Abstract
Background and Objectives
Sporadic late-onset nemaline myopathy (SLONM) is a treatable or otherwise fatal myopathy. Diagnosis of SLONM is still challenging, and no therapeutic consensus has been achieved. Here, we reported the clinicopathologic features and long-term follow-up data of SLONM in a Chinese cohort.
Methods
We performed a retrospective evaluation of clinical, pathologic, and treatment outcomes of 17 patients with SLONM diagnosed between March 1986 and April 2021 at our neuromuscular center. Immunohistochemistry (IHC) with antibodies against 5 Z-disc–associated proteins was performed in the muscle biopsies of SLONM to identify a potential pathologic marker in aid of diagnosis. In comparison, we also performed muscle IHC in patients with selective type II fiber atrophy (n = 22), neurogenic atrophy (n = 22), mitochondrial myopathy (n = 5), immune-mediated necrotizing myopathy (n = 5), and normal controls (n = 5).
Results
Most of the patients exhibited asymmetric limb muscles weakness (71%, 12/17) and neck extensor weakness (53%, 9/17). Immunofixation electrophoresis was performed in 11 patients, and 4 of them were identified with monoclonal gammopathy of undetermined significance (MGUS). EMG from 16 patients demonstrated a myopathic pattern with spontaneous activities in 69% (11/16) of them. Muscle MRI showed preferential involvement of paraspinal, gluteus minimus and medius, semimembranosus, and soleus muscles. Suspected nemaline bodies on modified Gomori trichrome were confirmed by IHC using anti–α-actinin antibody (100%, 17/17), anti-myotilin antibody (94%, 16/17), anti-desmin antibody (94%, 16/17), anti–α-B crystallin antibody (65%, 11/17), and anti-telethonin antibody (18%, 3/17) with various positive rates. Notably, anti–α-actinin IHC showed the highest percentage of strongly positive staining (77%, 13/17), being the only one without negative results. Moderate improvement following autologous stem cell transplantation (ASCT) was noted in 3/4 patients with MGUS; favorable outcomes were also achieved in 6/7 patients without MGUS, including 3 patients with complete recovery who were given a combined treatment of prednisone and another immunosuppressant.
Discussion
SLONM is a treatable myopathy with ASCT or traditional immunotherapy, especially when combined with steroids and immunosuppressants. Anti–α-actinin immunostaining is the most reliable pathologic marker to identify rod-bearing fibers, and it should be performed routinely in adult patients with undiagnosed nonnecrotic myopathies.
Sporadic late-onset nemaline myopathy (SLONM) is a rare, acquired disease presenting with an aggressive disease course in adulthood. It was first described in 1966.1 The association of this condition with a monoclonal gammopathy of undetermined significance (MGUS)2 and HIV infection3 was noticed in 1975 and 1987, respectively. After reports of favorable responses to plasma exchanges in patients with SLONM-MGUS4 and to corticosteroid in patients with SLONM-HIV,5 an autoimmune mechanism for this disease has been recognized and discussed. The hematologic therapy in the form of autologous stem cell transplantation (ASCT) or chemotherapy seems to be preferred for SLONM-MGUS.6,7 However, therapeutic experience in non-MGUS SLONM was limited, and the outcomes with traditional immunotherapy varied among different reports.8-10
Histopathologically, nemaline bodies tend to present as aggregates of reddish-purple granules,8,11 typically on modified Gomori trichrome (MGT) staining observed under light microscope with a high magnification. However, it is still challenging to identify the atypical nemaline rods when these structures are very tiny and ambiguous on MGT staining. On hematoxylin and eosin (HE) staining, the rod-bearing fibers may be coarsely basophilic and mistaken for regenerating fibers, which may lead to an underdiagnosis of SLONM.8,10 The nemaline rods originate from the muscle Z-disc, which is composed of several proteins such as α-actinin, myotilin, desmin, telethonin, and α-B crystallin.12 Immunostaining with myotilin or α-actinin has been used to show the nemaline bodies in some previously reported cases.8 However, systematic evaluation of the diagnostic value of these Z-disc-related proteins in muscle specimens from patients with SLONM is lacking.
We analyzed the detailed clinicopathologic characteristics and long-term treatment outcomes of 17 Chinese patients with SLONM. Meanwhile, we further evaluated the validity of IHC with anti–α-actinin as a pathologic marker in the diagnosis of SLONM and its role in distinguishing SLONM from other mimicking conditions.
Methods
Patients
This is a retrospective observational study. This study included 17 patients with clinically and histologically diagnosed SLONM at our neuromuscular center (NMD) from March 1986 to April 2021. For comparison, we also examined the following control groups of patients with pronounced muscle atrophy: 22 cases with selective type II fiber atrophy, 22 cases of neurogenic atrophy, 5 cases of a mitochondrial myopathy with m. 3243A>G variation, 5 cases with immune-mediated necrotizing myopathy (IMNM), and 5 cases without pathologic findings (normal controls).
In regard to the clinical assessment, muscle strength was evaluated by the ordinal 6-point (0–5) manual muscle testing (MMT) scale; asymmetric muscle weakness was defined as no less than 1 grade measured by MMT between 2 sides of the same muscle group. Functional ability was assessed by the Health Assessment Questionnaire Disability Index (HAQ-DI).13 The treatment response was graded as follows: no improvement, mild improvement (1 grade improvement in 1–2 muscle groups, persistently requiring assistance in daily activities), moderate improvement (>1 grade in multiple muscle groups, occasionally requiring assistance in daily activities), marked improvement (only mild weakness without functional impairment), and complete recovery (no symptoms or signs of muscle weakness); a favorable outcome was defined as marked improvement or complete recovery.14 Relapse was defined as an increase of more than 30% in HAQ-DI compared with the best after improvement,13 as used in assessing myositis. Hematologic response was assessed according to the International Myeloma Working Group criteria.15
Laboratory and Instrumental Examinations
All patients underwent blood cell count, creatine kinase (CK), and anti-HIV antibody tests. EMG data were obtained in 16 patients. Immunofixation electrophoresis was tested in 11 patients, and 3 of them had bone marrow biopsies. We also examined a complete panel of myositis specific antibodies (MSAs, Immunodot-Blot; MyBiotech, Überherrn, Germany) including anti–aminoacyl-tRNA synthetase, anti–Mi-2, anti–signal recognition particle, anti–3-hydroxy-3-methylglutaryl CoA reductase, anti–melanoma differentiation-associated protein 5, anti–transcription intermediary factor 1γ, anti–nuclear matrix protein 2, anti–small ubiquitin-like modifier activating enzyme, and anti–cytosolic 5′-nucleotidase 1A (cN1A) in the above 11 patients. Echocardiography and pulmonary function test (PFT) were performed in 11 patients, whereas ECG was performed in all 17 patients. MRI of bilateral lower limbs was performed in 11 patients, 3 of whom also had MRI of the paraspinal muscles.
