Abstract
In spite of a major public health burden with increasing prevalence, current osteoarthritis (OA) management is largely palliative with an unmet need for effective treatment. Both industry and academic researchers have invested a vast amount of time and financial expense to discover the first diseasing-modifying osteoarthritis drugs (DMOADs), with no regulatory success so far. In this narrative review, we discuss repurposed drugs as well as investigational agents which have progressed into phase II and III clinical trials based on three principal endotypes: bone-driven, synovitis-driven and cartilage-driven. Then, we will briefly describe the recent failures and lessons learned, promising findings from predefined post hoc analyses and insights gained, novel methodologies to enhance future success and steps underway to overcome regulatory hurdles.
Keywords: disease-modifying drugs, DMOADs, endotype, intra-articular therapy, osteoarthritis
Introduction
Impact and burden of osteoarthritis
Osteoarthritis (OA) is the most prevalent age-related articular disease and commonly manifests in the ageing population with a spectrum of signs and symptoms such as chronic articular pain, brief morning stiffness, limitation of functional activities, joint line tenderness, bony enlargement, joint deformity, coarse crepitus and muscle wasting. 1 Its global prevalence is estimated at 22.9% in persons over 40 years of age in 2020 (correspondingly 654.1 million individuals). 2 Increased prevalence in the next decades is expected due to the increasing age of the population and the obesity epidemic. Furthermore, the socioeconomic costs of OA are considerable 3 as 1–2.5% of the gross national product (GNP) has to be utilized as the direct and indirect costs for OA management in most Western countries, 4 constituting a major public health challenge for coming decades.
Drug development and clinical unmet needs
Current OA management focuses on symptomatic improvement only 5 and is largely palliative, although OA disease course is usually progressive over many years. 6 In addition, the existing pharmacological or non-pharmacological treatments have shown only modest efficacy at best 7 and adverse effects in the gastrointestinal (GI), cardiac or renal systems prohibit the long-term use of commonly used analgesics as OA patients are often elderly with comorbid diseases. 1 Hence, there exists an immense unmet need in the current therapies, with more than half of the patients with moderate and severe OA reporting unsatisfactory pain relief. 8
Despite such an enormous impact and disease burden on OA patients, no effective disease-modifying osteoarthritis drugs (DMOADs) have been approved by regulatory bodies. 9 A DMOAD is a pharmacological agent that will delay or reverse the progression of the structural damage of the joint, thereby leading to clinical translation of improvement in symptoms, manifested either by pain reduction or benefits in physical function. 9 Therefore, both structural and symptomatic benefits are needed for an agent to be considered a DMOAD. 10 It is crucial to discover innovative, effective DMOAD therapies to mitigate the disease burden.
Phenotypes/endotypes in OA
OA is a heterogeneous and multifaceted disease that manifests first as a molecular derangement (abnormal metabolism in the joint tissue) leading to anatomic and/or physiologic malfunction such as osteophyte formation, cartilage damage, synovitis and loss of normal joint function that can culminate in illness and whole joint organ failure. 11 Therefore, classification of the OA patients into subgroups that possess distinct characteristics or pathogenic pathways will enable clinical trials to identify appropriate patients who may have benefits from a particular investigational agent. Broadly, OA disease can involve different ‘clinical phenotypes’ or ‘molecular endotypes’. The term ‘clinical phenotype’ should represent the clinical manifestations of a disease and is based on observable traits such as aetiologic factors and risk factors, whereas ‘molecular endotype’ should pertain to the molecular drivers of disease pathogenesis via cellular, molecular and biomechanical signalling pathways and is irrespective of its clinical manifestations. 12
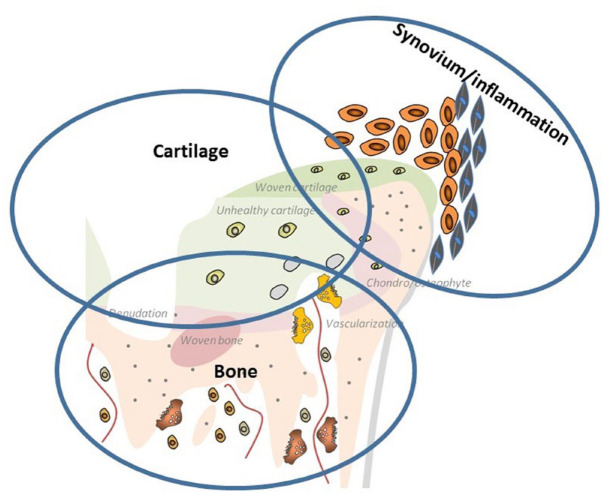
As an important qualification, OA patients may have overlapping clinical phenotypes, and a given clinical OA phenotype may possess a variety of molecular endotypes at different stages of pathogenesis pathways, complicating the task of identifying the distinct OA subgroups.9,13 However, generally, a consensus seems to exist regarding the presence of three endotypes (Figure 1): (1) bone-driven endotype, (2) synovitis-driven endotype and (3) cartilage-driven endotype.9,14
Figure 1.
The three endotypes of OA: (1) bone-driven endotype, (2) synovitis-driven endotype and (3) cartilage-driven endotype.
Bone-driven endotype
An uncoupled remodelling process between bone formation and resorption in the subchondral bone results in microstructural changes, depending on the spontaneous stimulation (in early-stage OA) or inhibition (in late-stage OA) of osteoclastic bone resorption. 15 Furthermore, bone pain can be induced by an acidic microenvironment created via H+ ions which bone-resorbing osteoclasts generated as shown in animal models of bone metastasis. 16 Osteoclasts can produce Netrin-1, leading to sensory innervation and pain via its receptor DCC (deleted in colorectal cancer). 17 These findings indicate the potential of bone-protective agents in DMOAD research.
Synovitis-driven endotype
In OA patients, there is biochemical and histological evidence 18 of infiltration of mononuclear cells in the synovium, 19 proliferation of synovial cells as well as inflammatory cytokines such as tumour necrosis factor (TNF)-α, interleukin (IL)-1β and IL-6 20 supported by the findings with imaging modalities. 21 In addition, an association of synovitis with symptoms and radiological progression was reported, 22 suggesting the potential role of anti-inflammatory agents in an OA subgroup with predominant inflammatory changes.
Cartilage-driven endotype
The hallmark of OA pathogenesis seems to be a failure to maintain cartilage homeostasis resulting in an imbalance between synthesis and degradation of extracellular matrix components. This phenomenon can lead to cartilage softening, fibrillation and fissuring of the superficial cartilage layers, and diminished cartilage thickness. 23
Our review will focus on the DMOAD candidates currently undergoing or having completed the active phase II and III clinical trials since 2017 by conducting electronic and manual searches on the https://clinicaltrials.gov/ (Tables 1 and 2). Although the assignment of a specific drug on account of its predominant activity was made only to one specific endotype, some drugs may have broader endotype effects, and where present, these are duly described.
Table 1.
Summary of repurposed DMOAD clinical trials which are active or completed since 2017 (phases II and III).
| Targeted endotype | Drug class | Name of investigational drug | Route | OA site | Active trial IDs/estimated completion date | Completed trial IDs/completed date |
|---|---|---|---|---|---|---|
| Bone-driven | PTH | Teriparatide | S/C | Knee | NCT03072147 (October 2022) | |
| Antiresorptives | Zoledronic acid | IV | Hip | NCT04303026 (March 2022) | ||
| Denosumab | S/C | Hand | NCT02771860 (May 2021) | |||
| Vitamin | Vitamin D | Oral | Knee | NCT04739592 (July 2024) | ||
| Synovitis-driven | DMARD | Methotrexate | Oral | Knee | NCT03815448 (December 2022) | ISRCTN77854383 (2018) |
| Hydroxychloroquine | Oral | Hand | ISRCTN46445413 (July 2018) | |||
| Anti-TNF | Etanercept | S/C | Hand | NTR1192 | ||
| Anti-IL-6 | Tocilizumab | IV | Hand | NCT02477059 (February 2019) | ||
| Other mechanism | Biguanides | Metformin | Oral | Knee |
NCT04767841 (December 2022) NCT05034029 (December 2024) |
|
| Antiobesity agents | Liraglutide | S/C | Knee | NCT02905864 (March 2019) |
DMARD, disease-modifying antirheumatic drug; DMOAD, disease-modifying osteoarthritis drug; IL, interleukin; IV, intravenous; OA, osteoarthritis; PTH, parathyroid hormone; S/C, subcutaneous; TNF. tumour necrosis factor.
Table 2.
Summary of investigational DMOAD clinical trials which are active or completed since 2017 (phases II and III).
| Targeted endotype | Drug class | Name of investigational drug | Route | OA site | Active trial IDs/estimated completion date | Completed trial IDs/completed date |
|---|---|---|---|---|---|---|
| Bone-driven | Cathepsin K inhibitors | MIV-711 | Oral | Knee |
NCT02705625 (May 2017) NCT03037489 (November 2017) |
|
| Matrix extracellular phosphoglycoprotein | TPX-100 | IA | Knee | NCT02837900 (August 2017) | ||
| Synovitis-driven | DNA plasmid with IL-10 transgene | XT-150 | IA | Knee | NCT04124042 (February 2022) | |
| Anti-IL-1 | Diacerein | Oral | Knee | NCT02688400 (December 2019) | ||
| Cartilage-driven | ADAMTS-5 inhibitors | GLPG1972/S201086 | Oral | Knee | NCT03595618 (July 2020) | |
| Fibroblast growth factor (FGF18) | Sprifermin (AS902330) | IA | Knee | NCT01919164 (May 2019) | ||
| Gene therapy | TissueGene-C | IA | Knee |
NCT03291470 (September 2021) NCT03203330 (October 2024) |
||
| Wnt/β-catenin signalling pathway inhibitors | Lorecivivint SM04690 |
IA | Knee |
NCT03928184 (August 2021) NCT03727022 (September 2021) NCT04385303 (September 2021) NCT03706521 (December 2021) NCT04520607 (September 2022) |
NCT02536833 (April 2017) NCT03122860 (April 2018) |
|
| Senolytic agents | UBX0101 | IA | Knee |
NCT04129944 (August 2020) NCT04349956 (November 2020) |
||
| ANGPTL3 protein | LNA043 | IA | Knee | NCT03275064 (September 2022) | ||
| LRX712 | IA | Knee | NCT04097379 (January 2024) |
ADAMTS, a disintegrin and metalloproteinases with thrombospondin motifs; ANGPTL3, angiopoietin-like 3; DMOAD, disease-modifying osteoarthritis drug; FGF, fibroblast growth factor; IA, intra-articular; IL, interleukin; OA, osteoarthritis.
Recent phase II and III clinical trials
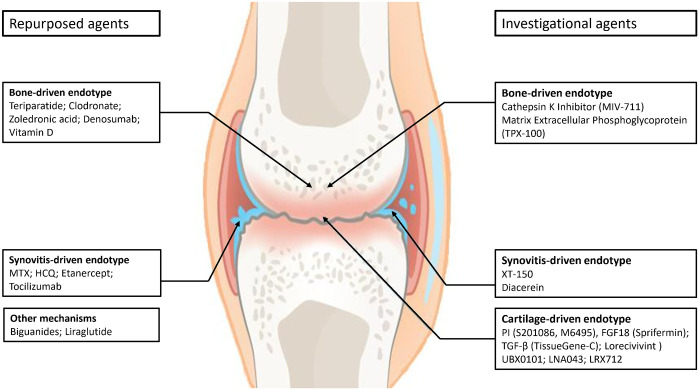
These agents will be broadly divided into two major groups: (1) repurposed drugs and (2) investigational drugs. The individual drugs included are illustrated in Figure 2.
Figure 2.
Repurposed or invesigational drugs related to the three main OA endotypes (phase II and III RCTs).
Repurposed drugs already in phase II and III trials, either active or completed since 2017
The costs of bringing new drugs to the market increased ninefold from 1979 (US$92 million) to 2010 (US$883.6 million), 24 and were reported at US$1335.9 million (in 2018 US dollars). 25 Due to high attrition rates, escalating costs and regulatory hurdles, drug repurposing strategy is often used for rediscovering new uses for existing approved therapeutic agents outside the scope of the original indication. 26 This approach offers a better risk-versus-reward trade-off over the de novo drug discovery as the safety and pharmacokinetic profiles have already been examined in preclinical models and humans, and the time frame for drug development is much reduced. 27 This leads to substantial savings in preclinical and phase I and II costs, 28 and reduces the development cost to US$300 million on average. 29 Repositioning success stories were evidenced in history (e.g. sildenafil in erectile dysfunction 30 ) and recent pharmacological treatment for COVID-19 disease. 31 With the proposed biochemical pathway scientifically validated, several existing drugs have been examined for the repositioning idea, some of which are in advanced stages of OA clinical trials (Table 3).
Table 3.
