Abstract
Background
The ZF2001 vaccine, which contains a dimeric form of the receptor-binding domain of severe acute respiratory syndrome coronavirus 2 and aluminum hydroxide as an adjuvant, was shown to be safe, with an acceptable side-effect profile, and immunogenic in adults in phase 1 and 2 clinical trials.
Methods
We conducted a randomized, double-blind, placebo-controlled, phase 3 trial to investigate the efficacy and confirm the safety of ZF2001. The trial was performed at 31 clinical centers across Uzbekistan, Indonesia, Pakistan, and Ecuador; an additional center in China was included in the safety analysis only. Adult participants (≥18 years of age) were randomly assigned in a 1:1 ratio to receive a total of three 25-μg doses (30 days apart) of ZF2001 or placebo. The primary end point was the occurrence of symptomatic coronavirus disease 2019 (Covid-19), as confirmed on polymerase-chain-reaction assay, at least 7 days after receipt of the third dose. A key secondary efficacy end point was the occurrence of severe-to-critical Covid-19 (including Covid-19–related death) at least 7 days after receipt of the third dose.
Results
Between December 12, 2020, and December 15, 2021, a total of 28,873 participants received at least one dose of ZF2001 or placebo and were included in the safety analysis; 25,193 participants who had completed the three-dose regimen, for whom there were approximately 6 months of follow-up data, were included in the updated primary efficacy analysis that was conducted at the second data cutoff date of December 15, 2021. In the updated analysis, primary end-point cases were reported in 158 of 12,625 participants in the ZF2001 group and in 580 of 12,568 participants in the placebo group, for a vaccine efficacy of 75.7% (95% confidence interval [CI], 71.0 to 79.8). Severe-to-critical Covid-19 occurred in 6 participants in the ZF2001 group and in 43 in the placebo group, for a vaccine efficacy of 87.6% (95% CI, 70.6 to 95.7); Covid-19–related death occurred in 2 and 12 participants, respectively, for a vaccine efficacy of 86.5% (95% CI, 38.9 to 98.5). The incidence of adverse events and serious adverse events was balanced in the two groups, and there were no vaccine-related deaths. Most adverse reactions (98.5%) were of grade 1 or 2.
Conclusions
In a large cohort of adults, the ZF2001 vaccine was shown to be safe and effective against symptomatic and severe-to-critical Covid-19 for at least 6 months after full vaccination. (Funded by the National Science and Technology Major Project and others; ClinicalTrials.gov number, NCT04646590.)
Control of the coronavirus disease 2019 (Covid-19) pandemic, caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), remains a global priority.1 Vaccination, with a supply of multiple vaccines based on different platforms, is the cornerstone of pandemic control.2,3 The results of clinical studies of the efficacy of Covid-19 vaccines based on different platforms have been reported,4-15 and those that use messenger RNA (mRNA) or adjuvanted recombinant protein were shown to have an efficacy of 90 to 96%.8,9,13 These vaccines target either the whole virus or the spike protein. Although the receptor-binding domain (RBD) has been widely used as the Covid-19 vaccine target (four vaccines have been approved for emergency use and several are in late stages of clinical trials3,16), data regarding the efficacy of RBD-based vaccines are lacking.
Currently, B.1.617.2 (delta) and B.1.1.529 (omicron), the highly transmissible SARS-CoV-2 variants of concern, have brought new waves of infection worldwide and have accounted for breakthrough infections owing to their reduced sensitivity to the currently available vaccines.17 Although vaccine effectiveness has been reported in real-world studies,18 clinical efficacy against the prevalent delta variant of concern has been reported for only two vaccines in two randomized, controlled phase 3 trials. In one trial, the BBV152 vaccine (Bharat Biotech International) was shown to have an efficacy of 65.2%,5 and in another, the SCB-2019 vaccine was shown to have an efficacy of 78.7%.15
We developed a protein subunit Covid-19 vaccine, ZF2001, using the tandem-repeat dimeric RBD of the SARS-CoV-2 spike protein (from the original Wuhan-Hu-1 strain) as the antigen.19 The antigen protein is produced in Chinese hamster ovary cells and then adjuvanted with aluminum hydroxide to produce the vaccine. ZF2001 requires less stringent cold-chain transport and storage, a factor that facilitates its availability in the global supply. Preclinical studies of ZF2001 have shown the immunogenicity and protective efficacy of the vaccine in rodents and macaques.20 The phase 1 and 2 clinical trials showed that the ZF2001 vaccine is safe, with an acceptable side-effect profile, and immunogenic in humans, and a three-dose regimen was selected for use in a phase 3 trial.21 In serum samples, the majority of vaccine-elicited neutralizing activities against the B.1.351 (beta) variant of concern22 and pseudovirus expressing a panel of spikes from variants of concern and variants of interest, including B.1.1.7 (alpha), beta, delta, B.1.617.1 (kappa), and omicron,23,24 were shown to be preserved. ZF2001 has been approved in China, Uzbekistan, Indonesia, and Colombia.3 Here, we report the results of a multinational, randomized, double-blind, placebo-controlled, phase 3 trial of the efficacy and safety of ZF2001 in adults 18 years of age or older during a period when the delta variant was prevalent.
Methods
Trial Design and Oversight
The trial was conducted at 31 centers in Uzbekistan, Indonesia, Pakistan, and Ecuador (see the Supplementary Appendix, available with the full text of this article at NEJM.org). An additional center in China was included only in the safety evaluation because of the difficulty in efficacy evaluation posed by the dynamic zero–Covid-19 strategy used in China. All the participants provided written informed consent. The trial protocol (available at NEJM.org), the written informed consent form, and other information related to participants were approved by the clinical research ethics board of the Hunan Provincial Center for Disease Control and Prevention (China) and the corresponding ethics committees in the other four countries. The trial was conducted according to the principles of the Declaration of Helsinki, the International Council for Harmonisation Good Clinical Practice guidelines, and the International Ethical Guidelines on Biomedical Research Involving Human Subjects.
Cases of Covid-19 meeting the definition of the primary end point (i.e., the occurrence of symptomatic Covid-19, as confirmed on polymerase-chain-reaction [PCR] assay, at least 7 days after the third dose [hereafter, primary end-point cases]) and the classification of disease severity were verified by an independent end-point adjudication committee. Safety oversight was performed by an independent data safety and monitoring board.
The conduct of the trial was overseen by Anhui Zhifei Longcom Biopharmaceutical (Zhifei), which manufactured the investigational products and used contract clinical research organizations to coordinate interactions between regulatory authorities and staff at the clinical sites regarding operations. Zhifei and collaborators designed the trial. The trial-site investigators collected the data, and representatives from Beijing Keytech Statistical Technology analyzed the data. The first author wrote the initial draft of the manuscript with input from the other authors. The data were available to all the authors, who vouch for the accuracy and completeness of the data and the analysis and for adherence of the trial to the protocol. All the authors had final responsibility for the decision to submit the manuscript for publication. No confidentiality agreements were in place between Zhifei and the authors or affiliated institutions.
