Abstract
Drug lag refers to the difference in the time of a new drug's approval in different countries; the dissemination of the new drug after approval within the countries is another problem. We examined the nationwide dissemination of 11 cancer drugs approved in Japan between 2011 and 2015 using the National Database of Health Insurance Claims data. We extracted data on the number of cancer drug prescriptions from 47 prefectures and associated demographic information, such as age and sex. Eight diabetes drugs were also examined for comparison. We observed a lag between the marketing approval date of the drugs and their first use. To further explore the rise and pattern of each drug’s dissemination, we analyzed the trend of the cumulative number and total of new prescriptions for each prefecture. The results showed that the first month of new cancer drug prescriptions varied across prefectures. On average, they lagged by up to 2 months in the slowest prefectures, whereas the variation was almost nonexistent for diabetes drugs. The patterns of dissemination varied more among cancer drugs across the seven Japanese geographical regions. After the initial prescription, the number of prescriptions showed a steep rise for most cancer drugs, whereas the increase was gradual for diabetes drugs. In conclusion, the dissemination of cancer drugs had a greater lag time than that of diabetes drugs. Further research is needed to explore the causative factors to ensure that all effective drugs are equally accessible for those who need them.
Keywords: cancer drug, diabetes drug, drug dissemination, drug lag, national database
This study aimed to examine the nationwide dissemination of 11 cancer drugs approved in Japan between 2011 and 2015. The dissemination of cancer drugs had a greater lag time than that of diabetes drugs. Further research is needed to explore the causative factors to ensure that all effective drugs are equally accessible for those who need them.

1. INTRODUCTION
Drug lag refers to the lag time between the development of a new drug and its use in clinical practice. Drug lag has long been considered an issue in Japanese pharmaceutical regulation. 1 , 2 The situation regarding newly approved cancer drugs, in particular, gained huge attention because of the severity of the disease. 2 For example, cancer drugs such as erlotinib or gemcitabine took as long as five additional years to be approved in Japan, despite being some of the only effective drugs for pancreatic cancer. 3 Therefore, in collaboration with the Pharmaceutical and Medical Devices Agency, the Japanese government took several significant measures to alleviate the situation. 4
Currently, the lag between Japan and other countries has significantly diminished due to the swift start of clinical trials and a reduction in reviewing time. 2 , 5 , 6 , 7 However, drug approval is just a prerequisite for use in clinical practice. Little is known about how quickly drugs are included in frontline clinical practice, and geographical disparities. As equitable health‐care delivery becomes a priority, vis‐à‐vis legislative efforts to ensure easy access to medicines, it is now imperative that all effective drugs are equally accessible for those who need them.
Therefore, this study aimed to investigate the nationwide dissemination of selected newly approved cancer drugs between 2011 and 2015 using the National Database of Health Insurance Claims (NDB). We used some diabetes drugs newly approved within the same period as the comparison group to illuminate the situation further.
2. MATERIALS AND METHODS
2.1. National database
We obtained data from the longitudinal database of electronic health insurance claims submitted from all insurance entities in Japan (NDB). In Japan, 99% of hospitals submit insurance claims electronically, which are accumulated in the NDB. 8 These data contain information on all medical services covered by public health insurance, including diagnostic tests, surgery, and prescription drugs. It also includes demographic information such as the prefecture of patient residence, age, and gender. A de‐identified unique number is allocated to each patient, allowing users to follow all medical procedures a patient has undertaken in chronological order within the defined period. We used NDB data between January 2010 and December 2017. The 2010 data was used to ensure that no drugs were prescribed before the first commercial sale.
This study protocol was approved by the institutional review board of the National Cancer Center, Japan (approval number: 2017–265).
