Abstract
Objectives:
To assess survival and prognostic factors among women with epithelial ovarian cancer in Western Saudi Arabia.
Methods:
A retrospective cohort study was carried out between October 2000 and May 2018, reviewing clinical and pathology data of all women who underwent staging or debulking surgery for epithelial ovarian cancer. Analysis of disease-free survival (DFS), overall survivals (OS) and the associated factors used Kaplan-Meier method in addition to cox multivariate regression.
Results:
A total of 144 patients were included (median age=49.5 years), with a median follow-up time was 3.4 years. Majority (59.7%) of the patients were diagnosed at an advanced stage (III or IV). The mean (95% CI) DFS was 82.3 (67.8-96.8) months, OS was 96.2 (81.3-111.2) months, and the 5-year survival rate was estimated as 38.9%. Univariate analysis showed that older age, clear cell or papillary carcinoma subtypes, serous type, advanced International Federation of Gynecology and Obstetrics (FIGO) stage and the presence of residual disease were associated with poorer DFS and OS (log rank <0.05). Cox regression showed FIGO stage and residual disease >1cm as the strongest prognostic factors independently associated with DFS and OS.
Conclusion:
Improving early diagnosis and achieving optimal cytoreduction are the most critical challenges to achieve significant positive impact on survival of women with epithelial ovarian cancer.
Keywords: epithelial, ovarian cancer, survival, Saudi Arabia
Epithelial ovarian cancers are the deadliest among all gynecological cancers, accounting for the fifth major cause of cancer-related death in European countries and the United States (US). 1,2 In 2018, the American Cancer Society reported as high as 22,240 new ovarian cancer cases and approximately, 14,070 disease-related deaths in the US. 3 Globally, 239,000 new cases of ovarian cancer are diagnosed, associated with over 150,000 annual deaths, which accounts for 4.3% of all cancer mortality. 4
Several efforts were made to improve early detection of epithelial ovary cancers, and several advances in therapeutic approaches have been achieved till date. However, due to their histological diversity and variable biological and molecular features, the origin and pathogenesis of these tumors are not elucidated well. Hence, efforts to improve survival and prognosis in these patients are barely saucerful. 5,6 In addition, ovarian cancer remains asymptomatic in early stages, which results in frequent presentation and diagnosis at an advanced stage. The disease onset is marked by nonspecific symptoms that are difficult to identify and are usually mistaken as physiological changes in the body due to pregnancy, menopause or aging. 7 Consequently, chances of permanent cure for patients are remarkably low, notably those diagnosed in the advanced stage. 8,9 The first line management of epithelial ovarian cancer relies on surgical cytoreduction aiming at complete tumor resection, which may be completed by platinum-based chemotherapy. 10
Considering the burdensome morbidity and mortality of epithelial ovarian cancers, it is critical to analyze patients’ survival locally and identify the associated prognostic factors predictive of relapse or mortality. Globally, the survival rates vary between the countries. 11 Further, according to the definition by the International Federation of Obstetrics and Gynecology (FIGO), few well-established prognostic factors include tumor staging, post-surgery residual lesion, tumor grade, and histopathological subtypes. 10,12
This study aimed to analyze survival in patients diagnosed with epithelial ovarian cancer residing in the western region of Saudi Arabia. The progression-free (PFS) and overall survivals (OS) were estimated and the associated prognostic factors were investigated. In addition, the study estimated the 5-year OS and PFS rates.
Methods
A retrospective chart review was conducted at King Abdulaziz University Hospital (KAUH), Jeddah, Saudi Arabia, between October 2000 and May 2018. The study was ethically approved by the Institutional Review Board of King Abdulaziz University. The study was conducted in accordance with the principles of Helsinki Declaration
Clinical data of all females who have benefited from primary staging or debulking surgery for serous and non-serous ovarian cancer, between October 2000 and May 2018 at KAUH was collected retrospectively. Patients who received neoadjuvant therapy and those with other histology types were excluded.
Clinical and histopathological data were extracted from the electronic patients’ records and histopathology reports. Patients’ data included age, marital status, height and weight, medical history, tumor histological type, FIGO stage, date of surgery, post-surgery residual disease, chemotherapy regimens, date of recurrence if any, and patient’s outcome at last follow-up (including last follow-up date). Histopathological diagnoses were based on the World Health Organization criteria.
Progression-free was defined as the time between primary surgery and recurrence, or death or last follow-up without recurrence while OS was defined as the time between primary surgery and death or last follow-up.
Statistical methods
Data was analyzed using SPSS version 21.0 for Windows (IBM Corp., Armonk, N.Y., USA). A p-value or log rank of <0.05 was considered for statistical significance. Categorical variables were correlated using Chi-square or Fisher’s exact test, as appropriate. Kaplan-Meier method was used to analyze PFS and OS, and the prognostic factors associated with PFS and OS were analyzed using Log Rank test for univariate analysis; results are presented as mean (95% confidence interval [95% CI]) PFS and OS, with graphic presentations of survival curves for most relevant prognostic factors. Multivariate models were built using Cox regression, by including significant prognostic factors; results are presented as hazard ratio (HR) with 95% CI.
