Abstract
Background and Objectives
Pure relapsing short myelitis with clinical and paraclinical features suggesting multiple sclerosis (MS) has been described recently. Here, we evaluated the existence of this potential new form of MS by retrospectively searching for similar cases in the databases of the French tertiary MS centers.
Methods
Patients were included based on the present criteria: at least 2 short (<3 vertebral segments) myelitis episodes; minimum follow-up of 3 years; no MS-like brain lesion during all the follow-up; tested negative for both anti–myelin oligodendrocyte glycoprotein and anti–aquaporin 4 antibodies in serum; presence of oligoclonal bands in CSF; and comprehensive workup to exclude alternative diagnoses.
Results
Eighteen patients fulfilled all criteria. The sex ratio (females/males) was 5/1; the median (range) age at first relapse was 35.5 (25–54) years, the disease duration was 80.5 (50–308) months, and the annualized relapse rate was 0.36 (0.1–0.5). The median (range) number of relapses per patient was 2 (2–5), and the median (range) Expanded Disability Status Scale score at last follow-up was 1 (0–7.5). In CSF, the median (range) protein level was 0.34 g/L (0.18–0.77), and the median (range) number of mononuclear cells was 3 (0–28). Spinal cord MRI demonstrated a median (range) number of 2 (1–5) lesions per examination and 3 [1–7] on the last examination. Fifty-five percent of lesions involved the cervical levels. Secondary progressive evolution occurred in 3 of 18 (17%) patients.
Discussion
Pure spinal MS could be a rare entity in the MS disease spectrum. However, the existence of a distinct entity in the inflammatory CNS disorders cannot be excluded.
Cases of pure relapsing short myelitis with clinical and paraclinical features suggesting multiple sclerosis (MS) have been reported recently.1 Better description of this potential new form of MS is of particular importance because nowadays these patients do not fulfill international diagnostic criteria of MS2 and could be consequently excluded from effective therapeutic strategies. Here, we evaluated the existence of this potential new form of MS by retrospectively searching for similar cases in the databases of the French centers involved in neuromyelitis optica and associated neurologic disorders (NOMADMUS) network.
Methods
Protocol and Participants
To be included, patients had to fulfill the following criteria: age >18 years at inclusion; evidence of at least 2 short (<3 vertebral segments) myelitis episodes; minimum follow-up of 3 years; no typical MS-like brain lesion during all the follow-up; no clinical history or visual evoked potential or eye examination suggesting prior optic neuritis; no history of clinical episode suggesting brain lesion; tested negative for both anti–myelin oligodendrocyte glycoprotein (anti-MOG) and anti–aquaporin 4 (anti-AQP4) antibodies in serum; presence of oligoclonal bands (OCBs) in CSF; and comprehensive workup to exclude alternative diagnoses of myelitis, namely, infections, vascular diseases, and subacute combined degeneration of spinal cord and autoimmune diseases.3-5 Particularly, other inflammatory causes of myelitis, including sarcoidosis, Behcet disease, paraneoplastic disorders, and connective tissue diseases, were excluded by using biological and imaging explorations. Anonymized centralized (Marseille) reinterpretation of brain MRIs by expert neurologists (consensus required among B.A., J.P., A.M., A.R., and C.B.) was performed to exclude all patients with any typical MS-like brain lesion.6
Standard Protocol Approvals, Registrations, and Patient Consent
The authors obtained ethical approval of national ethical authority (NOMADMUS cohort, CNIL decision DR-2014-558) to conduct the present study. Each participant gave free and informed written consent for anonymized use of clinical, MRI, and biological data for research purposes.
Data Availability
All data analyzed during this study will be shared anonymized by reasonable request of a qualified investigator to the corresponding author.
