Abstract
Muscle regeneration requires the coordination of several factors to mobilize satellite cells and macrophages, remodel the extracellular matrix surrounding muscle fibers, and repair existing and/or form new muscle fibers. In this review, we focus on insulin-like growth factor I and the matrix metalloproteinases, which are secreted proteins that act on cells and the matrix to resolve damage. While their actions appear independent, their interactions occur at the transcriptional and post-translational levels to promote feed-forward activation of each other. Together, these proteins assist at virtually every step of the repair process, and contribute significantly to muscle regenerative capacity.
Keywords: Insulin-like growth factor I, Matrix metalloproteinase, Satellite cell, IGF binding proteins, Single cell RNA-sequencing
1. Introduction
Skeletal muscle regeneration relies on a vast array of cells and secreted factors to remove damaged debris and to re-form innervated muscle fibers and the supporting vascular and extracellular meshwork. Communication between cells is required to coordinate regeneration and can be achieved through physical contact or via secreted proteins, termed myokines. The interplay of myokines secreted from and acting on multiple cell types within the muscle compartment is crucial for skeletal muscle growth and regeneration. Once released from a cell, myokines can drive autocrine or paracrine actions through binding to cell-surface receptors and triggering intracellular signaling pathways, which can ultimately lead to altered gene expression, cytoskeletal rearrangements, and cell maturation. Alternatively, myokines may act directly on the surrounding extracellular matrix (ECM) through enzymatic cleavage of ECM proteins. Together, these internal and external processes afford efficient resolution of damage.
Historically, several myokines were identified as key contributors to muscle regeneration, where global delivery of these agents could enhance regeneration, and global ablation of these factors impaired the healing process (reviewed in [1,2]). Less attention was paid to the importance of a particular cell source, in part, because myokine activity began within the extracellular environment. The development of more sophisticated tools, including single cell or single nucleus RNA-sequencing (RNA-Seq) [3-10], has enabled a closer examination of the cells contributing to myokine production. The spatial and temporal expression of myokines, their receptors, and their substrates provide clues to their actions, and combined with cell-specific targeting to eliminate single sources of myokines, we can begin to disentangle the multiple mechanisms underlying regeneration.
In this review, we will highlight the contribution of insulin-like growth factor-I (IGF-I), a well-known secreted factor driving intracellular processes for regeneration [11-13], and the matrix metalloproteinases (MMPs), which target extracellular matrix (ECM) components [14-19]. Muscle repair is initiated by the inflammatory response which includes the immune cell population, followed by satellite cell activation, proliferation, differentiation, and fusion, all while the fibrogenic cell population contributes to ECM remodeling [2]. We will focus on the sources and targets of these myokines, and how their activity is regulated during regeneration. In addition, because IGF and MMP actions are not mutually exclusive, we will also address the regulatory interactions between these myokines. Ultimately, we hope to illustrate the complex orchestration that must occur during regeneration, and the challenges faced in therapeutic development for improving regenerative capacity.
2. Insulin-like growth factor I
2.1. IGF-I structure
IGF-I is critical for growth in virtually all tissues, and skeletal muscle is no exception. Its role in muscle regeneration is also well-documented, where it can enhance both proliferation and differentiation of activated satellite cells, a property that sets this growth factor apart from other proteins in the regeneration process. Extensive reviews of the Igfl gene, its regulation, protein structure, and its signaling through the IGF-I receptor exist [20], and so only a limited description of these aspects are included here.
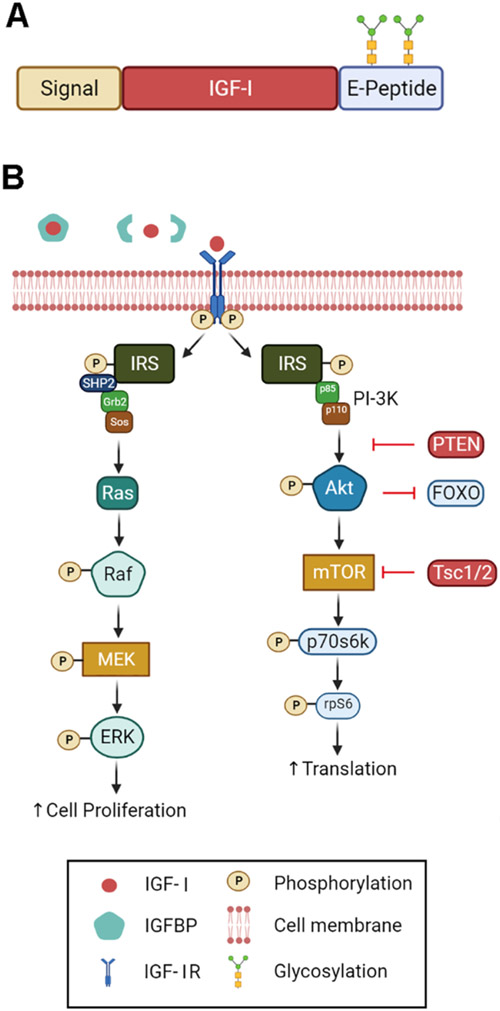
IGF-I is encoded by 6 exons, which undergo alternative splicing at both the 5- and 3-prime ends, leading to multiple isoforms (reviewed in [21]). Class I and II isoforms arise from the use of exons 1 and 2, respectively, which encodes the signal peptide (Fig. 1A). All isoforms include exons 3 and 4, which harbor the sequence for the mature peptide and a portion of the C-terminal E peptide. Heterogeneity of the E-peptide is the result of alternative splicing of exons 5 and 6. This splicing is species specific, where humans express transcripts with exon 6 only (Class A), exon 5 only (Class B), or a portion of exon 5 and an internal splice site causing a frame shift and premature termination in exon 6 (Class C). Rodent Class B mirrors human Class C, with inclusion of a 52-residue exon 5 leading to a similar frame shift in exon 6. All possible combinations between N-terminal signal peptide usage and C-terminal E peptides can occur in different IGF-I precursors. However, the most prevalent form expressed in skeletal muscle is class I A, encoded by exons 1-3-4-6.
Fig. 1. IGF-I structure and activity.
A. Domains of IGF-1. The N-terminus includes a signal peptide directing secretion. This is followed by the mature IGF-1 peptide, and a C-terminal extension celled the E-peptide. In the Igfla isoform, the E-peptide can undergo N-glycosylation. B. Signaling cascade of IGF-1. IGF-1 is normally sequestered by IGFBPs in the ECM. Upon release, it can bind to and activate the IGF-IR, which is a tyrosine kinase. The two arms of the pathways include the ras/raf/MEK/ERK pathway that can amplify satellite cell proliferation, and the Pl3K/Akt/mTOR pathway that increases protein translation. Figure was generated in BioRender.
Post-translational processing is also a significant contributor to IGF-I protein forms found in muscle. The 70 amino acid long mature IGF-I peptide must shed the C-terminal E peptide through a unique cleavage site recognized by subtilisin- related proprotein convertases [22,23], and it is this form that is stabilized by a family of IGF-I binding proteins (IGFBPs) in the extracellular space and in the circulation. On the other hand, retention of the E peptide, called pro-IGF-I, is also observed in muscle and in the circulation [24,25], calling into question whether this provides another way to stabilize more growth factor [26] independently on the IGFBPs. Further, pro-IGF-IA also has N-glycosylation sites that may also directly interact with the ECM. Hence, there are multiple forms of IGF-I found in the ECM, which may afford differential release mechanisms for ligand receptor interactions.
