Abstract
Sepsis caused by aggressive infection is a severe clinical problem with an increasing incidence worldwide. Toll-like receptors and their common adapter myeloid differentiation factor 88 (MyD88) can activate immune responses by recognizing a foreign microbe’s product. This study aimed to identify the different time expression of TLR four signaling pathway in an experimental rodent model of polymicrobial sepsis. A randomized animal study was investigated in rats with septic peritonitis induced by cecal ligation and puncture (CLP). The expressions of MyD88-dependent pathway biomarkers, including MyD88, nuclear factor-κB (NF-κB), and serum tumor necrosis factor-α (TNF-α), were analyzed and compared to the sham controls at the different time points after CLP surgery. CLP-induced sepsis increased liver MyD88 mRNA expression and protein expression compared to the control groups at 2 h after surgery. The MyD88 mRNA and protein expressions in rats with CLP-induced sepsis marked increased at 4 and 6 h, and their NF-κB activities and serum TNF-α levels also increased at 4 h after CLP surgery (both p < .05). The different serial expression of MyD88-ependent pathway during sepsis may be used as biomarkers during sepsis. These results may provide further helpful information for using pro-inflammatory biomarkers of innate immunity such as MyD88 and TNF-α in clinical sepsis or related abdominal surgical emergency in the future.
Keywords: myeloid differentiation factor 88, innate immunity, sepsis, rat
Background
Toll-like receptors (TLRs) can activate immune responses by recognizing a foreign microbe’s product.1–3 TLRs have been reported to recognize lipopolysaccharide, peptidoglycan, lipoteichoic acid, lipoproteins, flagellin, double/single strains RNA, and CpG-DNA from various pathogens.3–5 Among these receptors, TLR4 appears to be a prominent signal transduction component in response to Gram-negative infections, while TLR2 is for Gram-positive bacteria.2,5–8 In general, TLRs signal through two main pathways: a myeloid differentiation factor (MyD)88-dependent pathway that acts via NF-κB to induce pro-inflammatory cytokines and a MyD88-independent pathway that acts via type I interferons to increase the expression of interferon-inducible genes.5–8 MyD88 is a central adapter protein for the signal transduction of all TLRs, with the exception of TLR3 and the IL-1R family.1–5 TLR activation can initiate the signaling pathway, including nuclear factor-κB (NF-κB) signaling, to induce immune-related gene transcription and eliminate infecting organisms.7–9
Sepsis caused by aggressive infection is a severe clinical problem with an increasing incidence worldwide. TLR4 could control bacterial clearance, induction of pro-inflammatory immune response during bacterial sepsis. In some previous studies, TLR2 and TLR4 expression in the lungs of mice subjected to the cecal ligation and puncture (CLP)-induced polymicrobial sepsis increases within 24 h as compared to the sham group. 10 In addition, septic mice showed highly increased TLR2 and TLR4 expressions in both kidneys and intestine which indicated the pro-inflammatory critical role for the severity of the infections and organ damages. 11 Sepsis is thought to be regulated by various cytokines released from innate immune cells, including interleukin-6, interleukin-1, tumor necrosis factor (TNF) α, etc. Among the cytokines involved in sepsis, TNF-α is secreted immediately after infection and triggers the pathological process of septic shock. This study aimed to identify the different time expression of TLR4 signaling pathway in an experimental rodent model of polymicrobial sepsis induced by cecal ligation and puncture (CLP).
