Abstract
Background:
Decreasing the proinflammatory M1 macrophages or shifting the polarization status from M1 to M2 phenotype is thought to be beneficial for tendon-to-bone healing. In anterior cruciate ligament reconstruction (ACLR), using an insertion-preserved hamstring tendon (IP-HT) graft compared with a free hamstring tendon (FHT) graft has been shown to reduce graft necrosis and improve healing. However, the role of macrophage polarization at the tendon-to-bone interface is unclear.
Hypothesis:
ACLR using IP-HT graft would facilitate the phenotype shift from M1 to M2 macrophages at the tendon-to-bone interface.
Study Design:
Controlled laboratory study.
Methods:
Unilateral ACLR was performed on 42 healthy New Zealand White rabbits (study group, 21 rabbits with IP-HT graft; control group, 21 rabbits with FHT graft). At days 1, 3, and 7 and weeks 3, 6, 12, and 24 postoperatively, 3 rabbits in each group were sacrificed to investigate and compare the expression of surrogate markers for M1 macrophages (inducible nitric oxide synthase [iNOS] and tumor necrosis factor α [TNF-α]) and M2 macrophages (CD206 and transforming growth factor β [TGF-β]) via immunohistochemical staining and evaluation.
Results:
In the control group, the percentage of iNOS- and TNF-α–positive cells from postoperative day 7 and week 3 increased then decreased by week 6; positive expression of CD206 and TGF-β was weaker and peaked at 3 weeks postoperatively. In the study group, high CD206- and TGF-β–positive expression was observed from weeks 3 to 12 and peaked at week 6, and positive expression of iNOS- and TNF-α was weaker and peaked on day 7. At both 7 days and 3 weeks, the percentages of iNOS- and TNF-α–positive cells in the control group were both significantly higher than in the study group (P ≤ .04 for all). At 6 weeks, the percentages of CD206- and TGF-β–positive cells in the study group were both significantly higher than in the control group (P = .02 and P = .04, respectively).
Conclusion:
More expression of surrogate markers for M2 macrophages was observed in the tendon-to-bone healing process after ACLR using IP-HT versus FTP graft.
Clinical Relevance:
Using IP-HT grafts in ACLR may facilitate postoperative healing by shifting the local status of macrophage polarization at the tendon-to-bone interface.
Keywords: ACL, hamstring tendon, tendon-to-bone healing, macrophage polarization
Anterior cruciate ligament (ACL) injury has been treated surgically by replacing the function of the ACL with that of a graft. Autologous hamstring tendon graft is one of the most popular grafts for ACL reconstruction (ACLR). 4,7 Although the clinical outcomes of ACLR are generally good, delayed or incomplete tendon-to-bone healing can result in recurrent laxity and can also complicate revision surgery. 15,28 The slow process of tendon-to-bone healing contributes to the prolonged rehabilitation period, increased risk of reinjury, and risk of long-term failure after ACLR. 18,20
For now, most of the tendon grafts are avascular free grafts, which undergo graft necrosis in the early postoperative period after ACLR, and result in poor tendon-to-bone healing with the formation of a fibrovascular scar tissue interface. 18,23 To avoid graft necrosis, the insertion-preserved hamstring tendon (IP-HT) was proposed to provide a vascularized graft 22,30 and was proved to bypass the stages of avascular necrosis and revascularization 13,14,19,21 and improve the healing between tendon graft and bone tunnel after ACLR. 14,19 However, the underlying mechanism was still unclear.
