Abstract
Rationale and Objectives:
Increased levels of high-sensitivity C-reactive protein (CRP) are associated with mood symptoms in adults with bipolar disorder (BD). The few studies on this topic in youth with BD have not included controls. We, therefore, examined CRP levels in relation to symptomatic status in youth with and without BD.
Methods:
Participants included 154 youth (mean age 17 years; 48 asymptomatic BD, 39 symptomatic BD, 67 healthy controls (HC)). Rank analysis of covariance test examined group differences in CRP, controlling for age and sex. Correlation between CRP and mood symptom severity was examined using Spearman’s correlation within the BD group.
Results:
There were significant group differences in CRP levels (F(2,151) = 5.06, p = 0.007, ); post hoc analyses showed higher CRP levels in the symptomatic BD group compared with HC (p = 0.01). In sensitivity analyses, this finding was no longer significant after controlling for body mass index (BMI). CRP was not significantly associated with symptomatic severity.
Conclusions:
CRP levels are elevated among symptomatic youth with BD, partly related to BMI. As elevated BMI is associated with mood symptom burden, prospective studies are warranted to parse the associations among mood symptoms, BMI, and inflammation. Given the proportion of time that youth with BD are symptomatic, present findings raise concern about the long-term impact of elevated CRP on blood vessels, brain, and related clinical outcomes.
Keywords: Youth, bipolar disorder, C-reactive protein, symptomatology, symptom severity
Introduction
Bipolar disorder (BD) is a chronic, severe, and disabling medical illness that typically onsets during adolescence or young adulthood (GBD, 2018; Perlis et al., 2004). Approximately 60% of BD patients reported their first mood episode in childhood or adolescence, with BD youth spending approximately two-thirds of their time being symptomatic (Birmaher et al., 2009). The identification of reliable biomarkers for assisting with early disease diagnosis, treatment selection, and prognostication is a major priority in the field of BD (Frey et al., 2013; Roda et al., 2015). Evidence has pointed out the importance of inflammatory processes in the underlying pathology of BD, and the processes have been suggested to be associated with BD symptomatology (Goldstein et al., 2009; Miklowitz et al., 2016; Muneer, 2016; Ortiz-Domínguez et al., 2007).
High-sensitivity C-reactive protein (CRP) is one of the most commonly used, clinically relevant, and sensitive peripheral indicators of inflammatory activity (Pepys and Hirschfield, 2003). CRP is produced during inflammatory conditions primarily in the liver by the induction of pro-inflammatory cytokines such as interleukin-1 and interleukin-6 (Sproston and Ashworth, 2018). Upon release to the system, CRP activates the complement pathway to initialize pathogen opsonization (Sproston and Ashworth, 2018). Meta-analyses demonstrate that CRP levels are elevated in adults with BD, particularly during mania, and that CRP levels decreased following treatment and after symptomatic remission (Fernandes et al., 2016b; Uyanik et al., 2015). Moreover, studies that looked at the link between CRP and symptom severity demonstrate that elevated CRP levels are associated with higher mania and/or depressive severity score (De Berardis et al., 2008; Ford and Erlinger, 2004). Elevated CRP levels are not unique to BD and are also evident in other psychiatric disorders such as schizophrenia and major depressive disorder (Fernandes et al., 2016a, 2016b; Howren et al., 2009). While CRP is nonspecific and levels are readily influenced by environmental factors, CRP genetic factors are associated with the risk of BD and with elevated CRP levels in BD (Prins et al., 2016; Wium-Andersen et al., 2016).
Despite the burgeoning literature on CRP and BD symptomatology in adults, relatively few studies have examined this topic in youth, and no studies in youth with BD have included a healthy control (HC) comparison group. A preliminary study in 30 youth with BD reported an association between elevated CRP and greater hypomanic/manic symptom severity (Goldstein et al., 2011a). A subsequent study from the Course and Outcome of Bipolar Illness in Youth (COBY) study found that higher CRP is associated with higher maximum depression severity in the preceding months (Goldstein et al., 2015b).
In this study, we set out to build on the sparse literature regarding CRP among youth with BD by including an HC group and by evaluating symptomatic status categorically and dimensionally. Examining this topic in the youth population is advantageous since youth are less impacted by the allostatic load of BD, have fewer medical comorbidities, and less exposure to treatment. We hypothesized that CRP would be significantly higher in symptomatic BD youth compared with HC youth, with asymptomatic BD youth having intermediate CRP levels. We further hypothesized that depressive and hypomanic symptom severity will be positively associated with CRP levels.
Materials and methods
Participants
This study recruited a total of 154 English-speaking participants, including 87 BD (asymptomatic n = 48; symptomatic n = 39), and 67 HC between the ages of 13 and 20 years. BD youth with a diagnosis of BD-I (bipolar I disorder), BD-II, (bipolar II disorder), or BD-NOS (Not Otherwise Specified; akin to Other Specified Bipolar and Related Disorder) were recruited from the Centre for Youth Bipolar Disorder (CYBD), a tertiary subspecialty clinic in Toronto, Canada. HC participants were recruited through community advertisements on public transit and in local flyers. Exclusion criteria include use of anti-inflammatory medication, pre-existing cardiac, inflammatory, and/or autoimmune illness, infectious illness in the past 14 days, or inability to provide inform consent. HC participants underwent the Kiddie Schedule for Affective Disorders and Schizophrenia for School Age Children, Present and Lifetime version (K-SADS-PL) interview to confirm diagnoses (Kaufman et al., 1997). HC participants were excluded if they had lifetime mood or psychiatric disorders; first- or second-degree family history of BD; psychotic disorders; exposure to psychiatric medications in the past 3 months; or recent alcohol/drug dependence. Written informed consent was obtained from all participants and their parent/guardian prior to participation. All study procedures were approved by the research ethics board at Sunnybrook Health Sciences Centre.