Methods
Animal preparation and cecal ligation and puncture surgery
Eighty-five male Wistar rats were obtained from the National Science Council and maintained in the Animal Center of Chang Gung Memorial Hospital. Care and handling of the animals were in accordance with the National Institutes of Health guidelines. All experimental protocols were reviewed and approved by the Chang Gung Memorial Hospital Animal Care Review Board (Permit Number: 2015121517). The rats (250–300 g) were randomly assigned into two groups (the CLP surgery and the sham control). They were then anesthetized with an intramuscular dose of ketamine (75 mg/kg) and xylazine (5 mg/kg) at the surgical suite. A midline abdominal incision was made to expose the cecum. For the CLP modified from, 50–60% of the cecum and its associated mesentery was ligated with a 4-0 silk tie so as to prevent kinking or obstructing the bowel. The resulting sack of fecal material was punctured through both the proximal and distal surface with an 18 g needle. For the sham surgery, the cecum was exposed but was not ligated or punctured. The abdominal incision and all skin incisions were closed with 3-0 silk suture.
On the study day, the rats in each group (n = 5) were injected with ketamine (100 mg/kg, intramuscularly) at 0.5, 1, 2, 4, 6, 24, 72 h, and 7 days after CLP surgery. Within 1 min of death, blood samples were collected by aspiration from the left ventricle in heparin-coated vacutainer tubes and immediately placed on ice. One part of the liver tissue was immediately removed and frozen in liquid nitrogen to prevent degradation and stored at −80°C until further assay. The other part of the liver tissue was stored for histopathologic analysis.
Quantitative polymerase chain reaction analysis of MyD88 mRNA expression
The mRNA expression levels of Myd88 genes in the liver specimens were analyzed by quantitative real-time reverse transcription PCR. Briefly, total cellular RNA was extracted from liver tissue using the RNeasy Mini kit (Qiagen GmbH, Hilden, Germany). An aliquot of 1.0 μg of total RNA from each sample was subjected to assay using TaqMan One-Step RT-PCR Master Mix Reagent kit, and Applied Biosystems TaqMan Gene Expression Assays pre-designed primers and probes were used: The expression levels of the target gene Myd88 (Assay ID: Rn01640052_g1) were normalized with the control housekeeping gene, β-Actin (Assay ID: Rn01759928_g1) according to the manufacturer’s instructions. Q-PCR was performed on a ABI StepOne Real-Time PCR System (Applied Biosystems) with the following primers: MyD88 (forward): 5′ATCGAGGAGGACTGCCAGAAA3′, and MyD88 (reverse): 5′GCAGATGAAGGCGTCGAAAA3. Q-PCR was run under the following conditions: 15 s, 95°C; 30 s, 60°C; 15 s, 95°C for 40 cycles.
Immunohistochemistry
Tissue MyD88 protein expression of liver tissues in rats was analyzed by IHC staining. Cryostat sections were fixed for 10 min in ice-cold acetone and air dried at room temperature. IHC standard procedure was performed as previously described. 12 Anti-MyD88 Abs for IHC analysis in rodent liver tissues was from Santa Cruz Biotechnology. MyD88 protein expression in rodent livers were stained brown.
Western blot analysis
All tissues were sonicated in radioimmunoreactive protein extraction assay (RIPA) lysis buffer (protease and phosphatase inhibitor (150 mM NaCl, 20 mM NaH2PO4, 1% Triton-X-100, 1 M NaF, 100 mM phenylmethylsulfonyl fluoride, 100 mM sodium-orthovanadate, 10 μg/mL aprotinin, 10 μg/mL leupeptin). The homogenate was centrifuged at 10,000g at 4°C for 10 min, and the supernatant was collected and stored at −80°C. A total of 25 μg of protein was subjected to SDS-PAGE (12% polyacrylamide) and transferred to a polyvinylidene difluoride membrane (Amersham) that was incubated with anti-α-tubulin (SC-8035) and MyD88 (SC-136970) washed, and then incubated with a 1:10,000 dilution of horseradish peroxidase conjugated secondary antibody. Bands were visualized by development with the Amersham ECL-Plus detection regents (Amersham) for one-dimensional gel electrophoresis, and normalized with the internal control protein, α-tubulin.
