Abstract
Purpose:
The aim of this article was to compare longitudinal changes in hip morphology in cerebral palsy (hypertonic) and spinal muscular atrophy (hypotonic) to examine the influence of muscle tone on development of hip displacement.
Methods:
Children with spinal muscular atrophy (Types I and II) and cerebral palsy (Gross Motor Function Classification System IV and V) with hip displacement (migration percentage >30%) were included. Head shaft angle, migration percentage, and acetabular index were measured at T1 (1–2.5 years), T2 (3–5 years), and T3 (6–8 years). Analysis of variance testing and linear regression were utilized.
Results:
Sixty patients (cerebral palsy, N = 41; spinal muscular atrophy, N = 19) were included. Hip displacement occurred earlier in spinal muscular atrophy (34 months) than cerebral palsy (49 months) (p = 0.003). Head shaft angle was high and did not change between T1, T2, and T3, but significant changes in migration percentage were found (cerebral palsy: 23%, 36%, 45% (p < 0.01) and spinal muscular atrophy: 37%, 57%, 61% (p = 0.02)). Migration percentage increased by age in cerebral palsy (r = 0.41, p < 0.001), but not in spinal muscular atrophy (r = 0.18, p = 0.09). Acetabular index increased with migration percentage (cerebral palsy: r = 0.41, p < 0.001; spinal muscular atrophy: r = 0.48, p < 0.001).
Conclusion:
Persistent lateral physeal tilt by head shaft angle was found for both spinal muscular atrophy and cerebral palsy. Abnormal physeal alignment may be causally related to weakness of the hip abductor muscles rather than spasticity or muscle imbalance, resulting in coxa valga and secondary acetabular dysplasia.
Level of evidence:
III (case–control study).
Keywords: Spinal muscular atrophy, cerebral palsy, hip displacement, spasticity, head shaft angle, physeal tilt, muscle balance, diagnosis, acetabular dysplasia, neuromuscular
Introduction
Hip displacement (HD), joint instability associated with proximal femoral and acetabular dysplasia, is common in neuromuscular disorders, with a prevalence of approximately 35% in large population-based studies of children with cerebral palsy (CP).1,2 Most non-ambulant children with CP have hypertonia (most commonly spastic motor type) as well as a high prevalence of HD.1–6 Spasticity in the hip adductors and flexors has been suggested as a primary cause of HD, and has been a target for intervention both operatively and non-operatively. It has been theorized that the spastic hip adductors and flexors overpower the hip abductors and extensors, resulting in muscle imbalance that forces the hip out of the joint. However, a recent population-based study reports high rates of recurrence after adductor surgery for HD in children with CP. 7 Similarly, a randomized-controlled trial assessing the effects of botulinum toxin injection of the hip adductors and hamstrings reported no clinically relevant treatment effect. 8 These data suggest that the role of both spasticity and muscle imbalance in the development of HD may have been overstated.
Soo and colleagues 1 reported a linear relationship between Gross Motor Function Classification System (GMFCS) level and the risk of HD defined as migration percentage (MP) >30%, but they found no relationship to motor type or movement disorder. Children with hypotonia had the same risk of HD as those with hypertonia—the prevalence being predicted solely by GMFCS level. 1 These data suggest that impaired mobility may be a more important risk factor for the development of HD than muscle tone.
Children affected by spinal muscular atrophy (SMA) also develop HD, with radiographic features including progressive coxa valga (typified by lateral proximal femoral physeal tilt and increasing femoral neck shaft angle (NSA)) and associated acetabular dysplasia, being similar to CP. 9 Tönnis 10 highlighted the role of lateral physeal tilt as an etiologic factor in the development of coxa valga, with the physis aligning perpendicular to the direction of the resultant forces across the hip joint, especially where abductor insufficiency was present. Several other authors have discussed this mechanism as being a causative factor for HD in both CP and SMA.11,12
The purpose of this study was to compare the changes in proximal femoral and acetabular geometry over time for children with CP and SMA to help elucidate the influence of muscle tone on the development of HD. Our hypothesis was that hip morphology associated with HD is similar between hypertonic (CP) and hypotonic (SMA) neuromuscular disorders, typified by abnormalities in proximal femoral growth (lateral physeal tilt) leading to coxa valga and secondary acetabular dysplasia.
