Abstract
Obesity increases the risk of colorectal cancer (CRC) by 30%. The obese tumor microenvironment compromises antitumor immunity by eliciting exhausted T cells (Tex). Hypothesizing that Dahuang Fuzi Baijiang decoction (DFB) is a combined classical prescription from the “Synopsis of Prescriptions of the Golden Chamber”. We first determined that DFB regresses tumor growth in high‐fat diet–induced obese mice by expanding the TIM3− subset with intermediate expression of programmed cell death‐1 (PD‐1intTIM3−) and restricting the PD‐1hiTIM3+ subset. Transcription factor 1 (TCF1) is highly expressed in the PD‐1intTIM3− subset but is absent in PD‐1hiTIM3+ cells. We next confirmed that progenitor PD‐1intTCF+ cells robustly produce tumor necrosis factor‐α (TNFα) and interferon‐γ, whereas terminally differentiated PD‐1intTCF+ cells have defects in generating TNFα. With transgenic ob/ob mice, we found that DFB produces cooperative efficacy with anti‐PD‐1 (αPD‐1) by limiting the PD‐1hiTim3+ subset and amplifying the PD‐1intTCF+ population. Finally, we defined the recombinant chemokine C‐C‐motif receptor 2 (CCR2)+CD8+ subset as terminal Tex and identified that the differentiation from progenitor to terminal Tex is driven, at least in part, by the chemokine (C‐C motif) ligand 2 (CCL2)/CCR2 axis. The CCR2 inhibitor enhances the response to αPD‐1 by promoting the counts of progenitor Tex. Altogether, DFB dampens CCL2 and preserves progenitor Tex in the obese microenvironment to restrain CRC progression. These findings provide unambiguous evidence that the traditional Chinese formula DFB can prevent tumor progression by modulating adaptive immunity and establish a strong rationale for further clinical verification.
Keywords: CCL2, colorectal cancer, Dahuang Fuzi Baijiang decoction (DFB), microenvironment, obesity, T cell exhaustion
Dahuang Fuzi Baijiang decoction (DFB) produces cooperative efficacy with anti‐programmed cell death‐1 (PD‐1) by limiting the PD‐1hiTim3+ subset and amplifying the PD‐1intTCF+ population. DFB dampens CCL2 and preserves progenitor terminal exhausted T cells in the obese microenvironment to restrain colorectal cancer progression.
Abbreviations
- αPD‐1
anti‐PD‐1
- CCL2
chemokine (C‐C motif) ligand 2
- CCR2
recombinant chemokine C‐C‐motif receptor 2
- CRC
colorectal cancer
- DFB
Dahuang Fuzi Baijiang decoction
- DIO
diet‐induced obese
- GdCl3
gadolinium chloride
- HFD
high‐fat diet
- IFN‐γ
interferon‐γ
- PD‐1
programmed cell death‐1
- PD‐L1
PD‐1 ligand
- TCF1
transcription factor 1
- Tex
terminal exhausted T cell
- TGF‐β
transforming growth factor‐β
- TIL
tumor‐infiltrating lymphocyte
- TIM3
T‐cell immunoglobulin domain and mucin domain 3
- TNF‐α
tumor necrosis factor‐α
- TOX
thymocyte selection‐associated high mobility group box
1. INTRODUCTION
Obesity is associated with 55% of diagnosed cancers in women and 24% of diagnosed cancers in men. 1 Each 5 kg/m2 gain in body mass index can result in 13%–18% increase of CRC risk. 2 Obesity contributes to 35.4% of CRC cases in men and 20.8% in women. 3 Notably, approximately 40% of all cancer deaths are in large part associated with obesity. 1 Obesity represents a top risk factor and an independent prognostic variable of CRC.
Colorectal cancer is an obesity‐related cancer. 4 Cancer‐associated adipocytes secrete various adipokines, growth factors, and sex‐steroid hormones, including leptin, CCL2, and insulin‐like growth factor‐1, to create an immune privileged site. Chemokine (C‐C motif) ligand 2 is the pivotal chemokine that orchestrates the formation of an immunosuppressive tumor microenvironment by empowering adipocytes to maintain chronic inflammatory responses, to skew the inflammatory cells to differentiate into immature phenotypes, as well as to increase T cell tolerance and dysfunction. 5
As a highly mutated cancer, CRC progressively loses its tumor antigen and MHC‐1 contents until immune escape prevails. 6 Obesity tends to aid immune escape by conferring T cell populations with exhausted phenotypes. 7 Exhausted T cells are a discrete T cell subset characterized by progressive loss of effector cytokines and impaired cytotoxicity. 8 Actually, Tex represent a hyporesponsive state that stems from adaptation to persistent antigen and chronic T cell receptor stimulation. 9
Recently, it has been elegantly demonstrated that HFD‐induced obesity dampens the CD8+ TILs percentage and hampers their cytotoxicity. 10 In another study, obesity was reported to induce immune aging and promote tumor growth across multiple strains and tumor types. 11 Obesity results in T cell exhaustion by increasing PD‐1 expression and reducing IFN‐γ and TNF‐α production in TILs. Surprisingly, obesity augments the efficacy of the PD‐1/PD‐L1 blockade in various cancer patients and multiple tumor‐bearing mice, suggest a controversial impact of obesity on cancers. 11
Dahuang Fuzi Baijiang decoction is a combined classical prescription from the “Synopsis of Prescriptions of the Golden Chamber,” which has been praised as the ancestor of traditional Chinese medical formulary. Hypothesizing that combination of Dahuang Fuzi decoction and Yiyi Fuzi Baijiang powder ameliorates obesity‐induced T cell dysfunction, we tested this idea with a series of in vivo experiments in tumor‐bearing mice. Our results show that DFB expands progenitor Tex counts and maintains appropriate adaptive T cell responses in both transgenic and diet‐induced obese mice. These original findings would infuse novel meaning to the classical inheritance of traditional prescription and advance its clinical translation for tumor therapy.
2. MATERIALS AND METHODS
2.1. Cell lines
MC38 cell line (Heyuan Biotech) stably expressing luciferase (MC38‐luc, generated by stable transduction with RediFect Red‐Fluc lentivirus from Perkin Elmer per manufacturer recommendations; Figure S1). Cells were cultured in normal DMEM without pyruvate supplemented with 10% FBS and 1% penicillin/streptomycin. Cells were cultured at 37°C in a humidified 5% CO2 incubator.
2.2. Mice
Eight‐week‐old C57BL/6, C57BL/KS‐ob male mice were purchased from GemPharmatech Co., Ltd. For all experiments, 8‐week‐old mice were assigned to a normal diet. All mouse colonies and experimental animals were maintained in the same animal facility at Southern Hospital of Southern Medical University and housed in specific pathogen‐free conditions. All animals were used in accordance with animal care guidelines from the Southern Hospital Standing Committee on Animals and the National Institutes of Health. All mouse protocols were approved by the Southern Hospital Medical Area Standing Committee on Animals.
