Abstract
Background:The burn wound is one of the health problems in the world that affects physical and mental health. Today, adipose-derived mesenchymal stem cells (ADSCs) have received medical attention for their accessibility and the ability to reproduce and repair. The present study was designed to investigate the effect of ADSCs on burn wound healing.
Methods : The present experimental study was performed on 36 male Wistar rats divided into 1 control group and 3 experimental groups. The second-degree burns with a radius of 10 mm were induced after anesthesia. ADSCs and Dulbecco's Modified Eagle Medium (DMEM) were injected into the dermis around the burn area in the ADSCs and DMEM groups, respectively. Silver sulfadiazine (SSD) ointment was applied topically once daily as the SSD group. The control group did not receive any treatment. The rats were evaluated for 21 days. Wound healing rate, histopathological parameters, and the number of fibroblasts were evaluated by the immunofluorescence technique and vascular endothelial growth factor and transforming growth factor β (TGF-β) gene expression by reverse transcription-polymerase chain reaction. The results were entered into SPSS software (SPSS Inc) and analyzed with 1-way analysis of variance and repeated measures analysis.
Results: The number of fibroblasts, the number of vessels, TGF-β, and VEGF gene expression in the burn area were significantly higher in the ADSCs group than in the SSD, DMEM, and control groups. The results also showed that the amount of inflammation was significantly lower in the ADSCs group compared with the control group (p<0.001). Moreover, the percentage of wound recovery was significantly higher in the ADSCs group compared with other groups (p<0.001).
Conclusion: ADSCs accelerate and improve burn wound healing by affecting fibroblasts, keratinocytes, and inflammatory cells as well as increasing the expression of the TGF-β and VEGF genes, and thus increase in angiogenesis.
Keywords: Stem Cells, Second-Degree Burns, Silver Sulfadiazine, Wound Healing, Rat
What is “already known” in this topic
Mesenchymal stem cells are useful in the treatment of many wounds because of their high potential in cell division and differentiation.
What this article adds
Adipose-derived mesenchymal stem cells accelerate angiogenesis, stimulate regenerative cells, and reduce inflammation, which lead to faster and better-quality wound recovery.
Introduction
Burns is one of the challenges of the international community that has a great impact on the mental and physical health of people. Reducing burn treatment time to prevent infections and increase the quality of repair is highly important (1). Burn repair is a dynamic process that includes 4 stages: bleeding (open or closed), inflammation, proliferation and angiogenesis, and the regeneration and rearrangement of the skin surface (2). The wound healing process is performed by the interaction between skin cells, inflammatory cells, and cytokines. With positive interventions in each phase of wound healing, better quality healing, and a lower risk of infection can be achieved (2).
Silver sulfadiazine ointment is a topical and standard medicine for the treatment of burns in the world. Despite the many benefits, the release of high amounts of silver as a result of the use of silver sulfadiazine ointment can lead to silver poisoning and liver and kidney toxicities (3). Silver sulfadiazine can cause permanent discoloration of the skin and a reduction in lymphocyte counts (4, 5). Considering the shortcomings of existing therapies, various new remedies are emerging; the use of stem cells in wound healing is one of them (6).
Mesenchymal stem cells used in wound areas can help accelerate the wound healing process by secreting paracrine and affecting cells involved in the healing (7).
Mesenchymal stem cells can be collected from various sources, such as embryonic tissue, adipose tissue, and bone marrow. Adipose tissue has become the focus of considerable attention in wound healing and regenerative tissue engineering because of its convenient material acquisition and easy access, low damage, availability in large quantities, and abundant sources. Cells must be cultured in vitro to be proliferated under the appropriate conditions until they occupy all of the available substrates. In this regard, Dulbecco's Modified Eagle Medium (DMEM), which is rich in amino acids, vitamins, and nutrients provides a suitable environment for culturing the extracted ADSCs (8).
Stem cells can help speed up the ischemic wounds healing process by secreting cytokines, such as vascular endothelial growth factor (VEGF). In stem-cell therapy, the amount of VEGF is increased. Consequently, angiogenesis and tissue repair are performed to a greater extent and with better quality (9). Research has indicated that mesenchymal stem cells accelerate wound healing by affecting the migration of keratinocytes as well as matrix-forming fibroblasts (10, 11).
Studies have also shown that mesenchymal stem cells can affect the rate of inflammation in the healing process by affecting the secretion of specific types of cytokines and inflammatory cells (6, 12). Moreover, the effect of mesenchymal stem cells on the expression of genes, growth, and healing factors, including TGF-β, has been observed in a few studies evaluating the wound healing process (7).
So far, many genes, including TGF-β, have been discovered to be effective in wound healing. Studies conducted on cell pathway analysis in the field of wound healing has shown that an increase in TGF-β gene expression would ameliorate wound repairing by various mechanisms, including increasing fibroblast mitosis, angiogenesis, and myofibroblasts differentiation (13).
It has been proved that in the wound healing process, stem cells in the hair follicles and the basal layer divide and participate in healing, but the process of regeneration and division is slow and takes a long time to heal (14). To speed up the process of regeneration and division, more stem cells should be injected into the wound area.