2.2. Drugs and variables
We extracted prescription data from the NDB on 47 patient residence prefectures and associated demographic information such as age and sex. The targeted drugs were either cancer or diabetes drugs approved between January 2011 and December 2015. Information on the first month of prescription and changes in prescription volume were collected on the 23 cancer drugs and eight newly approved diabetes drugs. However, because the NDB Reporting Guidelines prevented us from reporting numbers less than 10 for privacy protection, any drugs with fewer than 10 prescriptions were excluded. The cancer drugs included in the analyses were degarelix acetate, axitinib, pazopanib, regorafenib, pertuzumab, ado‐trastuzumab emtansine, afatinib, trifluridine, enzalutamide, abiraterone acetate, nivolumab. The diabetes drugs included were ipragliflozin dapagliflozin, luseogliflozin, tofogliflozin, canagliflozin, empagliflozin, dulaglutide, and exenatide. We had to exclude cancer drugs carmustine, everolimus, ofatumumab, clofarabine, brentuximab vedotin, cabazitaxel, ruxolitinib, alectinib, alemtuzumab, bosutinib, streptozocin, and vemurafenib from this report.
2.3. Data analysis
We sought to determine whether there was a lag time between when the drugs first became available on the market and their prescription in practice from the perspective of geographical dissemination. To do this, we determined the month in which a drug was first prescribed. For each prefecture, we calculated the difference between the month the first prescription was observed anywhere and the month the first prescription appeared in each prefecture. We calculated the average lag time for all the analyzed cancer drugs to characterize the prefecture’s new drug uptake pattern.
Next, we examined the uptake of newly approved drugs and their geographic variation by the trend of the cumulative number of new prescriptions for each prefecture. For this analysis, we chose the drug with the largest number of prescriptions for cancer patients (degarelix acetate) and for diabetes patients (ipragliflozin). Since degarelix was only used for men, nivolumab, which had the largest number of prescription among those used for both males and females cancer patients were also analyzed.
To look at the trends in new drug uptake, we also analyzed the number of prescriptions for both cancer and diabetes drugs. The analysis included all patients administered newly approved cancer drugs and several diabetes drugs between January 2011 and December 2015.
A concave curve illustrates a rapid increase of new prescriptions in the early phase, while a convex curve shows slow uptake and later acceleration. As all sodium‐glucose cotransporter 2 (SGLT2) and glucagon‐like peptide‐1 drugs can be used interchangeably, we presented the aggregated result of all included diabetes drugs.
All NDB data extraction and processing were undertaken using Oracle SQL*plus (Oracle Corporation). Aggregated results were analyzed using Python 3.5.1.
3. RESULTS
The data of 346,729 patients with cancer and 1,436,990 patients with diabetes were analyzed. Of the patients with cancer, 211,876 (61.1%) were men, and the mean age was 68.6 ± 12.7 years. Similarly, 886,143 (61.6%) of the patients with diabetes were men, and the mean age was 60.0 ± 12.7 years. Sodium‐glucose cotransporter 2 was prescribed to 1,345,144 patients (mean age, 59.5 ± 12.5 years), and 62.2% of them were men. Glucagon‐like peptide 1 was prescribed to 146,858 patients (mean age, 64.9 ± 14.5 years), of which 55.2% were men. The patients’ characteristics by the respective drugs received are presented in Tables 1 and 2.
TABLE 1.
Demographics of patients prescribed new cancer drugs in Japan, January 2011‐December 2015
| Degarelix acetate | Axitinib | Pazopanib | Regorafenib | Pertuzumab | Ado‐trastuzumab emtansine | Afatinib | Trifluridine | Enzalutamide | Abiraterone acetate | Nivolumab | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| N | 55,083 | 12,387 | 11,226 | 17,737 | 15,808 | 7572 | 15,600 | 25,026 | 38,015 | 26,248 | 28,831 |
| Male (%) | All | 9294 (75.0) | 6805 (60.6) | 11,030 (62.2) | 58 (0.4) | 27 (0.4) | 6336 (40.6) | 15,012 (60.0) | All | All | 20,947 (72.7) |
| Age, y (SD) | 75.3 (8.2) | 67.5 (10.3) | 63.2 (15.3) | 65.8 (10.2) | 59.7 (11.7) | 59.9 (11.5) | 67.0 (10.2) | 67.0 (10.2) | 77.4 (7.7) | 77 (7.7) | 67.2 (10.2) |
| First prescription overall a | 2012/10 | 2012/8 | 2012/11 | 2013/5 | 2013/9 | 2014/4 | 2014/5 | 2014/5 | 2014/5 | 2014/9 | 2014/9 |
| First prescription in the slowest prefecture a | 2012/12 | 2013/8 | 2013/7 | 2013/8 | 2013/10 | 2014/8 | 2014/7 | 2014/6 | 2014/6 | 2014/9 | 2015/6 |
| Indication | Prostate | Renal cell | Renal cell, sarcoma |
Colorectal GIST Hepatic cell |
Breast | Breast | Lung |
Colorectal Gastric |
Prostate | Prostate |
Lung Melanoma Others |
Abbreviation: GIST, gastrointestinal stromal tumor.
