Abstract
Background
Coronavirus-like particles (CoVLP) that are produced in plants and display the prefusion spike glycoprotein of the original strain of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) are combined with an adjuvant (Adjuvant System 03 [AS03]) to form the candidate vaccine.
Methods
In this phase 3, multinational, randomized, placebo-controlled trial conducted at 85 centers, we assigned adults (≥18 years of age) in a 1:1 ratio to receive two intramuscular injections of the CoVLP+AS03 vaccine or placebo 21 days apart. The primary objective of the trial was to determine the efficacy of the CoVLP+AS03 vaccine in preventing symptomatic coronavirus disease 2019 (Covid-19) beginning at least 7 days after the second injection, with the analysis performed after the detection of at least 160 cases.
Results
A total of 24,141 volunteers participated in the trial; the median age of the participants was 29 years. Covid-19 was confirmed by polymerase-chain-reaction assay in 165 participants in the intention-to-treat population; all viral samples that could be sequenced contained variants of the original strain. Vaccine efficacy was 69.5% (95% confidence interval [CI], 56.7 to 78.8) against any symptomatic Covid-19 caused by five variants that were identified by sequencing. In a post hoc analysis, vaccine efficacy was 78.8% (95% CI, 55.8 to 90.8) against moderate-to-severe disease and 74.0% (95% CI, 62.1 to 82.5) among the participants who were seronegative at baseline. No severe cases of Covid-19 occurred in the vaccine group, in which the median viral load for breakthrough cases was lower than that in the placebo group by a factor of more than 100. Solicited adverse events were mostly mild or moderate and transient and were more frequent in the vaccine group than in the placebo group; local adverse events occurred in 92.3% and 45.5% of participants, respectively, and systemic adverse events in 87.3% and 65.0%. The incidence of unsolicited adverse events was similar in the two groups up to 21 days after each dose (22.7% and 20.4%) and from day 43 through day 201 (4.2% and 4.0%).
Conclusions
The CoVLP+AS03 vaccine was effective in preventing Covid-19 caused by a spectrum of variants, with efficacy ranging from 69.5% against symptomatic infection to 78.8% against moderate-to-severe disease. (Funded by Medicago; ClinicalTrials.gov number, NCT04636697.)
Since its emergence in 2019,1 severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has caused more than 497 million cases of coronavirus disease 2019 (Covid-19) and resulted in 6.1 million deaths globally.2 The scientific community responded to this threat by developing an unprecedented diversity of vaccines.3 The spike (S) glycoprotein is the antigen in most of these vaccines, and S-specific neutralizing antibodies have been correlated with protection against infection.4 Trials that were conducted early in the pandemic generally showed high vaccine efficacy against the original Wuhan-Hu-1 strain of SARS-CoV-2.5,6 Several vaccines have since been deployed with success7 and with acceptable safety profiles despite rare, platform-related adverse events.8,9 More recently, reduced protection due to waning immunity and the emergence of variants have been reported.10 Booster doses are being deployed to restore levels of neutralizing antibodies and improve cross-protection against a range of variants.11
Tensions that have been created by the demand for boosters in populations with high levels of vaccination and the need for primary vaccination in unvaccinated populations12 suggest that additional vaccines are needed to meet the global demand. Vaccines that remain stable at refrigerator temperatures or that can overcome concerns in vaccine-hesitant populations13 could also be useful.
A coronavirus-like particle (CoVLP) vaccine is being produced in a plant-based platform, which has been used to generate a number of viral vaccines that have shown substantial immunogenicity and efficacy.14,15 In the cells of the plant leaves, the expression of the SARS-CoV-2 S protein leads to the formation of viruslike particles measuring 100 to 150 nm. After harvesting and purification, these particles are stable for at least 6 months at 2 to 8°C. Adjuvant System 03 (AS03, GlaxoSmithKline) initiates a transient innate response16 and increases the magnitude, quality, and durability of adaptive responses.17 AS03 has been used in pandemic influenza vaccines as well as in other licensed and candidate vaccines.18 In early studies, the CoVLP+AS03 vaccine (Covifenz, Medicago) induced strong and durable levels of neutralizing antibodies and a balanced T-cell response (interferon-γ and interleukin-4),19,20 both of which could contribute to protection.21 Here, we report the early results of a pivotal phase 3 trial of the CoVLP+AS03 vaccine to evaluate its efficacy and safety.
Methods
Trial Design and Oversight
Enrollment for this ongoing randomized, placebo-controlled trial was conducted from March 15 to September 2, 2021, at 85 sites in Argentina, Brazil, Canada, Mexico, the United Kingdom, and the United States. The trial, which was funded by Medicago with financial support from the Canadian Innovation, Science and Economic Development Strategic Innovation Fund, was designed by company representatives with input from GlaxoSmithKline. Syneos Health Canada provided trial-management services. Site investigators were responsible for the recruitment of trial participants, local trial conduct, and data collection. Key laboratory analyses were performed by Viroclinics. All the participants provided written informed consent.
The protocol and protocol amendments (available with the full text of this article at NEJM.org) were reviewed by the appropriate institutional review board or ethics committee at each trial site. At the time of the vaccine efficacy and safety analyses, the investigators, trial staff members, and participants remained unaware of trial-group assignments; a limited number of team members had unblinded access to the data to facilitate submission of clinical safety findings to regulatory agencies and the data and safety monitoring committee. Representatives of Medicago and GlaxoSmithKline were responsible for the writing and review of the manuscript and for the decision to submit the manuscript for publication. These representatives vouch for the integrity and completeness of the data and for the fidelity of the trial to the protocol. Details regarding the trial oversight are provided in the Supplementary Appendix, available at NEJM.org.