Results
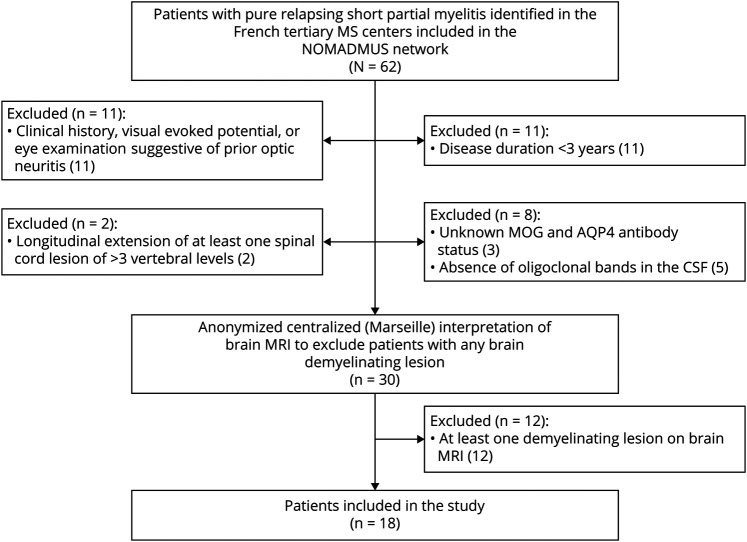
Among 62 patients first screened in the French tertiary MS centers, 18 fulfilled all inclusion criteria (Figure 1).
Figure 1. Flowchart of the Patients Included.
Clinical Features
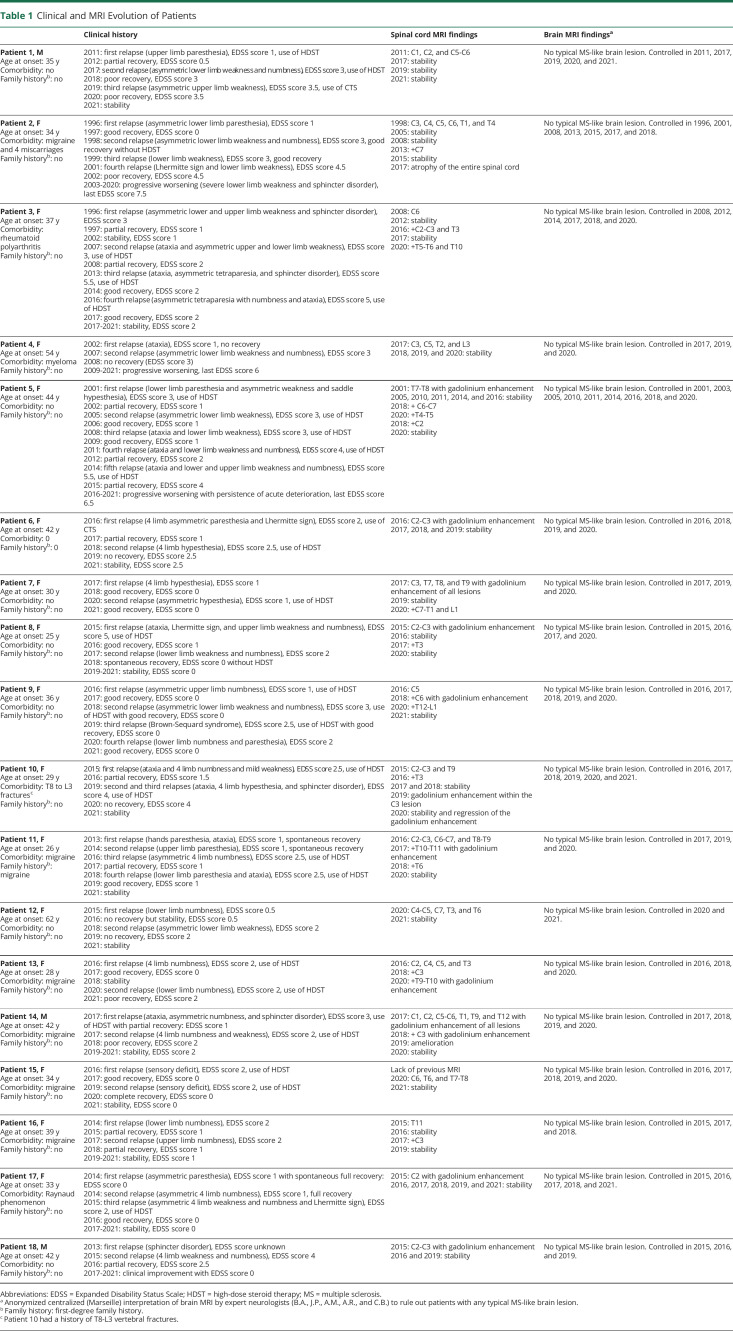
The sex ratio (females/males) was 5/1; the median (range) age at first relapse was 35.5 (25–54) years, the disease duration was 80.5 (50–308) months, and the annualized relapse rate (ARR) 0.36 (0.1–0.5) (Table 1). The median (range) number of relapses per patient was 2 (2–5), and the median (range) Expanded Disability Status Scale (EDSS) score during relapse and at last follow-up was 2.5 (0.5–5.5) and 1 (0–7.5), respectively. Among the 50 relapse cases, 24 (48%) showed pure sensitive signs (paresthesia, numbness, or proprioceptive ataxia); 11 (22%) sensitive and motor signs (arm or lower limb weakness); 7 (14%) sensitive, motor, and sphincter signs; 4 (8%) pure motor signs; 2 (4%) motor and sphincter signs; 