Results
In this study, 144 eligible women were included. The median (range) age was 49.5 years (20.0-85.0 years) and the percentage of patients below 40 (28.5%) and above 60 years (25.7%) were similar. The median body mass index (BMI) was calculated as 27.9 kg/m 2 and 70.1% were overweight or obese.
Majority patients (76.4%) had serous tumor including papillary carcinoma. Approximately, half of the patients were at stage III (47.9%) while 11.8% were at stage IV. Further, majority (56.3%) had grade 3 tumor. Subsequent to surgery, majority patients had no residual disease (53.5%), while 35.4% had residual disease >1cm. Chemotherapy was used by 69.4% of the patients with carboplatin + paclitaxel being the most common regimen (56.9%). Recurrence rate is 34.0% and mortality rate was 47.2%, with an overall median follow up of 3.4 years. All participants’ demographic and clinical characteristics are depicted in Table 1.
Table 1.
- Demographic, clinical, and tumor characteristics of patients with ovarian cancer (N=144).
| Variable | Median (range) | n | (%) |
|---|---|---|---|
| Age (years) | |||
| 20-39 | 41 | (28.5) | |
| 40-59 | 66 | (45.8) | |
| 60-85 | 37 | (25.7) | |
| Median (range) | 49.5 (20.0-85.0) | ||
| Marital status | |||
| Never married | 22 | (15.3) | |
| Married | 122 | (84.7) | |
| Body mass index (kg/m2) | |||
| Underweight (<18.5) | 5 | (3.5) | |
| Normal (18.5-24.9) | 38 | (26.4) | |
| Overweight (25.0-29.9) | 49 | (34.0) | |
| Obese (30+) | 52 | (36.1) | |
| Median (range) | 27.9 (14.6-44.73) | ||
| Parity | |||
| Nullipara | 38 | (26.4) | |
| 1-3 | 55 | (38.2) | |
| >4 | 51 | (35.4) | |
| Median (range) | 3 (0-13) | ||
| Medical disease | |||
| No | 69 | (47.9) | |
| Yes | 75 | (52.1) | |
| Histopathology | |||
| Serous | 110 | (76.4) | |
| Non-serous | 34 | (23.6) | |
| Mucinous | 24 | (16.7) | |
| Endometroid | 8 | (5.6) | |
| Clear cell | 2 | (1.4) | |
| Stage | |||
| I | 42 | (29.2) | |
| II | 16 | (11.1) | |
| III | 69 | (47.9) | |
| IV | 17 | (11.8) | |
| Grade | |||
| 1 | 37 | (25.7) | |
| 2 | 26 | (18.1) | |
| 3 | 81 | (56.3) | |
| Residual disease | |||
| 0 | 77 | (53.5) | |
| <1 | 16 | (11.1) | |
| >1 | 51 | (35.4) | |
| Chemotherapy | |||
| No | 44 | (30.6) | |
| Yes | 100 | (69.4) | |
| Carboplatin and paclitaxel | 82 | (56.9) | |
| Carboplatin alone | 18 | (18.0) | |
| Disease progression | |||
| No | 90 | (62.5) | |
| Recurrence | 49 | (34.0) | |
| Persistent disease | 5 | (3.5) | |
| Last follow up status | |||
| Alive | 76 | (52.8) | |
| Deceased | 68 | (47.2) | |
| Follow up period (years) | |||
| Median (range) | 3.4 (2.2-17.1) | ||
| Mean (SD) | 4.43 (3.90) | ||
Disease-free survival (DFS) and OS
Mean (95% CI) DFS was 82.3 months (67.8-96.8 months), while median (95% CI) DFS was 47.0 months (29.5-64.5 months). Mean (95% CI) OS was 96.2 months (81.3-111.2 months) and median (95% CI) DFS was 72.0 months (51.9-92.1 months). On the other hand, the 5-year DFS rate was 31.3% while the 5-year OS rates was 38.9%.
Prognostic factors for DFS and OS
Prognostic factors for DFS and OS are depicted in Table 2 and the most significant factors are depicted in Figure 1 for DFS and Figure 2 for OS. Kaplan-Meier survival analysis showed that older age (60 years and older), clear cell or serous type, advanced FIGO stage and the presence of residual disease were associated with a shorter DFS (log rank <0.05). Similarly, OS was significantly shorter among patients with older age, clear cell or serous type, advanced FIGO stage and presence of residual disease (log rank <0.05).
Table 2.
