Abstract
Propolis is a resinous beehive product that has a wide range of biological activities, namely antimicrobial, antioxidant, and anti-inflammatory properties. Propolis is collected by the bees from plant resin and exudates to protect hives and maintain hive homeostasis. The aim of the present systematic scoping review is to explore the potential and suitability of propolis as an adjunctive treatment in breast cancers, based on the latest available experimental evidence (2012-2021). After applying the exclusion criteria, a total of 83 research publications were identified and retrieved from Scopus, Web of Science, and Pubmed. Several relevant key themes identified from the included studies were cytotoxicity, synergistic/combination treatment, improvement in bioavailability, human clinical trials, and others. A majority of the studies identified were still in the in vitro and in vivo stages. Nonetheless, we managed to identify 4 human clinical trials that demonstrated the successful use of propolis in alleviating side effects of chemotherapy and radiotherapy while increasing the quality of life of breast cancer patients, with minimal adverse effects. In conclusion, propolis, as an adjunctive treatment, may have therapeutic benefits in alleviating symptoms related to breast cancers. However, further clinical trials, preferably with higher number of participants/subjects/patients, are urgently needed.
Keywords: propolis, adjunct therapy, supportive care, systematic review, nutraceutical, complementary medicine
Introduction
The burdensome nature of cancers is considered to be a challenging issue in modern healthcare and scientific discussions. Many epidemiological studies, psychosocial impact investigations, and economic burden analyses delineate the impact of cancers, not only on the medical and healthcare fields, but also its extensive influence on other important social structures.1,2 As an example, a report by National Cancer Institute in 2021 evaluated the US national economic burden for cancer care and found breast cancers were ranked first for top net economic burden with the total out-of-pocket cost and indirect expenditure of US$3.1 billion and $1.1 billion, respectively. 3 Additionally, a recent GLOBOCAN report had placed breast cancers as the most commonly diagnosed malignancy worldwide with more than 2.2 million new cases in 2020, accounting for 11.7% incidence of cancer worldwide, surpassing lung cancer rates, and also ranked first in term of cancer-related mortality in females. Statistically, breast cancer is found in 1 in 4 diagnosed cancer cases and 1 in 6 cancer-related deaths among women, which are remarkably alarming considering the changes of global pattern toward heavier cancer burden in upcoming years.4-6
Furthermore, the improvement in breast cancer management should be considered since the emerging issues of treatment-resistant breast cancers in certain subtypes notably the triple-negative breast cancer (TNBC). The therapeutic approaches often consist of a plethora of molecular/physiological targets, ranging from endocrine-based regiments, cytotoxic chemotherapy, radiotherapy, the targeted therapies such as the development of combination treatments.7-10 In addition, the anti-endocrine treatment is the principal approach in managing steroid-receptor positive breast cancers (ER+/PR+). However, the resistance toward these hormonal approaches due to genetic and/or epigenetic mutations may alter the ER gene expressions of the breast cancers and subsequently activates alternative signaling pathways to prevent estrogen blocking; treatment resistance will therefore eventually develop.10-12 The resistance issues toward conventional treatments illustrate the need to establish other therapeutic approaches, such as novel alternative or adjunctive treatments.
Although the earliest known written records of cancers were obtained from the ancient Egyptians’ manuscripts circa 1500 to 1600 BCE, and possibly were based on much older records; some notable advancements of cancer treatment options were only apparent after the early 20th century. Throughout the entire human history, the utilization of natural remedies to alleviate numerous diseases is unquestionably important and arguably safe to be implemented, and can be adapted to the current modern treatment strategies.13,14 Natural products have long been shown to be promising sources of anti-cancer compounds and/or therapeutics, for example, the Catharanthus roseus-derived vincristine and Taxus brevifolia-derived paclitaxel. 15 Propolis, or bee glue, with its diverse range of chemical compounds, has frequently been demonstrated to exhibit various biological activities, including immunomodulatory, anti-inflammatory, and anti-cancer properties.16-18 The aim of the present scoping review is to analyze the potential use and suitability of propolis as a supportive or adjunctive therapeutic substance for breast cancer management.
Methods
The present systematic review was performed in accordance with the guidelines provided by Peters et al 19 and Munn et al. 20 The guiding question was as follows: Can propolis be used as an adjunctive therapy in breast cancers? Two independent reviewers (F. Z. and K. C.) performed the search for articles dated January 2012 up to December 2021. The databases searched were Scopus, Pubmed, and Web of Science. Supplemental Table S1 shows the terms used in the search process. We intentionally did not include the terms that describe individual bioactive compounds of propolis such as caffeic acid phenethyl ester (CAPE), quercetin, galangin, kaempferol, and so on, as we focused on propolis as a whole. However, if during the search and screening process we encountered relevant studies that described the individual propolis bioactive compounds and met the inclusion criteria, we included them in the final list of the included studies. Only articles that were written in English were included. All articles that describe the potential use of propolis in treating breast cancers were selected; in vitro, in silico, animal models, and human clinical trials. However, we excluded any article that describes the use of synthetic derivatives of propolis bioactive compounds. The studies were recorded in Mendeley and the duplicates subsequently removed. We also excluded review articles as they might impart biases.
Subsequently, 2 reviewers (F. Z. and K. C.) assessed the search results independently. The articles were screened based on the titles, keywords, abstracts, and full texts. The articles that did not fit in the guiding question and the set criteria were then removed. If any disagreement arose on the eligibility of a particular article, the disagreement was resolved through discussion with another reviewer (D. H.). The following data were subsequently tabulated in Microsoft Excel: geographic locations of the propolis source and types of bees, types of propolis extract and/or propolis bioactive compounds, types of study, concentration of the propolis extract and/or bioactive compounds, outcome of the study, and references. The reviewers subsequently categorized the included studies based on the objectives of the studies into the appropriate themes.
Characteristics of the Studies
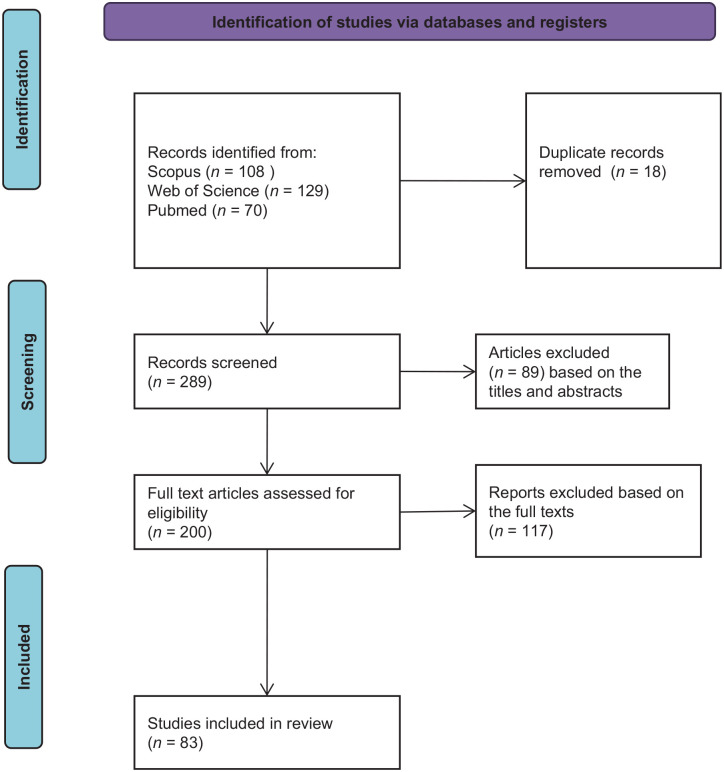
There were 307 scientific articles found in the initial search. The articles were initially screened based on the titles and abstracts. Further screening based on the full texts resulted in 83 articles. Figure 1 illustrates the screening process. Table 1 summarizes the themes, types of study, types of propolis extract and/or bioactive compounds, the measured outcome of the included studies, and the references. Figure 2 summarizes the characteristics of the included studies: percentages of the types of extract, the percentages of the study types, themes, and types of bees identified in the included studies.
Figure 1.
The screening process of the studies adapted from Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA).
Table 1.
The Summary of the Included Studies Demonstrating the Potential Use of Propolis in Breast Cancers.
| Geographical locations of the propolis source/bee species | Types of extract/bioactive compounds | Types of study | Concentration | Measured outcome | References |
|---|---|---|---|---|---|
| Cytotoxicity | |||||
| Indonesia/not specified—A. mellifera assumed | Bioactive compound | In vitro | IC50 = 4.57 μg/mL and 10.23 μg/mL | α-Amyrin isolated from Indonesian propolis had IC50 values of 4.57 μg/mL and 10.23 μg/mL against MCF-7 and T-47D breast cancer cells | Syamsudin and Simanjuntak 21 |
| Brazil, China/not specified—A. mellifera assumed | Ethanolic extract | In vitro | Propolis: 0.1-20 μg/mL | Propolis and its bioactive compound CAPE had cytotoxic effect against MCF-7 breast cancer cells. Brazilian red propolis extract had superior effect compared to Chinese and other Brazilian propolis extracts. | Kamiya et al 22 |
| CAPE | CAPE: 0.1-2 μM | Propolis extracts and CAPE induced apoptosis of MCF-7 cells by upregulating caspase-3 activity, DNA fragmentation, and CCAAT/enhancer-binding protein homologous protein (CHOP) expression in MCF-7 cells. It was also evident that propolis and CAPE promoted mitochondrial dysfunction and endoplasmic reticulum stress. | |||
| India/not specified—A. mellifera assumed | Hydroethanolic extract | In vitro | IC50 = 10 μg/mL | Cytotoxic activity against MCF-7 cells | Thirugnanasampandan et al 23 |
| China/not specified—A. mellifera assumed | Ethanolic extract followed by n-hexane and ethyl acetate fractionation | In vitro | Propolis extracts: 20 μg/mL | Cytotoxic activity against MDA-MB-231 cells | Sun et al 24 |
| CAPE | In vivo | CAPE: 17-34 μM | CAPE induced cell cycle arrest in the G0/G1 phase | ||
| Chrysin | Chrysin: 20-60 μM and 90 mg/kg/d (in vivo) | Chrysin inhibited HDAC8 and significantly increased the expression of p21 (waf1/cip1). Chrysin inhibited tumor growth in mice. | |||
| Malta/A. mellifera | Methanolic extract | In vitro | IC50 = 21-67 μg/mL | Cytotoxic activity against MCF-7 cells. Cytotoxicity appeared to be correlated with totarol content. | Zammit et al 25 |
| USA/A. mellifera | Ethanolic extract | In vitro | Propolis extract: | Propolis extracts and CAPE had a dose-dependent cytotoxic activity against MDA-MB-231, MCF-7, and SK-BR-3 cells | Omene et al 26 |
| CAPE | 5-50 μM standardized to CAPE content | Propolis extracts and CAPE appeared to have anti-cancer effects: | |||
| CAPE: 10-80 μM | 1. Promoted accumulation of acetylated histone proteins (epigenetic effects) | ||||
| 2. Downregulated the expression of estrogen receptor and progesterone receptor | |||||