Materials and methods
The study design was a retrospective case–control, conducted at an academic tertiary level children’s hospital. Children presenting between June 2005 and July 2020, with diagnostic codes specific to CP (the International Statistical Classification of Diseases and Related Health Problems–Tenth Edition ICD-10 (G80.1-G80.0) and International Statistical Classification of Diseases and Related Health Problems–Ninth Edition ICD-9 (343.0)) and SMA (ICD-10 (G12.9) and ICD-9 (335.10)) diagnoses were identified from our institution’s electronic medical record for potential inclusion in the study. Inclusion criteria were children with SMA (Types I and II) and CP (spastic motor type, GMFCS 13 IV and V) who developed HD, with regular hip surveillance radiographs over three specified age intervals (T1: 1–2.5 years old, T2: 3–5 years old, and T3: 6–8 years old), and no previous bony reconstructive surgery. Exclusion criteria were other neuromuscular diagnoses and/or functional levels and a lack of hip surveillance radiographs in the age intervals specified for this study. HD was defined as an MP greater than or equal to 30% in one or both hips during the radiographic hip surveillance period. 1
The primary outcome variable was the extent of proximal femoral physeal tilt by head shaft angle (HSA). The HSA previously has been shown to have good intra- and inter-rater reliability, is less affected by hip rotation than femoral NSA, 14 and is correlated with MP. 15 Secondary outcome variables included age at first hip surveillance radiograph, duration of radiographic hip surveillance follow-up, number of hip surveillance radiographs, and age at onset of HD. Other radiographic outcome variables included MP and acetabular index (AI), both found to have good to excellent intra- and inter-observer reliability in CP. 16 All radiographic measurements were performed by the lead author (A.C.U.), after a dedicated training period with the senior author (J.J.H.), a sub-specialized pediatric orthopedic surgeon with extensive experience treating children with CP and SMA. Data were collected and analyzed longitudinally for age intervals T1, T2, and T3 for each diagnostic group.
Statistical analysis
The collected data were analyzed using both descriptive and inferential statistics. As this was a retrospective chart review without any sample size calculation, inferential statistics were performed on an exploratory basis only. Descriptive statistics, including mean values and standard deviations (SDs) for outcome variables within both diagnostic groups, were tabulated. Analysis of variance testing was used to compare outcomes between time points. The HSA/MP/AI versus age and HSA/MP versus AI were analyzed by linear regression, with a significance level of p < 0.05.
The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines were utilized in preparing this article. No funding was required or utilized in this investigation.
Results
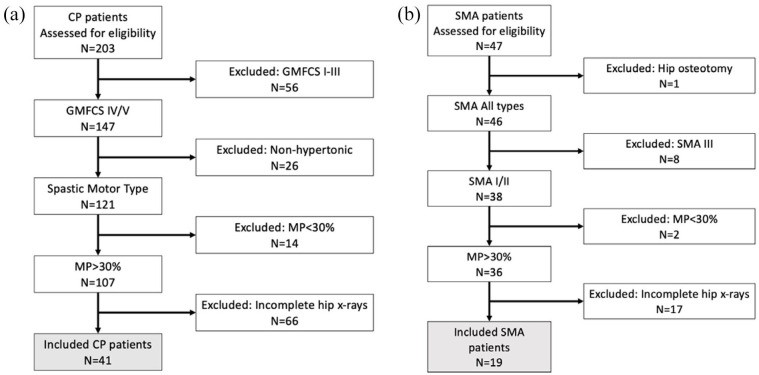
Two hundred and three children with CP and 47 with SMA were initially identified for inclusion in the study. After applying the inclusion criteria, 60 patients (CP, N = 41 (52 hips) and SMA, N = 19 (37 hips)) were available for analysis (Figure 1). The ages at initial presentation for the CP and SMA groups were 7.2 (SD = 5.1, range = 1–14) and 6.9 (SD = 6.0, range = 0–14) months, respectively. The ages at first pelvis radiograph for the CP and SMA groups were 22.6 (SD = 5.0, range = 12–30) and 24.1 (SD = 5.1, range = 12–30) months, respectively. The mean numbers of hip surveillance radiographs for the CP and SMA groups were 3.6 (SD = 0.9) and 2.2 (SD = 0.8), respectively. The total clinical follow-up duration for the entire cohort was 53.7 (SD = 16.5) months. The mean age for the entire cohort at the time of audit was 72.1 (SD = 18.3, range = 52–94) months. Table 1 summarizes the demographic characteristics by diagnostic group.