2.2. IGF-I activity
IGF-I signaling is mediated on most cells by the IGF-I receptor (IGF-IR), which is a heterotetrametric tyrosine kinase IGF-IR. IGF-I can also activate hybrid receptors, which are formed from hemireceptors of IGF-I and insulin receptors and found on muscle fibers [27]. While the biological significance of the receptor population is an area of active investigation, we will restrict discussion to the signaling pathway mediated by the IGF-IR. During muscle growth and regeneration, both satellite cells and myofibers expresses IGF-IR. Upon ligand binding, IGF-IR undergoes phosphorylation to mediate 2 arms of downstream signaling: 1) the ras/raf/MEK/ERK and 2) the PI3K/Akt/mTOR pathway (Fig. 1B). This dual signaling underlies the capacity for IGF-I to promote satellite cell proliferation and differentiation. The ras/raf/MEK/ERK pathway serves to amplify satellite cell proliferation while the PI3K/Akt/mTOR pathway has been shown to primarily increase protein translation and inhibit apoptosis, ultimately driving myofiber hypertrophy.
Insight into the role of IGF-IR in satellite cells was possible when an Igflr floxed mouse was crossed with the MyoD-Cre mouse, where only heterozygous (IGF-IR+/−) mice could be generated for analysis, suggesting developmental lethality when there is total loss of IGF-IR in MyoD positive cells [28]. These mice exhibited smaller muscle masses and fiber areas during active growth and muscle regeneration, which was attributed to the inability of satellite cells to proliferate and differentiate. Naturally, p-Akt was lower in the IGF-IR+/− mice, and interestingly a fibrotic phenotype was produced in the muscle post-injury. This could be due to the trans-differentiation of myoblasts into muscle fibroblasts with the increased levels of α-smooth muscle actin in damaged IGF-IR+/− muscles.
In contrast to satellite cell ablation, effects of targeting IGF-IRs on myofibers are dependent on the method of deletion. Ablation in adult muscle fibers by a skeletal muscle actin regulated Cre results in a minor phenotype, where only smaller muscle fiber areas are observed [29,30]. Use of the Mef2c promoter for IGF-IR ablation causes further decreases in myofiber size possibly due to the lack of myoblast fusion [31]. Use of the MKR mouse, which harbors muscle-specific dominant negative IGF-IR, exhibits an impaired ability to repair after cardiotoxin injection and points to the importance of IGF-I signaling in muscle regeneration [32]. However, the transgene hampers activity of both IGF-IRs and the hybrid receptors, supporting the fact that both receptor populations may be required for IGF-I dependent regenerative capacity.
2.2.1. The role of ras/raf/MEK/ERK signaling for satellite cell and myofiber activity
The distinction that the two arms of the IGF-I signaling pathway mediates mitogenesis and myogenesis was substantiated 25 years ago in culture [33]. Since then, the field has taken advantage of cell specific tools to gain more insight into the process. For example, the interplay of additional signaling molecules with the ERK pathway are necessary for satellite cell proliferation. Satellite cell specific ablation of Ptpnll encoding the tyrosine phosphatase, SHP2, prevents satellite cell proliferation, and instead promotes satellite cell quiescence [34]. As a consequence, acute injury caused by cardiotoxin injection does not resolve, and the muscle remains in a damaged state. The inability for satellite cells to proliferate is attributed to loss of sustained ERK phosphorylation, as the phenotype can be rescued by cross-breeding with mice expressing constitutively active mitogen-activated protein kinase (MAPK). Complementary to its role in satellite cells, modulation of the ERK pathway in fibers can also alter their physiological properties. In a mouse model where MEK1, the kinase upstream of ERK, is constitutively active in the muscle fiber, improvements in metabolic phenotype and protection from muscular dystrophy were observed [35]. The fast-to-slow fiber-type switching in skeletal muscle was primarily driven by ERK2, supporting the overall need for this pathway in muscle maintenance. While in both the above scenarios ERK activation can be driven by multiple ligand-receptor triggers, these studies demonstrate that IGF-I can harness these benefits for muscle growth and regeneration.
2.2.2. The role of PI3K/Akt/mTOR signaling for satellite cell and myofiber activity
Modulation of the myogenic arm of the IGF-I pathway illustrates the necessary balance needed among satellite cell activation, proliferation and differentiation. Using a MyoD Cre driver, mice with satellite cell ablation of phosphatase and tensin homolog (PTEN) have been evaluated (PTENmk0 mice) [36]. PTEN is a negative regulator to phosphatidylinositol 3-kinase (PI3K), reversing the actions of PI3K, and so loss of PTEN de-represses the PI3K/Akt/mTOR pathway. Mice display muscle hypertrophy (larger muscle mass, fiber area and myonuclei/myofiber) and hyperplasia (higher fiber number). Deletion of PTEN also promotes satellite cell proliferation and differentiation, with a propensity of these cells to exit the cell cycle. PTEN ablation in myofibers does not produce a major phenotype [37]. While the PTENMK0 mouse shows reduced atrophy following denervation and increased efficiency in muscle regeneration, the price to pay is the inability of PTENMK0 satellite cells to self-renew. The authors showed that proliferation (Ki67 + /MyoD + /Pax7 + cells) and differentiation (MyoG+ nuclei) were enhanced, but quiescent satellite cells (Ki67-/MyoD-/Pax7 + ) were significantly lower in PTENMK0 muscles at 10 days post-injury.Utilizing the MyoD promoter for Cre-induction only targets committed myogenic progenitor cells, complicating experiments in measuring the sternness of satellite cells. However, utilizing the Pax7CreER mice in driving PTEN deletion specifically in satellite cells (PTENPK0) [38] replicates the findings from PTENMKO, where PTENPK0 muscles are unable to efficiently regenerate and have depleted quiescent satellite cell numbers. The inability of PTENPK0 satellite cells to self-renew is not due to advanced apoptosis, but instead, accelerated satellite cell differentiation, which leads to enhanced activation of Akt and mammalian target of rapamycin (mTOR) pathways evidenced by higher numbers of MyoG+, p-Akt+, and p-S6 + satellite cells, which are markers of differentiation.
The crucial role of the PI3K/Akt/mTOR pathway has been illustrated through similar manipulation of many pathway components. For instance, mice with satellite cell-specific deletion of p110a, a catalytic subunit of PI3K, exhibit impaired regenerative capacity due to an inability of satellite cells to exit quiescence, as both in vivo and in vitro assessments demonstrate a failure to enter the cell cycle [39]. Partial rescue can occur through ablation of tuberous sclerosis protein 1/2 (Tscl), an mTORCl repressor, thus releasing the breaks on mTORCl and placing PI3K as an important checkpoint for satellite cells to enter the cell cycle. The serine/threonine kinase, mTOR, also plays a major role in cell development that is associated with raptor (mTORCl) and rictor (mTORC2). Deletion in mTOR specifically in satellite cells results in impaired muscle regeneration due to defective satellite cell proliferation and differentiation [40]. Specific deletion of mTORC2 in the satellite cells poses a less deleterious effect where muscle regeneration impairment is only observed after repeated bouts of cardiotoxin-induced injury or in aging mice [41]. Thus, the intracellular signaling pathways instigated by IGF-I activation modulate several of the key steps taken by satellite cells during muscle growth and regeneration.
Work on a zebrafish model with loss of myogenin (MyoG) further reinforces the role of IGF-I in satellite cell activation. The authors reported that deletion of MyoG (MyoGKO) in zebrafish increased Igfl mRNA by 130% and decreased myostatin mRNA by 50% in the muscle [42]. In terms of signaling, p-Akt was unchanged, but phosphorylation of ribosomal protein S6 (p-rpS6) was increased 4-fold in MyoGKO bulk muscle lysates. There was a similar trend following satellite cell specific MyoG ablation, where 2-fold increases in p-rpS6 were observed. The finding that expression of Tscl was also reduced helped to explain the absence of coupling between Akt and rpS6 and led to the conclusion that de-repression of mTORCl underlies satellite cell activation. The consensus is that IGF-I and its signaling pathway contribute to satellite cell activation; however, the IGF-I source has yet to be established.