Data are shown as year/month.
TABLE 2.
Demographics of patients prescribed new diabetes drugs in Japan, January 2011‐December 2015
| Ipragliflozin | Dapagliflozin | Luseogliflozin | Tofogliflozin | Canagliflozin | Empagliflozin | Dulaglutide | Exenatide | |
|---|---|---|---|---|---|---|---|---|
| N | 390,778 | 306,257 | 167,649 | 217,154 | 222,021 | 232,433 | 132,049 | 22,197 |
| Male (%) | 238,485 (61.0) | 189,311 (61.8) | 101,551 (60.6) | 131,872 (60.7) | 139,938 (63.0) | 151,509 (65.2) | 73,205 (55.4) | 11,750 (52.9) |
| Age, y (SD) | 59.3 (12.5) | 59.4 (12.4) | 60 (12.5) | 59.1 (12.5) | 59.6 (12.5) | 60.4 (12.7) | 65.3 (14.4) | 63.2 (15.2) |
| First prescription overall a | 2014/4 | 2014/5 | 2014/5 | 2014/5 | 2014/9 | 2015/2 | 2015/9 | 2013/5 |
| First prescription in the slowest prefecture a | 2014/4 | 2014/5 | 2014/5 | 2014/5 | 2014/9 | 2015/3 | 2015/11 | 2013/6 |
| Indication | Type I and II diabetes | Type II diabetes | ||||||
Data are shown as year/month.
3.1. First month of prescription
For all drugs, the earliest prescriptions were observed in the same month as the drug approval. The slowest cancer drug uptake was for axitinib and nivolumab, which took 12 months and 8 months, respectively. The slowest for a diabetes drug, dulaglutide, was 2 months.
The average lag time in prefectures ranged from 0 to 2.3 months. The lag time for each prefecture was plotted against the prefecture population for cancer drugs (Figure 1A) and diabetes drugs (Figure 1B). Regarding the 21 cancer drugs, the gap tended to diminish as the population increased (Figure 1A). The graph also showed that, especially for cancer drugs, the average lag time varied even among prefectures with similar population sizes. For the eight diabetes drugs, the gap was very small or almost nonexistent, regardless of the population size (Figure 1B).
FIGURE 1.
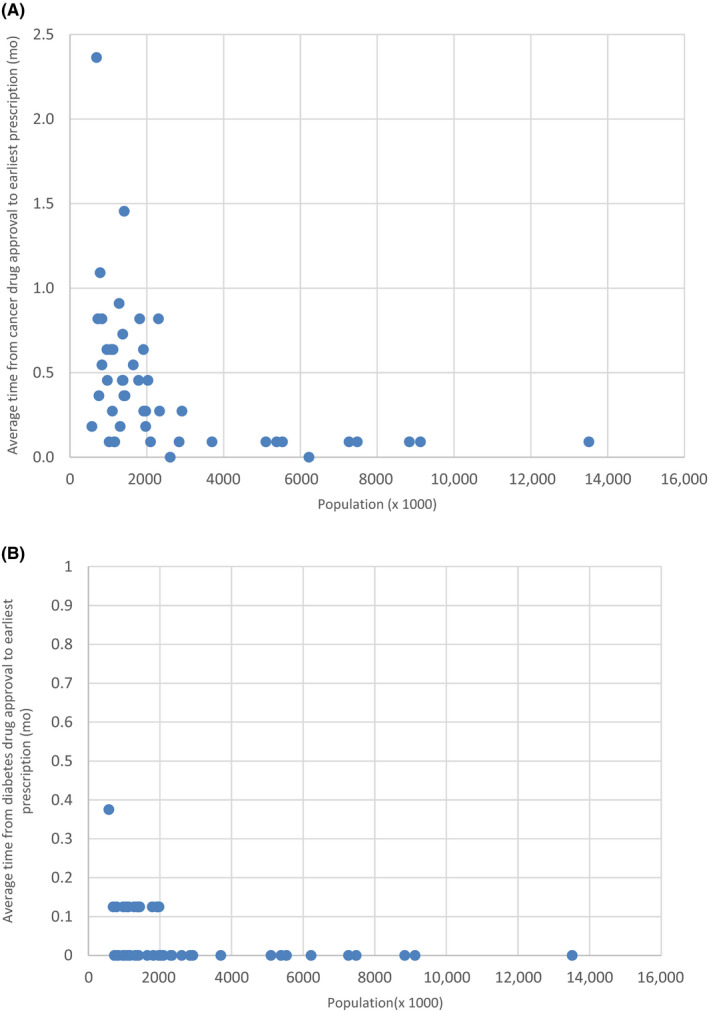
Relationship between population size and average time lag (months) from approval to earliest prescriptions across (A) 11 cancer drugs and (B) eight diabetes drugs in each Japanese prefecture. Each dot represents a prefecture
3.2. Cumulative number of new prescriptions in each region
