Abstract
Due to the increasing complexity of cancer treatment, ensuring safety and maintaining the quality of life during treatment are important issues. Patient‐reported outcomes (PROs) in oncology are essential for assessing patient symptoms. A feasibility study was undertaken on breast cancer patients by building a PRO data collection system based on LINE, one of the most popular social network service applications in Japan. In this study, one or more predefined PRO questions for each breast cancer patient's clinical situation were sent to the patient’s LINE application daily. The patient selected a predefined answer by tapping the screen, but no free‐text answers were allowed. Seventy‐three patients were enrolled. The median observation period was 435 days (84‐656 days), and the total number of PROs collected was 16,417, with a mean of 224.9 reports per patient. Patients on adjuvant endocrine therapy were notified of 2.5 questions per week, and the median number of responses per week and response rate were 2.387 (1.687‐11.627) and 95.5%, respectively. Analyzing the results by age group, the number of responses from those aged 60 and above was equal to or higher than that of the younger age group. It was also possible to track each patient’s PROs accurately. These results suggested that the design of the system, based on an application used daily, instead of using specifically prepared applications for collecting electronic PROs, was the reason for the favorable acceptance from patients and the satisfactory response rate from all age groups, including the elderly.
Keywords: breast cancer, electronic patient‐reported outcome, patient‐reported outcome
This study aimed to determine the feasibility of the LINE electronic patient‐reported outcome (ePRO) system to be accepted by breast cancer patients and to successfully collect ePRO data using LINE, which is the most popular social network service application in Japan. Our study suggests that the acceptance of the system by the patients was favorable, and the response rate was satisfactory. Therefore, building a PRO reporting system based on the applications frequently used by the citizens of a country is likely to contribute to improving the response rate.

Abbreviations
- ePRO
electronic patient‐reported outcome
- HRQOL
health‐related quality of life
- PRO‐CTCAE
Patient‐Reported Outcomes version of the Common Terminology Criteria for Adverse Events
- PRO
patient‐reported outcome
- QOL
quality of life
- SNS
social network service
1. INTRODUCTION
The importance of PROs, which are subjective evaluations by patients themselves, has been recently recognized both in clinical trials and clinical practices. Advancements in cancer therapies have prolonged survival, 1 which has drawn attention to the importance of patient survivorship and subjective experience. This has led to the recognition of the importance of PROs in the field of oncology, as the evaluation of tolerability and safety of the treatment is now considered an essential factor. In the past, PRO data from outpatients were collected using interviews and paper questionnaires. Recently, it has become possible to implement ePRO, a system that allows patients to report their symptoms electronically in real‐time using their smartphones or tablet devices. The usefulness of the ePRO system has been reported in several clinical studies that prospectively collected PRO data from patients with solid tumors. 2 , 3 , 4 , 5 , 6 In addition, ePRO was found to be equally reliable as a tool for assessing the European Organization for Research and Treatment of Cancer Quality of Life Questionnaire‐C30 (EORTC QLQ‐C30) and Functional Assessment of Cancer Therapy‐Breast (FACT‐B), which are frequently used QOL in breast cancer clinical trials. 7 , 8 However, it is necessary to be proficient in operating ePRO‐specific applications, which may be difficult, especially for the elderly who are not accustomed to handling digital devices.
Given this background, the aim of our study was to determine the feasibility of the LINE‐ePRO system to be accepted by breast cancer patients and to successfully collect ePRO data using LINE, which is the most popular SNS application in Japan. Patients answer their PROs by responding to push‐type notifications sent to LINE applications that they use frequently in their daily lives. This system was operated as LINE‐ePRO, and symptom and QOL data from patients with breast cancer were collected depending on their situation, such as receiving treatment in the adjuvant setting or metastatic setting. This study aims to verify whether PRO data collection can be feasible without participants’ special operation proficiency in smartphone applications and whether the LINE‐ePRO reporting can be incorporated into patients’ routine medical activities.