| India/stingless bee (Trigona spp.) | Hydroethanolic extract | In vitro | 10-250 μg/mL | Cytotoxic activity against MCF-7 cells | Choudhari et al 27 |
| Indonesia/stingless bee (Trigona spp.) | Hydroethanolic extract | In vitro | 100 μg/mL | Cytotoxic activity against MCF-7 cells | Hasan et al 28 |
| India/A. mellifera | Hydroethanolic extract | In vitro | IC50 = 27-104 μg/mL | Cytotoxic activity against MCF-7 cells | Shubharani et al 29 |
| Portugal/A. mellifera | Hydroethanolic extract | In vitro | GI50 (sample concentration achieving 50% of growth inhibition) = 36-182 μg/mL | Cytotoxic activity against MCF-7 cells | Calhelha et al 30 |
| China/A. mellifera | Ethanolic extract | In vitro | 25-200 μg/mL | Cytotoxic activity against MCF-7 and MDA-MB-231 cells | Xuan et al 31 |
| Propolis induced apoptosis by upregulating the expression of ANXA7, ROS level, and NF-κB p65 level, while simultaneously reducing the mitochondrial membrane potential. Propolis extract had little cytotoxicity on normal human umbilical vein endothelial cells (HUVECs). | |||||
| Brazilian Green Propolis/A. mellifera | Methanolic extract followed by hexane, chloroform, and n-butanol fractionation | In vitro | IC50: | Cytotoxicity against MCF-7 cells | de Oliveira et al 32 |
| Propolis extract = 246 μg/mL | Baccharin appeared to be the anticancer compound in Brazilian green propolis extract | ||||
| Artepillin C (not cytotoxic) | |||||
| Artepillin C | Baccharin = 23 μg/mL | ||||
| Baccharin | |||||
| Turkey/A. mellifera | Ethanolic extract | In vitro | IC50: | Cytotoxicity against MCF-7 cells | Turan et al 33 |
| Quercetin | Propolis = 28 μg/mL | ||||
| Quercetin = 9 μg/mL | |||||
| Thailand/A. mellifera | Cardanol | In vitro | IC50 = 15.6 ± 1.76 μg/mL | Cytotoxicity against BT-474 cell | Buahorm et al 34 |
| Cardanol induced apoptosis by causing cell cycle arrest at the G1 subphase and cell death at late apoptosis | |||||
| Cardanol modulated the expression of genes related to apoptosis: increased the expression of DR5 and Bcl-2 and reduced the expression of Mcl-1, MADD, and c-FLIPP | |||||
| Cardanol also affected the expression of genes related to cell division: increased p21, E2FI, p21 p-ERK, p-JNK, and p-p38 and decreased the expression of cyclin D, cyclin D1, cyclin E, CDK4, and CDK2, resulting in the failure to progress from the G1 to the S subphase | |||||
| Poland/A. mellifera | Hydroethanolic extract | In vitro | IC50: | Cytotoxicity against MDA-MB-231 and Hs578T cells. Based on MTT and LDH assays, and morphological changes, it appeared CAPE and propolis induced mitochondrial damage and subsequent apoptosis in breast cancer cells. | Rzepecka-Stojko et al 35 |
| CAPE | CAPE = 11.69-22.93 μg/mL (MDA-MB-231), 4.82-32.80 μg/mL (Hs578T) | ||||
| Propolis = 40.40-731.68 μg/mL (MDA-MB-231), 31.03->3000 μg/mL (Hs578T) | |||||
| Serbia/A. mellifera | Ethanolic extract | In vitro | IC50: | Cytotoxicity against MDA-MB-231 cells | Milosevic-Djordjevic et al 36 |
| Propolis = 81.65-96.57 μg/mL | Synergistic activity with mitomycin C | ||||
| In combination with 0.5 µg/mL MMC = 19.13-23.79 μg/mL | |||||
| Turkey/A. mellifera | Ethanolic extract | In vitro | 50 μg/mL | Propolis acted as antioxidant and reduced the cytotoxicity of homocysteine in MCF-7 cells | Tartik et al 37 |
| Bioactive compounds | CAPE | In vitro | 0.1-100 µM | Cytotoxicity against MDA-MB-231 and MDA-MB-468 | Fraser et al 38 |
| CAPE had anti-metastatic properties by interfering with and inhibiting the voltage-gated sodium channels and ion channel | |||||
| Bioactive compounds | CAPE and caffeic acid | In vitro | IC50: | CAPE and caffeic acid had cytotoxicity activity against MDA-MB-231 cells | Kabała-Dzik et al39,40 |
| CAPE = 55.79-68.82 µM | |||||
| Caffeic acid = 103.23-135.85 µM | CAPE and caffeic acid inhibited the migration rate of the cancer cells | ||||
| CAPE and caffeic acid induced cell cycle arrest in S phase, G0/G1 phase, and eliminated G2/M phase | |||||
| CAPE had significantly better efficacy compared to caffeic acid | |||||
| Cameroon/A. mellifera | Hydroethanolic extract | In vitro | 10−8 to 10−5 μg/mL | Did not appear to be cytotoxic to MCF-7 cells but reduced the proliferation of MCF-7 cells | Zingue et al 41 |
| China/A. mellifera | Ethanolic extract | In vitro | Propolis: 25, 50, and 100 μg/mL | Propolis and CAPE inhibited LPS-stimulated MDA-MB-231 cell proliferation by inducing apoptosis through upregulating caspase 3 and PARP. Propolis and CAPE also induced autophagy by upregulating LC3-II and downregulating p62 level. In addition, Propolis and CAPE downregulated TLR4 signaling pathway molecules such as TLR4, MyD88, IRAK4, TRIF, and NF-κB p65. | Chang et al 42 |
| CAPE | CAPE: 25 μg/mL | ||||
| Bioactive compounds | CAPE | In vitro | 10 and 25 µM | In MCF-7 cells, CAPE inhibited mitochondrial oxygen consumption rate (OCR) by reducing basal, maximal, and spare respiration rate and consequently inhibiting ATP production | Bonuccelli et al 43 |
| In addition, CAPE also inhibited mammosphere formation (3-D sphere formation) of MCF-7 cells | |||||
| Lebanon/A. mellifera assumed | Hydroethanolic extract followed with hexane, methylene chloride, and ethyl acetate fractionation | In vitro | IC50: 61-75 μg/mL | Cytotoxicity activity against MDA-MB-231 cells by apoptosis | Noureddine et al 44 |
| Iran/A. mellifera assumed | Ethanolic extract | In vitro | IC50 = 65-96 μg/mL | Cytotoxicity against MCF-7 cells by inducing intracellular ROS production | Asgharpour et al 45 |
| Malaysia/Geniotrigona thoracica | Hydroethanolic extract | In vitro | IC50 = 38.9 µg/mL | Cytotoxicity against MCF-7 cells | Ismail et al 46 |
| Serbia/A. mellifera assumed | Methanolic extract | In vitro | IC50 = 115->500 µg/mL | Eleven flavonoids were identified: chrysin, galangin, tectochrysin, apigenin, kaempferol, isohamnetin, luteolin, myricetin, pinocembrin, naringenin, hesperetin | Vukovic et al 47 |
| Myricetin, luteolin, galangin, and pinocembrin had the highest cytotoxicity activity against MDA-MB-231 cells. The flavonoids induced apoptosis in the cancer cells. | |||||
| Turkey/A. mellifera | Hydroethanolic extract | In vitro | 50-200 μM | Anti-proliferative effect on MDA-MB-231 and UACC-3199 breast cancer cell lines | Ozdal et al 48 |
| Turkish phenolics profile: pinocembrin, galangin, pinobanksin, pinostrobin, chrysin, caffeic acid, p-coumaric acid, ferullic acid, t-cinnamic acid | |||||
| Bioactive compounds | Apigenin, genistein, hesperidin, naringin, and quercetin | In vitro | IC50 = 9.39-130.10 μM | The flavonoids were more cytotoxic toward MCF-7 compared to MDA-MB-231 breast cancer cells | Kabała-Dzik et al 49 |
| Cytotoxicity: | |||||
| MCF-7: Hesperidin > Apigenin > Naringin > Genistein > Quercetin | |||||
| MDA-MB-231: Genistein > Hesperidin > Apigenin > Quercetin > Naringin | |||||
| Bioactive compounds | CAPE and caffeic acid | In vitro | IC50: | CAPE and caffeic acid inhibited the migration rate of MCF-7 cells | Kabała-Dzik et al 50 |
| Caffeic acid = 65.05-84.87 µM | CAPE > caffeic acid | ||||
| CAPE = 29.05-69.05 µM | |||||
| China, Argentina, Turkey/A. mellifera assumed | Hydroethanolic extract | In vitro | Propolis: 2.5-500 μg/mL | Propolis extracts were cytotoxic against MCF-7, SK-BR-3, and MDA-MB-231 cells with various degree of efficacy. The cytotoxicity did not correlate with the total phenolics/flavonoids but rather with the diversity of phenolics/flavonoids. The propolis extracts induced apoptosis in cancer cells. | Seyhan et al 51 |
| Galangin, caffeic acid, apigenin, and quercetin | Phenolics: 5-70 μg/mL | Galangin, caffeic acid, apigenin, and quercetin were cytotoxic against MCF-7 cells | |||
| Brazil/A. mellifera | Volatile oil | In vitro | IC50 = 62-85 μg/mL | Cytotoxicity activity against MCF-7 cells | de Lima et al 52 |
| Turkey/A. mellifera | Dulbecco’s Modified Eagle Medium (DMEM) extract | In vitro | 2.5-10 mg/mL | DMEM extract of propolis induced cytotoxic effect on MDA-MB-231 cells. The propolis extract appeared to induce morphological changes in cancer cells. | Uçar and Değer53,54 |
| Morocco/A. mellifera assumed | Hydroethanolic extract | In vitro | 6.25-400 µg/mL | Cytotoxicity against MCF-7 cells | Falcão et al 55 |
| Indonesia/Tetragonula biroi | Hydroethanolic extract | In vitro | 250 ppm | Cytotoxicity against MCF-7 cells | Diva et al 56 |
| Bioactive compounds identified: xanthoxyletin, curcumine, derrubone, arenobufagin, furanodiene, zerumbone, 6-dehydrogingerdione, and bufotalin | |||||
| Cuba/A. mellifera assumed | Hydroethanolic extract | In vitro | IC50 = 67.3 ± 12.8 µg/mL | Propolis had antiproliferative and cytotoxic activities against MDA MB-231 cells | Frión-Herrera et al 57 |
| Propolis induced mitochondrial dysfunction and lactate dehydrogenase release indicating the occurrence of ROS-associated necrosis. Propolis also reduced cell migration rate. Interestingly, a reduced expression of apoptosis-related genes such as TP53, CASP3, BAX, and P21) was observed, whereas the expressions of BCL-2, BCL-XL, NOXA, and PUMA were not affected. | |||||
| Bioactive compounds | CAPE | In vitro | 100 μM | CAPE had cytotoxic activity against MDA-MB-231 cells by inducing oxidative stress through upregulation of e-NOS and i-NOS levels | Fırat et al 58 |
| Turkey (Trabzon area). Not specified—A. mellifera assumed | Ethanolic extracts of propolis (EEP) | In vitro | IC50 for EEP was 61 µg/mL | EEP reduced cell viability in a dose-dependent manner. EEP displayed selective cytotoxicity against MCF-7 cells compared to normal foreskin fibroblast cells. EEP cause considerable number of apoptotic cells and reduce the number of viable cells in a dose dependent manner in MCF-7 cells. | Misir et al 59 |
| Indonesia (South Sulawesi). stingless bee of the Trigona spp. | Ethanolic extract (EEP) | In vitro | IC50 = 10.8 ± ± 0.06 μg/mL against MCF-7 | The water-insoluble propolis (wax fraction) had a strong cytotoxic activity on MCF-7 cells, with IC50 values of 0.04 ± 0.003 mg/mL | Amalia et al 60 |
| Iran/not mentioned—A. mellifera assumed | Ethanolic extracts of sirch propolis | In vitro | IC50 of EESP (24 h, 1% FBS): | Against human breast cancer cell lines of MDA-MB-231, SKBR-3, MCF-7 | Amalia et al 61 |
| —50.58 μg/mL for MDA-MB-231 | BrdU assay for proliferation inhibition of EESP at 200 μg/mL and 1% FBS (P < .0001) | ||||
| —60.98 μg/mL for SKBR-3 | Apoptotic effect and cell cycle analysis of EESP assessed by flow cytometry | ||||
| —198 μg/mL for MCF-7 | |||||
| The IC50 values were classified further (24 h/48 h and 1%/10% FBS) | |||||
| Brazil/not mentioned/A. mellifera assumed | Ethanolic extracts of propolis | In vitro | IC50 of EEP = 18.06 μg/mL against BT-20 cells (control: 17.02 and 20.10) | Against human breast cancer cell lines of BT-20, BT-549, MDA-MB-231, and MDA-MB-436 (triple-negative breast cancer cells line) | Assumpção et al 62 |