Figure 2A,B show the dissemination of the cancer drugs nivolumab and degarelix acetate in seven administrative regions in Japan by the cumulative percentage of new prescriptions in proportion to all prescriptions up to 36 months. We used 36 months as it was observed to best illustrate the trend for most drugs. Figure 2C shows the same graphs for ipragliflozin, the first SGLT2 inhibitor sold. However, when we compared the dissemination patterns of the graphs between cancer and diabetes drugs, there was more variation for cancer drugs, with similar trends across the region. Furthermore, densely populated areas such as the Kanto or Kinki areas had less variation in dissemination. Figure 2D shows the same curve for all diabetes drugs, as most diabetes drugs shared the same effect. Figure 2C,D shows that there was little variance in diabetes drug dissemination across the regions. The trend was even more apparent in Figure 2D after the diabetes drugs were grouped.
FIGURE 2.
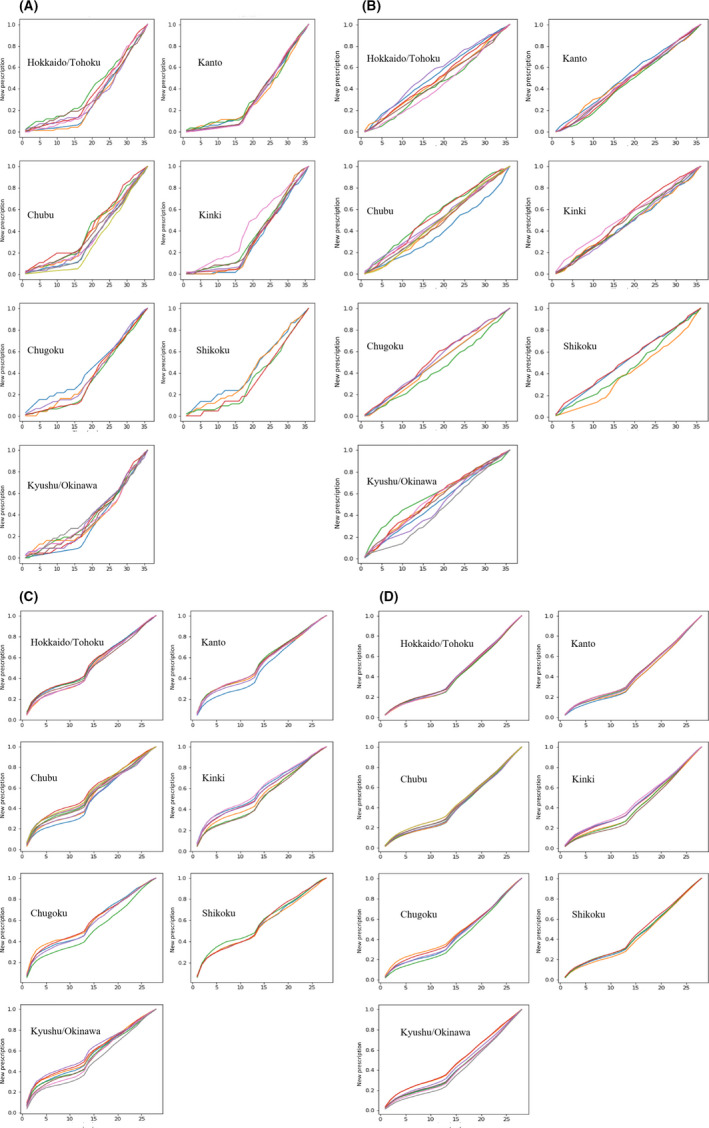
(A) Cumulative number of new nivolumab prescriptions in each geographic region in Japan (first month, Sep 2014). Each line represents a prefecture in the region. (B) Cumulative number of new degarelix acetate prescriptions in each geographic region (first month, Dec 2012). Each line represents a prefecture in the region. (C) Cumulative number of new ipragliflozin prescriptions in each geographic region (first month, Apr 2014). Each line represents a prefecture in the region. (D) Cumulative number of all new diabetes drug prescriptions in each geographic region. Each line represents a prefecture in the region
3.3. Trends in number of prescriptions
The monthly prescriptions for cancer and diabetes drugs are shown in Figure 3. While a steep rise of new prescriptions is seen in the initial phase for cancer drugs, there is a gradual rise in prescriptions over 20 months for diabetes drugs after 12 months.
FIGURE 3.
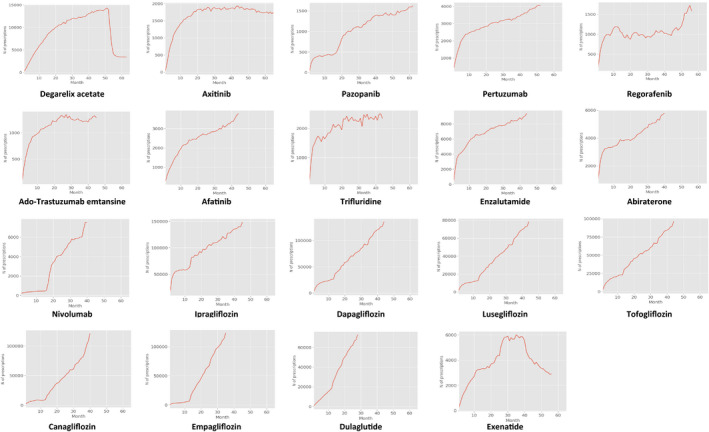
Trend of prescriptions of drugs introduced in Japan, January 2011‐December 2015
4. DISCUSSION