2. MATERIALS AND METHODS
2.1. Study design
This multicenter exploratory study was designed to evaluate the feasibility of LINE‐ePRO, as an SNS‐based communication tool, whether it can successfully collect PRO data from breast cancer patients. To gauge patients’ willingness to report their PROs, we evaluated the extent to which patients who were enrolled in the protocol used the LINE‐ePRO system to self‐report symptoms. Thus, the objectives of this study were to investigate the patients’ PRO reporting rates responding to inquiries received through the LINE‐ePRO system and to examine whether PRO reporting rates are different depending on patients’ age groups. The number of PRO reports and ratio of symptoms were analyzed both in the entire group of patients and in situation‐specific groups of patients (ie, adjuvant chemotherapy, adjuvant endocrine therapy, or treatment for metastatic breast cancer).
2.2. Eligibility criteria
Women who met the following criteria were eligible for inclusion: (a) patients with histologically or cytologically confirmed breast cancer in either Keio University Hospital or Teikyo University Hospital from whom free consent could be obtained; (b) patients who could enter data using a digital device such as a smartphone or tablet device; and (c) patients aged 20 years or older. Patients participating in a different clinical trial were excluded from the study. The criteria for discontinuation were as follows: (a) when the patient requested discontinuation; (b) when the patient withdrew consent on the input site; and (c) when the attending physician or principal investigator deemed discontinuation to be necessary. The study was carried out in compliance with the principles of the Declaration of Helsinki, and the study protocol was approved by the Institutional Review Board of Keio University School of Medicine (Approval No. 20170304) and by the local research ethics committee of Teikyo University Hospital. Written informed consent was obtained from all patients before enrollment.
2.3. Patient enrollment and PRO collection methods
In this study, patients undergoing adjuvant chemotherapy, adjuvant endocrine therapy, and treatment for metastatic breast cancer were included. The timing of patient registration was not limited to the start of treatment and was also open during the treatment. After obtaining written informed consent, the patient was issued with an ID and password to register with the PRO storage database system set up at the Keio University Tonomachi Campus. In parallel, this system was registered on LINE, a social networking application installed on the patient’s smartphone, to enable the patient to receive messages asking PRO questions. One or more predefined PRO questions were sent to the patient’s LINE at a regular time (6:00 pm). The patient selected a predesigned answer by tapping the smartphone screen (Figure 1). No free‐text responses were allowed. All of these message exchanges were encrypted, stored on a server, and accessible only to a specific researcher undertaking the analysis. If there were any predefined symptoms that the patient wished to report, they could be reported as PROs at any given time. In other words, the patient was allowed to enter PROs, in addition to the responses given to the timed standardized questions. In this case too, the patient responded to a predefined question format with a tap operation, and no free‐text responses were allowed.
FIGURE 1.
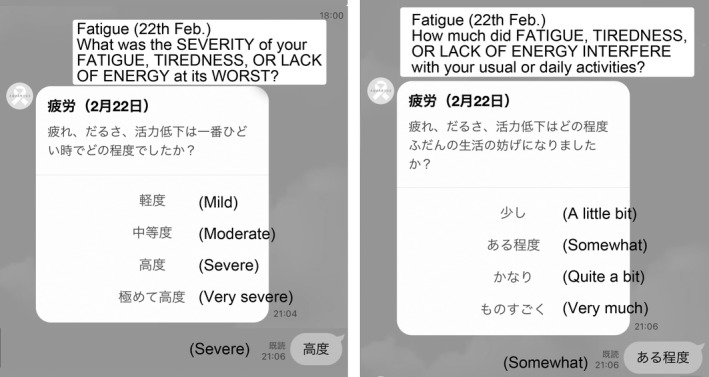
Screen capture of the client software of the LINE‐electronic patient‐reported outcome (ePRO) system (Japanese text in the image is translated into English). Questions about “fatigue” using the Japanese version of PRO‐Common Terminology Criteria for Adverse Events are notified, and PROs are stored in the server by selecting the predesigned answers with tap operation
2.4. Patient‐reported outcome questions and timing for asking
All predefined PRO questions and answers were made using PRO‐CTCAE version 1.0, which was developed by the US NCI, to establish an evaluation system for more accurate grading of adverse events in clinical trials. The PRO‐CTCAE system tool can measure adverse events based on a patient’s self‐assessment by introducing PRO elements based on CTCAE.