| IC50 of EEP = 25.45 μg/mL against BT-549 cells (control 13.94 and 19.16) | Compared against phenolic acids and EGCG in the same cell lines in cell viability analysis after in vitro treatment (P < .05; P < 2 h of exposure) | ||||
| IC50 of EEP against MDA-MB-231 and MDA-MB-436 weren’t mentioned | Analysis of global DNA methylation content to test the newly reported small molecules of DNMTi in propolis (as compared to control), significant P value of <.05 | ||||
| Brazil/Apis mellifera L. | Ethanolic extract | In vitro | IC50 against DPPH (for its scavenging activity) was 492.2 μg/mL (for Central group) and >1000 μg/mL (for 3 others group) | Against human breast cancer cell lines (MCF-7) | Costa et al 63 |
| Cytotoxic assay by assessing the growth inhibition of the cancer cell lines | |||||
| DPPH-radical-scavenging assay of PE EtOH | |||||
| Algeria/not specified—A. mellifera assumed | Ethanolic extract | In vitro | IC50 = 45 μg/mL | Propolis caused a strong dose dependent inhibition of cell growth in MDA-MB-231 cells. Propolis had a synergistic effect on Doxorubicin, which at 0.048 μM, in combination with propolis at 30 μg/mL significantly (P < .001) inhibited the growth of tumor cells (35%). | Rouibah et al 64 |
| Cameroon/A. mellifera | Freeze-dried hydroethanolic (70:30) extract (EEP) | In vitro | Cytotoxic effect (CC50) on human breast carcinomas MCF-7 and MDA-MB-231 cells and murine breast carcinoma were 88.7 ± 4.6, 69.1 ± 1.3, and 54.4 ± 2.1 μg/mL, respectively | Melanoma SK-MEL-28 cells were the most sensitive to EEP with a CC50 value of 33.1 ± 2.4 μg/mL. Average CC50 in cancerous cells was 60 μg/mL compared to the average CC50 of 127.5 μg/mL in nontumoral cells, leading to a Selectivity Index (SI) of ~2.1, indicating selectivity of EEP for cancer cells. | Zingue et al 65 |
| North China//poplar, not specified—A. mellifera assumed | Oven dried EEP | In vitro | Best inhibition of cell viability: 100 μg/mL | Propolis treatment of MDA-MB-231 cells in an inflammatory microenvironment was able to inhibit tumor cell proliferation by targeting key enzymes of glycolysis | Li et al 66 |
| Egypt/A. mellifera assumed | Ethanolic extract of propolis (EEP) | In vitro | IC50 = 11.95 ± 0.01 μg/mL against MCF-7 | Quercetin was reported to suppress viability and proliferation of MCF-7 cells by activation of both apoptosis and necrosis signaling pathways. The Egyptian propolis extract exhibited more potent cytotoxic activity than well-known cytotoxic agents such as platinum nanocatalysts 56 and even propolis from other regions such as Moroccan and Indian propolis. | Hamed et al 67 |
| Brazil/A. mellifera | Chemically derivatized from green propolis | In vitro | IC50 = 9.6 ± ± 3 µM | Best inhibitory activity was found in a compound derived from drupanin isolated from propolis | Rodrigues et al 68 |
| Selectivity Index = 5.5 against MCF-7 | |||||
| Indonesia/Homotrigona fimbriata, Heterotrigona itama, Heterotrigona bakeri, Tetragonula sarawakensis, Tetragonula testaceitarsis, Tetragonula fuscobalteata, Tetragonula laeviceps | Ethanolic extract | In vitro | 75 µg/mL | Propolis extracts of H. fimbriata and T. laeviceps were more cytotoxic toward MCF-7 cells compared to T. testaceitarsis, T. sarawakensis, H. bakeri, H. itama, and T. fuscobalteata in term of MCF-7 cell | Arung et al 69 |
| Bioactive compound that was found to be the most effective was mangiferonic acid (IC50 = 96.76 µM in MCF-7) | |||||
| Egypt/A. mellifera assumed | Hydroethanolic extract | In silico | Not determined | Propolis bioactive compounds genistein, luteolin, benzoic acid, quercetin, and vanillic acid, were shown to interfere with cancer-associated targets (estrogen signaling pathway) CYP1A1, CYP19A1, ESR1, NOS3, CASP3, and AKT1 | Ibrahim and El-Banna 70 |
| In vitro | IC50 = 11.95 µg/mL | Hydroethanolic extract of propolis was cytotoxic toward MCF-7 cells | |||
| Australia, Brazil, China/A. mellifera assumed | Ethanolic extract | In vitro | 6.25-200 µg/mL | Cytotoxicity against MCF-7 and MDA-MB-231 cells | Bhuyan et al 71 |
| Combination treatment | |||||
| Bioactive compounds | CAPE | In vitro | 1-100 µM | Cytotoxocity against MDA-MB-231 and T47D | Khoram et al 72 |
| CAPE improved the efficacy of radiotherapy by sensitizing the cancer cells through impairing DNA damage repair in cancer cells | |||||
| Bioactive compounds | CAPE | In vitro | 0.1-200 mM | Synergistic activity of tamoxifen and CAPE against MCF-7 cells by significantly inducing apoptosis and dowregulating the levels of Bcl-2 and beclin-1, and endothelial growth factor. More importantly, combination of TAM and CAPE increased the life span of the tumor-bearing mice compared to TAM or CAPE alone. | Motawi et al 73 , 74 |
| In vivo | 0.75 mg/kg BW/3 times a day for 12 d | ||||
| Not determined/A. mellifera assumed | Not determined | In vivo | 0.128 mg/kg BW of mangostin and 0.32 mg/kg BW propolis extract daily for 14 d | Propolis alone decelerated the growth of mammary tumor. However, the effect of combination of mangostin and propolis was more pronounced. | Tan and Hayati 75 |
| The combination of propolis and mangostin significantly reduced the expression of Wnt2, FAK, and HIF-1α, when compared to propolis or mangostin alone | |||||
| Bioactive compounds | Chrysin | In vitro | IC50: 43.4-72.2 µM | Cytotoxicity against T47D breast cancer cells linked to the downregulation of the mRNA levels of hTERT and cyclin D1 | Maasomi et al 76 |
| Combination with silibinin 24.4 µM | |||||
| Romania/A. mellifera assumed | Aqueous extract | In vitro | 0.072-0.09 mg/mL | Cytotoxicity against MCF-7 and Hs578T | Drigla et al 77 |
| Synergism with bee venom was observed | |||||
| Bioactive compounds | CAPE and Cucurbitacin I | In vitro | 20 µM + 20 nM concentrations | Synergistic effect of CAPE and cucurbitacin I against MCF-7 and MDA-MB-231 cells | Karakuş et al 78 |
| Turkey/A. Mellifera carnica | 70% Ethanol extract | In vitro | IC50 for cisplatin = 3.12 μg/mL, IC50 for curcumin = 0.31 μg/mL, IC50 for propolis = 160 μg/mL | Statistically significant decrease was found in the MCF-7 cell viability 48 h after applying different combinations of cisplatin (3.12 μg/mL) and curcumin (0.31 μg/mL) and propolis (160 μg/mL) extracts at the closest doses to the respective IC50 doses (P) | Yilmaz and Erdal 79 |
| Australian/A. mellifera | Ethanolic extracts of Australian propolis (AEEP) | In vitro | IC50 for AEEP was 177.2 µg/mL against MCF10A | Strong synergy between AEEP and DOX against MCF 7 cells. AEEP showed an MCF7 selectivity index of 2.81 and >2.85 compared with MCF10A and RAW 264.7 macrophages, respectively. | Alsherbiny et al 80 |
| IC50 for HPLC fractionated AEEP (fraction 3) was 10.62 µg/mL against MCF7 | |||||
| Croatia/A. mellifera | Water-soluble derivative of ethanolic extract of propolis | In vivo | Propolis extract: 50 mg/kg BW | Propolis enhanced the tumor-inhibiting effect of cisplatin and survivability of mice with Ehrlich ascites tumor (murine breast carcinoma) | Oršolić et al 81 |
| Propolis increased the cytotoxic activity of macrophage to tumor cells, sensitivity of tumor cells to hyperthermal intraperitoneal chemotherapy (HIPEC),and reduces cisplatin toxicity to normal cells | |||||
| Turkey/A. mellifera assumed | Aqeuous extract | In vitro | IC50 = 129.25 µg/mL | Cytotoxicity against 4 T1 cells (murine breast cancer cells) | Onur et al 82 |
| In vivo | 66 mg/kg BW of propolis daily and combination of 66 mg/kg BW of propolis and 108 CFU/mL/mouse of acidophilus milk | The treatment of propolis extract, acidophilus and the combination of both treatments inhibited the tumor volumes by 59.16%, 28.29%, and 63.39%, respectively | |||
| Propolis extract and combination treatments upregulated the ConA-, LPS-, and PHA-induced splenocyte proliferation | |||||
| The combination treatment stimulated IFN-γ production | |||||
| Improvement in bioavailability | |||||
| Bioactive compounds | Nanoencapsulation of CAPE using sucrose fatty acid ester (SFAE) | In vitro | 0.2-20 µg/mL | Nanoencapsulation with sucrose fatty acid ester and thymol increased CAPE dispersion and cytotoxicity against MCF-7 cells | Guan et al 83 |
| Bioactive compounds | Nanoparticles of chrysin | In vitro | IC50 = 40 μM | Nanoparticles of chrysin had significantly higher cytotoxicity against MCF-7 cells, compared to chrysin | Norouzi et al 84 |
| Bioactive compounds | Chrysin-loaded poly (D,L-lactic-co-glycolic acid) and polyvinyl alcohol nanoparticles | In vitro | IC50 = 50-155 μg/mL | Nanoparticles of chrysin had significantly higher cytotoxicity against MCF-7 cells, compared to chrysin | Sulaiman et al 85 |
| Bioactive compounds | CAPE-γ cyclodextrin complex | In vitro | 1-20 µM | Cytototoxicity against MCF-7 and MDA-MB-231 cells. The CAPE-γ cyclodextrin complex had higher activity compared to CAPE. | Wadhwa et al 86 |
| Egypt/A. mellifera assumed | Aqueous, hydroethanolic, ethanolic, and hexane extracts | In vitro | IC50 = 222.4-302 μg/mL | Cytotoxic activity against MCF-7 cells. Nano-encapsulation increased the IC50. | Sherif et al 87 |
| Nanoparticles (liposome) of the organic solvent extracts | |||||
| Indonesia/stingless bee (Trigona spp.) | Hydroethanolic extract | In vivo | Propolis extract: 233 µg/mL | 7,12-Dimethylbenz(a)anthracene (DMBA)—induced mammary tumor in rats treated with propolis | Hasan et al 88 |
| Nanopropolis | Nanopropolis: 8-56 µg/mL | Propolis treatment reduced tumor size and healed the wounds caused by the tumor. Nanopropolis appeared to be more efficacious probably due to a more efficient delivery of propolis bioactive compounds. | |||
| India/A. mellifera assumed | Ethanolic extract of Propolis—Loaded | In vitro | 10-80 μg/mL | Propolis nanoparticles appeared to increase cytotoxicity of propolis against MCF-7 cells | Kapare et al 89 |
| Poly (ε -Caprolactone) nanoparticles | |||||
| Others | |||||
| Bioactive compounds | CAPE | In vitro | 1-40 μM | CAPE reduced the malignancy of MDA-MB-231 cells by inducing changes in breast cancer stem cells characteristics such as inhibition of self renewal, progenitor formation, and clonal growth; and reduction of CD44 content | Omene et al 90 |
| Bioactive compounds | Baccharin | In vitro | 1-100 μM | Baccharin and artepillin C reduced the activity of Aldo-keto reductase family 1 member C3 (AKR1C3) in MCF-7 cells | Endo et al 91 |
| Artepillin C | |||||
| Thailand/A. mellifera | Methanolic-dichloromethane extraction and fractionation | In vitro and in vivo | Propolis extracts: 10-100 μg/mL | Propolis extracts and the bioactive compounds significantly reduced the hypoxic survival rate of 4T1 cells. Chrysin also inhibited the hypoxia-induced STAT3 tyrosine phosphorylation suggesting the mechanism of action was through STAT3 inhibition. | Lirdprapamongkol et al 92 |