Psychiatric and anthropometric measures
The K-SADS-PL semistructured diagnostic interview was used to assess present and lifetime history of psychiatric diagnoses (Kaufman et al., 1997). The Diagnosis and Statistical Manual of Mental Disorders, 4th Edition criteria (DSM-IV) was used for the clinical diagnosis of BD-I and BD-II (Kaufman et al., 1997). The Diagnosis and Statistical Manual of Mental Disorders, 5th Edition (DSM-5) criteria were not used since the DSM-5 version of the K-SADS-PL was published in 2016, whereas recruitment for this study started in 2012. The K-SADS-PL is a semistructured diagnostic interview designed for use with children and adolescents for the assessment of present and lifetime history of psychiatric diagnoses (Kaufman et al., 1997). The KSADS Depression Rating Scale (DRS) and the Mania Rating Scale (MRS) were used to assess related diagnosis and mood symptom severity scores (Axelson et al., 2003; Chambers et al., 1985). Diagnosis criteria of BD-NOS were established using operationalized criteria defined in the COBY study (Birmaher et al., 2006). Age of onset was defined as the age when the participant first experienced an episode of hypomania or mania. The Adolescent Longitudinal Interval Follow-Up Evaluation (A-LIFE) Psychiatric Status Rating (PSR) scale was used to rate depressive, manic, and hypomanic symptom severity in weekly intervals for the past 3 months (Keller et al., 1987). PSR was scored using a 6-point rating scale with a higher number indicating increasing symptom severity. Individuals with depression, and/or mania, and/or hypomania with a PSR score ≥4 from the last week were defined as symptomatic, whereas individuals with a score <4 were defined as asymptomatic. Family psychiatric history for participant’s first- and second-degree relatives was assessed using the Family History Screen Interview (Weissman et al., 2000). Information regarding psychotropic medication use and tobacco use was collected during the K-SADS-PL interview. Pubertal Developmental Scale was used to evaluate physical development with its five stages equivalent to Tanner stages (Petersen et al., 1988). All interviews were conducted by trained research staff under the supervision of the senior author (B.I.G); all diagnoses were confirmed during consensus conferences by a licensed child-adolescent psychiatrist prior to inclusion in the study.
Participant’s weight in kilograms and height in centimeters were measured using a digital scale and a wall-mounted stadiometer, respectively. For each participant, weight and height were measured twice and reported as an average for accuracy consideration (Krebs et al., 2007). Body mass index (BMI) was computed as the ratio between weight and squared height. Blood pressure was measured using the Life Source Digital Pressure Monitor.
Biochemical assay
Participants were instructed to refrain from smoking tobacco, consuming alcohol, and/or any illicit drugs for 24 h prior to study visit. Fasting blood samples were collected via antecubital venipuncture. CRP levels (mg/L) in serum were measured by the hospital blood laboratory using the Tina-Quant C-Reactive Protein (CRPL3) Gen.3 immunoturbidimetric assay on a Roche Cobas® 702 analyzer (Roche Diagnostics, IN, USA). The detection limit for CRP was 0.20 mg/L.
Statistical analysis
All statistical analyses were performed using the SPSS statistic software version 26 (IBM; NY, USA). Normality and homogeneity of variance assumptions were checked for all continuous variables using Shapiro–Wilk’s and Levene’s test, respectively. Group differences in demographic and clinical characteristics were examined using t-tests or one-way analysis of variance (ANOVA) as appropriate for continuous variables. Chi-square tests were used to examine groups difference for categorical variables. Nonparametric tests were used for continuous variables that were not normally distributed.
Both raw and log-transformed CRP values were not normally distributed; therefore, the Kruskal–Wallis test was used on the raw CRP data (Supplementary Figure 1). Quade’s rank analysis of covariance (ANCOVA) test was used to examine group differences in CRP levels, controlling for age and sex (Quade, 1967). Briefly, dependent and independent variables were ranked prior to inclusion in the ANCOVA model. Linear regression was performed with the dependent variable’s rank and the covariates’ ranks to obtain the adjusted CRP ranks. The final step involves between-group comparison based on the adjusted CRP rank. In sensitivity analysis, BMI was included as an additional covariate. Rank correlation coefficients (Spearman’s r) were computed to examine the association between CRP and PSR rating for (1) depression, (2) hypomania, or (3) depression and hypomania combined symptom severity scores. Within the BD group, the associations between the current medication use of antidepressants (combining selective serotonin reuptake inhibitor (SSRI) and non-SSRI antidepressants), lithium, and second-generation antipsychotic (SGA) with CRP levels were investigated using Mann–Whitney U test. Effect sizes are reported as Cramér’s V (V), Cohen’s d (d), or partial eta/eta squared ( ). Statistical significance was set at α = 0.05 (two-tailed), and Bonferroni correction was used to correct for multiple group comparisons.