2.3. Mouse tumor models
Orthotopic tumor transplantation was carried out as described previously. 12 , 13 Briefly, tumor tissue was prepared by injecting MC38‐luc cells into the abdominal flank of mice. The tumor slices were transplanted into the ileocecal area of the mice in situ. Mice were killed at humane end‐points or day 10‐14 for tissue harvest. Animals were kept in a sterile environment and treatment with DFB (intragastric administration) from day 3 after surgery, daily, or αPD‐1 (i.p), CCR2‐RA‐[R] (i.p.) every 2 days from day 5 after surgery. Weight was measured every 2 days. The mice were killed at the end of the experiments. Tumor, serum, liver, and spleen were collected for further experiments.
2.4. Drug preparation
Dahuang Fuzi Baijiang decoction is comprised of five commonly used herbs (Table S1): Radix et Rhizoma Rhei (root or rhizome of perennial herbaceous plant Rheum palmatum L.), Radix Aconiti Lateralis Praeparata (root of perennial herbaceous plant Aconitum carmichaelii Debeaux), Herba Asari (root of Asarum sieboldii Miq.), Semen Coicis (nut of Coix lacryma‐jobi L. var. ma‐yuen Stapf), Herba Patriniae (entire plants of Patrinia scabiosifolia Fisch. ex Link. and Patrinia villosa Juss). The formula and amount of the prescription are listed in Table S1.
The raw herbs for DFB were purchased from Beijing Tongrentang Co. Ltd. The voucher specimens were deposited in the storage cabinet of Chinese traditional medicine at the School of Traditional Chinese Medicine, Southern Medical University. A voucher specimen was deposited in the herbarium of the School of Traditional Chinese Medicine. These were mixed in the ratio of 12:6:30:15:3 (dry weight). Aqueous extracts of DFB were extracted at 80°C by stirring it for 1 hour using 10 volumes of distilled water (v/m). Then we centrifuged the extract at 1500 g at room temperature. To obtain the semisolid DFB solution, the supernatant was collected and subjected to condensation under reduced pressure of 70°C. The DFB extracts were quality controlled by HPLC analysis with five ingredients (Figures S2 and S3, Table S2). This was undertaken on a Shimadzu LC‐20A HPLC system using a C18 column (Shimadzu VP ODS; 250 × 4.6 mm; particle size 5 μm). The mobile phases comprised eluent A (water) and eluent B (methanol). The gradient flow was as follows: 0.00‐125.00 minutes, 20%‐90% B. The analysis was carried out at a flow rate of 1.0 mL/minute with photo‐diode array detection at 254 nm. The injection volume was 20 μL. Rhein (PubChem CID: 10168), fuziline (PubChem CID: 14163819), asarinin (PubChem CID: 11869417), coixol (PubChem CID: 10772), and quercetin (PubChem CID:5280343) in the DFB sample were determined to be the five active compounds in Radix et Rhizoma Rhei, Radix Aconiti Lateralis Praeparata, Herba Asari, Semen Coicis and Herba Patriniae, respectively.
The αPD‐1 (in vivo mAb anti‐mouse PD‐1) was purchased from BioX Cell and dissolved in PBS (200 μg/kg/2 d). The CCR2 inhibitor CCR2‐RA‐[R] was purchased from MedChemExpress and dissolved in solvent (10% DMSO + 90% corn oil; 5 mg/kg/2 d). The HFD composition is listed in Table S3. The major reagents are listed in Tables S4‐S7.
2.5. Tumor‐infiltrating leukocyte isolation
Tumor tissue (250 mg) was weighed, thoroughly cleaned, and gently cut it into small pieces. The chopped tissue was transferred to a 15‐mL centrifuge tube and 1 mL PBS was added (protease inhibitor 1 mM PMSF, 0.01 mg/mL leupeptin, and 0.01 mg/mL aprotinin). The centrifuge tube was gently shaken (100 g at 4°C) and centrifuged for 1 minute, then the supernatant was aspirated (mainly damaged tissue and cell debris) with a Pasteur pipette. One milliliter of PBS was added to the precipitation sample and incubated in a 37°C, 5% CO2 incubator for 1 hour. After incubation, the sample was centrifuged at 1000 g at 4°C for 3 minutes, the supernatant was removed, and the precipitate discarded. The sample was then centrifuged at 2000 g for 8 minutes at 4°C; the supernatant was removed and the precipitate discarded. Finally, the sample was centrifuged at 20,000 g at 4°C for 30 minutes; the supernatant was removed and the precipitate discarded.
2.6. Enzyme‐linked immunosorbent assay
Levels of TGF‐β1, IL‐6, and CCL2 in TILs and serum were measured by ELISA (Table S6) following the manufacturer’s instructions. We used a microplate reader (Multiskan; Thermo Fisher Scientific) to measure the absorbance at 450 nm.
2.7. Flow cytometry
Primary mouse cells isolated from tumor were stained with fluorescent Abs and analyzed by flow cytometry. Briefly, the tissue samples were first mechanically dissociated using a scalpel, then enzymatically dissociated in digestion medium (2 mg/mL Collagenase I [Sigma] and 0.2 mg/mL DNAse I [Sigma] in DMEM [Gibco]), and incubated for 30 minutes at 37°C with gentle rocking. Red blood cells were removed from the cell suspension using red blood cell lysis buffer (Beyotime), and the cells were filtered using a 70‐µm Flowmi tip strainer (VWR). Subsequent surface marker staining was undertaken in MACS buffer containing 1× PBS supplemented with 1% FBS and 2 mM EDTA. Intracellular staining for flow panels containing nuclear proteins was carried out using TCF1 (R&D Systems) and TOX (Miltenyi Biotech) transcription factor staining buffer set. For intracellular staining of cytoplasmic proteins, such as cytokines, the Fixation/Permeabilization Solution Kit (BD Biosciences) was used. Intracellular cytokine staining was carried out after 6 hours of stimulation with PMA (100 ng/mL) and ionomycin (500 ng/mL) in the presence of Golgi Stop at 37°C. Please see Table S7 for the fluorescently labeled Abs used for staining.
2.8. Antibodies targeting surface or intracellular proteins
Intracellular cytokine staining was undertaken after 5 hours of ex vivo stimulation with GP33‐41 peptide in the presence of Golgi Plug, Golgi Stop, and anti‐CD107a. After stimulation, cells were stained with surface Abs, followed by fixation with Fixation/Permeabilization Buffer and then stained with intracellular Abs for TNF‐α and IFN‐γ using Permeabilization Wash Buffer according to the manufacturer’s instructions. Data collection was carried out on a BD FACSymphony and analyzed using FlowJo version 10.4.1.
2.9. Statistical analysis
Statistical analyses were undertaken using Graphpad Prism 8.0.2 (GraphPad Software). All assays were repeated at least three times and data are presented as the mean ± SD. Differences between groups were calculated utilizing ANOVA or a Student’s t test for continuous variables. P < .05 was considered statistically significant.