In the early necrosis phase after ACLR, inflammatory cell infiltration can be observed around the graft. 12 Recently, the roles of inflammation regulation and macrophage polarization at the tendon-to-bone interface have been clarified. It has been shown that the fibrous scar tissue at the tendon-to-bone interface could result from the infiltrating macrophages, while the macrophage elimination would inhibit scar formation and improve fibrocartilage growth in the interface. 9,12 Further studies have confirmed that the tendon-to-bone healing could be inhibited by M1 macrophage (the main mediator of inflammation) and could be improved by M2 macrophage. 8,10,24,27 M1 macrophages usually appear early after tissue necrosis and are involved in the process of early inflammatory response and angiogenesis, mainly by secreting interleukin (IL)-1α, IL-6, tumor necrosis factor α (TNF-α), and other proinflammatory factors, and the production of nitric oxide via the induced synthesis of inducible nitric oxide synthase (iNOS). 3,11 Persistent M1 activation will result in chronic inflammation and play a negative role in the healing process. 2 M2 macrophages, mainly secreting IL-10, insulin-like growth factor 1 (IGF-1), transforming growth factor β (TGF-β), and other anti-inflammatory cytokines or growth factors, are thought to play important roles in tissue remodeling and healing. 25,26 According to a previous study, CD206, a cell surface protein, is normally expressed on the M2 but not M1 subtype and therefore serves as a useful marker to identify the M2 phenotype. 29 The regulation of shifting macrophages from M1 to M2 phenotype seemed to successfully promote tendon-to-bone healing in animal models. 5,16
Based on the evidence that the IP-HT could bypass the necrosis phase and improve the tendon-to-bone healing, we hypothesized that using IP-HT grafts in ACLR would influence the local status of macrophage polarization at the tendon-to-bone interface, specifically, facilitating the shift from M1 to M2 phenotype. This study investigated and compared the expression of surrogate markers for M1 and M2 at the tendon-to-bone interface after ACLR using IP-HT or free hamstring tendon (FHT) graft.
Methods
Study Design
After random enrollment into a study group or a control group, ACLR was performed on 42 male, skeletally mature (age, 8 months) New Zealand White rabbits (weight, 3.3 ± 0.3 kg) using the semitendinosus tendon autografts at the right knees. All animals were preoperatively confirmed to be healthy by a veterinarian. The 21 rabbits in the study group underwent ACLR using the semitendinosus tendons with intact tibial insertions, and the 21 rabbits in the control group underwent ACLR using the detached semitendinosus tendons. Postoperatively, 3 rabbits in each group were sacrificed to harvest the specimens at days 1, 3, and 7 and weeks 3, 6, 12, and 24. The experimental design was approved by an institutional animal care and use committee, and the study was conducted according to the Guide for the Care and Use of Laboratory Animals. All animals were procured from Shanghai Jiaotong University.
Surgical Procedure
All rabbits underwent unilateral ACLR using semitendinosus tendon autograft as previously described. 14 After the rabbit underwent general anesthesia and its knee was shaved, disinfected, and draped, the semitendinosus tendon was exposed and harvested at its proximal musculotendinous junction. Its tibial insertion was either detached or preserved according to the group. The original ACL was exposed and removed. The harvested semitendinosus tendon was advanced through the tibial and femoral tunnels, which were created using 2.0 mm–diameter K-wire at the footprints of native ACL. In the control group, both the tibial-side and femoral-side free ends of the graft were fixed via suture. The free end of the graft was sutured to the adjacent periosteum outside the femoral tunnel using 3-0 Ethibond (Ethicon, USA) for fixation on the tibial side. Then, after the graft on the femoral side was pulled under maximum manual tension, its free end was also fixed via suture. In the study group, only the free end of the graft on the femoral side was fixed via suture after the graft was pulled under maximum manual tension. After being flushed with normal saline, the wound was closed in layers. After being disinfected again and injected with penicillin, the animal was returned to its cage without any immobilization.