Trial Participants and Procedures
Volunteers 18 years of age or older who had provided written informed consent underwent screening for eligibility. Key exclusion criteria were a history of Covid-19, positive tests for SARS-CoV-2 exposure or vaccination, and a history of congenital or acquired immune deficiency or autoimmune disease. Full details regarding the inclusion and exclusion criteria are provided in the trial protocol.
Details of the trial procedures are provided in the trial protocol. Briefly, the participants were randomly assigned in a 1:1 ratio to receive three intramuscular injections in the deltoid region, administered 30 days apart, of the ZF2001 vaccine (with each dose containing 25 μg of RBD-dimer protein with 0.25 mg of aluminum hydroxide in 0.5 ml of buffer) or placebo (with each dose containing 0.25 mg of aluminum hydroxide in 0.5 ml of buffer) (Table S2). Randomization was stratified according to trial center and age (18 to 59 years or ≥60 years) and was performed with the use of an interactive Web-response system (version 5.0, Shanhu Health). The statisticians who conducted randomization were not involved in other work relevant to this clinical trial. The trial participants, field investigators, and nurses who administrated the vaccine or placebo were unaware of the trial-group assignments during the trial.
Efficacy Assessments
The primary efficacy end point was real-time PCR–confirmed symptomatic Covid-19 occurring at least 7 days after the third dose. Key secondary efficacy end points were severe-to-critical Covid-19 (including Covid-19–related death) occurring at least 7 days after the third dose and Covid-19 of any severity occurring at least 7 days after the third dose in subgroups defined according to age (18 to 59 years vs. ≥60 years). A key exploratory objective was the determination of vaccine efficacy against SARS-CoV-2 variants.
Participants with Covid-19 were monitored by the investigators through both symptom-driven passive surveillance and active follow-up visits (every 3 to 7 days) (see the Supplementary Methods section in the Supplementary Appendix). After the first dose of vaccine or placebo, a participant with any symptom of suspected Covid-19 would contact or be contacted by the investigators by telephone, text message, or email or be interviewed face -to-face. Details of the diagnostic criteria and definition of Covid-19 cases are provided in the trial protocol. If the case was confirmed by the investigators, examinations would be performed for further verification, including real-time PCR assay for SARS-CoV-2 RNA in nasopharyngeal swab samples and measurements of the respiratory rate, oxygen saturation, and oxygenation index. Samples were obtained again for testing within 24 to 48 hours after the PCR assay for case confirmation. At least one positive real-time PCR assay was required for the adjudicated cases. The S gene of SARS-CoV-2 in the positive samples was genotyped (see the Supplementary Methods section). Data regarding all suspected Covid-19 cases that were positive on real-time PCR assay, as well as the severity of disease, were reviewed and confirmed by the end-point adjudication committee.
Safety Assessments
Incident adverse events occurring within 30 minutes after each inoculation were recorded in the observation room. Adverse events (both solicited and unsolicited) occurring at 0 to 7 days after each inoculation were recorded by the participants on diary cards, which were collected by the investigators at the trial visits. Unsolicited adverse events occurring 8 to 30 days after each inoculation were recorded by the participants on contact cards. Serious adverse events occurring from the time after the first inoculation through 12 months after receipt of the third dose were documented by the investigators during telephone calls; the monitoring of serious adverse events was ongoing at the time of publication.
Statistical Analysis
In accordance with the guidelines of the World Health Organization (WHO) regarding vaccine efficacy,25,26 we aimed to detect a target vaccine efficacy of 60%, with the lower boundary of the 95% confidence interval of more than 30%. Assuming that the incidence of Covid-19 of any severity would be 1% during the trial period, we estimated that a sample of 22,144 participants (11,072 in each group) and 156 Covid-19 cases of any severity (as calculated by means of the exact conditional method according to the large-sample hypothesis of Poisson distribution of Chan and Bohidar27) would be required to provide the trial with 90% power to detect the target vaccine efficacy at a two-sided significance level of 5%. Therefore, considering the number of patients who would drop out, be involved in a protocol violation, or not adhere to the trial regimen, we planned to randomly assign 14,000 participants to each trial group.
Two interim analyses were planned, with the first to be conducted when at least one third of the estimated 156 Covid-19 cases of any severity had been reported and the second to be conducted when at least two thirds of the cases had been reported. The overall family-wise type I error was controlled at a two-sided significance level of 5% with the use of an O’Brien–Fleming alpha-spending function.28
A Poisson regression model was used to calculate the vaccine efficacy and the 95% confidence interval on the basis of the number of cases per person-year. Although two interim analyses were planned in this trial, more than 156 Covid-19 cases were reported and confirmed by the end-point adjudication committee as of June 30, 2021. The final analysis was conducted directly without any interim evaluation at the data cutoff date of June 30, 2021. Thus, the significance level of the test was not adjusted. An updated analysis with a second data cutoff date of December 15, 2021, was conducted 6 months after receipt of the third dose. Our hypothesis that the vaccine would provide better protection against Covid-19 of any severity than placebo at least 7 days after the third dose would be confirmed when the lower boundary of the 95% confidence interval for vaccine efficacy was more than 30%.
Analyses of vaccine efficacy beginning after the first dose included all the participants who had undergone randomization and received at least one dose of vaccine or placebo (the full analysis set), and analyses of vaccine efficacy beginning 7 days after the third dose (the primary efficacy analysis) included all the participants who completed the three-dose regimen of vaccine or placebo (the modified full analysis set). Participants who had received the incorrect trial regimen were included in the analysis according to their trial-group assignment on the basis of the intention-to-treat principle. The safety analysis set included all the participants who had undergone randomization and received at least one dose of vaccine or placebo; the participants were evaluated according to the trial regimen they had actually received. Additional exclusion criteria regarding the analysis populations are listed in Table S3 in the Supplementary Appendix.
Results
Participants
Between December 12, 2020, and December 15, 2021, a total of 28,904 participants were enrolled and underwent randomization at 32 clinical centers across 5 countries (China, Uzbekistan, Indonesia, Pakistan, and Ecuador). The demographic and clinical characteristics of the participants were similar in the two trial groups and in the subgroups defined according to age, body-mass index, race, and the presence of coexisting medical conditions (Table 1). Among these participants, 27,065 (93.6%) were 18 to 59 years of age, 1839 (6.4%) were 60 years of age or older, and 9383 (32.5%) were female. A total of 78.5% of the participants were Asian, and 17.7% were multiracial. More than 99.9% of the participants were negative for SARS-CoV-2 exposure or vaccination. At baseline, 13.2% of the participants had a coexisting medical condition.