1 (2%) sensitive and sphincter signs; and 1 (2%) pure sphincter signs. Of the 50 relapse cases, 30 (60%) involved the 4 limbs and 20 (40%) the lower limbs only.
Table 1.
Clinical and MRI Evolution of Patients
Laboratory Findings
No patient presented atypical CSF findings for MS. In CSF, the median (range) protein level was 0.34 (0.18–0.77) g/L, and the median (range) number of mononuclear cells was 3 (0–28). Nine of 18 patients were tested at least twice for anti-MOG and anti-AQP4 antibodies, 5 of 9 were tested twice, 3 of 9 three times, and 1 of 9 four times.
Brain and Spinal Cord MRI
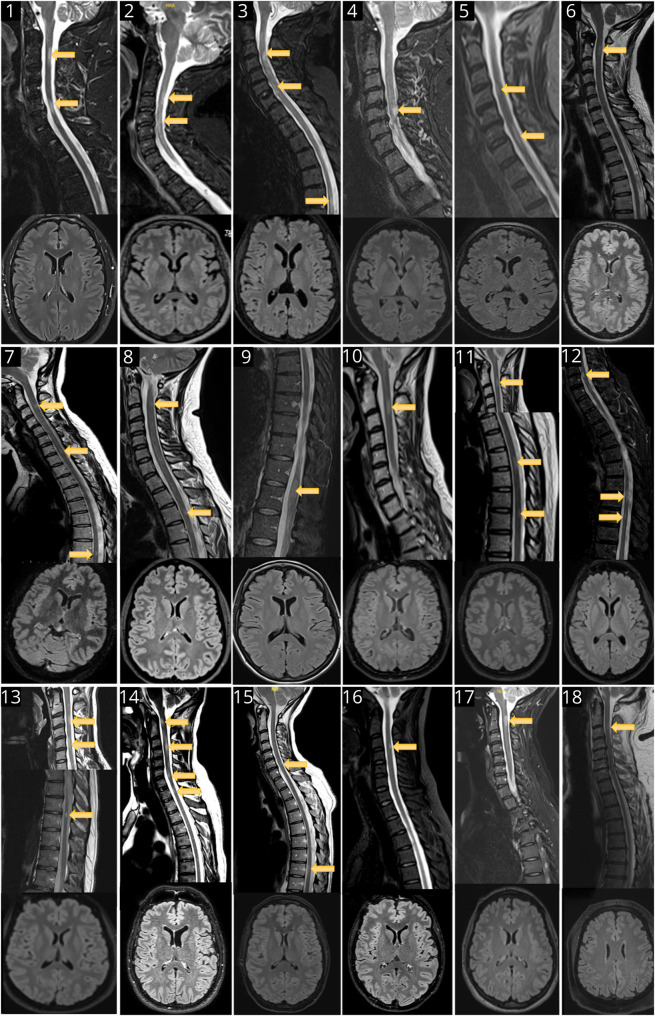
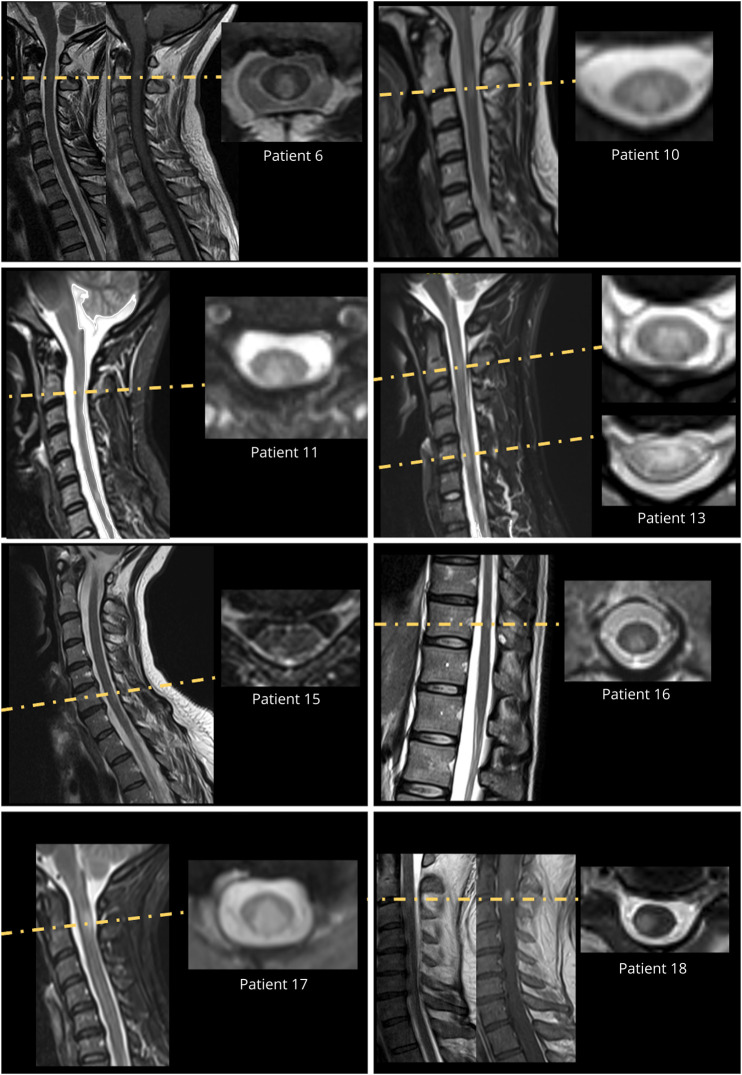
No typical MS-like brain lesion was detected in any patient despite repeat examination (median [range] number of brain MRI examinations per patient 4 [2–9]) (Figure 2). Importantly, 3D fluid-attenuated inversion recovery imaging was available for 15 of 18 patients. A median of 5 (2–6) spinal cord MRI examinations were performed per patient. All spinal cord MRI examinations included sagittal T2/STIR sequences. At least 1 series of axial sequences was available for 8 of 18 patients. Spinal cord MRI demonstrated a median (range) number of 2 (1–5) lesions per examination and a median (range) number of 3 (1–7) lesions on the last examination. The median (range) sagittal extension of the spinal cord lesions was 1 (0.5–2) vertebral segments. Among all spinal cord lesions (n = 67) depicted, 37 (55%) and 30 (45%) involved the cervical and thoracolumbar levels, respectively. Gadolinium injection was performed in 45 examinations, and 21 of 46 (45%) lesions showed gadolinium enhancement. Overall, 18 lesions in 9 patients were explored on the axial plane, and 12 (67%) showed partial myelitis (Figure 3).
Figure 2. Last Brain and Spinal Cord MRI of the Patients.
The patient's number is displayed in each image.
Figure 3. Axial View of Spinal Cord Lesion Performed in 8 of 18 Patients.