3. RESULTS
3.1. Dahuang Fuzi Baijiang decoction suppresses rapid growth of CRC in DIO mice by restricting terminal Tex
To explore the suitable dosage of well quality‐controlled DFB extracts, the size of murine colon adenocarcinoma (MC38‐luc; Figure S1) xenografts was recorded every other day (Figure S4A‐C). Both middle dosage and high dosage significantly restricted tumor growth, whereas the middle dosage showed better performance in regressing growth kinetics (Figure S4D,E). Hence, DFB middle dosage was chosen for subsequent research because of the best curative efficacy among the three different doses.
High‐fat diet–fed obese mice closely mimic the natural development of human obesity. We then monitored tumor growth in DIO mice (60% fat diet) vs control diet mice (control, standard chow diet). MC38 adenocarcinoma grew significantly faster in DIO mice, which showed an obvious increase of body weight compared with control counterparts (Figure 1A,B). Dahuang Fuzi Baijiang decoction enormously limited the growth of MC38 tumors, as indicated by luciferase‐based biochemiluminescence imaging (Figure 1C), suggested that DFB is highly effective in suppressing tumor growth even under a protumoral obese microenvironment.
FIGURE 1.
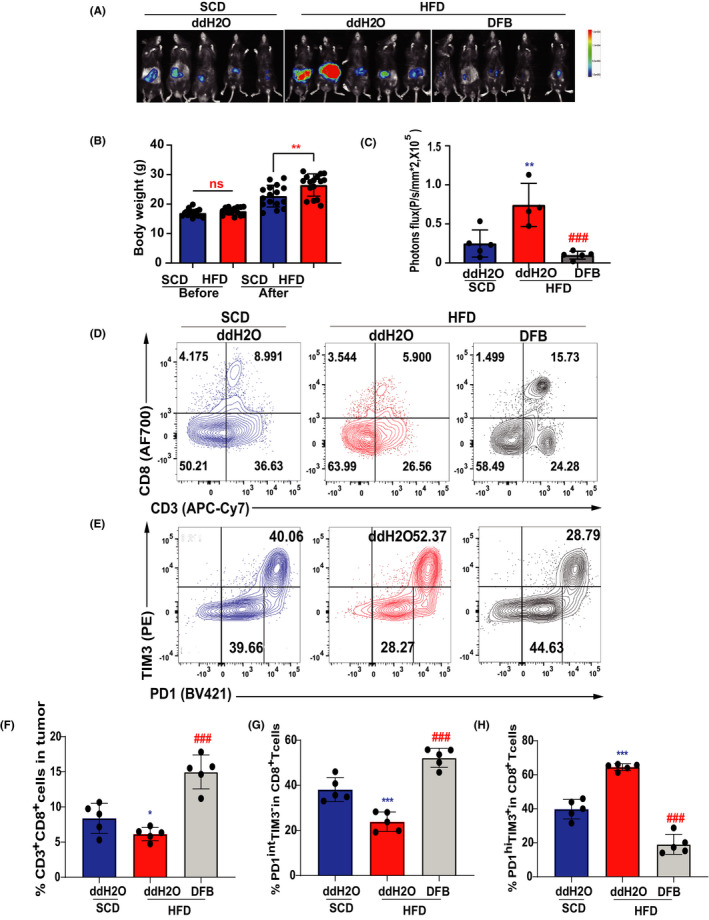
Dahuang Fuzi Baijiang decoction (DFB) suppresses rapid growth of colorectal cancer in diet‐induced obese (DIO) mice by restricting terminal exhausted T cells. (A) In vivo imaging of small animals in the colorectal orthotopic model. (B) Body weights of 8‐ to 16‐week‐old male control and DIO mice (n = 5 per group). (C) Fluorescence statistics of (A). (D) Representative flow plots and frequency of CD3+ CD8+ in tumor infiltration of mice in each group (top panel). Representative flow plots and frequency of PD‐1 and TIM3 in tumor infiltration of mice in each group (bottom panel). (E) Statistical chart of the expression of CD3+ CD8+ in tumor infiltration each group of mice (n = 5 per group). (F) Statistical chart of the expression of PD‐1midTIM3− and PD‐1hiTIM3+ on the tumor‐infiltrating CD8+ T cells of each group of mice (n = 5 per group). *P < .05, **P < .01, ***P < .001 compared with standard chow diet (SCD) (ddH2O) group. # P < .05, ## P < .01, ### P < .001 compared with the high‐fat diet (HFD) (ddH2O) group. Data in this figure are depicted as mean ± SEM, with all individual points shown. Ordinary one‐way ANOVA P values are shown. ns, not significant
We next studied the function of DFB on T cell phenotype in the obese microenvironment (Figure S5A,B). Obesity slightly reduced the ratio of CD8+ TILs in the tumor bed, whereas DFB significantly increased the recruitment of CD8+ TILs (Figure 1D,F). Specifically, HFD resulted in a markedly higher frequency of PD‐1hiTim3+ CD8+ TILs concomitant with an obvious decrease of the PD‐1intTim3− subset compared to control mice (Figure 1E,G,H). The PD‐1hiTim3+ subset is generally defined as terminally differentiated exhausted T cells, whereas intermediate expression of PD‐1 (PD‐1int) and coexistence of TCF‐1 confer Tex with stem cell‐like properties. 9 , 14 Dahuang Fuzi Baijiang decoction increased the PD‐1int subset and lowered the number of PD‐1hiTim3+ cells in the tumor. Taken together, DFB suppresses HFD‐accelerated tumor progression by reserving the PD‐1int subset and decreasing Tex counts.
3.2. TOX and TCF1 control Tex differentiation status
Terminally differentiated Tex is the driving force to form immunosuppressive microenvironment, whereas progenitor Tex subset is critical for generating response to PD‐1 blockade.15–17 To characterize the differentiation state of the two subsets, we sorted and analyzed transcription activity of TCF1 and thymocyte selection‐associated high mobility group box (TOX) (Figure S6C). PD‐1intTIM3‐ cells are characterized by strong expression of TCF1 which is absent in PD‐1hiTim3+ cells (Figure 2A, C). These TCF1+PD‐1int progenitor cells can expand and yield terminal exhausted PD‐1hiTim3+ cells. We observed a highly expression of TOX in the PD‐1hiTim3+ subset (Figure 2A, D). TOX transcriptionally activates co‐inhibitory receptor gene Pdcd1 and stimulates the transcription of Tcf7 which is the encoding gene for TCF1. We also noticed a overlap of TOX and TCF1 expression in TCF1+PD‐1int subset (Figure 2B), suggested that TOX may act upstream of TCF1 to drive the differentiation from progenitor to terminal Tex.
FIGURE 2.