Published results of phase II/III clinical trials related to the repurposed DMOAD agents since 2017 by OA endotypes.
| Authors/References | ClinicalTrials.gov Identifier/trial phase |
OA site | Dosage, route of interventions | n | Longest follow-up | Efficacy in symptomatic modification | Efficacy in structural modification | ||
|---|---|---|---|---|---|---|---|---|---|
| Pain (0–50) (WOMAC if not denoted otherwise) | Function (0–170) (WOMAC if not denoted otherwise) | Plain X-rays | MRI | ||||||
| Bone-driven endotype | |||||||||
| Clodronate | |||||||||
| Frediani et al. 32 | – | Knee | Clodronate IM 200 mg/day for 15 days and then once weekly for next 2.5 months Clodronate IM 200 mg/day for 15 days and then once weekly for next 11.5 months |
37 37 |
12 months | 50.3 ± 31.9 (SD) 15.6 ± 9.8 (SD)* (VAS = 0–100) |
24.0 ± 11.9 13.5 ± 5.7* |
NA | NA |
| Zoledronic acid | |||||||||
| Cai et al. 33 | ACTRN12613000039785 Phase III |
Knee | Placebo saline IV Zoledronic acid IV 5 mg baseline and 12 months |
110 113 |
24 months | −16.8 (−22.0 to −11.6) −11.5 (−16.9 to −6.2) (VAS) |
NA | NA | NA |
| Vitamin D | |||||||||
| Perry et al. 34 | – | Knee | Placebo Cholecalciferol oral 800 IU/day |
26 24 |
24 months | NA | NA | Change from baseline 61.5 (−1085.6 to 1208.6) 155.4 (−1097.3 to 1408.0) (synovial volume mm3) − 193.4 (−2845.7 to 2459.0) −506.9 (−3395.6 to 2381.9) (subchondral BML volume mm3) |
|
| MacFarlane et al. 35 | NCT01351805 | Knee | Placebo Cholecalciferol oral 2000 IU/day |
630 591 |
4 years | 34.6 ± 0.9 (SE) 32.7 ± 0.9 |
34.6 (0.9) (SE) 34.1 (1.0) |
||
| Synovitis-driven endotype | |||||||||
| Methotrexate | |||||||||
| Kingsbury et al. 36 | ISRCTN77854383 Phase III |
Knee | Placebo 10–25 mg over 8 weeks and then maintenance at 25 mg |
68 66 |
6 months | −0.83 (−1.55, −0.10) (mean difference, NRS pain) | –5.01 (−8.74, −1.29) | NA | 14.89 (–18.19, 47.96) (mean difference, mm3) |
| Ferrero et al. 37 |
NCT01068405 Phase III |
Hand | Placebo 10 mg MTX for 12 months |
32 32 |
12 months | 17.8 (29) 14.3 (19) Mean change from baseline (SD), VAS pain range (0–100) |
0.2 (5) 0.33 (5) Change from baseline (SD) FIHOA score range (0–30) |
No significant change in Verbruggen-Veys score and GUSS | No significant difference in MRI synovitis and other lesions |
| Hydroxychloroquine | |||||||||
| Lee et al. 38 |
NCT 01148043 Phase III |
Hand | Placebo HCQ 400 mg once a day for 24 weeks |
98 98 |
24 weeks | +1.1 mm (95% CI: −4.2 to 6.4) 1.1 mm (95% CI: −6.7 to 4.1) Mean change from baseline, VAS pain range 0–100 |
No significant change for AUSCAN and AIMS2-SF | NA | NA |
| Kingsbury et al. 39 | ISRCTN91859104 | Hand | Placebo HCQ (200–400 mg) for 12 months (maximum 6.5 mg/kg per day) |
119 113 |
12 months | 5.51 (95% CI: 4.98 to 6.04) 5.39 (95% CI: 4.83 to 5.92) Mean score, NRS pain 0–10 |
18.74 (17.30 to 20.18) 19.72 (18.24 to 21.20) AUSCAN |
48.30 (47.50 to 49.10) 48.14 (47.32 to 48.96) Kallman total radiographic score range (0–220) |
NA |
| Kedor et al. 40 | ISRCTN46445413 | Hand | Placebo 200 mg/day, 200 and 400 mg every other day or 200 mg two times a day according to body weight |
78 75 |
52 weeks | 26.5 (23.9 to 29.1) 26.7 (23.9 to 29.4) Mean score at 52 weeks |
51.3 (46.6 to 56.0) 48.1 (43.0 to 53.2) |
46.8 (95% CI: 45.7 to 47.8) 47.1 (95% CI: 4.60 to 4.82) |
|
| Etanercept | |||||||||
| Kloppenburg et al. 41 | Hand | Placebo Etanercept SC 50 mg weekly for the first 24 weeks, followed by 25 mg weekly thereafter |
45 45 |
1 year | 45.4 (25.7) 35.7 (25.1) Mean score (SD) VAS pain range 0–100 |
10.9 (9.9) 9.9 (5.9) FIHOA |
288 (34) (n = 31) 287 (36) (n = 23) GUSS |
Synovitis (n = 10 each) 1.4 (0.3) 1.0 (0.5) BMLs (n = 10 each) 0.7 (0.3) 0.6 (0.3) |
|
| Tocilizumab | |||||||||
| Richette et al. 42 |
NCT02477059 Phase III |
Hand | Placebo Tocilizumab 8 mg/kg IV at weeks 0 and 4 |
41 42 |
6 weeks | −9.9 (SD: 20.1) −7.9 (SD: 19.4) VAS pain range 0–100 |
0.2 ± 0.6 −0.04 ± 0.6 FIHOA (0–30) |
||
| Other mechanisms | |||||||||
| Liraglutide | |||||||||
| Gudbergsen et al. 43 |
NCT02905864 Phase IV |
Knee | Placebo liraglutide 3 mg/day for 52 weeks |
76 80 |
52 weeks | −0.6 (−4.4, 3.3) 0.4 (−3.3, 4.0) Mean change from baseline, KOOS symptom |
−1.6 (−5.1, 1.9) 1.4 (−2.0, 4.8) Mean change from baseline, KOOS function |
NA | NA |
denotes P ≤ 0.05, active vs control. AIMS2-SF, Arthritis Impact Measurement Scale 2–Short Form; AUSCAN, Australian Canadian Hand Osteoarthritis Index; BML, bone marrow lesion; CI, confidence interval; DMOAD, disease-modifying osteoarthritis drug; FIHOA, Functional Index for Hand Osteoarthritis; GUSS, Ghent University Scoring System; HCQ, hydroxychloroquine; IM, intramuscular; IV, intravenous; KOOS, Knee injury and Osteoarthritis Outcome Score; MRI, magnetic resonance imaging; MTX, methotrexate; NA, non-available; NRS, Numerical Rating Scale; OA, osteoarthritis; SC, subcutaneous; SD, standard deviation; VAS, Visual Analogue Scale; WOMAC, Western Ontario and McMaster Universities Osteoarthritis Index.
Bone-driven endotype
Antiresorptive agents, which are administered as the standard treatment in osteoporosis, 44 may be promising therapies as subchondral remodelling on which these agents act is involved in OA pathogenesis, including magnetic resonance imaging (MRI)–detected bone marrow lesions (BMLs). 45 BMLs are observed as an altered signal pattern related to increased vascularization, bone marrow necrosis, fibrosis and less mineralized bone on MRI 46 and as an abundance of matrix metalloproteinases (MMPs), TNF-α and substance P on histochemical analysis. 47 BMLs have an established association with OA pathogenesis and symptoms.48,49 In addition, compared with normal joints, the elastic modulus of the trabecular subchondral bone was reduced by 60% in early OA, 50 perhaps due to incomplete mineralization sequence because of the high rate of bone remodelling. 51 Therefore, antiresorptive agents seem to be a promising therapy due to their implications in pathogenesis and clinical manifestations in OA. Recently, teriparatide, clodronate, zoledronic acid, denosumab and vitamin D have been investigated as the potential agents targeted at the bone-driven OA endotypes.
Teriparatide
Teriparatide is a recombinant human parathyroid hormone (PTH) and acts on bone-forming osteoblasts, resulting in an anabolic effect within active remodelling sites (remodelling-based osteogenesis), and on surfaces of previously inactive bone (modelling-based osteogenesis). 52 Following completion of the Fracture Prevention Trial (FPT), 53 it was granted approval by the US Food and Drug Administration (FDA) in 2002 and later by the European Medicines Agency (EMA) in 2003. 54 It showed an increase in proteoglycan content, reducing cartilage damage in OA animal models 55 as well as pain reduction in temporomandibular joint OA changes in ageing mice. 56 Intermittent administration of teriparatide improved pain by reducing prostaglandin E2 and sensory innervation of subchondral bone. 57 A phase II clinical trial in knee OA is currently ongoing (NCT03072147).
In the context of long-term OA treatment, one of the potential safety issues related with teriparatide is osteosarcoma due to toxicology findings of osteosarcoma in rats. 58 However, administering non-human primates (cynomolgus monkeys) with the drug as eight times large as the dose used in the human dose did not lead to osteosarcoma development. 59 In addition, recent analysis of Forteo Patient Registry based on the estimated 242,782 person-years of observation 60 as well as a 15-year US Postmarketing Surveillance Study 61 revealed no incident cases of osteosarcoma among teriparatide users after 8 years of follow-up. In November 2020, FDA approved labelling changes such as removing the 2-year limitation and the boxed warning about the osteosarcoma risk. 62
Clodronate
Clodronate is a first-generation non-nitrogenous bisphosphonate and possesses anti-inflammatory and analgesic effects 63 in addition to osteoclast inhibition and apoptosis. 64 It reduced the generation of cytokines (IL-1, TNF-α) and metalloproteases (MMP 2/3/9) in the synovium and cartilage in animal models. 65 In a small study including 74 patients with knee OA, a reduction of pain and BMLs was reported after providing a higher dose of intramuscular clodronate than used for osteoporosis (200 mg daily for 15 days and then once weekly for the next 11.5 months), compared with a shorter maintenance regimen (2.5 months). 32 In non-overweight individuals in whom weight-bearing damage is not a driving cause of OA progression, a bone-related abnormality and remodelling may play a crucial role. 66 Non-overweight female patients (body mass index, BMI < 25 kg/m2) with early radiographic knee OA [baseline Kellgren-Lawrence (KL) grade <2] revealed a 51% reduction of 2-year radiographic progression after bisphosphonate exposure by utilizing a propensity-matched retrospective cohort analysis of the Osteoarthritis Initiative (OAI; n = 346) with no significant effects in patients with advanced OA disease. 67
Zoledronic acid
Zoledronic acid is a third-generation nitrogen-containing bisphosphonate, and inhibits farnesyl pyrophosphate synthase, which is critical for osteoclast function. 68 In in vitro studies, its inhibition is 3-, 7-, 17- and 67-fold more potent than risedronate, ibandronate, alendronate and pamidronate, respectively.69,70 It also showed the highest binding affinity for the hydroxyapatite preferentially at sites of high bone turnover. 71 It was first approved in 2002 for adjunctive treatment for multiple myeloma and bone metastases to reduce skeletal-related events 72 and later in 2007 for postmenopausal osteoporosis, 73 based on the findings of the landmark Health Otcomes and Reduced Incidence with Zoledronic Acid Once Yearly (HORIZON) Pivotal Fracture Trial. 74
In a recent 2-year Zoledronic Acid for Osteoarthritis Knee Pain (ZAP2) study targeted at the bone-driven endotypes, knee OA patients with significant knee pain and MRI-detected BMLs were recruited and provided with twice yearly administration of 5 mg of zoledronic acid (n = 223). Despite methodological strengths such as adequate power to detect disease-modifying effects on BMLs, utilization of the more sensitive MRI-detected cartilage volume as the primary outcome, and the 2-year length of the follow-up, the study failed to reveal no significant improvement in knee pain, BML size and cartilage volume loss. 33 A greater symptomatic improvement was detected in early knee OA patients [i.e. without radiographic joint space narrowing (JSN)] on the exploratory subgroup analysis. 33 There is neither structure-modifying benefit nor reduction of knee replacement (KR; 9% in the zoledronic acid group versus 2% in the placebo group). Currently, one more clinical trial is examining the effects of Zoledronic Acid in hip OA (NCT04303026).
Denosumab
Denosumab is a fully human monoclonal immunoglobulin G2 (IgG2) antibody that binds and inhibits the receptor activator of nuclear factor-κB ligand (RANKL), which selectively inhibits osteoclastogenesis with a profound decrease in the rate of bone remodelling. 75 Its use for postmenopausal osteoporosis was approved by the FDA in the United States and by the European Medicines Agency in Europe since 2010, 76 based on the results of the pivotal Fracture Reduction Evaluation of Denosumab in Osteoporosis Every 6 Months (FREEDOM) trial. 77 In terms of bone density and remodelling, denosumab is more efficacious than the alendronate, risedronate and ibandronate. 78 Denosumab might lead to a dramatic increase in bone mass, perhaps through anabolic action on bone remodelling. 79 However, there is no clue regarding the effect of this phenomenon on the quality of subchondral bone in early OA patients. 50 There is one clinical trial evaluating the benefits of denosumab in hand OA (NCT02771860).
Vitamin D
Vitamin D is a fat-soluble vitamin that is essential for calcium homeostasis and bone metabolism, such as subchondral remodelling. Both early-stage increased remodelling and bone loss, and the late-stage slow remodelling and subchondral sclerosis are implicated in different stages of the OA pathogenetic process. 80 It promotes proteoglycan production in chondrocytes and reduces the release of inflammatory cytokine via stimulation of 5′-adenosine monophosphate-activated protein kinase (AMPK)/mTOR signalling. 81 In a Vitamin D and Omega-3 Trial (VITAL) in patients with chronic knee pain, vitamin D supplementation showed no improvement in both pain and function at 4-year follow-up. 35 Vitamin D supplementation provided for symptomatic knee OA caused no structural benefits in the MRI outcomes such as synovial volume and subchondral BML volume at 2-year follow-up (n = 50). 34 In a recent meta-analysis, there is no structure-modifying effect of vitamin D in knee OA. 82 A small phase IV clinical trial is ongoing (NCT04739592).
Synovitis-driven endotype
A variety of conventional and biological disease-modifying antirheumatic drugs (DMARDs), which are being widely utilized in the treatment of inflammatory arthritides such as RA83,84 and psoriatic arthritis, 85 were recently examined in patients with inflammation-driven OA endotypes due to their impressive anti-inflammatory action. In a meta-analysis published in 2018, DMARDs failed to show clinically significant symptomatic improvement in either hand (both erosive and non-erosive) or knee OA. 86 Recently, methotrexate (MTX), hydroxychloroquine (HCQ), etanercept and tocilizumab were examined for repurposing agents in OA.
Methotrexate (MTX)
MTX was studied initially as a chemotherapeutic agent for childhood leukaemia in 1948 87 and then in rheumatoid arthritis (RA) around 1970, 88 leading to FDA approval as a conventional DMARD in 1988. 89 RA is a prototype of chronic inflammatory autoimmune disease with florid inflammation of the synovial joints involving proinflammatory cytokines such as IL-1 and TNF-α. 90 MTX is a folate analogue that binds dihydrofolate reductase to interfere with DNA synthesis in actively dividing cells and induces inhibition of IL, TNF-α and so on. 91 Currently, MTX is the anchor drug for RA treatment.