The three different patterns of the PRO collection algorithm were designed depending on the treatment circumstances: adjuvant endocrine therapy, chemotherapy, or metastatic treatment. The types and timing of questions for PRO data collection were prepared for each of the three clinical situations. The following 15 symptoms were used for PRO data collection: general pain, decreased appetite, constipation, diarrhea, swelling, fever, fatigue, numbness/tingling, nausea, vomiting, joint pain, hot flashes, increased sweating, anxiety, insomnia, and cough. For each clinical situation, 10 of these symptoms were selected and notified to the patient’s LINE at a predetermined time. For example, for patients undergoing adjuvant chemotherapy, we set the timing of notifications for a 21‐day cycle, for the 10 symptoms (ie, general pain, decreased appetite, constipation, diarrhea, swelling, fever, fatigue, numbness/tingling, nausea, and vomiting) and collected PRO data (Table 1). In this way, the types of symptoms to be asked and the timing of notifications were set for all clinical situations and operated (Table S1).
TABLE 1.
Symptom types and timing of questions using the electronic patient‐reporting outcomes LINE application for breast cancer patients receiving adjuvant chemotherapy
| No. | Symptoms | Day 1 | Day 2 | Day 3 | Day 4 | Day 5 | Day 6 | Day 7 | Day 8 | Day 9 | Day 10 | Day 11 | Day 12 | Day 13 | Day 14 | Day 15 | Day 16 | Day 17 | Day 18 | Day 19 | Day 20 | Day 21 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | General pain | ○ | ○ | ○ | ||||||||||||||||||
| 2 | Decreased appetite | ○ | ○ | ○ | ○ | ○ | ○ | |||||||||||||||
| 3 | Constipation | ○ | ○ | ○ | ||||||||||||||||||
| 4 | Diarrhea | ○ | ○ | ○ | ||||||||||||||||||
| 5 | Swelling | ○ | ○ | ○ | ||||||||||||||||||
| 6 | Fever | ○ | ○ | ○ | ||||||||||||||||||
| 7 | Fatigue | ○ | ○ | ○ | ||||||||||||||||||
| 8 | Numbness and tingling | ○ | ○ | ○ | ||||||||||||||||||
| 9 | Nausea | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ||||||||||||||
| 10 | Vomiting | ○ | ○ | ○ | ○ | ○ | ○ | ○ |
3. RESULTS
3.1. Patient characteristics
In this study, 73 patients with breast cancer from Keio University Hospital and Teikyo University Hospital were enrolled between June 2018 and October 2020. The median age was 47.5 years (range, 34‐68 years) and median duration of observation was 435 days (84‐656 days). Furthermore, 16 of the 73 patients received adjuvant chemotherapy and 15 of these patients were subsequently transitioned to endocrine therapy but continued to have their PRO data collected; the types of symptoms to be asked and the timing of notifications for them were accordingly changed on transitioning. Therefore, PRO data were collected from 65 patients in the endocrine therapy group, including 15 patients who were transferred from the adjuvant chemotherapy group. Seven patients were enrolled in the group that received treatment for metastatic breast cancer.
3.2. Number of PROs collected with or without symptoms
The total number of PROs collected from 73 patients was 16,417 (Figure 2A). The average number of reports per patient was 224.9 (Figure 2B). The highest and lowest numbers of PROs collected from individual patients stood at 803 in 656 days and 54 in 237 days, respectively. Patients on adjuvant endocrine therapy were notified with 10 questions in 28 days, which translates to 2.5 questions per week. With the exception of 15 patients transitioning from adjuvant chemotherapy, 40 patients received only adjuvant endocrine therapy, and the median number of responses per week for these patients was 2.387 (1.687‐11.627). This result suggests that patient adherence to LINE‐ePROs system reporting was favorable.
FIGURE 2.