Electrophoretic mobility shift assay
A Chemiluminescent Nucleic Acid Detection Module was used for EMSA. For binding reaction, 6 μg nuclear protein was incubated with 2 μL of 3′ end-labeled probe and 1 μg poly (dI-dC) for 20 min. Electrophoresis was carried out on a 4.8% polyacrylamide gel at 4°C. Samples were transferred to (+) nylon membranes blocked in blocking buffer and incubated with anti-biotin fragments (1:10,000) conjugated with horseradish peroxidase. The bands were visualized by enhanced chemiluminescence.
Analysis of cytokine production
Blood samples of sham-operated rats and CLP rats were collected at 0.5, 1, 2, 4, and 6 h after surgery by aspiration from the left ventricle in heparin-coated vacutainer tubes and immediately placed on ice. Rat cytokine concentrations were measured by enzyme-linked immunosorbent assay (ELISA) specific for tumor necrosis factor-α (TNF-α) by using Quantikine® Rat TNF-α Immunoassay (Catalog Number RTA00, R&D).
Statistical analysis
The experimental rats were divided into two groups: CLP surgery and sham control group. Statistical analyses were performed using the Mann–Whitney U-test and the Kruskal–Wallis test. Data were presented as mean ± standard derivation (SD). Possibility levels of less than .05 were taken as significant. Statistical analyses were performed with SPSS software (version 15.0, SPSS, Inc., Chicago, IL, USA).
Results
To evaluate the role of MyD88-dependent pathway in rodent sepsis, MyD88 mRNA and protein expressions in the CLP surgery and sham control rats were investigated. Both experimental and control groups were sacrificed at 0.5, 1, 2, 4, and 6 h after surgery for further analysis.
Gross findings and pathologic findings
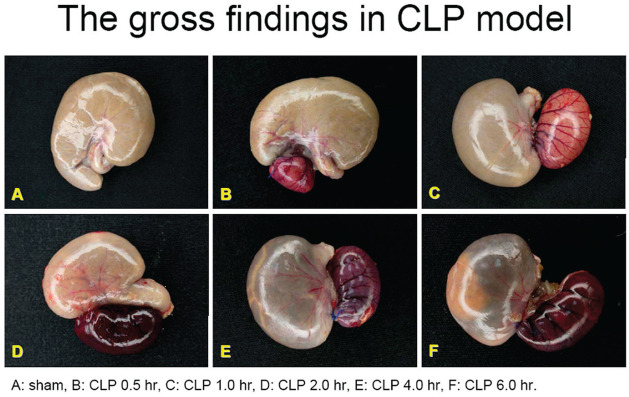
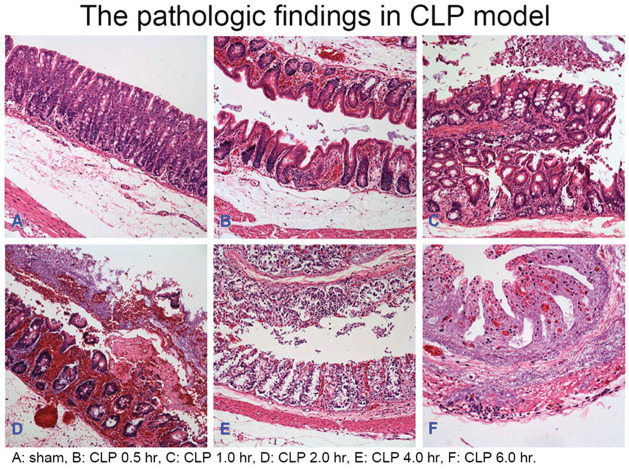
The gross findings and pathologic findings of the cecum tissues in the CLP surgery group and the sham control group were shown in Figure 1 and 2. The severity of inflammation and ischemia of the cecum tissues shown in gross and pathological pictures appeared predominant in the CLP surgery group compared to the sham control group, and showed much more predominant at 6 h after CLP surgery compared to other time points after CLP surgery.
Figure 1.