As the synovitis-driven OA endotype has increased levels of proinflammatory cytokines, modulating the inflammatory response by using MTX may be beneficial theoretically. In the phase III PROMOTE trial published as a 2019 Osteoarthritis Research Society International (OARSI) conference abstract (n = 134), oral MTX showed significant improvement in knee pain and function at 6-month follow-up but not at 9 and 12 months with no change in synovial volume on contrast-enhanced MRI at 6 months. 36 A recent study on 64 participants with symptomatic erosive hand OA revealed no significant difference over the pain VAS (Visual Analogue Scale) score and functional outcomes on Functional Index for Hand Osteoarthritis [FIHOA] score at both 3- and 12-month follow-ups. 37 There was also no significant difference in structural progression evaluated by plain radiographs and MRI BMLs, erosions and synovitis. Currently, a phase III knee study is active for symptomatic OA patients with effusion-synovitis grade of ⩾2 (NCT03815448).
Hydroxychloroquine (HCQ)
HCQ belongs to the group of antimalarial agents, was synthesized in 1946 and is currently used in various rheumatic and skin diseases since its first approval in 1955 by FDA.92,93 It possesses anti-inflammatory actions such as decreasing cytokine production from T cells and monocytes, especially IL-1 and IL-6. 94 In the first randomized controlled trial (RCT) conducted in hand OA and published in 2018, administration of oral 400 mg HCQ once a day for 24 weeks is not superior to placebo in improving symptomatic hand OA at weeks 6, 12 and 24 (n = 196). 38 Similarly, another bigger HERO study revealed no significant treatment differences at 3, 6 or 12 months in hand OA (n = 248). 39 Recently, in the OA-TREAT clinical trial conducted in erosive hand OA for 52 weeks (n = 153), HCQ failed to show any difference in symptomatic and radiographic outcomes, confirming the findings of the previous two studies. 40
Etanercept
Etanercept was the first TNF inhibitor approved to treat RA in the United States in 1998 and Europe in 2000. 95 In a 1-year, Etanercept in patients with inflammatory hand osteoarthritis (EHOA) clinical trial in participants with ⩾4 interphalangeal joints (IPJs) with osteoarthritic nodes, ⩾1 IPJ with soft tissue swelling or erythema, ⩾1 IPJ with positive power Doppler activity on ultrasound and ⩾1 IPJ with radiographic pre-erosive or erosive disease (n = 90), etanercept (24 weeks 50 mg/week, thereafter 25 mg/week) did not improve pain and function at 24 weeks or 1 year. However, on subgroup analyses of participants with active inflammation, such as the presence of soft tissue swelling or power Doppler signals, etanercept revealed an improvement in radiographic scores 41 measured by the validated Ghent University Scoring System. 96 In addition, etanercept reduced serum MMP3-levels but not other soluble biomarkers of inflammation, cartilage and bone damage. 97
Tocilizumab
Tocilizumab is a genetically engineered humanized neutralizing antibody inhibiting the binding of IL-6 to its receptors 98 and blocking both classic (anti-inflammatory) and trans-signalling (proinflammatory) pathways. 98 Its use in RA was approved in Europe in 2009 and in the United States in 2010. 96 However, in a recent 12-week phase III clinical trial (n = 83), two infusions 4 weeks apart (weeks 0 and 4) of tocilizumab (8 mg/kg intravenous) revealed no significant benefits in pain and function in refractory hand OA with at least three painful joints, compared with the placebo. 42 This might suggest that removing IL-6 signalling alone in the short term is not sufficient for pain reduction in human OA.
Agents with other mechanisms
Biguanides (metformin)
Metformin is a member of the biguanides and the first-line therapy for type 2 diabetes mellitus (DM) patients with obesity. While it was clinically available in the United Kingdom in 1958 and in Canada in 1972, the FDA approved metformin in 1994 for type 2 DM. 100 In murine arthritis models, administration with metformin showed a reduction of arthritis scores and bone destruction as well as an anti-inflammatory effect by lowering serum levels of proinflammatory cytokines mediated via indirect activation of AMPK pathways.101,102 Treatment with metformin increased antinociceptive activity, and anti-inflammatory and chondroprotective effects in OA mice with monosodium iodoacetate (MIA) model 103 as well as with collagenase-induced osteoarthritis (CIOA) model.104,105
A combination of metformin with COX-2 inhibitors (n = 968) reduced the risk of joint replacement surgery (12.81% versus 16.22%) over 10 years compared with COX-2 inhibitors alone (n = 1936) in type 2 DM patients with OA in a nationwide, retrospective, matched-cohort study. 106 However, data on the OA severity, disease duration and other assessment scores are unavailable, limiting the validity of study findings. In OAI participants with radiographic knee OA and obesity (BMI ⩾ 30 kg/m2), metformin users (n = 56) showed slower MRI-detected cartilage volume loss (0.71% versus 1.57% per annum) in the medial compartment of the joint over 4 years compared with non-user (n = 762) despite no significant symptomatic improvement. 107 Currently, the first two RCTs are ongoing (NCT04767841, NCT05034029).
Liraglutide
Liraglutide is a glucagon-like peptide-1 (GLP-1) that regulates glucose and energy homeostasis via GLP-1R binding. Liraglutide was approved by the FDA in 2014, and EMA in 2015, for chronic weight management in overweight people with a BMI ⩾ 27 kg/m2. 108 It attenuated cartilage degeneration in an OA model of knee joints in vivo by exerting antiapoptotic and anti-inflammatory effects on chondrocytes.109,110 Administration of Liraglutide 3 mg/day for 52 weeks in participants with KOA and overweight/obesity (BMI ⩾ 27 kg/m2) revealed no difference in pain between the liraglutide and placebo group despite a significant loss in body weight (n = 156). 43 More GI adverse events were reported in the liraglutide group (50.2% versus 39.2%).
Investigational DMOAD agents already in phase II and III trials, either active or completed since 2017
In the United States, federal law required that a drug must have proven safety as well as efficacy before marketing for the prescription. 111 If a drug appears promising in preclinical studies in a laboratory, a drug sponsor or sponsor-investigator can submit an investigational new drug (IND) application for collecting the data required to establish that the product will not expose humans to unreasonable risks when used in limited, early-stage clinical studies. 112 Therefore, an investigational drug is an experimental drug that is being examined in clinical trials to detect whether it is safe in humans and effective for a particular disease.
Very recently, we have extensively reviewed these investigational agents, and we will briefly discuss and update, if new findings are published in the interim, these drugs in this article (Table 4). Therefore, the readers who are keen on a more detailed coverage are suggested to read the recent reviews.9,13
Table 4.
Published results of phase II/III clinical trials related to the investigational DMOAD agents since 2017 by OA endotypes.
| Authors/References | ClinicalTrials.gov Identifier/trial phase |
OA site | Dosage, route of interventions | n | Longest follow-up | Efficacy in symptomatic modification | Efficacy in structural modification | ||
|---|---|---|---|---|---|---|---|---|---|
| Pain (0–50) (WOMAC if not denoted otherwise) | Function (0–170) (WOMAC if not denoted otherwise) | Plain X-rays | MRI | ||||||
| Bone-driven endotype | |||||||||
| Cathepsin K inhibitors | |||||||||
| Conaghan et al. 113 |
NCT02705625 NCT03037489 Phase II |
Knee | Placebo MIV-711, 100 mg/day MIV-711, 200 mg/day |
80 82 82 |
26 weeks | −1.4 (−1.9 to −0.8) −1.7 (−2.3 to −1.2) −1.5 (−2.0 to −0.9) NRS (0–10) |
NA | NA | 23.3 (15.7 to 30.9) 7.9 (0.5 to 15.3)** 8.6 (1.1 to 16.1)** (Bone area, mm2) −0.066 (−0.119 to −0.013) 0.011 (−0.042 to 0.063)* −0.022 (−0.074 to 0.031) (Cartilage thickness, mm) |
| Matrix extracellular phosphoglycoprotein | |||||||||
| McGuire et al. 114 | – Phase II |
Knee | Placebo TPX-100 IA 200 mg 4 weekly injections |
93 93 |
12 months | – Significant WOMAC scores (No numerical data) |
– Significant WOMAC scores (No numerical data) |
NA | No significance in cartilage thickness/volume on quantitative MRI (No numerical data) |
| McGuire et al. 115 | – Phase II |
Knee | Placebo TPX-100 IA 200 mg 4 weekly injections |
78 78 |
12 months | NA | Significant decrease in pathologic bone shape change in the femur (No numerical data) |
||
| Synovitis-driven endotype | |||||||||
| Diacerein | |||||||||
| Pelletier et al. 116 |
NCT02688400 Phase II |
Knee | Diacerein 50 mg OD for 1 month and then BD celecoxib 200 mg OD |
140 148 |
6 months | −11.1 (0.9) −11.8 (0.9) |
−27.2 (39.0) −29.3 (39.8) |
||
| Cartilage-driven endotype | |||||||||
| Proteinase inhibitors | |||||||||
| Galapagos and Servier 117 |
NCT03595618 Phase II |
Knee | Placebo S201086/GLPG1972 low dose S201086/GLPG1972 medium dose S201086/GLPG1972 high dose (no numerical report) |
932 (total) | 52 weeks | No significance (no numerical report) |
No significance (No numerical report) |
NA | −0.116 (0.27) −0.068 (0.20) −0.097 (0.27) 0.085 (0.22) Change in cartilage thickness (in mm (SD)) |
| Fibroblast growth factor 18 | |||||||||
| Lohmander et al. 118 |
NCT01033994 Phase Ib |
Knee | Placebo Sprifermin IA 10 µg 3 once weekly Sprifermin IA 30 µg 3 once weekly Sprifermin IA 100 µg 3 once weekly |
42 21 42 63 |
12 months | −5.56 (4.17) −4.10 (5.11) −3.54 (3.67) −2.87 (4.76)* (Mean change from baseline, SD) |
−17.02 (13.56) −15.76 (13.72) −12.12 (12.06) −11.28 (15.30)* (Mean change from baseline, SD) |
−0.02 (0.90) 0.05 (1.00) 0.03 (0.72) −0.04 (0.90) (Mean change, MFTC JSW, mm) −0.18 (0.74) −0.02 (0.86) 0.0 (0.73) 0.34 (0.90)* (Mean change, LFTC JSW, mm) |
−0.11 (−0.20, −0.02) 0.02 (−0.18, 0.23) −0.11 (−0.18, −0.03) −0.03 (−0.11, 0.04) (Mean change, cMFTC cartilage thickness, mm) −0.03 (−0.04, 0.01) 0.00 (−0.08, 0.08) −0.01 (−0.02, 0.01) 0.01 (0.00, 0.03)* (Mean change, TFTC cartilage thickness, mm) |
| Hochberg et al. 119 |
NCT01919164 Phase II |
Knee | Placebo Sprifermin IA 30 µg 3 once weekly q6mo Sprifermin IA 30 µg 3 once weekly q12mo Sprifermin IA 100 µg 3 once weekly q6mo Sprifermin IA 100 µg 3 once weekly q12mo |
108 111 110 110 110 |
24 months | NA 2.58 (–3.47, 8.64) 1.29 (–4.53, 7.10) −0.06 (–5.76, 5.65) 3.65 (–1.99, 9.28) (Difference with placebo; total WOMAC score) |
No numerical data for individual WOMAC scores | NA 0.08 mm (−0.08, 0.25) 0.04 mm (−0.13, 0.20) 0.26 mm (0.12, 0.40)** 0.26 mm (0.12, 0.41)** (Difference versus placebo, LFTC JSW, mm) |
−0.02 (−0.04, −0.01) 0.00 (−0.02, 0.02) −0.01 (−0.03, 0.00) 0.03 (0.01, 0.04) 0.02 (0.00, 0.03)*** (Mean change, LFTC cartilage thickness, mm) |
| Eckstein et al. 120 |
NCT01919164 Phase II |
Knee | Placebo Sprifermin IA 30 µg 3 once weekly q12mo Sprifermin IA 30 µg 3 once weekly q6mo Sprifermin IA 100 µg 3 once weekly q12mo Sprifermin IA 100 µg 3 once weekly q6mo |
108 110 111 110 110 |
60 months | −22.38 (22.19) −24.41 (22.48) −20.38 (22.49) −24.94 (19.95) −24.00 (22.38) (Change from baseline at 5 years) |
−17.03 (24.15) −18.74 (21.87) −18.55 (23.76) −18.82 (21.62) −18.56 (23.60) |
−0.38 (0.72); mean (SD) −0.47 (1.02) −0.31 (0.75) −0.27 (0.75) −0.16 (0.77) Change from baseline at 5 years; MFTC JSW, mm −0.13 (0.65) −0.05 (0.60) −0.03 (0.69) 0.03 (0.88) 0.02 (0.76) Change from baseline at 5 years; LFTC JSW, mm |
– – – –* No numerical data reported for LFTC cartilage thickness |
| Transforming growth factor-β | |||||||||
| Guermazi et al. 121 |
NCT01221441 Phase II |
Knee | placebo IA (2 ml normal saline 0.9%) TissueGene-C IA | 29 57 |
12 months | NA | NA | – | 47.9% 34.6% Progression of cartilage morphology in any subregion 21.1% 9.6% Any worsening in Hoffa-synovitis/effusion-synovitis combined 60.6% 66.2% Any BML progression 32.4% 31.6% Any meniscal damage progression |
| Lee et al. 122 |
NCT01221441 Phase II |
Knee | Placebo IA (2 ml normal saline 0.9%) TissueGene-C IA | 35 67 |
12 months | Significant, improvement in VAS pain | Significant, improvement in IKDC scores | – | 47.9% 34.6% Progression of cartilage morphology in any subregion 21.1% 9.6% Any worsening in Hoffa-synovitis/effusion-synovitis combined |
| Kim et al. 123 | – Phase III |
Knee | Placebo IA (2 ml normal saline 0.9%) TissueGene-C IA | 81 78 |
12 months | –10 –25*** (Change from baseline, VAS pain score) |
NA | Not significant (JSW) | No significant change in any of WORMS subscore |
| Wnt/β-catenin signalling pathway inhibitors | |||||||||
| Yazici et al. 124 |
NCT02536833 Phase IIa |
knee | Placebo Lorecivivint (SM04690) 0.03 mg Lorecivivint (SM04690) 0.07 mg Lorecivivint (SM04690) 0.23 mg |
114 112 117 109 |
13 weeks | −22.1 ± 2.1 −23.3 ± 2.2 −23.5 ± 2.1 −23.5 ± 2.1 (Mean ± SD change from baseline) |
NA | −0.20 mm −0.07 mm −0.11 mm −0.02 mm* (Mean ± SD change from baseline, medial JSW) |
NA |
| Yazici et al. 125 |
NCT03122860 Phase IIb |
Knee | Dry needle Placebo Lorecivivint (SM04690) 0.03 mg Lorecivivint (SM04690) 0.07 mg Lorecivivint (SM04690) 0.15 mg Lorecivivint (SM04690) 0.23 mg |
117 116 116 115 115 116 |
24 weeks | NA 6.2 (1.0) 6.2 (1.1)* 6.1 (1.1) 6.1 (1.0) 6.1 (1.0)* (Mean ± SD change from baseline, NRS pain) |
NA 59.2 (9.8) 59.0 (10.9) 58.1 (11.2) 57.7 (11.1) 57.3 (11.4)* (Mean ± SD change from baseline) |
NA 3.44 (1.31) 3.30 (1.26) 3.16 (1.10) 3.26 (1.24) 3.27 (1.08) (Mean ± SD change from baseline, medial JSW) |
NA |
| Senolytic agents | |||||||||
| UNITY Biotechnology 126 |
NCT04129944 Phase II |
Knee | Placebo UBX0101 IA 0.5 mg UBX0101 IA 2.0 mg UBX0101 IA 4.0 mg |
46 45 46 46 |
12 weeks | −1.017 −0.924 −1.052 −1.019 (Mean change from baseline) |
NA | NA | NA |
, **, and *** denote P ≤ 0.05, P ≤ 0.01, and P ≤ 0.001, respectively, active vs control. BD, bis die; BML, bone marrow lesion; cMFTC, central medial femorotibial compartment; DMOAD, disease-modifying osteoarthritis drug; IA, intra-articular; IKDC, international knee documentation committee; JSW, joint space width; LFTC, lateral femorotibial compartment; MFTC, medial femorotibial compartment; MRI, magnetic resonance imaging; NA, non-available; NRS, Numerical Rating Scale; OA, osteoarthritis; OD, omne in die; SD, standard deviation; VAS, Visual Analogue Scale; WOMAC, Western Ontario and McMaster Universities Osteoarthritis Index. WORMS, whole organ magnetic resonance imaging score.