Results
Demographic characteristics are summarized in Table 1. The HC group was more likely to be of South East/Eastern Asian ancestry (χ2 = 27.59, p = 0.02, V = 0.30) than the other two groups. The percentage of Tanner stage 4 was lower, and that of stage 5 was higher in the symptomatic BD compared with HC (χ2 = 20.33, p = 0.002, V = 0.26). BMI (H = 14.34, p = 0.001, η2 = 0.08) was greater in the symptomatic BD group compared with HC. There was significantly greater proportion of participants in the asymptomatic and symptomatic BD groups with CRP levels ⩾1 or ⩾3 mg/L compared with the HC group. In addition, the proportion of participants with CRP ⩾5 mg/L was significantly greater in the symptomatic BD group compared with the other two groups. There were no significant between-group differences in age or sex.
Table 1.
Demographic and physical characteristics.
| HC (n = 67) |
Asymptomatic
BD (n = 48) |
Symptomatic
BD (n = 39) |
F/H/ χ2 | p-value | Effect size | ||
|---|---|---|---|---|---|---|---|
| Age | 17.06 ± 1.38 | 17.51 ± 1.60 | 17.43 ± 1.64 | 1.42 | 0.25 | 0.02 | |
| Sex (n, % female) | 39 (58%) | 31 (65%) | 27 (69%) | 1.36 | 0.51 | 0.09 | |
| Race (n, %) c | European | 36 (54%) | 37 (77%) | 29 (74.5%) | 27.59 | 0.02* | 0.30 |
| South East/Eastern Asian | 12 (18%) | 1 (2%) | 2 (5.1%) | ||||
| Black or African | 7 (10.5%) | 1 (2%) | 0 (0%) | ||||
| South Asian | 5 (7.5%) | 0 (0%) | 1 (2.6%) | ||||
| Hispanic | 2 (3%) | 2 (4%) | 2 (5.1%) | ||||
| Aboriginal | 0 (0%) | 0 (0%) | 1 (2.6%) | ||||
| West Asian or Middle Eastern | 0 (0%) | 1 (2%) | 1 (2.6%) | ||||
| Other | 5 (7%) | 6 (13%) | 3 (7.5%) | ||||
| Intact family (n, % yes) | 45 (67%) | 32 (67%) | 25 (64%) | 0.11 | 0.95 | 0.03 | |
| BMI (adjusted)a,b | 22.20 ± 3.69 | 23.28 ± 3.57 | 25.66 ± 5.60 | 14.34 | 0.001* | 0.08 | |
| Blood pressure diastolic | 68.16 ± 7.69 | 69.49 ± 7.35 | 70.29 ± 9.10 | 0.95 | 0.39 | 0.01 | |
| Blood pressure systolic | 107.60 ± 12.30 | 108.63 ± 12.60 | 110.55 ± 13.34 | 0.67 | 0.51 | 0.01 | |
| Tanner Stage d | 5 | 20 (30%) | 25 (53%) | 26 (70%) | 20.33 | 0.002* | 0.26 |
| 4 | 41 (62%) | 19 (41%) | 9 (24%) | ||||
| 3 | 5 (8%) | 3 (6%) | 1 (3%) | ||||
| 2 | 0 (0%) | 0 (0%) | 1 (3%) | ||||
| CRP (mg/L) ⩾ 1 (n, %) e | 13 (19%) | 20 (42%) | 17 (46%) | 9.27 | 0.01* | 0.25 | |
| CRP (mg/L) ⩾ 3 (n, %) e | 4 (6%) | 9 (19%) | 9 (23%) | 7.03 | 0.03* | 0.21 | |
| CRP (mg/L) ⩾ 5 (n, %) f | 1 (1%) | 2 (4%) | 5 (13%) | 6.57 | 0.04* | 0.21 | |
BD: bipolar disorder; HC: healthy control; BMI: body mass index. CRP: high-sensitivity C-reactive protein.
Non-normally distributed, examined using nonparametric Kruskal–Wallis test.
Results are reported as mean ± standard deviation (SD) unless otherwise specified.
Effect size was reported as partial eta square = for ANOVA, eta square = η2 for Kruskal–Wallis, and Cramér’s V = V for Crosstab
Missing cases (n): blood pressure diastolic (2); blood pressure systolic (1); Tanner stage (4).
Symptomatic showed higher value compared with HC.
Asymptomatic showed higher value compared with HC.
HC showed higher percentage of South East/Eastern Asian ancestry than the other two groups.
Symptomatic showed lower percentage of stage 4 and higher percentage of stage 5 compared with HC.
HC showed lower percentage compared with the other two groups.
Symptomatic group showed higher percentage than the other two groups.
Significant difference.
Clinical characteristics in the asymptomatic and symptomatic BD groups are summarized in Table 2. Severity of hypomania symptom (t = 2.91, p = 0.005, d = 0.60), severity of depression symptom (t = 9.27, p < 0.001, d = 1.94), MRS (t = 2.57, p = 0.01, d = 0.55), and DRS (t = 4.98, p < 0.001, d = 1.08) were higher in symptomatic BD group. There was a higher percentage of BD-II participants (χ2 = 10.36, p = 0.01, V = 0.35), greater lifetime suicide attempts (χ2 = 4.41, p = 0.04, V = 0.23), lifetime prevalence of attention deficit-hyperactivity disorder (χ2 = 4.55, p = 0.03, V = 0.23), and current use of SSRI (χ2 = 6.37, p = 0.01, V = 0.27) in the symptomatic versus asymptomatic BD group.