Immunohistochemistry Staining and Analyses
To test whether IP-HT grafts could influence the phenotype of macrophages, we carried out immunohistochemistry according to the previously reported standard method. 6 The tendon-to-bone interface at a depth of 5 mm from the tibial joint surface was evaluated. After deparaffinzing and rehydrating, the paraffin section underwent antigen retrieval. Then 3% hydrogen peroxide was used to block endogenous peroxidase activity. After serum sealing, primary and secondary antibody incubations, DAB chromogenic reaction, and nucleus counterstaining, the section was dehydrated and mounted. According to the results of antibody activity in our preliminary experiment, anti-CD206 (DF7523; Affinity), anti-iNOS (DF7688; Affinity), anti–TNF-α (AF5202; Affinity), and anti–TGF-β (AF6330; Affinity) were the primary antibodies utilized. According to a previous report, 31 the positive area at the tendon-to-bone interface was quantified (the percentage of positive cells) using ImageJ software (US National Institutes of Health) by the third author (Z.L.), who was blind to group allocation. Three slices of each sample were quantified to obtain an average value, representing the value of this sample.
Statistical Analysis
All continuous data were reported as mean and standard deviation. The Student t test or nonparametric Mann-Whitney U test was used to analyze the difference between the 2 groups at each time point. Data analysis was performed using Stata 13.0 software (Stata Corp). The statistical significance level was set at .05.
Results
Expression of the M1 Marker (iNOS)
The results showed that there was no obvious positive expression of the M1-marker (iNOS) in both groups on postoperative days 1 and 3. From day 7 to week 3, a significantly greater positive expression was observed in the control group versus the study group. At 6, 12, and 24 weeks, there was no obvious positive expression in the 2 groups (Figure 1, A and B). The percentage of iNOS-positive cells was significantly higher in the control group versus the study group on both day 7 (67.7% ± 12.9% vs 22.0% ± 8.0%; P = .04) and week 3 (47.0% ± 8.0% vs 5.3% ± 3.5%; P = .01) (Figure 1C).
Figure 1.
Immunohistochemical staining of inducible nitric oxide synthase (iNOS) in the study groups at postoperative (A) days 1, 3, and 7 and (B) weeks 3, 6, 12, and 24. Scale bars indicate 100 µm. (C) Percentage of iNOS-positive cells at the tendon-to-bone interface; error bars represent SDs. Part A: A-1 day study group; B-3 day study group; C-7 day study group; D-1day control group; E-3 day control group; F-7 day control group. Part B: A-3 week study group; B-6 week study group; C-12 week study group; D-24 week study group; E-3 week control group; F-6 week control group; G-12 week control group; H-24 week control group. *Significant difference between groups (P < .05). ACLR, anterior cruciate ligament reconstruction; FHT, free hamstring tendon; IP-HT, insertion-preserved hamstring tendon.
Expression of M1-Related Inflammation Factor (TNF-α)
The immunohistochemical results of inflammation factor (TNF-α) showed that there was no significant positive expression in either group from 1 day to 3 days after surgery. The positive expression was found in the control group from 7 days to 6 weeks after surgery. In the study group, the positive ratio was always lower than that in the control group from 7 days to 24 weeks after surgery, with the peak centered at 3 weeks (Figure 2, A and B). Quantitative analyses showed that the percentage of TNF-α–positive cells was significantly higher in the control group versus the study group at 7 days (79.3% ± 11.6% vs 26.3% ± 3.2%; P = .01) and 3 weeks (66.0% ± 10.5% vs 28.7% ± 6.5%; P = .03) (Figure 2C).
Figure 2.
Immunohistochemical staining of tumor necrosis factor α (TNF-α) in the study groups at postoperative (A) days 1, 3, and 7 and (B) weeks 3, 6, 12, and 24. Scale bars indicate 100 µm. (C) Percentage of TNF-α–positive cells at the tendon-to-bone interface; error bars represent SDs. Part A: A-1 day study group; B-3 day study group; C-7 day study group; D-1day control group; E-3 day control group; F-7 day control group. Part B: A-3 week study group; B-6 week study group; C-12 week study group; D-24 week study group; E-3 week control group; F-6 week control group; G-12 week control group; H-24 week control group. *Significant difference between groups (P < .05). ACLR, anterior cruciate ligament reconstruction; FHT, free hamstring tendon; IP-HT, insertion-preserved hamstring tendon.