| Bioactive compounds | Tectochrysin and chrysin | Tectochrysin and chrysin: 20-100 μM | In animal models, chrysin was shown to have anti-metastatic effect | ||
| Iran/A. mellifera | Hydroethanolic extract | In vivo | 100 mg/kg BW daily | Spontaneous mouse mammary tumor (SMMT)-bearing mice | Khosravi et al 93 |
| C. albicans infection significantly increased the tumor size and propolis appeared to ameliorate the increase in tumor-bearing mice infected with C. albicans. Propolis reduced the expression of TIMP-1, IL-4, and IL-10. Interestingly, propolis appeared to increase TNF-α in tumor bearing-mice infected with C. albicans. | |||||
| Brazil/A. mellifera assumed | Ethanolic extract | In vitro | Cell cultures = 5.5 μg/mL | In MCF-7 cells, propolis induced the gene expression of estrogen-inducible genes; PR and TFF-1 at the highest concentration tested; 5.5 μg/mL | Okamoto et al 94 |
| In vivo | In vivo = 55 and 550 mg/kg BW daily for 3 d | In overiectomized rats, propolis induced the ductal cell proliferation in the mammary glands | |||
| Bioactive compounds | Nemorosone | In vitro | 5-40 μg | Nemorosone inhibited the activity of 17-β-estradiol (E2) in MCF-7 BUS cells | Camargo et al 95 |
| Not determined | Hydroethanolic extract | In vivo | 50 mg/kg BW, 100 mg/kg BW, and 200 mg/kg BW daily for 4 wk | Propolis significantly reduced the relative number of CD4+ CD25+ FoxP3+ regulatory T cells expressing IL-10 or TGF-β in mice with breast cancer | Kusnul et al 96 |
| The suppression of IL-10, which is an immunosuppressive cytokine, is thought to be beneficial in cancers | |||||
| Romania/not mentioned/A. mellifera assumed | Ethanolic extracts of propolis (EEP) or PE as elaborated in the study | In vivo | PE dose was 1.05 mg/kg BW/d in experimental group | Flavones and flavonols content assessment of PE (based on aluminum chloride complex formation) | Gal et al 97 |
| Chemo-preventive effects (in vivo, as observed in MNU-exposed rats); represented by occurrence of the developed tumor tissues in exposed-MNU only, MNU and PE applied, etc. | |||||
| Antioxidative status of propolis by assessing 3 antioxidant enzyme levels. In hepatic antioxidative markers of rat, the P values were statistically significant (<.05) | |||||
| Turkey/A. mellifera caucasica from Ardahan and Erzurum provinces | EEP of 70% ethanolic extract rotor vacuum evaporated | In vitro | On MCF-7 human breast cancer cell line: 65 μg/mL (Erzurum propolis) and 125 μg/mL (Ardahan propolis) | The Erzurum propolis was significantly more potent at these concentrations than even MMC (mitomycin C), let alone the Ardahan propolis | Arslan et al 98 |
| Regardless of origin of propolis and the presence of mitomycin C in the culture medium, propolis enhanced human peripheral lymphocyte viability, which depended on the duration and propolis concentration | |||||
| Human trials | |||||
| Not determined/A. mellifera assumed | Propolis capsules | Human clinical trial | 400 mg, 3 times daily for 10 d pre-, during, and post | Propolis alleviated the negative impact associated with radiotherapy in breast cancer patients: Propolis prevented the increase in Comet tail parameters (Tail length, % Tail DNA, Tail moment) in peripheral blood mononuclear cells, serum malonaldehyde (MDA). Propolis prevented the decrease of total antioxidant capacity, hemoglobin (Hb) concentration, white blood cells (WBCs), and platelets counts. | Ebeid et al 99 |
| More importantly, patients supplemented with propolis had significantly longer median disease free survival time | |||||
| Not determined/A. mellifera assumed | Not determined | Observational study | Not determined | Observational study to investigate the use of complementary and alternative medicine (CAM) in cancer patients. Total included patients were 316 patients. A total of 173 patients were female and 32.3% breast cancers. A total of 38.5% of the included participants reported the use of natural remedies, where 11.4% reported the use of propolis as CAM. | Juanbeltz Zurbano et al 100 |
| A total of 65% of the patients reported improvements, especially in terms of physical and psychological well-being | |||||
| Not determined/A. mellifera assumed | Dry extract (Natur Farma S.A.S) titrated in 8% to 12% galangin | Human clinical trial (n = 60) | 8-10 mg/kg BW/d for 15 d + mouth rinsing with sodium bicarbonate | In breast cancer patients subjected to chemotherapy and treated with propolis and sodium bicarbonate, none developed oral mucositis >G1 | Piredda et al 101 |
| In the control arm (treated only with sodium bicarbonate), 16.7% developed oral mucositis >G1, OM graded G1 to G3 was 43.3% and that of severe OM (G3) was 3.3% | |||||
| Western Iran/A. mellifera | Dried in liquid N2 and powdered | Human intervention study | 250 mg propolis administrated to breast cancer patients twice a day | Chemotherapy significantly increased the serum protein carbonyl as a biomarker of oxidative tress and the pro-inflammatory factors of TNF-α and IL-2, but with the use of Propolis capsules plus chemotherapy, there was no significant change in the serum levels of these markers and the oxidant-antioxidant balance after 3 mo | Darvishi et al 102 |
| Iran/A. mellifera | Propolis capsules 250 mg | Human intervention | Used as a supplement with chemotherapy | Oral consumption of propolis increased the energy and nutrient intake of breast cancer patients under chemotherapy, and had a positive impact on the emotional functioning, quality of life from the patient’s perspective, and the reduction of economic problems caused by illness and treatment | Davoodi et al 103 |
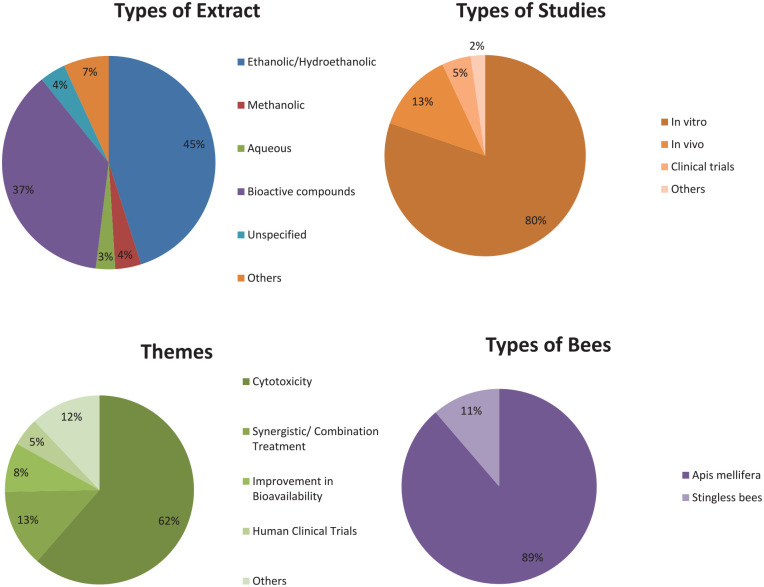
Figure 2.
Characteristics of the included studies. (A) Types of extract. (B) Types of studies. (C) Themes. (D) Types of bees.
The largest body of experimental evidence in the included studies was cytotoxicity against breast cancers (61%), followed by synergistic/combination treatment (13%), improvement of bioavailability (8%), human clinical trials (5%), and others (12%). In terms of types of study, in vitro studies were the largest category at 80%, followed by animal models/in vivo (13%), human clinical trials (5%), and others (2%). The majority of the included studies identified the types of extract, geographical locations of the propolis source, and the bioactive compounds. Only 4% of the included studies did not identify the types of extract and/or the geographical locations where the raw propolis was sourced. All percentages were rounded to the nearest whole number. A majority of the studies utilize propolis sourced from Apis mellifera (89%) whereas 11% of the studies use propolis from stingless bees.
Cytotoxicity Against Breast Cancer Cells
Propolis has been shown to be cytotoxic against a wide range of breast cancer cells, namely BT-20, BT-474, BT-549, SKBR-3, MCF-7, MDA-MB-231, MDA-MB-436, T47D, Hs578T, and so on (Table 1). The mechanisms of action of propolis and its bioactive compounds with regards to breast cancer cytotoxicity have been extensively elucidated by various studies. The main mode of action appears to be through inducing apoptosis. Kamiya et al 22 demonstrated that ethanolic extracts of propolis and its bioactive compound, caffeic acid phenethyl ester (CAPE) induced apoptosis of MCF-7 breast cancer cells by upregulating caspase-3 activity, DNA fragmentation, and CCAAT/enhancer-binding protein homologous protein (CHOP) expression. Propolis also promotes mitochondrial dysfunction and endoplasmic reticulum stress. In addition, propolis induces apoptosis in MCF-7 and MDA-MB-231 cancer cells by upregulating the expression of Annexin A7 (ANXA7), reactive oxygen species (ROS) level, and NF-κB p65 level, while simultaneously reducing the mitochondrial membrane potential.31,45 More importantly, these studies demonstrate that propolis extracts have little cytotoxicity on normal human umbilical vein endothelial cells (HUVECs) and normal fibroblast cells.31,45
In addition, Chang et al demonstrated that propolis inhibited lipopolysaccharide (LPS)-stimulated MDA-MB-231 cell proliferation by inducing apoptosis through the upregulation of caspase 3 and poly (ADP-ribose) polymerase (PARP). They also showed that propolis induced autophagy by increasing the expression of LC3-II and reducing the expression of p62 level. Furthermore, propolis downregulates the inflammatory TLR4 signaling pathway molecules such as TLR4, MyD88, IRAK4, TRIF, and NF-κB p65. 42 This study reported contradictory results compared to studies by Asgharpour et al 45 and Xuan et al 31 where propolis was shown to promote inflammatory and oxidative stresses on the cancer cells whereby apoptosis was consequently induced. This suggests propolis may act differentially depending upon types of cancer and probably their stages .
Moreover, Frión-Herrera et al showed that propolis affected the expression of apoptosis-related genes in PI3K/Akt and ERK1/2 pathways, namely TP53, CASP3, BAX, and P21. They also found that propolis induced mitochondrial dysfunction and lactate dehydrogenase release indicating ROS-associated necrosis in MDA MB-231cancer cells. 104 Li et al demonstrated that propolis was able to inhibit the proliferation of MDA-MB-231 cells by targeting key enzymes of glycolysis, namely glycolysis-hexokinase 2 (HK2), phosphofructokinase (PFK), pyruvate kinase muscle isozyme M2 (PKM2), and lactate dehydrogenase A (LDHA), in an inflammatory microenvironment. There is also evidence that propolis affects immune response in the presence of breast cancers. 66 Kusnul et al 96 reported that propolis significantly reduced the relative number of CD4+, CD25+, FoxP3+ regulatory T cells expressing IL-10 and TGF-β in mice with breast cancer. The suppression of IL-10, which is an immunosuppressive cytokine, is thought to be beneficial in treating cancers.
Furthermore, Seyhan et al investigated propolis extracts from 3 countries, China, Argentina, and Turkey, and found that cytotoxicity against 3 cancer cell lines namely, MCF-7, SK-BR-3, and MDA-MB-231 did not correlate with the total phenolics and/or flavonoids but rather with the diversity of the phenolics and/or flavonoids, suggesting that the biological activities of propolis are due to the synergy of the bioactive compounds. 51 Nevertheless, several propolis-derived bioactive compounds, such as chrysin, caffeic acid phenethyl ester (CAPE), caffeic acid, quercetin, hesperidin, apigenin, naringin, myricetin, luteolin, galangin, artepillin C, pinocembrin, baccharin, cardanol, α-amyrin, and mangiferonic acid have been shown to have anti-breast cancer activities (Table 1).