- Prognostic factors affecting overall survival and progression free survival among ovarian cancer patients (N=144).
| Parameters | n | PFS (months) | OS (months) | ||||
|---|---|---|---|---|---|---|---|
| % censored | Mean (95% CI) | Log rank | % censored | Mean (95% CI) | Log rank | ||
| Age (years) | |||||||
| 20-39 | 41 | 75.6 | 141.9 (115.6-168.2) | <0.001* | 80.5 | 152.6 (128.0-177.2) | <0.001* |
| 40-59 | 66 | 37.9 | 72.7 (53.3-92.2) | 50.0 | 89.9 (70.1-109.8) | ||
| 60-85 | 37 | 21.6 | 42.4 (24.1-60.7) | 27.0 | 50.2 (31.5-68.8) | ||
| BMI levels (kg/m2) | |||||||
| Underweight | 5 | 40.0 | 85.2 (8.0-162.4) | 0.942 | 40.0 | 89.0 (14.5-163.5) | 0.791 |
| Normal | 38 | 50.0 | 89.0 (61.9-116.1) | 63.2 | 106.8 (78.7-134.9 | ||
| Overweight | 49 | 44.9 | 67.6 (49.9-85.4) | 51.0 | 75.6 (58.3-92.9) | ||
| Obese | 52 | 40.4 | 74.9 (53.3-96.4) | 48.1 | 88.7 (66.7-110.7) | ||
| Parity | |||||||
| Nullipara | 38 | 52.6 | 76.5 (56.4-96.5) | 0.388 | 57.9 | 82.0 (63.0-101.1) | 0.126 |
| 1-3 | 55 | 49.1 | 88.0 (64.4-111.7) | 63.6 | 114.5 (89.0-140.0) | ||
| > 4 | 51 | 33.3 | 65.8 (45.3-86.2) | 37.3 | 73.8 (54.1-93.4) | ||
| Marital status | |||||||
| Single | 22 | 54.5 | 67.7 (44.7-90.7) | 0.567 | 59.1 | 79.7 (55.5-103.9) | 0.912 |
| Married | 111 | 44.1 | 82.9 (66.6-99.2) | 52.3 | 95.5 (78.8-112.2) | ||
| Divorced | 5 | 0.0 | 34.2 (19.2-49.2) | 40.0 | 91.8 (24.4-159.2) | ||
| Widow | 6 | 50.0 | 47.2 (9.4-85.0) | 50.0 | 48.2 (11.0-85.4) | ||
| Histopathology | |||||||
| Clear cell | 2 | 50.0 | 35.0 (0.0-72.4) | 0.004* | 100.0 | NC | 0.025* |
| Endometroid | 8 | 50.0 | 119.2 (68.1-170-2) | 62.5 | 126.1 (73.3-178.9) | ||
| Mucinous | 24 | 79.2 | 145.2 (109.7-180.7) | 79.2 | 145.3 (109.9-180.7) | ||
| serous | 110 | 36.4 | 64.7 (50.6-78.8) | 45.5 | 80.7 (65.5-95.9) | ||
| Type | |||||||
| Serous | 110 | 36.4 | 64.7 (50.6-78.8) | <0.001* | 45.5 | 80.7 (65.5-95.9) | 0.003* |
| Non-serous | 34 | 70.6 | 134.1 (105.6-162.6) | 76.5 | 145.2 (117.1-173.2) | ||
| Stage | |||||||
| I | 42 | 85.7 | 163.6 (143.9-183.3) | <0.001* | 88.1 | 169.7 (152.2-187.3) | <0.001* |
| II | 16 | 50.0 | 67.3 (46.6-88.0) | 62.5 | 81.1 (62.7-98.6) | ||
| III | 69 | 23.2 | 42.5 (28.1-56.9) | 34.8 | 54.4 (38.5-70.3) | ||
| IV | 17 | 23.5 | 36.6 (11.6-61.7) | 29.4 | 49.9 (24.3-75.5) | ||
| Grade | |||||||
| 1 | 37 | 83.8 | 141.8 (124.6-158.9) | <0.001* | 83.8 | 141.8 (124.8-158.9) | <0.001* |
| 2 | 26 | 57.7 | 110.6 (74.8-146.3) | 61.5 | 119.1 (84.7-153.4) | ||
| 3 | 81 | 22.2 | 43.6 (29.9-57.4) | 35.8 | 60.5 (44.6-76.4) | ||
| Residual disease (cm) | |||||||
| 0 | 77 | 74.0 | 137.2 (118.4-156.1) | <0.001* | 77.9 | 145.7 (127.1-164.3) | <0.001 |
| <1 | 16 | 12.5 | 23.7 (18.2-29.3) | 31.3 | 48.1 (36.1-60.0) | ||
| >1 | 51 | 9.8 | 21.2 (12.9-29.4) | 21.6 | 38.2 (26.5-50.0) | ||
| Chemotherapy | |||||||
| No | 44 | 77.3 | 147.3 (123.3-171.3) | <0.001* | 77.3 | 147.3 (123.3-171.3) | <0.001* |
| Carboplatin and taxane | 82 | 32.9 | 55.8 (40.5-71.1) | 46.3 | 69.4 (53.2-85.6) | ||
| Carboplatin | 15 | 20.0 | 58.3 (23.8-92.8) | 26.7 | 72.4 (34.5-110.2) | ||
| Recurrence | |||||||
| No | 90 | - | - | - | 71.1 | 128.8 (109.2-148.4) | <0.001* |
| Yes | 49 | - | - | 24.5 | 55.3 (40.7-69.9) | ||
| Persistent disease | 5 | - | - | 0.0 | 9.0 (3.9-14.1) | ||
Kaplan-Meier survival analysis. *Statistically significant result (log rank<0.05). NC: Statistics were not calculated because all observations were censored. DFS: disease-free survival, OS: overall survival, BMI: body mass index
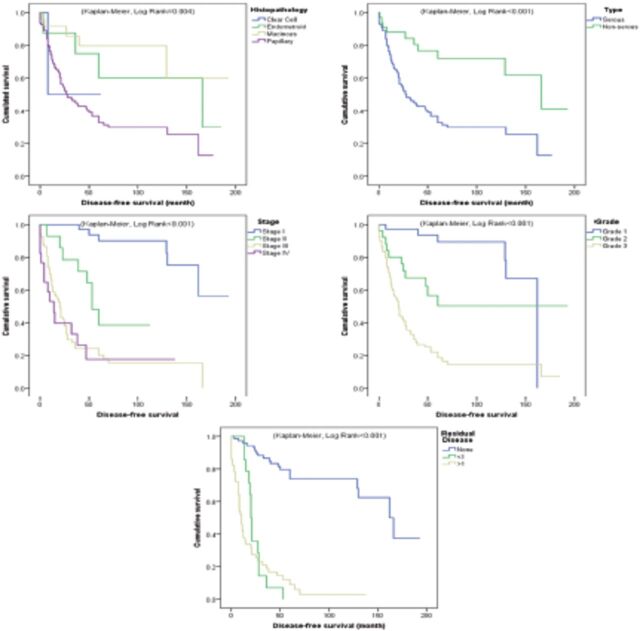
Figure 1.