Table 1. Demographic and Clinical Characteristics of the Participants at Baseline (Intention-to-Treat Population).*.
| Characteristic | ZF2001 (N=14,453)† |
Placebo (N=14,451)‡ |
All Participants (N=28,904) |
|---|---|---|---|
| Age — yr | 37.0±13.1 | 36.7±13.1 | 36.8±13.1 |
| Median (range) — yr | 35.0 (18–92) | 35.0 (17–88) | 35.0 (17–92) |
| Distribution | |||
| 18–59 yr — no. (%) | 13,534 (93.6) | 13,531 (93.6) | 27,065 (93.6) |
| Mean — yr | 35.0±11.0 | 34.7±10.9 | 34.8±10.9 |
| Median (range) — yr | 34.0 (18–59) | 34.0 (17–59) | 34.0 (17–59) |
| ≥60 yr | 919 (6.4) | 920 (6.4) | 1,839 (6.4) |
| Mean — yr | 66.2±5.6 | 65.8±5.0 | 66.0±5.3 |
| Median (range) — yr | 65.0 (60–92) | 65.0 (60–88) | 65.0 (60–92) |
| Sex — no. (%) | |||
| Male | 9,766 (67.6) | 9,755 (67.5) | 19,521 (67.5) |
| Female | 4,687 (32.4) | 4,696 (32.5) | 9,383 (32.5) |
| Body-mass index§ | 25.53±4.7 | 25.55±4.7 | 25.54±4.7 |
| Race or ethnic group — no. (%)¶ | |||
| Ethnic Chinese | 503 (3.5) | 503 (3.5) | 1,006 (3.5) |
| Asian but not ethnic Chinese | 11,251 (77.8) | 11,225 (77.7) | 22,476 (77.7) |
| Black or African or Caribbean | 5 (<0.1) | 8 (<0.1) | 13 (<0.1) |
| White | 41 (0.3) | 59 (0.4) | 100 (0.3) |
| Multiracial | 2,650 (18.3) | 2,655 (18.4) | 5,305 (18.4) |
| Other | 3 (<0.1) | 1 (<0.1) | 4 (<0.1) |
| SARS-CoV-2 status — no. (%) | |||
| Positive for IgG, IgM, or antigen or had positive real-time PCR assay‖ | 3 (<0.1) | 4 (<0.1) | 7 (<0.1) |
| Negative for IgG, IgM, and antigen and had negative real-time PCR assay | 14,437 (99.9) | 14,429 (99.8) | 28,866 (99.9) |
| Data missing | 13 (<0.1) | 18 (0.1) | 31 (0.1) |
| Any coexisting medical condition — no. (%)** | 1,904 (13.2) | 1,925 (13.3) | 3,829 (13.2) |
| Distribution according to age group | |||
| 18–59 yr | 1,601 (11.1) | 1,617 (11.2) | 3,218 (11.1) |
| ≥60 yr | 303 (2.1) | 308 (2.1) | 611 (2.1) |
Plus–minus values are means ±SD. PCR denotes polymerase chain reaction, and SARS-CoV-2 severe acute respiratory syndrome coronavirus 2.
Among the participants who had been randomly assigned to receive ZF2001, placebo was incorrectly administered to 8 at the first dose, 9 at the second dose, and 4 at the third dose. These participants were included in the protocol violation report.
Among the participants who had been randomly assigned to receive placebo, ZF2001 was incorrectly administered to 4 at the first dose, 3 at the second dose, and 3 at the third dose. These participants were included in the protocol violation report.
The body-mass index is the weight in kilograms divided by the square of the height in meters.
Race or ethnic group was reported by the participants.
Seven participants who were positive for IgG or IgM antibodies or SARS-CoV-2 antigen or had a positive real-time PCR assay at baseline were recruited by the site investigators in error. These participants were included in the protocol violation report.
Coexisting medical conditions were diseases that had started before the first dose and ended after the first dose or persisted during the trial. Coexisting conditions were classified according to the Medical Dictionary for Regulatory Activities, version 24.0.
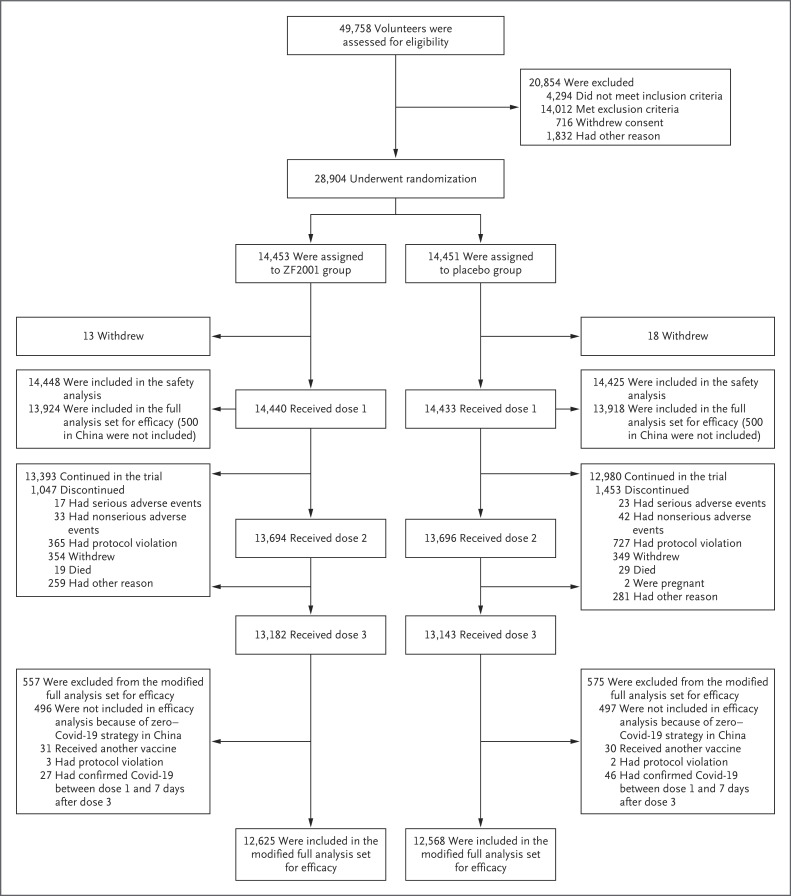
At the first data cutoff date (June 30, 2021), a total of 28,470 participants had received at least one dose of vaccine or placebo, and 14,681 were included in the modified full analysis set for efficacy (Fig. S1 and Tables S3 and S6). At the second data cutoff date (December 15, 2021), a total of 28,873 participants had received at least one dose of ZF2001 or placebo and were included in the safety analysis set. The modified full analysis set for efficacy included 25,193 participants (Figure 1 and Table S7).
Figure 1. Screening, Randomization, Follow-up, and Analyses Performed up to the Second Data Cutoff Date of December 15, 2021.
Eight participants who had been randomly assigned to receive placebo and instead received at least one dose of ZF2001 (protocol violation) were included in the ZF2001 group in the safety analysis. Covid-19 denotes coronavirus disease 2019.