Bone-driven endotype
Cathepsin K inhibitor
Cathepsin K is a lysosomal cysteine protease present in activated osteoclasts for degrading collagen and other matrix proteins during bone resorption. 127 MIV-711 is a potent selective cathepsin K inhibitor that revealed reduced subchondral bone loss and cartilage damage in animal models. 128 In a 26-week phase II human trial in knee OA (n = 244), significant reductions in bone and cartilage OA progression were detected but with no symptomatic benefits. Skin disorders were more common in the active groups (100 mg/day: 7.3%; 200 mg/day: 12.2%, placebo: 2.5%). 113
Matrix extracellular phosphoglycoprotein
Matrix extracellular phosphoglycoprotein (MEPE) is largely detected in normal osteocytes and downregulated in OA. 129 TPX-100 is a synthetic 23mer peptide fragment of MEPE (AC-100, region 242-264). 130 Intra-articular injections of TPX-100 stimulate articular cartilage proliferation in goats. 115 A phase II study involving 93 participants with bilateral patellofemoral OA, 4 weekly injections of 200 mg TPX-100, demonstrated a significant difference in pain when ascending and descending stairs at 12 months but no structural benefits on quantitative MRI, 114 perhaps due to the small sample size. On a post hoc clinical ‘responder’ analysis for participants having bilateral tibiofemoral cartilage defects (73%), more TPX-100-treated knees met the responder criteria than placebo-exposed knees. 131 Using a retrospective MRI study, TPX-100 (n = 78) demonstrated a statistically significant decrease in pathologic bone shape change in the femur and associations with less tibiofemoral cartilage loss. 115
Synovitis-driven endotype
XT-150
Due to a short half-life and low target concentrations in the joint when systemically administered, plasmid DNA-based technology was used to generate XT-150 as a long-acting human IL-10 variant. 132 It is well tolerated and provides a symptomatic improvement in canine OA model. 133 On exploratory analyses of the combined phase I studies by using a WOMAC (Western Ontario and McMaster Universities Osteoarthritis Index) pain responder analysis (30% reduction from baseline versus day 180), a significant difference in efficacy was observed for 150 µg XT-150 compared with the placebo (67% versus 21%). 123
Currently, its efficacy is being evaluated in a phase II clinical trial for knee OA (NCT04124042). It was reported that 215 subjects have already been enrolled out of planned enrolment of 270 subjects as of February 2021. 134
Diacerein
Diacerein inhibits the IL-1b system and related downstream pathways in mice. 135 Administration of diacerein 50 mg once per day for 1 month and twice daily thereafter (n = 140) in moderate and severe knee OA showed comparable efficacy in symptomatic improvement with celecoxib 200 mg once per day for 6 months (n = 148) despite more prevalence of GI side effects (10.2% versus 3.7%). The study was also limited as no structural outcomes were evaluated. 116 The EMA’s Pharmacovigilance Risk Assessment Committee recommended restrictions of its use to limit risks of severe diarrhoea and hepatotoxicity in 2014. 136 In a recent meta-analysis, diacerein was significantly related to GI disorders (OR: 2.53; 95% CI: 1.43 to 4.46) and renal disorders (OR: 3.16; 95% CI: 1.93 to 5.15) even when concomitant OA medications were not allowed. 137
Cartilage-driven endotype
Proteinase inhibitors
Collagenases such as MMPs and aggrecanase such as a disintegrin and metalloproteinases with thrombospondin motifs (ADAMTS) digest triple-helical type II collagen fibrils (collagenolysis) and aggrecan, the major proteoglycan in articular cartilage. 138 Due to severe adverse events such as musculoskeletal syndrome (arthralgia, myalgia, tendinitis) and GI disorders, further development of broad-spectrum MMP inhibitors such as PG-116800 has been terminated. 139
Although the involvement of MMP-13 in OA pathogenesis is suggested by preclinical studies, 140 MMP-13 inhibitors with superior selectivity profiles by occupying themselves deeper in the S1′ pocket have not reached the phase II clinical trials due to poor solubility, metabolic stability or bioavailability. 141
As ADAMTS-5 inhibition reduces joint damage in mice and human chondrocytes. 142 S201086/GLPG1972 has been developed as an inhibitor of ADAMTS-5 142 with an eightfold selectivity over ADAMTS-4 in preclinical studies. 143 Neither symptomatic nor disease-modifying benefits detected by MRI were found in a phase II study (Roccella study) for knee OA (n = 932) 117 despite the fact that the study design was optimized for capturing cartilage changes. 144 Phase I studies for an ADAMTS-5 nanobody (M6495) showed overall acceptable safety at single doses up to 300 mg for further clinical development. 145
Fibroblast growth factor 18
Sprifermin is a recombinant human fibroblast growth factor 18 (FGF18), derived from Escherichia coli expression system. 146 It increased the synthesis of extracellular matrix in animal studies. 147 In 2007, a First-in-Human (FiH) trial (NCT00911469) revealed its beneficial effects on the cartilage samples taken from 73 end-stage knee OA participants during KR surgery. 148 In 2008, a significant dose-dependent disease-modifying response was detected in prespecified secondary radiograph and MRI outcomes over 12 months but with no symptomatic benefits (n = 168).118,149,150
The third phase II clinical trial [FGF18 Osteoarthritis Randomized Trial with Administration of Repeated Doses (FORWARD) study] (NCT01919164) confirmed the previous findings at 2- and 3-year follow-ups, especially with the dose of 100 µg sprifermin (n = 549). 119 In post hoc analyses, location-independent reduction of cartilage loss was reported, 151 and a symptomatic benefit on WOMAC score at 3-year follow-up [−8.8 (−22.4, 4.9)] was observed in a ‘subgroup at risk’ with narrower medial or lateral minimum joint space width (mJSW) and higher WOMAC pain, identifying a potential target group for future sprifermin clinical trials. 152
Recently, the results of the 5-year FORWARD study (n = 494) demonstrated the maintenance of structure-protective effects of 2-year administration of sprifermin 100 µg every 6 months despite a treatment-free period of 3 years, with a good safety profile. 120 A recent meta-analysis pooling data from eight reports of three original trials confirmed its disease-modifying properties such as improvement in cartilage thickness, volume and morphology. 153 However, it failed to exhibit symptomatic improvement measured by WOMAC subscores. As a note, structure-protective effects may prevent or delay KOA patients from reaching levels of debilitating pain in the long term despite lack of initial symptomatic improvement.
Transforming growth factor-β
Transforming growth factor-β (TGF-β) is involved in the extracellular matrix protein synthesis 154 and regulation of subchondral bone remodelling. 155 TissueGene-C (TG-C) is a retrovirally transduced agent for stimulating TGF-beta1 transcription (hChonJb#7 cells). 121 Its cartilage-regeneration potential was confirmed in a recent study in OA rat model. 156
In a phase II trial, the IA TG-C administration (n = 57) reduced the cartilage damage and inflammation markers compared with the placebo (n = 29) over 12 months. 121 In a 2017 poster abstract, a single IA administration of the TG-C showed symptomatic benefits but inconclusive effects on cartilage measures at 12-month follow-up (n = 156). 157 These findings were confirmed by another study in 102 OA participants. 122 A 1-year phase III trial revealed a reduction in pain of 25% with TG-C treatment compared with 10% with the placebo group and no significant structural benefits on the JSN and MRI outcomes as the secondary endpoints (n = 163), 123 despite exhibiting a clear trend towards slower bone area progression in the TG-C group. The failure to meet the structural endpoints may be perhaps due to the limited image quality and statistical power. The temporary clinical hold in April 2019 over the concerns of the potential mislabelling of ingredients was lifted in April 2020. 158 An analysis of observational long-term safety follow-up data showed no evidence of its association with increased risk of cancer over an average 10 years. 159 There are two ongoing pivotal phase III trials (NCT03203330, NCT03291470).
Wnt signalling inhibitors
Increased Wnt signalling in the chondrocytes, subchondral bone and synovium leads to cartilage damage, bone sclerosis and production of MMPs, respectively.160–162 Lorecivivint (SM04690) is a small-molecule CLK/DYRK1A Wnt signalling inhibitor that reduced cartilage damage in preclinical models. 163 In a 52-week, phase IIa trial in 455 participants with knee OA, any Lorecivivint dose group did not meet the primary endpoint, significant improvement in the WOMAC pain score at week 13 in comparison with the placebo group. However, in the post hoc analyses in subgroup of participants with unilateral symptomatic knee OA (n = 164) or unilateral symptomatic knee OA but without widespread pain (n = 128), administration of 0.07 mg resulted in significant symptomatic and radiographic improvements at 52 weeks, 124 identifying a potential responsive target phenotype. In another phase IIb clinical trial (NCT03122860) (n = 700), the lowest optimal dose was determined as 0.07 mg, 125 supporting the previous findings. The analysis of the combined data of these two trials showed a favourable safety profile (848 = Lorecivivint-treated and 360 = control subjects). 164 There are several ongoing trials on the https://clinicaltrials.gov/: two small phase II (NCT03727022, NCT03706521) and three phase III (NCT03928184, NCT04385303, NCT04520607) trials.
UBX0101
Recently, senescence (termination of cell division) caused by proinflammatory cytokines and dysfunction of neighbouring cells165,166 is focused on the pathogenesis of ageing-related OA. 167 As a senotherapeutic, UBX0101 is a p53/MDM2 interaction inhibitor and promoted chondrogenesis in animal models. 168 Although it showed encouraging results in a phase I study (n = 48), 169 a 12-week phase II study failed to reveal symptomatic improvement (n = 183), 126 leading to the termination of a long-term follow-up observational study. As senolytics appear to be cell type specific, a single senolytic drug may not be able to eliminate all types of senescent cells, 170 attributing to possible reasons for failures of single-drug RCT to meet the trial endpoints. Future research to improve the specificity and potency of senolytics by using delivery system such as galactose conjugation examines the efficacy and safety profiles of intermittent versus continuous administration, and a combination of senolytic agents should be considered. 171
LNA043
LNA043 is a modified human angiopoietin-like 3 (ANGPTL3) protein. A single IA injection of 20 mg LNA043 had anabolic effects on the cartilage via forming hyaline-like tissue detected on the high-field 7-T MRI. 172 In a dose-finding phase I study (NCT02491281), it showed dose-dependent modulation of cartilage-repair genes in the damaged cartilage tissue acquired from knee OA patients scheduled for total knee replacement (TKR), with a favourable safety profile (n = 28). LNA043 possessed rapid penetration predominantly into the damaged cartilage within 2 h after IA injection and was undetectable in the articular cartilage or synovial fluid 7 days post injection. 173
A phase IIa/b study (NCT03275064) is ongoing. In the analysis of the phase IIa part of this clinical trial, significant regeneration of damaged cartilage was detected up to week 28 on the femoral lesions but not on the patellar lesions after 4 weekly IA injections of 20 mg LNA043 in participants with a partial thickness cartilage lesion. There was higher incidence of joint swelling (9.3% versus 0%) and arthralgia (11.6% versus 6.7%) in the LNA043 group (n = 43), compared with the placebo (n = 15). 172 On the https://www.clinicaltrialsregister.eu/, a recent record for a 5-year clinical trial (ONWARDS) was found (EudraCT number: 2020-004897-22). It was granted FDA fast track designation (a process used to expedite the progress and review of new drugs which demonstrates the potential by theoretical or mechanistic rationale to address unmet medical needs 174 ) for knee OA in September 2021. 175
LRX712
LRX712 stimulates differentiation of chondroprogenitor cells to generate new extracellular matrix. 176 In a phase I clinical trial (NCT03355196) completed in 2019, it had a favourable safety profile with most of the adverse events being the injection site pain and swelling in the active group (n = 28) compared with the placebo (n = 14) (up to 75% in the highest dose versus 7%). 177 A phase II study is underway to examine its cartilage morphometrics detected by 7-T MRI and estimated to be completed in early 2024 (NCT04097379).