The gross findings of cecum tissues in rats with sepsis at different time points after CLP surgery.
Figure 2.
The pathologic findings of cecum in rats with sepsis at different time points after CLP surgery.
qPCR analysis of MyD88 mRNA expression
The results of qPCR indicated that MyD88 mRNA expression in the liver tissues increased at 2 h after CLP surgery and highly increased at 4 and 6 h after CLP surgery while MyD88 mRNA expression remained steady in the sham control groups (Figure 3). As such, MyD88 protein expression was also expressed early at 4 h after CLP surgery. However, MyD88 mRNA expressions were not elevated after 24 h after CLP surgery.
Figure 3.
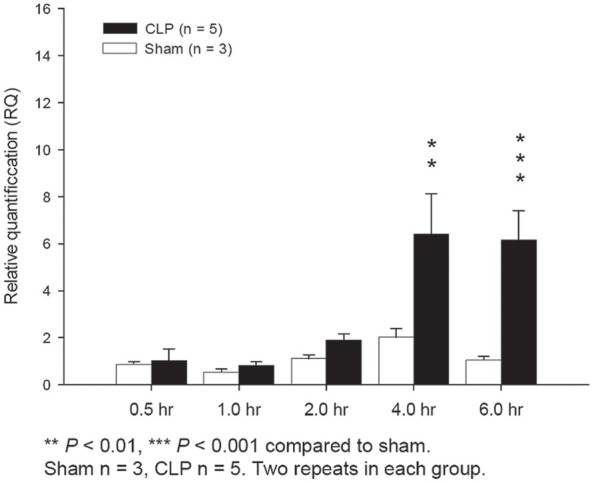
MyD88 QPCR showed MyD88 mRNA expression in the CLP group was statistically significantly higher than the sham control group at 4 h (p < .01) and 6 h after surgery (p < .001).
MyD88 protein expression
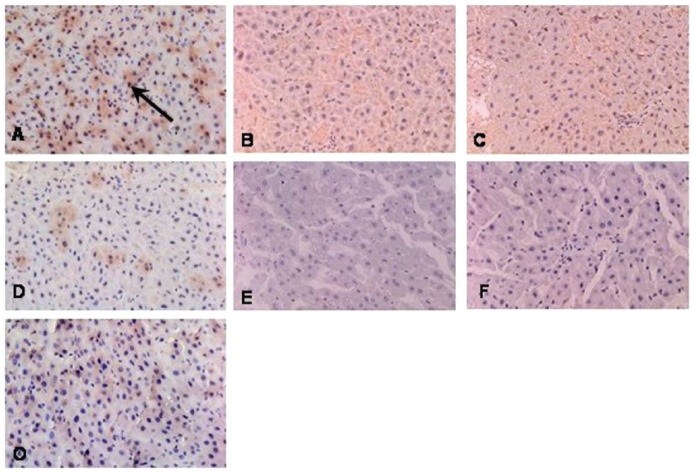
MyD88 protein expression in the CLP surgery group was higher increase at 6 h after surgery compared to that at 2 and 4 h after surgery (Figure 4). In addition, MyD88 showed higher increase in the CLP surgery group than that in the sham control group at 6 h after surgery, but there was not different between the two groups at 2 and 4 h after surgery. MyD88 protein expressions decreased after 24 h after CLP surgery
Figure 4.
IHC staining of rat liver tissues revealed that MyD88 protein expression in the CLP group at 6 h (a), 4 h (b), and 2 h (c) after surgery; MyD88 protein expression in the sham control group was not different at 6 (d), 4 (e), and 2 (f) hours after surgery, compared to no-surgery controls (o).
NF-κB
EMSA showed that activity of NF-κB, a MyD88 downstream factor, obviously increased at 4 h after CLP surgery (Figure 5). The activity of NF-κB increased at 4 h after CLP surgery, but obviously increased at 6 h after CLP surgery. The activity of NF-κB obviously decreased after 24 h after CLP surgery.