Trial Objective
The primary objective of the trial was to determine the efficacy of the CoVLP+AS03 vaccine. After the detection of at least 160 laboratory-confirmed Covid-19 cases at least 7 days after the second trial injection, the primary vaccine efficacy results were calculated after a median safety follow-up of at least 2 months. The data-cutoff dates for the efficacy and safety analyses were August 20, 2021, and October 25, 2021, respectively.
Participants and Randomization
Trial participants were adults (≥18 years of age) who had not received previous vaccination against SARS-CoV-2 and who had no history of confirmed Covid-19. Full inclusion and exclusion criteria are provided in the Supplementary Appendix and the protocol. Participants were randomly assigned in a 1:1 ratio to receive two sequential intramuscular injections of the CoVLP+AS03 vaccine or placebo, administered 21 days apart. Staff members who were unaware of trial-group assignments performed all safety evaluations. The intention-to-treat population included all the participants who had undergone randomization. The safety population included all the participants in the intention-to-treat population who had received at least one dose of vaccine or placebo. The per-protocol population included all the participants who had received two doses of vaccine or placebo as scheduled and who had no major protocol deviations.
Trial Injections
The CoVLP vaccine, which has been described previously,20,22 displays full-length, prestabilized S glycoprotein trimers from SARS-CoV-2 (hCoV-19/USA/CA2/2020) expressed in Nicotiana benthamiana. The vaccine contained 3.75 μg of CoVLP combined with AS03, which contains DL-α-tocopherol and squalene, in a final volume of 0.5 ml. The placebo injection contained 0.5 ml of phosphate-buffered saline with polysorbate-80. Both the vaccine and placebo were injected in two doses administered 21 days apart.
Safety Assessments
At the time of this report, data regarding solicited adverse events were available for 4136 participants in the vaccine group and 3683 in the placebo group who had received two doses of the vaccine or placebo according to the protocol and completed at least 2 months of follow-up after the second dose. Local and systemic solicited adverse events within 7 days after each dose were collected by means of written or electronic diaries. Unsolicited adverse events were monitored for 21 days after each dose.
Throughout the trial, an unblinded medical monitor (Syneos Health Canada) and the pharmacovigilance group at Medicago are reviewing all serious adverse events, medically attended adverse events, adverse events leading to withdrawal from the trial, adverse events of special interest (including vaccine-associated enhanced respiratory disease, anaphylaxis or severe allergic reactions, and potential immune-mediated disorders), and deaths. Methods for the grading of adverse events and for the implementation of trial-stopping rules are detailed in the Supplementary Appendix. Unsolicited adverse events were coded according to the terms used in the Medical Dictionary for Regulatory Activities (MedDRA), version 24.0.
Efficacy Assessments
The primary vaccine efficacy end point was the prevention of symptomatic Covid-19 detected at least 7 days after the second dose. Cases were adjudicated in a blinded fashion by a subcommittee of the data and safety monitoring committee. A positive case was defined as the presence of at least one symptom that was compatible with Covid-19 and positive results for SARS-CoV-2 on a nasopharyngeal or nasal swab by reverse-transcriptase–polymerase-chain-reaction (RT-PCR) assay (Viroclinics). This assay provided both qualitative and quantitative results, including viral load in copies per milliliter.
Additional efficacy assessments were the prevention of severe or moderate-to-severe Covid-19 (a post hoc analysis), viral load at diagnosis, and variant-specific efficacy. Assessments of disease severity were based on the criteria of the Food and Drug Administration.23 A full list of the primary, secondary, and exploratory objectives of the trial are provided in Table S1 in the Supplementary Appendix.
Statistical Analysis
Vaccine efficacy was calculated as 100×(1–incidence rate ratio), in which the incidence rate ratio is defined as the ratio of the rate of Covid-19 cases per person-year in the vaccine group to the rate in the placebo group. The null hypothesis of no between-group difference in vaccine efficacy was rejected if the point estimate for vaccine efficacy was at least 50% and the lower limit of the 95% confidence interval was more than 30% with the use of a binomial probability conditional on the observed case margin. Descriptive safety analyses were summarized as counts and percentages. Details regarding the statistical analysis are provided in the Supplementary Appendix.
Results
Characteristics of the Participants
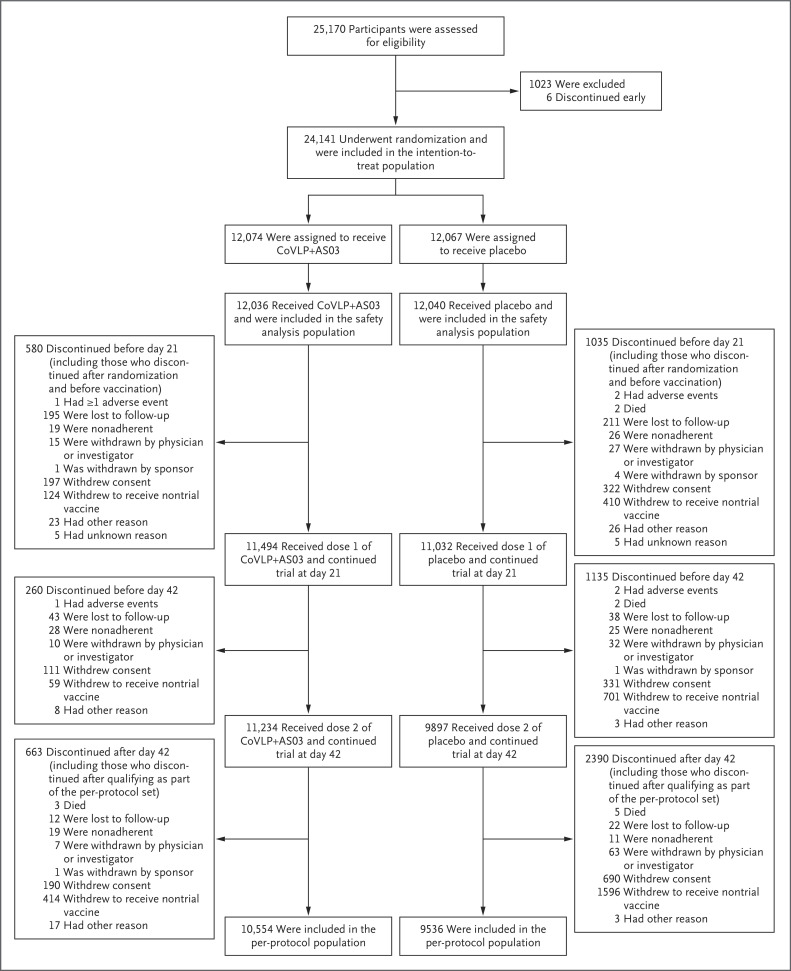
Details regarding the enrollment and randomization of the participants are provided in Figure 1. A total of 24,141 participants underwent randomization and were included in the intention-to-treat population. Demographic information and details after randomization were not available for 2 participants, who were not included in either efficacy or safety analyses.