Histopathologic Examinations
Open-muscle biopsies had been performed for the diagnostic purposes in all patients (Table 1, P1-P17, including P16 with a second biopsy due to an inconclusive result of the first biopsy). Serial frozen sections from the muscle specimens were stained with the following routine histochemical and immunohistochemical (IHC) stainings: HE, MGT, nicotinamide adenine dinucleotide tetrazolium reductase, adenosine triphosphatase (ATPase) at pH 4.3 and 10.8, Congo red, anti-CD3 antibody (clone LN10; Zhongshan Golden Bridge Biotechnology, Beijing, China), anti–membrane attack complex (MAC) antibody (clone aE11; Dako, Santa Clara, CA), anti–Class I major histocompatibility complex (MHC-I) antibody (clone EP1395Y; Abcam, Cambridge, United Kingdom), anti–neural cell adhesion molecule 1 (NCAM1) antibody (polyclonal; Proteintech, Rosemont, IL), anti–α-actinin antibody (clone EA-53; MilliporeSigma, Burlington, MA), anti-desmin antibody (clone Y66; Abcam), anti-myotilin antibody (Polyclonal; Proteintech), anti–α-B crystallin antibody (clone 1B6.1-3G4; Abcam), and anti-telethonin antibody (clone G-11; Santa Cruz Biotechnology, Dallas, TX). IHC of kappa light chain (clone CH15; Zhongshan Golden Bridge Biotechnology) and lambda light chain (clone SHL53, Zhongshan Golden Bridge Biotechnology) was examined in 4 patients with MGUS. Electron microscopy (EM) was performed in 7 cases.
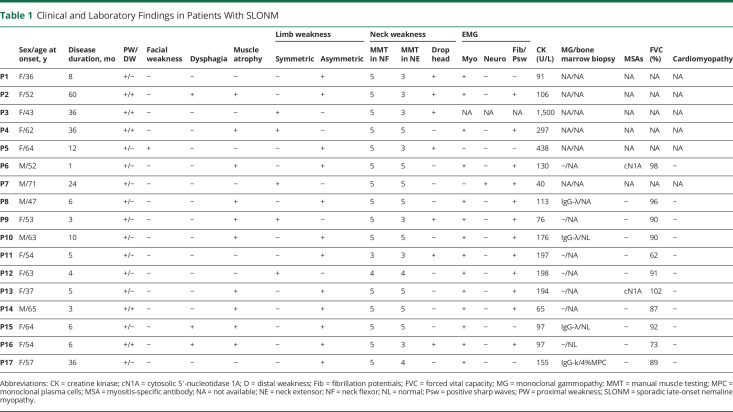
Table 1.
Clinical and Laboratory Findings in Patients With SLONM
The quantitative evaluations were performed manually in 10 randomly different fields at 40× magnification. CD3+ lymphocytic infiltrates were classified as scattered or focal: scattered infiltrates were defined as more than 15 cells scattered in the endomysium, perivascular, and/or perimysial region per field; focal infiltrates referred to more than 15 cells clustered in the above regions. Myofiber expression of MHC-I was defined as sarcolemmal staining associated or not with sarcoplasmic staining.16 The staining intensity of α-actinin, desmin, myotilin, α-B crystallin, and telethonin was assessed semiquantitatively as follows: 0, negative; 1, positive but without granular deposition; and 2, strongly positive with granular deposition in the involved fibers. All specimens were assessed by 2 neuromuscular pathologists. We used the STROBE cohort reporting guidelines to check.
Statistical Analysis
Qualitative variables were reported as percentages and absolute frequencies. Quantitative data were expressed as median (interquartile). The Friedman rank-sum test was used for a comparison of the 5 Z-disc–related proteins. The χ2 test and Kruskal-Wallis test were performed to compare the qualitative and quantitative variables between SLONM and other groups, respectively. All analyses were performed using SPSS 26 (IBM Corp., Armonk, NY) and Microsoft Excel for MAC version 16.49. p < 0.05 was considered statistically significant.
Standard Protocol Approvals, Registrations, and Patient Consents
This study was approved by the Ethics Committee of Qilu Hospital, Shandong University, China (KYLL-202011-135). Written informed consent for tests and publication was obtained from all the participants in the present study.
Data Availability
Individual participant data will not be made publicly available because of potential confidentiality concerns related to the rarity of the condition and the small study population. Further information can be obtained by qualified researchers from the corresponding author on reasonable request.
Results
Clinical and Laboratory Findings
The baseline demographic and clinical characteristics of the subjects in this study were displayed in eTable 1 (links.lww.com/NXI/A718). A total of 17 patients with clinically and pathologically diagnosed SLONM were selected from the database of 4,650 muscle biopsy specimens at our NMD center. The detailed clinical and laboratory data of the 17 patients with SLONM were summarized in Table 1. Our cohort consisted of 12 women and 5 men. The median age at onset was 54 years, with a range of 36–71 years. The median time from the onset to final diagnosis was 6 months, with a range of 3–60 months, suggestive of a definite delay in the diagnosis. Seventy-one percent (12/17) of them exhibited asymmetric proximal muscle weakness and 59% (10/17) of them presented with muscle atrophy. Five patients also presented with mild distal muscle weakness. Axial weakness was also prominent in our cohort, as 9 of them (53%) exhibited weakness in the neck extensor, and 7 of them (41%) showed a head drop sign at their first evaluation. Facial weakness was found in only 1 patient, and mild dysphagia was noticed in 3 patients. None of the patients showed remarkable abnormality in ECG, echocardiogram, or PFT.