Perspectives
Recent failures and potential reasons (DMARDs, GLPG1972)
Despite the immense success of DMARDs in the remission of disease activity in inflammatory arthritides such as RA, 83 neither conventional (i.e. MTX, 37 HCQ 40 ) nor biological DMARDs (i.e. etanercept, 96 tocilizumab 42 ) in recent clinical trials have brought success in improving either symptomatic or structural benefits or both in the case of predominantly inflammatory OA endotypes. These failures may be attributable to the involvement of more complex interactions among various inflammatory cytokines in developing this endotype, 1 an insufficient number of participants, too short follow-up duration, recruiting participants with low disease activity or insufficient inflammatory activity to capture the symptomatic or structure-modifying efficacy of these medications. 9 However, there is no existing consensus on the threshold of optimal follow-up duration which is sufficient for detecting DMOAD effects 13 and the recent exploratory analysis of Canakinumab Anti-inflammatory Thrombosis Outcomes Study (CANTOS) trial suggests that to see the benefits for modulating inflammation may take longer (averaged follow-up duration of 3.7 years). 178 One of the other limitations may be due to the disease definition itself as the commonly used inclusion criteria in DMOAD trials [i.e. the American College of Rheumatology (ACR) criteria and radiographic criteria such as KL grade 2 or 3 on plain radiograph] may miss an early responsive window period where the best disease-modifying opportunities may be available for diagnosis and initiation of DMOAD therapy 13 as evidenced in paradigm shift in RA management in the last decade. 179 Therefore, a consensus definition for identifying either an ‘early OA’ or the pre-stages of OA for the purpose of DMOAD clinical trials may improve the chances of success in future trials.
In the case of proteinase inhibitors (PIs) such as GLPG1972, a major issue in targeted therapies is the high degree of conservation of proteinases in the sequence and structure of the active site, causing undesirable cross-inactivation of multiple proteinases and off-target effects when systemically administered. 180 As an example, while inhibition of the aggrecan degradation is directed to the articular cartilage, 181 simultaneous inhibition in tendon and aorta leads to decreased mechanical properties 182 and aortic dissection and rupture. 183 Therefore, linkage of the molecular target with the disease and fulfilments of the target agent with the attributes of receptor or functional selectivity, specificity and potency are crucial 184 potentially benefitted by long-acting intra-articular administration.
Recent insights/successes (sprifermin, Lorecivivint)
Sprifermin seems to have structure-modifying capability as well as long-term maintenance of its effects, based on the 5-year phase II data. One major strength of this study is using quantitative MRI as the primary outcome, resulting in an observed difference in MRI-detected cartilage thickness of significant results of pre-planned and post-hoc the medial femorotibial compartment while there was no difference in change of mJSW in the medial compartment. 119 Although the study did not meet its secondary endpoint, change in WOMAC score, it was not primarily designed as a pain trial with 90% in each treatment group taking additional pain medications. In addition, the participants were heterogeneous (32% with WOMAC pain subscale score <40/100) and relatively high mJSW (50% >3.7 mm) at baseline. IA saline injections were used as the placebo, masking any symptomatic benefits.
The post hoc analysis in a more homogeneous OA patient subgroup provided further insight that a symptomatic response to treatment is more likely in homogeneous symptomatic subgroups with rapid progression of the disease. 152 The time lag of symptomatic improvement translated from structural improvement is more than 2 years which may explain the failure of some clinical trials with shorter duration or with heterogeneous patient populations. In addition, a trial population with low mJSW and high WOMAC pain at baseline may be more effective to fulfil the definition of a DMOAD.
Although the Lorecivivint clinical trial did not meet the primary endpoint of WOMAC score, 124 the significant results of pre-planned and post hoc analyses in participants with unilateral symptoms of knee OA provided further insight for designing future clinical trials. 125 As the ability of a participant to discriminate pain between target and non-target knees is crucial, selection of participants can be limited to the unilateral OA symptoms, based on pain NRS (Numerical Rating Scale) cut-off points between knees during screening (i.e. the contralateral knee must have had a daily average NRS intensity score <4 for 4 of 7 days). In this way, a potential symptomatic improvement could be more clearly delineated.
Novel methodologies that may enhance success (placebo response tools, drug delivery system)
For obtaining regulatory approval, RCTs are the ‘gold standard’ to prove the efficacy and safety of a medical intervention. In an RCT, a placebo has to be administered in the control group to compare with the active agents in terms of benefits and adverse events for evidence generation. 185 As observed in many of the DMOAD RCT discussed above, the control group showed symptomatic improvement in the longitudinal follow-ups. A recent meta-analysis showed that the placebo effects of IA saline at 6-month follow-up [−13.4 (−21.7, −5.1) on a 0–100 VAS pain score and −10.1 (−12.2,-8.0) WOMAC function subscore] are higher than the ‘minimal clinically important difference’ (13.7/100 for VAS pain score and 4.6/68 for WOMAC function score). 186 In addition, the placebo response tends to increase in proportion to the effect size of the active treatment. 187 This high long-lasting placebo response seems a challenge for designing a clinical trial and may mask the efficacy of the potential DMOADs. 14
The use of needles or injections gives rise to greater placebo (contextual) effects [proportion attributable to contextual effects (PCE) = 0.81, 95% CI: 0.75 to 0.88], compared with oral medications. 188 To increase the accuracy of the IA drugs, ultrasound guidance is frequently used in DMOAD RCT. The use of such a sophisticated imaging tool may provide greater placebo response 189 as observed as high placebo response in invasive procedures. 190 Therefore, the placebo effects of IA saline should be accounted for in planning the trial design when pain and function endpoints are used as the primary measures.13,191 Furthermore, placebo response tools for predicting the placebo response for a particular intervention in a specific disease are being developed. 192
Due to the higher prevalence of slowly progressive OA in the fragile elderly with multiple comorbidities, 7 systemic administration could cause off-target effects and undesirable systemic effects over the long term. On the contrary, local therapy such as the IA administration will directly reach the targeted organ and requires a lower dosage. 193 Still, the short half-life/residence times of the agents in the joints is a major barrier to progress leading to frequent IA injections and burden for both participants and medical practitioners. Several drug delivery systems (DDS) which are capable of controlled and/or sustained drug release are being developed to prolong the residence time in the joint. 194 New smart drug delivery strategies, using nanoparticle, microparticle and hydrogel methods, may maximize the efficacy and safety of intra-articular agents. 195
Steps underway to enhance approval (Foundation for NIH qualification efforts, accelerated approval regulations)
Due to the FDA’s formal recognition of OA as a serious disease, utilization of surrogate outcome measures becomes feasible to submit the findings to the regulatory bodies for accelerated approval regulations. However, there remain two challenges ahead: (1) selection and evaluation of relevant surrogate outcome measures, and (2) appropriate designs for postmarketing confirmatory studies. The Foundation for NIH (FNIH) OA Biomarkers Consortium initiative was established to address the first challenge. 196 Two major study design scenarios were put forward to address the second challenge: (1) prospective trial continuation, which permits all participants on initial drug allocation to continue into the postmarketing approval trial until reaching a failure threshold; and (2) separate postmarketing approval study which recruits different study population to be treated with active medication only. 197
Conclusion
With the increasing combined trends of aeging, and rising epidemic of obesity, OA will lead to a major public health issue in the coming decades but with an immense unmet need for effective and safe therapies despite massive efforts and investments in R&D pipelines. To cut off the escalating costs, drug repurposing strategies are being used for finding a DMOAD but without success in OA so far. Recently, a few investigational drugs revealed promising findings in late clinical trials, paving the way for selecting a population at risk and developing more novel trial designs. Lessons learned from past failed clinical trials and insights gained through the successful progression of drug development phases, utilization of novel methodologies/techniques such as placebo response tools/DDS and steps to overcome barriers to regulatory hurdles will facilitate the emergence of the first DMOAD to fulfil the unmet need of patients with OA.
Acknowledgments
D.J.H. is supported by an National Health and Medical Research Council (NHMRC) Investigator Grant. W.M.O. was supported by the Presidential Scholarship of Myanmar for his PhD course.
Footnotes
Author contribution(s): Win Min Oo: Conceptualization; Data curation; Methodology; Visualization; Writing – original draft; Writing – review & editing.
David J. Hunter: Conceptualization; Methodology; Supervision; Writing – review & editing.
Conflict of interest statement: The authors declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: D.J.H. provides consulting advice on scientific advisory boards for Pfizer, Lilly, TLCBio, Novartis, Tissuegene and Biobone. W.M.O. has no conflict of interest.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: David J. Hunter  https://orcid.org/0000-0002-4065-7395
https://orcid.org/0000-0002-4065-7395
Contributor Information
Win Min Oo, Rheumatology Department, Royal North Shore Hospital, and Institute of Bone and Joint Research, Kolling Institute, Faculty of Medicine and Health, The University of Sydney, Sydney, NSW, Australia; Department of Physical Medicine and Rehabilitation, Mandalay General Hospital, University of Medicine, Mandalay, Mandalay, Myanmar.
David J. Hunter, Rheumatology Department, Royal North Shore Hospital, and Institute of Bone and Joint Research, Kolling Institute, Faculty of Medicine and Health, The University of Sydney, Sydney, NSW 2065, Australia.
References
- 1. Oo WM, Yu SP, Daniel MS, et al. Disease-modifying drugs in osteoarthritis: current understanding and future therapeutics. Expert Opin Emerg Drugs 2018; 23: 331–347. [DOI] [PubMed] [Google Scholar]
- 2. Cui A, Li H, Wang D, et al. Global, regional prevalence, incidence and risk factors of knee osteoarthritis in population-based studies. EClinicalMedicine 2020; 29–30: 100587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lo J, Chan L, Flynn S. A systematic review of the incidence, prevalence, costs, and activity and work limitations of amputation, osteoarthritis, rheumatoid arthritis, back pain, multiple sclerosis, spinal cord injury, stroke, and traumatic brain injury in the United States: a 2019 update. Arch Phys Med Rehabil 2021; 102: 115–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Leifer VP, Katz JN, Losina E. The burden of OA-health services and economics. Osteoarthritis Cartilage 2022; 30: 10–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bannuru RR, Osani MC, Vaysbrot EE, et al. OARSI guidelines for the non-surgical management of knee, hip, and polyarticular osteoarthritis. Osteoarthritis Cartilage 2019; 27: 1578–1589. [DOI] [PubMed] [Google Scholar]
- 6. Driban JB, Harkey MS, Barbe MF, et al. Risk factors and the natural history of accelerated knee osteoarthritis: a narrative review. BMC Musculoskelet Disord 2020; 21: 332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hunter DJ, Bierma-Zeinstra S. Osteoarthritis. Lancet 2019; 393: 1745–1759. [DOI] [PubMed] [Google Scholar]
- 8. Castro-Domínguez F, Vargas-Negrín F, Pérez C, et al. Unmet needs in the osteoarthritis chronic moderate to severe pain management in Spain: a real word data study. Rheumatol Ther 2021; 8: 1113–1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Oo WM, Little C, Duong V, et al. The development of disease-modifying therapies for osteoarthritis (DMOADs): the evidence to date. Drug Des Devel Ther 2021; 15: 2921–2945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Katz JN, Neogi T, Callahan LF, et al. Disease modification in osteoarthritis; pathways to drug approval. Osteoarthritis Cartilage Open 2020; 2: 100059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kraus VB, Blanco FJ, Englund M, et al. Call for standardized definitions of osteoarthritis and risk stratification for clinical trials and clinical use. Osteoarthritis Cartilage 2015; 23: 1233–1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mobasheri A, van Spil WE, Budd E, et al. Molecular taxonomy of osteoarthritis for patient stratification, disease management and drug development: biochemical markers associated with emerging clinical phenotypes and molecular endotypes. Curr Opin Rheumatol 2019; 31: 80–89. [DOI] [PubMed] [Google Scholar]
- 13. Oo WM. Prospects of disease-modifying osteoarthritis drugs (DMOADs). Clin Geriatr Med 2022; 38(2): 397–432. [DOI] [PubMed] [Google Scholar]