- Progression free survival. Kaplan-Meier survival curves of the most significant prognostic factors for disease-free survival in epithelial ovarian cancer.
Figure 2.
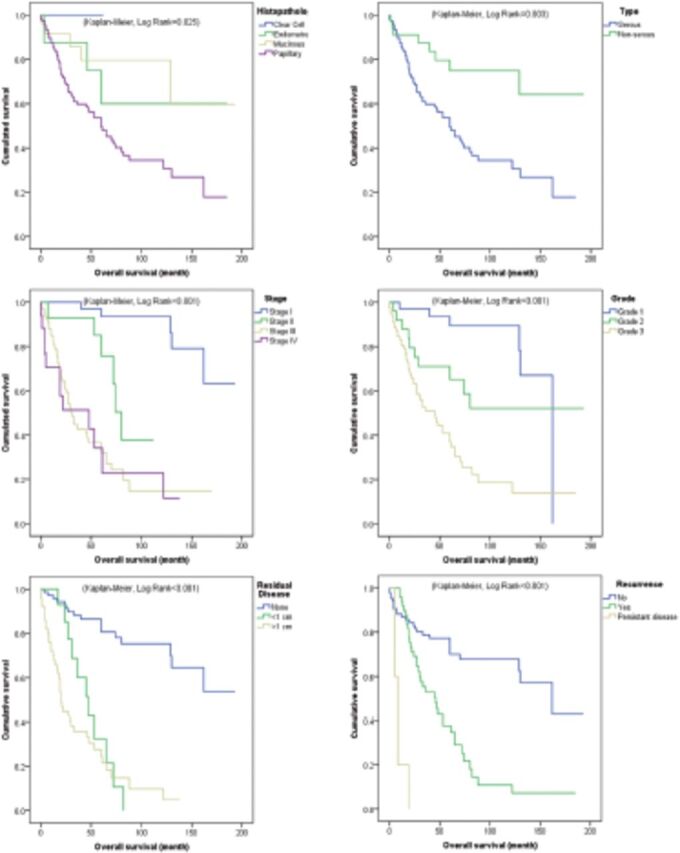
- Overall survival. Kaplan-Meier survival curves of the most significant prognostic factors for overall survival in epithelial ovarian cancer.
Independent factors of DSF and OS
Multivariate Cox regression models showed FIGO stage to be an independent factor of both DFS and OS. Regarding DFS, the adjusted HRs of recurrence or death for tumor stage II, III and IV as compared with tumor stage I were 4.43 (p=0.044), 13.08 (p=0.001), and 6.15 (p=0.024) respectively; while in OS the hazard ratio of death were 10.34 (p=0.005), 39.14 (p=0.001), and 15.14 (p=0.003) respectively. Residual disease >1 cm was independently associated with DFS (HR=4.78; p<0.001) but not with OS (HR=2.92; p=0.032, with reference category not significant [p=0.079]). Regarding disease progression, only persistent disease was independently associated with OS (HR=3.90; p=0.039), while recurrence was not a significant predictor of OS (HR=1.01; p=0.988); however, the reference category (no progression) was not significant at the assumed level of significance (p=0.068). Patient’s age and tumor histological subtype were not independently associated with DFS or OS (Table 3).