Because of the advantages of the mesenchymal stem cells in the process of wound healing, we decided to evaluate the effects of locally administrated adipose-derived mesenchymal stem cells (ADSCs) on histopathological parameters in all phases of burn repair. Therefore, this study was performed to investigate the effect of ADSCs on the healing process of second-degree burn wounds in an animal model.
Methods
Animals
36 adult male Wistar rats, weighing 200- 250 g and aged 3 months old, were used in the study and randomly divided into 4 groups. Rats werepurchased fromPastor Institute (Karaj, Iran). Rats were housed individually in separate cages, under a 12-hour light/dark cycle, the temperature of the animal room was set to 25 °C to 30 °C and humidity (55 ∀ 5%), with ad libitum access to food and water. The study protocols were in compliance with the Ethical Committee of Tehran University of Medical Sciences (IR.TUMS.MEDICINE.REC.1399.609) and were according to the ‘‘Principles of Laboratory Animal Care’’ NIH publication 82-23, revised in 1985 and further improved in 1996.
Isolation and Culture of ADSCs
The ADSCs were harvested from the subcutaneous area of the rat abdomen and exposed to collagenase type 1 (collagenase SCR-Merck KGaA) at 37 °C in an incubator shaker for 1 hour to take out extra tissues. The solution of ADSCs was centrifuged at 3,500 rpm for 45 minutes. The stem cells were then placed in flasks containing DMEM medium (GIBCO), 15% FBS, 100 μg/mL streptomycin (Sina Darou), and 100 IU/mL penicillin (Sina Darou) and were incubated (37 °C and 5% CO2) for 24 hours. Afterward, the cells were washed with phosphate-buffered saline, placed in a pure DMEM medium (GIBCO), and incubated under standard conditions (15, 16).
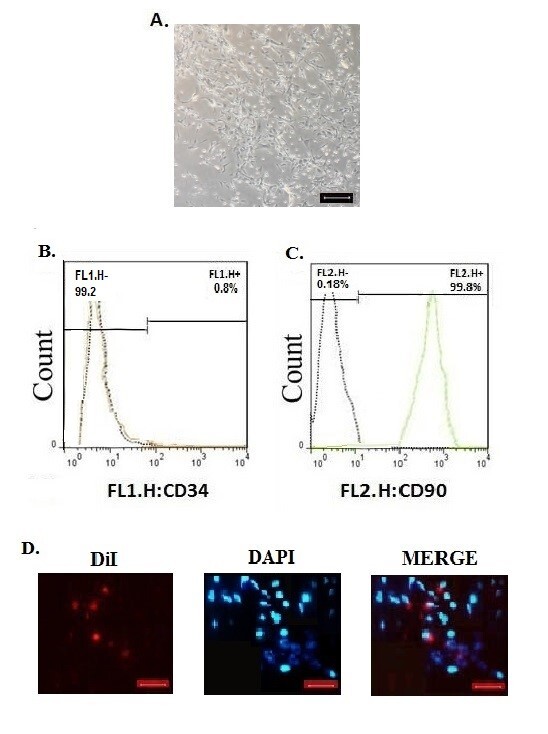
The surface markers of cells were examined by conjugated antibodies of markers and the flow cytometry method to find the cells for and CD90 for injection after third passages and at a confluency of 80%.
Labeling of Stem Cells and Injection
Adipose-derived stem cells at passage 3 were labeled by lipophilic Dil stain (1,1′-Dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate, Molecular Probes) . The stem cells were stained with a concentration of 1 mg/μL in 0.2 mL lipophilic Dil/DMEM(GIBCO) and incubated (5 min: 37 °C and 15 min: 4 °C, respectively).
On the day of surgery, 106 stem cells were divided into 4 sections and injected intradermally into the area around the burn with a 31 gauge syringe (17). On the last day of study, tissue samples were collected from the healing areas and investigated for the presence of labeled stem cells with a fluorescent microscope (CX31-OLYMPUS, Japan).
Burn Wound Model
On day zero, rats were anesthetized by injecting ketamine hydrochloride (Ketalar; Gedeon Richter) at a dose of 50 mg/kg, diazepam (Valium; Chemidarou, Iran) at a dose of 4.5 mg/kg, and pentazocine (Pentazocine; Toliddaru) at a dose of 0.4 mg/kg. Then, the hairs on the back of the animal's neck were removed and the skin of that area was disinfected with povidone-iodine (Betadine; Chemidarou, Iran) (18). To create a second-degree burn wound on the anesthetized rats, based on previous research (19), an aluminum piece with a radius of 10 mm (314 mm2) heated at 100 °C for 30 seconds was immediately placed on the back of the animal's neck for 10 seconds without any pressure (19). The development of second-degree burn was confirmed by the pathology team.