Our study shows that the dissemination of cancer drugs lags more than that of diabetes drugs and has greater geographic variation. While the drugs are made available for prescription simultaneously nationwide, there was a geographic variation in the first month of prescriptions across the prefectures. The gap in the first prescription between the fastest and slowest prefectures was larger for cancer drugs than for diabetes drugs. While drug lag usually refers to the difference in the time of a new drug's availability in different countries, our finding underscores the importance of monitoring the dissemination of new drugs within the country. Although pharmaceutical regulation is uniform throughout Japan, and the new drug can be used simultaneously, the actual usage can lag due to reasons other than market availability. Hence, future studies should explore how new drugs can be swiftly delivered to patients through an understanding of practice patterns of frontline physicians. The dissemination patterns were also different between cancer and diabetes drugs. After the initial prescription, there was a steep rise in prescriptions for most cancer drugs, while the increase was more gradual among diabetes drugs.
The lags in the first prescription were analyzed at the prefecture level on the premise that the patients should have equal access to the new drugs regardless of where they live. There were limited data on the factors that influenced the differential diffusion of the new drugs in this study. A recent systematic review of new drug uptake for, but not limited to, cancer drugs, summarized the influencing factors into broad categories of patient, prescriber, medicine, and organizational and environmental factors. 9 The geographic variation observed in our study could represent a combination of these factors. Additionally, the review found that the influence of each factor appeared to be different across drug types. Hence, health policy and future research should investigate the factors that contribute to this variation, especially for cancer drugs. Although the prefectures with smaller populations had the longest average lag time, the lag varied even among those with similar populations. Also, the dissemination of nivolumab varied across prefectures, even within the same regions, to a great extent. This trend could reveal different expectations or levels of trust toward the newly approved drugs. A further study should elucidate the mechanisms of how physicians adopt new drugs into their practice.