CK levels were normal or mildly elevated (<2× the upper limit) in all except P3, whose CK was 1,500 U/L. A myopathic pattern with spontaneous activities on EMG was demonstrated in 69% (11/16) of them. Immunofixation electrophoresis disclosed a monoclonal gammopathy in 4/11 patients (3 immunoglobulin [Ig] G-λ and 1 IgG-k). Bone marrow biopsy revealed monoclonal plasma cell infiltration (4% of the total cellular count) in 1 patient (P17). We screened a complete MSAs profile in 11/17 patients: anti-cN1A antibodies were detected in 2 patients, and no other MSA was found in our cohort. Anti-HIV antibody testing was negative in all cases.
Muscle MRI
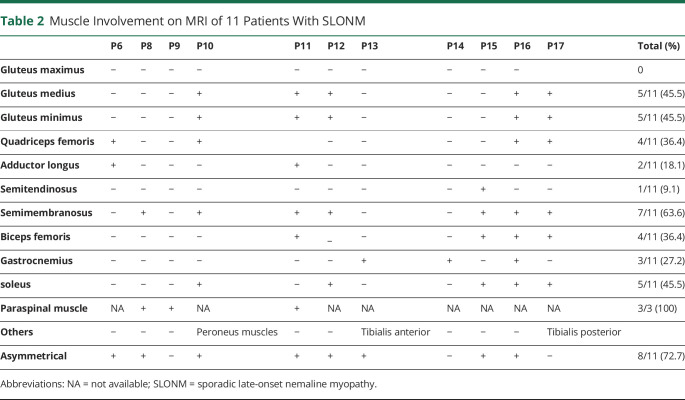
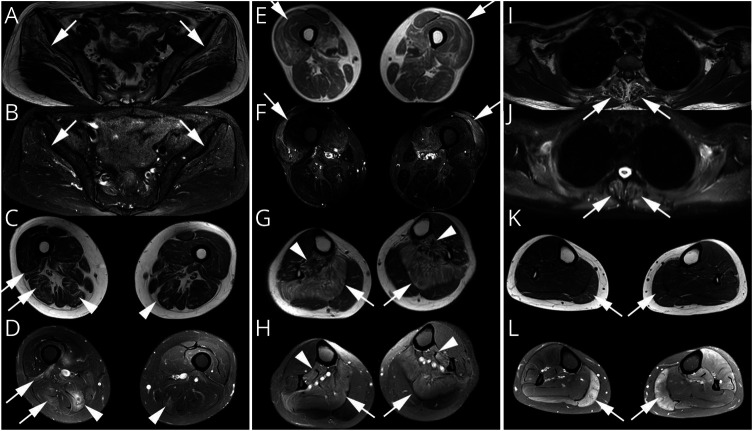
Table 2 summaries the results of MRI of bilateral lower limbs and paraspinal muscles from 11 and 3 patients, respectively. Typical images were shown in Figure 1. The asymmetry of muscle involvement is a main feature found in 73% of our patients. In the pelvis, the most frequently involved muscles were gluteus minimus and medius, with gluteus maximus relatively spared (46%, 5/11; Figure 1, A and B). In the thigh, semimembranosus was usually involved with relative sparing of semitendinosus (55%, 6/11; Figure 1, C and D). Quadriceps femoris, the most frequently involved anterior muscle group in lower limbs, was involved in 36% (4/11) of the patients (Figure 1, E and F). In the calf, soleus was the most strikingly involved (46%, 5/11; Figure 1, G and H). Axial muscle MRI was performed in 3 patients, in whom 2 exhibited head drop sign and 1 had normal neck strength. Apparent paraspinal muscle involvement was seen on MRI in all of them (Figure 1, I and J). Tibialis anterior and peroneus muscles involvement were observed in 1 patient. Generally, the affected muscles exhibited hyperintensity on both T1-weighted images and T2-weighted short-tau inversion recovery (T2W-STIR) sequences, whereas gastrocnemius involvement was detected only on T2W-STIR in P13 (Figure 1, K and L).
Table 2.
Muscle Involvement on MRI of 11 Patients With SLONM
Figure 1. Representative Muscle MRI on T1WI and STIR.
(A and B) Selective involvement in the gluteus medius and minimus with maximus spared in P10. (C and D) Bilateral semimembranosus muscle (arrowhead) and the right biceps femoris (arrow) asymmetrically involved in P16. (E and F) Quadriceps femoris involvement in P6. (G and H) Soleus (arrow) and tibialis posterior (arrowhead) distinctively affected in P17. (I and J) Paraspinal muscle atrophy in P8. (K and L) Gastrocnemius involvement more visible on STIR in P13. T1WI = T1-weighted images; STIR = short-tau inversion recovery.