Table 2.
Psychiatric clinical characteristics.
| Asymptomatic
BD (n = 48) |
Symptomatic
BD (n = 39) |
t/χ2 | p-value | Effect size | |
|---|---|---|---|---|---|
| BD-I | 19 (40%) | 8 (20%) | 10.36 | 0.01* | |
| BD-II | 10 (20%) | 21 (54%) | 0.35v | ||
| BD-NOS | 19 (40%) | 10 (26%) | |||
| Age of onset | 14.95 ± 2.98 | 15.00 ± 2.36 | 0.09 | 0.93 | 0.02d |
| PSR hypomania symptom severity | 1.31 ± 0.66 | 2.05 ± 1.61 | 2.91 | 0.005* | 0.60d |
| PSR depression symptom severity | 1.69 ± 0.88 | 3.97 ± 1.41 | 9.27 | < 0.001* | 1.94d |
| MRS | 8.00 ± 10.38 | 14.59 ± 13.5 | 2.57 | 0.01* | 0.55d |
| DRS | 12.19 ± 10.31 | 22.77 ± 9.26 | 4.98 | < 0.001* | 1.08d |
| Lifetime clinical characteristics | |||||
| Lifetime psychosis | 10 (21%) | 5 (13%) | 0.97 | 0.33 | 0.11v |
| Lifetime suicide attempts | 4 (8%) | 10 (26%) | 4.41 | 0.04* | 0.23v |
| Lifetime self-injurious behavior | 23 (48%) | 20 (51%) | 0.01 | 0.91 | 0.01v |
| Lifetime suicidal ideation | 22 (48%) | 26 (67%) | 3.05 | 0.08 | 0.19v |
| Legal history (police contact/arrest) | 7 (16%) | 9 (23%) | 0.77 | 0.38 | 0.10v |
| Lifetime physical and/or sexual abuse | 5 (11%) | 5 (13%) | 0.13 | 0.72 | 0.04v |
| Lifetime psychiatric hospitalization | 21(44%) | 15 (38%) | 0.25 | 0.62 | 0.05v |
| Lifetime comorbid diagnoses | |||||
| ADHD | 27 (56%) | 13 (33%) | 4.55 | 0.03* | 0.23v |
| Anxiety disorder | 39 (81%) | 32 (82%) | 0.01 | 0.92 | 0.01v |
| Number of anxiety disorders | 1.69 ± 1.26 | 1.82 ± 1.27 | 0.49 | 0.63 | 0.10v |
| Conduct disorder | 1 (2%) | 1 (3%) | 0.02 | 0.88 | 0.02v |
| Oppositional defiant disorder | 12 (25%) | 10 (26%) | 0.01 | 0.95 | 0.01v |
| Substance use disorder | 11 (23%) | 9 (23%) | 0.001 | 0.97 | 0.004v |
| Nicotine use | 5 (11%) | 7 (21%) | 1.45 | 0.23 | 0.14v |
| Current medications | |||||
| Second-generation antipsychotics | 29 (60%) | 27 (69%) | 0.73 | 0.39 | 0.09v |
| Lithium | 12 (25%) | 10 (26%) | 0.01 | 0.95 | 0.01v |
| Non-SSRI antidepressants | 1 (2%) | 2 (5%) | 0.60 | 0.44 | 0.08v |
| SSRI antidepressants | 3 (6%) | 10 (26%) | 6.37 | 0.01* | 0.27v |
| Stimulants | 5 (10%) | 4 (10%) | 0.001 | 0.98 | 0.003v |
| Any medication | 41 (85%) | 36 (92%) | 1.00 | 0.32 | 0.11v |
| Family psychiatric history | |||||
| Mania/hypomania | 22 (46%) | 20 (51%) | 0.26 | 0.61 | 0.05v |
| Depression | 35 (73%) | 30 (77%) | 0.18 | 0.67 | 0.05v |
| Anxiety | 23 (48%) | 21 (54%) | 0.30 | 0.58 | 0.06v |
| ADHD | 16 (33%) | 17 (44%) | 0.96 | 0.33 | 0.11v |
BD: bipolar disorder; NOS: not otherwise specified; HC: healthy control; PSR: Psychiatric Status Rating; DRS: KSADS Depression Rating Scale; MRS: KSADS Mania Rating Scale; ADHD: attention deficit-hyperactivity disorder; SSRI: selective serotonin reuptake inhibitor.
Results are reported as mean ± standard deviation (SD) or percentage (%) unless otherwise specified.
Missing cases (n): age of onset (4); lifetime suicidal ideation (2); lifetime physical and/or sexual abuse (2); legal history (3); lifetime substance use disorder (1); nicotine use (7).
Effect size was reported as Cohen’s d = d for t-test and Cramér’s V = V for Crosstab.
Significant difference.