Experimental Groups
Animals were classified into 4 main groups: control, ADSCs, DMEM, and SSD groups (Table 1). The control Group: 9 healthy rats with no treatment were separated to be used as a reference group. ADSCs group: four areas around the wound were injected intradermally once with ADSCs in this group. The DMEM group: rats were intradermally injected with 100 μL DMEM once in 4 areas around the wound. SSD: The top of the ulcer was treated once daily with 1% silver sulfadiazine cream. Each group was divided into 3 subgroups (days 7, 14, and 21) with 3 rats in each subgroup. It should also be mentioned that DMEM and ADSCs were intradermally injected around the burn wound on day 0.
Table 1. The Classification of Studied Animals .
| Main Groups | Number | Interventions |
|---|---|---|
| 1 | 9 rats | 10 6(ADSCs) in 100 μl of DMEM |
| 2 | 9 rats | 100 μl of DMEM |
| 3 | 9 rats | 1% silver sulfadiazine cream once a day |
| Control | 9 rats | No treatment |
Rate of Wound Closure
To measure wound closure on days 4, 7, 10, 14, 17, and 21 photos were taken; the burn wound area was measured using Image J software. Also, the wound closure rate was calculated with the following formula (20).
X is the wound surface measured on the given day.
Histopathological Examination
At the end of each of the study periods on days 7, 14, and 21 the rats of the respective subgroup were sacrificed by inhaling CO2 gas. Then, a sample of wound bed with epidermis and dermis and adjacent healthy skin was prepared and placed in 10% neutral buffered formalin, dehydrated with ethanol, and cleaned with xylol; after covering with paraffin, they were cut into 5 μm thick pieces. Finally, hematoxylin-eosin and Masson trichrome staining were performed. Tissue samples were examined using an optical microscope (CX31-OLYMPUS) and ImageJ software. Inflammation was measured by counting inflammatory cells, including neutrophils and macrophages. Furthermore, to obtain the number of blood vessels, 3 areas of each tissue piece were evaluated and the number of blood vessels was counted. The rate ofcollagen deposition was measured according to Abramov's histological scoring system (21) (Table 2).
Table 2. The histological Scoring for Collagen Deposition Based on Abramov's Histological Scoring System (21) .
| Parameter | Score | |||
|---|---|---|---|---|
| 0 | 1 | 2 | 3 | |
| Collagen deposition | None | Scant | Moderate | Abundant |
Reverse Transcription Polymerase Chain Reaction (RT-PCR) for Transforming Growth Factor β (TGF-β) and VEGF
We evaluated TGF-β and VEGF gene expression using the RT-PCR technique. On days 7 and 14, tissue samples were isolated and Ribonucleic acid (RNA) was extracted using RNX Plus Solution (Cinnagen). After a quantitative measurement and a qualitative analysis of the RNA, the complementary deoxyribonucleic acid was synthesized using the PreMixkit kit (Bioneer). Sequences of TGF-β forward and reverse primers were 5-AGGAGACGGA ATACAGGGCT-3 ´ and 5-GGATCCACTTCCAACCC AGG-3, respectively. Sequences of VEGF forward and reverse primers were 5-TACCTCCACCATGCCA AGT-3 and 5-TGCATTCACATTTGTTGTGC-3, respectively. The PCR program consisted of 30 cycles for the following program: First, denaturation was performed at 94 °C for 3 minutes, secondary denaturation at 94 °C for 1 minute, annealing at 57 °C for 1 minute, elongation at 72 °C for 1 minute, and final elongation at 72 °C for 10 minutes. Moreover, 1% agarose gel was used to detect bands associated with the PCR products at 150 volts for 25 minutes. The agarose gel was stained with ethidium bromide. Gel Doc (UVITEC) imaging was used to observe and interpret resultant bands (18, 9).
Immunofluorescence Technique
Immunofluorescent staining with a specific marker of Vimentin was used to determine the number of fibroblasts. The formalin pigment was removed by exposing the fixed samples to 10% ammonium in 95% ethanol for 10 minutes. To inhibit intracellular peroxidase activity, sections were treated with oxygenated water (DCytomation, Denmark). To identify bound antibodies, sections were further incubated at room temperature with mouse antivimentin antibody (ab8978, dilution 1:00) as the primary antibody for 120 minutes and the goat anti-mouse antibody (ab150113, dilution 1:150) as the secondary antibody for 30 minutes. Cells were finally stained using the 4′,6-diamidino-2-phenylindole and allowed to stand for 20 minutes in a dark condition. Finally, morphological changes (the percentage of vimentin markers) were viewed using fluorescence microscopy (Olympus BX61) and ImageJ software (22).
Statistical Analysis
Data are reported as means ± standard deviation. Statistical evaluation was done by 1-way analysis of variance using SPSS 24 (Chicago). For all tests, P<0.05 were considered as statisticallysignificant. Bar charts were created for the rate of collagen deposition, VEGF, and TGF-β gene expression , and frequency of vimentin marker of fibroblasts. A line chart was created for demonstrating the percentage of wound closure using GraphPad Prism.