Chrysin, a propolis bioactive compound, inhibits HDAC8 and significantly increases the expression of p21 (waf1/cip1) in breast cancer cells, leading to apoptosis. 24 Chrysin also inhibits the hypoxia-induced STAT3 tyrosine phosphorylation leading to the significant reduction of hypoxia survival rate of 4T1 breast cancer cells. 105 In addition, CAPE can reduce the malignancy of MDA-MB-231 cells by inducing changes in breast cancer stem cell characteristics such as inhibition of self-renewal, progenitor formation, and clonal growth, and reduction of CD44 content. 90 CAPE also has cytotoxicity activity against breast cancer cells through various mechanisms such as by promoting mitochondrial dysfunction and endoplasmic reticulum stress, 22 inducing cell cycle arrest in the in S, G0/G1, and G2/M phase,24,39 promoting the accumulation of acetylated histone proteins (epigenetic effects), downregulating the expression of estrogen receptor and progesterone receptor, 26 inducing autophagy and downregulating TLR4 signaling pathway molecules, 42 interfering with and inhibiting the voltage-gated sodium channels, 38 inhibiting mitochondrial oxygen consumption rate and mammosphere formation, 43 and inducing oxidative stress by promoting endothelial nitric oxide synthase (eNOS) and inducible nitric oxide synthase (iNOS) levels. 58
Buahorm et al demonstrated that a phenolic lipid cardanol, isolated from Thai propolis caused BT-474 cell apoptosis by inducing cell cycle arrest at the G1 subphase and cell death at late apoptosis stage and modulating the expression of genes related to apoptosis: upregulating the expression of DR5 and Bcl-2 (apoptosis regulator) and downregulating the expression of Mcl-1, MADD, and c-FLIPP. They also found that cardanol modulated the expression of genes related to cell division: it increased the expression of p21, E2FI, p21 p-ERK, p-JNK, and p-p38 and decreased the expression of cyclin D, cyclin D1, cyclin E, CDK4, and CDK2, resulting in the failure to progress from the G1 to the S subphase. 34 Moreover, other propolis-derived compounds such as genistein, luteolin, benzoic acid, quercetin, and vanillic acid, are shown, in silico and in vitro, to interfere with cancer-associated targets such as CYP1A1, CYP19A1, ESR1, NOS3, CASP3, and AKT1. 70
Combination Effects of Propolis With Other Anti-Cancer Treatments
In addition to affecting the cancer cells directly, propolis has also been demonstrated to work synergistically with other compounds. Tan and Hayati found that propolis decelerated the growth of mammary tumor in mice. However, the combination of propolis extract and mangostin had a more pronounced effect. The combination of propolis and mangostin significantly reduced the expression of Wnt2, FAK, and HIF-1α, when compared to propolis or mangostin alone. 75 Oršolić et al showed that propolis enhanced the tumor-inhibiting effect of cisplatin and improved the survivability of mice with Ehrlich ascites tumor (murine breast carcinoma). They also found that propolis increased the cytotoxic activity of macrophages to tumor cells and sensitivity of tumor cells to hyperthermal intraperitoneal chemotherapy (HIPEC). Interestingly, propolis also reduced cisplatin toxicity to normal cells. 81
Propolis has synergistic activity with doxorubicin, a standard drug for breast cancer. Alsherbiny et al 80 demonstrated that propolis significantly improved the proliferation inhibitory effect of doxorubicin in MCF-7 cells in a dose-dependent manner. Propolis also upregulated the expression of catalase, HTRA2/Omi, FADD, and TRAIL-associated DR5 and DR4 which significantly enhanced the cytotoxicity of doxorubicin in MCF-7 cells. They also found the differential expression in 21 proteins in the combination treatment compared to single treatments of either propolis or doxorubicin. The differentially expressed proteins were associated with TP53/ATM-regulated non-homologous end-joining pathway and double-strand breaks repairs, recruitment of overexpressed BRCA1, and the suppression of RIF1 encoded proteins. Perhaps more importantly, there was an overexpression of UPF2 in the combination treatment, indicating that it could potentially treat doxorubicin resistance-associated long non-coding RNA and the subsequent metastasis of the MCF7 cells. The study also elucidated the potential protective effect of propolis against the side effects of doxorubicin by reversing doxorubicin-mediated necrosis. 80
Moreover, propolis has synergistic activities with beneficial lactic acid bacterium Lactobacillus acidophilus LA-5. Onur et al demonstrated that propolis extract, L. acidophilus LA-5, and the combination of both treatments inhibited the tumor volumes by 59.16%, 28.29%, and 63.39%, respectively, when given to mice with murine breast carcinoma 4 T1. Propolis extract and the combination treatment upregulated the ConA-, LPS-, and PHA-induced splenocyte proliferation. Additionally, the combination treatment stimulated IFN-γ production. 82
Propolis bioactive compounds have also been shown to improve breast cancer therapies. CAPE improves the efficacy of radiotherapy by sensitizing breast cancer cells through impairing DNA damage repair mechanisms in cancer cells. 72 CAPE also works synergistically with tamoxifen, a selective estrogen receptor modulator, by significantly downregulating the levels of Bcl-2 and beclin-1, and endothelial growth factor and consequently inducing apoptosis. Notably, the combination of tamoxifen and CAPE increased the life span of the tumor-bearing mice compared to tamoxifen or CAPE alone. 72 Maasomi et al 76 demonstrated that chrysin acted synergistically with silibinin, a bioactive compound of Silybum marianum, in inducing cytotoxicity of T47D breast cancer cells through enhancing the downregulation of the expression of telomerase reverse transcriptase and cyclin D1.
Potential Improvement of Delivery Through Encapsulation
During the search process, we also found several interesting studies exploring the methods to improve the bioavailability of propolis and its bioactive compounds through encapsulation. Hasan et al demonstrated that by encapsulating propolis extract into nanoparticles, the effective concentration, to reduce the tumor size, heal tumor-associated wounds, and eliminate cancer cells of mammary gland tumors in rats, was significantly reduced from 233 to 32 µg/mL. However, in this particular study, it was not clear what encapsulation materials were used. 88 Kapare et al encapsulated ethanolic extract of propolis with poly (ε-caprolactone), a biodegradable polymer, into ~190 nm particles. They found that the concentration of the encapsulated propolis required for total growth inhibition of MCF-7 cancer cells in a designated time period was reduced by 33.06%, compared to non-encapsulated propolis. Furthermore, the solubility and sustained drug release were also enhanced. 89
However, Sherif et al reported a negative effect of nano-encapsulation of propolis in terms of cytotoxicity efficacy. It was shown that encapsulation using 1,2-dioleoyl-sn-glycero-3-phosphocholine (DOPC) liposomes actually increased the IC50 against MCF-7 breast cancer cells of hexane extract of propolis from ~222.4to 333.3 µg/mL. More significantly, the encapsulation using 1,2-dipalmitoyl-sn-glycero-3-phosphocholine (DPPC) liposomes fully removed the cytotoxic effect of the propolis extract against MCF-7 cells. 87 These studies illustrate the need to extensively investigate the types of materials in the preparation of the propolis-infused nanoparticles/nanoencapsulation.
Additionally, several studies reported the improved biological activities of propolis bioactive compounds through encapsulation and/or other molecular complexes. Gamma-cyclodextrin appears to be a promising molecule for propolis bioactive compound complex formation. Wadhwa et al demonstrated that γ-cyclodextrin greatly enhanced the heat stability, chemical stability, and oxidative stability of CAPE. Gamma-cyclodextrin did not reduce the anti-cancer properties of CAPE. 86 Furthermore, nano-encapsulation of CAPE with sucrose fatty acid esters appears to enhance anti-cancer properties of CAPE. Guan et al found that nano-encapsulation of CAPE with sucrose fatty acid esters, polyethylene glycol, and/or thymol as co-surfactants enhanced the storage stability. The encapsulation also further enhanced the antioxidant and cytotoxicity of CAPE against MCF-7 cells. The authors postulated that the enhanced biological activities were due to better dispersion of nano-encapsulated CAPE in the aqueous solution compared to free CAPE. 83 A similar trend is also observed for another propolis bioactive compound, chrysin. Encapsulation using chitosan, poly (D,L-lactic-co-glycolic acid), and polyvinyl alcohol result in enhanced cytotoxicity against breast cancer cells.84,85
Human Clinical Trials
The most important thing for the development for any therapeutic is the translation of pre-clinical data to the application in humans. Propolis has been extensively studied for decades and therapeutically used for thousands of years as folk medicine. However, there is still relatively limited human clinical data, especially in the sphere of the treatment of cancers. In the present scoping review, we managed to identify 3 groups of researchers that performed human clinical trials investigating the effect of propolis in breast cancer patients.
Ebeid et al investigated the potential protective effect of propolis in breast cancer patients who were undergoing chemotherapy and radiotherapy. The total patients included in the clinical trial were 135 females who were divided into 3 groups. Group I (control group) consisted of 45 healthy females who were age and menopausal status-matched to the cancer patients in the subsequent groups. Group II consisted of 45 breast cancer patients who received chemotherapy followed by radiation therapy. Group III consisted of 45 breast cancer patients who received chemotherapy followed by radiation therapy and 400 mg propolis extract, 3 times daily for 10 consecutive days before radiotherapy and during the course of radiotherapy, and 10 days after completing the radiotherapy session. It was found that propolis alleviated the negative impact associated with radiotherapy in breast cancer patients, namely the increase in Comet tail parameters (Tail length, % Tail DNA, Tail moment) in peripheral blood mononuclear cells, and serum malonaldehyde (MDA). Propolis also prevented the decrease in total antioxidant capacity, hemoglobin (Hb) concentration, white blood cells (WBCs), and platelets counts associated with radiotherapy. More importantly, patients supplemented with propolis had significantly longer median disease free survival time. No adverse effect linked to propolis consumption was reported in this study. However, the study did not describe the randomization of the patients and any adverse effect experienced by the patients. Furthermore, the phytochemical analyses and the source of the propolis extract used in the study were not reported. 99
In addition, Piredda et al investigated the effect of propolis consumption in reducing the incidence of oral mucositis in breast cancer patients receiving chemotherapy, in a pilot randomized controlled trial with 60 patients. The intervention trial was carried out in the first chemotherapy cycle and lasted for 15 days. The included patients were randomized into a control group and an intervention group. The control group received the treatment of mouth rinsing with sodium bicarbonate 3 times a day, whereas the intervention group received the exact treatment of mouth rinsing with sodium bicarbonate 3 times a day in addition to being instructed to consume tablets of a dry extract of propolis, 2 to 3 times/day between meals. The propolis tablets (80 mg/tablet) were supplied by Natur Farma S.A.S. and they contained 8% to 12% galangin. The total daily number of tablets consumed by the patients was calculated according to patients’ body weight and ranged from 8 to 10 mg/kg BW/day of propolis. Incidence of oral mucositis was then evaluated at days 5, 10, 15, and 21. The patients were also followed up at the end of each chemotherapy cycle over a 6-month period. The incidence of oral mucositis was measured in accordance to the National Cancer Institute Scale (NCI-CTCAE) version 4.0. The patients in the intervention group recorded no incidence of oral mucositis that was more severe than grade G1, whereas in the control arm, 13.3% of patients had G2 and 3.3% of the patients had severe oral mucositis of grade G3. However, compliance with the propolis therapy was not completed for 6 patients due to emesis, suspected allergy, and complaints of the consumption many other oral drugs. In addition, the blinding of assessors could not be achieved as they were also involved in evaluating participants’ compliance with the treatments. 101
Moreover, a group of Iranian researchers investigated the effect of propolis on the antioxidant, inflammation, nutritional status, and quality of life of breast cancer patients treated with chemotherapy in a randomized, double-blind, placebo-controlled trial.102,103 Propolis used in the study was collected from the bee hives in the Kurdistan province, Iran. The harvested raw propolis was put in the water bath. Wood and paint particles were subsequently removed from the raw propolis. The relatively pure propolis gum was subsequently subjected to liquid nitrogen and crushed. The powdered propolis was then obtained. The resulting propolis powder was placed in gelatin capsules as 250 mg doses. Placebo capsules with the similar look, shape, and size were also prepared. No phytochemical analysis was carried out. A total of 50 patients with newly diagnosed breast cancers were included: 26 patients in the intervention arm and 24 patients in the placebo arm. The patients in the intervention arm were instructed to consume the 250 mg propolis capsules twice a day with breakfast and lunch for the duration of chemotherapy (3 months). The intervention started 1 week before the chemotherapy. The patients in the placebo arm received the exact same treatment. They reported no side effect and propolis in the study was well tolerated.