Routine Muscle Pathologies
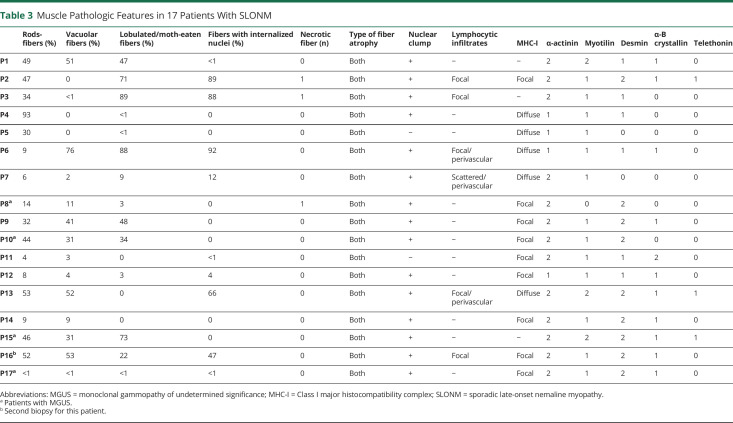
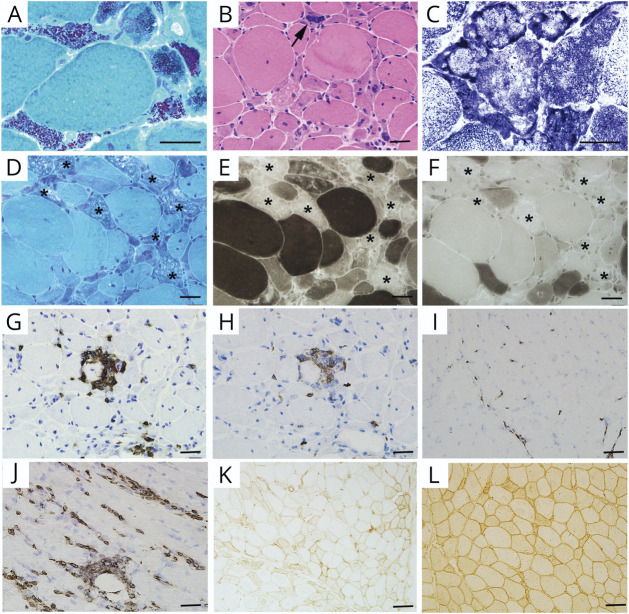
The histopathologic features of 17 patients are summarized in Table 3. The percentage of rod-bearing fibers varied greatly from patient to patient (1%–95%), irrespective of the severity of muscle weakness. Rods were almost exclusively accumulated in the atrophic type I or II fibers. The rod-containing fibers frequently showed multiple small vacuoles, basophilic cytoplasm, and disorganized fibrillar network (Figure 2, A and B). Lobulated or moth-eaten fibers were presented in 88% of the specimens (Figure 2C). Pyknotic and internalized nuclei were observed in 94% and 65% of the specimens, respectively. In addition, the rod-bearing fibers demonstrated the loss of myosin ATPase enzyme activity at pH levels of both 4.3 and 10.4 in 71% (12/17) of the muscles (Figure 2, D–F).
Table 3.
Muscle Pathologic Features in 17 Patients With SLONM
Figure 2. Routine Muscle Pathology.
(A) MGT staining showing typical reddish-purple granular rods accumulated in the atrophic fibers (P15). (B) HE staining demonstrating the angulated fibers with basophilic sarcoplasm, and pyknotic nuclei (arrow; P9). (C) Disorganized intermyofibrils presenting as lobulated or moth-eaten fibers (P15). (D–F) Atrophy of both type I and II fibers, and some rod-bearing fibers exhibiting loss of ATPase reactivity at both pH 10.4 (E) and 4.3 (F, asterisk; P8). (G and H) Focal endomysial CD3+ cells (G) and CD8+ cell (H) infiltrates in P13. (I and J) Scattered CD3+ cell infiltrates in P7 (I) and perivascular CD3+ cell infiltrates in P6 (J). (K and L) Focal MHC-I expression in P12 (K) and diffuse MHC-I expression in P7 (L). Scale bars: A–F, 10 μm; G–L, 50 μm. ATPase = adenosine triphosphatase; HE = hematoxylin and eosin; MGT = modified Gomori trichrome; MHC-I = Class I major histocompatibility complex.
Lymphocytic infiltrates were seen in 35% (6/17) of the specimens. The infiltrates were scattered in 1 specimen and focal in the other 5 specimens (Figure 2, G–I). Perivascular lymphocytic infiltration was found in 3 specimens (Figure 2J). Sarcolemmal MHC-I expression was commonly seen in 76% (13/17) of the cases: focal in 8 specimens and diffuse in the other 5 specimens (Figure 2, K and L). None of our patients demonstrated MAC deposition on sarcolemma of the myofibers or endomysial capillaries. There was no evidence of kappa, lambda light chain, or amyloid deposition in the 4 patients with SLONM-MGUS (data not shown).
Ultrastructural and Immunopathologic Features of the Rods
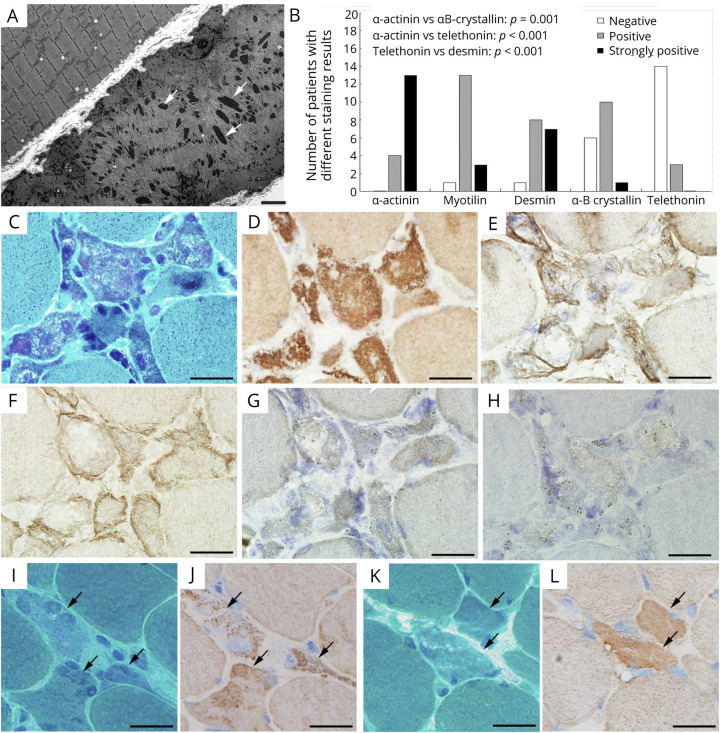
In 7 patients, EM revealed intrasarcoplasmic rods in 5 cases (Figure 3A) and intranuclear rods in 1 case (P2). Some other ultrastructural features included clustered mitochondria, smeared Z-line materials, and disoriented thin filaments (data not shown).
Figure 3. Ultrastructure and Immunopathologic Features of the Rods.