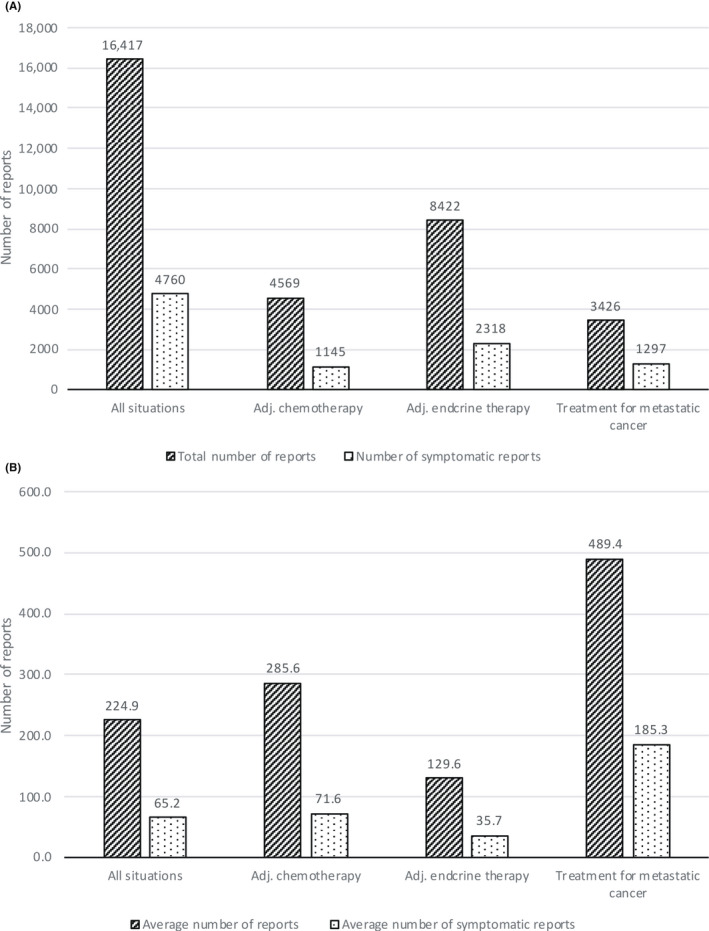
Number of patient‐reported outcome (PRO) reports and symptomatic PROs, overall and by condition, among breast cancer patients receiving adjuvant (Adj.) chemotherapy, adjuvant endocrine therapy, or metastatic cancer treatment. (A) Total number of PROs. (B) Average number of PROs per patient
Of the total number of PROs, 4760 (29.0%) were symptomatic (Figure 2A) and 65.2 was the average number of symptomatic PROs per person. The average number of PROs per patient by status was 285.6 in the adjuvant chemotherapy group, 129.6 in the adjuvant endocrine therapy group, and 489.4 in the metastatic cancer treatment group. The metastatic cancer treatment group had the highest symptom rate among the three groups with 185.3 (37.9%) symptomatic PROs (Figure 2B).
By analyzing the symptomatic reports depending on the treatment phase, different patterns or PROs were identified. In the group undergoing treatment for advanced breast cancer, more than 50% of the symptomatic PROs observed were general pain, swelling, fatigue, and numbness/tingling. The symptom rate of general pain was also high in the adjuvant chemotherapy group (60.3%), suggesting the importance of pain control during chemotherapy (Figure 3).
FIGURE 3.