Results
Wound Closure
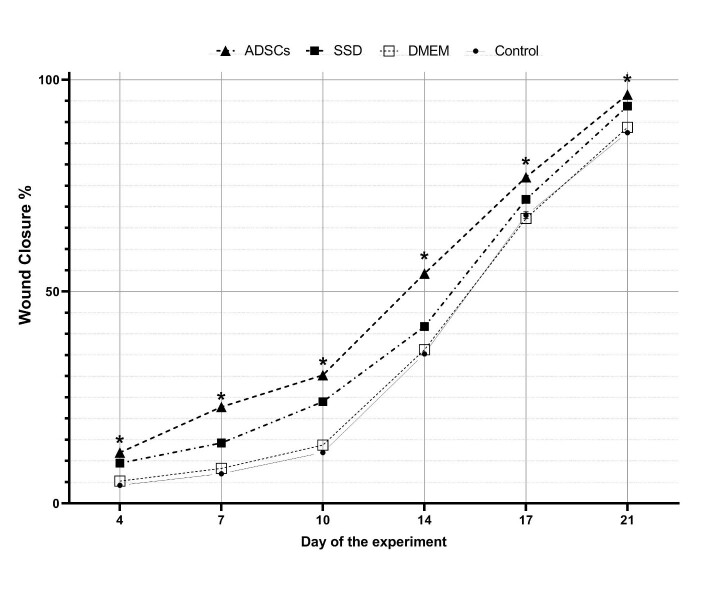
The percentage of wound closure in all groups is shown in Figure 1. The percentage of wound closure increased in all groups from the 4th day. The stem cells which are evaluated by surface markers (CD34 and CD90) andobserved at the wound site on day 21 (Figure 2), provided the most wound surface coverage in ADSCs group. The wound closure rate in the ADSCs group was highest on the 21st day comparing with other groups (97% vs 93% in SSD, 88% in DMEM and 87% in the control group; p<0.001). The results also showed that there was no significant difference between DMEM and control groups (p>0.05).
Fig. 1.
Percentage of wound closure on days 4, 7,10, 14,17, and 21 post burns. There is significant difference between the control and the ADSCs is indicated as * P < .05. (1-way analysis of variance; values are presented as mean ± SD.)
Fig. 2.
A. Image of adipose stem cells at the third passage of culture (scale bar = 100 μm). B, C. Representative flow cytometry analysis of surface markers of ADSCs (CD34 and CD90). D. Fluorescence microscopy showed Dil-labelled, red ADSCs on day 21, Nuclei were stained with DAPI, blue. (magnification 400× ,scale bar = 100 μm)
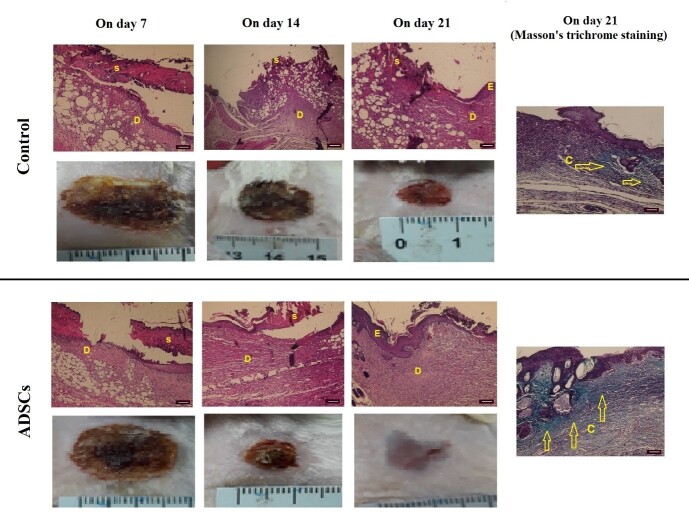
Histopathological Examinations
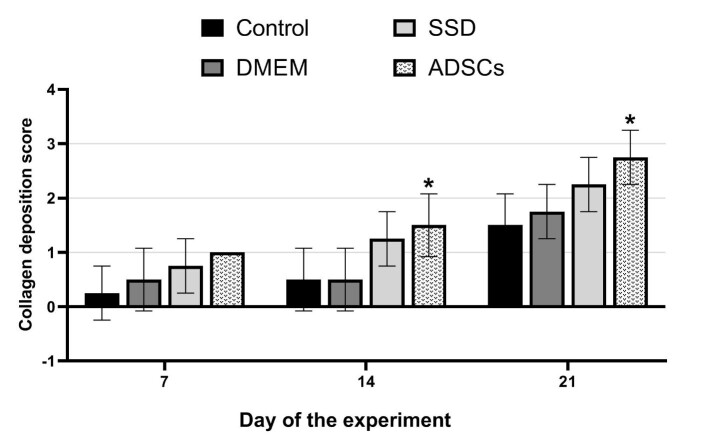
Figure 3 illustrates macroscopic and microscopic view of the wound surface of control and ADSCs groups on days 7, 14, and 21 . The rate of collagen deposition on day 21 is ranked according to the histological scoring system (Table 2) and Figure 4). The highest amount of collagen belongs to the ADSCs group (score: 2.7). Besides, there was a significant difference between the ADSCs and control group on days 14 and 21(p=0.043 and p=0.007, respectively).