(A) Typical electron-dense rods accumulating in the atrophic fiber (P15, arrows). (B) Comparison of the diagnostic value among 5 common Z-disc–associated proteins in SLONM. IHC intensity scores significantly (p < 0.05) higher in anti–α-actinin, anti-desmin, and anti-myotilin staining than those in anti–α-B crystallin and anti-telethonin staining. Anti–α-actinin showing the highest percentage of strongly positive results and being the only staining without negative results. (C–H) Serial frozen sections of P10: rod-bearing fibers on MGT (C), strongly positive for α-actinin (D), faintly positive for desmin (E) and myotilin (F), and negative for telethonin (G) and α-B crystallin (H). (I and J) Serial frozen sections of muscle from P11: atrophic fibers containing obscure rod-like structures on MGT (I), with score 2 on anti–α-actinin staining (J). (K and L) Serial frozen sections of muscle from P12: atrophic fibers containing obscure sand-like structures and small vacuoles on MGT (K), with score 1 on anti–α-actinin staining (L). Scale bars: A, 2 μm; C–L, 10 μm. IHC = immunohistochemistry; MGT = modified Gomori trichrome; SLONM = sporadic late-onset nemaline myopathy
To further characterize the molecular features and evaluate their diagnostic value in SLOMN, we next performed IHC staining of 5 antibodies against Z-disc–associated proteins. Our analysis revealed that the IHC intensity scores were significantly (p < 0.05) higher in anti–α-actinin, anti-desmin, and anti-myotilin staining than those in anti–α-B crystallin and anti-telethonin staining (Figure 3B). In particular, the percentage of strongly positive specimens was significantly higher with anti–α-actinin staining (77%, 13/17) than those with anti-myotilin (18%, 3/17) and anti-desmin (41%, 7/17). Representative microphotographs were shown in Figure 3, C–H. Especially, in P11 and P12, the rod-bearing fibers were easily identified on anti–α-actinin immunostaining, whereas they were suspected but uncertain on MGT staining (Figure 3, I–L). No sarcoplasmic α-actinin accumulation was found in specimens with neurogenic atrophy, type II fiber atrophy, IMNM, mitochondrial myopathy, or normal controls, except the target fibers in 5 specimens with neurogenic atrophy showing limited positivity in the concentric zone (eFigures 1 and 2, links.lww.com/NXI/A718), which may be explained by the focal accumulation of Z-line materials on EM. Of interest, some basophilic atrophic fibers in SLONM were also positive for NCAM staining, in keeping with the regenerative fibers in IMNM, but definitely negative for α-actinin staining (eFigure 2, A–F).
Treatments and Follow-up
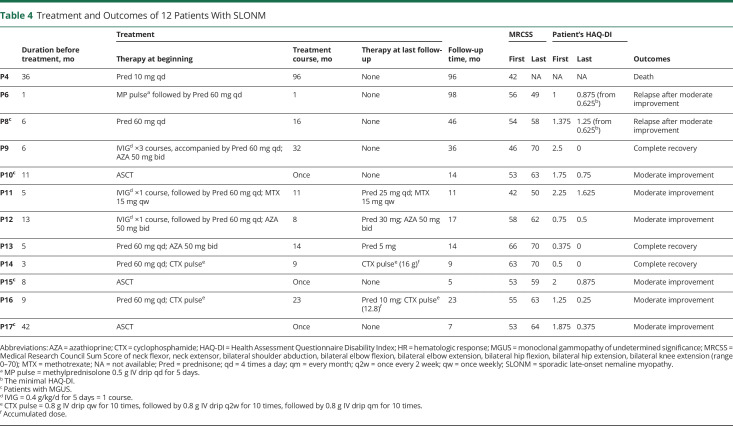
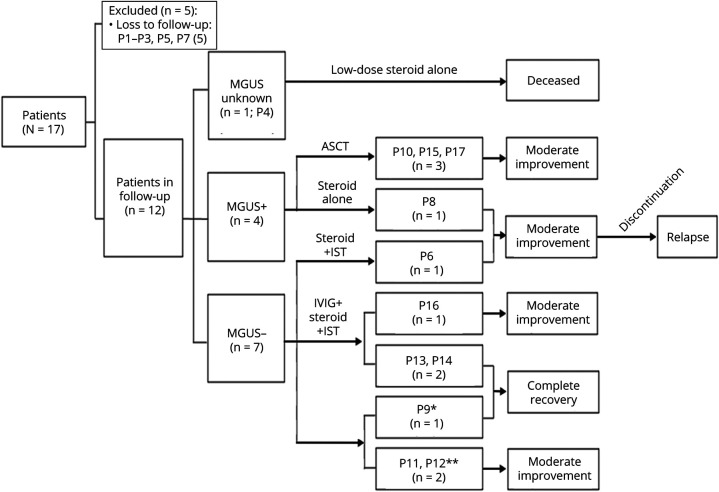
In the present study, 71% (12/17) of the patients were followed up. The median follow-up time was 15.5 months, with a range of 5–98 months. The detailed drug therapy and treatment outcomes of 12 patients are summarized in Table 4 and Figure 4. Three of 4 patients with MGUS received ASCT, and all of them achieved moderate improvement in MMT and complete remission in hematology. Six of 7 patients without MGUS were treated with steroids and additional immunotherapies: 3 patients treated with prednisone and another immunosuppressant achieved moderate improvement (P16) or complete recovery (P13 and P14); the other 3 patients in whom IVIGs (0.4 g/kg/d for 5 days) were used for 1–3 courses as an initial therapy, followed by the maintenance treatment with prednisone and immunosuppressant, also achieved moderate improvement (P11 and P12) or complete recovery (P9). Steroids alone were given to 3 other patients: P4 with unknown M protein status was given low doses of prednisone (10 mg/d) and died 8 years later; both P6 without MGUS who was given methylprednisolone pulse followed by oral prednisone for 1 month and P8 with MGUS who was given sufficient prednisone for 16 months had achieved moderate improvement despite relapse after the discontinuation of steroids several months later.
Table 4.
Treatment and Outcomes of 12 Patients With SLONM
Figure 4. Treatment and Follow-up of Patients With SLONM.