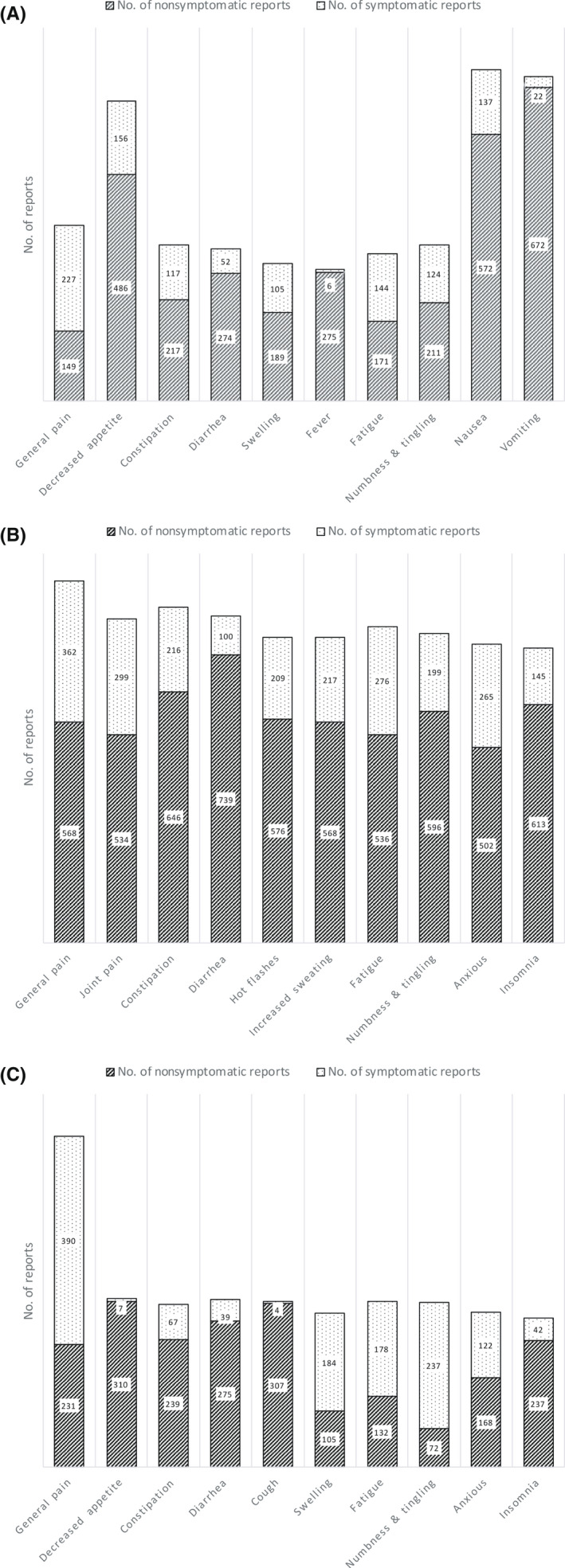
Number of patient‐reported outcomes reported and symptomatic reports by type of symptoms among breast cancer patients. (A) Adjuvant chemotherapy group. (B) Adjuvant endocrine therapy group. (C) Treatment for metastatic cancer group
3.3. Number of PRO reports classified by age
The number of PROs collected by age was investigated because elderly women might not be as proficient in operating electronic devices as younger women and might have more difficulties in PRO data collection. The observation period for each age band was almost the same, except 309.5 days for those above 65 years of age. The median number of PROs per person was 376 and 304 for age bands 60‐64 and 65 years or above, respectively. Despite the shorter observation period for age band 65 years or above, PROs per person from this band were higher than those in patients in the age bands 40‐44, 45‐49, 50‐59, and 55‐59 years (Figure 4). Significant differences in PRO responses were observed between the 26 patients aged 49 years or younger and the 7 patients aged 60 years or older, with a median of 184 (54‐803) and 376 (149‐554) PRO responses, respectively (P = .012, Mann–Whitney U test). These results indicate that the LINE‐ePRO system can be successfully used for PRO data collection, even in relatively elderly people over 60 years of age.
FIGURE 4.
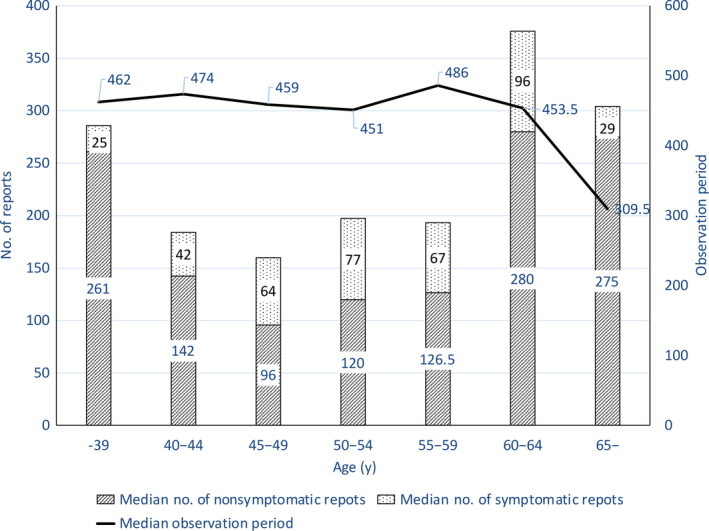
Median number of patient‐reported outcome (PRO) reports and observation period by age category in breast cancer patients. Bars show the median number of PRO reports and the symptomatic reports included by age category. The line graph shows the median observation period for each age category
3.4. Tracking the symptoms of a single patient
Using the LINE‐ePRO system, it was possible to track the symptoms of an individual patient. Figure 5 can be considered here for an illustration: it shows PRO data of a patient who received docetaxel once every 3 weeks as adjuvant chemotherapy. A record describing the change in symptoms between the first and fourth courses of docetaxel treatment can be seen for 10 different symptoms. Although the PRO‐CTCAE system was designed to record both the frequency and severity of symptoms, only the severity is listed in this table under Figure 5. Grade 3 general pain is frequently observed in the first course, but by the fourth course, the general pain improves to grade 1 or 2; numbness/tingling is almost absent in the first course, but worsens markedly in the fourth course with grade 3 or 4. Other side‐effects, except constipation, were rarely observed during docetaxel treatment. It was possible to track the details of a single patient’s symptoms and analyze the side‐effect information in a timeline.