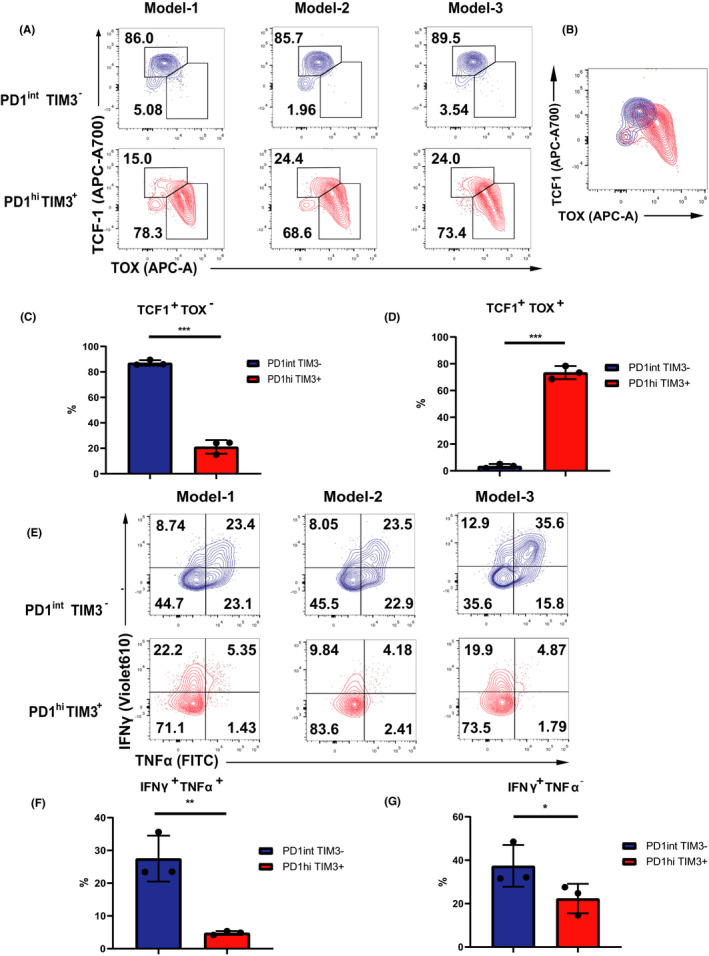
TOX and TCF1 control T ex differentiation status. (A) Representative flow plots of TOX and TCF1 ratio in PD1int TIM3‐ and PD1hi TIM3+ cells (n = 3 per group). (B) The overlap of TOX and TCF1 expression in TCF1+PD‐1int subset. (C, D) Statistical chart of A (n = 3 per group). (E) Representative flow plots and frequency of IFNγ and TNFα expression among CD8+ TILs after Stimulation 6h with CD3/CD28 Ab in PD1int TIM3‐ and PD1hi TIM3+ cells (n = 3 per group). (F–G) Statistical chart of E (n = 3 per group). Statistical significance was assessed by Student’s t test (C–D; F–G). (not significant [ns], p > 0.05, *p < 0.05, **p < 0.01)
As Tex progressively lose its effector function in a hierarchical pattern, we examined the alterations of effector cytokines with an ex vivo experiment. The sorted TCF1+PD‐1int and PD‐1hiTim3+ cells were stimulated with CD3 and CD28 antibody (Figure S6C). TCF1+PD‐1int cells displayed robust ability to produce TNFɑ and IFNγ. However, PD‐1hiTim3+ exhibited defects in generating IFN‐γ while showed residue competence to produce TNFɑ (Figure 2E–F). Loss of IFNγ has been considered occurring at the late stage of exhaustion14, our results suggested that the cytotoxity defect in PD‐1hiTim3+ cells is corresponding to the terminally exhausted state (Figure 2E, G). These distinct fingerprints of Tex suggested that TOX and TCF1 drive the evolution between progenitor and terminal Tex which determine the exhausted status and their response to checkpoint blockade.
3.3. Dahuang Fuzi Baijiang decoction reserves progenitor Tex to elicit immune response to αPD‐1
As PD‐1intTCF‐1+ progenitor TILs are poised to respond to PD‐1 blockade, we reasoned that DFB might coordinate αPD‐1 response by modulating PD‐1 expression in CD8+ T cells. Ob/ob mice carry a spontaneous mutation at the leptin locus that leads to obesity, hyperglycemia, and elevated plasma insulin. Ob/ob mice were visibly obese with elevated body weight and abdominal circumference (Figure S6A,B). Mirroring the protumorigenic activity of DIO, the ob/ob phenotype drastically accelerated MC38 tumor growth compared to its corresponding C57BL/6J background (Figure S7). We established a subcutaneous ectopic transplanted tumor model in ob/ob mice (Figure 3A). Dahuang Fuzi Baijiang decoction not only moderately attenuated tumor growth (Figure 3B,C), but displayed prominent cooperative efficacy in limiting tumor size in ob/ob mice (Figure S1B,C).
FIGURE 3.
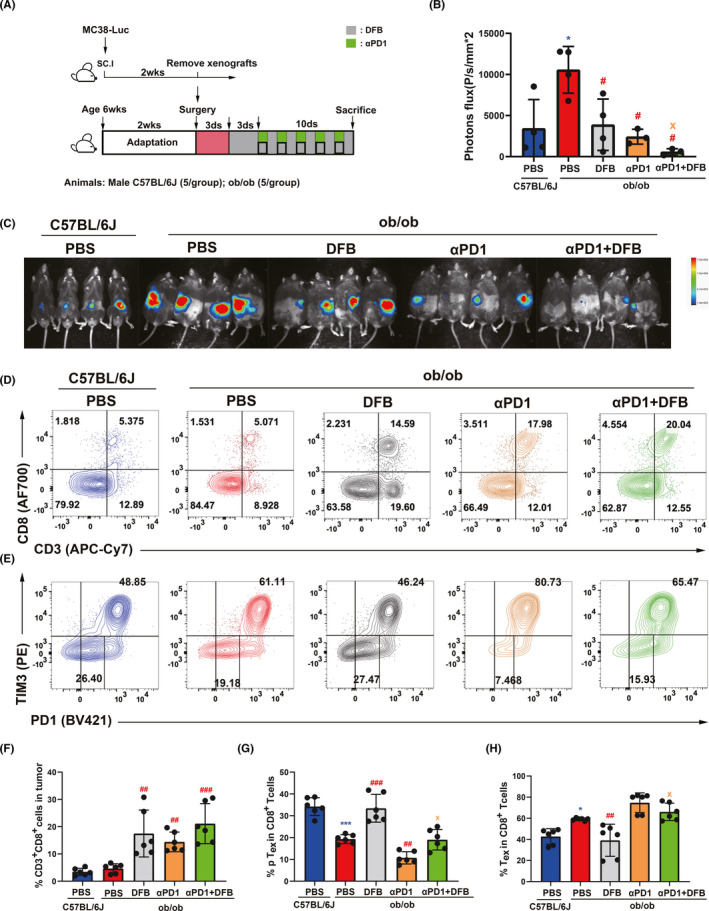
Dahuang Fuzi Baijiang decoction (DFB) reserves progenitor terminal exhausted T cells (Tex) to elicit immune response to anti‐programmed cell death‐1 (αPD‐1). (A) Schema depicting experimental setup. (B) Body weights of WT C57BL/6J and ob/ob mice during the experiment. (C) In vivo imaging of small animals in the colorectal orthotopic model. (D) Representative flow plots and frequency of CD3+ CD8+ in tumor infiltration of mice in each group (top panel); Representative flow plots and frequency of PD‐1 and TIM3 in tumor infiltration of mice in each group (top panel). Representative flow plots and frequency of PD‐1 and TIM3 in tumor infiltration of mice in each group (bottom panel). (E) Statistical chart of the expression of CD3+ CD8+ in tumor infiltration each group of mice (n = 5 per group); (F) Statistical chart of the expression of tTex and pTex on the tumor‐infiltrating CD8+ T cells of each group of mice (n = 5 per group). *P < .05; **P < .01; ***P < .001, compared with C57BL/6J (PBS) group. # P < .05; ## P < .01; ### P < .001, compared with ob/ob (PBS) group. x p < .05, compared with ob/ob (αPD1 + DFB) group. Data in this figure are depicted as mean ± SEM, with all individual points shown. Ordinary one‐way ANOVA P values are shown
To investigate whether reduced growth rates of tumors by DFB was due to control by T cells, we regenotyped the T cells in the tumor with PD‐1 and TIM3 (Figure S5A,B). Dahuang Fuzi Baijiang decoction augmented the number of CD8+ T cells and synergized the effects of αPD‐1 (Figure 3D,F). As expected, ob/ob mice increased the percentage of PD‐1hiTim3+ cells, corresponding to a reduction of PD‐1int cells (Figure 3E,G,H). Dahuang Fuzi Baijiang decoction substantially decreased the ratio of terminal PD‐1hiTim3+ Tex to ameliorate the immunosuppressive condition. It also increased the number of PD‐1int T cells that serve to respond to αPD‐1 immunotherapy. Together, DFB curbs the tumor growth by limiting the PD‐1hiTim3+ subset and amplifying PD‐1int population to enhance the efficacy of PD‐1 checkpoint blockade.