Discussion
This retrospective single-center study showed that women afflicted with epithelial ovarian cancer have an average DFS of ~6.9 years and OS of 8 years following primary staging or debulking surgery. Further, the 5-year DFS rates was ~31% and OS was 39%. The FIGO stage was found to be the most significant prognostic factor in these patients, with advanced stages being independently associated with high HR in both DFS (HR >4.4) and OS (HR >10.3), depending on the stage and with reference to FIGO stage I. Kaplan-Meier survival analysis showed that both PFS and OS were inversely associated with tumor stage; that is, mean PFS and OS decreased from 163 and 169.7 months in women with stage I disease to 23.5 and 49.9 months in those with stage IV disease, respectively (log rank <0.001) (Figures 1&2). The significance of tumor stage was further demonstrated in multivariate analysis, with hazard ratios in stage II (4.43 and 10.34), stage III (13.08 and 39.14), and stage IV (6.15 and 15.14), by reference to stage I for relapse and death respectively.
Other independent prognostic factors included residual disease >1 cm and carboplatin alone chemotherapy regimen, which were associated with ~4.8 and 3.2 HR in DFS, respectively; besides persistent disease, which was associated with 3.9 HR in OS and by considering a margin error of 0.1 for the reference category. On the other hand, older age, serous tumor including papillary and clear cell carcinoma type were associated with shorter DFS and OS in unadjusted analysis but did not show any significance in adjusted survival analysis.
Survival in epithelial ovarian cancer and the prognostic value of FIGO stage
Although epithelial ovarian cancer is characterized by poor prognosis and survival, findings from the present study showed relatively lower survival results compared to international data. An American study by Baldwin et al, 13 which included 40.690 patients, showed a 5-year and 10-year survival rates as high as 44% and 36% that increased to 53% and 42% in patients who had surgery as first treatment, respectively. Besides, being perceptively higher than the 39% survival rate found in the present study, these figures still denote poor prognosis of the disease. Indeed, despite advancements in surgical techniques and chemotherapy of epithelial ovarian cancer, no significant improvement in the long-term survival has been achieved in the last decades. Additionally, the rate of recurrence among survivors is very high, resulting in chronic cancer disease. 14 A recent report from Netherlands showed a 5-year survival fluctuating between 31% and 35% between 1989 and 2014, which dropped to ~16%-25% for FIGO stages IIB-IV. The other remarkable observation was the higher percentage of patients diagnosed at advanced stage (II or higher), representing the majority of cases, such as approximately 80%, and explaining the poor survival in this population of patients (15). These findings are closely comparable to the present study which showed as low as 39% survival rate at 5 years with a mean survival dropping from 8.3 years in stage I to 5.2 years in stage II; besides approximately 70% of the patients had FIGO stage II or higher at surgery. This is also consistent with the study by Baldwin et al, 13 showing that 80% of American women with epithelial ovary cancer are diagnosed and treated at stage II or higher, resulting in substantial decrease in the 5 year survival from 89% in stage I to 70% in stage II, 36% in stage III, and 17% in stage IV. 13 Comparably, a Turkish study by Ercelep et al 16 reported remarkably better survival figures, with a 5-year survival being approximately 80% among a cohort of 378 patients. However, with reference to the other studies, the Turkish cohort comprised a higher percentage (~38%) of patients who were diagnosed and treated at stage I, along with a high percentage (89%) having no or minimal residual disease after surgery, which may explain the high survival figures. 16 Altogether, these observations put forward the burden of delayed diagnosis on the prognosis of epithelial ovarian cancers and highlight the important of optimal cytoreductive surgery to improve survival.
Prognostic value of histological features of epithelial ovarian cancers
This study showed a set of other prognostic factors that were associated with poor survival. Of interest, residual disease >1cm was associated with approximately 5 hazard ratios in in DFS and 3 hazard ratios in OS, while other expected factors including older age, tumor grade, and histological subtype were only significant in unadjusted analysis.
Overall, tumor stage continues to be a strong predictor for survival and prognosis in epithelial ovarian cancers, with no change in this trend over the past 2 decades. Literature shows that advanced stages notably stage III and stage IV were independent predictors for clinical outcome and associated with poor survival. 17,18 However, the prognosis of advanced tumor stage may vary by histological subtype. A Japanese study on women with stage IV epithelial ovary cancers demonstrated worse OS among those with mucinous and clear-cell subtypes versus those with serous subtype. 19 Further, Oliver et al 20 analyzed data from 9,531 ovarian cancer patients with respect to the histology and cancer stage, and observed a statistically significant interaction between stage and histology for both PFS and OS. Stage I-II patients had significantly better PFS in clear cell carcinoma than in serous histopathology, while OS was numerically better but not significantly. In stage III and IV, patients with clear cell carcinomas were at significantly increased risk of disease progression or death than serous types. 20