Figure 1.
Patient flow diagrams for study eligibility for the (a) CP and (b) SMA groups.
CP: cerebral palsy; SMA: spinal muscular atrophy.
Table 1.
Patient demographics.
| Demographic | CP | SMA |
|---|---|---|
| Number of patients: n (% of total cohort) | 41 (68.3) | 19 (31.7) |
| Sex (male/female, % female) | 25/16, 39 | 7/12, 63 |
| Functional severity | ||
| CP: GMFCS (n (IV/V), % V) | 7/34, 83 | 10/9, 52 |
| SMA: type (n (I/II), % I) | ||
| Age at first hip surveillance radiograph (months, mean (SD)) | 22.6 (5.0) | 24.1 (5.1) |
| Age at last hip surveillance radiograph (months, mean (SD)) | 82.6 (6.7) | 79.4 (5.3) |
| Number of hip surveillance radiographs (mean (SD)) | 3.6 (0.9) | 2.2 (0.8) |
| Final follow-up (months) (mean (SD)) | 59.5 (9.3) | 59.6 (11) |
| Hip adductor tenotomy during T1 to T3 intervals (n (%)) | 17 (41.5) | 1 (5.3) |
CP: cerebral palsy; GMFCS: Gross Motor Function Classification System; SD: standard deviation; SMA: spinal muscular atrophy.
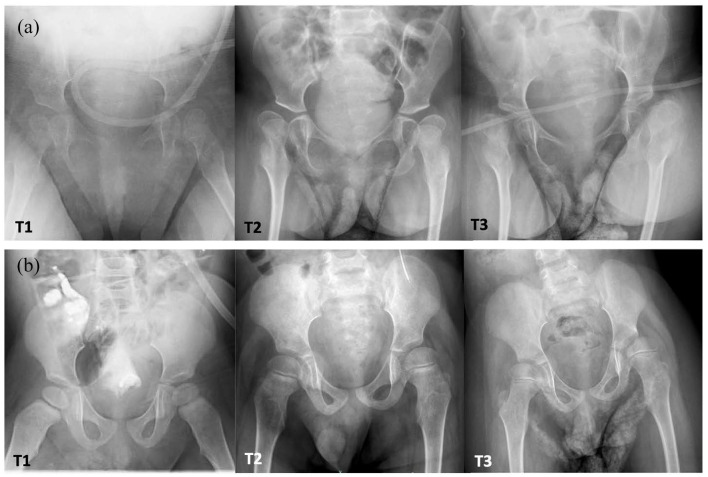
Figure 2.
(a) Type I spinal muscular atrophy and (b) quadriplegic cerebral palsy (Gross Motor Function Classification System V), with bilateral progressive hip displacement from T1 to T3. Note persistent lateral proximal femoral physeal tilt, progressive coxa valga, acetabular dysplasia, and migration percentage for both diagnoses. The spinal muscular atrophy hips demonstrate faster migration percentage progression and proximal femoral deformity over time compared with cerebral palsy.