Treatment and Progress
In all, 30 of 50 (60%) relapse cases were treated with high-dose IV corticosteroids. The median (range) EDSS score during relapse and after recovery was 2.5 (0.5–5.5) and 1 (0–4.5), respectively.
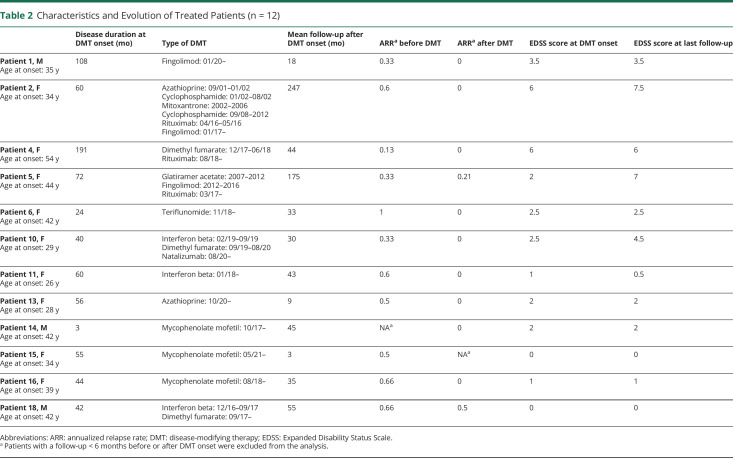
Disease-modifying therapy (DMT) was used in 12 of 18 (67%) patients (Table 2). In these patients, the median (range) follow-up before and after DMT onset was 55.5 (3–191) and 40 (4–248) months, respectively. The median (range) ARR before and after DMT onset was 0.5 (0.1–1) and 0 (0–0.5), respectively, after excluding patients with a follow-up <6 months before or after DMT onset. Seven of 11 (63%) patients were free from relapse after DMT onset. The median EDSS (range) score at DMT onset and at last follow-up was 2 (0–6) and 2.5 (0–7.5), respectively.
Table 2.
Characteristics and Evolution of Treated Patients (n = 12)
Secondary progressive evolution occurred in 3 of 18 (17%) patients. The median (range) follow-up of these patients was 242 (236–308) vs 64 (36–296) months for other patients. The median (range) EDSS score of these patients was 7 (6–7.5) vs 1.5 (0–4.5) for other patients.
Discussion
The present study including the French centers involved in the NOMADMUS network reports 18 cases of pure relapsing short myelitis. According to the retrospective design of the study, the number of cases is probably underestimated, which prevents any conclusion about the prevalence of pure relapsing short myelitis. In addition, some cases were probably not reported because they never experienced a second relapse in that treatment onset occurred just after the first myelitis. However, this therapeutic attitude is highly unusual in France, which limits the number of potential underreported cases.
Because all patients included in the present study had to have OCBs in the CSF, we paid careful attention to searching and excluding all known causes of inflammatory myelitis. Moreover, we also excluded vitamin B12 deficiency in terms of its frequency. However, if a search for other rare metabolic causes of myelopathy such as copper deficiency was not available in the medical chart, patients were not excluded because radiologic and CSF findings did not suggest metabolic disorders.
Several features of these cases argue for the existence of a pure spinal form of MS. First, the characteristics of myelitis highly suggest relapsing MS: no clinical presentation suggested transverse myelitis, clinical presentations mostly suggested an involvement of the posterior part of the spinal cord, spinal cord MRI demonstrating most partial and posterior myelitis in the axial plane. Second, all patients showed typical CSF findings for MS. Third, in all patients receiving MS DMTs, disease activity decreased. Fourth, 17% of patients showed secondary progression—an evolution highly suggestive of MS—several years after disease onset. Finally, we were not able to provide a better explanation than MS in all patients despite extensive explorations. In that way, we recommend preferentially using DMTs with demonstrated efficacy in MS to treat pure relapsing short myelitis.
Nevertheless, several features may argue for the existence of a possible distinct inflammatory entity. First, the sex ratio was more imbalanced in favor of females as compared with MS. Second, it is unexpected in a pathologic perspective that brain involvement could be totally absent after several years of evolution with MS.