*IVIG ×1 course initially; **IVIG ×3 courses initially. ASCT = autologous stem cell transplantation; Ig = immunoglobulin; IST = immunosuppressant; MGUS = monoclonal gammopathy of undetermined significance; SLONM = sporadic late-onset nemaline myopathy.
Discussion
Our present study is the first report of SLONM clinicopathologic profiles in a Chinese cohort. The present group of patients with SLONM was clinically characterized by a subacute or chronic, asymmetric muscle weakness and atrophy in a limb-girdle distribution as well as a normal or mildly elevated serum CK, and a myopathic pattern with spontaneous activities on EMG. Another clinical indication of SLONM is neck extensor weakness, which was seen in more than half (9/17) of our patients, and 7 of these patients presented with a typical head drop sign, similar to the report in a recent review.11 Mild involvement of facial and bulbar muscles was observed only in 1 and 3 individuals, respectively, which was less common than that of other reports.11 Although several reports indicated that the pulmonary and cardiac involvement in SLONM was the leading cause of death,10,17-19 none of our patients had subjective symptoms or objective evidence indicating cardiopulmonary dysfunction. Generally, a combination of myogenic changes with spontaneous activities on EMG examination is more common in necrotic myopathy, such as myositis or muscular dystrophy, in which the CK level is always remarkably elevated. This unique EMG pattern in patients with nonnecrotic myopathy strongly indicates the diagnosis of SLONM. MRI findings in this Chinese cohort were consistent with those in an Italian study.10 Posterior muscle groups were more likely to be affected than the anterior ones. More specifically, gluteus minimus and medius, semimembranosus, and soleus seem to be preferentially involved. This distinctive pattern of muscle involvement has not been described in other neuromuscular disorders.10 The paraspinal muscle involvement was found in all 3 patients who had a neck muscle MRI scanning, including 2 patients with a head drop sign and 1 with normal neck muscle strength. MRI may be useful to find the subclinical involvement of neck muscles.
To explore a reliable pathologic marker for the diagnosis of SLONM, we compared 5 most common Z-disc–associated proteins12 for the identification of rod-containing fibers. Although there was no significant difference in the total staining intensity scores of IHC staining among anti–α-actinin, anti-desmin, and anti-myotilin, anti–α-actinin staining showed the highest percentage of strongly positive fibers and was the only one without negative results. This finding can be explained by the fact that α-actinin is the major component of Z-disc cross-linking actin filaments to maintain the structural support and tension during muscle contraction.20 In addition, the present study indicated the high specificity of anti–α-actinin staining for recognition of nemaline rods, based on the fact that α-actinin accumulation was not markedly detected in other neuromuscular disorders with basophilic or atrophic fibers except the target fibers due to neurogenic atrophy. Therefore, we propose diffuse/granular α-actinin expression in myofibers as the best pathologic marker for the diagnosis of SLONM in adult patients. In one of our present patients (P6), the diagnosis of SLONM was not established until 8 years later when we revisited the muscle pathology by anti–α-actinin immunostaining. Two other patients (P11 and P12) were confirmed as having SLONM after anti–α-actinin immunostaining since the rods on MGT were very obscure. We speculate that SLONM may be underdiagnosed because of the difficulty in identifying nemaline rods under routine histologic evaluations. Thus, we recommend that anti–α-actinin immunostaining should be routinely performed in adult patients with undiagnosed nonnecrotic myopathy.
Recently, SLONM has been recognized as an acquired and treatable autoimmune myopathy.21 However, a therapeutic consensus for SLONM is not available. IVIG has been favored as the first-line therapy for all patients because it showed the best response and overall survival in SLONM with or without MGUS.9 Emerging evidence has suggested that consolidative chemotherapy combined with or without ASCT was more effective than the traditional immunotherapy (including IVIG) for SLONM-MGUS.22-25 In keeping with the previous observation, 3 of our patients with MGUS (P10, P15, and P17) treated with ASCT alone achieved moderate improvement in MMT and complete remission in hematology. As for the patients with SLONM without MGUS, traditional immunotherapy was still the most common choice, but the efficacy varied among different reports.8-10 Our follow-up data highlighted that traditional immunotherapy was effective for most patients with SLONM even in 1 with MGUS. IVIG was used only for 1–3 courses as an initial treatment in 3 patients, in whom the constant improvement and complete recovery should be also attributed to the maintenance treatment of prednisone and another immunosuppressant. Furthermore, the fact that 3 patients (P9, P13, and P14) achieved complete recovery after receiving a combination of steroids and another immunosuppressant, compared with 2 other patients (P6 and P8) who achieved moderate improvement before the relapse after receiving steroids alone, indicated that the combination therapy may be more likely to achieve better clinical outcomes. In addition, the dose of steroids should be sufficient in the treatment of SLONM. As for P4, the long-term treatment with a low dosage of prednisone (10 mg/d) showed no improvement in her condition before death. Thus, our results strongly suggested that a sufficient dose of steroids combined with another immunosuppressant might be the most economic and effective therapy for SLONM, especially in patients without MGUS, and could be the first-choice option for SLONM treatment until a widely accepted consensus exists.
An autoimmune pathomechanism in SLONM should be considered, given the clinical profile of progressive limb-girdle weakness with a subacute adult-onset, good responsiveness to immunotherapy, and pathologic features with increased MHC-1 expression as well as endomysial and/or perivascular lymphocytic infiltration, which may be similar to that of classical autoimmune inflammatory myopathies (AIM).26 However, no specific or related antibody has been identified in SLONM up to now. MGUS could be detected up to 53% of SLONM patients,11,27 which should not be a coincidence. Moreover, the muscle strength improved steadily, and the rods disappeared after ASCT.7 These findings imply that there may be a causal relationship between M protein and the formation of nemaline bodies. The muscle fiber injury in SLONM is unlikely to be caused by direct M protein deposition, as suggested by some previous studies,28 and also by our study showing no light chain or amyloid deposition in 4 cases with MGUS. We hypothesize that some potential antibodies, such as M protein or the neutralizing antibodies along with HIV-1 infection,29 combined with other humoral mediators against the sarcomere's proteins,10,22 might play an important role in the pathophysiology of this rare disease. Unlike typical subtypes of AIM, SLONM seems to have some silent damage to the muscle without obvious necrosis and active phagocytosis despite the rod-bearing process, and SLONM shows the infiltration of CD68+ macrophages preferentially in the endomysium or perimysium but no direct infiltration of T cells or macrophages into myofibers.30 We speculate that this type of silent damage to myofibers in SLONM could be caused by the pathogenic antibodies directed against proteins associated with the Z-disc or its related structures, resulting in sarcomere destabilization, disorganization of the contractile component of myofibrils, abnormal protein accumulation and degradation, rod formation, and ultimately myofiber atrophy. In addition, IVIG may remove the circulating antibodies through saturating the protective neonatal Fc receptor.31 ASCT or steroids may reduce the production of pathogenic antibodies through eliminating or inhibiting the involvement of vicious B cells or plasma cells.