FIGURE 5.
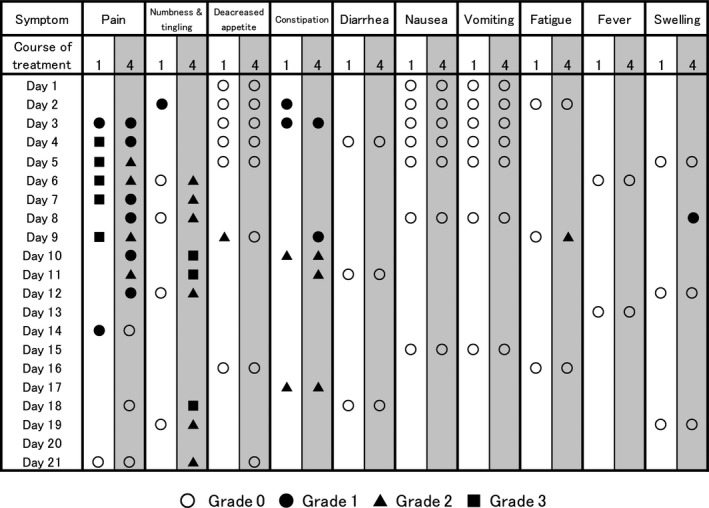
Patient‐reported outcome (PRO) tracking of a breast cancer patient who received docetaxel once every 3 weeks in adjuvant chemotherapy. The PROs for each symptom in the first and fourth courses of chemotherapy are shown, with the first day of treatment as Day 1. Each symbol represents a grade of the symptom: ○, grade 0; ●, grade 1; ▲, grade 2; and ■, grade 3
4. DISCUSSION
To achieve patient‐centered care, decisions must be made with respect to individual preferences, needs, and values. Due to this growing awareness, the number of clinical study designs that include QOL as an end‐point in randomized controlled trials of cancer treatment is increasing. 9 , 10 The value of health, which comprises various factors such as physical, mental, and social role and function, is defined as HRQOL. One of the methods used to assess HRQOL is PRO, which is represented by patient self‐administered questionnaires. The PRO‐CTCAE was developed to form a consensus on how to analyze, interpret, and report PRO data on adverse symptoms in cancer patients. It is a system tool that can measure adverse events based on patient self‐assessment, utilizing existing CTCAE scales but introducing elements of PROs. 11 We undertook a feasibility study to determine whether the ePRO system, which is based on PRO‐CTCAE, would be accepted by breast cancer patients. From the results of this study, the median number of responses divided by the number of questions asked was 95.5% in 40 patients with adjuvant endocrine therapy; therefore, the PRO data collection using LINE‐ePRO was considered to have a satisfactory response rate. Response rates in clinical trials using other ePRO systems were relatively low, with a 75% response rate in a clinical trial in which patients answered questions involving PRO‐CTCAEs that used a Web‐based system. 5 In a trial using an application built to answer ePROs, the response rate was 68%. 12 Although it was assumed that the elderly would not be proficient in operating electronic devices, contrary to the expectation, it was found from the age‐specific analysis that they responded through LINE‐ePRO in the same number or more, as the younger age group. As it is difficult for all patients to be familiar with the use of a specialized application for ePRO, the response rate decreases. However, LINE‐ePRO is considered to have achieved a favorable response rate by constructing a system that allows patients to answer ePRO questions on the LINE application that they use daily. A bias to consider, however, is that the participants in this study were breast cancer patients who owned smartphones and were familiar with the use of social networking sites, including the LINE application. The oldest patient who participated in this study was 68 years old. According to statistics from Japan’s Ministry of Internal Affairs and Communications in 2020, the smartphone ownership rate among people aged in their 60s is reported to be 67.4% and is increasing every year. 13 Therefore, the results cannot be universally applied to all patients immediately, but as more patients become proficient with digital devices soon, it is possible to expect high reproducibility of these results. Even for patients with a limited understanding of information technology, obtaining PROs provides important information for clinical practice. Bennett et al. examined the equivalence and acceptability of the three data collection modes (Web‐enabled touchscreen tablet computer, interactive voice response system, and paper) available within the PRO‐CTCAE measurement system. They observed moderate to high levels of agreement across modes and provided evidence of the acceptability of each mode of administration to a majority of respondents. 