Figure 1. Enrollment, Randomization, and Analysis Populations.
The data-cutoff date for the primary vaccine efficacy analysis was August 20, 2021. Of the 25,170 participants who were recruited, 24,141 underwent randomization in the intention-to-treat population. These participants had no virologic evidence of Covid-19 before receiving the trial injection. The safety population included 24,076 participants who had received one or more trial injections. The per-protocol population included 20,090 participants who had received two trial injections as scheduled and had no major protocol deviations. Participants may have discontinued their involvement in the trial after qualification as part of the per-protocol population (shown in the bottom set of boxes). An additional 10 participants withdrew from the study (4 in the vaccine group and 6 in the placebo group), and the timing of their discontinuation (by day 21, by day 42, or after day 42) could not be ascertained with confidence.
The demographic and clinical characteristics of the participants in the intention-to-treat and per-protocol populations are provided in Tables S2 and S3. The median age of the participants was 29 years (range, 18 to 86), and 14.8% of the participants were seropositive at baseline. The intention-to-treat population included 21,651 healthy adults younger than 65 years of age (89.7%), 127 healthy adults 65 years of age or older (0.5%), and 2361 adults with coexisting illnesses (9.8%) (Table S4). The participants included 12,293 men (50.9%) and 11,846 women (49.1%). Participants were predominantly White (88.8%), and 82.0% reported having Hispanic or Latinx ethnicity. The high representation of Hispanic or Latinx participants reflected contributions from multiple clinical sites in Central and South America.
Despite an effort to enroll older adults and those with coexisting illnesses, these populations were underrepresented because of the timing of the trial during a surge of the B.1.617.2 (delta) and P.1 (gamma) variants in the trial areas, which increased the risk of participating in a clinical trial in those populations. The timing of the trial also led many participants to exercise their protocol-sanctioned option to withdraw or undergo unblinding to access another vaccine. This factor resulted in a progressive imbalance between the vaccine and placebo groups that was managed with the use of person-year denominators to calculate efficacy outcomes. The relevance and representativeness of the trial populations are discussed in Table S5. The participants who discontinued were more likely to be male or to identify as White, but there were no major differences between the groups (Table S6).
Efficacy
As of August 20, 2021, a total of 176 cases of Covid-19 that were predicted to contribute to the primary vaccine efficacy end point had been identified. Adjudication subsequently confirmed 165 cases. Data for 10 participants (9 in the vaccine group) were removed according to the protocol owing to unblinding before symptom onset; data for 1 participant did not meet the criteria for inclusion in the primary analysis of vaccine efficacy. An additional 7 participants in the placebo group who were subsequently adjudicated as having met the primary analysis criteria were not included in the analysis because of incomplete information by the data-cutoff date. Thus, the primary vaccine efficacy analysis included 165 cases in the intention-to-treat analysis and 157 cases in the per-protocol analysis.
In the intention-to-treat population, the median duration of time until data censoring for the efficacy analysis was 1.5 months (interquartile range [IQR], 0.8 to 2.0) in the vaccine group and 1.4 months (IQR, 0.8 to 1.9) in the placebo group. Among the 165 adjudicated cases, 40 occurred in the vaccine group and 125 in the placebo group, for an incidence rate of 0.025 (95% confidence interval [CI], 0.018 to 0.033) and 0.080 (95% CI, 0.068 to 0.096) per person-year, respectively.