Efficacy after Short-Term Follow-up
At the first data cutoff date, 663 Covid-19 cases were confirmed on real-time PCR assay after the first dose; 224 of these cases had an onset at least 7 days after the third dose and were analyzed as primary end-point cases. The mean (±SD) duration of follow-up, starting 7 days after the third dose, was 50.4±37.1 days in the ZF2001 group and 50.6±37.1 days in the placebo group. A total of 36 cases occurred among 7359 participants in the ZF2001 group, and 188 cases occurred among 7322 participants in the placebo group. These results yielded a vaccine efficacy of 81.4% (95% confidence interval [CI], 73.3 to 87.3) (Table 2).
Table 2. Vaccine Efficacy of ZF2001 against Covid-19 According to Analysis Groups.
| Efficacy Analysis | At First Data Cutoff: June 30, 2021 | At Second Data Cutoff: Dec. 15, 2021 | ||||||
|---|---|---|---|---|---|---|---|---|
| Total Cases | ZF2001 | Placebo | Vaccine Efficacy (95% CI) |
Total Cases | ZF2001 | Placebo | Vaccine Efficacy (95% CI) |
|
| no. | no. of cases/no. at risk | % | no. | no. of cases/no. at risk | % | |||
| Analyses in the modified full analysis set * | ||||||||
| Primary end point: symptomatic Covid-19 occurring ≥7 days after the third dose | 224 | 36/7359 | 188/7322 | 81.4 (73.3 to 87.3) | 738 | 158/12,625 | 580/12,568 | 75.7 (71.0 to 79.8) |
| Secondary end point: severe-to-critical Covid-19 occurring ≥7 days after the third dose | 14 | 1/7359 | 13/7322 | 92.9 (52.4 to 99.8) | 49 | 6/12,656 | 43/12,568 | 87.6 (70.6 to 95.7) |
| Secondary end-point component: Covid-19–related death occurring ≥7 days after the third dose | 5 | 0/7359 | 5/7322 | 100 (−8.4 to 100) | 14 | 2/12,656 | 12/12,568 | 86.5 (38.9 to 98.5) |
| Subgroup analyses in the modified full analysis set | ||||||||
| Analysis according to age | ||||||||
| 18–59 yr | 216 | 35/7153 | 181/7111 | 81.2 (72.8 to 87.3) | 710 | 150/11,921 | 560/11,846 | 76.0 (71.2 to 80.1) |
| ≥60 yr | 8 | 1/206 | 7/211 | 87.6 (2.5 to 99.7) | 28 | 8/704 | 20/722 | 67.6 (21.9 to 87.8) |
| Analysis according to country† | ||||||||
| Uzbekistan | 53 | 7/3185 | 46/3180 | 84.8 (66.2 to 94.2) | 191 | 35/3226 | 156/3221 | 80.2 (71.3 to 86.7) |
| Indonesia | 159 | 28/1908 | 131/1895 | 79.4 (68.9 to 86.9) | 352 | 77/1927 | 275/1902 | 76.0 (68.9 to 81.6) |
| Ecuador | 12 | 1/1323 | 11/1293 | 91.2 (39.5 to 99.8) | 130 | 33/2280 | 97/2249 | 67.6 (51.4 to 78.9) |
| Pakistan | 0 | 0/943 | 0/954 | NA | 65 | 13/5192 | 52/5196 | 75.1 (53.7 to 87.6) |
| Analysis according to race | ||||||||
| Asian | 211 | 35/5997 | 176/5964 | 80.7 (72.1 to 87.0) | 601 | 124/10,303 | 477/10,252 | 77.2 (72.2 to 81.5) |
| Other race | 13 | 1/1362 | 12/1358 | 91.7 (43.4 to 99.8) | 137 | 34/2322 | 103/2316 | 68.2 (52.8 to 79.1) |
| Analysis according to coexisting conditions that were risk factors for severe Covid-19 | ||||||||
| Cardiovascular disease | 12 | 1/203 | 11/242 | 93.1 (45.4 to 99.9) | 38 | 11/401 | 27/454 | 64.0 (20.2 to 84.9) |
| Diabetes | 5 | 1/88 | 4/95 | 31.7 (−1264.8 to 98.9) | 17 | 5/279 | 12/299 | 55.4 (−60.4 to 90.1) |
| Chronic lung disease | 3 | 1/42 | 2/27 | 74.0 (−435.3 to 99.6) | 7 | 2/54 | 5/43 | 86.7 (−13.9 to 99.0) |
| Severe obesity | 1 | 0/8 | 1/11 | 100 (−3275.7 to 100) | 1 | 0/9 | 1/14 | 100 (−4353.8 to 100) |
| Any of the above | 19 | 3/308 | 16/344 | 84.4 (41.9 to 97.2) | 58 | 18/661 | 40/716 | 61.6 (29.5 to 79.9) |
| Analysis according to SARS-CoV-2 variant‡ | ||||||||
| B.1.617.2, AY.4, AY.6, or AY.12 (delta) | 130 | 21/7359 | 109/7322 | 81.4 (70.1 to 88.9) | 454 | 96/12,625 | 358/12,568 | 76.1 (70.0 to 81.2) |
| B.1.1.7 (alpha) | 29 | 2/7359 | 27/7322 | 92.7 (70.9 to 99.2) | 35 | 4/12,625 | 31/12,568 | 88.3 (66.8 to 97.0) |
| B.1.617.1 (kappa) or B.1.617.3 | 15 | 2/7359 | 13/7322 | 84.8 (32.9 to 98.3) | 68 | 15/12,625 | 53/12,568 | 75.2 (55.3 to 87.0) |
| Other variants or not identified§ | 50 | 11/7359 | 38/7322 | 71.3 (42.7 to 86.8) | 181 | 43/12,625 | 138/12,568 | 71.9 (60.1 to 80.5) |
| Analyses in the full analysis set ¶ | ||||||||
| Symptomatic Covid-19 since the first dose | 663 | 249/13,669 | 414/13,664 | 40.2 (29.9 to 49.1) | 1255 | 405/13,909 | 850/13,899 | 55.4 (49.7 to 60.4) |
| Severe-to-critical Covid-19 since the first dose | 59 | 26/13,669 | 33/13,664 | 21.6 (−35.1 to 55.0) | 101 | 36/13,909 | 65/13,899 | 47.4 (19.8 to 66.0) |
| Covid-19–related death since the first dose | 6 | 0 | 6/13,664 | 100 (15.2 to 100) | 18 | 4/13,909 | 14/13,898 | 75.5 (21.5 to 94.1) |
The modified full analysis set for efficacy included all the participants who had undergone randomization and completed the three-dose regimen (additional exclusion criteria are listed in Table S3 in the Supplementary Appendix). Participants who had received the incorrect trial regimen were included in the analysis according to their trial-group assignment on the basis of the intention-to-treat principle. Covid-19 denotes coronavirus disease 2019, and NA not applicable.