14 Based on these results, it is possible to obtain clinically useful information similarly by considering voice or paper‐based PRO acquisition. Moreover, we used LINE, the most popular SNS application in Japan, but it is difficult to make the results universal because the share of SNS and messenger applications in each country is different. However, this study suggests that building a PRO reporting system based on the applications frequently used by the citizens of a country is likely to contribute to improving the response rate.
The LINE‐ePRO system used in this study allowed us to obtain the symptom patterns of multiple patients in similar clinical situations, such as those undergoing chemotherapy. It was also possible to observe the symptom trends of a single patient in detail. Although we retrospectively analyzed PROs in this study, we constructed a system to monitor these PROs in real time daily. Basch et al. undertook a prospective clinical study in which patients receiving chemotherapy were monitored in real time for various symptoms. A total of 766 patients with metastatic solid tumors were randomly assigned to the PRO group for Web‐based, patient‐reported, symptom monitoring and to the usual care group for conventional care. They found that proactive intervention in the event of certain adverse events improved patients’ QOL and prolonged the duration of cancer chemotherapy and overall survival. 2 , 15 The LINE‐ePRO system was shown to be capable of tracking the PROs of a single patient in detail. In the future, it will be possible to apply the LINE‐ePRO system to prospective interventional studies and use it not only to evaluate adverse symptoms and QOL, but also to assess OS and relative dose intensity.
In summary, we undertook a feasibility study on the LINE‐ePRO system in breast cancer patients. The acceptance of the system by the patients was favorable, and the response rate was satisfactory. In particular, the number of PROs obtained from older patients was comparable to or higher than that of younger patients, suggesting that the system was well designed based on applications that are used daily. In this context, ePROs will enable screening and monitoring of patients’ symptoms and other factors, which will lead to a safer and more accurate response to cancer drug therapy that is becoming more complex. To utilize ePRO more efficiently in daily clinical practice, it is desirable to analyze the huge amount of data collected and to link it with electronic medical records. The concept of ePRO has been recognized, and clinical studies of ePRO using internet‐connected devices have begun to be carried out worldwide. In the future, intervention by medical professionals according to the symptom monitoring system will be considered important, and a multidisciplinary approach to intervention will be required.
DISCLOSURE
T. Hayashida and H. Miyata received research funding from SHIONOGI Inc. T. Hayashida and Y. Kitagawa are editorial board members of Cancer Science. There are no other disclosures to be made for this research.
Supporting information
Table S1
ACKNOWLEDGMENTS
The LINE‐ePRO system used in this study was established with research funding from SHIONOGI Inc. This study was carried out as part of Cabinet Office of Japan, the Cross‐ministerial Strategic Innovation Promotion Program, “Innovative AI Hospital System” using the provided research funds. This work was also supported by JSPS KAKENHI, Grant‐in‐Aid for Scientific Research (C), Grant Number 19K09082.