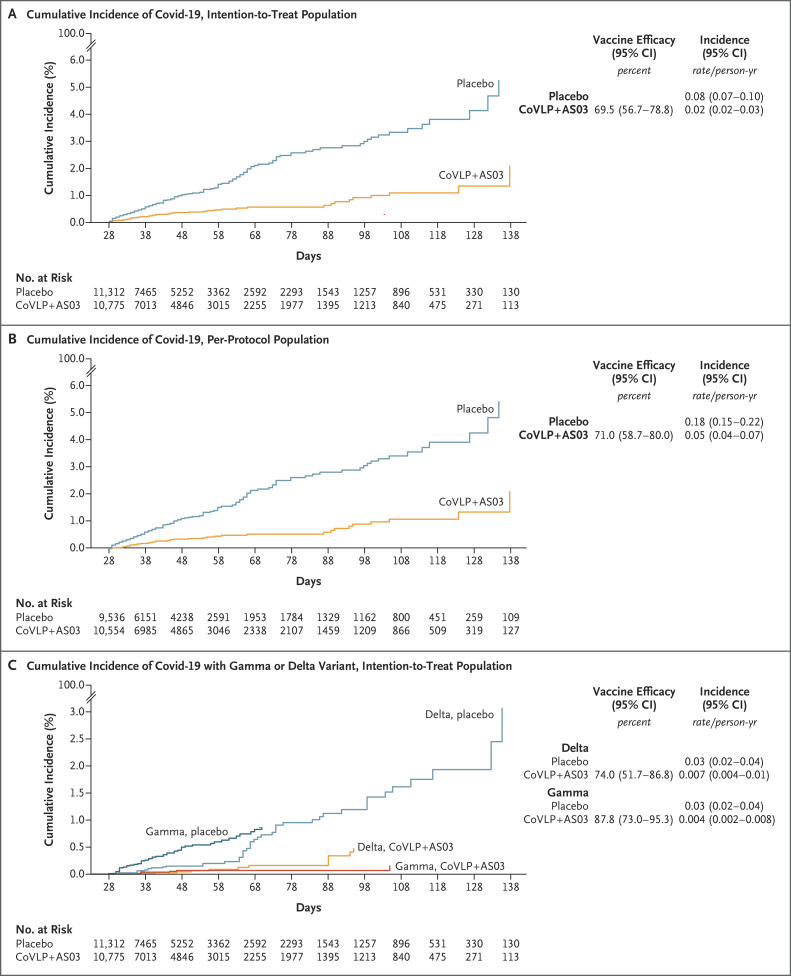
The overall vaccine efficacy was 69.5% (95% CI, 56.7 to 78.8) in the intention-to-treat population, regardless of serostatus at baseline. In the per-protocol population, 39 cases occurred in 10,554 participants in the vaccine group and in 118 of 9536 participants in the placebo group, for a vaccine efficacy of 71.0% (95% CI, 58.7 to 80.0) (Table S7).
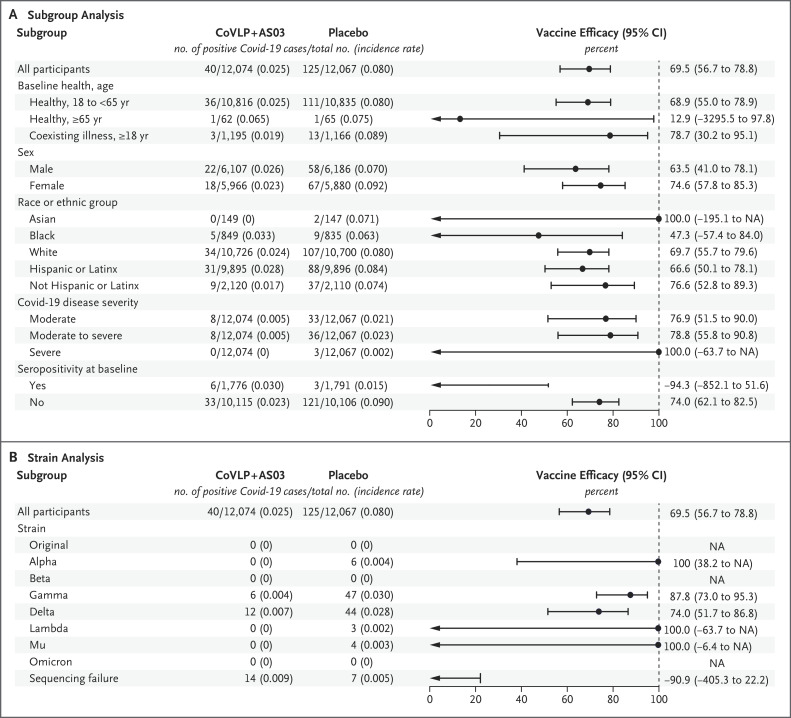
In the intention-to-treat population, the vaccine efficacy was 68.9% (95% CI, 55.0 to 78.9) in healthy adults younger than 65 years of age and 78.7% (95% CI, 30.2 to 95.1) in those with coexisting illnesses (Figure 2A). In the per-protocol population, the vaccine efficacy was 70.9% (95% CI, 57.7 to 80.4) and 76.8% (95% CI, 21.5 to 94.8), respectively. In both the intention-to-treat and per-protocol populations, among healthy adults who were 65 years of age or older, Covid-19 was detected in only 1 participant in each group, so the vaccine efficacy could not be determined. In the overall intention-to-treat population, 3 severe cases that led to 2 hospitalizations occurred in the placebo group.
Figure 2. Vaccine Efficacy, According to Subgroup and Variant.
Shown are the results of the efficacy analysis of the CoVLP+AS03 vaccine in preventing Covid-19 according to subgroup of participants (Panel A) and according to variant (Panel B) in the intention-to-treat population; 22 samples had not yet been sequenced. In both panels, the incidence rate was calculated as the number of cases per person-year. Variant-specific values probably overestimate the true vaccine efficacy, since cases that were positive on polymerase-chain-reaction (PCR) assay but had no available sequencing data were asymmetrically distributed (14 in the vaccine group and 7 in the placebo group). The implications of these missing data for the variant-specific efficacy estimates are discussed in the Supplementary Appendix. Race or ethnic group was reported by the participants. NA denotes not applicable.
Overall vaccine efficacy in preventing moderate-to-severe disease (post hoc analysis) was 78.8% (95% CI, 55.8 to 90.8) in the intention-to-treat population. Among the participants who were seronegative at baseline, the vaccine efficacy against moderate-to-severe disease was 86.0% (95% CI, 66.2 to 95.1). There were no Covid-19–related deaths. The cumulative incidence curves for all cases and for cases caused by the delta and gamma variants are presented in Figure 3.
Figure 3. Cumulative Incidence of Covid-19, According to Population and Presence of Variants.
Shown is the cumulative incidence of adjudicated Covid-19 in the intention-to-treat population (Panel A), in the per-protocol population (Panel B), and according to the presence of the delta or gamma variant in the intention-to-treat population (Panel C), starting 7 days after the second vaccination. Covid-19 cases were confirmed on the basis of PCR-positive nasopharyngeal swabs and were independently adjudicated by a subcommittee of the data and safety monitoring committee.