Expression of the M2 Marker (CD206)
The results of immunohistochemical staining and quantitative analyses for the M2 macrophage marker (CD206) showed that there was no obvious positive expression in the 2 groups at 1 day and 3 days after surgery. The control group showed obvious positive expression at 7 days and 3 weeks after surgery. There was no obvious positive expression in the control group at 12 and 24 weeks. The positive expression was found in the study group from 7 days to 12 weeks after surgery, peaking at 3 and 6 weeks (Figure 3, A and B). Quantitative analyses showed that the percentage of CD206-positive cells at 6 weeks was significantly higher in the study group versus the control group (46.3 ± 7.5 vs 12.7 ± 5.7; P = .02) (Figure 3C).
Figure 3.
Immunohistochemical staining of CD206 in the study groups at postoperative (A) days 1, 3, and 7 and (B) weeks 3, 6, 12, and 24. Scale bars indicate 100 µm. (C) Percentage of CD206-positive cells at the tendon-to-bone interface; error bars represent SDs.
Part A: A-1 day study group; B-3 day study group; C-7 day study group; D-1day control group; E-3 day control group; F-7 day control group. Part B: A-3 week study group; B-6 week study group; C-12 week study group; D-24 week study group; E-3 week control group; F-6 week control group; G-12 week control group; H-24 week control group. *Significant difference between groups (P < .05). ACLR, anterior cruciate ligament reconstruction; FHT, free hamstring tendon; IP-HT, insertion-preserved hamstring tendon.
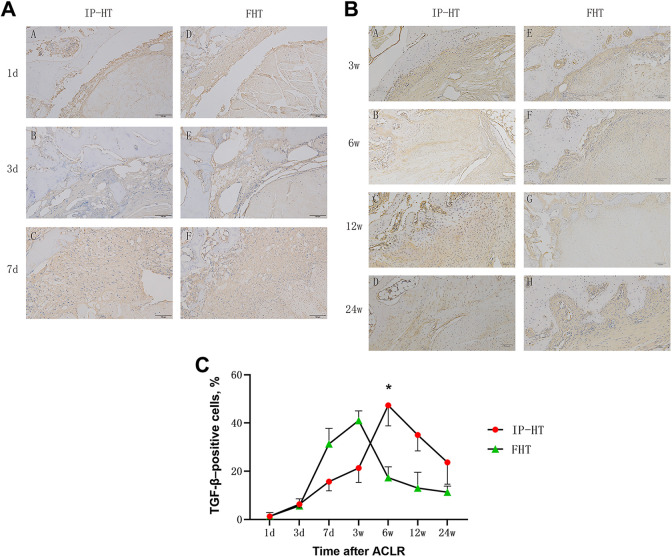
Expression of M2-Related Growth Factor (TGF-β)
There was no significant positive expression of M2-related growth factor (TGF-β) in either group between 1 day and 3 days after surgery. At 7 days and 3 weeks after surgery, there was strong positive expression in the control group. After that, the positive expression in the control group decreased. At 7 days and 3 weeks after surgery, the positive ratio in the study group was lower than that in the control group. At 6 weeks after surgery, there was strong positive expression in the study group. At 12 and 24 weeks after surgery, positive expression was still visible in the study group (Figure 4, A and B). Quantitative analyses showed that the percentage of TGF-β–positive cells in the study group was significantly higher than that in the control group at 6 weeks after surgery (47.3 ± 8.5 vs 17.3 ± 4.5; P = .04) (Figure 4C).
Figure 4.
Immunohistochemical staining of transforming growth factor β (TGF-β) in the study groups at postoperative (A) days 1, 3, and 7 and (B) weeks 3, 6, 12, and 24. Scale bars indicate 100 µm. (C) Percentage of TGF-β–positive cells at the tendon-to-bone interface; error bars represent SDs. Part A: A-1 day study group; B-3 day study group; C-7 day study group; D-1day control group; E-3 day control group; F-7 day control group. Part B: A-3 week study group; B-6 week study group; C-12 week study group; D-24 week study group; E-3 week control group; F-6 week control group; G-12 week control group; H-24 week control group. *Significant difference between groups (P < .05). ACLR, anterior cruciate ligament reconstruction; FHT, free hamstring tendon; IP-HT, insertion-preserved hamstring tendon.