Fig. 3.
Macroscopic and microscopic view; Hematoxylin-Eosin staining and Masson's trichrome staining of burn wounds in the control and ADSCs groups: E, epidermis; S, scar. D, dermis, C, collagen. On day 21 complete epithelialization, considerable collagen fibers, as well as granulation tissue are observed in the ADSCs group. (×10 scale bar = 100 µm).
Fig. 4.
Rate of collagen deposition in experimental groups based on the histological scoring system. (1-way analysis of variance; values are presented as mean ± SEM vs control group [*P < .05])
The highest number of inflammatory cells belonged to the control group in all examined phases (Table 3), while the lowest number of inflammatory cells was observed in the ADSCs group on days 7, 14 (25.5 ± 0.57, 18 ± 0.81; mean ± SD; p<0.001 compared with control and DMEM; p=0.019 compared with SSD). The results indicated that the rate of angiogenesis peaked in all groups on day 14 and then decreased (Table 3). The greatest rate of angiogenesis was observed in the ADSCs group on days 7, 14, and 21, which was statistically significant compared with the control group.
Table 3. Histopathological evaluation of the number of blood vessels and inflammatory cells on days 7, 14, and 21 (1-way ANOVA, mean ± SD, multiple comparisons with control group; *P <0.05).
| Control | DMEM | SSD | ADSCs | |
|---|---|---|---|---|
| The number of blood vessels on day 7 | 7.25±0.5 | *10±0.816 | *10.5±0.577 | *13.5±1 |
| The number of blood vessels on day 14 | 12.75±0.957 | 14±1.411 | *17±0 | *18.5±0.577 |
| The number of blood vessels on day 21 | 3.25 ±0.5 | *5.5 ± 0.577 | *7.75±0.5 | *8.75±0.5 |
| The number of inflammatory cells on day 7 | 40.5±0.57 | *37.5±0.57 | *30.25±0.5 | *25.5±0.57 |
| The number of inflammatory cells on day 14 | 30.5±0.57 | *27.75±0.5 | *19.75±0.95 | *18±0.81 |
| The number of inflammatory cells on day 21 | 21.75±1.5 | 21.75±1.5 | *17±1.414 | *14.75±0.5 |
Investigation of TGF- β and VEGF Genes Expression
As shown inFigure 5, the TGF-β gene expression in all groups was higher on day 14 than on day 7. The VEGF gene expression was higher on day 14 than on day 7 in ADSCs and SSD groups, conversely, and it was lower on day 14 than day 7 in DMEM and control groups. TGF- βgene expression was highest in the ADSCs group evaluated on days 7 and 14 (p<0.001 compared with other groups). Also, VEGF gene expression was highest in the ADSCs group (p< 0.001 compared with other groups).
Fig. 5.
Rate of collagen deposition in experimental groups based on the histological scoring system. (1-way analysis of variance; values are presented as mean ± SEM vs control group [*P < .05])
Immunofluorescence Evaluation of Fibroblasts
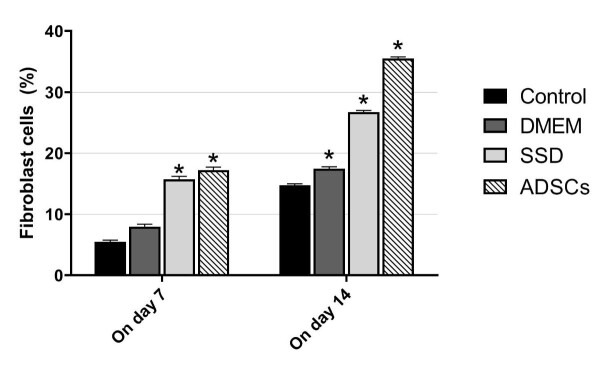
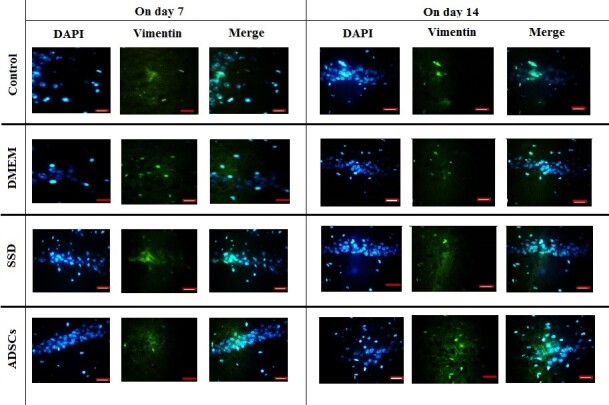
The immunofluorescence examinations revealed that the percentage of fibroblasts in the ADSCs group was significantly higher than other groups on day 14 (p<0.001). The second highest number of fibroblasts were observed in the SSD group (on day 7: compared with control and DMEM; p<0.001; on day 14: compared with other groups; p<0.001) and the lowest number of fibroblasts were observed in the control group (compared with other groups; p< .001) (Figure 6 and Figure 7).
