e123–e155. [DOI] [PubMed] [Google Scholar]
- 8.Valgimigli M, Costa F, Lokhnygina Y. et al. Trade-off of myocardial infarction vs. bleeding types on mortality after acute coronary syndrome: lessons from the Thrombin Receptor Antagonist for Clinical Event Reduction in Acute Coronary Syndrome (TRACER) randomized trial. Eur Heart J 2017;38:804–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aradi D, Kirtane A, Bonello L. et al. Bleeding and stent thrombosis on P2Y12-inhibitors: collaborative analysis on the role of platelet reactivity for risk stratification after percutaneous coronary intervention. Eur Heart J 2015;36:1762–1771. [DOI] [PubMed] [Google Scholar]
- 10.Sibbing D, Aradi D, Alexopoulos D. et al. Updated expert consensus statement on platelet function and genetic testing for guiding P2Y12 receptor inhibitor treatment in percutaneous coronary intervention. JACC Cardiovasc Interv 2019;12:1521–1537. [DOI] [PubMed] [Google Scholar]
- 11.Galli M, Capodanno D, Andreotti F, Crea F, Angiolillo DJ.. Safety and efficacy of P2Y12 inhibitor monotherapy in patients undergoing percutaneous coronary interventions. Expert Opin Drug Saf 2021;20:9–21. [DOI] [PubMed] [Google Scholar]
- 12.Pereira NL, Rihal C, Lennon R. et al. Effect of CYP2C19 genotype on ischemic outcomes during oral P2Y12 inhibitor therapy: a meta-analysis. JACC Cardiovasc Interv 2021;14:739–750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Galli M, Franchi F, Rollini F. et al. Genetic testing in patients undergoing percutaneous coronary intervention: rationale, evidence and practical recommendations. Expert Rev Clin Pharmacol 2021;14:963–978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Galli M, Benenati S, Capodanno D. et al. Guided versus standard antiplatelet therapy in patients undergoing percutaneous coronary intervention: a systematic review and meta-analysis. Lancet 2021;397:1470–1483. [DOI] [PubMed] [Google Scholar]
- 15.Sibbing D, Kastrati A.. Guided P2Y12 inhibitor therapy after percutaneous coronary intervention. Lancet 2021;397:1423–1425. [DOI] [PubMed] [Google Scholar]
- 16.Moher D, Shamseer L, Clarke M. et al. ; PRISMA-P Group. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev 2015;4:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Higgins JPT, Altman DG, Gøtzsche PC. et al. ; Cochrane Bias Methods Group, Cochrane Statistical Methods Group. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011;343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tonin FS, Rotta I, Mendes AM, Pontarolo R.. Network meta-analysis: a technique to gather evidence from direct and indirect comparisons. Pharm Pract (Granada) 2017;15:943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Borenstein M, Higgins JP, Hedges LV, Rothstein HR.. Basics of meta-analysis: I2 is not an absolute measure of heterogeneity. Res Synth Methods 2017;8:5–18. [DOI] [PubMed] [Google Scholar]
- 20.Krahn U, Binder H, König J.. A graphical tool for locating inconsistency in network meta-analyses. BMC Med Res Methodol 2013;13:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rücker G, Schwarzer G.. Ranking treatments in frequentist network meta-analysis works without resampling methods. BMC Med Res Methodol 2015;15:58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cayla G, Cuisset T, Silvain J. et al. ; ANTARCTIC Investigators. Platelet function monitoring to adjust antiplatelet therapy in elderly patients stented for an acute coronary syndrome (ANTARCTIC): an open-label, blinded-endpoint, randomised controlled superiority trial. Lancet 2016;388:2015–2022. [DOI] [PubMed] [Google Scholar]
- 23.Notarangelo FM, Maglietta G, Bevilacqua P. et al. Pharmacogenomic Approach to Selecting Antiplatelet Therapy in Patients With Acute Coronary Syndromes: the PHARMCLO Trial. J Am Coll Cardiol 2018;71:1869–1877. [DOI] [PubMed] [Google Scholar]
- 24.Claassens DMF, Vos GJA, Bergmeijer TO. et al. A genotype-guided strategy for oral P2Y12 inhibitors in primary PCI. N Engl J Med 2019;381:1621–1631. [DOI] [PubMed] [Google Scholar]