During the course of the study, they found that the patients in the placebo arm had a significant increase in the serum pro-inflammatory cytokines, namely TNF-α and IL-2, and oxidative stress marker protein carbonyl. Conversely, the propolis arm patients did not record any significant increase in pro-inflammatory and oxidative markers. In addition, the intervention group patients had a statistically significant reduction in serum prooxidant-antioxidant balance (PAB), whereas the placebo arm patients did not. 102 The same group of researchers also found that the patients in the intervention arm tended to have improvements in their quality of life, assessed using EORTC QLQ30 questionnaire. At the end of the 3 months of intervention, the patients in the propolis arm had increased energy intake and significant improvements especially in terms of emotional functioning and global quality of life, relative to the patients in the placebo arm. Interestingly, they also found the patients in the placebo group had increased incidence of financial difficulties. 103 These sets of human clinical trials appeared to support the use of propolis as an adjunctive nutraceutical in breast cancer patients. However, larger clinical trials are needed to confirm the therapeutic benefit of propolis in clinical settings.
General Discussion and Future Direction
The present systematic review found that most experimental studies investigating the potential therapeutic use of propolis in breast cancers were in vitro studies, followed by in vivo, in silico, and clinical trials. Majority of the studies demonstrated cytotoxicity activity of propolis and its bioactive compounds against various breast cancer cells. Some studies also investigated the potential synergistic activity of propolis with other therapeutics and more importantly, 3 sets of human clinical trials were identified with no serious adverse event recorded.
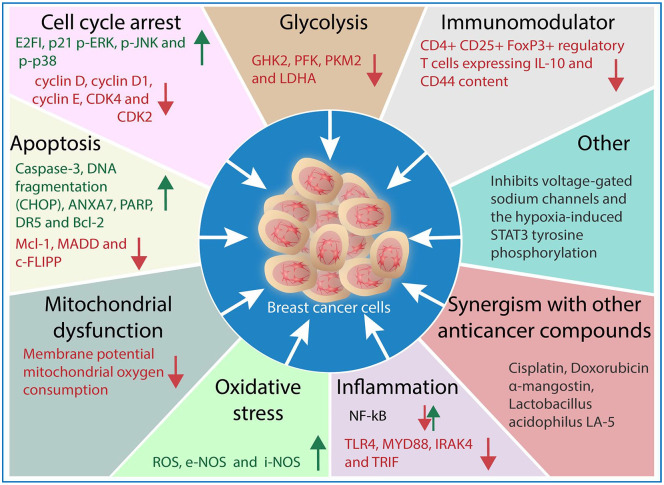
Figure 3 illustrates the potential mechanisms of action of propolis against breast cancers summarized in accordance to the studies in the present systematic review. Propolis induces cytotoxicity in breast cancer cells (in vitro and in vivo) through various mechanisms, namely apoptosis, cell cycle arrest, glycolysis inhibition, mitochondrial dysfunction, oxidative stress promotion, and immunomodulatory and inflammation pathways. Propolis induces apoptosis and oxidative stress by inducing Caspase-3, ANXA7, PARP, DR5, Bcl-2, DNA fragmentation, iNOS, and eNOS levels while downregulating Mcl-1, MADD, c-FLIPP. In addition, cell cycle arrest is promoted by propolis through the upregulation of p21, E2FI, p21 p-ERK, p-JNK, and p-p38 and the downregulation of cyclin D, cyclin D1, cyclin E, CDK4, and CDK2. Propolis also inhibits glycolysis by downregulating the activity of glycolysis-hexokinase 2 (HK2), phosphofructokinase (PFK), pyruvate kinase muscle isozyme M2 (PKM2), and lactate dehydrogenase A (LDHA). Propolis negatively impacts the mitochondrial functions of the breast cancer cells by affecting membrane potential and oxygen consumption. Additionally, propolis also works through immune system and inflammation pathway modulation such as CD4+ CD25+ FoxP3+ regulatory T-cells expressing IL-10, NF-κB, TLR4, MYD88, IRAK4, and TRIF.
Figure 3.
Summary of the mechanisms of action of propolis against breast cancer cells based on in vitro and in vivo studies.
The therapeutic benefits of propolis have also been observed in breast cancer patients. In these pilot clinical studies, propolis appears to reduce the negative impact of chemotherapy, such as the reduction in the incidence of oral mucositis, inflammation, and oxidative stress. Propolis also appears to maintain the quality of life the breast cancer patients. Furthermore, propolis reduces the adverse effect of radiotherapy, namely DNA damage, while maintaining total antioxidant capacity, hemoglobin (Hb) concentration, white blood cells (WBCs), and platelet counts of the breast cancer patients subjected to radiotherapy (Figure 4). More importantly, these clinical trials reported minimal adverse effect with regards to the consumption of propolis. Perhaps not surprisingly since propolis has been used therapeutically as traditional/folk medicine for thousands of years in many civilizations. 106 However, these clinical trials should be considered preliminary and future research with larger number of participants needs to be conducted.
Figure 4.
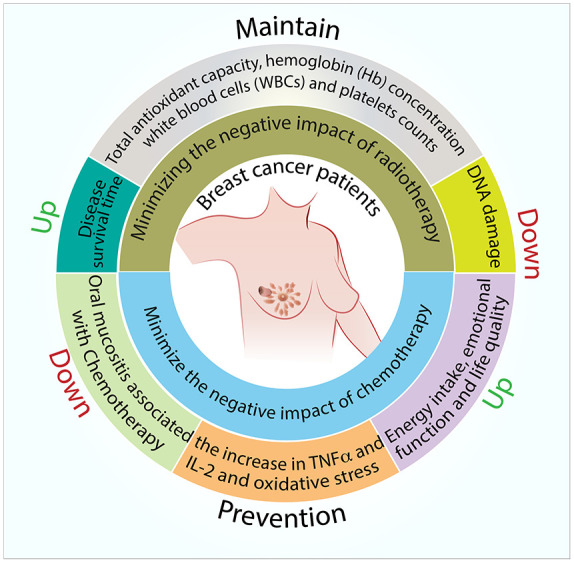
Summary of proposed areas of research on potential clinical benefits of propolis in breast cancer.
In conclusion, the present systematic review demonstrates that propolis may be a useful therapeutic substance to be used as an adjunctive therapy for treating breast cancers. However, more human clinical trials are needed to find the optimum therapeutic concentrations and further explore its potential.
Study Limitations
In the present review, the authors adopted a comprehensive and systematic search strategy in order to objectively fulfill the aim of the study. A broad range of studies from all fields of science and technology was collected and analyzed. The reviewers limited the search to studies that were published in the last 10 years, to provide coverage of the latest experimental evidence in the field. However, the reviewers only assessed and included English language articles, which could potentially lead to missing studies from non-English databases, as it is apparent most studies originated from non-English speaking countries. In addition, the reviewers did not perform a meta-analysis as it is not appropriate due to the heterogeneity of the included studies.
Supplemental Material
Supplemental material, sj-docx-1-ict-10.1177_15347354221096868 for The Potential Use of Propolis as an Adjunctive Therapy in Breast Cancers by Dedy Hermansyah, Felix Zulhendri, Conrad O. Perera, Naufal N. Firsty, Kavita Chandrasekaran, Rizky Abdulah, Herry Herman and Ronny Lesmana in Integrative Cancer Therapies
Acknowledgments
We are thankful to Fuad Bahram, PhD for the production Figures 3 and 4.
Footnotes
Author Contributions: Conceptualization, Methodology, Writing—original draft: D.H., F.Z., N.N.F., C.O.P., K.C. Supervision, Writing—review and editing: R.A., H.H., R.L. All authors have read and agreed to the published version of the manuscript.
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Kebun Efi produces propolis extracts of the Indonesian stingless bees. All other authors declare no competing financial interests and no conflict of interest.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This research received no external funding. If accepted, the APC is funded by Universitas Sumatera Utara.
ORCID iDs: Felix Zulhendri  https://orcid.org/0000-0002-7881-1845
https://orcid.org/0000-0002-7881-1845
Naufal N. Firsty  https://orcid.org/0000-0003-1668-6660
https://orcid.org/0000-0003-1668-6660
Ronny Lesmana  https://orcid.org/0000-0002-7425-915X
https://orcid.org/0000-0002-7425-915X
Supplemental Material: Supplemental material for this article is available online.
References
- 1. Zettler ME, Feinberg BA, Jeune-Smith Y, Gajra A. Impact of social determinants of health on cancer care: a survey of community oncologists. BMJ Open. 2021;11:e049259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Pilleron S, Soto-Perez-de-Celis E, Vignat J, et al. Estimated global cancer incidence in the oldest adults in 2018 and projections to 2050. Int J Cancer. 2021;148:601-608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Yabroff KR, Mariotto A, Tangka F, et al. Annual report to the nation on the status of cancer, part 2: patient economic burden associated with cancer care. JNCI. 2021;113:1670-1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lima SM, Kehm RD, Terry MB. Global breast cancer incidence and mortality trends by region, age-groups, and fertility patterns. EClinicalMedicine. 2021;38:100985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hu K, Ding P, Wu Y, Tian W, Pan T, Zhang S. Global patterns and trends in the breast cancer incidence and mortality according to sociodemographic indices: an observational study based on the global burden of diseases. BMJ Open. 2019;9:e028461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sung H, Ferlay J, Siegel RL. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 71(3):209-249. [DOI] [PubMed] [Google Scholar]
- 7. Cao J, Zhang M, Wang B, Zhang L, Zhou F, Fang M. Corrigendum: chemoresistance and metastasis in breast cancer molecular mechanisms and novel clinical strategies. Front Oncol. 2021;11:745052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ji X, Lu Y, Tian H, Meng X, Wei M, Cho WC. Chemoresistance mechanisms of breast cancer and their countermeasures. Biomed Pharmacother. 2019;114:108800. [DOI] [PubMed] [Google Scholar]
- 9. Nedeljković M, Damjanović A. Mechanisms of chemotherapy resistance in triple-negative breast cancer—how we can rise to the challenge. Cell. 2019;8:957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Luque-Bolivar A, Pérez-Mora E, Villegas VE, Rondón-Lagos M. Resistance and overcoming resistance in breast cancer. Breast Cancer Target Ther. 2020;12:211-229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hartkopf AD, Grischke EM, Brucker SY. Endocrine-resistant breast cancer: mechanisms and treatment. Breast Care. 2020;15:347-354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Rani A, Stebbing J, Giamas G, Murphy J. Endocrine resistance in hormone receptor positive breast cancer from mechanism to therapy. Front Endocrinol. 2019;10:245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Eteraf-Oskouei T, Najafi M. Traditional and modern uses of natural honey in human diseases: a review. Iran J Basic Med Sci. 2013;16:731-742. [PMC free article] [PubMed] [Google Scholar]
- 14. Faguet GB. A brief history of cancer: age-old milestones underlying our current knowledge database. Int J Cancer. 2015;136:2022-2036. [DOI] [PubMed] [Google Scholar]
- 15. Lichota A, Gwozdzinski K. Anticancer activity of natural compounds from plant and marine environment. Int J Mol Sci. 2018;19:3533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zhou GP, Jiang YZ, Sun LY, Zhu ZJ. Therapeutic effect and safety of stem cell therapy for chronic liver disease: a systematic review and meta-analysis of randomized controlled trials. Stem Cell Res Ther. 2020;11:419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Forma E, Bryś M. Anticancer activity of propolis and its compounds. Nutrients. 2021;13:2594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chiu HF, Han YC, Shen YC, Golovinskaia O, Venkatakrishnan K, Wang CK. Chemopreventive and chemotherapeutic effect of propolis and its constituents: a mini-review. J Cancer Prev. 2020;25:70-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Peters MD, Godfrey CM, Khalil H, McInerney P, Parker D, Soares CB. Guidance for conducting systematic scoping reviews. Int J Evid Based Healthc. 2015;13:141-146. [DOI] [PubMed] [Google Scholar]