Fig. 6.
Frequency of vimentin marker of fibroblasts on days 7 and 14. The highest number of fibroblasts was observed in the ADSCs group. (1-way analysis of variance; values are presented as mean ± SEM vs control group [*P < 0.05])
Fig. 7.
Fluorescence microscopy images to confirm vimentin marker to evaluate the number of fibroblasts on days 7 and 14 in the studied groups. DAPI: the nuclei that are blue using DAPI staining. Vimentin: Fibroblast cells using vimentin marker. Merged: the merged image shows the nucleus conforming to the cytoplasm of the cells. Magnification: 400×, Scale Bar: 20 µm.
Discussion
Burn wound healing is a dynamic process with the cooperation of cells and various factors (1). By interfering in this process, the speed and quality of therapy can be adjusted. In this study, the efficacy of ADSCs injection in burn wound healing was evaluated. According to our findings, burn wound treatment with ADSCs increases TGF-β and VEGF gene expression, the number of fibroblasts, the number of blood vessels, collagen deposition, and decreases inflammation. In our study, ADSCs treatment in rats promoted wound healing by leading to an integrated epidermis and a mature dermis, which made it preferable to conventional treatment with silver sulfadiazine cream.
In one study, the wound healing rate was measured after injecting ADSCs locally into the dermal area of both groups of healthy rats and diabetic rats. They reported that labeled ADSCs were not only observed in the dermis but also the epidermis, indicating the ability of these cells to differentiate into various cells during wound healing and having a positive effect on the healing of diabetic lesions (23).
The paracrine effects of mesenchymal stem cells on keratinocytes and fibroblasts, known as repair cells, was examined by Lee et al. They investigated the extent of repair and increase in keratinocyte and fibroblasts after exposure to ADSCs. The results showed that the proximity of mesenchymal stem cells increased the fibroblasts and covering keratinocytes leading to overall healing improvement. The study also showed that the expression of alpha 1 procollagen gene (COL1A1), one of the most important collagen protein building chains, was significantly higher in ADSCs treated group (7).
Wang et al examined the effect of ASC-Exosomes on the wound healing process of diabetic models. The results showed that in the final stages of repair, the amount of collagen in the ASC-Exosomes group was significantly higher than in the control group. It turned out that the labeled exosomes enter the fibroblasts to help increase their proliferation (24).
In another study, Smith et al discovered that paracrine effects of stem cells cause the collection of fibroblasts to the repair area, increase the proliferation of fibroblast,s and the expression of its fundamental proteins, thus contributing to the wound healing process. Furthermore, it was found that stem cells give rise to the expression of integrin genes and a decline in the expression of matrix metalloproteinase genes in fibroblasts (25).
A few studies (23- 25) have shown a significant increase in healing rate and fibroblast cells in stem cell-treated burn wounds, which is congruent with our study. Subsequently, fibroblast cells produce extracellular matrix and dermal proteins and accelerate the wound healing process.
Accordingly, in our research ADSCs group was associated with more collagen deposition than the other groups. Our study showed that the presence of injected stem cells significantly increased the expression of TGF-β and VEGF genes compared with other groups. The effect of stem cells on TGF-β levels and other factors and genes involved in the wound healing process had already been measured (26). One of the investigations performed on Wharton's jelly mesenchymal stem cells proved the positive impact of mesenchymal stem cells on the secretion of angiogenic and growth factors, such as TGF-β, VEGF, and platelet-derived growth factor (27).
In line with our results, which showed that treatment with ADSCs increases the levels of VEGF and the number of blood vessels, Lu et al reported that stem cells significantly increased the healing capacity of the skin flaps by increasing blood flow through participation in new endothelial tissues and increasing angiogenic factors (28).
Several investigations had shown a positive effect of ADSCs in increasing hematopoietic factors, such as fibroblast growth factor 2, hepatocyte growth factor, and VEGF, all of which increased vascular buds in the wound bed, resulting in more angiogenesis (9, 29).
This study showed that treatment with ADSCs significantly reduces the neutrophils and macrophages in the burn repair area (p<0.001). In this regard, various studies evaluated inflammatory cells and factors, including Tumor Necrosis Factor-α and interleukin-1 during the wound healing process. The results showed that stem cells modulated inflammation more efficiently along with repair (12).
In general, the results of our study showed that the ADSCs therapy can accelerate the burn healing process compared with other groups. Finally, near the end of the study in the ADSCs group, the wound surface was totally covered and a higher quality repair was performed. We did not investigate the synergistic effects of ADSCs and silver sulfadiazine cream in this study because of the budget limits. We suggest future studies to investigate the synergistic effects of ADSCs and silver sulfadiazine cream in the burn wound healing.
Conclusion
Our study showed that ADSCs injection to the burn wound increases TGF-β and VEGF gene expression, the number of fibroblasts, the number of blood vessels, collagen deposition, and decreases inflammation of the wound area comparing to SSD, DMEM, or control groups. We conclude that ADSCs ameliorate histopathological parameters, accelerate the burn healing process, and ultimately lead to better quality and faster healing.