On the other hand, literature shows inconsistent relationship of survival and prognosis with tumor histology and grade. 21-23 A previous study on a larger sample reported statistically significant association of survival with tumor histology in multivariate analysis, besides age and residual disease, while tumor grade showed significance only in univariate analysis. 10 Furthermore, authors of the latter study observed a better PFS and OS in patients with serous histology compared with mucinous and clear-cell carcinomas, and mucinous subtype was found to carry 2-fold risk of death compared to clear-cell. 10 This is discrepant from our findings showing mucinous subtype being associated with the best survival and DFS outcomes. In a Chinese study, ovarian cancer patients with early-stage disease and younger age at diagnosis were observed to have better OS in multivariable analysis. However, for advanced-stage patients, histology, platinum-based adjuvant chemotherapy, tumor stage, and age at diagnosis were significantly associated with OS. 18 In another study, the tumor grade, residual disease and age were found significant prognostic factors of survival in univariate analysis; whereas in multivariate analysis, tumor grade and stage showed to be the only independent predictors for survival. 17
Prognostic value of residual disease
Besides histological features, the most important factor for improving survival among ovarian, tubal and peritoneal cancer patients is the complete macroscopic resection, which is determined by minimal residual disease. 24-26 In line with our findings a large meta-analysis has shown that there is an increase of 5.5% in median survival time for each 10% increase in the amount of cytoreduction. Achieving complete cytoreduction until absence of residual disease significantly contributes in favourable survival. 27 Researchers further noted that optimal cytoreduction had carried a 3 and half fold decreased risk of death compared with the suboptimal cytoreduction surgery. 28,29
Chemotherapy and survival
In the present study, survival analysis showed that survival was poorer among patients who had chemotherapy, accounting for more than 60% reduction in mean DFS and 50% reduction in OS in unadjusted analysis. However, adjusted analysis showed no significance for chemotherapy, except for carboplatin regimen in DFS. This is plausibly explained by chemotherapy being selectively indicated for patients with advanced stage, which is confirmed by further analysis showing chemotherapy being used in 88%-100% of stage II-IV cases versus only 12% in stage I cases. This is supported by another study showing that taxane-based adjuvant chemotherapy was significant as a prognostic factor for OS in advanced-stage patients, besides other clinical and pathological factors. 18 Further, time frame between surgery and chemotherapy was not investigated in the present study, extraction. 30-34 Studies on experimental animal models have observed an increased metastatic activity after the surgical removal of primary tumor, it suggests that a short duration between surgery and the beginning of chemotherapy is beneficial for survival. 12,35
Other prognostic factors in epithelial ovarian cancer
The association between age at diagnosis and prognosis has been evaluated in various studies of ovarian cancer. It is usual observation that younger patients present more frequently with borderline or well differentiated invasive forms of epithelial ovarian cancer than older patients. 17,36 Although such observation seems to be explained by life expectancy in the general population, in this study PFS and OS was significantly shorter among patients with age 60 years old and older. The effect of age is often combined with that of other associated pathological factors. For example, a study conducted in stage III ovarian cancer patients found that younger patients had better survival than the older patients. 10 Another study showed that patients >65 years, with serous ovarian cancer were more typically characterized by higher tumor grade and poor survival, as compared with younger patients. 37
We have not found any association between survival and BMI levels of ovarian cancer patients. Previous investigations also reported that there is no association between BMI levels and OS or disease-specific survival in ovarian cancer. However, a strong effect was evident when BMI levels were viewed according to stage. 38 In our study, marital status of patients was also not a significant predictor of survival in univariate or multivariate analysis. Contradictory results have been reported about the marital status and survival among ovarian cancer patients. Wang et al 39 have observed similar risk of mortality for married and unmarried women. 39 while other data suggested that unmarried women were at significantly higher risk of metastatic cancer and death compared to the married patients. 40
Another prognostic factor in epithelial ovarian cancer the the present study did not investigate is lymphadenectomy. Lymphadenectomy was reported to significantly improve the PFS by 72% and OS by 61%, notably after the dissection of both pelvic and paraaortic lymph nodes. 16
Study limitations
The 2 major limitations for the present study include the retrospective design and small sample size. The former resulted in some unobserved variables while the later resulted in high percentage of censoring in the survival analysis reducing the power of analysis. Small sample size had led to poor subgroup analysis; notably in comparing the prognostic values of different histological subtypes and chemotherapy regimens, and stratifying the respective effects of these 2 factors by tumor stage.
In conclusion, epithelial ovarian cancers are characterized by poor prognosis including reduced survival and DFS, with majority patients not achieving 5-year survival. Although, these local figures are in concordance with international data, further efforts should be made to enhance early detection as the disease stage at surgery was of major prognostic value in patients’ survival. By considering residual disease as one of the most critical modifiable prognostic factors, we emphasize the importance of utilizing state-of-the-art surgical techniques and enhancing surgeons’ performance to achieve optimal cytoreduction and improve the chances for long term survival of patients. Further local studies with longer follow up are warranted to provide sufficient meta-data for adequate analysis of the long-term survival and the relevant prognostic factors, thereby enabling the establishment of a national strategy in the diagnosis and management of epithelial ovarian cancers.
Acknowledgment
The authors gratefully acknowledge WAX Clinical Research Skills Limited for English language editing.