3.4. Dahuang Fuzi Baijiang decoction reduces tumor interstitial and serum CCL2 production
Obesity has been demonstrated to induce a chronic, low‐grade inflammatory status with increased adipokines, such as IL‐6, TGF‐β, leptin, and TNF‐α, as well as elevated levels of CCL2. Ob/ob genotype elevated IL‐6 in tumor interstitial fluid, but had no obvious impact on TGF‐β secretion compared to age‐matched WT mice (Figure 4A,D). Dahuang Fuzi Baijiang decoction substantially depressed tumor interstitial IL‐6 content but displayed no synergistic effects with αPD‐1 on IL‐6 levels (Figure 4B,E).
FIGURE 4.
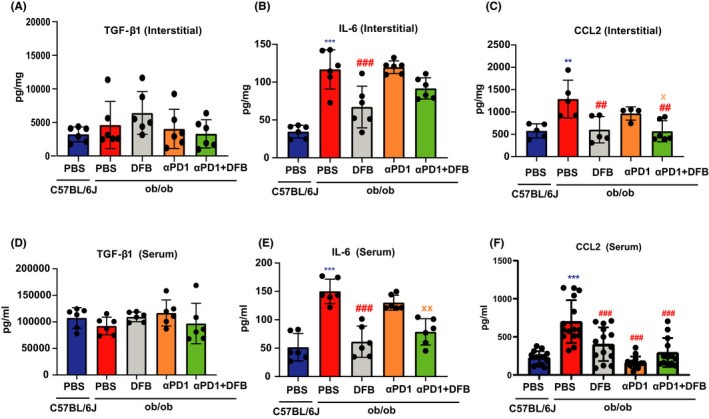
Dahuang Fuzi Baijiang decoction (DFB) reduces tumor interstitial and serum chemokine (C‐C motif) ligand 2 (CCL2) production. Contents of key adipokines in tumor interstitial fluid and serum. Transforming growth factor‐β1 (TGF‐β1) in tumor interstitial fluid (A) and serum (D). Interleukin‐6 (IL‐6) in tumor interstitial fluid (B) and serum (E). CCL2 in tumor interstitial fluid (C) and serum (F). *P < .05, **P < .01, ***P < .001, compared with ob/ob (PBS) group. # P < .05, ## P < .01, ### P < .001, compared with ob/ob (anti‐programmed cell death‐1 [αPD‐1] + DFB) group,. Data in this figure are depicted as mean ± SEM, with all individual points shown. Ordinary one‐way ANOVA P values are shown
Chemokine (C‐C motif) ligand 2, which is symbolically presented at high levels in obesity, gives rise to immune evasion through PD‐1 signaling. 18 We first assessed the effects of DFB on CCL2 in ob/ob mice. Dahuang Fuzi Baijiang decoction reversed obesity‐raised stromal CCL2 content. Although αPD‐1 alone could not lower CCL2 secretion, DFB helped αPD‐1 inhibitor to reduce stromal CCL2 levels (Figure 4C,F). We next evaluated the impact of DFB in DIO mice and observed a remarkably similar pattern. Dahuang Fuzi Baijiang decoction attenuated interstitial CCL2 content in HFD‐feeding obese mice. Additionally, we also measured the alteration of serum CCL2 contents. As expected, either DFB or αPD‐1, or the two together, decreased serum CCL2 elevation that is induced by the obese background. Collectively, DFB dampens obesity‐driven tumor interstitial and serum CCL2 production both in DIO and transgenic obese mice.
3.5. Recombinant chemokine C‐C‐motif receptor 2 inhibitor blocks shift from progenitor Tex to terminal Tex
As elevated CCL2 expression drives PD‐1 inhibitor resistance 19 and CCR2 antagonism enhances tumor response to αPD‐1 monotherapy, 20 we then tested whether the CCL2‐CCR2 axis contributes to Tex differentiation with a specific inhibitor, CCR2‐RA‐[R]. As we found earlier, MC38 tumor growth was accelerated in ob/ob mice compared with control C57BL6/J mice (Figure 5A,B). There was no significant change in the weight of the spleen or liver (Figure S8). The CCR2 inhibitor restrained tumor growth and increased CD8+ T cell infiltration (Figure 5C,E).
FIGURE 5.