HD occurred at an earlier age (months) in SMA (mean = 34, range = 6–51) versus CP (mean = 49, range = 27–61) (p = 0.003) (Figure 1). For age intervals T1, T2, and T3, the mean HSA values for CP were 169°, 170°, and 169°, respectively (not significant (NS)), and for SMA, they were 173°, 174°, and 173°, respectively (NS). For these same intervals, the mean MP values for CP were 23%, 36%, and 45%, respectively (p < 0.01), and for SMA, they were 37%, 57%, and 61%, respectively (p = 0.02).
Regression analysis showed significant increases in MP by age for CP (r = 0.41, p < 0.001), but not for SMA (r = 0.18, p = 0.09), while HSA by age for both diagnoses was NS (CP: r = 0.20, p = 0.8 and SMA: r = 0.14, p = 0.18). AI increased linearly with MP for both diagnoses (CP: r = 0.41, p < 0.001 and SMA: r = 0.48, p < 0.001), but not with HSA. AI was not found to be significantly correlated with age for either CP (p = 0.7) or SMA (p = 0.35).
With respect to hip surgical interventions during or before age intervals T1 to T3, soft-tissue releases (typically adductor longus +/− adductor brevis, gracilis, and iliopsoas) were performed in 17 of 41 patients (41.5%) and in 1 of 19 patients (5.3%) for the CP and SMA groups, respectively (at mean age (years) for CP: 6.9 (SD = 1.8, range = 4.9–8.8) and SMA: 5.0 (SD = N/A, range= N/A)). Following the last hip surveillance radiograph during the T3 age interval period, 29 (70.7%) underwent hip surgery procedures at a mean age of 7.8 (SD = 1.6, range = 6.1–9.7) years in the CP group. Procedures included one (2.4%) preventive (hip adductor/flexor/proximal hamstring releases) alone, six (14.6%) reconstructive (proximal femoral osteotomy and/or acetabuloplasty) alone, and 22 (53.7%) both preventive and reconstructive. For the SMA group, two patients (10.5%) underwent hip surgery procedures at a mean age of 10 (SD = 3.6, range = 7.4–12.6) years following the last hip surveillance radiograph during the T3 age interval period. Procedures included two (10.5%) reconstructive (proximal femoral osteotomy and/or acetabuloplasty) alone.
Discussion
Identifying the cause of HD in neuromuscular disorders may be important to help elucidate the most effective treatment options, including preventive strategies. For HD in children with CP, the “traditional view” is that spasticity results in muscle imbalance, fixed contractures, and progressive HD.17,18 In this view, there is little emphasis on understanding changes in proximal femoral geometry, in which coxa valga has a linear relationship to GMFCS level. 19 As such, early treatment of HD has been focused on surgical lengthening of spastic hip adductors, flexors, and proximal hamstrings. This approach is supported by favorable results in the early literature, which reported good survivorship after soft-tissue releases alone in studies with short-term follow-up and not stratified by the GMFCS level.20,21 However, a more recent population-based study, the largest to date with the longest mean follow-up, reported high rates of recurrent HD in non-ambulatory children with CP (GMFCS levels IV or V) after adductor surgery. 7 The authors proposed that lack of weight-bearing and weakness in the hip abductors may be causative for recurrent HD in non-ambulatory children with CP.