The trends are influenced by regulatory events such as the expansion of indications and the end of limits on prescription drug dispensation for oral prescriptions. For all oral medications in Japan, prescription drug dispensation is limited to a maximum of 2 weeks for new drugs for 12 months after approval. 10 Nivolumab was initially indicated for melanoma only. However, 15 months after initial approval, its indication was expanded to other cancers, including non‐small‐cell lung cancers. Graphs show two‐phased upturns, at 15 months (December 2015) for nivolumab and 12 months for diabetes drugs. Pazopanib's indication was expanded from soft tissue sarcoma to renal cell carcinoma 17 months after approval. Its curve is like nivolumab's, as shown in Figure 3.
The availability of substitutive drugs can also affect prescription volumes. Generally, the trend of diabetes drug prescriptions after 12 months–after the long‐term prescription is issued–is much slower than for cancer drugs. For instance, drugs such as dapagliflozin, luseogliflozin, and tofogliflozin, which have a similar effect to ipragliflozin, were approved 1 month after its introduction in May 2014. In response to this, the prescription volume of ipragliflozin flattened after the first month. Unlike most diabetes drugs, fewer alternatives are available for most cancer drugs, which can explain the difference in the steepness of the rise in cancer drug prescriptions.
This is the first study in Japan that investigated the geographic variation in the dissemination of new drugs. In contrast, prior studies that examined the dissemination of new cancer drugs were from other countries, and they focused on practice (eg, practice size and teaching status) and drug characteristics (eg, indication of the drugs and promotion by the manufacturer). 11 , 12 , 13 Geographic variations are important from the perspective of access to quality care. 14 , 15 , 16 Such variation suggests that location and the number of medical providers affect patients’ access to cancer drugs. Prior studies have shown that the geographic variation is associated with access to optimal treatment and consequently mortality 17 , 18 , 19 ; therefore diminishing the gap in accessibility becomes pertinent.
Our research has several limitations. First, dissemination patterns for cancer and diabetes drugs in this study may be similar, but they do not necessarily represent the dissemination of other types of drugs. However, we believe the comparison highlights factors such as the effect of prescription drug dispensation, changes in prescription volume due to expanded drug indications, and the influence of substitutive drugs. Second, the number of patients in each prefecture can affect the timing of the first drug use; a larger population increases the chance of encountering a patient for whom the new drugs are indicated. However, we still found variations between prefectures with similar populations. Third, some patients might have traveled to a neighboring prefecture to receive treatment, which may affect our results to some extent. However, we expect most patients to prefer to access care near their residence. Finally, we could not explore the factors that affected the geographic variation because the geographic variable was only available at the prefectural level due to the privacy policy of the NDB. More detailed information, such as per capita provider volumes and distance to the capital city in a smaller area unit, would have enabled more detailed analysis.
The drug approval lag between Japan and other countries has improved, but drug dissemination patterns remain relatively unknown. Our study showed that the dissemination of cancer drugs appeared to lag more than for diabetes drugs. This finding is worth attention because cancer drugs have fewer alternatives than diabetes drugs. Further research is needed to ascertain the factors that contribute to lags in drug dissemination to ensure that effective drugs are equally accessible for those who need them.
DISCLOSURE
The authors have no conflict of interest.
ETHICAL APPROVAL
This study protocol was approved by the institutional review board of the National Cancer Center, Japan (approval number: 2017–265).
ACKNOWLEDGMENT
This study was funded by the National Cancer Center Research and Development Grant (31‐A‐21).
Watanabe T, Sugiyama T, Imai K, Higashi T. How are new drugs disseminated in Japan? Analysis using the National Database of Health Insurance Claims of Japan. Cancer Sci. 2022;113:1771–1778. doi: 10.1111/cas.15322
Funding information
National Cancer Center, Grant/Award Number: 31‐A‐21.