Variant-Specific Efficacy
During the trial period, circulating variants differed in the participating countries, according to genomic sequences that were shared through GISAID, a global science initiative that provides open access to genomic data (Fig. S1).24 The delta and gamma variants dominated in Argentina and Brazil, and the B.1.1.7 (alpha) and delta variants dominated in North America and the United Kingdom. Participants with positive results for Covid-19 were largely from Argentina (59 cases), Brazil (53 cases), and the United States (47 cases).
Of the 165 cases in the intention-to-treat population that were included in the primary vaccine efficacy analysis, sequencing data were available for 122 participants (73.9%); samples that were obtained from an additional 21 participants with confirmed cases (12.7%) could not be sequenced. All sequenced strains were variants of the original strain: delta in 56 participants (45.9%), gamma in 53 (43.4%), alpha in 6 (4.9%), B.1.621 (mu) in 4 (3.3%), and C.37 (lambda) in 3 (2.5%).
When the viral load in the upper respiratory tract was sufficient to permit sequencing, the overall variant-specific efficacy estimates were 87.8% (95% CI, 73.0 to 95.3) for the gamma variant, 74.0% (95% CI, 51.7 to 86.8) for the delta variant, and 100% for the alpha, lambda, and mu variants (Figure 2B). However, these values probably overestimate the true variant-specific efficacy, since cases that were PCR-positive but had negative results on sequencing were asymmetrically distributed (14 in the vaccine group and 7 in the placebo group). The implications of the missing data for the variant-specific efficacy estimates are discussed in the Supplementary Appendix.
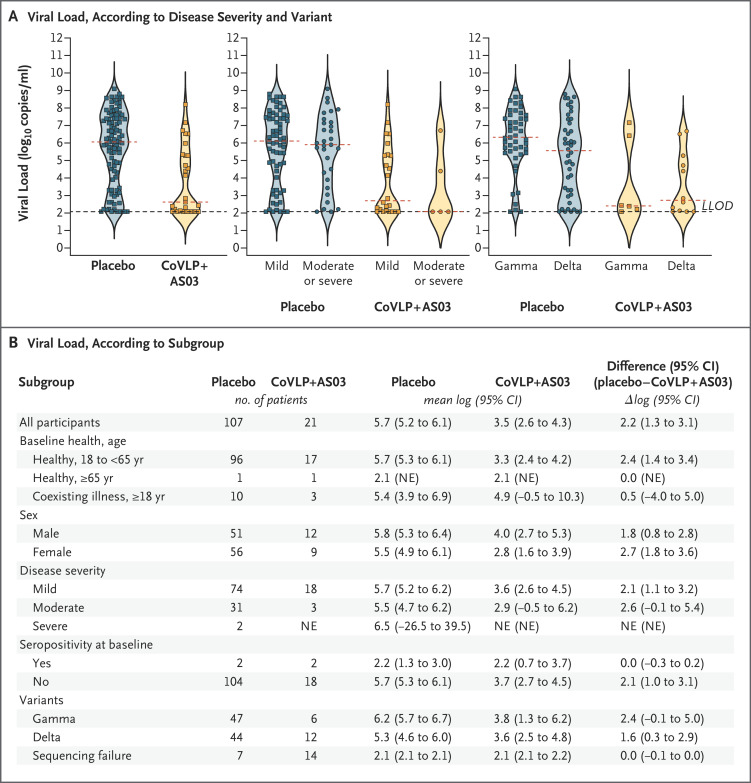
Measurement of viral load over time after diagnosis was a prespecified outcome of the trial. However, the between-group difference in sequencing success (nearly twice as high in the placebo group as in the vaccine group) prompted analysis of viral load only at the time of Covid-19 diagnosis. Such analysis revealed a higher viral load in the placebo group by a factor of more than 100 (3.46 log10 copies per milliliter in the vaccine group vs. 5.65 log10 copies per milliliter in the placebo group) (Figure 4). The median viral load in cases that were PCR-positive but sequence-negative was at the limit of detection (2.08 log10 [or 120] copies per milliliter) as compared with more than 500,000 copies per milliliter in cases that were PCR- and sequence-positive. Viral loads in the breakthrough cases with the delta variant in the vaccine group were lower by a factor of 42 than those in the placebo group (3.65 log10 vs. 5.27 log10 copies); the corresponding values in the breakthrough cases with the gamma variant were lower by a factor of 269 (3.78 log10 vs. 6.21 log10 copies). A similar trend of lower viral loads in the vaccine group was observed in breakthrough cases that were classified as mild (by a factor of 138) or moderate (by a factor of 426).
Figure 4. Viral Load at the Time of Covid-19 Diagnosis.
Panel A shows viral loads for patients for whom data were available in the two groups, arranged in violin plots. Within each plot, the red dashed line indicates the median, and the black dashed line indicates the lower limit of detection (LLOD). Panel B shows mean viral loads, presented as log10 virus copies per milliliter, according to subgroup. All analyses were performed in the intention-to-treat population. NE denotes not estimable.