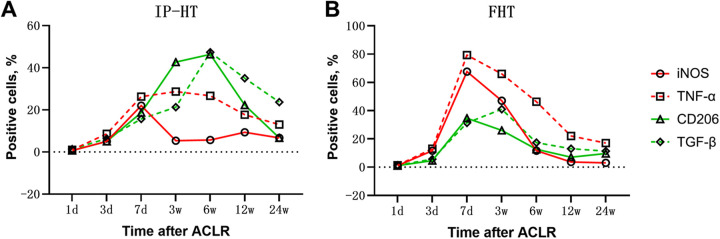
Changes in Expression Over Time
We further analyzed the trends of positive expression in the 2 groups with time after surgery, respectively, and found that the CD206 and TGF-β–positive expression predominated in the study group. More significant expression was observed between 3 and 12 weeks after surgery, with the peak centered at 6 weeks. Positive expression of iNOS and TNF-α was overall weaker than that of CD206 and TGF-β, peaked at 7 days, and then declined to a relatively low level (Figure 5A). In the control group, the expression of iNOS and TNF-α predominated, and more significant expression was observed at 7 days after surgery and decreased to a relatively low level by 6 weeks. The CD206 and TGF-β expression was weaker than the iNOS and TNF-α expression, peaking at 7 days and up to 3 weeks after surgery, after which the expression was not obvious (Figure 5B).
Figure 5.
Percentage of positive cells of inducible nitric oxide synthase (iNOS), CD206, tumor necrosis factor α (TNF-α), and transforming growth factor β (TGF-β) after anterior cruciate ligament reconstruction (ACLR) using (A) insertion-preserved hamstring tendon (IP-HT) graft and (B) free hamstring tendon (FHT) graft at postoperative days 1, 3, and 7 and weeks 3, 6, 12, and 24.
Discussion
This study compared immunohistochemistry results of iNOS, CD206, TNF-α, and TGF-β after ACLR using FHT versus IP-HT graft at days 1, 3, and 7 and weeks 3, 6, 12, and 24. The most important finding was that the use of an FHT graft resulted in significantly positive expression of surrogate markers for M1 (iNOS and TNF-α) at the tendon-to-bone interface in the early time after ACLR while the use of an IP-HT graft led to less expression of surrogate markers of M1 and changed the positive ratio and existence duration of surrogate markers of M2 (CD206 and TGF-β).
After ACLR using FHT graft, the graft undergoes a necrosis phase with excessive local inflammation reaction and inflammatory cell accumulation with macrophages in the majority. 12 Generally, macrophages can be classified into 2 subpopulations according to their polarization status: classically activated M1 macrophages and alternatively activated M2 macrophages. 1,17 M1 macrophages usually appear early after tissue necrosis; are involved in the process of early inflammatory response; and also have an important role in initiating angiogenesis, mainly by secreting IL-1α, IL-6, TNF-α, and other proinflammatory factors. 3 Persistent M1 activation will result in chronic inflammation and play a negative role in the healing process. 3 M2 macrophages, mainly secreting IL-10, IGF-1, TGF-β, and other anti-inflammatory cytokines or growth factors, are thought to play important roles in tissue remodeling and healing. 25,26 Our previous study reported the histologic, radiologic, and biomechanical results after ACLR using a semitendinosus tendon, with or without the tibial insertion preserved, in a rabbit model at postoperative weeks 3, 6, 12, and 24 and showed that an IP-HT graft in ACLR sustained enough blood supply to bypass the graft avascular necrosis stage and result in better tendon-to-bone healing, which ensured a higher biomechanical strength. 14 In this study, we found that the use of an FHT graft resulted in a higher ratio of iNOS- or TNF-α–positive (M1-like) cells at the tendon-to-bone interface in the early time after ACLR while the use of an IP-HT graft led to fewer M1-like cells and a higher proportion of CD206- or TGF-β–positive (M2-like) cells. Several studies have proved that decreasing the proinflammatory M1 macrophages or shifting the polarization status from M1 to M2 phenotype is beneficial to tendon-to-bone healing. 5,10,16,24 Song et al 27 proved that M1 macrophage played a positive role in aseptic inflammation–related graft loosening after ACLR. Gulotta et al 8 observed a significant decrease of M1 macrophages and improved tendon-to-bone healing after administering TNF-α antibodies in a rat model. Hays et al 9 eliminated macrophage in the early postoperative phase in rats with liposomal clodronate and found less fibrovascular scar tissue and more fibrocartilage between graft and bone tunnel. Chen et al 5 found that conditioned medium of bone marrow stem cells could improve tendon-to-bone healing by inhibiting the M1 phenotype and promoting the M2 phenotype. Considering these studies with our results, the shifting from the M1 to M2 phenotype might be an important reason for the improvement of tendon-to-bone healing.