Footnotes
References
- 1. Siegel R, Ma J, Zou Z, Jemal A.. Cancer statistics, 2014. CA Cancer J Clin 2014;64:9–29. [DOI] [PubMed] [Google Scholar]
- 2. Arbyn M, Weiderpass E, Bruni L, de Sanjosé S, Saraiya M, Ferlay J, et al. Estimates of incidence and mortality of cervical cancer in 2018: a worldwide analysis. Lancet Glob Health 2020; 8: e191–e203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Torre LA, Trabert B, DeSantis CE, Miller KD, Samimi G, Runowicz CD, et al. Ovarian cancer statistics, 2018. CA Cancer J Clin 2018; 68: 284–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A.. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018; 68: 394–424. [DOI] [PubMed] [Google Scholar]
- 5. Kurman RJ, Shih IM.. The origin and pathogenesis of epithelial ovarian cancer: a proposed unifying theory. Am J Surg Pathol 2010; 34: 433–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lheureux S, Braunstein M, Oza AM.. Epithelial ovarian cancer: Evolution of management in the era of precision medicine. CA Cancer J Clin 2019; 69: 280–304. [DOI] [PubMed] [Google Scholar]
- 7. Bankhead CR, Kehoe ST, Austoker J.. Symptoms associated with diagnosis of ovarian cancer: a systematic review. BJOG 2005; 112: 857–865. [DOI] [PubMed] [Google Scholar]
- 8. Goff BA, Mandel LS, Melancon CH, Muntz HG.. Frequency of symptoms of ovarian cancer in women presenting to primary care clinics. JAMA 2004; 291: 2705–2712. [DOI] [PubMed] [Google Scholar]
- 9. Ozols RF. Systemic therapy for ovarian cancer: current status and new treatments. Semin Oncol 2006; 33: S3–S11. [DOI] [PubMed] [Google Scholar]
- 10. Winter WE III, Maxwell GL, Tian C, Carlson JW, Ozols RF, Rose PG, et al. Prognostic factors for stage III epithelial ovarian cancer: a Gynecologic Oncology Group Study. J Clin Oncol 2007; 25: 3621–3627. [DOI] [PubMed] [Google Scholar]
- 11. Wong KH, Mang OWK, Au KH, Law SCK.. Incidence, mortality, and survival trends of ovarian cancer in Hong Kong, 1997 to 2006: a population-based study. Hong Kong Med J 2012; 18: 466–474. [PubMed] [Google Scholar]
- 12. Tewari KS, Java JJ, Eskander RN, Monk BJ, Burger RA.. Early initiation of chemotherapy following complete resection of advanced ovarian cancer associated with improved survival: NRG Oncology/Gynecologic Oncology Group study. Annal Oncol 2016; 27: 114–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Baldwin LA, Huang B, Miller RW, Tucker T, Goodrich ST, Podzielinski I, et al. Ten-year relative survival for epithelial ovarian cancer. Obstet Gynecol 2012; 120: 612–618. [DOI] [PubMed] [Google Scholar]
- 14. Markman M. Optimal management of recurrent ovarian cancer. Int J Gynecol Cancer 2009; 19: S40–S43. [DOI] [PubMed] [Google Scholar]
- 15. Timmermans M, Sonke GS, van de Vijver KK, van der Aa MA, Kruitwagen RFPM.. No improvement in long-term survival for epithelial ovarian cancer patients: A population-based study between 1989 and 2014 in the Netherlands. Eur J Cancer 2018; 88: 31–37. [DOI] [PubMed] [Google Scholar]
- 16. Ercelep O, Ozcelik M, Gumus M.. Association of lymphadenectomy and survival in epithelial ovarian cancer. Curr Probl Cancer 2019; 43: 151–159. [DOI] [PubMed] [Google Scholar]
- 17. Duska LR, Chang Y, Flynn CE, Chen AH, Goodman A, Fuller AF, et al. Epithelial ovarian carcinoma in the reproductive age group. Cancer 1999; 85: 2623–2629. [DOI] [PubMed] [Google Scholar]
- 18. Chang LC, Huang CF, Lai MS, Shen LJ, Wu FLL, Cheng WF.. Prognostic factors in epithelial ovarian cancer: a population-based study. PLoS One 2018; 13: e0194993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Akahira J-I, Yoshikawa H, Shimizu Y, Tsunematsu R, Hirakawa T, Kuramoto H, et al. Prognostic factors of stage IV epithelial ovarian cancer: a multicenter retrospective study. Gynecol Oncol 2001; 81: 398–403. [DOI] [PubMed] [Google Scholar]
- 20. Oliver KE, Brady WE, Birrer M, Gershenson DM, Fleming G, Copeland LJ, et al. An evaluation of progression free survival and overall survival of ovarian cancer patients with clear cell carcinoma versus serous carcinoma treated with platinum therapy: an NRG Oncology/Gynecologic Oncology Group experience. Gynecol Oncol 2017; 147: 243–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gates MA, Rosner BA, Hecht JL, Tworoger SS.. Risk factors for epithelial ovarian cancer by histologic subtype. Am J Epidemiol 2010; 171: 45–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Melin A, Lundholm C, Malki N, Swahn M, Sparen P, Bergqvist A.. Endometriosis as a prognostic factor for cancer survival. Int J Cancer 2011; 129: 948–955. [DOI] [PubMed] [Google Scholar]