Lot 1 vaccine was used in the Uzbekistan cohort, and lot 2 vaccine was used in the other countries.
Vaccine efficacy against each severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) variant was determined on the basis of symptomatic cases in the modified full analysis set for efficacy that were confirmed on real-time PCR assay and had SARS-CoV-2 genotyped with nasopharyngeal swabs.
Nasopharyngeal swabs with a cycle threshold value of 30 or more were not genotyped. We were able to retrieve sequences or genotypes from 185 case samples in the short-term follow-up and 626 case samples in the long-term follow-up. Shown are the efficacies against three major variants. The other 11 confirmed SARS-CoV-2 variants identified in the short-term follow-up and 69 in the long-term follow-up are shown in Table S14.
The full analysis set for efficacy included all the participants who had undergone randomization and received at least one dose of vaccine or placebo. The full analysis set was used to evaluate protective efficacy after the first dose. The end-point cases were counted since the receipt of the first dose of vaccine or placebo. The current analysis did not include data from 7 participants who had a positive test for IgG or IgM antibodies or SARS-CoV-2 antigen or had a positive real-time PCR assay at baseline and those who had not yet completed at least one case-surveillance follow-up visit (121 patients at the first data cutoff date and 27 at the second data cutoff date).
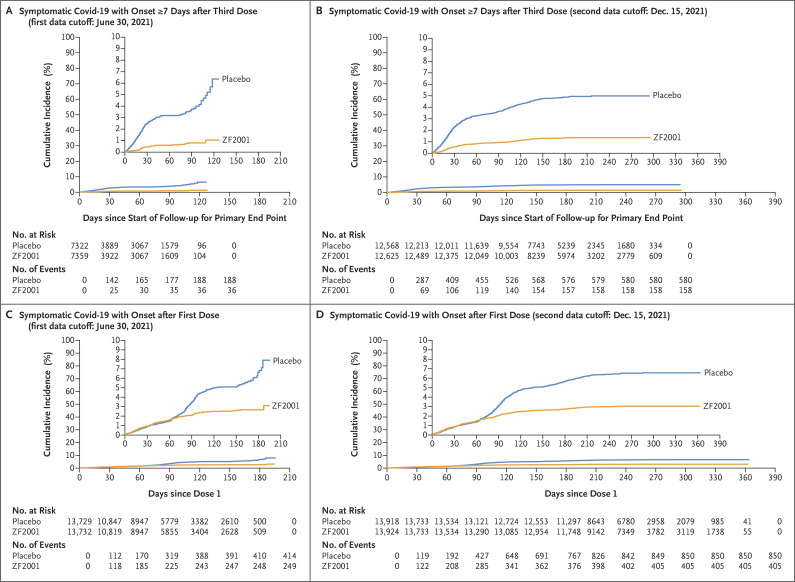
The cumulative incidence of Covid-19 events over time among the participants in the ZF2001 group and those in the placebo group diverged immediately after the beginning of follow-up for the primary end-point analysis, a result indicating the start of protection (Figure 2A). Among the participants who received at least one dose of vaccine or placebo in the full analysis set for efficacy, a late onset of protection and low vaccine efficacy were observed before the third dose (Figure 2C and Tables S10 and S11).
Figure 2. Kaplan–Meier Plots of the Cumulative Incidence of Symptomatic Covid-19 in the Trial Groups.
The cumulative incidence of symptomatic Covid-19, as confirmed on real-time polymerase-chain-reaction (PCR) assay, with an onset of at least 7 days after the third dose of ZF2001 or placebo (the primary end point) in the modified full analysis set for efficacy is shown at the first data cutoff date of June 30, 2021 (Panel A) and at the second data cutoff date of December 15, 2021 (Panel B). The cumulative incidence of real-time PCR–confirmed symptomatic Covid-19, with an onset after the first dose of ZF2001 or placebo, in the full analysis set for efficacy is shown at the first data cutoff date of June 30, 2021 (Panel C) and at the second data cutoff date of December 15, 2021 (Panel D). In each panel, the inset shows the same data on an enlarged y axis.
A total of 14 cases met the criteria for severe-to-critical Covid-19 in the modified full analysis set for efficacy. One case occurred in the ZF2001 group, and 13 occurred in the placebo group. These results yielded a vaccine efficacy of 92.9% (95% CI, 52.4 to 99.8). A total of five Covid-19–related deaths occurred — all in the placebo group (Table 2). Among the participants with coexisting medical conditions that were risk factors for severe Covid-19, vaccine efficacy was 84.4% (95% CI, 41.8 to 97.2). Among the participants 18 to 59 years of age, vaccine efficacy was 81.2% (95% CI, 72.8 to 87.3), and among those 60 years of age or older, vaccine efficacy was 87.6% (95% CI, 2.5 to 99.7). Two lots of ZF2001 vaccine were used in this trial. Lot 1 was used in the Uzbekistan cohort, and lot 2 was used in the other countries. Both lots had an efficacy of more than 80%.
In a post hoc exploratory analysis of the primary end-point cases, 185 SARS-CoV-2–positive swab samples yielded genotyping results, all of which showed variants. The three major variants were the delta (B.1.617.2, AY.4, AY.6, and AY.12) variant of concern (130 cases), the alpha (B.1.1.7) variant of concern (29 cases), and the kappa (B.1.617.1) variant of interest plus the B.1.617.3 variant (15 cases). Vaccine efficacy was 81.4% (95% CI, 70.1 to 88.9) against the delta variant, 92.7% (95% CI, 70.9 to 99.2) against the alpha variant, and 84.8% (95% CI, 32.9 to 98.3) against the kappa variant plus the B.1.617.3 variant (Table 2).
Efficacy after Long-Term Follow-up
At the second data cutoff date, 1255 Covid-19 cases had been confirmed after the first dose. Among these confirmed cases, 738 had an onset of at least 7 days after the third dose and were evaluated as primary end-point cases. The mean (±SD) duration of follow-up, starting 7 days after the third dose, was 178.6±56.9 days in the ZF2001 group and 177.8±56.4 days in the placebo group. A total of 158 cases occurred among 12,625 participants in the ZF2001 group, and 580 cases occurred among 12,568 participants in the placebo group. These results yielded a vaccine efficacy of 75.7% (95% CI, 71.0 to 79.8) (Table 2).
A time-to-event plot showed that the incidence of Covid-19 in the ZF2001 group and in the placebo group diverged immediately after the beginning of follow-up for the primary end-point analysis, a result indicating the start of protection (Figure 2B). Among the participants who received at least one dose in the full analysis set for efficacy, a late onset of protection and low efficacy before the third dose were observed (Figure 2D and Tables S10 and S11).