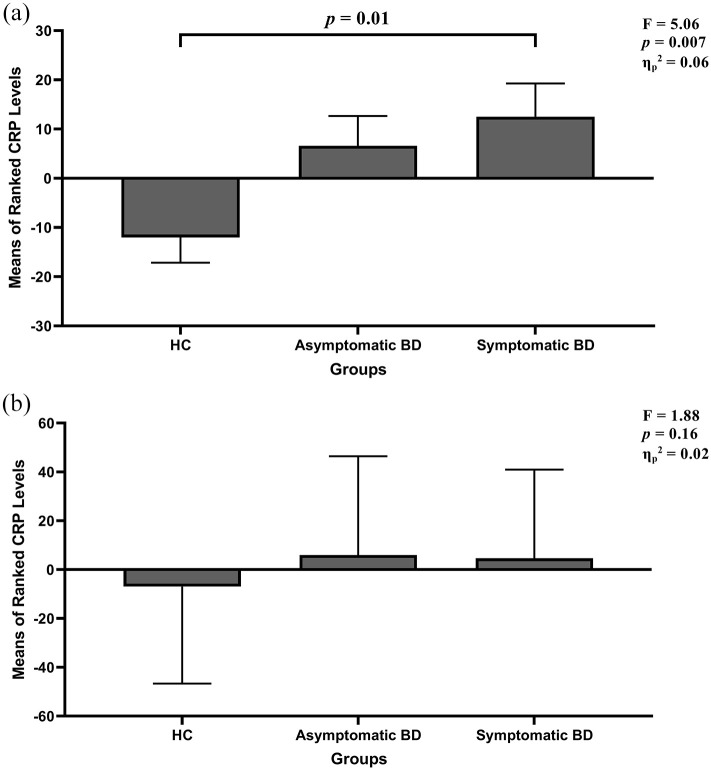
There were significant differences in CRP levels between groups (H(2) = 11.59, p = 0.003, η2 = 0.07) with a medium effect size, and this finding remained significant after controlling for age and sex (F(2,151) = 5.06, p = 0.007, ; Figure 1(a)). Post hoc analyses revealed higher CRP levels in the symptomatic BD group compared with HC both before (p = 0.006) and after (p = 0.01) controlling for age and sex. CRP levels were also higher in the asymptomatic BD group compared with the HC group (p = 0.04), but this finding did not remain significant (p = 0.06) after controlling for age and sex. Sensitivity analysis further controlling for BMI was no longer significant (F(2,151) = 1.88, p = 0.16, ; Figure 1(b)).
Figure 1.
(a) Rank-ANCOVA analysis for CRP group comparison controlling for age and sex. There was a significant group difference in CRP levels (F(2,151) = 5.06, p = 0.007, ). Post hoc analyses revealed significantly higher CRP levels in the symptomatic BD group (p = 0.01) compared with the HC group. Results are reported as mean ranks ± standard error. (b) Sensitivity analysis further controlling for BMI (F(2,151) = 1.88, p = 0.16, ).
There were no significant associations between CRP levels and depression, hypomania, or combined depression and hypomania symptom severity scores within BD. No significant group differences were observed for CRP levels between the BD groups with and without current use of antidepressants, lithium, or SGA.
Discussion
This study adds to the limited literature regarding the association between blood CRP levels and mood symptoms in youth with BD. We found significantly higher CRP levels in the symptomatic BD group compared with HC, with a medium effect size, explained in part by BMI. There were no significant associations between symptom severity and CRP levels, and CRP was not associated with medication use.
CRP predicts future cardiovascular disease (CVD) over and above traditional cardiovascular risk factors (Collaboration et al., 2010; Ridker et al., 1997, 2000). This is particularly relevant for youth with BD, who are at increased risk for early onset of CVD and increased CVD mortality (Goldstein et al., 2015a; Miller and Bauer, 2014; Weiner et al., 2011). Given that youth with BD spend approximately 60% of the time in symptomatic intervals, this raises concerns regarding the long-term impact of elevated CRP levels on the brain and body in this population (Birmaher et al., 2009).
There are a number of putative mechanisms through which inflammation may impact mood symptoms, and these have been elaborated in several reviews on this topic (Goldstein and Young, 2013; Jones et al., 2020; Leonard, 2010; Lima Giacobbo et al., 2019; Rosenblat and McIntyre, 2017). Specifically, these includes the role of CRP in increasing the permeability of the brain–blood barrier, activating microglia, increasing oxidative stress, and alternating neurotransmitter production and metabolism (Hsuchou et al., 2021; Jones et al., 2020; Savitz et al., 2015a, 2015b; Van den Ameele et al., 2020; Watkins et al., 2014).
This study found elevated CRP levels in the symptomatic youth with BD compared with the HC group. Higher CRP levels were also observed in the asymptomatic BD group compared with HC, but this finding did not remain significant after controlling for age and sex. These findings align with literature in adults with BD. A recent meta-analysis in a large sample of 2161 adults with BD across 27 studies demonstrated that CRP levels are substantially elevated during mania, with more moderate but significant elevations during depression and euthymia compared with HC (Fernandes et al., 2016b). However, individual studies on this topic have yielded variable findings (Bai et al., 2014; Chang et al., 2017; De Berardis et al., 2008; Dickerson et al., 2015; Hope et al., 2011; Jacoby et al., 2016).