- 20. Munn Z, Peters MDJ, Stern C, Tufanaru C, McArthur A, Aromataris E. Systematic review or scoping review? Guidance for authors when choosing between a systematic or scoping review approach. BMC Med Res Methodol. 2018;18:143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Syamsudin Simanjuntak P. Cytotoxic activity of triterpenoid fraction of Indonesian propolis on human breast carcinoma cell lines. Asian J Pharm Clin Res. 2012;5:168-169. [Google Scholar]
- 22. Kamiya T, Nishihara H, Hara H, Adachi T. Ethanol extract of Brazilian red propolis induces apoptosis in human breast cancer MCF-7 cells through endoplasmic reticulum stress. J Agric Food Chem. 2012;60:11065-11070. [DOI] [PubMed] [Google Scholar]
- 23. Thirugnanasampandan R, Raveendran SB, Jayakumar R. Analysis of chemical composition and bioactive property evaluation of Indian propolis. Asian Pac J Trop Biomed. 2012;2:651-654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sun L-P, Chen A-L, Hung H-C, et al. Chrysin: a histone deacetylase 8 inhibitor with anticancer activity and a suitable candidate for the standardization of Chinese propolis. J Agric Food Chem. 2012;60:11748-11758. [DOI] [PubMed] [Google Scholar]
- 25. Zammit EJ, Theuma KB, Darmanin S. Totarol content and cytotoxicity varies significantly in different types of propolis. Res J Pharm Biol Chem Sci. 2013;4:1047-1058. [Google Scholar]
- 26. Omene C, Kalac M, Wu J, Marchi E, Frenkel K, O’Connor OA. Propolis and its active component, caffeic acid phenethyl ester (CAPE), modulate breast cancer therapeutic targets via an epigenetically mediated mechanism of action. J Cancer Sci Ther. 2013;5:334-342. [PMC free article] [PubMed] [Google Scholar]
- 27. Choudhari MK, Haghniaz R, Rajwade JM, Paknikar KM. Anticancer activity of Indian stingless bee propolis: an in vitro study. Evid Based Complement Altern Med. 2013;2013:928280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hasan A, Mangunwidjaja D, Sunarti T, Suparno O, Setiyono A. Investigating the antioxidant and anticytotoxic activities of propolis collected from five regions of Indonesia and their abilities to induce apoptosis. Emirates J Food Agric. 2014;26:390-398. [Google Scholar]
- 29. Shubharani R, Sivaram V, Kishore BR. In-vitro cytotoxicity of Indian bee Propolis on cancer cell lines. Int J Pharma Bio Sci. 2014;5:698-706. [Google Scholar]
- 30. Calhelha RC, Falcão S, Queiroz MJ, Vilas-Boas M, Ferreira IC. Cytotoxicity of Portuguese propolis: the proximity of the in vitro doses for tumor and normal cell lines. Biomed Res Int. 2014;2014:897361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Xuan H, Li Z, Yan H, et al. Antitumor activity of Chinese propolis in human breast cancer MCF-7 and MDA-MB-231 cells. Evid Based Complement Alternat Med. 2014;2014:280120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. de Oliveira PF, de Souza Lima IM, Munari CC, Bastos JK, da Silva Filho AA, Tavares DC. Comparative evaluation of antiproliferative effects of Brazilian green propolis, its main source Baccharis dracunculifolia, and their major constituents artepillin C and baccharin. Planta Med. 2014;80:490-492. [DOI] [PubMed] [Google Scholar]
- 33. Turan I, Demir S, Misir S, et al. Cytotoxic effect of Turkish propolis on liver, colon, breast, cervix and prostate cancer cell lines. Trop J Pharm Res. 2015;14:777-782. [Google Scholar]
- 34. Buahorm S, Puthong S, Palaga T, et al. Cardanol isolated from Thai Apis mellifera propolis induces cell cycle arrest and apoptosis of BT-474 breast cancer cells via p21 upregulation. Daru. 2015;23:55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Rzepecka-Stojko A, Kabała-Dzik A, Moździerz A, et al. Caffeic acid phenethyl ester and ethanol extract of propolis induce the complementary cytotoxic effect on triple-negative breast cancer cell lines. Molecules. 2015;20:9242-9262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Milosevic-Djordjevic O, Grujicic D, Radovic M, Vukovic N, Zizic J, Markovic S. In vitro chemoprotective and anticancer activities of propolis in human lymphocytes and breast cancer cells. Arch Biol Sci. 2015;67:571-581. [Google Scholar]
- 37. Tartik M, Darendelioglu E, Aykutoglu G, Baydas G. Turkish propolis supresses MCF-7 cell death induced by homocysteine. Biomed Pharmacother. 2016;82:704-712. [DOI] [PubMed] [Google Scholar]
- 38. Fraser SP, Hemsley F, Djamgoz MBA. Caffeic acid phenethyl ester: inhibition of metastatic cell behaviours via voltage-gated sodium channel in human breast cancer in vitro. Int J Biochem Cell Biol. 2016;71:111-118. [DOI] [PubMed] [Google Scholar]
- 39. Kabała-Dzik A, Rzepecka-Stojko A, Kubina R, et al. Comparison of two components of propolis: caffeic acid (CA) and caffeic acid phenethyl ester (CAPE) induce apoptosis and cell cycle arrest of breast cancer cells MDA-MB-231. Molecules. 2017;22:1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kabała-Dzik A, Rzepecka-Stojko A, Kubina R, et al. Migration rate inhibition of breast cancer cells treated by caffeic acid and caffeic acid phenethyl ester: an in vitro comparison study. Nutrients. 2017;9:1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Zingue S, Nde CBM, Michel T, et al. Ethanol-extracted Cameroonian propolis exerts estrogenic effects and alleviates hot flushes in ovariectomized Wistar rats. BMC Complement Altern Med. 2017;17:65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Chang H, Wang Y, Yin X, Liu X, Xuan H. Ethanol extract of propolis and its constituent caffeic acid phenethyl ester inhibit breast cancer cells proliferation in inflammatory microenvironment by inhibiting TLR4 signal pathway and inducing apoptosis and autophagy. BMC Complement Altern Med. 2017;17:471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Bonuccelli G, De Francesco EM, de Boer R, Tanowitz HB, Lisanti MP. NADH autofluorescence, a new metabolic biomarker for cancer stem cells: identification of Vitamin C and CAPE as natural products targeting “stemness.” Oncotarget. 2017;8:20667-20678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Noureddine H, Hage-Sleiman R, Wehbi B, et al. Chemical characterization and cytotoxic activity evaluation of Lebanese propolis. Biomed Pharmacother. 2017;95:298-307. [DOI] [PubMed] [Google Scholar]
- 45. Asgharpour F, Moghadamnia AA, Kazemi S, et al. Chemical composition analysis and in vitro investigation of cytotoxic and antioxidative activities of Iranian propolis against breast cancer cell line, MCF-7. Chem Sel. 2018;3:10857-10863. [Google Scholar]
- 46. Ismail WIW, Hussin NN, Mazlan SNF, Hussin NH, Radzi MNFM. Physicochemical analysis, antioxidant and anti proliferation activities of honey, propolis and beebread harvested from stingless bee. IOP Conf Ser Mater Sci Eng. 2018;440:012048. [Google Scholar]
- 47. Vukovic NL, Obradovic AD, Vukic MD, Jovanovic D, Djurdjevic PM. Cytotoxic, proapoptotic and antioxidative potential of flavonoids isolated from propolis against colon (HCT-116) and breast (MDA-MB-231) cancer cell lines. Food Res Intern. 2018;106:71-80. [DOI] [PubMed] [Google Scholar]
- 48. Ozdal T, Sari-Kaplan G, Mutlu-Altundag E, Boyacioglu D, Capanoglu E. Evaluation of Turkish propolis for its chemical composition, antioxidant capacity, anti-proliferative effect on several human breast cancer cell lines and proliferative effect on fibroblasts and mouse mesenchymal stem cell line. J Apic Res. 2018;57:627-638. [Google Scholar]
- 49. Kabała-Dzik A, Rzepecka-Stojko A, Kubina R, et al. Flavonoids, bioactive components of propolis, exhibit cytotoxic activity and induce cell cycle arrest and apoptosis in human breast cancer cells MDA-MB-231 and MCF-7—a comparative study. Cell Mol Biol. 2018;64:1-10. [PubMed] [Google Scholar]
- 50. Kabała-Dzik A, Rzepecka-Stojko A, Kubina R, Wojtyczka RD, Buszman E, Stojko J. Caffeic acid versus caffeic acid phenethyl ester in the treatment of breast cancer MCF-7 cells: migration rate inhibition. Integr Cancer Ther. 2018;17:1247-1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Seyhan MF, Yılmaz E, Timirci-Kahraman, et al. Different propolis samples, phenolic content, and breast cancer cell lines: variable cytotoxicity ranging from ineffective to potent. IUBMB Life. 2019;71:619-631. [DOI] [PubMed] [Google Scholar]
- 52. de Lima VHM, Almeida KDCR, Alves CCF, et al. Biological properties of volatile oil from Brazilian brown propolis. Rev Bras Farm J Pharmacogn. 2019;29:807-810. [Google Scholar]
- 53. Uçar M, Değer O. Morphological evaluation of MDA-MB-231 human breast cancer cells treated with DMEM extract of Turkish propolis. Trop J Pharm Res. 2021;18:935-939. [Google Scholar]
- 54. Uçar M, Değer O. Evaluation of cytotoxic and wound healing effect of DMEM extracts of Turkish propolis in MDA-MB-231 cell lines. Trop J Pharm Res. 2019;18:321-325. [Google Scholar]
- 55. Falcão SI, Calhelha RC, Touzani S, Lyoussi B, Ferreira ICFR, Vilas-Boas M. In vitro interactions of Moroccan propolis phytochemical’s on human tumor cell lines and anti-inflammatory properties. Biomolecules. 2019;9:315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Diva AN, Pratami DK, Wijanarko A, Hermansyah H, Sahlan M. Effect of ethanolic propolis extract from Tetragonula biroi bees on the growth of human cancer cell lines HeLa and MCF-7. AIP Conf Proc. 2019;2092:030002. [Google Scholar]
- 57. Frión-Herrera Y, Gabbia D, Cuesta-Rubio O, De Martin S, Carrara M. Nemorosone inhibits the proliferation and migration of hepatocellular carcinoma cells. Life Sci. 2019;235:116817. [DOI] [PubMed] [Google Scholar]
- 58. Fırat F, Özgül M, Türköz Uluer E, Inan S. Effects of caffeic acid phenethyl ester (CAPE) on angiogenesis, apoptosis and oxidative stress in various cancer cell lines. Biotech Histochem. 2019;94:491-497. [DOI] [PubMed] [Google Scholar]
- 59. Misir S, Aliyazicioglu Y, Demir S, Turan I, Hepokur C. Effect of Turkish Propolis on miRNA expression, cell cycle, and apoptosis in human breast cancer (MCF-7) cells. Nutr Cancer. 2020;72:133-145. [DOI] [PubMed] [Google Scholar]
- 60. Amalia E, Diantini A, Subarnas A. Water-soluble propolis and bee pollen of Trigona spp. From South Sulawesi Indonesia induce apoptosis in the human breast cancer MCF-7 cell line. Oncol Lett. 2020;20:274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Soltaninejad V, Kazemipour N, Yaghoobi MM, Pardakhty A. Ethanolic extract of propolis from Kerman area triggers apoptosis and arrests cell cycle in three human breast cancer cell lines MDA-MB-231, SKBR and MCF-7. J Kerman Univ Med Sci. 2020;27:120-133. [Google Scholar]
- 62. Assumpção JHM, Takeda AAS, Sforcin JM, Rainho CA. Effects of propolis and phenolic acids on triple-negative breast cancer cell lines: potential involvement of epigenetic mechanisms. Molecules. 2020;25:1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Costa Jr, Yoshida NC, Garcez WS, Perdomo RT, Matos MFC, Garcez FR. Metabolomics approach expands the classification of propolis samples from midwest Brazil. J Nat Prod. 2020;83:AG-343. [DOI] [PubMed] [Google Scholar]