Vaccine efficacy against severe-to-critical Covid-19 was 87.6% (95% CI, 70.6 to 95.7), with confirmed cases of Covid-19 occurring in 6 participants in the ZF2001 group and in 43 participants in the placebo group. Vaccine efficacy against Covid-19–related death was 86.5% (95% CI, 38.9 to 98.5), with death occurring in 2 participants in the ZF2001 group and in 12 participants in the placebo group. Among the participants with coexisting medical conditions that were risk factors for severe Covid-19, vaccine efficacy was 61.6% (95% CI, 29.5 to 79.9). Among younger participants (18 to 59 years of age), vaccine efficacy was 76.0% (95% CI, 71.2 to 80.1), and among older participants (≥60 years of age), vaccine efficacy was 67.6% (95% CI, 21.9 to 87.8).
In a post hoc exploratory analysis of the 738 primary end-point cases, 626 swab samples yielded genotyping results, all of which showed SARS-CoV-2 variants. The three major variants were the delta (454 cases) and alpha (35 cases) variants of concern and the kappa variant of interest plus the B.1.617.3 variant (68 cases). Vaccine efficacy was 76.1% (95% CI, 70.0 to 81.2) against the delta variant, 88.3% (95% CI, 66.8 to 97.0) against the alpha variant, and 75.2% (95% CI, 55.3 to 87.0) against the kappa variant (Table 2).
Safety
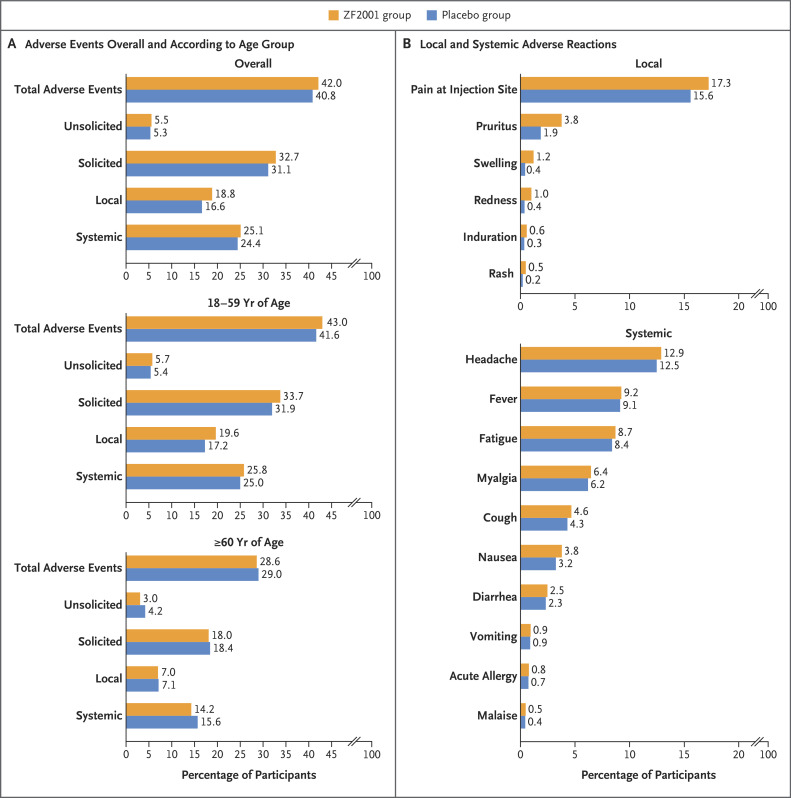
A total of 28,873 participants were included in the safety analysis set (14,448 in the ZF2001 group and 14,425 in the placebo group); 8 participants who had been randomly assigned to receive placebo and instead received at least one dose of ZF2001 (protocol violation) were included in the ZF2001 group in the safety analysis. At least one adverse event was reported by 11,957 (41.4%) participants — 6073 (42.0%) in the ZF2001 group and 5884 (40.8%) in the placebo group (Figure 3A). Adverse events reported by 9588 participants (33.2%) were related to the investigational products, as determined by the trial investigators; the incidence of these events was similar in the trial groups (34.1% [4922 participants] in the ZF2001 group and 32.4% [4666 participants] in the placebo group). Within 7 days after each dose, 4730 participants (32.7%) in the ZF2001 group and 4481 participants (31.1%) in the placebo group reported having solicited reactions. Among the 4730 participants in the ZF2001 group, the incidence of local reactions was 18.8%, and the incidence of systemic reactions was 25.1%. The incidence of unsolicited reactions was low and similar in the two groups (5.5% in the ZF2001 group and 5.3% in the placebo group). In the ZF2001 group, the most common solicited local and systemic reactions were injection-site pain (in 17.3%) and headache (in 12.9%) (Figure 3B).
Figure 3. Adverse Events at the Second Data Cutoff Date of December 15, 2021.
Panel A shows the incidence of adverse events, both overall and according to age group, among the participants who received at least one dose of ZF2001 or placebo. Panel B shows the overall incidence of local and systemic adverse reactions. A total of 28,873 participants were included in the safety analysis set (14,448 in the ZF2001 group and 14,425 in the placebo group); 8 participants who had been randomly assigned to receive placebo and instead received at least one dose of ZF2001 (protocol violation) were included in the ZF2001 group for the safety analysis.
The overall reactogenicity was consistent with that reported in a phase 2 clinical trial of ZF2001.12 In the current trial, 98.5% of the adverse reactions in the ZF2001 group were of grade 1 and 2, according to the criteria of the National Medical Products Administration (Tables S17 through S20), and 1.5% were of grade 3 or higher. The second and third injections did not further increase the incidence of adverse reactions (Table S21).
Serious adverse events were reported by 463 participants (1.6%) — 199 (1.4%) in the ZF2001 group and 264 (1.8%) in the placebo group (Table S23). A total of 4 participants (2 ZF2001 recipients and 2 placebo recipients) reported serious adverse events that were determined by the trial investigators to be related to the investigational products in terms of hypersensitivity. All symptoms resolved after medical treatment without sequelae (Table S24). A total of 48 deaths were documented, but none were attributed to the investigational products (Table S25). No cases of antibody-dependent enhancement or vaccine-enhanced disease were confirmed.
The incidence of adverse events was lower among the participants 60 years of age or older than among those 18 to 59 years of age (28.8% vs. 42.3%), as were the incidences of unsolicited and solicited adverse events and local and systemic reactions (Figure 3A). Among the participants 60 years of age or older in the ZF2001 group, reactogenicity events were mainly grade 1 and 2 (96.7% of events), and no vaccine-related serious adverse events occurred.
Discussion
Whole inactivated virion, full-length spike, and RBD are the three major targets used in the currently approved Covid-19 vaccines.2 RBD is a favorable vaccine target because it focuses the immune response on interference with receptor-binding activities.2 Many RBD-based Covid-19 vaccines are under development, with some having been approved and some being evaluated in late stages of clinical trials.3,16,29 Here, we report the clinical efficacy of an RBD-based Covid-19 vaccine and add a piece to the puzzle in understanding one of the major viral targets used in Covid-19 vaccines.