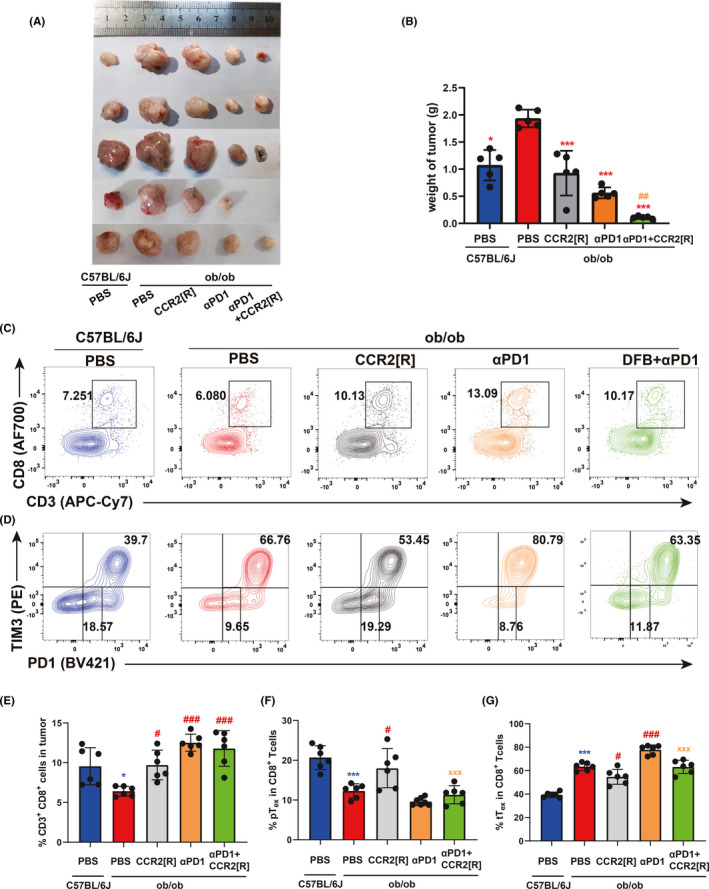
CCR2 inhibitor suppresses the shift from pTex to tTex. (A) Tumor weight of MC38 subcutaneously inoculated in 8‐week‐old C57BL/6J (n = 5) and ob/ob (n = 5) male mice. Tumor weight depicted as mean ± SEM. (B) Statistical chart of the tumor weight in each group (n = 5 per group). (C) Representative flow plots of CD3+ CD8+ ratio in tumor‐bearing mice in each group (Above); Representative flow plots of PD1 and TIM3 ratio in tumor‐bearing mice in each group (Below). (D) Statistical chart of the ratio of CD3+ CD8+ in tumor infiltration each group (n = 5 per group). (E) Statistical chart of the ratio of terminal exhausted T cells (tTex) and progenitor exhausted T cells (pTex) in the tumor‐infiltrating CD8+ T cells of each group (n = 5 per group). (F) Statistical chart of the ratio of tTex and pTex in the tumor‐infiltrating CD8+ T cells of each group (n = 5 per group). Compared with C57BL/6J (PBS) group, *p < 0.05; **p < 0.01; ***p < 0.001. Compared with ob/ob (PBS) group, # p < 0.05; ## p < 0.01; ### p < 0.001. Compared with ob/ob (αPD1+CCR2‐RA‐[R]) group, xx p < 0.01. Data in this figure are all depicted as mean ± SEM, with all individual points shown. Ordinary one‐way ANOVA p values are shown
We also observed a reduction of progenitor PD‐1int number in ob/ob mice compared to their WT counterparts. Importantly, CCR2 inhibitor enhanced the percentage of progenitor PD‐1int cells, and also expanded this subset when combined with αPD‐1 immunotherapy (Figure 5D,F,G). Corresponding to its protumor phenotype, ob/ob mice also increased the ratio of terminal PD‐1hiTIM3+ CD8+ TILs, whereas CCR2 inhibitor reduced the counts of this subset. Hence, obesity enhanced tumor interstitial CCL2 to facilitate the differentiation from progenitor to terminal Tex. Dahuang Fuzi Baijiang decoction exerts it antitumor activity through disturbing CCL2/CCR2‐mediated terminal T cell exhaustion. 21 Together, DFB decreases obesity‐driven CCL2 secretion and reverses the Tex differentiation shift to regress CRC progression.
3.6. Dahuang Fuzi Baijiang decoction inhibits terminal Tex differentiation by disrupting T cell CCR2 signaling
Apart from T cells, CCR2 is predominantly expressed by monocytes/macrophages. 22 To elucidate the role of macrophages on CD8+ T cell differentiation, a monocyte/macrophage depleting agent, GdCl3, was used. GdCl3 restricts tumor growth in ob/ob mice, similar to the effects of CCR2 antagonist. Our results also show that combining Gdcl3 and CCR2 antagonist does not result in an additive or synergistic effect in suppressing the growth of MC38 xenografted tumor (Figure 6A,B), which is consistent with a previous report. 23
FIGURE 6.
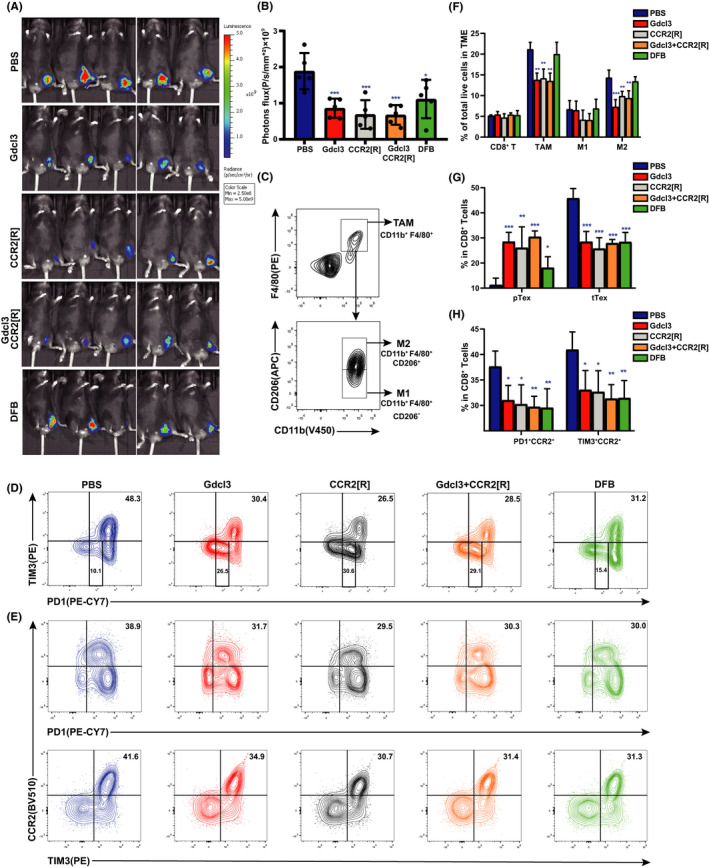
Dahuang Fuzi Baijiang decoction (DFB) inhibits shift from progenitor exhausted T cells (pTex) to terminal exhuasted T cells (tTex) independent of macrophages. (A) In vivo imaging of small animals in the colorectal subcutaneous model. (B) Fluorescence statistics of figure (A). (C) Flow cytometry gating strategies used to define tumor‐associated macrophages (TAM) (CD11b+ F4/80+), M1‐TAM (CD11b+F4/80+CD206−) and M2‐TAM (CD11b+F4/80+CD206+). (D) Representative flow plots and frequency of PD‐1 and TIM3 in tumor infiltration of mice in each group. (E) Representative flow plots and frequency of recombinant chemokine C‐C‐motif receptor 2 (CCR2) on the tumor‐infiltrating CD8+ Tex cells of each group. (F) Relative percentages of the tumor‐infiltrating inflammatory cells, as determined by FACS, in the tumor tissues of mice subjected to various treatments. These include CD8+ tumor‐infiltrating lymphocytes, TAM, M1‐TAM, and M2‐TAM (n = 5 per group). (G) Statistical chart of the expression of tTex and pTex on the tumor‐infiltrating CD8+ T cells of each group of mice (n = 5 per group). (H) Statistical chart of the expression of CCR2 on the tumor‐infiltrating CD8+ Tex cells of each group of mice (n = 5 per group). *P < .05, **P < .01, ***P < .001, compared with C57BL/6J (PBS) group. Data in this figure are depicted as mean ± SEM, with all individual points shown. Ordinary one‐way ANOVA P values are shown. GdCl3, gadolinium chloride
Treatment with Gdcl3 or/and CCR2 inhibitor significantly reduced the infiltration of tumor‐associated macrophages (CD11b+ F4/80+), especially the proportion of M2 (CD11b+ F4/80+ CD206+; Figure 6C,F). CD8+ T cell genotyping shows that DFB, Gdcl3, and/or CCR2 inhibitor restrains the differentiation from progenitor to terminal Tex (Figure 6D,G). However, DFB does not cause appreciable changes in macrophage subsets, suggested that progenitor‐prone differentiation of CD8+ T cells caused by DFB is not dependent on macrophages. Further genotyping shows that the CCR2+CD8+ T cell subset is mainly composed of PD‐1+TIM3+ cells, which is defined as terminal Tex (Figure 6E,H). Taken together, the CCR2+CD8+ subset is characterized as terminal Tex; DFB inhibits terminal Tex differentiation by disrupting the CCL2/CCR2 axis in T cells.