- 64. Rouibah H, Kebsa W, Lahouel M, et al. Algerian propolis: between protection of normal cells and potentialisation of the anticancer effects of doxorubicin against breast cancer cells via P-glycoprotein inhibition and cell cycle arrest in the S phase. J Physiol Pharmacol. 2021;72:239-248. [DOI] [PubMed] [Google Scholar]
- 65. Zingue S, Cisilotto J, Fogang RCM, et al. The antimammary tumor effects of ethanolic extract of propolis from Adamawa region (Cameroon) are by apoptosis via reactive oxygen species-mediated mitochondrial pathway. Environ Toxicol. 2021;36:861-873. [DOI] [PubMed] [Google Scholar]
- 66. Li J, Liu H, Liu X, Hao S, Zhang Z, Xuan H. Chinese poplar propolis inhibits MDA-MB-231 cell proliferation in an inflammatory microenvironment by targeting enzymes of the glycolytic pathway. J Immunol Res. 2021;2021:6641341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Hamed MT, Bakr BA, Shahin YH, et al. Novel synthesis of titanium oxide nanoparticles: biological activity and acute toxicity study. Bioinorg Chem Appl. 2021;2021:8171786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Rodrigues DM, Portapilla GB, Silva GM, et al. Synthesis, antitumor activity and in silico analyses of amino acid derivatives of artepillin C, drupanin and baccharin from green propolis. Bioorg Med Chem. 2021;47:116372. [DOI] [PubMed] [Google Scholar]
- 69. Arung ET, Ramadhan R, Khairunnisa B, et al. Cytotoxicity effect of honey, bee pollen, and propolis from seven stingless bees in some cancer cell lines. Saudi J Biol Sci. 2021;28:7182-7189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Ibrahim RS, El-Banna AA. Network pharmacology-based analysis for unraveling potential cancer-related molecular targets of Egyptian propolis phytoconstituents accompanied with molecular docking and in vitro studies. RSC Adv. 2021;11:11610-11626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Bhuyan DJ, Alsherbiny MA, Low MN, et al. Broad-spectrum pharmacological activity of Australian propolis and metabolomic-driven identification of marker metabolites of propolis samples from three continents. Food Funct. 2021;12:2498-2519. [DOI] [PubMed] [Google Scholar]
- 72. Khoram NM, Bigdeli B, Nikoofar A, Goliaei B. Caffeic acid phenethyl ester increases radiosensitivity of estrogen receptor-positive and -negative breast cancer cells by prolonging radiation-induced DNA damage. NPJ Breast Cancer. 2016;19:18-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Motawi TK, Abdelazim SA, Darwish HA, Elbaz EM, Shouman SA. Could caffeic acid phenethyl ester expand the antitumor effect of tamoxifen in breast carcinoma? Nutr Cancer. 2016;68:435-445. [DOI] [PubMed] [Google Scholar]
- 74. Motawi TK, Abdelazim SA, Darwish HA, Elbaz EM, Shouman SA. Modulation of tamoxifen cytotoxicity by caffeic acid phenethyl ester in MCF-7 breast cancer cells. Oxid Med Cell Longev. 2016;2016:3017108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Tan MI, Hayati I. Inhibition of mammary gland cancer development by propolis and mangostin in female mice Balb/C. J Math Fundam Sci. 2017;49:40-50. [Google Scholar]
- 76. Maasomi ZJ, Soltanahmadi YP, Dadashpour M, Alipour S, Abolhasani S, Zarghami N. Synergistic anticancer effects of silibinin and chrysin in T47D breast cancer cells. Asian Pac J Cancer Prev. 2017;18:1283-1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Drigla F, Balacescu O, Visan S, Bisboaca SE, Berindan-Neagoe I, Marghitas LA. Synergistic effects induced by combined treatments of aqueous extract of propolis and venom. Clujul Med. 2016;89:104-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Karakuş F, Yılmaz K, Eyol E, Ünüvar S. Combination of 2 bioactive compounds for treatment of breast cancer: triterpenoid cucurbitacin I and phenolic CAPE. Nat Prod Commun. 2019;14:1934578X1985749. [Google Scholar]
- 79. Yilmaz B, Erdal B. Anti-cancer activities of curcumin and propolis extracts on MCF-7 breast cancer cell line model. Med Sci Int Med J. 2020;9:877. [Google Scholar]
- 80. Alsherbiny MA, Bhuyan DJ, Radwan I, Chang D, Li CG. Metabolomic identification of anticancer metabolites of Australian propolis and proteomic elucidation of its synergistic mechanisms with doxorubicin in the MCF7 cells. Int J Mol Sci. 2021;22:7840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Oršolić N, Car N, Lisičić D, et al. Synergism between propolis and hyperthermal intraperitoneal chemotherapy with cisplatin on Ehrlich ascites tumor in mice. J Pharm Sci. 2013;102:4395-4405. [DOI] [PubMed] [Google Scholar]
- 82. Onur E, Gökmen GG, Nalbantsoy A, Kışla D. Investigation of the supportive therapy potential of propolis extract and Lactobacillus acidophilus LA-5 milk combination against breast cancer in mice. Cytokine. 2022;149:155743. [DOI] [PubMed] [Google Scholar]
- 83. Guan Y, Chen H, Zhong Q. Nanoencapsulation of caffeic acid phenethyl ester in sucrose fatty acid esters to improve activities against cancer cells. J Food Eng. 2019;246:125-133. [Google Scholar]
- 84. Norouzi M, Rezazadeh SL, Moghadamnia AA, Bahramifar N, Barari L, Kazemi S. The effect of chrysin nanoparticles in preventing the growth of MCF-7 cancer cells. J Babol Univ Med Sci. 2018;20:56-62. [Google Scholar]
- 85. Sulaiman GM, Jabir MS, Hameed AH. Nanoscale modification of chrysin for improved of therapeutic efficiency and cytotoxicity. Artif Cells Nanomed Biotechnol. 2018;46:S708-S720. [DOI] [PubMed] [Google Scholar]
- 86. Wadhwa R, Nigam N, Bhargava P, et al. Molecular characterization and enhancement of anticancer activity of caffeic acid phenethyl ester by γ cyclodextrin. J Cancer. 2016;7:1755-1771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Sherif MS, Rehab TA, Hamdia ZA, Torchilin VP. Cytotoxicity of propolis nanopreparations in cancer cell monolayers: multimode of action including apoptotsis and nitric oxide production. Gen Physiol Biophys. 2018;37:101-110. [DOI] [PubMed] [Google Scholar]
- 88. Hasan AEZ, Mangunwidjaja D, Sunarti TC, Suparno O, Setiyono A. Antibreast cancer activity of nanopropolis Indonesia on induced mammary gland tumor by DMBA in virgin Sprague-Dawley rats. Biotropia. 2016;23:35-41. [Google Scholar]
- 89. Kapare H, Sathiyanarayanan L, Arulmozhi S, Mahadik K. Design and development of Indian propolis loaded poly (ε-caprolactone) nanoparticles for improved anticancer efficacy. Int J Pharm Res. 2017;9:73-80. [Google Scholar]
- 90. Omene CO, Wu J, Frenkel K. Caffeic acid phenethyl ester (CAPE) derived from propolis, a honeybee product, inhibits growth of breast cancer stem cells. Investig New Drugs. 2012;30:1279-1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Endo S, Matsunaga T, Kanamori A, et al. Selective inhibition of human type-5 17β-hydroxysteroid dehydrogenase (AKR1C3) by baccharin, a component of Brazilian propolis. J Nat Prod. 2012;75:716-721. [DOI] [PubMed] [Google Scholar]
- 92. Lirdprapamongkol K, Sakurai H, Abdelhamed S, et al. Chrysin overcomes TRAIL resistance of cancer cells through Mcl-1 downregulation by inhibiting STAT3 phosphorylation. Internet J Oncol. 2013;43:329-337. [DOI] [PubMed] [Google Scholar]
- 93. Khosravi AR, Shokri H, Darvishi S, Taghavi M. Immunomodulatory efficacy of ethanol extract of propolis on tumor-bearing mice with disseminated candidiasis. J Mycol Med. 2014;24:e143-e148. [DOI] [PubMed] [Google Scholar]
- 94. Okamoto Y, Tobe T, Ueda K, Takada T, Kojima N. Oral administration of Brazilian propolis exerts estrogenic effect in ovariectomized rats. J Toxicol Sci. 2015;40:235-242. [DOI] [PubMed] [Google Scholar]
- 95. Camargo MS, Prieto AM, Resende FA, et al. Evaluation of estrogenic, antiestrogenic and genotoxic activity of nemorosone, the major compound found in brown Cuban propolis. BMC Complement Altern Med. 2013;13:201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Kusnul Z, Rahayu P, Rifa’i M, Widjajanto E. Immunomodulatory effect of propolis extract on population of IL-10 and TGF-β expression in CD4+CD25+ regulatory T cells in DMBA-induced breast cancer in female Sprague-Dawley rats. Turk J Immunol. 2017;5:69-76. [Google Scholar]
- 97. Gal AF, Stan L, Tăbăran F, Rugină D, Cătoi AF, Andrei S. Chemopreventive effects of propolis in the MNU-induced rat mammary tumor model. Oxid Med Cell Longev. 2020;2020:4014838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Arslan M, Sevgiler Y, Güven C, et al. Chemical and biological characteristics of propolis from Apis mellifera caucasica from the Ardahan and Erzurum provinces of Turkey: a comparative study. Arh Hig Rada Toksikol. 2021;72:53-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Ebeid SA, Abd El, Moneim NA, El-Benhawy SA, Hussain NG, Hussain MI. Assessment of the radioprotective effect of propolis in breast cancer patients undergoing radiotherapy: new perspective for an old honey bee product. J Radiat Res Appl Sci. 2016;9:431-440. [Google Scholar]
- 100. Juanbeltz Zurbano R, Pérez-Fernández MD, Tirapu Nicolás B, Vera García R, De la Cruz Sánchez S, Sarobe Carricas MT. Complementary medicine use in cancer patients receiving intravenous antineoplastic treatment. Farm Hosp. 2017;41:589-600. [DOI] [PubMed] [Google Scholar]
- 101. Piredda M, Facchinetti G, Biagioli V, et al. Propolis in the prevention of oral mucositis in breast cancer patients receiving adjuvant chemotherapy: a pilot randomised controlled trial. Eur J Cancer Care. 2017;26:e12757. [DOI] [PubMed] [Google Scholar]
- 102. Darvishi N, Yousefinejad V, Akbari ME, et al. Antioxidant and anti-inflammatory effects of oral propolis in patients with breast cancer treated with chemotherapy: a randomized controlled trial. J Herb Med. 2020;23:100385. [Google Scholar]
- 103. Davoodi SH, Yousefinejad V, Ghaderi B, et al. Oral propolis, nutritional status and quality of life with chemotherapy for breast cancer: a randomized, double-blind clinical trial. Nutr Cancer. 2021;8:1-9. [DOI] [PubMed] [Google Scholar]
- 104. Frión-Herrera Y, Díaz-García A, Ruiz-Fuentes J, Rodríguez-Sánchez H, Sforcin JM. The cytotoxic effects of propolis on breast cancer cells involve PI3K/Akt and ERK1/2 pathways, mitochondrial membrane potential, and reactive oxygen species generation. Inflammopharmacology. 2019;27:1081-1089. [DOI] [PubMed] [Google Scholar]
- 105. Lirdprapamongkol K, Sakurai H, Abdelhamed S, et al. A flavonoid chrysin suppresses hypoxic survival and metastatic growth of mouse breast cancer cells. Oncol Rep. 2013;30:2357-2364. [DOI] [PubMed] [Google Scholar]
- 106. Kuropatnicki AK, Szliszka E, Krol W. Historical aspects of propolis research in modern times. Evid Based Complement Altern Med. 2013;2013:964149. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-ict-10.1177_15347354221096868 for The Potential Use of Propolis as an Adjunctive Therapy in Breast Cancers by Dedy Hermansyah, Felix Zulhendri, Conrad O. Perera, Naufal N. Firsty, Kavita Chandrasekaran, Rizky Abdulah, Herry Herman and Ronny Lesmana in Integrative Cancer Therapies