In addition, our results revealed the different trends of macrophage polarization in the 2 groups after ACLR. Previously, Kawamura et al 12 observed the interface between free flexor digitorum longus tendon and bone tunnel and found that M1 macrophages accumulated early after surgery and lasted for 4 weeks while M2 macrophages did not emerge until 11 days after surgery and were highly expressed until 4 weeks. In the FHT group, we found that the expression of surrogate markers for M1 macrophage predominated at 7 days after surgery and lasted for 3 weeks. The expression of surrogate markers for M2 macrophage also peaked at 7 days and up to 3 weeks after surgery but was significantly less than the expression of those for M1 macrophage. In the IP-HT group, we found that the expression of surrogate markers for M1 macrophage, also peaking at 7 days after surgery, was overall less than the expression of surrogate markers for M2 macrophage. Predominated expression of surrogate markers for M2 macrophage was observed between 3 and 12 weeks after surgery, with the peak centered at 6 weeks. These results showed that the use of an IP-HT graft in ACLR might decrease the percentage of M1 macrophages in the early postoperative time at the tendon-to-bone interface and raise the ratio and existence duration of M2 macrophages. This could be the reason for the improvement of tendon-to-bone healing after ACLR using IP-HT graft.
Limitations
There are some limitations to this current study, which follow. (1) It is an animal study based on the rabbit, and the resource of antibodies was limited; therefore, we only detected the expression of iNOS, CD206, TNF-α, and TGF-β. (2) A small number of specimens at each time period may have contributed to a small number of significant differences. (3) Only 1 observer performed quantitative analyses. (4) Other possible reasons that could have made a difference in our findings, other than keeping insertion, are different tensions and fixations in tibia. (5) The underlying reasons why retaining the tendon attachment can alter the local status of macrophage polarization and how macrophages influenced the tendon-to-bone healing still need to be explored and confirmed.
Conclusion
More expression of surrogate markers for M1 rather than M2 macrophages was observed in the tendon-to-bone healing process after ACLR using an FHT graft, whereas use of an IP-HT graft led to reduced M1 macrophages in the early stages and changes in the duration of M2 macrophages.
Footnotes
Final revision submitted December 22, 2021; accepted January 21, 2022.
This study was funded by National Natural Science Funding (81972062). The authors declared that they have no conflicts of interest in the authorship and publication of this contribution. AOSSM checks author disclosures against the Open Payments Database (OPD). AOSSM has not conducted an independent investigation on the OPD and disclaims any liability or responsibility relating thereto.
Ethical approval for this study was obtained from Jiaotong University (reference No. 20150105001).