Our study has several limitations. First, it is a retrospective analysis from a single neuromuscular center, and the number of patients is limited with a possible referral bias contributing to the findings. Second, only 1 patient (P9) had the genetic screening to exclude a hereditary cause. Nevertheless, in our patients, the clinical progression patterns and treatment outcomes were not suggestive of congenital myopathies. Third, in some patients, the clinical information, particularly from several years ago, was incomplete. Fourth, the treatment and follow-up in our study were not standardized. Finally, the relapse should be assessed by the patents' HAQ-DI as MMT could not be obtained at their best response. However, given the rarity and aggressive disease course of SLONM, a large randomized controlled trial is unlikely to be conducted. At present, our findings further characterize the clinical and pathologic features of this orphan disease, and we offer an available database of therapy for SLONM with or without MGUS.
In conclusion, SLONM is a fatal but treatable myopathy that remains underrecognized on pathologic examination. Anti–α-actinin staining should be included in the routine IHC panel to identify nemaline bodies for the diagnosis of SLONM, especially in patients with clinically suspected SLONM, regardless of the MGT and other histologic stains. Our study further confirms the favorable efficacy of ASCT for SLONM-MGUS and, for the first time, suggests that corticosteroids combined with another immunosuppressant should be the first-line choice of treatment for patients without MGUS. Further investigation is needed to understand the molecular immunopathogenesis of SLONM.
Acknowledgment
The authors thank the patients with SLONM and controls for their participations in this study.
Glossary
- AIM
autoimmune inflammatory myopathies
- ASCT
autologous stem cell transplantation
- ATPase
adenosine triphosphatase
- CK
creatine kinase
- cN1A
cytosolic 5′-nucleotidase 1A
- EM
electron microscopy
- HAQ-DI
Health Assessment Questionnaire Disability Index
- HE
hematoxylin and eosin
- Ig
immunoglobulin
- IHC
immunohistochemistry
- IMNM
immune-mediated necrotizing myopathy
- MAC
membrane attack complex
- MGT
modified Gomori trichrome
- MGUS
monoclonal gammopathy of undetermined significance
- MHC-I
Class I major histocompatibility complex
- MMT
manual muscle testing
- MSA
myositis specific antibody
- NCAM
neural cell adhesion molecule
- NMD
neuromuscular disorder
- PFT
pulmonary function test
- SLONM
sporadic late-onset nemaline myopathy
- T2W-STIR
T2-weighted short-tau inversion recovery
Appendix. Authors
Contributor Information
Bing Zhao, Email: zhaobing1122@126.com.
Tingjun Dai, Email: tingjundai@sdu.edu.cn.
Dandan Zhao, Email: 997626225@qq.com.
Xiaotian Ma, Email: mxt_qlqd@sina.com.
Cuiping Zhao, Email: zhaocuipingzsu@126.com.
Ling Li, Email: liling5313@sina.com.
Yuan Sun, Email: 44937209@qq.com.
Yongqing Zhang, Email: dryqzhang@163.com.
Yaping Yan, Email: yaping.yan@snnu.edu.cn.
Jian-Qiang Lu, Email: luj85@mcmaster.ca.
Fuchen Liu, Email: fuchen.liu@email.sdu.edu.cn.
Study Funding
This study was supported by the National Key Research and Development Program of China (2021YFC2700904), the National Natural Science Foundation of China (No. 82071412), the Taishan Scholars Program of Shandong Province, Qingdao Technology Program for Health and Welfare (20–3-4–42-nsh), and the 20-policy supported projects of collaborative innovation and achievement transformation in universities and research institutes of Jinan (2019GXRC050).
Disclosure
The authors report no disclosures. Go to Neurology.org/NN for full disclosures.