Whatever the nosological classification, the existence of patients with pure relapsing short myelitis argues for systematically adding spinal cord MRI to brain MRI for the imaging surveillance of patients followed after an isolated myelitis episode with OCBs in the CSF. For patients with recurrent myelitis, we recommend performing imaging at least annually as recommended for MS but systematically adding spinal cord imaging to brain imaging. According to the relative low disease activity evidenced in the patients reported here, we do not recommend exceeding annual imaging in the absence of relapse or therapeutic considerations.
Pure spinal MS could be a rare entity in the MS disease spectrum. However, the existence of a distinct entity in the inflammatory CNS disorders cannot be excluded. Future studies are needed to disentangle these 2 interpretations.
Glossary
- anti-AQP4
anti–aquaporin 4
- anti-MOG
anti–myelin oligodendrocyte glycoprotein
- ARR
annualized relapse rate
- DMT
disease-modifying therapy
- EDSS
Expanded Disability Status Scale
- MS
multiple sclerosis
- NOMADMUS
Neuromyelitis Optica Study Group in France
- OCB
oligoclonal band
Appendix. Authors
Contributor Information
Zélia Poullet, Email: zelia.poullet@ap-hm.fr.
Julie Pique, Email: julie.pique@chu-lyon.fr.
Adil Maarouf, Email: adil.maarouf@ap-hm.fr.
Clemence Boutiere, Email: clemence.boutiere@ap-hm.fr.
Audrey Rico, Email: audrey.rico@ap-hm.fr.
Sarah Demortiere, Email: sarah.demortiere@ap-hm.fr.
Pierre Durozard, Email: pierre.durozard@ap-hm.fr.
Caroline Papeix, Email: caroline.papeix@aphp.fr.
Elisabeth Maillart, Email: elisabeth.maillart@aphp.fr.
Nicolas Collongues, Email: nicolas.collongues@chru-strasbourg.fr.
Xavier Ayrignac, Email: xavier.ayrignac@yahoo.fr.
Helene Zephir, Email: helene.zephir@chru-lille.fr.
Romain Deschamps, Email: rdeschamps@for.paris.
Jonathan Ciron, Email: ciron.j@chu-toulouse.fr.
Jean Pelletier, Email: jean.pelletier@ap-hm.fr.
Romain Marignier, Email: romain.marignier@chu-lyon.fr.
Study Funding
No targeted funding reported.
Disclosure
Dr. Poullet reports no disclosures relevant to the manuscript. Go to Neurology.org/NN for full disclosures.
References
- 1.Schee JP, Viswanathan S. Pure spinal multiple sclerosis: a possible novel entity within the multiple sclerosis disease spectrum. Mult Scler. 2019;25(8):1189-1195. [DOI] [PubMed] [Google Scholar]
- 2.Thompson AJ, Banwell BL, Barkhof F, et al. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol. 2018;17(2):162-173. [DOI] [PubMed] [Google Scholar]
- 3.Charil A, Yousry TA, Rovaris M, et al. MRI and the diagnosis of multiple sclerosis: expanding the concept of “no better explanation”. Lancet Neurol. 2006;5(10):841-852. [DOI] [PubMed] [Google Scholar]
- 4.Hardy TA, Reddel SW, Barnett MH, Palace J, Lucchinetti CF, Weinshenker BG. Atypical inflammatory demyelinating syndromes of the CNS. Lancet Neurol. 2016;15(9):967-981. [DOI] [PubMed] [Google Scholar]
- 5.Calabrese M, Gasperini C, Tortorella C, et al. Better explanations” in multiple sclerosis diagnostic workup. Neurology. 2019;92(22):e2527-e2537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wattjes MP, Ciccarelli O, Reich DS, et al. MAGNIFY-CMSC-NAIMS consensus recommendations on the use of MRI in patients with multiple sclerosis. Lancet Neurol. 2021;20(8):653-670. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data analyzed during this study will be shared anonymized by reasonable request of a qualified investigator to the corresponding author.