References
- 1. Amitava D, Mithun S, Soma D, et al. Monocyte and macrophage plasticity in tissue repair and regeneration. Am J Pathol. 2015;185(10):2596–2606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Anca S, Thorsten P, Stefan W, et al. An unrestrained proinflammatory M1 macrophage population induced by iron impairs wound healing in humans and mice. J Clin Invest. 2011;121(3):985–997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Barrientos S, Stojadinovic OM, Brem H, Tomic-Canic M. Growth factors and cytokines in wound healing. Wound Repair Regen. 2010;16(5):585–601. [DOI] [PubMed] [Google Scholar]
- 4. Budny J, Fox J, Rauh M, Fineberg M. Emerging trends in anterior cruciate ligament reconstruction. J Knee Surg. 2017;30(1):63–69. [DOI] [PubMed] [Google Scholar]
- 5. Chen W, Sun Y, Gu X, et al. Conditioned medium of human bone marrow-derived stem cells promotes tendon-bone healing of the rotator cuff in a rat model. Biomaterials. 2021;271:120714. [DOI] [PubMed] [Google Scholar]
- 6. Deng X, Lebaschi A, Camp CL, et al. Expression of signaling molecules involved in embryonic development of the insertion site is inadequate for reformation of the native enthesis: evaluation in a novel murine ACL reconstruction model. J Bone Joint Surg Am. 2018;100(15):e102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Goradia VK, Rochat MC, Grana WA, Rohrer MD, Prasad HS. Tendon-to-bone healing of a semitendinosus tendon autograft used for ACL reconstruction in a sheep model. Am J Knee Surg. 2000;13(3):143–151. [PubMed] [Google Scholar]
- 8. Gulotta LV, Kovacevic D, Cordasco F, Rodeo SA. Evaluation of tumor necrosis factor α blockade on early tendon-to-bone healing in a rat rotator cuff repair model. Arthroscopy. 2011;27(10):1351–1357. [DOI] [PubMed] [Google Scholar]
- 9. Hays PL, Kawamura S, Deng X, et al. The role of macrophages in early healing of a tendon graft in a bone tunnel. J Bone Joint Surg Am. 2008;90(3):565–579. [DOI] [PubMed] [Google Scholar]
- 10. Huang Y, He B, Wang L, et al. Bone marrow mesenchymal stem cell-derived exosomes promote rotator cuff tendon-bone healing by promoting angiogenesis and regulating M1 macrophages in rats. Stem Cell Res Ther. 2020;11(1):496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Juhas U, Ryba-Stanisławowska M, Szargiej P, Myśliwska J. Different pathways of macrophage activation and polarization. Postepy Hig Med Dosw (Online). 2015;69:496–502. [DOI] [PubMed] [Google Scholar]
- 12. Kawamura S, Ying L, Kim HJ, Dynybil C, Rodeo SA. Macrophages accumulate in the early phase of tendon-bone healing. J Orthop Res. 2005;23(6):1425–1432. [DOI] [PubMed] [Google Scholar]
- 13. Liu S, Li H, Tao H, Sun Y, Chen S, Chen J. A randomized clinical trial to evaluate attached hamstring anterior cruciate ligament graft maturity with magnetic resonance imaging. Am J Sports Med. 2018;46(5):1143–1149. [DOI] [PubMed] [Google Scholar]
- 14. Liu S, Sun Y, Wan F, Ding Z, Chen S, Chen J. Advantages of an attached semitendinosus tendon graft in anterior cruciate ligament reconstruction in a rabbit model. Am J Sports Med. 2018;46(13):3227–3236. [DOI] [PubMed] [Google Scholar]
- 15. Lu H, Chen C, Xie S, Tang Y, Qu J. Tendon healing in bone tunnel after human anterior cruciate ligament reconstruction: a systematic review of histological results. J Knee Surg. 2019;32(5):454–462. [DOI] [PubMed] [Google Scholar]
- 16. Lu J, Chamberlain CS, Ji ML, et al. Tendon-to-bone healing in a rat extra-articular bone tunnel model: a comparison of fresh autologous bone marrow and bone marrow-derived mesenchymal stem cells. Am J Sports Med. 2019;47(11):2729–2736. [DOI] [PubMed] [Google Scholar]