- 14. Karsdal MA, Michaelis M, Ladel C, et al. Disease-modifying treatments for osteoarthritis (DMOADs) of the knee and hip: lessons learned from failures and opportunities for the future. Osteoarthritis Cartilage 2016; 24: 2013–2021. [DOI] [PubMed] [Google Scholar]
- 15. Hu W, Chen Y, Dou C, et al. Microenvironment in subchondral bone: predominant regulator for the treatment of osteoarthritis. Ann Rheum Dis 2020; 80: 413–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Nagae M, Hiraga T, Yoneda T. Acidic microenvironment created by osteoclasts causes bone pain associated with tumor colonization. J Bone Miner Metab 2007; 25: 99–104. [DOI] [PubMed] [Google Scholar]
- 17. Zhu S, Zhu J, Zhen G, et al. Subchondral bone osteoclasts induce sensory innervation and osteoarthritis pain. J Clin Invest 2019; 129: 1076–1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. de Lange-Brokaar BJ, Ioan-Facsinay A, van Osch GJ, et al. Synovial inflammation, immune cells and their cytokines in osteoarthritis: a review. Osteoarthritis Cartilage 2012; 20: 1484–1499. [DOI] [PubMed] [Google Scholar]
- 19. Prieto-Potin I, Largo R, Roman-Blas JA, et al. Characterization of multinucleated giant cells in synovium and subchondral bone in knee osteoarthritis and rheumatoid arthritis. BMC Musculoskelet Disord 2015; 16: 226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wojdasiewicz P, Poniatowski ŁA, Szukiewicz D. The role of inflammatory and anti-inflammatory cytokines in the pathogenesis of osteoarthritis. Mediators Inflamm 2014; 2014: 561459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Oo WM, Linklater JM, Hunter DJ. Imaging in knee osteoarthritis. Curr Opin Rheumatol 2017; 29: 86–95. [DOI] [PubMed] [Google Scholar]
- 22. Collins JE, Losina E, Nevitt MC, et al. Semiquantitative imaging biomarkers of knee osteoarthritis progression: data From the Foundation for the National Institutes of Health Osteoarthritis Biomarkers Consortium. Arthritis Rheumatol 2016; 68: 2422–2431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Man GS, Mologhianu G. Osteoarthritis pathogenesis – a complex process that involves the entire joint. J Med Life 2014; 7: 37–41. [PMC free article] [PubMed] [Google Scholar]
- 24. Morgan S, Grootendorst P, Lexchin J, et al. The cost of drug development: a systematic review. Health Policy 2011; 100: 4–17. [DOI] [PubMed] [Google Scholar]
- 25. Wouters OJ, McKee M, Luyten J. Estimated research and development investment needed to bring a new medicine to market, 2009-2018. JAMA 2020; 323: 844–853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Pushpakom S, Iorio F, Eyers PA, et al. Drug repurposing: progress, challenges and recommendations. Nat Rev Drug Discov 2019; 18: 41–58. [DOI] [PubMed] [Google Scholar]
- 27. Ashburn TT, Thor KB. Drug repositioning: identifying and developing new uses for existing drugs. Nat Rev Drug Discov 2004; 3: 673–683. [DOI] [PubMed] [Google Scholar]
- 28. Parvathaneni V, Kulkarni NS, Muth A, et al. Drug repurposing: a promising tool to accelerate the drug discovery process. Drug Discov Today 2019; 24: 2076–2085. [DOI] [PubMed] [Google Scholar]
- 29. Nosengo N. Can you teach old drugs new tricks? Nature 2016; 534: 314–316. [DOI] [PubMed] [Google Scholar]
- 30. Renaud RC, Xuereb H. Erectile-dysfunction therapies. Nat Rev Drug Discov 2002; 1: 663–664. [DOI] [PubMed] [Google Scholar]
- 31. Sahoo BM, Ravi Kumar BVV, Sruti J, et al. Drug repurposing strategy (DRS): emerging approach to identify potential therapeutics for treatment of novel coronavirus infection. Front Mol Biosci 2021; 8: 628144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Frediani B, Toscano C, Falsetti P, et al. Intramuscular clodronate in long-term treatment of symptomatic knee osteoarthritis: a randomized controlled study. Drugs R D 2020; 20: 39–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Cai G, Aitken D, Laslett LL, et al. Effect of intravenous zoledronic acid on tibiofemoral cartilage volume among patients with knee osteoarthritis with bone marrow lesions: a randomized clinical trial. JAMA 2020; 323: 1456–1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Perry TA, Parkes MJ, Hodgson R, et al. Effect of Vitamin D supplementation on synovial tissue volume and subchondral bone marrow lesion volume in symptomatic knee osteoarthritis. BMC Musculoskelet Disord 2019; 20: 76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. MacFarlane LA, Cook NR, Kim E, et al. The effects of vitamin d and marine omega-3 fatty acid supplementation on chronic knee pain in older US adults: results from a randomized trial. Arthritis Rheumatol 2020; 72: 1836–1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kingsbury SR, Tharmanathan P, Keding A, et al. Significant pain reduction with oral methotrexate in knee osteoarthritis; results from the promote randomised controlled phase iii trial of treatment effectiveness. Osteoarthritis Cartilage 2019; 27: S84–S85. [Google Scholar]
- 37. Ferrero S, Wittoek R, Allado E, et al. Methotrexate treatment in hand osteoarthritis refractory to usual treatments: a randomised, double-blind, placebo-controlled trial. Semin Arthritis Rheum 2021; 51: 831–838. [DOI] [PubMed] [Google Scholar]
- 38. Lee W, Ruijgrok L, Boxma-de Klerk B, et al. Efficacy of hydroxychloroquine in hand osteoarthritis: a randomized, double-blind, placebo-controlled trial. Arthritis Care Res 2018; 70: 1320–1325. [DOI] [PubMed] [Google Scholar]
- 39. Kingsbury SR, Tharmanathan P, Keding A, et al. Hydroxychloroquine effectiveness in reducing symptoms of hand osteoarthritis: a randomized trial. Ann Intern Med 2018; 168: 385–395. [DOI] [PubMed] [Google Scholar]
- 40. Kedor C, Detert J, Rau R, et al. Hydroxychloroquine in patients with inflammatory and erosive osteoarthritis of the hands: results of the OA-TREAT study-a randomised, double-blind, placebo-controlled, multicentre, investigator-initiated trial. RMD Open 2021; 7: e001660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kloppenburg M, Ramonda R, Bobacz K, et al. Etanercept in patients with inflammatory hand osteoarthritis (EHOA): a multicentre, randomised, double-blind, placebo-controlled trial. Ann Rheum Dis 2018; 77: 1757–1764. [DOI] [PubMed] [Google Scholar]
- 42. Richette P, Latourte A, Sellam J, et al. Efficacy of tocilizumab in patients with hand osteoarthritis: double blind, randomised, placebo-controlled, multicentre trial. Ann Rheum Dis 2021; 80: 349–355. [DOI] [PubMed] [Google Scholar]
- 43. Gudbergsen H, Overgaard A, Henriksen M, et al. Liraglutide after diet-induced weight loss for pain and weight control in knee osteoarthritis: a randomized controlled trial. Am J Clin Nutr 2021; 113: 314–323. [DOI] [PubMed] [Google Scholar]
- 44. Camacho PM, Petak SM, Binkley N, et al. American Association of Clinical Endocrinologists/American College of Endocrinology clinical practice guidelines for the diagnosis and treatment of postmenopausal osteoporosis-2020 update. Endocr Pract 2020; 26: 1–46. [DOI] [PubMed] [Google Scholar]
- 45. Roemer FW, Neogi T, Nevitt MC, et al. Subchondral bone marrow lesions are highly associated with, and predict subchondral bone attrition longitudinally: the MOST study. Osteoarthritis Cartilage 2010; 18: 47–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Zanetti M, Bruder E, Romero J, et al. Bone marrow edema pattern in osteoarthritic knees: correlation between MR imaging and histologic findings. Radiology 2000; 215: 835–840. [DOI] [PubMed] [Google Scholar]
- 47. Singh V, Oliashirazi A, Tan T, et al. Clinical and pathophysiologic significance of MRI identified bone marrow lesions associated with knee osteoarthritis. Arch Bone Jt Surg 2019; 7: 211–219. [PMC free article] [PubMed] [Google Scholar]
- 48. Crema MD, Roemer FW, Zhu Y, et al. Subchondral cystlike lesions develop longitudinally in areas of bone marrow edema-like lesions in patients with or at risk for knee osteoarthritis: detection with MR imaging – the MOST study. Radiology 2010; 256: 855–862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Hunter DJ, Zhang W, Conaghan PG, et al. Systematic review of the concurrent and predictive validity of MRI biomarkers in OA. Osteoarthritis Cartilage 2011; 19: 557–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Day JS, Ding M, van der Linden JC, et al. A decreased subchondral trabecular bone tissue elastic modulus is associated with pre-arthritic cartilage damage. J Orthop Res 2001; 19: 914–918. [DOI] [PubMed] [Google Scholar]
- 51. Herrero-Beaumont G, Roman-Blas JA, Mediero A, et al. Treating osteoporotic osteoarthritis, or the art of cutting a balding man’s hair. Osteoarthritis Cartilage 2020; 28: 239–241. [DOI] [PubMed] [Google Scholar]
- 52. Lindsay R, Krege JH, Marin F, et al. Teriparatide for osteoporosis: importance of the full course. Osteoporos Int 2016; 27: 2395–2410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Neer RM, Arnaud CD, Zanchetta JR, et al. Effect of parathyroid hormone (1-34) on fractures and bone mineral density in postmenopausal women with osteoporosis. N Engl J Med 2001; 344: 1434–1441. [DOI] [PubMed] [Google Scholar]
- 54. Minisola S, Cipriani C, Grotta GD, et al. Update on the safety and efficacy of teriparatide in the treatment of osteoporosis. Ther Adv Musculoskelet Dis 2019; 11: 1759720X19877994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Sampson ER, Hilton MJ, Tian Y, et al. Teriparatide as a chondroregenerative therapy for injury-induced osteoarthritis. Sci Transl Med 2011; 3: 101–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Cui C, Zheng L, Fan Y, et al. Parathyroid hormone ameliorates temporomandibular joint osteoarthritic-like changes related to age. Cell Prolif 2020; 53: e12755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Sun Q, Zhen G, Li TP, et al. Parathyroid hormone attenuates osteoarthritis pain by remodeling subchondral bone in mice. eLife 2021; 10: e66532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Vahle JL, Sato M, Long GG, et al. Skeletal changes in rats given daily subcutaneous injections of recombinant human parathyroid hormone (1-34) for 2 years and relevance to human safety. Toxicol Pathol 2002; 30: 312–321. [DOI] [PubMed] [Google Scholar]
- 59. Vahle JL, Anderson U, Blomme EAG, et al. Use of toxicogenomics in drug safety evaluation: current status and an industry perspective. Regul Toxicol Pharmacol 2018; 96: 18–29. [DOI] [PubMed] [Google Scholar]
- 60. Gilsenan A, Harding A, Kellier-Steele N, et al. The Forteo Patient Registry linkage to multiple state cancer registries: study design and results from the first 8 years. Osteoporos Int 2018; 29: 2335–2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Gilsenan A, Midkiff K, Harris D, et al. Teriparatide did not increase adult osteosarcoma incidence in a 15-year US postmarketing surveillance study. J Bone Miner Res 2021; 36: 244–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Miller PD, Lewiecki EM, Krohn K, et al. Teriparatide: label changes and identifying patients for long-term use. Cleve Clin J Med 2021; 88: 489–493. [DOI] [PubMed] [Google Scholar]
- 63. Montanaro R, D’Addona A, Izzo A, et al. In vitro evidence for the involvement of H2S pathway in the effect of clodronate during inflammatory response. Sci 2021; 11: 14811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Saviola G, Abdi-Ali L, Comini L, et al. Use of clodronate in the management of osteoarthritis: an update. J Biol Regul Homeost Agents 2019; 33: 1315–1320. [DOI] [PubMed] [Google Scholar]
- 65. Moretti A, Paoletta M, Liguori S, et al. The rationale for the intra-articular administration of clodronate in osteoarthritis. Int J Mol Sci 2021; 22: 2693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Karsdal MA, Bay-Jensen AC, Lories RJ, et al. The coupling of bone and cartilage turnover in osteoarthritis: opportunities for bone antiresorptives and anabolics as potential treatments? Ann Rheum Dis 2014; 73: 336–348. [DOI] [PubMed] [Google Scholar]
- 67. Hayes KN, Giannakeas V, Wong AKO. Bisphosphonate use is protective of radiographic knee osteoarthritis progression among those with low disease severity and being non-overweight: data from the Osteoarthritis Initiative. J Bone Miner Res 2020; 35: 2318–2326. [DOI] [PubMed] [Google Scholar]
- 68. Li EC, Davis LE. Zoledronic acid: a new parenteral bisphosphonate. Clin Ther 2003; 25: 2669–2708. [DOI] [PubMed] [Google Scholar]
- 69. Dunford JE, Thompson K, Coxon FP, et al. Structure-activity relationships for inhibition of farnesyl diphosphate synthase in vitro and inhibition of bone resorption in vivo by nitrogen-containing bisphosphonates. J Pharmacol Exp Ther 2001; 296: 235–242. [PubMed] [Google Scholar]
- 70. Rackoff P. Efficacy and safety of risedronate 150 mg once a month in the treatment of postmenopausal osteoporosis. Clin Interv Aging 2009; 4: 207–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Nancollas GH, Tang R, Phipps RJ, et al. Novel insights into actions of bisphosphonates on bone: differences in interactions with hydroxyapatite. Bone 2006; 38: 617–627. [DOI] [PubMed] [Google Scholar]
- 72. Ibrahim A, Scher N, Williams G, et al. Approval summary for zoledronic acid for treatment of multiple myeloma and cancer bone metastases. Clin Cancer Res 2003; 9: 2394–2399. [PubMed] [Google Scholar]
- 73. Schumann S-A, Hickner J. Annual zoledronic acid infusion lowers risk of fracture, death. J Fam Pract 2007; 56: 1013–1016. [PMC free article] [PubMed] [Google Scholar]
- 74. Black DM, Delmas PD, Eastell R, et al. Once-yearly zoledronic acid for treatment of postmenopausal osteoporosis. N Engl J Med 2007; 356: 1809–1822. [DOI] [PubMed] [Google Scholar]
- 75. Lewiecki EM. New and emerging concepts in the use of denosumab for the treatment of osteoporosis. Ther Adv Musculoskelet Dis 2018; 10: 209–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Charopoulos I, Orme S, Giannoudis PV. The role and efficacy of denosumab in the treatment of osteoporosis: an update. Expert Opin Drug Saf 2011; 10: 205–217. [DOI] [PubMed] [Google Scholar]
- 77. Cummings SR, Martin JS, McClung MR, et al. Denosumab for prevention of fractures in postmenopausal women with osteoporosis. N Engl J Med 2009; 361: 756–765. [DOI] [PubMed] [Google Scholar]