- 25.Pereira NL, Farkouh ME, So D. et al. Effect of genotype-guided oral P2Y12 inhibitor selection vs conventional clopidogrel therapy on ischemic outcomes after percutaneous coronary intervention: the TAILOR-PCI randomized clinical trial. JAMA 2020;324:761–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sibbing D, Aradi D, Jacobshagen C. et al. ; TROPICAL-ACS Investigators. Guided de-escalation of antiplatelet treatment in patients with acute coronary syndrome undergoing percutaneous coronary intervention (TROPICAL-ACS): a randomised, open-label, multicentre trial. Lancet 2017;390:1747–1757. [DOI] [PubMed] [Google Scholar]
- 27.Roe MT, Armstrong PW, Fox KAA. et al. ; TRILOGY ACS Investigators. Prasugrel versus clopidogrel for acute coronary syndromes without revascularization. N Engl J Med 2012;367:1297–1309. [DOI] [PubMed] [Google Scholar]
- 28.Galli M, Angiolillo DJ.. Antiplatelet therapy in percutaneous coronary intervention: latest evidence from randomized controlled trials. Curr Opin Cardiol 2021;36:390–396. [DOI] [PubMed] [Google Scholar]
- 29.D’Ascenzo F, Biolè C, Raposeiras-Roubin S. et al. Average daily ischemic versus bleeding risk in patients with ACS undergoing PCI: insights from the BleeMACS and RENAMI registries. Am Heart J 2020;220:108–115. [DOI] [PubMed] [Google Scholar]
- 30.Michelson AD, Frelinger AL 3rd, Braunwald E. et al. Pharmacodynamic assessment of platelet inhibition by prasugrel vs. clopidogrel in the TRITON-TIMI 38 trial. Eur Heart J 2009;30:1753–1763. [DOI] [PubMed] [Google Scholar]
- 31.Gimbel M, Qaderdan K, Willemsen L. et al. Clopidogrel versus ticagrelor or prasugrel in patients aged 70 years or older with non-ST-elevation acute coronary syndrome (POPular AGE): the randomised, open-label, non-inferiority trial. Lancet 2020;395:1374–1381. [DOI] [PubMed] [Google Scholar]
- 32.You SC, Rho Y, Bikdeli B. et al. Association of ticagrelor vs clopidogrel with net adverse clinical events in patients with acute coronary syndrome undergoing percutaneous coronary intervention. JAMA 2020;324:1640–1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Turgeon RD, Koshman SL, Youngson E. et al. Association of ticagrelor vs clopidogrel with major adverse coronary events in patients with acute coronary syndrome undergoing percutaneous coronary intervention. JAMA Intern Med 2020;180:420–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schüpke S, Neumann FJ, Menichelli M. et al. ; ISAR-REACT 5 Trial Investigators. Ticagrelor or prasugrel in patients with acute coronary syndromes. N Engl J Med 2019;381:1524–1534. [DOI] [PubMed] [Google Scholar]
- 35.Navarese EP, Khan SU, Kołodziejczak M. et al. Comparative efficacy and safety of oral P2Y(12) inhibitors in acute coronary syndrome: network meta-analysis of 52 816 patients from 12 randomized trials. Circulation 2020;142:150–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cattaneo M, Schulz R, Nylander S.. Adenosine-mediated effects of ticagrelor: evidence and potential clinical relevance. J Am Coll Cardiol 2014;63:2503–2509. [DOI] [PubMed] [Google Scholar]
- 37.AlMukdad S, Elewa H, Al-Badriyeh D.. Economic evaluations of CYP2C19 genotype-guided antiplatelet therapy compared to the universal use of antiplatelets in patients with acute coronary syndrome: a systematic review. J Cardiovasc Pharmacol Ther 2020;25:201–211. [DOI] [PubMed] [Google Scholar]
- 38.Galli M, Franchi F.. Guided selection of antiplatelet therapy in acute coronary syndrome: Impact on outcomes and resource utilization. Int J Cardiol 2021;345:36–38. [DOI] [PubMed] [Google Scholar]
- 39.Valina C, Neumann FJ, Menichelli M. et al. Ticagrelor or prasugrel in patients with non-ST-segment elevation acute coronary syndromes. J Am Coll Cardiol 2020;76:2436–2446. [DOI] [PubMed] [Google Scholar]
- 40.Benenati S, Galli M, Marzo V. et al. Very short vs. long dual antiplatelet therapy after second generation drug-eluting stents in 35 785 patients undergoing percutaneous coronary interventions: a meta-analysis of randomized controlled trials. Eur Heart J Cardiovasc Pharmacother 2021;7:86–93. [DOI] [PubMed] [Google Scholar]
- 41.Koo BK, Kang J, Park KW. et al. ; HOST-EXAM investigators. Aspirin versus clopidogrel for chronic maintenance monotherapy after percutaneous coronary intervention (HOST-EXAM): an investigator-initiated, prospective, randomised, open-label, multicentre trial. Lancet 2021;397:2487–2496. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article are available in the individual trials included in the analysis and have been fully reported in the article and in its online supplementary material.