SMA is a lower motor neuron disorder, secondary to deterioration of the anterior horn cells in the spinal cord, with symmetric proximal muscle weakness and atrophy being more severe in Types I and II. 22 Zenios et al. 11 suggest that the development of HD in SMA is secondary to diminished weight-bearing and “profound gluteal weakness,” which depressed the stimulus for trochanteric growth, resulting in coxa valga and joint subluxation. A similar etiology for CP HD is proposed by Phelps, 23 who deduces that delayed weight-bearing and abductor insufficiency are causative, and that adductor contracture plays a lesser role. This view is echoed by Minear and Tachdjian, 24 who claim that abductor weakness causes a lateral realignment of the proximal femoral physis, leading to coxa valga and hip subluxation in CP. Indeed, the negative features of the CP upper motor neuron syndrome—including weakness, poor selective motor control, and balance—have been implicated as the primary determinants of gross motor function, including the acquisition of functional weight-bearing. 25
Pauwels 26 was the first to theorize that proximal femoral geometry is modulated by functional loading, affected by physeal stimulation proportional to the magnitude of imparted stress. Tönnis 10 further claims that “valgus angulation of the femoral neck is caused by a relative predominance of adductors over abductors.” In their population-based study of proximal femoral geometry in CP, Robin et al. 27 confirm a step-wise increase in femoral NSA with decreasing functional mobility by GMFCS. They suggest that the resulting coxa valga is an acquired deformity proportional to ambulatory ability, with GMFCS levels IV and V having the highest NSA. Along with a persistence in femoral anteversion, like NSA, the MP also increases with the GMFCS level, confirming that the combination of both deformities predisposes to the development of HD. In this study, we chose HSA rather than NSA as our primary measure of proximal femoral geometry. This decision was made given the lack of reliability in NSA measurement due to inconsistency in hip rotation during radiographic positioning. 28 In addition, NSA does not allow for a direct assessment of physeal tilt.
For both SMA and CP, we found a persistence in lateral proximal femoral physeal tilt that did not significantly decrease throughout the age intervals up to 8 years old. Our results for CP are consistent with van der List and colleagues 12 who also found HSA to be high and unchanged over similar age intervals for GMFCS levels IV and V. They attribute imbalance between hip adductors and abductors, with associated physeal realignment, to be responsible for their findings. A second study from these authors, using typically developing children as a comparison group, found a significant decrease in HSA over time compared with age-matched children with CP. 29 By contrast, they found that HSA in GMFCS levels I to III significantly decreases over time, although to a lesser extent than for typically developing children. With similar findings in children with CP under age 5 years, Terjesen and Horn 30 report persistently high and unchanged HSA for GMFCS levels IV and V, but significant decreases in HSA over time for GMFCS III. These findings support the role of functional mobility in proximal femoral development.
Significant increases in MP over time, positively correlated with progressive acetabular dysplasia, were found for both diagnoses in the current study. Coupled with the presence of a persistently laterally tilted proximal femoral physis in SMA and CP, these similarities suggest a more unifying cause of HD for both hypertonic and hypotonic disorders, which, given the prior discussion, would seem to point to a lack of functional weight-bearing and associated abductor weakness. Although we did not have hip abductor strength data for review in this study, Darras et al. 31 investigated lower extremity weakness in children with CP compared with typically developing controls. They found that the hip abductor muscles are significantly weaker in children with CP compared with typically developing children overall, and those in GMFCS levels III/IV are significantly weaker than I/II. This suggests that hip abductor strength decreases in proportion to functional mobility. Given the muscle imbalance theory proposed by Pauwel and others,10,17,26,32,33 it would seem plausible that the presence of both adductor spasticity and abductor weakness would lead to an earlier and more severe progression of HD, but we did not find this to be the case. Typified by proximal muscle weakness, children with SMA had an earlier age of onset of HD compared with CP and displayed a higher MP at all age intervals. These results suggest that either SMA has more profound abductor weakness than CP, or that the presence of adductor spasticity may be less influential than previously thought.
Progressive distortion of proximal femoral and acetabular geometry requires bony surgery for correction, and this may be the reason why bony hip reconstruction is so much more effective in long-term CP studies than either chemodenervation with botulinum toxin A or hip adductor lengthening surgery. In addition, if abductor weakness is a primary cause of progressive changes in proximal femoral geometry, there may be a role for guided growth in the proximal femur to counteract the associated coxa valga and secondary acetabular dysplasia.