Figure 5.
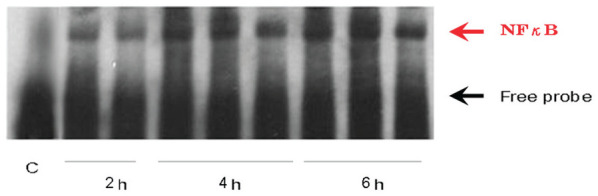
Septic peritonitis induced by CLP surgery stimulated NF-κB activation in an electrophoretic mobility gel shift assay (EMSA). Activity of NF-κB in response to the MyD88-dependent pathways stimulated early 4 h after CLP surgery.
Serum TNF-α levels
Serum TNF-α levels in the CLP group increased as early as 2 h after surgery and highly increased at 4 and 6 h compared to 0.5 h after CLP surgery (both p < .001) (Figure 6). In addition, serum TNF-α levels increased significantly at 4 and 6 h after CLP surgery than those in the sham control group (p < .05 and p = .001, respectively). Therefore, the MyD88-dependent pathway was activated as early as 2 h after CLP surgery in the experimental rodent sepsis model.
Figure 6.
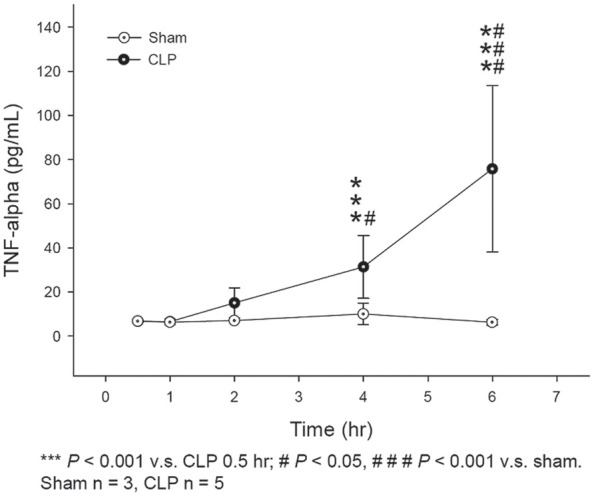
ELISA analysis for TNF-alpha levels at different time points after surgery in the CLP group and sham control group.
Discussion
A number of studies suggest that the innate immune system is the first line of the host defense against invading pathogens.1–4 TLRs are now generally accepted as receptors that recognize pathogen-associated molecular patterns. The stimulation of TLRs by agonists can trigger the activation of two downstream signaling pathways: MyD88-dependent and MyD88-independent pathways.13–15 The stimulation of TLRs by agonists can trigger the activation of two downstream signaling pathways: MyD88-dependent and MyD88-independent pathways.16–18 The CLP model is the most representative animal model of human sepsis. 19 The CLP sepsis consists of both Gram-negative and Gram-positive bacteremia and may activate the TLR responses.
In this investigation, the different time points of expression of MyD88 and its downstream factors in the CLP-induced sepsis animal model were determined. The experimental animal study demonstrated the serial expression of MyD88 in the CLP surgery group compared to the sham control group. We observed that CLP surgery significantly increased tissue MyD88 mRNA concentrations at 2 h after induction of sepsis. Accompanying the activation of MyD88-dependent TLR signaling pathway, NF-κB can be subsequently activated and serum cytokines including TNF-α also can be highly elevated.16–18 Cytokines play a pivotal role in both the pro-inflammatory and the anti-inflammatory reaction to sepsis. Various kinds of pro-inflammatory and anti-inflammatory cytokines are involved in sepsis pathology. 20 The pro-inflammatory cytokine TNF-α is one of the first cytokines to be released after infections. 21 Peak concentrations of TNF-α can be observed early within 2 h after infections or trauma.22–24 Previous studies have demonstrated a positive correlation between elevated TNF-α and poor outcome.22–24 In this study, we only measured serum levels of TNF-α as the representative cytokine for the early stage of sepsis. In the current study, we noticed that NF-κB activity and the levels of serum TNF-α both increased at 4 h after CLP surgery. Notably, the MyD88-dependent pathway is activated early in CLP-induced sepsis, before 4 h after CLP surgery.