2.3. IGF-I sources and targets during muscle regeneration
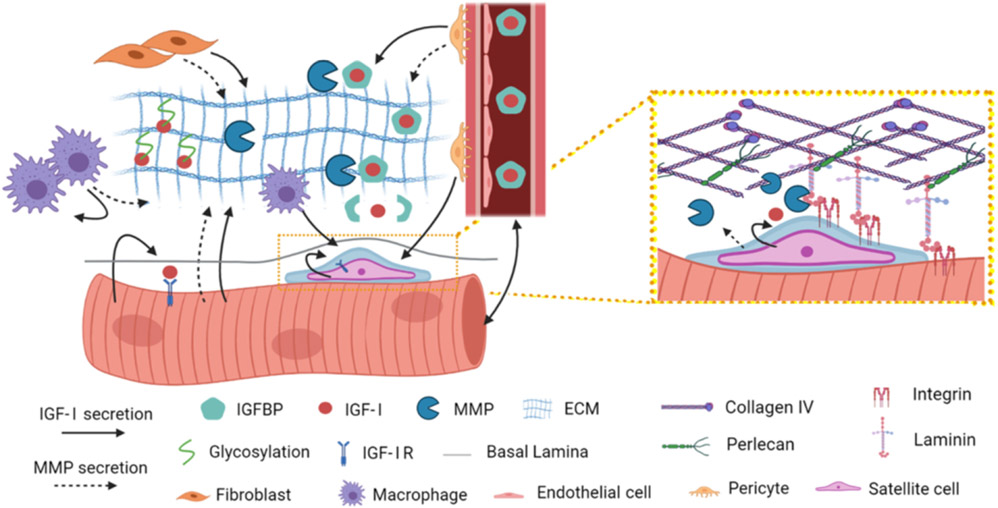
There are multiple sources of IGF-I within the skeletal muscle compartment (Fig. 2). At the onset of skeletal muscle repair, inflammatory cells, such as macrophages, secrete IGF-I that can activate satellite cells and instigate cell proliferation [43]. Even though there may be IGF-I stored in the ECM, myeloid cell-specific deletion of Igfl in mice leads to lower levels of IGF-I in injured muscles [44], suggesting that inflammatory cells contribute significantly to the muscle IGF-I pool. Phenotypic defects in these mice include reduced macrophage polarization and satellite cell proliferation as well as smaller muscle fiber areas later in the regeneration process. Macrophage IGF-I actions seem restricted to early stages of regeneration, as myoblast fusion is not disrupted [44]. Therefore, IGF-I from the macrophage population has the capacity to drive Ml to M2 polarization via autocrine regulation, plus provide a paracrine source for satellite cell expansion, which further benefits downstream muscle regenerative processes. Other models of injury, such as tourniquet-induced ischemia reperfusion (TK-I/R) also display increased Igfl expression in macrophages, which is highly correlated to Emrl (F4/80) expression in whole muscle [45]. Further, injection of Ml macrophages into the gastrocnemius of mice with TK-I/R leads to heightened muscle Igfl expression unlike treatment with unpolarized (MO) macrophages or saline [46]. These studies help to pinpoint the macrophage subpopulation most relevant for IGF-I production.
Fig. 2.
Sources of IGF-1 and MMPs in muscle. IGF-1 (solid arrow) can be secreted by many cell types in muscle, including muscle fibers, macrophages, fibroblasts, pericytes, and satellite cells, as well as from the circulation. IGF-1 is stabilized in the ECM through IGFBPs and direct interaction with ECM proteins. MMPs (dotted arrow) are also secreted from these cell sources to act on ECM proteins. The satellite cell niche (Inset) may include high local concentrations of MMPs that specifically cleave proteins of the basement membrane. Figure was generated in BioRender.
Muscle satellite cells also produce IGF-I as they emerge from quiescence (Fig. 2). In a study of muscle biopsies obtained from human subjects, co-localization of IGF-I and Pax7, a marker for satellite cells, was detected 72 h post-resistance exercise in both type 1 and type 2 muscle fibers, accompanying the bulk muscle increase in Igfl a expression [47]. Given the time between exercise and biopsy, it is likely that the satellite cells had been activated. However, it raises the question as to when satellite cells begin to produce and/or respond to IGF-I. A recent study assessed the differences in satellite cell ability to retain sternness, termed a genuine state with high CD34, in contrast to a primed state with low CD34, where a satellite cell had restricted commitment to myogenic differentiation [48]. The authors found that primed satellite cells had higher Akt phosphorylation (p-Akt) levels and lower FOXO3a within the nucleus when compared to genuine satellite cells, supporting that signaling through the IGF-I pathway was part of the switch for priming. Upon entry into the primed state, satellite cells exhibit decreased sternness, suggesting that IGF-I signaling underlies the switch. However, even though IGF-I must be within the satellite cell niche to stimulate them, where this factor is produced is unclear. Satellite cells undergo several stages during the regeneration process (discussed in other reviews in this issue) after leaving their niche by a muscle fiber, and IGF-I also serves as a major factor in their differentiation and fusion. A recent innovative study provided a closer look at myoblast fusion, demonstrating an IGF-I dose- and time-dependent enhancement of myonuclear accretion and myotube formation in culture [49], pointing to the necessity of IGF-I for myoblast fusion.
In comparison to other cell types, myofiber-derived IGF-I has been well studied and is the primary source of IGF-I in homeostatic muscle. Glycosylated pro- and pro-IGF-I appear to be the predominant forms generated by skeletal muscle fibers, as transgenic or viral overexpression of Igfl a leads to accumulation of the two higher molecular weight bands [24]. In contrast, muscle-specific deletion of Igfl (MID mice) or its chaperone Grp94 decreases bulk muscle IGF-I content and results in the disappearance of glycosylated pro-IGF-I [25,50]. These longer forms of IGF-I retaining the C-terminal E peptide extension have been shown to bind to the ECM [26], potentially creating an additional IGF binding protein independent reservoir of this factor. Regenerative capacity is enhanced in mice with muscle-specific transgenic overexpression of IGF-I [51]. Thus, the increased local IGF-I provided by the muscle fibers serves as an active source of the growth factor. The target of this IGF-I reservoir could be any cell type within the muscle compartment. In a mouse model of disuse atrophy followed by reloading, viral delivery of IGF-I caused an increase in Ki67 + cells, a marker of proliferation, during the recovery phase, most of which were not Pax7 + [52], supporting that myofiber-secreted IGF-I plays a role in the proliferation of other cell types in the muscle.
During acute muscle regeneration, the fibroadipogenic progenitor (FAP) population also secretes IGF-I [53,54]. When FAPs differentiate into fibroblasts at the later stages of regeneration, IGF-I from these fibroblasts contributes to myoblast differentiation and myotube formation, taking over from the earlier macrophage source [44]. Chronic degeneration, however, leads to a reduction in fibroblast production of IGF-I: FAPs purified from old mdx mice have lower Igfl than young mdx mice [55]. FAP Igfl expression also appears dependent upon their metabolic fuel, where FAPs from mdx mice treated with high fat diet leads to increased Igfl mRNA [56]. Hence, modulation of this cell source occurs by processes beyond the direct steps of regeneration.
IGF-I from the myovascular niche is provided by pericytes [57], and this source can also act on satellite cells. Conditioned media from pericytes enhance myoblast differentiation, which is prevented by the addition of an IGF-I blocking antibody. Further, pericyte specific deletion of Igfl in mice at birth results in smaller muscles and a lower number of myonuclei per muscle fiber 3 weeks later. Thus, while pericyte derived IGF-I levels pale in comparison to that from the muscle fibers, the proximity of these cells afford communication through boosting the local concentration of secreted factors.