In accordance with the above discussion, reversal of coxa valga by inferomedial screw epiphysiodesis of the proximal femoral physis has been investigated as a potential treatment of HD in CP. 34 Hsieh et al. 35 reported significant decreases in HSA, MP, and AI in their cohort of children with CP HD, with longer follow-up duration and smaller preoperative MP associated with larger HSA reduction. The reduction in AI they identified at final follow-up supports the view that pressure from a laterally displaced femoral head is causative in the development of acetabular dysplasia. 36 Furthermore, in this study, AI was significantly correlated with MP but not to HSA, likely due to the persistence of excessive physeal tilt over the age intervals investigated.
Given its retrospective nature, there were limitations inherent to the study design, most importantly its small sample size and not being population-based. This resulted from our inclusion criteria, which required regular hip surveillance radiographs in each of the designated age intervals. We attempted to account for this statistically by doubling our sample of CP versus SMA patients, and age-matching the groups within designated temporal intervals. Given the lack of consistent availability of more advanced imaging, we did not assess three-dimensional aspects of hip dysplasia in this study. We acknowledge that an assessment of femoral anteversion and three-dimensional acetabular deficiency for both diagnoses would be even more comprehensive. Owing to its resistance to positional changes, we used HSA as a primary measure of proximal femoral geometry and physeal alignment rather than NSA.
Another limitation was the exclusion of patients who underwent bony reconstructive surgery before/within the time intervals of interest. This was necessary to allow for the assessment of changes in hip geometry over time. This approach may have introduced some bias by excluding patients who have a more severe phenotype, requiring early surgery. That said, in a population-based study extending to skeletal maturity, patients with CP underwent hip reconstruction at a mean age of 7 years 11 months, at the upper limit of the T3 age interval used in this study. 3
In summary, persistent proximal femoral lateral physeal tilt was found for both SMA and CP, with progression to HD being earlier and more severe in SMA, despite the lack of spasticity in that diagnosis. This physeal tilt may represent a more unifying cause for the development of HD, leading to progressive coxa valga with acetabular dysplasia secondary to lateral pressure from the femoral head. Thus, rather than spasticity, the development of HD may be more related to features that are common to both hypertonic and hypotonic disorders, with abductor muscle weakness and a lack of functional weight-bearing being probable causes of persistent proximal femoral lateral physeal tilt. As such, strategies aimed at early modulation of proximal femoral physeal growth may be warranted to help prevent or treat HD.
Footnotes
Author contributions: A.C.U. contributed to the conception and design, acquisition of the data, analysis and interpretation of the data, drafting of the article, critical revision of the article for important intellectual content, statistical expertise, collection and assembly of data, final approval of the version to be published and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. A.A. contributed to the conception and design, acquisition of the data, analysis and interpretation of the data, critical revision of the article for important intellectual content, statistical expertise, collection and assembly of data, final approval of the version to be published and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. K.J.R. contributed to the conception and design; acquisition of the data; analysis and interpretation of the data; critical revision of the article for important intellectual content; statistical expertise; administrative, technical, or logistic support; collection and assembly of data, final approval of the version to be published and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. M.W.S. contributed