In this study, we found no significant associations between CRP levels and depression, hypomania, or combined symptom severity scores. In contrast, a prior study in youth with BD observed a positive association between CRP levels with hypomanic/manic symptom severity score (Goldstein et al., 2011a). This difference may be attributed to methodological differences; the prior study used mood scores during the worst week in the past month, whereas we used mood scores during the week preceding blood draw. Literature regarding CRP levels and symptom severity among adults with BD is inconsistent; while a recent meta-analysis found that CRP levels were not significantly correlated with mood symptom severity (Fernandes et al., 2016b), there is a mix of positive and null findings (Cunha et al., 2008; De Berardis et al., 2008; Ford and Erlinger, 2004).
Finally, the findings of this study did not remain significant after further controlling for BMI, suggesting that this association might be partly related to BMI. While BMI may have a confounding effect on the observed associations, the relationship among BMI, CRP, and mood is complex. BMI is strongly associated with CRP and also significantly associated with symptom burden (Goldstein et al., 2011b; Luppino et al., 2010; McElroy and Keck, 2012; Tashakori et al., 2016). As such, controlling for BMI renders the variance between the symptomatic and asymptomatic groups insignificant.
This study has a few limitations to consider. First, the cross-sectional study design precludes examination of the temporal association between CRP and mood. Second, the sample was heterogeneous, with variability related to numerous potentially confounding factors such as specific medications, BD subtypes, alcohol and drug use, comorbidity, family history, and childhood adversity. While this is among the larger studies on the topic, the study was not powered to examine all of these covariates. Similarly, we combined depressive and hypomanic/manic groups into one large symptomatic group due to the limited number of participants with hypomania at the time of blood draw in this outpatient sample. Finally, although we did not identify significant medication-related differences in sensitivity analyses, the vast majority of BD participants were naturalistically treated with psychotropic medication and we cannot completely eliminate its possible confounding effect. Integrating CRP within placebo-controlled clinical trials in youth with BD is warranted to extend upon current findings.
To our knowledge, no prior studies have examined symptomatic status both categorically and dimensionally with CRP levels among youth with BD with an inclusion of an HC group. Overall, this study, with its limitations acknowledged, adds to the sparse literature regarding the association between CRP levels and mood symptoms in youth with BD. Our findings lend further support for the potential use of CRP as a clinically relevant biomarker in youth BD and raise concern regarding the long-term impact of persistently elevated CRP levels on the brain and vascular health of youth with BD. Future longitudinal studies examining CRP across mood states and examining CRP in relation to brain and cardiovascular outcomes are warranted.
Supplementary Material
Acknowledgments
The authors thank all study participants, their families, and the staff at the Centre for Youth Bipolar Disorder.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The research was supported by the Canadian Institutes of Health Research (CIHR) under grant (MOP-119525) and grant (MOP-136947).
ORCID iD: Yi Zou  "/>
https://orcid.org/0000-0001-9288-7275
"/>
https://orcid.org/0000-0001-9288-7275
References
- Axelson D, Birmaher BJ, Brent D, et al. (2003) A preliminary study of the Kiddie Schedule for Affective Disorders and Schizophrenia for School-Age Children mania rating scale for children and adolescents. Journal of Child and Adolescent Psychopharmacology 13(4): 463–470. [DOI] [PubMed] [Google Scholar]
- Bai YM, Su TP, Tsai SJ, et al. (2014) Comparison of inflammatory cytokine levels among type I/type II and manic/hypomanic/euthymic/depressive states of bipolar disorder. Journal of Affective Disorders 166: 187–192. [DOI] [PubMed] [Google Scholar]
- Birmaher B, Axelson D, Goldstein B, et al. (2009) Four-year longitudinal course of children and adolescents with bipolar spectrum disorders: The Course and Outcome of Bipolar Youth (COBY) study. The American Journal of Psychiatry 166(7): 795–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birmaher B, Axelson D, Strober M, et al. (2006) Clinical course of children and adolescents with bipolar spectrum disorders. Archives of General Psychiatry 63(2): 175–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers WJ, Puig-Antich J, Hirsch M, et al. (1985) The assessment of affective disorders in children and adolescents by semistructured interview. Test-retest reliability of the schedule for affective disorders and schizophrenia for school-age children, present episode version. Archives of General Psychiatry 42(7): 696–702. [DOI] [PubMed] [Google Scholar]
- Chang HH, Wang TY, Lee IH, et al. (2017) C-reactive protein: A differential biomarker for major depressive disorder and bipolar II disorder. The World Journal of Biological Psychiatry 18(1): 63–70. [DOI] [PubMed] [Google Scholar]
- Collaboration ERF, Kaptoge S, Di Angelantonio E, et al. (2010) C-reactive protein concentration and risk of coronary heart disease, stroke, and mortality: An individual participant meta-analysis. The Lancet 375(9709): 132–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunha AB, Andreazza AC, Gomes FA, et al. (2008) Investigation of serum high-sensitive C-reactive protein levels across all mood states in bipolar disorder. European Archives of Psychiatry and Clinical Neuroscience 258(5): 300–304. [DOI] [PubMed] [Google Scholar]