2.4. Temporal regulation of IGF-1 expression during muscle growth and regeneration
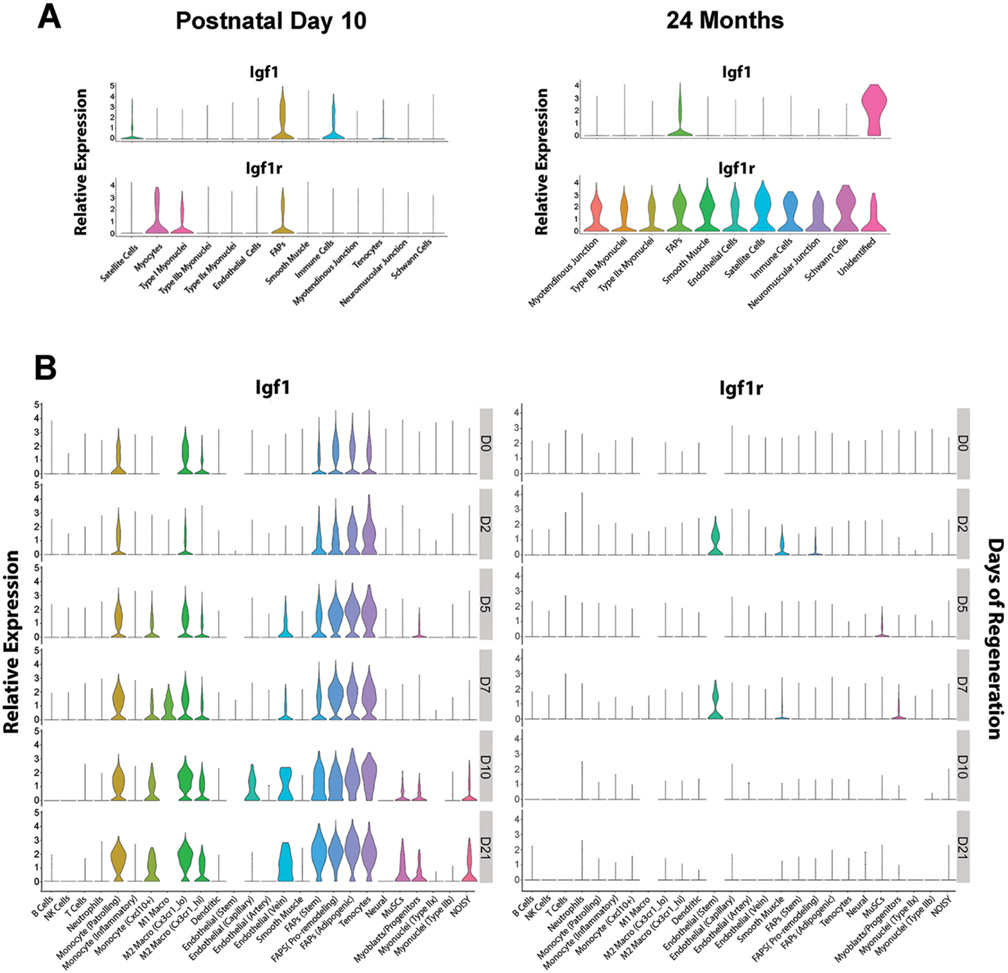
The studies above identify several sources of IGF-I, but the timing in IGF-I production during skeletal muscle growth and regeneration also requires consideration. For instance, several sources of IGF-1 may diffuse into the satellite cell niche for satellite cell activation and proliferation, although we cannot discount the possibility of autocrine regulation of IGF-1 from satellite cells themselves. To gain a handle on the coordination of these sources as well as the presence of IGF-1 receptors, delving into single cell analysis is key. The timing of IGF-I expression during skeletal muscle growth and regeneration can be observed using publicly available platforms that provides single cell and single nucleus transcriptomic evaluation (sc/sn RNA-seq) within the muscle. The Myoatlas [8] platform (https://research.cchmc.org/myoatlas/) enables the evaluation of IGF-I expression during murine muscle development and aging (Fig. 3A). In addition, the scMuscle [3,58] platform (http://scmuscle.bme.comell.edu/) provides cell-specific expression during muscle regeneration (Fig. 3B). At day 10 of life (P10), Igfl expression in muscle is most prominent in FAP cells, satellite cells and immune cells (Fig. 3A). On the receiving end, IGF-I receptor (Igflr) expression appears higher in FAP cells, myocytes and type 1 myofibers. This points to the potential autocrine regulation of IGF-I in FAP cells, while the myocytes and type 1 myofibers may rely on IGF-I from the satellite cell and immune cell populations. A different scenario emerges in muscles from 24 month old mice, where the onset of sarcopenic decrements in muscle mass and force have been documented [59]. Igfl expression is predominantly from FAP cells and a small population of cells of unknown identity [8]. In contrast to PI0 muscles, Igflr is highly expressed in all cell types within the aged muscle, possibly indicating high demand of IGF-I signaling in these muscles. At this age, total IGF-I levels are modestly changed, but serum IGF-I levels have decreased, and mice display muscle atrophy, neuromuscular junction (NMJ) fragmentation accompanied by fast to slow fiber transition, lower fiber number and decreased exercise tolerance [60]. Nevertheless, transgenic overexpression of muscle-specific IGF-I can rescue the aging phenotype including its regenerative capacity [13], implying that it is the local source of IGF-I that is lacking in terms of bioavailability.
Fig. 3. Cell specific expression of Igfl and Igflr.
A. Age dependent expression of Igfl and Igflr [8], demonstrates the decrease in Igfl expression in muscle fibers and satellite cells with age, and a persistence of Igfl expression in FAPs with age, and the general increase in Igflr expression in old murine muscle. B. Timecourse of Igfl and Igflr expression [3] from Day 0 (DO), representing no damage, through Day 21 (D21) following acute injury, shows the transient appearance of these transcripts, where macrophages and the FAP population have persistent Igfl expression, and muscle stems cells and myoblasts express Igfl at greater levels late in the regeneration process. Muscle stems cells and myoblasts show upregulation of Igflr expression at D5 and D7, respectively, preceding the rise in their own Igfl expression.
In acute muscle regeneration, Igfl expression begins with inflammatory and fibrogenic cells (Fig. 3B). From day 5 post-injury onwards, Igfl expression expands to additional cell populations, including Ml and M2 macrophages, endothelial cells, satellite cells, and myoblasts. Using a more severe freeze injury in contrast to chemical injections [61], Igfl expression is most pronounced at the 3 day timepoint. Presence of Igflr expression occurs in endothelial and smooth muscle cells as well as myogenic cells including satellite cells and myoblasts, suggesting that all of these cells can respond to IGF-I in the early stages of muscle regeneration (Fig. 3B). Taken together, muscle regeneration expression patterns replicate some aspects of early muscle growth, such as Igfl expression from muscle and fibroadipogenic progenitors, but also expand to include transient IGF-I production by inflammatory cells, which may be key to triggering satellite cells.
3. Matrix metalloproteinases
3.1. MMP family and stTucture
The matrix metalloproteinases (MMPs) are aptly named for their site, regulation, and mode of action. The MMPs are key enzymes for degrading components of the ECM, enabling cell migration, and releasing growth factors [62,63]. As all of these actions are central features of muscle regeneration, the family of more than 2 dozen MMPs coordinate the process through highly regulated temporal and cell specific changes in expression and production, activation through cross talk between members, and preferential substrate specificity (reviewed in [64]). Control of MMP activity occurs at several levels: transcription, activation of the precursor zymogen, and inhibition by endogenous inhibitors (tissue inhibitors of metalloproteinases, or TIMPs). Without regulation, active MMPs can cause extensive ECM remodeling and result in a spectrum of pathological conditions, including arthritis, cancer, atherosclerosis, and fibrosis. However, the absence of MMP activity results in unresolved repair in bone [62], stalled development [65], and impaired wound healing (reviewed in [66]). Therefore, appropriately timed activity of MMPs is essential for proper ECM remodeling during development and tissue repair.