to the conception and design, analysis and interpretation of the data, critical revision of the article for important intellectual content, provision of study materials or patients, final approval of the version to be published and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. H.K.G. contributed to the analysis and interpretation of the data, drafting of the article, critical revision of the article for important intellectual content, final approval of the version to be published and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. J.J.H. contributed to the conception and design, acquisition of the data, analysis and interpretation of the data, critical revision of the article for important intellectual content, statistical expertise, collection and assembly of data, provision of study materials or patients, final approval of the version to be published and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical approval: All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. This retrospective study was reviewed and approved by the institutional review board.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Soo B, Howard JJ, Boyd RN, et al. Hip displacement in cerebral palsy. J Bone Joint Surg Am 2006; 88: 121–129. [DOI] [PubMed] [Google Scholar]
- 2. Hägglund G, Lauge-Pedersen H, Wagner P. Characteristics of children with hip displacement in cerebral palsy. BMC Musculoskelet Disord 2007; 8: 101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Penner M, Xie WY, Binepal N, et al. Characteristics of pain in children and youth with cerebral palsy. Pediatrics 2013; 132(2): e407–e413. [DOI] [PubMed] [Google Scholar]
- 4. Wawrzuta J, Willoughby KL, Molesworth C, et al. Hip health at skeletal maturity: a population-based study of young adults with cerebral palsy. Dev Med Child Neurol 2016; 58(12): 1273–1280. [DOI] [PubMed] [Google Scholar]
- 5. Masłoń A, Jóźwiak M, Pawlak M, et al. Hip joint pain in spastic dislocation: aetiological aspects. Dev Med Child Neurol 2011; 53(11): 1019–1023. [DOI] [PubMed] [Google Scholar]
- 6. Howard J, Soo B, Graham HK, et al. Cerebral palsy in Victoria: motor types, topography and gross motor function. J Paediatr Child Health 2005; 41(9–10): 479–483. [DOI] [PubMed] [Google Scholar]
- 7. Shore BJ, Yu X, Desai S, et al. Adductor surgery to prevent hip displacement in children with cerebral palsy: the predictive role of the Gross Motor Function Classification System. J Bone Joint Surg Am 2012; 94: 326–334. [DOI] [PubMed] [Google Scholar]
- 8. Graham HK, Boyd R, Carlin JB, et al. Does botulinum toxin A combined with bracing prevent hip displacement in children with cerebral palsy and “hips at risk”? A randomized, controlled trial. J Bone Joint Surg Am 2008; 90(1): 23–33. [DOI] [PubMed] [Google Scholar]
- 9. Sporer SM, Smith BG. Hip dislocation in patients with spinal muscular atrophy. J Pediatr Orthop 2003; 23(1): 10–14. [PubMed] [Google Scholar]
- 10. Tönnis D. Development of the hip joint. In: Tönnis D. (ed.) Congenital dysplasia and dislocation of the hip children and adults. Berlin; Heidelberg: Springer, 1987, pp. 13–22. [Google Scholar]
- 11. Zenios M, Sampath J, Cole C, et al. Operative treatment for hip subluxation in spinal muscular atrophy. J Bone Joint Surg Br 2005; 87(11): 1541–1544. [DOI] [PubMed] [Google Scholar]
- 12. van der List JP, Witbreuk MM, Buizer AI, et al. The prognostic value of the head-shaft angle on hip displacement in children with cerebral palsy. J Child Orthop 2015; 9(2): 129–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Palisano R, Rosenbaum P, Walter S, et al. Development and reliability of a system to classify gross motor function in children with cerebral palsy. Dev Med Child Neurol 1997; 39(4): 214–223. [DOI] [PubMed] [Google Scholar]
- 14. Foroohar A, McCarthy JJ, Yucha D, et al. Head-shaft angle measurement in children with cerebral palsy. J Pediatr Orthop 2009; 29(3): 248–250. [DOI] [PubMed] [Google Scholar]
- 15. Lee KM, Kang JY, Chung CY, et al. Clinical relevance of valgus deformity of proximal femur in cerebral palsy. J Pediatr Orthop 2010; 30(7): 720–725. [DOI] [PubMed] [Google Scholar]