- De Berardis D, Conti CM, Campanella D, et al. (2008) Evaluation of C-reactive protein and total serum cholesterol in adult patients with bipolar disorder. International Journal of Immunopathology and Pharmacology 21(2): 319–324. [DOI] [PubMed] [Google Scholar]
- Dickerson F, Katsafanas E, Schweinfurth LA, et al. (2015) Immune alterations in acute bipolar depression. Acta Psychiatrica Scandinavica 132(3): 204–210. [DOI] [PubMed] [Google Scholar]
- Fernandes BS, Steiner J, Bernstein HG, et al. (2016. a) C-reactive protein is increased in schizophrenia but is not altered by antipsychotics: Meta-analysis and implications. Molecular Psychiatry 21(4): 554–564. [DOI] [PubMed] [Google Scholar]
- Fernandes BS, Steiner J, Molendijk ML, et al. (2016. b) C-reactive protein concentrations across the mood spectrum in bipolar disorder: A systematic review and meta-analysis. The Lancet. Psychiatry 3(12): 1147–1156. [DOI] [PubMed] [Google Scholar]
- Ford DE, Erlinger TP. (2004) Depression and C-reactive protein in US adults: Data from the Third National Health and Nutrition Examination Survey. Archives of Internal Medicine 164(9): 1010–1014. [DOI] [PubMed] [Google Scholar]
- Frey BN, Andreazza AC, Houenou J, et al. (2013) Biomarkers in bipolar disorder: A positional paper from the International Society for Bipolar Disorders Biomarkers Task Force. The Australian and New Zealand Journal of Psychiatry 47(4): 321–332. [DOI] [PubMed] [Google Scholar]
- GBD (2018) 2017 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. The Lancet 10(392): 10159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein BI, Carnethon MR, Matthews KA, et al. (2015. a) American Heart Association Atherosclerosis; Hypertension and Obesity in Youth Committee of the Council on Cardiovascular Disease in the Young. Major Depressive Disorder and Bipolar Disorder Predispose Youth to Accelerated Atherosclerosis and Early Cardiov. Circulation 132(10): 965–986. [DOI] [PubMed] [Google Scholar]
- Goldstein BI, Collinger KA, Lotrich F, et al. (2011. a) Preliminary findings regarding proinflammatory markers and brain-derived neurotrophic factor among adolescents with bipolar spectrum disorders. Journal of Child and Adolescent Psychopharmacology 21(5): 479–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein BI, Kemp DE, Soczynska JK, et al. (2009) Inflammation and the phenomenology, pathophysiology, comorbidity, and treatment of bipolar disorder: A systematic review of the literature. The Journal of Clinical Psychiatry 70(8): 1078–1090. [DOI] [PubMed] [Google Scholar]
- Goldstein BI, Liu SM, Zivkovic N, et al. (2011. b) The burden of obesity among adults with bipolar disorder in the United States. Bipolar Disorders 13(4): 387–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein BI, Lotrich F, Axelson DA, et al. (2015. b) Inflammatory markers among adolescents and young adults with bipolar spectrum disorders. The Journal of Clinical Psychiatry 76(11): 1556–1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein BI, Young LT. (2013) Toward clinically applicable biomarkers in bipolar disorder: Focus on BDNF, inflammatory markers, and endothelial function. Current Psychiatry Reports 15(12): 425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hope S, Dieset I, Agartz I, et al. (2011) Affective symptoms are associated with markers of inflammation and immune activation in bipolar disorders but not in schizophrenia. Journal of Psychiatric Research 45(12): 1608–1616. [DOI] [PubMed] [Google Scholar]
- Howren MB, Lamkin DM, Suls J. (2009) Associations of depression with C-reactive protein, IL-1, and IL-6: A meta-analysis. Psychosomatic Medicine 71(2): 171–186. [DOI] [PubMed] [Google Scholar]
- Hsuchou H, Kastin AJ, Mishra PK, et al. (2021) C-reactive protein increases BBB permeability: Implications for obesity and neuroinflammation. Cellular Physiology and Biochemistry 30(5): 1109–1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacoby AS, Munkholm K, Vinberg M, et al. (2016) Cytokines, brain—derived neurotrophic factor and C—reactive protein in bipolar I disorder—Results from a prospective study. Journal of Affective Disorders 197: 167–174. [DOI] [PubMed] [Google Scholar]
- Jones BDM, Daskalakis ZJ, Carvalho AF, et al. (2020) Inflammation as a treatment target in mood disorders: Review. BJPsych Open 6(4): e60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman J, Birmaher B, Brent D, et al. (1997) Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL): Initial reliability and validity data. Journal of the American Academy of Child and Adolescent Psychiatry 36(7): 980–988. [DOI] [PubMed] [Google Scholar]
- Keller MB, Lavori PW, Friedman B, et al. (1987) The Longitudinal Interval Follow-up Evaluation. A comprehensive method for assessing outcome in prospective longitudinal studies. Archives of General Psychiatry 44(6): 540–548. [DOI] [PubMed] [Google Scholar]
- Krebs NF, Himes JH, Jacobson D, et al. (2007) Assessment of child and adolescent overweight and obesity. Pediatrics 4: S193–S228. [DOI] [PubMed] [Google Scholar]
- Leonard BE. (2010) The concept of depression as a dysfunction of the immune system. Current Immunology Reviews 6(3): 205–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lima Giacobbo B, Doorduin J, Klein HC, et al. (2019) Brain-derived neurotrophic factor in brain disorders: Focus on neuroinflammation. Molecular Neurobiology 56(5): 3295–3312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luppino FS, deWit LM, Bouvy PF, et al. (2010) Overweight, obesity, and depression: A systematic review and meta-analysis of longitudinal studies. Archives of General Psychiatry 67(3): 220–229. [DOI] [PubMed] [Google Scholar]