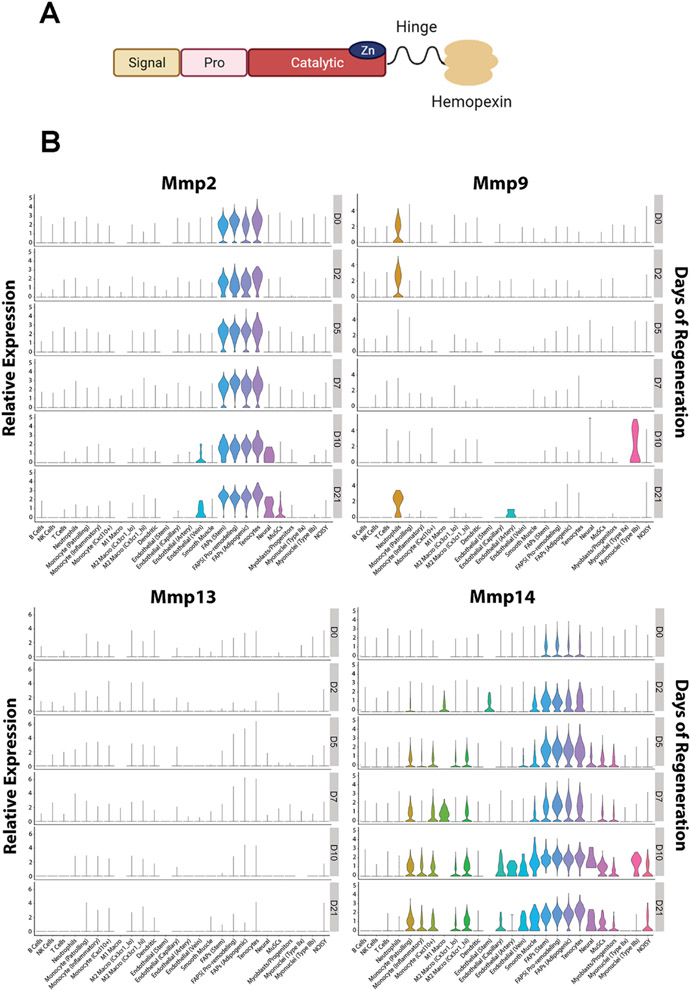
The family of MMPs are classified by their structural domains and their substrates (collagenases, gelatinases, stromelysins and matrilysins) although extensive substrate overlap exists (reviewed in [67]). MMPs share a number of domains (Fig. 4A), which include a signal peptide that directs nascent MMPs to the endoplasmic reticulum for processing, a regulatory propeptide, and a catalytic domain where zinc binding is required for endopeptidase activity [68]. Most MMPs also have a hemopexin-like domain connected to the catalytic domain via a proline rich linker. The propeptide inhibits zymogen activity, as it is docked in the active site on the catalytic domain and prevents substrate entry [69]. Removal of the propeptide converts latent MMPs to active enzymes [70], affording the active site to cleave substrates. Substrate specificity is aided by the hemopexin-like domain, which forms a 4-bladed propeller that contributes to substrate binding and also interacts with tissue inhibitors of metalloproteinases (TIMPs) [71]. Most MMPs are secreted and remain in the ECM as latent proenzymes awaiting activation by other activated MMPs or by serine proteinases. A small group of members are membrane bound via a transmembrane domain at the carboxy-terminus [16,63].
Fig. 4. MMP protein domains and expression during muscle regeneration.
A. Domains of MMPs include a signal peptide conferring secretion, a pro domain that can regulate activity, the catalytic domain, a hinge linker, and a hemopexin-like domain, which can tune substrate specificity. Figure was generated in BioRender. B. Timecourse of Mmp expression [3] from Day 0 (DO), representing no damage, through Day 21 (D21) following acute injury. Mmp2 shows stable expression by the FAP population throughout regeneration. Mmp9 shows early upregulation in neutrophils, and an increase in muscle fibers expression at day 10 (DIO). Mmpl3 has the lowest level of expression in comparison to the other MMPs shown. Mmp14 has the broadest expression, including muscle stems cells (MUSCs) and myoblasts from Days 5 - 21.
3.2. MMP actions during regeneration
Even though there is much overlap in the specificity for ECM substrates, there is a wide range of substrate cleavage potency across the MMP family and is an important part of the regeneration process (Fig. 2) [72]. This allows for a high level of regulation of the ECM degradation process, not only in the targets for removal, but also in the localization of matrix remodeling. Generalized MMP inhibition prevents resolution of muscle damage, supporting the necessity of the MMP family for regeneration [73]. Conceptually, there are two major interleaved processes governed by MMP activity: ECM remodeling and cell migration. Degrading specific protein components “loosens” the meshwork of the ECM, affording greater ability for cells to move; however, the cells themselves can also secrete MMPs, or harbor them on their surface, to clear paths through the ECM and increase the distance and/or speed of cell movement.
In skeletal muscle, only a few of the many MMPs have been deeply investigated. The most prevalent MMPs reported in homeostatic muscle include MMP-2, a gelatinase and MTl-MMP/MMP-14, a membrane bound MMP [14,74]. In regenerating muscle, additional MMPs emerge, including MMP-9, a second gelatinase, and MMP-1 and MMP-13, members of the collagenases. The following sections will highlight features of these most prominent MMPs with respect to their temporal expression and varied sources during regeneration, as well as their targets upon activation.
3.3. Gelatinases
The gelatinase, MMP-2, is the most prevalent MMP in muscle, but has minimal activity in healthy muscle. It resides as a latent zymogen in the ECM, and further inhibited by TIMP2 binding. It is activated by several of the MMP family members as well mechanical loading [75]. One of the most well-studied MMP2 activation pathways is via MMP-14 (reviewed in [63]). One molecule of MMP-14 bound and inhibited by TIMP2 serves as a tether for pro-MMP-2, after which an uninhibited MMP-14 molecule can cleave and activate MMP-2. Given the highly regulated activation of MMP-2, it is not surprising that transcriptional regulation is minimal. The greatest proportion of expression is from the FAP cell population (Fig. 4B), which remains fairly stable during regeneration; however, other cell types express Mmp2, including muscle stem cells.
In synergist ablation models, increased MMP-2 activity is associated with ECM remodeling and muscle hypertrophy, and the hypertrophic response is impaired with the global loss of MMP-2 [76]. This study points to the need for ECM remodeling not only with regeneration but also to allow changes in muscle fiber size. One can consider the ECM structure as a corset, restricting muscle fiber expansion much like Victorian era waistlines. While MMP activity is most well characterized in the extracellular space, several MMPs have been detected inside cells, including MMP-2, where they may also cleave intracellular targets. MMP-2 is detectable within muscle fibers, concentrated at the z-line, in mitochondria, and surrounding the nucleus [77], distinct from compartments involved in protein secretion. In situ zymography indicates that MMP-2 is active within the cell and supports the observation that MMP-2 cleaves titin [78]. Given MMP-2 can act both inside and outside of the muscle fiber, it may also coordinate intracellular protein turnover as the fibers expand. Thus, similar to the ECM remodeling ascribed to MMPs, they can also assist in remodeling inside fibers.
The gelatinase MMP-9 shares many substrates with MMP-2, but is transiently produced during regeneration, and elevated in chronically regenerating dystrophic muscle [79,80]. It is expressed early in the regeneration process by neutrophils, with a peak of expression by muscle fibers at day 10 post injury (Fig. 4B). Hence, its actions may be beneficial for remodeling muscle tissue after acute damage, but detrimental to muscle stability in chronic damage, exemplifying the double-edged sword of MMP actions [17]. Inhibition of MMP-9 through genetic ablation or pharmacologic inhibition improved the dystrophic phenotype in mdx mice [81,82], with multi-pronged actions on macrophage polarization and satellite cell proliferation. However, active MMP-9 increased muscle fiber hypertrophy with a commensurate improvement in strength [83]. In the same study, regenerative capacity was improved in the presence of active MMP-9, with enhanced Igfl expression, and improved satellite cell fusion. These contradictory findings with regard to MMP9 suggest that timing and location may underly the differences. One recent observation supports that cell specific actions may be important to regulate when and where MMP-9 is present and active. In a model of mechanical overload, the ablation of satellite cells resulted in enhance expression, implicating satellite cells as a repressive force on MMP-9 production by muscle [84]. In sum, MMP-9 clearly plays a role in muscle regeneration, but grappling with its dual actions warrants further investigation.