- 16. Pons C, Remy-Neris O, Medee B, et al. Validity and reliability of radiological methods to assess proximal hip geometry in children with cerebral palsy: a systemic review. Dev Med Child Neurol 2013; 55: 1089–1102. [DOI] [PubMed] [Google Scholar]
- 17. Lovell WW, Winter RB. Lovell and Winter’s pediatric orthopaedics. Philadelphia, PA: Lippincott, 1990. [Google Scholar]
- 18. Howard JJ, Herzog W. Skeletal muscle in cerebral palsy: from belly to myofibril. Front Neurol 2021; 12: 620852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chung MK, Zulkarnain A, Lee JB, et al. Functional status and amount of hip displacement independently affect acetabular dysplasia in cerebral palsy. Dev Med Child Neurol 2017; 59(7): 743–749. [DOI] [PubMed] [Google Scholar]
- 20. Bleck EE. Management of the lower extremities in children who have cerebral palsy. J Bone Joint Surg Am 1990; 72(1): 140–144. [PubMed] [Google Scholar]
- 21. Miller F, Cardoso Dias R, Dabney KW, et al. Soft-tissue release for spastic hip subluxation in cerebral palsy. J Pediatr Orthop 1997; 17(5): 571–584. [DOI] [PubMed] [Google Scholar]
- 22. Messina S, Sframeli M, Maggi L, et al. Spinal muscular atrophy: state of the art and new therapeutic strategies. Neurol Sci. Epub ahead of print 19 April 2021. DOI: 10.1007/s10072-021-05258-3. [DOI] [PubMed] [Google Scholar]
- 23. Phelps WM. Prevention of acquired dislocation of the hip in cerebral palsy. J Bone Joint Surg Am 1959; 41-A(3): 440–448. [PubMed] [Google Scholar]
- 24. Minear WL, Tachdjian MO. Hip dislocation in cerebral palsy. J Bone Joint Surg Am 1956; 38-A: 1358–1364. [PubMed] [Google Scholar]
- 25. Graham HK, Rosenbaum P, Paneth N, et al. Cerebral palsy. Nat Rev Dis Primers 2016; 2: 15082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Pauwels F. Funktionelle Anpassung des Knochens durch Langenwachstum. Verh Dtsch Orthop Ges, 45. Kongr 1957. Z Orthop 1965; 90: 34–56 (Beilage-heft). [Google Scholar]
- 27. Robin J, Graham HK, Selber P, et al. Proximal femoral geometry in cerebral palsy: a population-based cross-sectional study. J Bone Joint Surg Br 2008; 90(10): 1372–1379. [DOI] [PubMed] [Google Scholar]
- 28. Kay RM, Jaki KA, Skaggs DL. The effect of femoral rotation on the projected femoral neck-shaft angle. J Pediatr Orthop 2000; 20(6): 736–739. [DOI] [PubMed] [Google Scholar]
- 29. van der List JP, Witbreuk MM, Buizer AI, et al. The head-shaft angle of the hip in early childhood: a comparison of reference values for children with cerebral palsy and normally developing hips. Bone Joint J 2015; 97-B(9): 1291–1295. [DOI] [PubMed] [Google Scholar]
- 30. Terjesen T, Horn J. The femoral head-shaft angle is not a predictor of hip displacement in children under 5 years with cerebral palsy: a population-based study of children at GMFCS levels III–V. J Pediatr Orthop 2021; 41: e659–e663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Darras N, Nikaina E, Tziomaki M, et al. Development of lower extremity strength in ambulatory children with bilateral spastic cerebral palsy in comparison with typically developing controls using absolute and normalized to body weight force values. Front Neurol 2021; 12: 617971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Inman VT. Functional aspects of the abductor muscles of the hip. J Bone Joint Surg Am 1947; 29(3): 607–619. [PubMed] [Google Scholar]
- 33. Samilson RL, Tsou P, Aamoth G, et al. Dislocation and subluxation of the hip in cerebral palsy. Pathogenesis, natural history and management. J Bone Joint Surg Am 1972; 54: 863–873. [PubMed] [Google Scholar]
- 34. McCarthy JJ, Noonan KJ, Nemke B, et al. Guided growth of the proximal femur: a pilot study in the lamb model. J Pediatr Orthop 2010; 30(7): 690–694. [DOI] [PubMed] [Google Scholar]
- 35. Hsieh HC, Wang TM, Kuo KN, et al. Guided growth improves coxa valga and hip subluxation in children with cerebral palsy. Clin Orthop Relat Res 2019; 477: 2568–2576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Sauser DD, Hewes RC, Root L. Hip changes in spastic cerebral palsy. AJR Am J Roentgenol 1986; 146: 1219–1222. [DOI] [PubMed] [Google Scholar]