This trial was designed as a case-driven study. From the end of May to June 30, 2021, primary end-point cases accrued rapidly and quickly exceeded the case numbers required for both the interim and final analyses. Therefore, we decided to conduct the final analysis directly without interim evaluations and set the data cutoff date at June 30, 2021, although approximately 40% of the participants had not yet completed the assigned three-dose regimen. After June 30, 2021, these participants continued to complete the assigned trial regimen, and they were followed up for vaccine efficacy by the trial investigators over time. When the mean duration of follow-up for the primary end-point analysis reached 6 months, a second data cutoff date for an updated analysis, December 15, 2021, was set. At this date, most of the participants (87.3%) had been included in the primary efficacy analysis.
The three-dose ZF2001 regimen had a vaccine efficacy against Covid-19 of any severity of 81.4% in the short-term follow-up and 75.7% in the long-term follow-up. The waning of efficacy was small (Table S12). With an efficacy of more than 75% and a lower boundary of the 95% confidence interval of more than 30%, this vaccine met our prespecified criteria for success and exceeded the preferred criteria (≥70%) of WHO Target Product Profiles for Covid-19 vaccines.30 Vaccine efficacy against severe-to-critical disease was 92.9% in the short-term follow-up and 87.6% in the long-term follow-up. Therefore, ZF2001 would provide fundamental immunity to relieve the pressure on health care facilities that could be overwhelmed by patients with severe-to-critical Covid-19.
Clinical efficacy of Covid-19 vaccines against SARS-CoV-2 variants of concern is a global priority. Clinical efficacy against the alpha variant of concern has been reported for two vaccines; NVX-CoV2373 showed an efficacy of 93.6%9 and CVnCoV an efficacy of 55.1%.6 Real-world investigations have shown that the efficacy of vaccines against the delta variant of concern is lower than that against the alpha variant.31-33 Clinical efficacy against the delta variant has been reported for two vaccines (BBV152 and SCB-2019).5,15 Our phase 3 trial was conducted when new waves of infection became dominated by SARS-CoV-2 variants. The alpha variant was prevalent at the beginning of 2021 but was replaced by the delta variant in the next few months. The high cross-protection conferred by ZF2001 (an antigen based on the Wuhan-Hu-1 sequence) against different SARS-CoV-2 variants is encouraging. The vaccine showed an efficacy against the alpha variant of 92.7% in the short-term follow-up and 88.3% in the long-term follow-up; an efficacy against the delta variant of 81.4% and 76.1%, respectively; and an efficacy against the kappa variant of interest of 84.8% and 75.2%, respectively. The reduced efficacy against the delta variant and the kappa variant plus the B.1.617.3 variant from that against the alpha variant was consistent with the trends shown in our previous research involving the use of a pseudovirus neutralization assay.23,24
This trial involving a large cohort of participants confirmed the acceptable safety and reactogenicity profile of ZF2001 that was shown in our previous phase 1 and 2 trials in smaller cohorts.21 The overall incidence of adverse events was similar to that in our phase 2 trial but was more balanced between the ZF2001 group and the placebo group, presumably owing to the larger population in the phase 3 trial. Adverse events of grade 3 or higher in the ZF2001 group were uncommon in both trials, with an incidence of 2.7% in the phase 2 trial and 1.5% in the phase 3 trial, and serious adverse events that were determined to be related to ZF2001 were rare, with no events in the phase 2 trial and 2 events in the phase 3 trial. In addition, older participants (≥60 years of age) were included in this phase 3 trial, and the incidence of adverse and reactogenicity events among them was lower than that among the younger participants (18 to 59 years of age). These findings support the good safety profiles of protein subunit vaccines that use an aluminum adjuvant, as has been reported for the licensed vaccines against hepatitis B and human papillomavirus infections.34,35
This trial has several limitations. First, most of the participants were Asian (78.5%). We welcome further investigation in a more diverse cohort. Second, recruitment of participants 60 years of age or older was lower than anticipated, and the vaccine efficacies of 87.6% in the short-term follow-up and 67.6% in the long-term follow-up of the older participants were insufficient, given the lower boundaries of the 95% confidence intervals of less than 30% (2.5% and 21.9%, respectively). Vaccine effectiveness in older populations should be further evaluated in real-world settings. Third, this trial was not designed to address the vaccine efficacy against asymptomatic infections. Given the possible importance of asymptomatic infections with respect to virus transmission, we welcome a new trial to test the efficacy of ZF2001 against these infections. Fourth, an imbalance of severe-to-critical Covid-19 cases was observed in favor of the placebo group in the early dosing period (Fig. S3), particularly between dose 1 and 2, as indicated by negative vaccine efficacy (Table S11). This imbalance could represent a risk of vaccine-enhanced disease or may simply be a chance finding related to the limited sample size. The results of post hoc landmark analyses did not support a significant incidence of vaccine-enhanced disease in the early dosing period (Fig. S4). However, postmarket surveillance for imbalances in cases of severe Covid-19 needs to be conducted thoroughly.
Overall, this trial provides clinical evidence that an RBD-based vaccine is a promising alternative in the prevention of symptomatic Covid-19.
Acknowledgments
We thank the members of the end-point adjudication committee and the data safety and monitoring board for their diligent review of the data; the participants who volunteered to be part of this study; the members of the clinical research teams at the participating sites; the members involved in contract clinical research organization (Chongqing Medleader Bio-Pharm, China; the Center for Advanced Technologies, Uzbekistan; DRK Pharma Solutions, Pakistan; and Accuracy Research, Ecuador); and Yuxuan Zheng (Institute of Microbiology, Chinese Academy of Sciences) and Jingxin Li and Fengcai Zhu (Jiangsu Provincial Center for Disease Control and Prevention) for their technical support in the data analyses.
Protocol
Supplementary Appendix
Disclosure Forms
Data Sharing Statement
This article was published on May 4, 2022, at NEJM.org.
A data sharing statement provided by the authors is available with the full text of this article at NEJM.org.
Footnotes
Supported by a grant from the National Science and Technology Major Project (2020YFA0907100), a grant from the National Natural Science Foundation of China (81991494), and an intramural special grant for SARS-CoV-2 research from the Chinese Academy of Sciences and by Anhui Zhifei Longcom Biopharmaceutical. Dr. Dai is supported by grants from the National Natural Science Foundation of China (82122031) and the Chinese Academy of Sciences (YSBR-010).
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
References
- 1.Gao GF. Science-based COVID-19 vaccine development. Natl Sci Rev 2021;8:nwab193-nwab193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dai L, Gao GF. Viral targets for vaccines against COVID-19. Nat Rev Immunol 2021;21:73-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.COVID-19 vaccine tracker. Approved vaccines (https://covid19.trackvaccines.org/vaccines/approved).