- 17. Mescher AL. Macrophages and fibroblasts during inflammation and tissue repair in models of organ regeneration. Regeneration. 2017;4(2):39–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Nebelung W, Becker R, Urbach D, Röpke M, Roessner A. Histological findings of tendon-bone healing following anterior cruciate ligament reconstruction with hamstring grafts. Arch Orthop Trauma Surg. 2003;123(4):158–163. [DOI] [PubMed] [Google Scholar]
- 19. Papachristou G, Nikolaou V, Efstathopoulos N, et al. ACL reconstruction with semitendinosus tendon autograft without detachment of its tibial insertion: a histologic study in a rabbit model. Knee Surg Sports Traumatol Arthrosc. 2007;15(10):1175–1180. [DOI] [PubMed] [Google Scholar]
- 20. Rodeo SA, Arnoczky SP, Torzilli PA, Hidaka C, Warren RF. Tendon-healing in a bone tunnel: a biomechanical and histological study in the dog. J Bone Joint Surg Am. 1993;75(12):1795–1803. [DOI] [PubMed] [Google Scholar]
- 21. Ruffilli A, Pagliazzi G, Ferranti E, Busacca M, Capannelli D, Buda R. Hamstring graft tibial insertion preservation versus detachment in anterior cruciate ligament reconstruction: a prospective randomized comparative study. Eur J Orthop Surg Traumatol. 2016;26(6):657–664. [DOI] [PubMed] [Google Scholar]
- 22. Ruffilli A, Traina F, Evangelisti G, Borghi R, Perna F, Faldini C. Preservation of hamstring tibial insertion in anterior cruciate ligament reconstruction: a review of the current literature. Musculoskelet Surg. 2015;99(2):87–92. [DOI] [PubMed] [Google Scholar]
- 23. Scheffler SU, Unterhauser FN, Weiler A. Graft remodeling and ligamentization after cruciate ligament reconstruction. Knee Surg Sports Traumatol Arthrosc. 2008;16(9):834–842. [DOI] [PubMed] [Google Scholar]
- 24. Shi Y, Kang X, Wang Y, et al. Exosomes derived from bone marrow stromal cells (BMSCs) enhance tendon-bone healing by regulating macrophage polarization. Med Sci Monit. 2020;26:e923328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Shiraishi M, Shintani Y, Shintani Y, et al. Alternatively activated macrophages determine repair of the infarcted adult murine heart. J Clin Invest. 2016;126(6):2151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Siamon G. Alternative activation of macrophages. Nat Rev Immunol. 2003;3(1):23–35. [DOI] [PubMed] [Google Scholar]
- 27. Song B, Jiang C, Luo H, et al. Macrophage M1 plays a positive role in aseptic inflammation-related graft loosening after anterior cruciate ligament reconstruction surgery. Inflammation. 2017;40(6):1815–1824. [DOI] [PubMed] [Google Scholar]
- 28. Webster KE, Feller JA, Hameister KA. Bone tunnel enlargement following anterior cruciate ligament reconstruction: a randomised comparison of hamstring and patellar tendon grafts with 2-year follow-up. Knee Surg Sports Traumatol Arthrosc. 2001;9(2):86–91. [DOI] [PubMed] [Google Scholar]
- 29. Xu ZJ, Gu Y, Wang CZ, et al. The M2 macrophage marker CD206: a novel prognostic indicator for acute myeloid leukemia. Oncoimmunology. 2020;9(1):1683347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Zaffagnini S, Golano P, Farinas O, et al. Vascularity and neuroreceptors of the pes anserinus: anatomic study. Clin Anat. 2003;16(1):19–24. [DOI] [PubMed] [Google Scholar]
- 31. Zhang X, Deng XH, Song Z, et al. Matrix metalloproteinase inhibition with doxycycline affects the progression of posttraumatic osteoarthritis after anterior cruciate ligament rupture: evaluation in a new nonsurgical murine ACL rupture model. Am J Sports Med. 2020;48(1):143–152. [DOI] [PubMed] [Google Scholar]