Safety
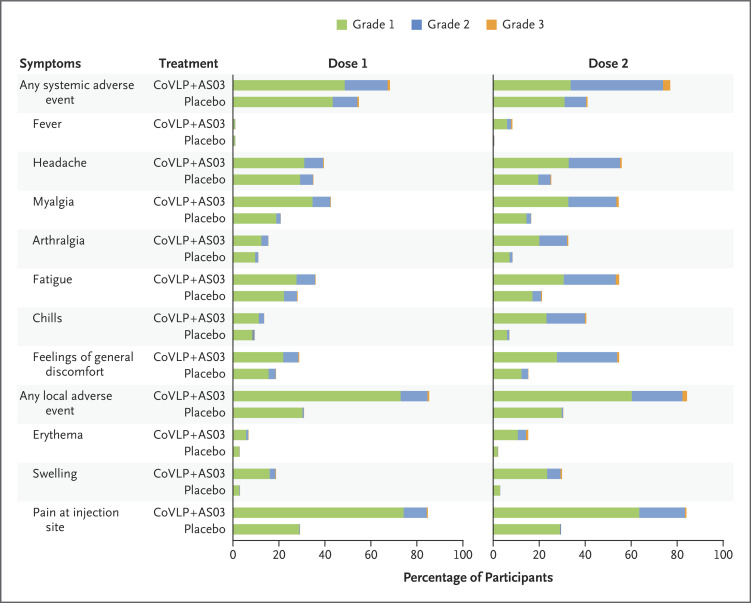
The safety population included 24,076 participants (12,036 in the vaccine group and 12,040 in the placebo group). Solicited adverse events up to 7 days after each dose were analyzed in 7819 participants (Figure 5). Both local and systemic solicited adverse events were predominantly mild-to-moderate and transient (duration, 1 to 3 days). More local and systemic adverse events were reported in the vaccine group than in the placebo group. Overall, local solicited adverse events were reported after the first and second doses combined in 3819 participants (92.3%) in the vaccine group and in 1677 (45.5%) in the placebo group. Systemic solicited adverse events were reported in 3612 participants (87.3%) and 2394 (65.0%), respectively.
Figure 5. Solicited Local and Systemic Adverse Events during 7 Days after the First or Second Dose.
Participants were monitored for local and systemic solicited adverse events through 7 days after administration of the trial injection. Participants who reported having no adverse events or who had missing data make up the remainder of the 100% calculation (not shown). For each category, adverse events are classified as follows: grade 1, mild; grade 2, moderate; and grade 3, severe. In addition, six grade 4 events (potentially life-threatening) were reported in 3 participants after the second injection: in 2 participants in the vaccine group (1 participant with chills, general discomfort, headache, and muscles aches and 1 participant with fever) and in 1 participant in the placebo group (headache) (proportional representation not visible in the graph). If a participant had different grades of the same adverse event, the highest grade was reported. If any of the solicited adverse events persisted beyond day 7 after administration of the trial injection, it was recorded as an unsolicited adverse event. Fever was defined as an oral temperature of at least 38.0°C.
In the two groups, local reactogenicity was primarily injection-site pain (Table S9). The most common systemic adverse events were headache, myalgia, fatigue, and a feeling of general discomfort (Table S10), with more participants in the vaccine group reporting these events. Grade 2 and 3 local and systemic adverse events occurred more frequently after the second dose. Grade 3 (severe) local adverse events were reported in 2.1% of the participants in the vaccine group and in less than 0.1% of those in the placebo group after the second dose; grade 3 systemic reactions were reported in 3.1% and 0.5%, respectively. No grade 4 (life-threatening) local adverse events were reported, but 3 participants reported grade 4 systemic adverse events after the second dose: 2 in the vaccine group (chills, headache, muscles aches, and a feeling of general discomfort in 1 participant and fever in the other) and 1 in the placebo group (headache).
The occurrence and intensity of unsolicited adverse events in each group are provided in Tables S11 and S12, along with data regarding serious and medically attended adverse events, events leading to withdrawal from the trial, events of special interest, and deaths. The incidence of unsolicited adverse events after the first or second dose was slightly higher in the vaccine group than in the placebo group (22.7% vs. 20.4% up to 21 days after the second dose; 4.2% vs. 4.0% from day 43 to day 201). Unsolicited adverse events with a frequency of at least 1% after the first or second dose according to the MedDRA preferred terms are listed in Table S13. The incidence of serious adverse events was similar in the two groups up to 21 days after the first or second doses (24 participants in the vaccine group [0.2%] vs. 16 in the placebo group [0.1%]) and between days 43 and 201 (19 [0.2%] vs. 22 [0.2%], respectively). One participant in the placebo group reported two serious adverse events (aortic thrombosis and peripheral artery thrombosis) that were considered by the site investigator to be related to the trial injection. No deaths were considered to be related to the vaccine.
Discussion
In this trial, we found that the CoVLP+AS03 vaccine provided substantial protection against Covid-19 caused by a range of variants. The overall vaccine efficacy was approximately 70% against any symptomatic infection in a young adult population; of these participants, almost 10% had high-risk coexisting illnesses, regardless of baseline serostatus. Vaccine efficacy among adults who were 65 years or older could not be determined because of the limited enrollment of participants in this age group. However, previous evidence suggests that the CoVLP+AS03 vaccine induced similar immune responses in both young and older adults.19
The prevention of severe disease and limiting transmission are critical objectives of ongoing vaccination efforts. Although few severe cases were noted in this trial, all such cases were in the placebo group, and overall vaccine efficacy against moderate-to-severe disease in post hoc analysis was 78.8% (86.0% among participants who were seronegative at baseline). Since the viral load in the upper respiratory tract is a determinant of both transmission risk and sequencing success, the finding that sequencing failed in approximately twice as many cases in the vaccine group as in the placebo group suggested that the viral load in vaccine breakthrough cases was probably low, a hypothesis that proved to be true (median level, 120 copies per milliliter).