Conflict of Interests
The authors declare that they have no competing interests.
Acknowledgment
The authors would like to thank Tehran University of Medical Sciences.
Ethical Approval
The study protocols were in compliance with the Ethical Committee of Tehran University of Medical Sciences (IR.TUMS.MEDICINE.REC.1399.609)
Cite this article as : Rezaei Yazdi F, Ghahary A, Mirdoraghi M, Sarvnaz H, Asgardoon MH, Rastegar T, Malek F, Abbasi Moayyer T, Ghaffari Dafchahi K, Takzaree N. Promotion of Burn Wound Healing by Local Application of Adipose-Derived Mesenchymal Stem Cells: An Experimental Study. Med J Islam Repub Iran. 2021 (22 Dec);35:172. https://doi.org/10.47176/.35.172
References
- 1.Li Y, Xia WD, Van Der, Dai WT, Lin C. Efficacy of stem cell therapy for burn wounds: a systematic review and meta-analysis of preclinical studies. Stem Cell Res Ther. 2020 doi: 10.1186/s13287-020-01839-9. [DOI] [PMC free article] [PubMed]
- 2.Jeschke MG, Rehou S, McCann MR, Shahrokhi S. Allogeneic mesenchymal stem cells for treatment of severe burn injury. Stem Cell Res Ther. 2019 doi: 10.1186/s13287-019-1465-9. [DOI] [PMC free article] [PubMed]
- 3.Razavi H, Darvishi MH, Janfaza S. Silver Sulfadiazine Encapsulated in Lipid-Based Nanocarriers for Burn Treatment. J Burn Care Res. 2018 doi: 10.1097/BCR.0000000000000602. [DOI] [PubMed]
- 4.Storm-Versloot MN, Vos CG, Ubbink DT, Vermeulen H. Topical silver for preventing wound infection. Cochrane Database Syst Rev. 2010 doi: 10.1002/14651858.CD006478.pub2. [DOI] [PubMed]
- 5.Sandri G, Bonferoni MC, Ferrari F, Rossi S, Aguzzi C, Mori M. et al. Montmorillonite-chitosan-silver sulfadiazine nanocomposites for topical treatment of chronic skin lesions: In vitro biocompatibility, antibacterial efficacy and gap closure cell motility properties. Carbohydr Polym. 2014 doi: 10.1016/j.carbpol.2013.10.029. [DOI] [PubMed]
- 6.Gundogan B, Orgill DP. Basic Science of Wound Healing and Management of Chronic Wounds. In: Grabb and Smith’s Plastic Surgery. 2020
- 7.Lee SH, Jin SY, Song JS, Seo KK, Cho KH. Paracrine effects of adipose-derived stem cells on keratinocytes and dermal fibroblasts. Ann Dermatol. 2012 doi: 10.5021/ad.2012.24.2.136. [DOI] [PMC free article] [PubMed]
- 8.Miana VV, Prieto González. Adipose tissue stem cells in regenerative medicine. Ecancermedicalscience. 2018 doi: 10.3332/ecancer.2018.822. [DOI] [PMC free article] [PubMed]
- 9.Schlosser S, Dennler C, Schweizer R, Eberli D, Stein J. , Enzmann V, et al. Paracrine effects of mesenchymal stem cells enhance vascular regeneration in ischemic murine skin. Microvasc Res doi: 10.1016/j.mvr.2012.02.011. [DOI] [PubMed]
- 10.Walter MNM, Wright KT, Fuller HR, MacNeil S, Johnson WEB. Mesenchymal stem cell-conditioned medium accelerates skin wound healing: An in vitro study of fibroblast and keratinocyte scratch assays. Exp Cell Res. 2010 doi: 10.1016/j.yexcr.2010.02.026. [DOI] [PubMed]
- 11.Jeon YK, Jang YH, Yoo DR, Kim SN, Lee SK, Nam MJ. Mesenchymal stem cells’ interaction with skin: Wound-healing effect on fibroblast cells and skin tissue. Wound Repair Regen. 2010 doi: 10.1111/j.1524-475X.2010.00636.x. [DOI] [PubMed]
- 12.Xu J, Wu W, Zhang L, Dorset-Martin W, Morris MW, Mitchell ME. et al. The role of MicroRNA-146a in the pathogenesis of the diabetic wound-healing impairment: Correction with mesenchymal stem cell treatment. Diabetes. 2012 doi: 10.2337/db12-0145. [DOI] [PMC free article] [PubMed]
- 13.Lu L, Chen-wen L, Qing W, Min H, San-jun S, Zi-wei L. et al. Silver nanoparticles/chitosan oligosaccharide/poly(vinyl alcohol) nanofiber promotes wound healing by activating TGFβ1/Smad signaling pathway. Int J Nanomedicine. 2016 Jan 21 doi: 10.2147/IJN.S91975. [DOI] [PMC free article] [PubMed]