References
- 1.Engel AG. Late-onset rod myopathy (a new syndrome?): light and electron microscopic observations in two cases. J Mayo Clin Proc. 1966;41(11):713-741. [PubMed] [Google Scholar]
- 2.Engel WK, Oberc MA. Abundant nuclear rods in adult-onset rod disease. J Neuropathol Exp Neurol. 1975;34(2):119-132. [DOI] [PubMed] [Google Scholar]
- 3.Dalakas MC, Pezeshkpour GH, Flaherty M. Progressive nemaline (rod) myopathy associated with HIV infection. N Engl J Med. 1987;317(25):1602-1603. [DOI] [PubMed] [Google Scholar]
- 4.Eymard B, Brouet JC, Collin H, Chevallay M, Bussel A, Fardeau M. Late-onset rod myopathy associated with monoclonal gammopathy. Neuromuscul Disord. 1993;3(5-6):557-560. [DOI] [PubMed] [Google Scholar]
- 5.Dwyer BA, Maye RF, Lee SC. Progressive nemaline (rod) myopathy as a presentation of human immunodeficiency virus infection. Arch Neurol. 1992;49(5):440. [DOI] [PubMed] [Google Scholar]
- 6.Eskazan AE, Gunduz A. What is the best treatment approach for sporadic late-onset nemaline myopathy associated with “monoclonal gammopathy of neurological significance”? Int J Cancer. 2021;148(11):2638-2639. [DOI] [PubMed] [Google Scholar]
- 7.Benveniste O, Laforet P, Dubourg O, et al. Stem cell transplantation in a patient with late-onset nemaline myopathy and gammopathy. Neurology. 2008;71(7):531-532. [DOI] [PubMed] [Google Scholar]
- 8.Chahin N, Selcen D, Engel AG. Sporadic late onset nemaline myopathy. Neurology. 2005;65(8):1158-1164. [DOI] [PubMed] [Google Scholar]
- 9.Naddaf E, Milone M, Kansagra A, et al. Sporadic late-onset nemaline myopathy: clinical spectrum, survival, and treatment outcomes. Neurology. 2019;93(3):e298-e305. [DOI] [PubMed] [Google Scholar]
- 10.Monforte M, Primiano G, Silvestri G, et al. Sporadic late-onset nemaline myopathy: clinical, pathology and imaging findings in a single center cohort. J Neurol. 2018;265(3):542-551. [DOI] [PubMed] [Google Scholar]
- 11.Schnitzler LJ, Schreckenbach T, Nadaj-Pakleza A, et al. Sporadic late-onset nemaline myopathy: clinico-pathological characteristics and review of 76 cases. Orphanet J Rare Dis. 2017;12(1):86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Laing NG. The Sarcomere and Skeletal Muscle Disease. Springer-Verlag, 2008. [Google Scholar]
- 13.Rider LG, Aggarwal R, Machado PM, et al. Update on outcome assessment in myositis. Nat Rev Rheumatol. 2018;14(5):303-318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kassardjian CD, Lennon VA, Alfugham NB, Mahler M, Milone M. Clinical features and treatment outcomes of necrotizing autoimmune myopathy. JAMA Neurol. 2015;72(9):996-1003. [DOI] [PubMed] [Google Scholar]
- 15.Durie BG, Harousseau JL, Miguel JS, et al. International uniform response criteria for multiple myeloma. Leukemia. 2006;20(9):1467-1473. [DOI] [PubMed] [Google Scholar]
- 16.Aouizerate J, De Antonio M, Bassez G, et al. Myofiber HLA-DR expression is a distinctive biomarker for antisynthetase-associated myopathy. Acta Neuropathol Commun. 2014;2:154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Truffert A, Iancu Ferfoglia R, Lobrinus JA, Samii K, Kohler A. Sporadic late onset nemaline myopathy with monoclonal gammopathy of undetermined significance: two cases with long term stability. Eur J Transl Myol. 2020;30(3):9225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Matsuzono K, Kumutpongpanich T, Kubota K, et al. Noteworthy cardiovascular involvement with sporadic late-onset nemaline myopathy. Intern Med. 2021;60(14):2327-2332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Turnquist C, Grogono JC, Hofer M, Pitcher A. Sporadic late-onset nemaline myopathy: a case report of a treatable cause of cardiac failure. Eur Heart J Case Rep. 2021;5(1):ytaa480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dubowitz V, Sewry C, Oldfors A, Lane RJ. Muscle Biopsy: A Practical Approach. Saunders Elsevier, 2013. [Google Scholar]
- 21.Sewry CA, Laitila JM, Wallgren-Pettersson C. Nemaline myopathies: a current view. J Muscle Res Cell Motil. 2019;40(2):111-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kotchetkov R, Susman D, Bhutani D, Broch K, Dispenzieri A, Buadi FK. Chemotherapy-based approach is the preferred treatment for sporadic late-onset nemaline myopathy with a monoclonal protein. Int J Cancer. 2021;148(11):2807-2814. [DOI] [PubMed] [Google Scholar]
- 23.Okhovat AA, Nilipour Y, Boostani R, et al. Sporadic late-onset nemaline myopathy with monoclonal gammopathy of undetermined significance: report of four patients. Neuromuscul Disord. 2021;31(1):29-34. [DOI] [PubMed] [Google Scholar]
- 24.Kotchetkov R, Dyszkiewicz-Korpanty A, Kukreti V. Chemotherapy with stem cell transplantation is more effective than immunotherapy in sporadic late onset nemaline myopathy with monoclonal gammopathy. Bone Marrow Transplant. 2018;53(7):895-899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kumutpongpanich T, Owattanapanich W, Tanboon J, Nishino I, Boonyapisit K. Sporadic late-onset nemaline myopathy with monoclonal gammopathy of undetermined significance (SLONM-MGUS): an alternative treatment using cyclophosphamide–thalidomide–dexamethasone (CTD) regimen. Neuromuscul Disord. 2018;28(7):610-613. [DOI] [PubMed] [Google Scholar]
- 26.Dalakas MC. Inflammatory muscle diseases. N Engl J Med. 2015;372(18):1734-1747. [DOI] [PubMed] [Google Scholar]
- 27.Dalakas MC, Smith SA. A “nema” of hope in the treatment of late-onset nemaline myopathy. Neurology. 2008;71(7):472-473. [DOI] [PubMed] [Google Scholar]
- 28.Doppler K, Knop S, Einsele H, Sommer C, Wessig C. Sporadic late onset nemaline myopathy and immunoglobulin deposition disease. Muscle Nerve. 2013;48(6):983-988. [DOI] [PubMed] [Google Scholar]
- 29.Pilgrim AK, Pantaleo G, Cohen OJ, et al. Neutralizing antibody responses to human immunodeficiency virus type 1 in primary infection and long-term-nonprogressive infection. J Infect Dis. 1997;176(4):924-932. [DOI] [PubMed] [Google Scholar]
- 30.Tanboon J, Uruha A, Arahata Y, et al. Inflammatory features in sporadic late-onset nemaline myopathy are independent from monoclonal gammopathy. Brain Pathol. 2021;31(3):e12962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dalakas MC. Update on intravenous immunoglobulin in neurology: modulating neuro-autoimmunity, evolving factors on efficacy and dosing and challenges on stopping chronic IVIg therapy. Neurotherapeutics. 2021;18(4):2397-2418. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Individual participant data will not be made publicly available because of potential confidentiality concerns related to the rarity of the condition and the small study population. Further information can be obtained by qualified researchers from the corresponding author on reasonable request.