3.4. Collagenases
MMP-13 is a member of the collagenase family that includes MMP-1 and MMP-8, and these can cleave native fibrillar collagens, proteoglycans, and fibronectin. MMP-13 is activated by MMP-2, −3, and −14, and also can activate MMP-2 and −9 [85], contributing to the intricate crosstalk among the MMP family. A primary action of MMP-13 is cell migration in virtually all cell types investigated. Modulation of MMP-13 activity in C2C12 myoblasts is directly correlated with their ability to move in transwell assays [86]. Fibroblasts in the dermal layer involved in migration toward the wound center express high levels of MMP-13 [87]. Even its original discovery in breast carcinomas points to its role in cell migration [88]. Thus, it comes as no surprise that targeted ablation of MMP-13 in satellite cells slows their migration in vitro resulting in diminished muscle growth and regenerative capacity in vivo [15]. The global knockout of MMP-13 displays similar defects in resolution from acute injury. In mdx mice, however, there is no apparent difference in muscle morphology between mice with or without MMP-13. The fact that there are heightened levels of several MMPs in dystrophic tissue [89,90] may mask any MMP-13 specific actions, and supports the concept of functional overlap between MMPs.
Unlike the more prevalent MMPs, Mmpl3 expression is almost undetectable in healthy muscle [15,86]. However, during regeneration, expression and MMP-13 activity transiently increase, with a peak at ~ 1 week following cardiotoxin injection [86]. Similar patterns have been documented in other models of healing, such as recovery from cutaneous wounds [87]. Cell specific expression of Mmpl3 illustrates the relatively low transcript abundance across all cell types compared to Mmp2 or Mmp9 (Fig. 4B). FAPs display the greatest level of expression, with maximal expression occurring at day 7 post cardiotoxin injection, similar to the findings in bulk muscle tissue. In contrast, myoblasts and muscle satellite cells express very low Mmpl3 levels. Bearing in mind the significant effects to regeneration caused by Mmpl3 ablation from Pax7 positive cells, this suggests that the cell source may be more critical than the amount produced, where local Mmpl3 increases in the immediate vicinity of satellite cells are more impactful on the regeneration process.
The collagenase family shares cell migration properties. MMP-1 has been considered as a therapeutic agent to boost resolution of damage after muscle injury. Exogenous delivery of recombinant MMP-1 combined with myoblasts into damaged muscle enhanced cell engraftment [91]. More recently, transduction of myoblasts to express MMP-1 enhanced migration in vitro and in vivo [92]. Thus, the collagenase family appears to be generally beneficial for muscle regeneration, directly enhancing cell migration, and likely indirectly enhancing myoblast fusion.
The third collagenase, MMP-8, is classically dubbed the “neutrophil collagenase”, as its initial identification was in peripheral leukocytes [93]. No evidence of skeletal muscle expression has been reported, but detection in additional cell types, including endothelial cells, smooth muscle cells, and mononuclear phagocytes associated with atherosclerotic plaques suggests a broader expression profile [94]. Whether these cell sources also produce MMP-8 in muscle is not known. However, because collagen types I and III are preferential substrates for MMP-8, and it is unable to cleave collagen IV [95], it is possible that MMP-8 could help to remodel the ECM, while sparing the basement membrane that is enriched with collagen IV.
3.5. Membrane bound MMPs
MTl-MMP/MMP-14 is one of the membrane bound MMPs. In addition to the domains shown in Fig. 4A, the carboxy-terminus also contains a 24 residue transmembrane domain and a cytoplasmic tail. The PEX domain of MMP-14 also includes unique loop structures that enable substrate specificity via the hemopexin blades for collagens I and III, non-collagenous substrates found in the basement membrane (e.g., fibronectin, laminins), other MMPs (MMP-2 and −13), and integrins (reviewed in [16]). The propeptide can be cleaved by MMP-2 affording its activation, whereas MMP-14 activity is negatively regulated by TIMPs, particularly TIMP2. Inactivation can occur through autocatalytic cleavage at the hinge domain, separating the catalytic domain from the membrane anchor.
MMP-14 is found in many tissues within mice, including the kidney, heart, lung, and developmental tissues. Further, adult mouse muscle has detectable MMP-14 protein [96] and transcription [15]. During regeneration, activated MMP-14 appears at day 4 following cardiotoxin injection, preceding the appearance of active MMP-2 in gelatin zymography [14]. Its absence impairs overall collagen turnover leading to a variety of connective tissue diseases in knockout mice, which are viable for only a few weeks after birth [74]. Despite the severe phenotype examination of skeletal muscles up to 4 weeks of age was possible [14]. The quadriceps exhibited fiber size heterogeneity, a modest increase in central nucleation, pockets of interstitial ECM, and thickened basement membrane. The authors went on to show that there was abnormal processing of laminin within the muscle, suggesting that MMP-14 has a specific cleavage site on laminin alpha2. Taken together, the direct actions of MMP-14 on the basement membrane as well as its activation of MMP-2 have significant effects on resolution of muscle damage.
More recent analysis of MMP-14 actions has been performed in human myoblasts [97], as there was no MMP-14 evident on murine primary myoblasts, even though it is found on the surfaces of the myoblast C2C12 cell line. Cell based 3D invasion assays with C2C12 and human myoblasts demonstrate that MMP-14 directs migration through Collagen I rich substrates [97], a property that is common to its actions in pericellular remodeling and invasion by mesenchymal stem cells and tumor cells. Whether murine satellite cells produce a different membrane bound MMP, or if the redundancy of MMP actions enables growth and regeneration to progress without MMP-14 on their surface is not clear.
To address the cell specificity of Mmpl4 expression, the single cell RNA-Seq data base was queried. FAPs, monocytes, and macrophages exhibit Mmpl4 expression throughout the regeneration process (Fig. 4). In addition, muscle stem cells and myoblast/myogenic progenitors express Mmpl4 in the later stages of regeneration, even though in cultured cells detection is minimal. This suggests that the properties described for human myoblasts in 3D invasion assays bear relevance to the in vivo remodeling process in mouse. Further, presence of Mmpl4 transcripts in type IIb myonuclei suggests that the membrane bound MMP could alter the basement membrane surrounding muscle fibers, supporting the earlier observations of the thickened basement membrane in the MMP-14 knockout mice.
4. Interactions between IGF-I and the MMPs
4.1. MMP actions on IGF-1
It should be borne in mind that presence of IGF-I is not the only requirement for its ability to enhance regeneration. Coordinated production of IGF-I and the MMPs during muscle regeneration is critical to afford both cells and their environment to undergo the changes needed for resolving damage. The most direct coupling between these factors is within the ECM. Given that a large reservoir of IGF-I is stored within the matrix, cleavage of its ECM anchors allows for bulk release of IGF-I to activate cells. IGF-I release could occur in multiple ways. First, cleavage of ECM proteins can release IGF:IGFBP complexes, or pro-IGF-I that may be bound directly to the ECM [26]. For example, IGFBP-5 has high affinity for a number of ECM proteins, including collagen III found in the interstitial matrix, as well as several basement membrane proteins, including collagen IV, fibronectin, and laminin [98]. Cleavage of these proteins untethers IGF:IGFBP, and in turn, enables proteases to cleave IGFBPs and release IGF-I. IGFBPs can associate with the ECM with or without IGF-I association, such as IGFBP3 and fibronectin [99]. Further, association with the ECM reduces the affinity between IGFBP-5 and IGF-I, suggesting that the localization of the IGF:IGFBP complex to the matrix may also lower the barrier for IGF-I release. Alternatively, the ECM-IGFBP docking provides the stabilization sites for IGF-I once it is produced by cells within the muscle.