- 23. Karpathiou G, Chauleur C, Corsini T, Venet M, Habougit C, Honeyman F, et al. Seromucinous ovarian tumor A comparison with the rest of ovarian epithelial tumors. Ann Diagn Pathol 2017; 27: 28–33. [DOI] [PubMed] [Google Scholar]
- 24. Chi DS, Liao JB, Leon LF, Venkatraman ES, Hensley ML, Bhaskaran D, et al. Identification of prognostic factors in advanced epithelial ovarian carcinoma. Gynecol Oncol 2001; 82: 532–537. [DOI] [PubMed] [Google Scholar]
- 25. Wimberger P, Lehmann N, Kimmig R, Burges A, Meier W, du Bois A.. Prognostic factors for complete debulking in advanced ovarian cancer and its impact on survival. An exploratory analysis of a prospectively randomized phase III study of the Arbeitsgemeinschaft Gynaekologische Onkologie Ovarian Cancer Study Group (AGO-OVA. Gynecol Oncol 2007; 106: 69–74. [DOI] [PubMed] [Google Scholar]
- 26. Chang SJ, Bristow RE, Ryu HS.. Impact of complete cytoreduction leaving no gross residual disease associated with radical cytoreductive surgical procedures on survival in advanced ovarian cancer. Ann Surg Oncol 2012; 19: 4059–4067. [DOI] [PubMed] [Google Scholar]
- 27. Chang SJ, Hodeib M, Chang J, Bristow RE.. Survival impact of complete cytoreduction to no gross residual disease for advanced-stage ovarian cancer: a meta-analysis. Gynecol Oncol 2013; 130: 493–498. [DOI] [PubMed] [Google Scholar]
- 28. Rajkumar S, Nath R, Lane G, Mehra G, Begum S, Sayasneh A.. Advanced stage (IIIC/IV) endometrial cancer: role of cytoreduction and determinants of survival. Eur J Obstet Gynecol Reprod Biol 2019; 234: 26–31. [DOI] [PubMed] [Google Scholar]
- 29. Tate S, Kato K, Nishikimi K, Matsuoka A, Shozu M.. Survival and safety associated with aggressive surgery for stage III/IV epithelial ovarian cancer: a single institution observation study. Gynecol Oncol 2017; 147: 73–80. [DOI] [PubMed] [Google Scholar]
- 30. Aletti GD, Long HJ, Podratz KC, Cliby WA.. Is time to chemotherapy a determinant of prognosis in advanced-stage ovarian cancer? Gynecol Oncol 2007; 104: 212–216. [DOI] [PubMed] [Google Scholar]
- 31. Gadducci A, Cosio S, Zizioli V, Notaro S, Tana R, Panattoni A, et al. Patterns of recurrence and clinical outcome of patients with stage IIIC to stage IV epithelial ovarian cancer in complete response after primary debulking surgery plus chemotherapy or neoadjuvant chemotherapy followed by interval debulking surgery: An Italian multicenter retrospective study. Int J Gynecol Cancer 2017; 27: 28–36. [DOI] [PubMed] [Google Scholar]
- 32. Larsen E, Blaakaer J.. Epithelial ovarian cancer: does the time interval between primary surgery and postoperative chemotherapy have any prognostic importance? Acta Obstet Gynecol Scand 2009; 88: 373–377. [DOI] [PubMed] [Google Scholar]
- 33. Xu F, Rimm AA, Fu P, Krishnamurthi SS, Cooper GS.. The impact of delayed chemotherapy on its completion and survival outcomes in stage II colon cancer patients. PLoS ONE 2014; 9: e107993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Nachiappan S, Askari A, Mamidanna R, Munasinghe A, Currie A, Stebbing J, et al. The impact of adjuvant chemotherapy timing on overall survival following colorectal cancer resection. Eur J Surg Oncol 2015; 41: 1636–1644. [DOI] [PubMed] [Google Scholar]
- 35. Kim YW, Choi EH, Kim BR, Ko WA, Do YM, Kim IY.. The impact of delayed commencement of adjuvant chemotherapy (eight or more weeks) on survival in stage II and III colon cancer: a national population-based cohort study. Oncotarget. 2017; 8: 80061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Tsai YT, Lozanski G, Lehman A, Sass EJ, Hertlein E, Salunke SB, et al. BRAFV600E induces ABCB1/P-glycoprotein expression and drug resistance in B-cells via AP-1 activation. Leuk Res 2015; 39: 1270–1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Deng F, Xu X, Lv M, Ren B, Wang Y, Guo W, et al. Age is associated with prognosis in serous ovarian carcinoma. J Ovarian Res 2017; 10: 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Bandera EV, Lee VS, Qin B, Rodriguez-Rodriguez L, Powell CB, Kushi LH.. Impact of body mass index on ovarian cancer survival varies by stage. Br J cancer 2017; 117: 282–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Wang X, Li X, Su S, Liu M.. Marital status and survival in epithelial ovarian cancer patients: a SEER-based study. Oncotarget 2017; 8: 89040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Mahdi H, Kumar S, Munkarah AR, Abdalamir M, Doherty M, Swensen R.. Prognostic impact of marital status on survival of women with epithelial ovarian cancer. Psychooncology 2013; 22: 83–88. [DOI] [PubMed] [Google Scholar]