REFERENCES
- 1. Tsuji K, Tsutani K. If Japan is to become a front‐runner in pharmaceutical development, it must not only speed up its approval of new drugs, but also enhance its own research capabilities. Nature. 2008;32:851‐852. [Google Scholar]
- 2. Maeda H, Kurokawa T. Recent trends for drug lag in clinical development of oncology drugs in Japan: does the oncology drug lag still exist in Japan? Int J Clin Oncol. 2015;20:1072‐1080. [DOI] [PubMed] [Google Scholar]
- 3. Pancreatic Cancer Action Network . Policy proposal: drug lag issues. (Japanese). Accessed January 24, 2022. https://www.pancan.jp/index.php?option=com_content&view=article&id=331
- 4. Ministry of Health, Labour and Welfare . Globalizing drug approval and development procedures (Japanese). Accessed January 24., 2022. https://www.mhlw.go.jp/wp/seisaku/jigyou/11jigyou01/dl/IV‐1‐7‐6.pdf
- 5. Maeda H, Kurokawa T. Regulatory review time for approval of oncology drugs in Japan between 2001 and 2014. Considerations of changes, factors that affect review time, and difference with the United States. J Clin Pharmacol. 2015;55:481‐489. [DOI] [PubMed] [Google Scholar]
- 6. Honig PK. Recent trends and success factors in reducing the lag time to approval of new drugs in Japan. Clin Pharmacol Ther. 2014;95:467‐469. [DOI] [PubMed] [Google Scholar]
- 7. Yonemori K, Hirakawa A, Ando M, et al. The notorious "drug lag" for oncology drugs in Japan. Invest New Drugs. 2011;29:706‐712. [DOI] [PubMed] [Google Scholar]
- 8. Ministry of Health, Labour and Welfare . National Database of Health Insurance Claim Information and Specified Medical Checkups (Japanese). Accessed January 24., 2022. http://www.mhlw.go.jp/stf/shingi/2r9852000002ss9z‐att/2r9852000002ssfg.pdf
- 9. Medlinskiene K, Tomlinson J, Marques I, et al. Barriers and facilitators to the uptake of new medicines into clinical practice: a systematic review. BMC Health Serv Res. 2021;21:1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Health and Global Policy Institute . Pharmaceuticals. Accessed January 24, 2022. https://japanhpn.org/en/section‐6‐1/
- 11. Keating NL, Huskamp HA, Schrag D, et al. Diffusion of bevacizumab across oncology practices: an observational study. Med Care. 2018;56:69‐77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. O'Connor JM, Fessele KL, Steiner J, et al. Speed of adoption of immune checkpoint inhibitors of programmed cell death 1 protein and comparison of patient ages in clinical practice vs pivotal clinical trials. JAMA Oncol. 2018;4:e180798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Schmidt C. What drives diffusion of new cancer therapies? J Natl Cancer Inst. 2015;107:djv162. [DOI] [PubMed] [Google Scholar]
- 14. Ward MM, Ullrich F, Matthews K, et al. Access to chemotherapy services by availability of local and visiting oncologists. J Oncol Pract. 2014;10:26‐31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lin CC, Bruinooge SS, Kirkwood MK, et al. Association Between geographic access to cancer care, insurance, and receipt of chemotherapy: geographic distribution of oncologists and travel distance. J Clin Oncol. 2015;33:3177‐3185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wan S, Jubelirer S. Geographic access and age‐related variation in chemotherapy use in elderly with metastatic breast cancer. Breast Cancer Res Treat. 2015;149:199‐209. [DOI] [PubMed] [Google Scholar]
- 17. Onega T, Duell EJ, Shi X, et al. Geographic access to cancer care in the U.S. Cancer. 2008;112:909‐918. [DOI] [PubMed] [Google Scholar]
- 18. Chan L, Hart LG, Goodman DC. Geographic access to health care for rural Medicare beneficiaries. J Rural Health. 2006;22:140‐146. [DOI] [PubMed] [Google Scholar]
- 19. Baldwin LM, Cai Y, Larson EH, et al. Access to cancer services for rural colorectal cancer patients. J Rural Health. 2008;24:390‐399. [DOI] [PMC free article] [PubMed] [Google Scholar]