A second way MMP can modulate IGF-I activity is via direct cleavage of IGFBPs [100]. Much of the evidence for MMP cleavage of IGFBPs has been collected in cell-based assays rather than in vivo. No studies, to date, have explicitly addressed the MMP-IGFBP interactions in muscle, but inferences from other experimental platforms provide clues to what may occur during muscle regeneration. A proteomic comparison of MMP-2 and MMP-9 substrates, or “degradomics”, revealed that IGFBP-4 was a shared substrate [101]. This may bear significance on the release of IGF-1 in muscle early in the regeneration process, as IGFBP-4 inhibits IGF-I activity [102]. MMP-2 can also cleave IGFBP-5 and −6 [17], and several MMPs can degrade IGFBP-3. In particular, MMP-9 mediated cleavage of IGFBP-3 underlies the paracrine signaling between endothelial cells and myoblasts, affording both angiogenic and myogenic actions in wound healing [103]. Thus, MMP actions on non-ECM substrates, such as the IGFBPs are also critical for regenerative processes.
4.2. IGF-1 actions on MMPs
IGF-I signaling can alter gene expression of MMPs and their inhibitors in multiple tissues, although less has been established in skeletal muscle. In our hands, we found that viral mediated expression of IGF-I in skeletal muscle dramatically increased Mmpl3 expression and also elevated Mmp9 expression [11]. Given the actions of these MMPs, this would enable activated satellite cells to produce MMP-13 and allow for efficient migration [15]. In addition, a feed-forward loop could occur through the MMP-9 mediated cleavage of IGFBP-3 [103]. IGF-1 can also promote Mmp2 transcription in vascular smooth muscle cells in an Akt dependent manner [104]. However, not all tissues exhibit the same IGF-1 dependence for MMP expression. In the heart, heightened IGF-IA can improve functional recovery following infarction and is associated with blunted Mmp2 and Mmp9 expression [105]. Alternatively, rather than a direct coupling to regulation of transcription, the differential pattern of Mmp expression may be due to the reduction of inflammatory monocytes entering the infarction area.
5. Clinical prospects and concluding remarks
There are several situations in which inefficient regeneration leads to muscle weakness and accumulation of fibrosis, and seeking ways to improve regenerative capacity is an active research area. In the simplest view, strategies to boost IGF-I and reduce MMP have been considered. For IGF-1, a major hurdle is seeking a way to restrict its activity to muscle, rather than have systemic pro-growth activity. As the acceptance of gene therapy to restore functional proteins lost in genetic disease grows, it is more plausible that a similar strategy could be adopted for muscle specific expression of IGF-I. Alternatively, strategies that target delivery of therapeutics through engagement with muscle specific receptors could reduce side effects associated with systemic alterations in IGF-1 or MMPs. Further, highly innovative biomaterials afford controlled release of IGF-1 and other growth factors from scaffolds laid directly on traumatic muscle wounds [106]. Importantly, many scaffolds contain or are composed of decellularized ECM [107], supporting the notion that the proteins retained within the ECM are also key for muscle healing. For the MMPs, the “protease web” [108] makes it challenging to specifically target a single MMP without compensatory changes in others. In chronic conditions, such as muscular dystrophies, toning down elevated MMPs, such as MMP-2 or −9, may be beneficial for patients [80,81,109]. Ultimately, muscle regeneration requires the coordinated and transient actions of the MMPs along with IGF-1 to efficiently resolve damage.
Acknowledgements
This work was supported by the National Institutes of Health (AR052646, AR074271); and the American Federation for Aging Research (Diana Jacobs Kalman Scholarship for Research).
Abbreviations:
- ECM
extracellular matrix
- FAP
fibroadipogenic progenitor
- IGF-I
insulin-like growth factor I
- IGF-IR
insulin-like growth factor I receptor
- IGFBP
insulin-like growth factor binding protein
- MMP
matrix metalloproteinase
- MyoG
myogenin
- PTEN
phosphatase and tensin homolog
- RNA-Seq
RNA sequencing
- TIMP
tissue inhibitor of metalloproteinases.
Footnotes
Conflict of Interest
Dr. Barton consults for Anagenesis, DTx Pharma, and REGENXBIO. Ms. Kok has no conflicts of interest.
References
- [1].Forcina L, Cosentino M, Musaro A, Mechanisms regulating muscle regeneration: insights into the interrelated and time-dependent phases of tissue healing, Cells 9 (5) (2020), 10.3390/cells9051297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Tidball JG, Regulation of muscle growth and regeneration by the immune system, Nat. Rev. Immunol 17 (3) (2017) 165–178, 10.1038/nri.2016.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].De Micheli AJ, Laurilliard EJ, Heinke CL, Ravichandran H, Fraczek P, Soueid-Baumgarten S, De Vlaminck I, Elemento O, Cosgrove BD, Single-cell analysis of the muscle stem cell hierarchy identifies heterotypic communication signals involved in skeletal muscle regeneration, Cell Rep. 30 (10) (2020) 3583–3595.e5, 10.1016/j.celrep.2020.02.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Dell’Orso S, Juan AH, Ko KD, Naz F, Perovanovic J, Gutierrez-Cruz G, Feng X, Sartorelli V, Single cell analysis of adult mouse skeletal muscle stem cells in homeostatic and regenerative conditions, Development Camb. 146 (12) (2019), 10.1242/dev.174177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Giordani L, He GJ, Negroni E, Sakai H, Law JYC, Siu MM, Wan R,Comeau A, Tajbakhsh S, Cheung TH, Le Grand F, High-dimensional single-cell cartography reveals novel skeletal muscle-resident cell populations, Mol. Cell 74 (3) (2019) 609–621.e6, 10.1016/j.molcel.2019.02.026. [DOI] [PubMed] [Google Scholar]
- [6].Kalucka J, de Rooij LPMH, Goveia J, Rohlenova K, Dumas SJ, Meta E, Conchinha NV, Taverna F, Teuwen LA, Veys K, García-Caballero M, Khan S, Geldhof V, Sokol L, Chen R, Treps L, Borri M, de Zeeuw P, Dubois C, Karakach TK, Falkenberg KD, Parys M, Yin X, Vinckier S, Du Y, Fenton RA,Schoonjans L, Dewerchin M, Eelen G, Thienpont B, Lin L, Bolund L, Li X,Luo Y, Carmeliet P, Single-cell transcriptome atlas of murine endothelial cells, Cell 180 (4) (2020) 764–779.e20, 10.1016/j.cell.2020.01.015. [DOI] [PubMed] [Google Scholar]
- [7].Kimmel JC, Hwang AB, Scaramozza A, Marshall WF, Brack AS, Aging induces aberrant state transition kinetics in murine muscle stem cells, Development Camb. Engl 147 (9) (2020), 10.1242/dev.183855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Petrany MJ, Swoboda CO, Sun C, Chetal K, Chen X, Weirauch MT, Salomonis N, Millay DP, Single-nucleus RNA-seq identifies transcriptional heterogeneity in multinucleated skeletal myofibers, Nat. Commun 11 (1) (2020) 1–12, 10.1038/s41467-020-20063-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Rubenstein AB, Smith GR, Raue U, Begue G, Minchev K, Ruf-Zamojski F, Nair VD, Wang X, Zhou L, Zaslavsky E, Trappe TA, Trappe S, Sealfon SC, Single-cell transcriptional profiles in human skeletal muscle, Sci. Rep 10 (1) (2020) 229, 10.1038/s41598-019-57110-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Stepien DM, Hwang C, Marini S, Pagani CA, Sorkin M, Visser ND, Huber AK, Edwards NJ, Loder SJ, Vasquez K, Aguilar CA, Kumar R, Mascharak S, Longaker MT, Li J, Levi B, Tuning macrophage phenotype to mitigate skeletal muscle fibrosis, J. Immunol 204 (8) (2020) 2203–2215, 10.4049/jimmunol.1900814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Barton ER, DeMeo J, Lei H, The insulin-like growth factor (IGF)-1 E-peptides are required for isoform-specific gene expression and muscle hypertrophy after local IGF-I production (1985), J. Appl. Physiol 108 (5) (2010) 1069–1076, 10.1152/japplphysiol.01308.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Barton-Davis ER, Shoturma DI, Musaro A, Rosenthal N, Sweeney HL, Viral mediated expression of insulin-like growth factor I blocks the aging-related loss of skeletal muscle function, Proc. Natl. Acad. Sci. USA 95 (26) (1998) 15603–15607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Musarò A, McCullagh K, Paul A, Houghton L, Dobrowolny G, Molinaro M, Barton ER, Sweeney HL, Rosenthal N, Localized Igf-1 transgene expression sustains hypertrophy and regeneration in senescent skeletal muscle, Nat. Genet 27 (2) (2001) 195–200, 10.1038/84839. [DOI] [PubMed] [Google Scholar]