- 78. Zaheer S, LeBoff M, Lewiecki EM. Denosumab for the treatment of osteoporosis. Expert Opin Drug Metab Toxicol 2015; 11: 461–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Portal-Núñez S, Mediero A, Esbrit P, et al. Unexpected bone formation produced by RANKL blockade. Trends Endocrinol Metab 2017; 28: 695–704. [DOI] [PubMed] [Google Scholar]
- 80. Burr DB, Gallant MA. Bone remodelling in osteoarthritis. Nat Rev Rheumatol 2012; 8: 665–673. [DOI] [PubMed] [Google Scholar]
- 81. Kong C, Wang C, Shi Y, et al. Active vitamin D activates chondrocyte autophagy to reduce osteoarthritis via mediating the AMPK-mTOR signaling pathway. Biochem Cell Biol 2020; 98: 434–442. [DOI] [PubMed] [Google Scholar]
- 82. Zhao ZX, He Y, Peng LH, et al. Does vitamin D improve symptomatic and structural outcomes in knee osteoarthritis? A systematic review and meta-analysis. Aging Clin Exp Res 2021; 33: 2393–2403. [DOI] [PubMed] [Google Scholar]
- 83. Chaplin S. Summary of the new EULAR rheumatoid arthritis guideline. Prescriber 2020; 31: 15–19. [Google Scholar]
- 84. Fraenkel L, Bathon JM, England BR, et al. 2021. American College of Rheumatology guideline for the treatment of rheumatoid arthritis. Arthritis Rheumatol 2021; 73: 1108–1123. [DOI] [PubMed] [Google Scholar]
- 85. Singh JA, Guyatt G, Ogdie A, et al. 2018. American College of Rheumatology/National Psoriasis Foundation guideline for the treatment of psoriatic arthritis. J Psoriasis Psoriatic Arthritis 2019; 4: 31–58. [Google Scholar]
- 86. Persson MSM, Sarmanova A, Doherty M, et al. Conventional and biologic disease-modifying anti-rheumatic drugs for osteoarthritis: a meta-analysis of randomized controlled trials. Rheumatology 2018; 57: 1830–1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Farber S, Diamond LK. Temporary remissions in acute leukemia in children produced by folic acid antagonist, 4-aminopteroyl-glutamic acid. N Engl J Med 1948; 238: 787–793. [DOI] [PubMed] [Google Scholar]
- 88. Hoffmeister RT. Methotrexate therapy in rheumatoid arthritis: 15 years experience. Am J Med 1983; 75: 69–73. [DOI] [PubMed] [Google Scholar]
- 89. Weinblatt ME. Methotrexate in rheumatoid arthritis: a quarter century of development. Trans Am Clin Climatol Assoc 2013; 124: 16–25. [PMC free article] [PubMed] [Google Scholar]
- 90. Gabay C. Cytokine inhibitors in the treatment of rheumatoid arthritis. Expert Opin Biol Ther 2002; 2: 135–149. [DOI] [PubMed] [Google Scholar]
- 91. Cutolo M, Sulli A, Pizzorni C, et al. Anti-inflammatory mechanisms of Anti-inflammatory mechanisms of methotrexate in rheumatoid arthritis. Ann Rheum Dis 2001; 60: 729–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Ben-Zvi I, Kivity S, Langevitz P, et al. Hydroxychloroquine: from malaria to autoimmunity. Clin Rev Allergy Immunol 2012; 42: 145–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Udupa A, Leverenz D, Balevic SJ, et al. Hydroxychloroquine and COVID-19: a rheumatologist’s take on the lessons learned. Curr Allergy Asthma Rep 2021; 21: 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Sperber K, Quraishi H, Kalb TH, et al. Selective regulation of cytokine secretion by hydroxychloroquine: inhibition of interleukin 1 alpha (IL-1-alpha) and IL-6 in human monocytes and T cells. J Rheumatol 1993; 20: 803–808. [PubMed] [Google Scholar]
- 95. Pelechas E, Drosos AA. Etanercept biosimilar SB-4. Expert Opin Biol Ther 2019; 19: 173–179. [DOI] [PubMed] [Google Scholar]
- 96. Verbruggen G, Wittoek R, Vander Cruyssen B, et al. Morbid anatomy of ‘erosive osteoarthritis’ of the interphalangeal finger joints: an optimised scoring system to monitor disease progression in affected joints. Ann Rheum Dis 2010; 69: 862–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Kroon F, Bay-Jensen AC, Wittoek R, et al. Etanercept therapy leads to reductions in matrix metalloproteinase-3 in patients with erosive hand osteoarthritis. Scand J Rheumatol 2020; 49: 167–168. [DOI] [PubMed] [Google Scholar]
- 98. Sheppard M, Laskou F, Stapleton PP, et al. Tocilizumab (Actemra). Hum Vaccin Immunother 2017; 13: 1972–1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Rose-John S. IL-6 trans-signaling via the soluble IL-6 receptor: importance for the pro-inflammatory activities of IL-6. Int J Biol Sci 2012; 8: 1237–1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Bailey CJ. Metformin: historical overview. Diabetologia 2017; 60: 1566–1576. [DOI] [PubMed] [Google Scholar]
- 101. Kang KY, Kim YK, Yi H, et al. Metformin downregulates Th17 cells differentiation and attenuates murine autoimmune arthritis. Int Immunopharmacol 2013; 16: 85–92. [DOI] [PubMed] [Google Scholar]
- 102. Son HJ, Lee J, Lee SY, et al. Metformin attenuates experimental autoimmune arthritis through reciprocal regulation of Th17/Treg balance and osteoclastogenesis. Mediators Inflamm 2014; 2014: 973986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Park MJ, Moon SJ, Baek JA, et al. Metformin augments anti-inflammatory and chondroprotective properties of mesenchymal stem cells in experimental osteoarthritis. J Immunol 2019; 203: 127–136. [DOI] [PubMed] [Google Scholar]
- 104. Belenska-Todorova L, Lambova SN, Stoyanova S, et al. Disease-modifying potential of metformin and alendronate in an experimental mouse model of osteoarthritis. Biomedicines 2021; 9: 1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Na HS, Kwon JY, Lee SY, et al. Metformin attenuates monosodium-iodoacetate-induced osteoarthritis via regulation of pain mediators and the autophagy-lysosomal pathway. Cells 2021; 10: 681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Lu CH, Chung CH, Lee CH, et al. Combination COX-2 inhibitor and metformin attenuate rate of joint replacement in osteoarthritis with diabetes: a nationwide, retrospective, matched-cohort study in Taiwan. PLoS ONE 2018; 13: e0191242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Wang Y, Hussain SM, Wluka AE, et al. Association between metformin use and disease progression in obese people with knee osteoarthritis: data from the Osteoarthritis Initiative – a prospective cohort study. Arthritis Res Ther 2019; 21: 127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Mehta A, Marso SP, Neeland IJ. Liraglutide for weight management: a critical review of the evidence. Obes Sci Pract 2017; 3: 3–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Chen J, Xie J-J, Shi K-S, et al. Glucagon-like peptide-1 receptor regulates endoplasmic reticulum stress-induced apoptosis and the associated inflammatory response in chondrocytes and the progression of osteoarthritis in rat. Cell Death Dis 2018; 9: 212–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Que Q, Guo X, Zhan L, et al. The GLP-1 agonist, liraglutide, ameliorates inflammation through the activation of the PKA/CREB pathway in a rat model of knee osteoarthritis. J Inflamm 2019; 16: 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Gieringer DH. The safety and efficacy of new drug approval. Cato J 1985; 5: 177–201. [PubMed] [Google Scholar]
- 112. U.S. Food & Drug Administration. Investigational new drug (IND) application, https://www.fda.gov/drugs/types-applications/investigational-new-drug-ind-application (2021, accessed 14 November 2021).
- 113. Conaghan PG, Bowes MA, Kingsbury SR, et al. Disease-modifying effects of a novel cathepsin K inhibitor in osteoarthritis: a randomized controlled trial. Ann Intern Med 2020; 172: 86–95. [DOI] [PubMed] [Google Scholar]
- 114. McGuire D, Lane N, Segal N, et al. TPX-100 leads to marked, sustained improvements in subjects with knee osteoarthritis: pre-clinical rationale and results of a controlled clinical trial [Abstract]. Osteoarthritis Cartilage 2018; 26: S243. [Google Scholar]
- 115. McGuire D, Bowes M, Brett A, et al. Study TPX-100-5: intra-articular TPX-100 significantly delays pathological bone shape change and stabilizes cartilage in moderate to severe bilateral knee OA. Arthritis Res Ther 2021; 23: 242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Pelletier JP, Raynauld JP, Dorais M, et al. An international, multicentre, double-blind, randomized study (DISSCO): effect of diacerein vs celecoxib on symptoms in knee osteoarthritis. Rheumatology 2020; 59: 3858–3868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Servier Ga. Galapagos and Servier report topline results for ROCCELLA Phase 2 clinical trial with GLPG1972/S201086 in knee osteoarthritis patients, 2020, https://servier.com/en/communique/galapagos-and-servier-report-topline-results-for-roccella-phase-2-clinical-trial-with-glpg1972-s201086-in-knee-osteoarthritis-patients/
- 118. Lohmander LS, Hellot S, Dreher D, et al. Intraarticular sprifermin (recombinant human fibroblast growth factor 18) in knee osteoarthritis: a randomized, double-blind, placebo-controlled trial. Arthritis Rheumatol 2014; 66: 1820–1831. [DOI] [PubMed] [Google Scholar]
- 119. Hochberg MC, Guermazi A, Guehring H, et al. Effect of intra-articular sprifermin vs placebo on femorotibial joint cartilage thickness in patients with osteoarthritis: the FORWARD randomized clinical trial. JAMA 2019; 322: 1360–1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Eckstein F, Hochberg MC, Guehring H, et al. Long-term structural and symptomatic effects of intra-articular sprifermin in patients with knee osteoarthritis: 5-year results from the FORWARD study. Ann Rheum Dis 2021; 80: 1062–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Guermazi A, Kalsi G, Niu J, et al. Structural effects of intra-articular TGF-beta1 in moderate to advanced knee osteoarthritis: MRI-based assessment in a randomized controlled trial. BMC Musculoskelet Disord 2017; 18: 461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Lee B, Parvizi J, Bramlet D, et al. Results of a phase II study to determine the efficacy and safety of genetically engineered allogeneic human chondrocytes expressing TGF-β1. J Knee Surg 2020; 33: 167–172. [DOI] [PubMed] [Google Scholar]
- 123. Kim MK, Ha CW, In Y, et al. A multicenter, double-blind, phase III clinical trial to evaluate the efficacy and safety of a cell and gene therapy in knee osteoarthritis patients. Hum Gene Ther Clin Dev 2018; 29: 48–59. [DOI] [PubMed] [Google Scholar]
- 124. Yazici Y, McAlindon TE, Gibofsky A, et al. Lorecivivint, a novel intraarticular CDC-like kinase 2 and dual-specificity tyrosine phosphorylation-regulated kinase 1A inhibitor and Wnt pathway modulator for the treatment of knee osteoarthritis: a phase II randomized trial. Arthritis Rheumatol 2020; 72: 1694–1706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Yazici Y, McAlindon TE, Gibofsky A, et al. A phase 2b randomized trial of Lorecivivint, a novel intra-articular CLK2/DYRK1A inhibitor and Wnt pathway modulator for knee osteoarthritis. Osteoarthritis Cartilage 2021; 29: 654–666. [DOI] [PubMed] [Google Scholar]
- 126. Unity Biotechnology I. UNITY Biotechnology announces 12-week data from UBX0101 phase 2 clinical study in patients with painful osteoarthritis of the knee, 2020, https://www.globenewswire.com/news-release/2020/08/17/2079116/0/en/UNITY-Biotechnology-Announces-12-week-data-from-UBX0101-Phase-2-Clinical-Study-in-Patients-with-Painful-Osteoarthritis-of-the-Knee.html
- 127. Costa AG, Cusano NE, Silva BC, et al. Cathepsin K: its skeletal actions and role as a therapeutic target in osteoporosis. Nat Rev Rheumatol 2011; 7: 447–456. [DOI] [PubMed] [Google Scholar]
- 128. Lindström E, Rizoska B, Tunblad K, et al. The selective cathepsin K inhibitor MIV-711 attenuates joint pathology in experimental animal models of osteoarthritis. J Transl Med 2018; 16: 56–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Hopwood B, Tsykin A, Findlay DM, et al. Microarray gene expression profiling of osteoarthritic bone suggests altered bone remodelling, WNT and transforming growth factor-β/bone morphogenic protein signalling. Arthritis Res Ther 2007; 9: R100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Hayashibara T, Hiraga T, Yi B, et al. A synthetic peptide fragment of human MEPE stimulates new bone formation in vitro and in vivo. J Bone Miner Res 2004; 19: 455–462. [DOI] [PubMed] [Google Scholar]
- 131. McGuire D, Segal N, Metyas S, et al. Intra-articular TPX-100 in knee osteoarthritis: robust functional response at 6 and 12 months is associated with increased tibiofemoral cartilage thickness [Abstract]. Arthritis Rheumatol 2018; 201870(Suppl. 10): 3405–3406. [Google Scholar]
- 132. Broeren MGA, de Vries M, Bennink MB, et al. Suppression of the inflammatory response by disease-inducible interleukin-10 gene therapy in a three-dimensional micromass model of the human synovial membrane. Arthritis Res Ther 2016; 18: 186–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Watkins LR, Chavez RA, Landry R, et al. Targeted interleukin-10 plasmid DNA therapy in the treatment of osteoarthritis: toxicology and pain efficacy assessments. Brain Behav Immun 2020; 90: 155–166. [DOI] [PubMed] [Google Scholar]
- 134. Grigsby E, Rickam M, Thewlis D, et al. XT-150 – a novel immunomodulatory gene therapy for osteoarthritis pain in phase 2b development. Osteoarthritis Cartilage 2021; 29: S12. [Google Scholar]
- 135. Gadotti VM, Martins DF, Pinto HF, et al. Diacerein decreases visceral pain through inhibition of glutamatergic neurotransmission and cytokine signaling in mice. Pharmacol Biochem Behav 2012; 102: 549–554. [DOI] [PubMed] [Google Scholar]
- 136. European Medicines Agency. PRAC re-examines diacerein and recommends that it remain available with restrictions, 2014, https://www.ema.europa.eu/en/news/prac-re-examines-diacerein-recommends-it-remain-available-restrictions