Recent evidence suggests that the relationship between viral load at diagnosis and disease progression or severity remains unclear.25 However, in the current trial, when sequencing failed in either group, the cases were all classified as mild. In these cases, the mean viral load was lower by a factor of approximately 3715 than that in cases in the placebo group in which sequencing had been successful. Although viral load at diagnosis has not been widely used in previous efficacy trials, a recent study in the United Kingdom suggested that cases of Covid-19 that have a low viral load can have a dilutive effect on vaccine efficacy estimates.26 In that study, during the delta wave, the efficacy of a messenger RNA (mRNA) vaccine in cases with a high viral load was 86% (cycle threshold [Ct] values, <30) but fell to 71% when the viral load was low (Ct values, ≥30). The use of a single Covid-19 symptom to initiate PCR testing in the current trial may have resulted in the inclusion of more mild cases than studies that used more restrictive criteria to trigger testing. Analysis of every-other-day swabs from these cases is ongoing, but the difference in viral load at diagnosis raises the possibility that CoVLP+AS03 had substantial virologic effect even in breakthrough cases, which has possible implications for disease severity and reduced transmission.
CoVLP+AS03, like all currently deployed vaccines, was designed to target the original viral strain, but no case caused by this strain was identified. Even though some vaccines that were tested early in the pandemic reported efficacies of more than 90%,5,6 more recent randomized, controlled trials and real-world effectiveness studies have shown lower vaccine efficacy, although prevention of severe disease has been better preserved. A recent meta-analysis of vaccine efficacy against the delta variant according to platform27 suggested a vaccine efficacy of 59% (95% CI, 26.1 to 100) for inactivated vaccines, 67.7% (95% CI, 62.3 to 72.5) for adenovirus-vectored vaccines, and 77.7% (95% CI, 68.22 to 88.59) for mRNA-based vaccines.
The results regarding vaccine effectiveness from randomized trials and from real-world evidence should be compared with caution (and the latter results are often influenced by both strain and interval after vaccination).10 However, the context in which vaccines are currently being tested has clearly changed since early in the pandemic. The overall vaccine efficacy for CoVLP+AS03 of 69.5% (74.0% in participants who were seronegative at baseline, with a strain-specific vaccine efficacy of at least 74% among participants with confirmed infection and availability of sequencing data) appears to be similar to the reported performance of other candidate and deployed vaccines against highly transmissible or immune-evasive strains.10,27 As noted above, the variant-specific vaccine efficacies reported here may be overestimates, since 12.7% of cases had viral loads that were too low to be sequenced. A sensitivity analysis that was performed to assess the possible effect of these missing data suggested that efficacy could have been as low as 63.8% for the delta variant and 71.6% for the gamma variant and ranged from 72 to 92.5% for the other variants (Table S8). Unfortunately, the viral diversity in the current trial continues to expand with the emergence of the B.1.1.529 (omicron) variant, and many of the cases that have been identified after the data-cutoff date for the primary analysis of vaccine efficacy probably were caused by this new variant. The omicron-specific efficacy of CoVLP+AS03 will be evaluated after sequencing and adjudication have been completed.
Overall, the safety profile of CoVLP+AS03 in the current trial confirmed observations from earlier studies.19,20 Most CoVLP+AS03 recipients reported having at least one local or systemic solicited adverse event; of these events, most were grade 1 or 2 and were transient and were consistent with previous findings regarding AS03-adjuvanted vaccines.18 As observed with several other vaccines,5,6,28,29 the frequency and severity of solicited adverse events increased with the second dose. No safety concerns were identified up to the cutoff date for the analysis. Although the number of participants who received CoVLP+AS03 (approximately 13,000) and the period of follow-up are both modest to date, there has been no evidence of vaccine-associated enhanced respiratory disease in either a primate challenge study30 or clinical trials,19,20 and no episodes of anaphylaxis or imbalances in myocarditis or thrombotic events have been observed.
CoVLP+AS03 is the first plant-based vaccine that has been approved for human use and is one of only a small number of plant-produced biopharmaceutical products. Although downstream processing and purification procedures are similar across all recombinant vaccine platforms, the upstream processes for plant-produced vaccines are based on sunlight, tightly controlled use of water, and growth substrate to support the living-plant “bioreactor.” As with several of the new vaccine platforms that have been introduced during the pandemic, plant-based vaccines targeting new variants can be produced within a few months and can potentially be manufactured at different scales of production.31 The potential effect of this plant-based technology in the current pandemic will be greatly influenced by the evolution of the pandemic itself. However, the availability and further development of this platform could have important implications for pandemic readiness. CoVLP is stable at refrigerator temperatures, which makes it easy to use in remote communities and in low- and middle-income countries.31 The more traditional format of CoVLP+AS0332 may be reassuring for persons who have conflicting beliefs or concerns about some currently available vaccines.13 CoVLP+AS03 may also have a role as a booster after primary immunization with other products,11 and booster studies in children and adults are either under way or planned.
Acknowledgments
We thank all the trial participants; the staff members at each site for their high degree of professionalism in the conduct of the trial; all the employees and contractors at Medicago and GSK for their support; and all the contributors to the GISAID initiative for generating the genetic sequences and metadata on which Figure S1 is based.
Protocol
Supplementary Appendix
Disclosure Forms
Data Sharing Statement
This article was published on May 4, 2022, at NEJM.org.
A data sharing statement provided by the authors is available with the full text of this article at NEJM.org.
Footnotes
Supported by Medicago with financial assistance from the Canadian Innovation, Science and Economic Development Strategic Innovation Fund.
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
References
- 1.Zhou P, Yang X-L, Wang X-G, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 2020;579:270-273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.WHO coronavirus disease (COVID-19) dashboard. 2021. (https://covid19.who.int/).