4. DISCUSSION
Obesity boosts TILs entering an exhausted state. T cell exhaustion represents a compromise to continuous antigen stimulation, which allows T cells to control tumor growth without causing detrimental immunological pathology. However, cancer cells have hijacked the exhaustion tactics during evolution, encouraging the adaptive immune system to “tolerate” them so as to gain the survival allowance. The “host‐pathogen stalemate” during obesity reveals that the “anergy”‐like hyporesponsive state of T cell and chronic inflammation can be coupled. 9 So, DFB might suppress obesity‐associated incidence of CRC by ameliorating the adaptive state of T cell hyporesponsiveness and dampening low‐grade inflammation. 24 , 25
Of particular note, DFB increases the infiltration of the PD‐1intTCF1+ subset, which can be ascribed to progenitor/stem‐like exhausted T cells. 26 The specific pool of exhausted T cells retains partial protective capability once reinvigorated by blockade of the PD‐1 pathway. 27 , 28 Importantly, these progenitor cells hold potential to proliferate and to yield terminally differentiated Tex. Dahuang Fuzi Baijiang decoction inhibits the shift of progenitor exhausted T cells to terminally differentiated T cells and augments the effects of PD‐1 checkpoint blockade. So, DFB provides synergistic therapeutic benefit with checkpoint blockade to enhance the enhancer in tumor immunity. 21
The hyperplasia and hypertrophy of adipocytes in obesity trigger chronic inflammation by increasing the secretion of CCL2. 29 It has been well demonstrated that the CCL2/CCR2 axis enhances tumor progression by amplifying the recruitment of myeloid‐derived suppressor cells and metastasis‐promoting monocytes. 22 , 30 Our results give additional evidence that CCL2 compels T cells to differentiate into a terminally exhausted phenotype which accelerates the progression of CRC.
Recombinant chemokine C‐C‐motif receptor 2 antagonism promotes tumor response to PD‐1 inhibitor in multiple cancer types, 19 , 31 indicating that the CCR2+CD8+ subset may be immunosuppressive. Dahuang Fuzi Baijiang decoction reverses CCL2 production in the obese microenvironment and holds Tex back into a progenitor state. So, targeting CCL2/CCR2 might be a suitable strategy in rescuing terminal T cell exhaustion.
Altogether, DFB dampens CCL2 and preserves progenitor Tex in the obese microenvironment to restrain CRC progression (Figure 7). Obesity enhances tumor progression by raising CCL2 secretion, which recruits terminally exhausted T cells to establish a healthy‐Qi deficient microenvironment. Dahuang Fuzi Baijiang decoction strengthens healthy‐Qi and eliminates cancerous lesions by restricting CCL2/CCR2‐mediated recruitment of exhausted T cells. Dahuang Fuzi Baijiang decoction also inhibits the polarization of progenitor T cells to terminal exhausted T cells. The preservation of progenitor Tex is indispensable for the response to PD‐1 checkpoint blockade. 17 Taken together, our outcomes pave a novel avenue to use classic prescriptions to cure cancerous disease through regulating adaptive immunology. Although the two constituent formulae of DFB have been used for thousands of years, the immunomodulatory properties of DFB still need further confirmation from clinical practice.
FIGURE 7.
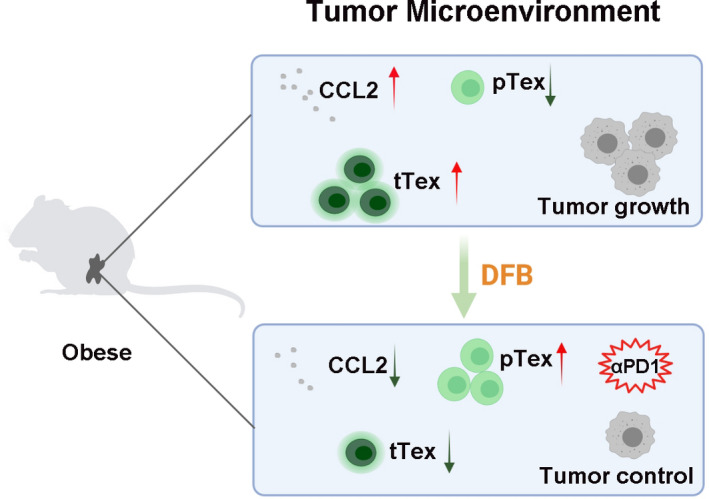
Summary illustration of the effect of Dahuang Fuzi Baijiang decoction (DFB) on colorectal cancer tumor growth in obese mice. αPD‐1, anti‐programmed cell death‐1; CCL2, chemokine (C‐C motif) ligand 2; pTex, progenitor exhausted T cells; tTex, terminal exhausted T cells
CONFLICT OF INTEREST
The authors declare that they have no competing interests.
Supporting information
Fig S1‐S8
Table S1‐S7
ACKNOWLEDGMENTS
This work was supported by the National Science Foundation of China (81974554, 81774172, 81904075), Key‐Area Research and Development Program of Guangdong Province, Modernization of Chinese Medicine in Lingnan (2020B1111100011), Planned Science Technology Project of Guangzhou (202002030111).
Xu Y, Wang H, Wang T, et al. Dahuang Fuzi Baijiang decoction restricts progenitor to terminally exhausted T cell differentiation in colorectal cancer. Cancer Sci. 2022;113:1739–1751. doi: 10.1111/cas.15311
Yihua Xu, Hao Wang, and Tao Wang contributed equally to this work.