Hayashida T, Nagayama A, Seki T, et al. Feasibility study on collecting patient‐reported outcomes from breast cancer patients using the LINE‐ePRO system. Cancer Sci. 2022;113:1722–1730. doi: 10.1111/cas.15329
Funding information
SHIONOGI Inc.; Cabinet Office of Japan, Cross‐ministerial Strategic Innovation Promotion Program, “Innovative AI Hospital System”; JSPS KAKENHI, Grant‐in‐Aid for Scientific Research (C), Grant/Award Number: 19K09082.
REFERENCES
- 1. Yoshimura A, Ito H, Nishino Y, et al. Recent improvement in the long‐term survival of breast cancer patients by age and stage in Japan. J Epidemiol. 2018;28:420‐427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Basch E, Deal AM, Kris MG, et al. Symptom monitoring with patient‐reported outcomes during routine cancer treatment: a randomized controlled trial. J Clin Oncol. 2016;34:557‐565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Girgis A, Durcinoska I, Arnold A, et al. Web‐based patient‐reported outcome measures for personalized treatment and care (PROMPT‐Care): multicenter pragmatic nonrandomized trial. J Med Internet Res. 2020;22:e19685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Iivanainen S, Alanko T, Vihinen P, et al. Follow‐up of cancer patients receiving anti‐PD‐(L)1 therapy using an electronic patient‐reported outcomes tool (KISS): prospective feasibility cohort study. JMIR Form Res. 2020;4:e17898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Taarnhøj GA, Lindberg H, Dohn LH, et al. Electronic reporting of patient‐reported outcomes in a fragile and comorbid population during cancer therapy – a feasibility study. Health Qual Life Outcomes. 2020;18:225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Tran C, Dicker A, Leiby B, Gressen E, Williams N, Jim H. Utilizing digital health to collect electronic patient‐reported outcomes in prostate cancer: single‐arm pilot trial. J Med Internet Res. 2020;22:e12689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Matthies LM, Taran F‐A, Keilmann L, et al. An electronic patient‐reported outcome tool for the FACT‐B (functional assessment of cancer therapy‐breast) questionnaire for measuring the health‐related quality of life in patients with breast cancer: reliability study. J Med Internet Res. 2019;21:e10004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wallwiener M, Matthies L, Simoes E, et al. Reliability of an e‐PRO tool of EORTC QLQ‐C30 for measurement of health‐related quality of life in patients with breast cancer: prospective randomized trial. J Med Internet Res. 2017;19:e322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hagstrom AD, Marshall PW, Lonsdale C, Cheema BS, Fiatarone Singh MA, Green S. Resistance training improves fatigue and quality of life in previously sedentary breast cancer survivors: a randomised controlled trial. Eur J Cancer Care (Engl). 2016;25:784‐794. [DOI] [PubMed] [Google Scholar]
- 10. Mukand NH, Ko NY, Nabulsi NA, et al. The association between physical health‐related quality of life, physical functioning, and risk of contralateral breast cancer among older women. Breast Cancer. 2022;29:287‐295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Basch E, Reeve BB, Mitchell SA, et al. Development of the National Cancer Institute's patient‐reported outcomes version of the common terminology criteria for adverse events (PRO‐CTCAE). J Natl Cancer Inst. 2014;106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zylla DM, Gilmore GE, Steele GL, et al. Collection of electronic patient‐reported symptoms in patients with advanced cancer using Epic MyChart surveys. Support Care Cancer. 2020;28:3153‐3163. [DOI] [PubMed] [Google Scholar]
- 13. Japanese Ministry of Internal Affairs and Communications . Survey on Trends in Telecommunications Usage in 2020. 2021 June 18; https://www.soumu.go.jp/johotsusintokei/statistics/data/210618_210611.pdf
- 14. Bennett AV, Dueck AC, Mitchell SA, et al. Mode equivalence and acceptability of tablet computer‐, interactive voice response system‐, and paper‐based administration of the U.S. National Cancer Institute's Patient‐Reported Outcomes version of the Common Terminology Criteria for Adverse Events (PRO‐CTCAE). Health Qual Life Outcomes. 2016;14:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Basch E, Deal AM, Dueck AC, et al. Overall survival results of a trial assessing patient‐reported outcomes for symptom monitoring during routine cancer treatment. JAMA. 2017;318:197‐198. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1