- 3.Funk CD, Laferrière C, Ardakani A. Target product profile analysis of COVID-19 vaccines in phase III clinical trials and beyond: an early 2021 perspective. Viruses 2021;13:418-418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Harvey WT, Carabelli AM, Jackson B, et al. SARS-CoV-2 variants, spike mutations and immune escape. Nat Rev Microbiol 2021;19:409-424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med 2020;383:2603-2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baden LR, El Sahly HM, Essink B, et al. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med 2021;384:403-416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rotshild V, Hirsh-Raccah B, Miskin I, Muszkat M, Matok I. Comparing the clinical efficacy of COVID-19 vaccines: a systematic review and network meta-analysis. Sci Rep 2021;11:22777-22777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Castiello T, Georgiopoulos G, Finocchiaro G, et al. COVID-19 and myocarditis: a systematic review and overview of current challenges. Heart Fail Rev 2022;27:251-261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Greinacher A, Thiele T, Warkentin TE, Weisser K, Kyrle PA, Eichinger S. Thrombotic thrombocytopenia after ChAdOx1 nCov-19 vaccination. N Engl J Med 2021;384:2092-2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fiolet T, Kherabi Y, MacDonald C-J, Ghosn J, Peiffer-Smadja N. Comparing COVID-19 vaccines for their characteristics, efficacy and effectiveness against SARS-CoV-2 and variants of concern: a narrative review. Clin Microbiol Infect 2022;28:202-221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Munro APS, Janani L, Cornelius V, et al. Safety and immunogenicity of seven COVID-19 vaccines as a third dose (booster) following two doses of ChAdOx1 nCov-19 or BNT162b2 in the UK (COV-BOOST): a blinded, multicentre, randomised, controlled, phase 2 trial. Lancet 2021;398:2258-2276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wouters OJ, Shadlen KC, Salcher-Konrad M, et al. Challenges in ensuring global access to COVID-19 vaccines: production, affordability, allocation, and deployment. Lancet 2021;397:1023-1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aw J, Seng JJB, Seah SSY, Low LL. COVID-19 vaccine hesitancy — a scoping review of literature in high-income countries. Vaccines (Basel) 2021;9:900-900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pillet S, Aubin É, Trépanier S, et al. Humoral and cell-mediated immune responses to H5N1 plant-made virus-like particle vaccine are differentially impacted by alum and GLA-SE adjuvants in a phase 2 clinical trial. NPJ Vaccines 2018;3:3-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ward BJ, Makarkov A, Séguin A, et al. Efficacy, immunogenicity, and safety of a plant-derived, quadrivalent, virus-like particle influenza vaccine in adults (18-64 years) and older adults (≥65 years): two multicentre, randomised phase 3 trials. Lancet 2020;396:1491-1503. [DOI] [PubMed] [Google Scholar]
- 16.Morel S, Didierlaurent A, Bourguignon P, et al. Adjuvant system AS03 containing α-tocopherol modulates innate immune response and leads to improved adaptive immunity. Vaccine 2011;29:2461-2473. [DOI] [PubMed] [Google Scholar]
- 17.Budroni S, Buricchi F, Cavallone A, et al. Antibody avidity, persistence, and response to antigen recall: comparison of vaccine adjuvants. NPJ Vaccines 2021;6:78-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cohet C, van der Most R, Bauchau V, et al. Safety of AS03-adjuvanted influenza vaccines: a review of the evidence. Vaccine 2019;37:3006-3021. [DOI] [PubMed] [Google Scholar]
- 19.Gobeil P, Pillet S, Séguin A, et al. Interim report of a phase 2 randomized trial of a plant-produced virus-like particle vaccine for Covid-19 in healthy adults aged 18-64 and older adults aged 65 and older. May 17, 2021. (https://www.medrxiv.org/content/10.1101/2021.05.14.21257248v1). preprint.
- 20.Ward BJ, Gobeil P, Séguin A, et al. Phase 1 randomized trial of a plant-derived virus-like particle vaccine for COVID-19. Nat Med 2021;27:1071-1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sette A, Crotty S. Adaptive immunity to SARS-CoV-2 and COVID-19. Cell 2021;184:861-880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.D’Aoust M-A, Couture MM-J, Charland N, et al. The production of hemagglutinin-based virus-like particles in plants: a rapid, efficient and safe response to pandemic influenza. Plant Biotechnol J 2010;8:607-619. [DOI] [PubMed] [Google Scholar]
- 23.FDA. COVID-19: developing drugs and biological products for treatment or prevention. Guidance for industry. May 2020. (https://www.fda.gov/regulatory-information/search-fda-guidance-documents/covid-19-developing-drugs-and-biological-products-treatment-or-prevention).
- 24.Khare S, Gurry C, Freitas L, et al. GISAID’s role in pandemic response. China CDC Wkly 2021;3:1049-1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.El Zein S, Chehab O, Kanj A, et al. SARS-CoV-2 infection: initial viral load (iVL) predicts severity of illness/outcome, and declining trend of iVL in hospitalized patients corresponds with slowing of the pandemic. PLoS One 2021;16(9):e0255981-e0255981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pouwels KB, Pritchard E, Matthews PC, et al. Effect of Delta variant on viral burden and vaccine effectiveness against new SARS-CoV-2 infections in the UK. Nat Med 2021;27:2127-2135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cai C, Liu Y, Zeng S, Shen H, Han Y. The efficacy of COVID-19 vaccines against the B.1.617.2 (delta) variant. Mol Ther 2021;29:2890-2892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Heath PT, Galiza EP, Baxter DN, et al. Safety and efficacy of NVX-CoV2373 Covid-19 vaccine. N Engl J Med 2021;385:1172-1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Voysey M, Clemens SAC, Madhi SA, et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet 2021;397:99-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pillet S, Arunachalam PS, Andreani G, et al. Safety, immunogenicity, and protection provided by unadjuvanted and adjuvanted formulations of a recombinant plant-derived virus-like particle vaccine candidate for COVID-19 in nonhuman primates. Cell Mol Immunol 2022;19:222-233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.AboulFotouh K, Cui Z, Williams RO III Next-generation COVID-19 vaccines should take efficiency of distribution into consideration. AAPS PharmSciTech 2021;22:126-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hotez PJ, Bottazzi ME. Whole inactivated virus and protein-based COVID-19 vaccines. Annu Rev Med 2021;73:55-64. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.