- McElroy SL, Keck PE., Jr (2012) Obesity in bipolar disorder: An overview. Current Psychiatry Reports 14(6): 650–658. [DOI] [PubMed] [Google Scholar]
- Miklowitz DJ, Portnoff LC, Armstrong CC, et al. (2016) Inflammatory cytokines and nuclear factor-kappa B activation in adolescents with bipolar and major depressive disorders. Psychiatry Research 241: 315–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller C, Bauer MS. (2014) Excess mortality in bipolar disorders. Current Psychiatry Reports 16(11): 499. [DOI] [PubMed] [Google Scholar]
- Muneer A. (2016) Bipolar disorder: Role of inflammation and the development of disease biomarkers. Psychiatry Investigation 13(1): 18–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortiz-Domínguez A, Hernández ME, Berlanga C, et al. (2007) Immune variations in bipolar disorder: Phasic differences. Bipolar Disorders 9(6): 596–602. [DOI] [PubMed] [Google Scholar]
- Pepys MB, Hirschfield GM. (2003) C-reactive protein: A critical update. The Journal of Clinical Investigation 111(12): 1805–1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlis RH, Miyahara S, Marangell LB, et al. (2004) Long-term implications of early onset in bipolar disorder: Data from the first 1000 participants in the systematic treatment enhancement program for bipolar disorder (STEP-BD). Biological Psychiatry 55(9): 875–881. [DOI] [PubMed] [Google Scholar]
- Petersen AC, Crockett L, Richards M, et al. (1988) A self-report measure of pubertal status: Reliability, validity, and initial norms. Journal of Youth and Adolescence 17(2): 117–133. [DOI] [PubMed] [Google Scholar]
- Prins BP, Abbasi A, Wong A, et al. (2016) Investigating the causal relationship of C-reactive protein with 32 complex somatic and psychiatric outcomes: A large-scale cross-consortium mendelian randomization study. PLoS Medicine 13(6): e1001976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quade D. (1967) Rank analysis of covariance. Journal of the American Statistical Association 62(320): 1187–1200. [Google Scholar]
- Ridker P, Hennekens CH, Buring JE, et al. (2000) C-reactive protein and other markers of inflammation in the prediction of cardiovascular disease in women. The New England Journal of Medicine 342(12): 836–843. [DOI] [PubMed] [Google Scholar]
- Ridker PM, Cushman M, Stampfer MJ, et al. (1997) Inflammation, aspirin, and the risk of cardiovascular disease in apparently healthy men. The New England Journal of Medicine 336(14): 973–979. [DOI] [PubMed] [Google Scholar]
- Roda Â, Chendo I, Kunz M. (2015) Biomarkers and staging of bipolar disorder: A systematic review. Trends in Psychiatry and Psychotherapy 37(1): 3–11. [DOI] [PubMed] [Google Scholar]
- Rosenblat JD, McIntyre RS. (2017) Bipolar disorder and immune dysfunction: Epidemiological findings, proposed pathophysiology and clinical implications. Brain Sciences 7(11): 144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savitz J, Dantzer R, Wurfel BE, et al. (2015. a). Neuroprotective kynurenine metabolite indices are abnormally reduced and positively associated with hippocampal and amygdalar volume in bipolar disorder. Psychoneuroendocrinology 52: 200–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savitz J, Drevets WC, Wurfel BE, et al. (2015. b) Reduction of kynurenic acid to quinolinic acid ratio in both the depressed and remitted phases of major depressive disorder. Brain, Behavior, and Immunity 46: 55–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sproston NR, Ashworth JJ. (2018) Role of C-reactive protein at sites of inflammation and infection. Frontiers in Immunology 9: 754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tashakori A, Riahi F, Mohammadpour A. (2016) The relationship between body mass index and depression among high school girls in Ahvaz. Advances in Medicine 2016: 3645493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uyanik V, Tuglu C, Gorgulu Y, et al. (2015) Assessment of cytokine levels and hs-CRP in bipolar I disorder before and after treatment. Psychiatry Research 228(3): 386–392. [DOI] [PubMed] [Google Scholar]
- Van den Ameele S, van Nuijs AL, Lai FY, et al. (2020) A mood state-specific interaction between kynurenine metabolism and inflammation is present in bipolar disorder. Bipolar Disorders 22(1): 59–69. [DOI] [PubMed] [Google Scholar]
- Watkins CC, Sawa A, Pomper MG. (2014) Glia and immune cell signaling in bipolar disorder: Insights from neuropharmacology and molecular imaging to clinical application. Translational Psychiatry 4(1): e350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner M, Warren L, Fiedorowicz JG. (2011) Cardiovascular morbidity and mortality in bipolar disorder. Annals of Clinical Psychiatry: Official Journal of the American Academy of Clinical Psychiatrists 23(1): 40–47. [PMC free article] [PubMed] [Google Scholar]
- Weissman MM, Wickramaratne P, Adams P, et al. (2000) Brief screening for family psychiatric history: The family history screen. Archives of General Psychiatry 57(7): 675–682. [DOI] [PubMed] [Google Scholar]
- Wium-Andersen MK, Ørsted DD, Nordestgaard BG. (2016) Elevated C-reactive protein and late-onset bipolar disorder in 78 809 individuals from the general population. The British Journal of Psychiatry: The Journal of Mental Science 208(2): 138–145. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.