- 137. Honvo G, Reginster J-Y, Rabenda V, et al. Safety of symptomatic slow-acting drugs for osteoarthritis: outcomes of a systematic review and meta-analysis. Drugs Aging 2019; 36(Suppl. 1): 65–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Yamamoto K, Wilkinson D, Bou-Gharios G. Targeting dysregulation of metalloproteinase activity in osteoarthritis. Calcif Tissue Int 2021; 109: 277–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Fields GB. The rebirth of matrix metalloproteinase inhibitors: moving beyond the dogma. Cells 2019; 8: 984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Wang M, Sampson ER, Jin H, et al. MMP13 is a critical target gene during the progression of osteoarthritis. Arthritis Res Ther 2013; 15: R5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Hu Q, Ecker M. Overview of MMP-13 as a promising target for the treatment of osteoarthritis. Int J Mol Sci 2021; 22: 1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Santamaria S. ADAMTS-5: a difficult teenager turning 20. Int J Exp Pathol 2020; 101: 4–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Brebion F, Gosmini R, Deprez P, et al. Discovery of GLPG1972/S201086, a potent, selective, and orally bioavailable ADAMTS-5 inhibitor for the treatment of osteoarthritis. J Med Chem 2021; 64: 2937–2952. [DOI] [PubMed] [Google Scholar]
- 144. vanderAar E, Deckx H, Van Der Stoep M, et al. Study design of a phase 2 clinical trial with a disease-modifying osteoarthritis drug candidate GLPG1972/S201086: the Roccella trial. Osteoarthritis Cartilage 2020; 28: S499–S500. [Google Scholar]
- 145. Guehring H, Balchen T, Goteti K, et al. Safety, tolerability, pharmacokinetics and pharmacodynamics of single ascending doses of the anti-ADAMTS-5 Nanobody®, M6495, in healthy male subjects: a phase I, placebo-controlled, First-in-Human study [Abstract]. Arthritis Rheumatol 2019; 71(Suppl. 10), https://acrabstracts.org/abstract/safety-tolerability-pharmacokinetics-and-pharmacodynamics-of-single-ascending-doses-of-the-anti-adamts-5-nanobody-m6495-in-healthy-male-subjects-a-phase-i-placebo-controlled-first-in-huma/ [Google Scholar]
- 146. Song L, Huang Z, Chen Y, et al. High-efficiency production of bioactive recombinant human fibroblast growth factor 18 in Escherichia coli and its effects on hair follicle growth. Appl Microbiol Biotechnol 2014; 98: 695–704. [DOI] [PubMed] [Google Scholar]
- 147. Hendesi H, Stewart S, Gibison ML, et al. Recombinant fibroblast growth factor-18 (sprifermin) enhances microfracture-induced cartilage healing. J Orthop Res 2022; 40: 553–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Muurahainen N. Cartilage repair and the sprifermin story: mechanisms, preclinical and clinical study results, and lessons learned. Osteoarthritis Cartilage 2016; 24: S4. [Google Scholar]
- 149. Eckstein F, Wirth W, Guermazi A, et al. Brief report: intraarticular sprifermin not only increases cartilage thickness, but also reduces cartilage loss: location-independent post hoc analysis using magnetic resonance imaging. Arthritis Rheumatol 2015; 67: 2916–2922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Roemer FW, Aydemir A, Lohmander S, et al. Structural effects of sprifermin in knee osteoarthritis: a post-hoc analysis on cartilage and non-cartilaginous tissue alterations in a randomized controlled trial. BMC Musculoskelet Disord 2016; 17: 267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Eckstein F, Kraines JL, Aydemir A, et al. Intra-articular sprifermin reduces cartilage loss in addition to increasing cartilage gain independent of location in the femorotibial joint: post-hoc analysis of a randomised, placebo-controlled phase II clinical trial. Ann Rheum Dis 2020; 79: 525–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Guehring H, Moreau F, Daelken B, et al. The effects of sprifermin on symptoms and structure in a subgroup at risk of progression in the FORWARD knee osteoarthritis trial. Semin Arthritis Rheum 2021; 51: 450–456. [DOI] [PubMed] [Google Scholar]
- 153. Zeng N, Chen X-Y, Yan Z-P, et al. Efficacy and safety of sprifermin injection for knee osteoarthritis treatment: a meta-analysis. Arthritis Res Ther 2021; 23: 107–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Zhai G, Dore J, Rahman P. TGF-beta signal transduction pathways and osteoarthritis. Rheumatol Int 2015; 35: 1283–1292. [DOI] [PubMed] [Google Scholar]
- 155. Dai G, Xiao H, Liao J, et al. Osteocyte TGFβ1-Smad2/3 is positively associated with bone turnover parameters in subchondral bone of advanced osteoarthritis. Int J Mol Med 2020; 46: 167–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Lee H, Kim H, Seo J, et al. TissueGene-C promotes an anti-inflammatory micro-environment in a rat monoiodoacetate model of osteoarthritis via polarization of M2 macrophages leading to pain relief and structural improvement. Inflammopharmacology 2020; 28: 1237–1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Cho J, Kim T, Shin J, et al. A phase III clinical results of INVOSSA™ (TissueGene C): a clues for the potential disease modifying OA drug. Cytotherapy 2017; 19: S148. [Google Scholar]
- 158. Kolon TissueGene cleared to resume US Phase III trial for Invossa. Thepharmaletter, 14 April 2020, https://www.thepharmaletter.com/article/kolon-tissuegene-cleared-to-resume-us-phase-iii-trial-for-invossa
- 159. Hunter D, Casper R, Wang M, et al. Overall safety of TG-C: safety analysis of phase-1, phase-2 and long-term safety trials [Abstract]. Osteoarthritis Cartilage 2020; 28: S549. [Google Scholar]
- 160. Lories RJ, Monteagudo S. Review article: is Wnt signaling an attractive target for the treatment of osteoarthritis? Rheumatol Ther 2020; 7: 259–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Kovács B, Vajda E, Nagy EE. Regulatory effects and interactions of the Wnt and OPG-RANKL-RANK signaling at the bone-cartilage interface in osteoarthritis. Int J Mol Sci 2019; 20: 4653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Cherifi C, Monteagudo S, Lories RJ. Promising targets for therapy of osteoarthritis: a review on the Wnt and TGF-β signalling pathways. Ther Adv Musculoskelet Dis 2021; 13: 1759720X211006959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Deshmukh V, O’Green AL, Bossard C, et al. Modulation of the Wnt pathway through inhibition of CLK2 and DYRK1A by Lorecivivint as a novel, potentially disease-modifying approach for knee osteoarthritis treatment. Osteoarthritis Cartilage 2019; 27: 1347–1360. [DOI] [PubMed] [Google Scholar]
- 164. Simsek I, Swearingen C, Kennedy S, et al. OP0188 Integrated safety summary of the novel, intra-articular agent Lorecivivint (SM04690), a CLK/DYRK1A inhibitor that modulates the WNT pathway, in subjects with knee osteoarthritis. Ann Rheum Dis 2020; 79: 117–117. [Google Scholar]
- 165. Coppé JP, Patil CK, Rodier F, et al. Senescence-associated secretory phenotypes reveal cell-nonautonomous functions of oncogenic RAS and the p53 tumor suppressor. PLoS Biol 2008; 6: 2853–2868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Ferreira-Gonzalez S, Lu WY, Raven A, et al. Paracrine cellular senescence exacerbates biliary injury and impairs regeneration. Nat Commun 2018; 9: 1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Loeser RF. Aging and osteoarthritis: the role of chondrocyte senescence and aging changes in the cartilage matrix. Osteoarthritis Cartilage 2009; 17: 971–979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Jeon OH, Kim C, Laberge R-M, et al. Local clearance of senescent cells attenuates the development of post-traumatic osteoarthritis and creates a pro-regenerative environment. Nat Med 2017; 23: 775–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Hsu B, Visich J, Lane NE, et al. Safety, tolerability, pharmacokinetics, and clinical outcomes following single-dose IA administration of UBX0101, a senolytic MDM2/p53 interaction inhibitor, in patients with knee OA [Abstract]. Osteoarthritis Cartilage 2020; 28: S479–480. [Google Scholar]
- 170. Zhu Y, Tchkonia T, Pirtskhalava T, et al. The Achilles’ heel of senescent cells: from transcriptome to senolytic drugs. Aging Cell 2015; 14: 644–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Zhang X-X, He S-H, Liang X, et al. Aging, cell senescence, the pathogenesis and targeted therapies of osteoarthritis. Front Pharmacol 2021; 12: 728100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Trattnig S, Scotti C, Laurent D, et al. POS0277 ANABOLIC EFFECT OF LNA043, A NOVEL DISEASE-MODIFYING OSTEOARTHRITIS DRUG CANDIDATE: RESULTS FROM AN IMAGING-BASED PROOF-OF-CONCEPT TRIAL IN PATIENTS WITH FOCAL ARTICULAR CARTILAGE LESIONS. Ann Rheum Dis 2021; 80: 363–363. [Google Scholar]
- 173. Scotti C, Gimbel J, Laurent D, et al. LNA043, a novel cartilage regenerative treatment for osteoarthritis: results from a First-in-Human trial in patients with knee osteoarthritis [Abstract]. Arthritis Rheumatol 2020; 72(Suppl. 10), https://acrabstracts.org/abstract/lna043-a-novel-cartilage-regenerative-treatment-for-osteoarthritis-results-from-a-first-in-human-trial-in-patients-with-knee-osteoarthritis/ [Google Scholar]
- 174. Kepplinger EE. FDA’s expedited approval mechanisms for new drug products. Biotechnol Law Rep 2015; 34: 15–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Novartis. Novartis receives FDA fast track designation for LNA043 in osteoarthritis of the knee, 2021, https://www.novartis.com/news/novartis-receives-fda-fast-track-designation-lna043-osteoarthritis-knee
- 176. Vrouwe JPM, Burggraaf J, Kloppenburg M, et al. Challenges and opportunities of pharmacological interventions for osteoarthritis: a review of current clinical trials and developments. Osteoarthritis Cartilage Open 2021; 3: 100212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Novartis. A clinical trial to learn about the safety of trial drug LRX712 for people with osteoarthritis, 2019. https://www.novctrd.com/ctrdweb/patientsummary/patientsummaries?patientSummaryId=381#:~:text=The%20clinical%20trial%20team%20concluded,the%20participants%20in%20this%20trial.&text=someone%20may%20need%20to%20take,a%20small%20number%20of%20people
- 178. Schieker M, Conaghan PG, Mindeholm L, et al. Effects of interleukin-1β inhibition on incident hip and knee replacement: exploratory analyses from a randomized, double-blind, placebo-controlled trial. Ann Intern Med 2020; 173: 509–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. Demoruelle MK, Deane KD. Treatment strategies in early rheumatoid arthritis and prevention of rheumatoid arthritis. Curr Rheumatol Rep 2012; 14: 472–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Rose KWJ, Taye N, Karoulias SZ, et al. Regulation of ADAMTS proteases. Front Mol Biosci 2021; 8: 701959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. Verma P, Dalal K. ADAMTS-4 and ADAMTS-5: key enzymes in osteoarthritis. J Cell Biochem 2011; 112: 3507–3514. [DOI] [PubMed] [Google Scholar]
- 182. Wang VM, Bell RM, Thakore R, et al. Murine tendon function is adversely affected by aggrecan accumulation due to the knockout of ADAMTS5. J Orthop Res 2012; 30: 620–626. [DOI] [PubMed] [Google Scholar]
- 183. Cikach FS, Koch CD, Mead TJ, et al. Massive aggrecan and versican accumulation in thoracic aortic aneurysm and dissection. JCI Insight 2018; 3: e97167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184. Berg KA, Clarke WP. Making sense of pharmacology: inverse agonism and functional selectivity. Int J Neuropsychopharmacol 2018; 21: 962–977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185. Downing NS, Aminawung JA, Shah ND, et al. Clinical trial evidence supporting FDA approval of novel therapeutic agents, 2005-2012. JAMA 2014; 311: 368–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186. Previtali D, Merli G, Di Laura Frattura G, et al. The long-lasting effects of ‘placebo injections’ in knee osteoarthritis: a meta-analysis. Cartilage 2021; 13: 185S–196S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187. Vase L, Riley JL, 3rd, Price DD. A comparison of placebo effects in clinical analgesic trials versus studies of placebo analgesia. Pain 2002; 99: 443–452. [DOI] [PubMed] [Google Scholar]
- 188. Zou K, Wong J, Abdullah N, et al. Examination of overall treatment effect and the proportion attributable to contextual effect in osteoarthritis: meta-analysis of randomised controlled trials. Ann Rheum Dis 2016; 75: 1964–1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189. Scales D. Ethical implications for potential placebo effects of point of care ultrasound. Perspect Biol Med 2019; 62: 717–736. [DOI] [PubMed] [Google Scholar]
- 190. Doherty M, Dieppe P. The ‘placebo’ response in osteoarthritis and its implications for clinical practice. Osteoarthritis Cartilage 2009; 17: 1255–1262. [DOI] [PubMed] [Google Scholar]
- 191. Enck P, Bingel U, Schedlowski M, et al. The placebo response in medicine: minimize, maximize or personalize? Nat Rev Drug Discov 2013; 12: 191–204. [DOI] [PubMed] [Google Scholar]
- 192. Feltner D, Hill C, Lenderking W, et al. Development of a patient-reported assessment to identify placebo responders in a generalized anxiety disorder trial. J Psychiatr Res 2009; 43: 1224–1230. [DOI] [PubMed] [Google Scholar]
- 193. Oo WM, Liu X, Hunter DJ. Pharmacodynamics, efficacy, safety and administration of intra-articular therapies for knee osteoarthritis. Expert Opin Drug Metab Toxicol 2019; 15: 1021–1032. [DOI] [PubMed] [Google Scholar]
- 194. Maudens P, Jordan O, Allémann E. Recent advances in intra-articular drug delivery systems for osteoarthritis therapy. Drug Discov Today 2018; 23: 1761–1775. [DOI] [PubMed] [Google Scholar]
- 195. Gambaro FM, Ummarino A, Torres Andón F, et al. Drug delivery systems for the treatment of knee osteoarthritis: a systematic review of in vivo studies. Int J Mol Sci 2021; 22: 9137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196. Hunter DJ, Nevitt M, Losina E, et al. Biomarkers for osteoarthritis: current position and steps towards further validation. Best Pract Res Clin Rheumatol 2014; 28: 61–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197. Kraus VB, Simon LS, Katz JN, et al. Proposed study designs for approval based on a surrogate endpoint and a post-marketing confirmatory study under FDA’s accelerated approval regulations for disease modifying osteoarthritis drugs. Osteoarthritis Cartilage 2019; 27: 571–579. [DOI] [PubMed] [Google Scholar]