- 4.Al Kaabi N, Zhang Y, Xia S, et al. Effect of 2 inactivated SARS-CoV-2 vaccines on symptomatic COVID-19 infection in adults: a randomized clinical trial. JAMA 2021;326:35-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ella R, Reddy S, Blackwelder W, et al. Efficacy, safety, and lot-to-lot immunogenicity of an inactivated SARS-CoV-2 vaccine (BBV152): interim results of a randomised, double-blind, controlled, phase 3 trial. Lancet 2021;398:2173-2184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kremsner PG, Ahuad Guerrero RA, Arana-Arri E, et al. Efficacy and safety of the CVnCoV SARS-CoV-2 mRNA vaccine candidate in ten countries in Europe and Latin America (HERALD): a randomised, observer-blinded, placebo-controlled, phase 2b/3 trial. Lancet Infect Dis 2022;22:329-340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Logunov DY, Dolzhikova IV, Shcheblyakov DV, et al. Safety and efficacy of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine: an interim analysis of a randomised controlled phase 3 trial in Russia. Lancet 2021;397:671-681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baden LR, El Sahly HM, Essink B, et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med 2021;384:403-416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dunkle LM, Kotloff KL, Gay CL, et al. Efficacy and safety of NVX-CoV2373 in adults in the United States and Mexico. N Engl J Med 2022;386:531-543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Falsey AR, Sobieszczyk ME, Hirsch I, et al. Phase 3 safety and efficacy of AZD1222 (ChAdOx1 nCoV-19) Covid-19 vaccine. N Engl J Med 2021;385:2348-2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Heath PT, Galiza EP, Baxter DN, et al. Safety and efficacy of NVX-CoV2373 Covid-19 vaccine. N Engl J Med 2021;385:1172-1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sadoff J, Gray G, Vandebosch A, et al. Safety and efficacy of single-dose Ad26.COV2.S vaccine against Covid-19. N Engl J Med 2021;384:2187-2201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med 2020;383:2603-2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Halperin SA, Ye L, MacKinnon-Cameron D, et al. Final efficacy analysis, interim safety analysis, and immunogenicity of a single dose of recombinant novel coronavirus vaccine (adenovirus type 5 vector) in adults 18 years and older: an international, multicentre, randomised, double-blinded, placebo-controlled phase 3 trial. Lancet 2022;399:237-248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bravo L, Smolenov I, Han HH, et al. Efficacy of the adjuvanted subunit protein COVID-19 vaccine, SCB-2019: a phase 2 and 3 multicentre, double-blind, randomised, placebo-controlled trial. Lancet 2022;399:461-472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.COVID-19 vaccine tracker and landscape. Geneva: World Health Organization, March 29, 2022 (https://www.who.int/publications/m/item/draft-landscape-of-covid-19-candidate-vaccines).
- 17.Li J, Lai S, Gao GF, Shi W. The emergence, genomic diversity and global spread of SARS-CoV-2. Nature 2021;600:408-418. [DOI] [PubMed] [Google Scholar]
- 18.Pormohammad A, Zarei M, Ghorbani S, et al. Effectiveness of COVID-19 vaccines against delta (B.1.617.2) variant: a systematic review and meta-analysis of clinical studies. Vaccines (Basel) 2021;10:23-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dai L, Zheng T, Xu K, et al. A universal design of betacoronavirus vaccines against COVID-19, MERS, and SARS. Cell 2020;182(3):722-733.e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.An Y, Li S, Jin X, et al. A tandem-repeat dimeric RBD protein-based covid-19 vaccine zf2001 protects mice and nonhuman primates. Emerg Microbes Infect 2022;11:1058-1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang S, Li Y, Dai L, et al. Safety and immunogenicity of a recombinant tandem-repeat dimeric RBD-based protein subunit vaccine (ZF2001) against COVID-19 in adults: two randomised, double-blind, placebo-controlled, phase 1 and 2 trials. Lancet Infect Dis 2021;21:1107-1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huang B, Dai L, Wang H, et al. Serum sample neutralisation of BBIBP-CorV and ZF2001 vaccines to SARS-CoV-2 501Y.V2. Lancet Microbe 2021;2(7):e285-e285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhao X, Zheng A, Li D, et al. Neutralisation of ZF2001-elicited antisera to SARS-CoV-2 variants. Lancet Microbe 2021;2(10):e494-e494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhao X, Li D, Ruan W, et al. Effects of a prolonged booster interval on neutralization of omicron variant. N Engl J Med 2022;386:894-896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.An international randomised trial of candidate vaccines against COVID-19. Geneva: World Health Organization, May 28, 2020 (https://www.who.int/publications/i/item/an-international-randomised-trial-of-candidate-vaccines-against-covid-19).
- 26.Design of vaccine efficacy trials to be used during public health emergencies — points of considerations and key principles. Geneva: World Health Organization, 2019 (https://www.who.int/docs/default-source/blue-print/working-group-for-vaccine-evaluation-(4th-consultation)/ap1-guidelines-online-consultation.pdf).
- 27.Chan ISF, Bohidar NR. Exact power and sample size for vaccine efficacy studies. Commun Stat Theory Methods 1998;27:1305-1322. [Google Scholar]
- 28.O’Brien PC, Fleming TR. A multiple testing procedure for clinical trials. Biometrics 1979;35:549-556. [PubMed] [Google Scholar]
- 29.Kleanthous H, Silverman JM, Makar KW, Yoon IK, Jackson N, Vaughn DW. Scientific rationale for developing potent RBD-based vaccines targeting COVID-19. NPJ Vaccines 2021;6:128-128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.WHO target product profiles for COVID-19 vaccines. Geneva: World Health Organization, April 9, 2020 (https://www.who.int/publications/m/item/who-target-product-profiles-for-covid-19-vaccines).
- 31.Lopez Bernal J, Andrews N, Gower C, et al. Effectiveness of Covid-19 vaccines against the B.1.617.2 (delta) variant. N Engl J Med 2021;385:585-594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bruxvoort KJ, Sy LS, Qian L, et al. Effectiveness of mRNA-1273 against delta, mu, and other emerging variants of SARS-CoV-2: test negative case-control study. BMJ 2021;375:e068848-e068848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tang P, Hasan MR, Chemaitelly H, et al. BNT162b2 and mRNA-1273 COVID-19 vaccine effectiveness against the SARS-CoV-2 delta variant in Qatar. Nat Med 2021;27:2136-2143. [DOI] [PubMed] [Google Scholar]
- 34.Van Damme P, Ward JW, Shouval D, Zanetti A. Hepatitis B vaccines. In: Plotkin SA, Orenstein WA, Offit PA, Edwards KM, eds. Plotkin’s vaccines. 7th ed. Philadelphia: Elsevier, 2018:342-374.e17. [Google Scholar]
- 35.Schiller JT, Markowitz LE, Hildesheim A, Lowy DR. Human papillomavirus vaccines. In: Plotkin SA, Orenstein WA, Offit PA, Edwards KM, eds. Plotkin’s vaccines. 7th ed. Philadelphia: Elsevier, 2018:430-455.e10. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.