Funding information
Key‐Area Research and Development Program of Guangdong Province, Modernization of Chinese medicine in Lingnan, Grant/Award Number: 2020B1111100011; National Natural Science Foundation of China, Grant/Award Number: 81974554, 81774172, 81904075; Planned Science Technology Project of Guangzhou, Grant/Award Number: 202002030111
REFERENCES
- 1. Steele CB, Thomas CC, Henley SJ, et al. Vital Signs: trends in incidence of cancers associated with overweight and obesity ‐ United States, 2005–2014. MMWR Morb Mortal Wkly Rep. 2017;66:1052‐1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Eheman C, Henley SJ, Ballard‐Barbash R, et al. Annual report to the nation on the status of cancer, 1975–2008, featuring cancers associated with excess weight and lack of sufficient physical activity. Cancer‐Am Cancer Soc. 2012;118:2338‐2366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Calle EE, Kaaks R. Overweight, obesity and cancer: epidemiological evidence and proposed mechanisms. Nat Rev Cancer. 2004;4:579‐591. [DOI] [PubMed] [Google Scholar]
- 4. Bardou M, Barkun AN, Martel M. Obesity and colorectal cancer. Gut. 2013;62:933‐947. [DOI] [PubMed] [Google Scholar]
- 5. Kanneganti TD, Dixit VD. Immunological complications of obesity. Nat Immunol. 2012;13:707‐712. [DOI] [PubMed] [Google Scholar]
- 6. Woodall MJ, Neumann S, Campbell K, Pattison ST, Young SL. The effects of obesity on anti‐cancer immunity and cancer immunotherapy. Cancers (Basel). 2020;12:1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Xie Q, Ding J, Chen Y. Role of CD8(+) T lymphocyte cells: interplay with stromal cells in tumor microenvironment. Acta Pharm Sin B. 2021;11:1365‐1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Yang Y, Liu F, Liu W, et al. Analysis of single‐cell RNAseq identifies transitional states of T cells associated with hepatocellular carcinoma. Clin Transl Med. 2020;10:e133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Blank CU, Haining WN, Held W, et al. Defining ‘T cell exhaustion'. Nat Rev Immunol. 2019;19:665‐674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ringel AE, Drijvers JM, Baker GJ, et al. Obesity shapes metabolism in the tumor microenvironment to suppress anti‐tumor immunity. Cell. 2020;183:1848‐1866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wang Z, Aguilar EG, Luna JI, et al. Paradoxical effects of obesity on T cell function during tumor progression and PD‐1 checkpoint blockade. Nat Med. 2019;25:141‐151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zhao L, Wang H, Liu C, et al. Promotion of colorectal cancer growth and metastasis by the LIM and SH3 domain protein 1. Gut. 2010;59:1226‐1235. [DOI] [PubMed] [Google Scholar]
- 13. Han Q, Xu L, Lin W, et al. Long noncoding RNA CRCMSL suppresses tumor invasive and metastasis in colorectal carcinoma through nucleocytoplasmic shuttling of HMGB2. Oncogene. 2019;38:3019‐3032. [DOI] [PubMed] [Google Scholar]
- 14. McLane LM, Abdel‐Hakeem MS, Wherry EJ. CD8 T cell exhaustion during chronic viral infection and cancer. Annu Rev Immunol. 2019;37:457‐495. [DOI] [PubMed] [Google Scholar]
- 15. Sakuishi K, Apetoh L, Sullivan JM, Blazar BR, Kuchroo VK, Anderson AC. Targeting Tim‐3 and PD‐1 pathways to reverse T cell exhaustion and restore anti‐tumor immunity. J Exp Med. 2010;207:2187‐2194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Dammeijer F, van Gulijk M, Mulder EE, et al. The PD‐1/PD‐L1‐checkpoint restrains T cell immunity in tumor‐draining lymph nodes. Cancer Cell. 2020;38:685‐700. [DOI] [PubMed] [Google Scholar]
- 17. Miller BC, Sen DR, Al AR, et al. Subsets of exhausted CD8(+) T cells differentially mediate tumor control and respond to checkpoint blockade. Nat Immunol. 2019;20:326‐336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Yang H, Zhang Q, Xu M, et al. CCL2‐CCR2 axis recruits tumor associated macrophages to induce immune evasion through PD‐1 signaling in esophageal carcinogenesis. Mol Cancer. 2020;19:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Choi J, Lee HJ, Yoon S, et al. Blockade of CCL2 expression overcomes intrinsic PD‐1/PD‐L1 inhibitor‐resistance in transglutaminase 2‐induced PD‐L1 positive triple negative breast cancer. Am J Cancer Res. 2020;10:2878‐2894. [PMC free article] [PubMed] [Google Scholar]
- 20. Tu MM, Abdel‐Hafiz HA, Jones RT, et al. Inhibition of the CCL2 receptor, CCR2, enhances tumor response to immune checkpoint therapy. Commun Biol. 2020;3:720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gu Y, Liu Y, Cao X. Evolving strategies for tumor immunotherapy: enhancing the enhancer and suppressing the suppressor. Natl Sci Rev. 2015;4:161‐163. [Google Scholar]
- 22. Hartwig T, Montinaro A, von Karstedt S, et al. The TRAIL‐induced cancer secretome promotes a tumor‐supportive immune microenvironment via CCR2. Mol Cell. 2017;65:730‐742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Li X, Yao W, Yuan Y, et al. Targeting of tumour‐infiltrating macrophages via CCL2/CCR2 signalling as a therapeutic strategy against hepatocellular carcinoma. Gut. 2017;66:157‐167. [DOI] [PubMed] [Google Scholar]
- 24. Sinicrope FA, Foster NR, Sargent DJ, O'Connell MJ, Rankin C. Obesity is an independent prognostic variable in colon cancer survivors. Clin Cancer Res. 2010;16:1884‐1893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Chen X, Pan X, Zhang W, et al. Epigenetic strategies synergize with PD‐L1/PD‐1 targeted cancer immunotherapies to enhance antitumor responses. Acta Pharm Sin B. 2020;10:723‐733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Chen Z, Ji Z, Ngiow SF, et al. TCF‐1‐centered transcriptional network drives an effector versus exhausted CD8 T cell‐fate decision. Immunity. 2019;51:840‐855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Pauken KE, Wherry EJ. SnapShot: T cell exhaustion. Cell. 2015;163:1038. [DOI] [PubMed] [Google Scholar]
- 28. Beltra JC, Manne S, Abdel‐Hakeem MS, et al. Developmental relationships of four exhausted CD8(+) T Cell subsets reveals underlying transcriptional and epigenetic landscape control mechanisms. Immunity. 2020;52:825‐841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Catalan V, Gomez‐Ambrosi J, Rodriguez A, et al. IL‐32alpha‐induced inflammation constitutes a link between obesity and colon cancer. Oncoimmunology. 2017;6:e1328338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Yang Y, Li L, Xu C, et al. Cross‐talk between the gut microbiota and monocyte‐like macrophages mediates an inflammatory response to promote colitis‐associated tumourigenesis. Gut. 2021;70(8):1495‐1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Shi L, Wang J, Ding N, et al. Inflammation induced by incomplete radiofrequency ablation accelerates tumor progression and hinders PD‐1 immunotherapy. Nat Commun. 2019;10:5421. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig S1‐S8
Table S1‐S7