Conclusion
The animal data demonstrates that the MyD88-dependent pathway is activated early in CLP-induced sepsis rats. These results may provide further helpful information for using pro-inflammatory biomarkers of innate immunity such as MyD88 and TNF-α in clinical sepsis or related abdominal surgical emergency in the future.
Supplemental Material
Supplemental material, sj-docx-1-iji-10.1177_03946320221090021 for Time-serial expression of toll-like receptor 4 signaling during polymicrobial sepsis in rats by En-Pei Lee, Mao-Jen Lin and Han-Ping Wu in International Journal of Immunopathology and Pharmacology
Appendix
TLRs: Toll-like receptors
MyD88: myeloid differentiation factor 88
NF-κB: nuclear factor-κB
CLP: cecal ligation and puncture
QPCR: quantitative polymerase chain reaction
ELISA: enzyme-linked immunosorbent assay
TNF-α: tumor necrosis factor-α
SD: standard deviation
Footnotes
Availability of data and material: De-identified data used in this study is available upon request of author HP-W.
Author contributions: HP-W and E-PL performed the experiment, analyzed, interpreted the data, designed and oversaw the study and revised the article. E-PL and M-JL drafted the article. All authors have read and approved the final article for publication.
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The study was supported in part by the China Medical University Hospital DMR-111-073.
Ethics approval: Ethical approval was not sought for the present study because *This is only an animal study*.
Animal welfare: The present study followed international, national, and institutional guidelines for humane animal treatment and complied with relevant legislation. All experimental protocols were reviewed and approved by the Chang Gung Memorial Hospital Animal Care Review Board (Permit Number: 2015121517).
ORCID iD: Han-Ping Wu  https://orcid.org/0000-0002-0069-4183
https://orcid.org/0000-0002-0069-4183
References
- 1. Gay NJ, Symmons MF, Gangloff M, et al. (2014) Assembly and localization of Toll-like receptor signalling complexes. Nature Reviews Immunology 14: 546–558. [DOI] [PubMed] [Google Scholar]
- 2. Bauer RN, Diaz-Sanchez D, Jaspers I. (2012) Effects of air pollutants on innate immunity: The role of Toll-like receptors and nucleotide-binding oligomerization domain-like receptors. Journal of Allergy and Clinical Immunology 129: 14–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Płóciennikowska A, Hromada-Judycka A, Borzęcka K, et al. (2015) Co-operation of TLR4 and raft proteins in LPS-induced pro-inflammatory signaling. Cell Mol Life Sci 72: 557–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hwang EH, Kim TH, Oh SM, et al. (2016) Toll/IL-1 domain-containing adaptor inducing IFN-β (TRIF) mediates innate immune responses in murine peritoneal mesothelial cells through TLR3 and TLR4 stimulation. Cytokine 77: 127–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Han J. (2006) MyD88 beyond Toll. Nature Immunology 7: 370–371. [DOI] [PubMed] [Google Scholar]
- 6. Xiong Y, Pennini M, Vogel SN, et al. (2013) IRAK4 kinase activity is not required for induction of endotoxin tolerance but contributes to TLR2-mediated tolerance. Journal of Leukocyte Biology 94: 291–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Yang JC, Wu SC, Rau CS, et al. (2014) Inhibition of the phosphoinositide 3-kinase pathway decreases innate resistance to lipopolysaccharide toxicity in TLR4 deficient mice. Journal of biomedical science 21(21): 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Liu J, Cao X. (2016) Cellular and molecular regulation of innate inflammatory responses. Cellular & Molecular Immunology 13: 711–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Takeuchi O, Akira S. (2010) Pattern recognition receptors and inflammation. Cell 140: 805–820. [DOI] [PubMed] [Google Scholar]