- 14.Worst PKM, Mackenzie IC, Fusenig NE. Reformation of organized epidermal structure by transplantation of suspensions and cultures of epidermal and dermal cells. Cell Tissue Res. 1982 doi: 10.1007/BF00216219. [DOI] [PubMed]
- 15.Davoodian N, Lotfi AS, Soleimani M, Mowla SJ. MicroRNA-122 overexpression promotes hepatic differentiation of human adipose tissue-derived stem cells. J Cell Biochem. 2014 doi: 10.1002/jcb.24822. [DOI] [PubMed]
- 16.Zuk PA, Zhu M, Mizuno H, Huang J, Futrell JW, Katz AJ. et al. Multilineage cells from human adipose tissue: Implications for cell-based therapies. In: Tissue Engineering. 2001 doi: 10.1089/107632701300062859. [DOI] [PubMed]
- 17.Zografou A, Tsigris C, Papadopoulos O, Kavantzas N, Patsouris E, Donta I. et al. Improvement of skin-graft survival after autologous transplantation of adipose-derived stem cells in rats. J Plast Reconstr Aesthetic Surg. 2011 doi: 10.1016/j.bjps.2011.07.009. [DOI] [PubMed]
- 18.Takzaree N, Hadjiakhondi A, Hassanzadeh G, Rouini MR, Manayi A, Zolbin MM. Transforming growth factor-β (TGF-β) activation in cutaneous wounds after topical application of aloe vera gel. Can J Physiol Pharmacol. 2016 Jul 14:1285. doi: 10.1139/cjpp-2015-0460. [DOI] [PubMed]
- 19.Oryan A, Alemzadeh E, Mohammadi AA, Moshiri A. Healing potential of injectable Aloe vera hydrogel loaded by adipose-derived stem cell in skin tissue-engineering in a rat burn wound model. Cell Tissue Res. 2019 doi: 10.1007/s00441-019-03015-9. [DOI] [PubMed]
- 20.Takzaree N, Hassanzadeh G, Rouini MR, Keshtkar A, Manayi A, Haajiakhondi A. Histological study of wound repair with topical aloe vera gel in rat. Tehran Univ Med J. 2015
- 21.Abramov Y, Golden B, Sullivan M, Botros SM, Miller JJR, Alshahrour A. et al. Histologic characterization of vaginal vs. abdominal surgical wound healing in a rabbit model. Wound Repair Regen doi: 10.1111/j.1524-475X.2006.00188.x. [DOI] [PubMed]
- 22.Fuyuhiro Y, Yashiro M, Noda S, Kashiwagi S, Matsuoka J, Doi Y. et al. Clinical significance of vimentin-positive gastric cancer cells. Anticancer Res. 2010 [PubMed]
- 23.Nie C, Yang D, Xu J, Si Z, Jin X, Zhang J. Locally administered Adipose-derived stem cells accelerate wound healing through differentiation and vasculogenesis. Cell Transplant. 2011 doi: 10.3727/096368910X520065. [DOI] [PubMed]
- 24.Wang J, Yi Y, Zhu Y, Wang Z, Wu S, Zhang J. et al. Effects of adipose-derived stem cell released exosomes on wound healing in diabetic mice. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2020 doi: 10.7507/1002-1892.201903058. [DOI] [PMC free article] [PubMed]
- 25.Smith AN, Willis E, Chan VT, Muffley LA, Isik FF, Gibran NS. et al. Mesenchymal stem cells induce dermal fibroblast responses to injury. Exp Cell Res. 2010 doi: 10.1016/j.yexcr.2009.08.001. [DOI] [PMC free article] [PubMed]
- 26.Lu L, Chen-wen L, Qing W, Min H, San-jun S, Zi-wei L. et al. Silver nanoparticles/chitosan oligosaccharide/poly(vinyl alcohol) nanofiber promotes wound healing by activating TGFβ1/Smad signaling pathway. Int J Nanomedicine. 2016 Jan 21 doi: 10.2147/IJN.S91975. [DOI] [PMC free article] [PubMed]
- 27.Choi M, Lee HS, Naidansaren P, Kim HK, Eunju O, Cha JH. et al. Proangiogenic features of Wharton’s jelly-derived mesenchymal stromal/stem cells and their ability to form functional vessels. Int J Biochem Cell Biol. 2013 doi: 10.1016/j.biocel.2012.12.001. [DOI] [PubMed]
- 28.Lu F, Mizuno H, Uysal CA, Cai X, Ogawa R, Hyakusoku H. Improved viability of random pattern skin flaps through the use of adipose-derived stem cells. Plast Reconstr Surg. 2008 doi: 10.1097/01.prs.0000293876.10700.b8. [DOI] [PubMed]
- 29.Cao Y, Sun Z, Liao L, Meng Y, Han Q, Zhao RC. Human adipose tissue-derived stem cells differentiate into endothelial cells in vitro and improve postnatal neovascularization in vivo. Biochem Biophys Res Commun. 2005 doi: 10.1016/j.bbrc.2005.04.135. [DOI] [PubMed]