- 10. Bakopoulos A, Kapelouzou A, Tsilimigras DI, et al. (2017) Expression of Toll-like receptors (TLRs) in the lungs of an experimental sepsis mouse model. Plos One 12: e0188050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Krivan S, Kapelouzou A, Vagios S, et al. (2019) Increased expression of Toll-like receptors 2, 3, 4 and 7 mRNA in the kidney and intestine of a septic mouse model. Sci Rep 9: 4010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Majetschak M, King DR, Krehmeier U, et al. (2005) Ubiquitin immunoreactivity in cerebrospinal fluid after traumatic brain injury: Clinical and experimental findings. Critical Care Medicine 33: 1589–1594. [DOI] [PubMed] [Google Scholar]
- 13. Deng M, Scott MJ, Loughran P, et al. (2013) Lipopolysaccharide clearance, bacterial clearance, and systemic inflammatory responses are regulated by cell type-specific functions of TLR4 during sepsis. The Journal of Immunology 190: 5152–5160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Tsao PN, Wei SC, Huang MT, et al. (2011) Lipopolysaccharide-induced Notch signaling activation through JNK-dependent pathway regulates inflammatory response. Journal of Biomedical Science 18: 56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Rosadini CV, Zanoni I, Odendall C, et al. (2015) A single bacterial immune evasion strategy dismantles both MyD88 and TRIF signaling pathways downstream of TLR4. Cell Host & Microbe 18: 682–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kerfoot SM, Long EM, Hickey MJ, et al. (2004) TLR4 contributes to disease-inducing mechanisms resulting in central nervous system autoimmune disease. The Journal of Immunology 173: 7070–7077. [DOI] [PubMed] [Google Scholar]
- 17. Ware CF, VanArsdale S, VanArsdale TL. (1996) Apoptosis mediated by the TNF-related cytokine and receptor families. Journal of Cellular Biochemistry 60: 47–55. [DOI] [PubMed] [Google Scholar]
- 18. Beutler B, Cerami A. (1989) The biology of cachectin/TNF–a primary mediator of the host response. Annual Review of Immunology 7: 625–655. [DOI] [PubMed] [Google Scholar]
- 19. Toscano MG, Ganea D, Gamero AM. (2011) Cecal ligation puncture procedure. Journal of Visualized Experiments: JoVE 7: 2860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Schulte W, Bernhagen J, Bucala R. (2013) Cytokines in sepsis: potent immunoregulators and potential therapeutic targets—an updated view. Mediators of Inflammation 2013: 165974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hietbrink F, Koenderman L, Rijkers G, et al. (2006) Trauma: The role of the innate immune system. World Journal of Emergency Surgery 1: 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Strieter RM, Kunkel SL, Bone RC. (1993) Role of tumor necrosis factor-α in disease states and inflammation. Critical Care Medicine 21: S447–S463. [DOI] [PubMed] [Google Scholar]
- 23. Ozturk H, Yagmur Y, Ozturk H. (2008) The prognostic importance of serum IL-1beta, IL-6, IL-8, and TNF-alpha levels compared to trauma scoring systems for early mortality in children with blunt trauma. Pediatric Surgery International 24: 235–239. [DOI] [PubMed] [Google Scholar]
- 24. Zedler S, Faist E. (2006) The impact of endogenous triggers on trauma-associated inflammation. Current Opinion in Critical Care 12: 595–601. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-iji-10.1177_03946320221090021 for Time-serial expression of toll-like receptor 4 signaling during polymicrobial sepsis in rats by En-Pei Lee, Mao-Jen Lin and Han-Ping Wu in International Journal of Immunopathology and Pharmacology