- [14].Ohtake Y, Tojo H, Seiki M, Multifunctional roles of MTl-MMP in myofiber formation and morphostatic maintenance of skeletal muscle, J. Cell Sci 119 (Pt 18) (2006) 3822–3832, 10.1242/jcs.03158. [DOI] [PubMed] [Google Scholar]
- [15].Smith LR, Kok HJ, Zhang B, Chung D, Spradlin RA, Rakoczy KD, Lei H, Boesze-Battaglia K, Barton ER, Matrix metalloproteinase 13 from satellite cells is required for efficient muscle growth and regeneration, Cell. Physiol. Biochem.: Int. J. Exp. Cell. Physiol. Biochem. Pharmacol 54 (3) (2020) 333–353, 10.33594/000000223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Snyman C, Niesler CU, MMP-14 in skeletal muscle repair, J. Muscle Res. Cell Motil 36 (3) (2015) 215–225, 10.1007/s10974-015-9414-4. [DOI] [PubMed] [Google Scholar]
- [17].Alameddine HS, Matrix Metalloproteinases in Skeletal Muscles: Friends or Foes? Academic Press, 2012, pp. 508–518. [DOI] [PubMed] [Google Scholar]
- [18].Davis ME, Gumucio JP, Sugg KB, Bedi A, Mendias CL, MMP Inhibition as a Potential Method to Augment the Healing of Skeletal Muscle and Tendon Extracellular Matrix, American Physiological Society, 2013, pp. 884–891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Deryugina EI, Quigley JP, Matrix metalloproteinases and tumor metastasis, Cancer Metastas. Rev 25 (1) (2006) 9–34, 10.1007/s10555-006-7886-9. [DOI] [PubMed] [Google Scholar]
- [20].Vassilakos G, Barton ER, Insulin-like growth factor I regulation and its actions in skeletal muscle, Compr. Physiol 9 (1) (2018) 413–438, 10.1002/cphy.c180010. [DOI] [PubMed] [Google Scholar]
- [21].Barton ER, The ABCs of IGF-I isoforms: impact on muscle hypertrophy and implications for repair, Appl. Physiol. Nutr. Metab 31 (6) (2006) 791–797, 10.1139/h06-054. [DOI] [PubMed] [Google Scholar]
- [22].Duguay SJ, Post-translational processing of insulin-like growth factors, Horm.Metab. Res 31 (2-3) (1999) 43–49, 10.1055/s-2007-978697. [DOI] [PubMed] [Google Scholar]
- [23].Duguay SJ, Lai-Zhang J, Steiner DF, Mutational analysis of the insulin-like growth factor I prohormone processing site, J. Biol. Chem 270 (29) (1995) 17566–17574, 10.1074/jbc.270.29.17566. [DOI] [PubMed] [Google Scholar]
- [24].Durzynska J, Philippou A, Brisson BK, Nguyen-McCarty M, Barton ER, The pro-forms of insulin-like growth factor I (IGF-1) are predominant in skeletal muscle and alter IGF-I receptor activation, Endocrinology 154 (3) (2013) 1215–1224, 10.1210/en.2012-1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Vassilakos G, Lei H, Yang Y, Puglise J, Matheny M, Durzynska J, Ozery M, Bennett K, Spradlin R, Bonanno H, Park S, Ahima RS, Barton ER, Deletion of muscle IGF-I transiently impairs growth and progressively disrupts glucose homeostasis in male mice, FASEB J. 33 (1) (2019) 181–194, 10.1096/fj.201800459R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Hede MS, Salimova E, Piszczek A, Perlas E, Winn N, Nastasi T, Rosenthal N, E-peptides control bioavailability of IGF-1, PLoS One 7 (12) (2012), e51152, 10.1371/joumal.pone.0051152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Bailyes EM, Nave BT, Soos MA, Orr SR, Hayward AC, Siddle K, Insulin receptor/IGF-I receptor hybrids are widely distributed in mammalian tissues: quantification of individual receptor species by selective immunoprecipitation and immunoblotting, Biochem J. 327 (Pt 1) (1997) 209–215, 10.1042/bj3270209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Dong Y, Lakhia R, Thomas SS, Dong Y, Wang XH, Silva KAS, Zhang L, Interactions between p-Akt and Smad3 in injured muscles initiate myogenesis or fibrogenesis, Am. J. Physiol. Endocrinol. Metab 305 (3) (2013) E367–E375, 10.1152/ajpendo.00644.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].O’Neill BT, Lee KY, Klaus K, Softie S, Krumpoch MT, Fentz J, Stanford KI, Robinson MM, Cai W, Kleinridders A, Pereira RO, Hirshman MF, Abel ED,Accili D, Goodyear LJ, Nair KS, Kahn CR, Insulin and IGF-1 receptors regulate FoxO-mediated signaling in muscle proteostasis, J. Clin. Investig 126 (9) (2016) 3433–3446, 10.1172/JCI86522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].O’Neill BT, Lauritzen HPMM, Hirshman MF, Smyth G, Goodyear LJ, Kahn CR, Differential role of insulin/IGF-1 receptor signaling in muscle growth and glucose homeostasis, Cell Rep. 11 (8) (2015) 1220–1235, 10.1016/j.celrep.2015.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Mavalli MD, DiGirolamo DJ, Fan Y, Riddle RC, Campbell KS, van Groen T, Frank SJ, Sperling MA, Esser KA, Bamman MM, Clemens TL, Distinct growth hormone receptor signaling modes regulate skeletal muscle development and insulin sensitivity in mice, J. Clin. Invest 120 (11) (2010) 4007–4020, 10.1172/JCI42447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Vijayakumar A, Buffin NJ, Gallagher EJ, Blank J, Wu Y, Yakar S, LeRoith D, Deletion of growth hormone receptors in postnatal skeletal muscle of male mice does not alter muscle mass and response to pathological injury, Endocrinology 154 (10) (2013) 3776–3783, 10.1210/en.2013-1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Coolican SA, Samuel DS, Ewton DZ, McWade FJ, Florini JR, The mitogenic and myogenic actions of insulin-like growth factors utilize distinct signaling pathways, J. Biol. Chem 272 (10) (1997) 6653–6662, 10.1074/jbc.272.10.6653. [DOI] [PubMed] [Google Scholar]
- [34].Griger J, Schneider R, Lahmann I, Schöwel V, Keller C, Spuler S, Nazare M, Birchmeier C, Loss of Ptpnl l (Shp2) drives satellite cells into quiescence, eLife 6 (2017), 10.7554/eLife.21552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Boyer JG, Prasad V, Song T, Lee D, Fu X, Grimes KM, Sargent MA, Sadayappan S, Molkentin JD, ERK1/2 signaling induces skeletal muscle slow fiber-type switching and reduces muscular dystrophy disease severity, JCI Insight 4 (10) (2019), 10.1172/jci.insight.127356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Yue F, Bi P, Wang C, Li J, Liu X, Kuang S, Conditional loss of pten in myogenic progenitors leads to postnatal skeletal muscle hypertrophy but age-dependent exhaustion of satellite cells, Cell Rep. 17 (9) (2016) 2340–2353, 10.1016/j.celrep.2016.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Hamilton DL, Philp A, MacKenzie MG, Baar K, Limited Role A, A limited role for PI(3,4,5)P3 regulation in controlling skeletal muscle mass in response to resistance exercise, ell624-ell624, PLoS One 5 (7) (2010), ell624, 10.1371/joumal.pone.0011624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Yue F, Bi P, Wang C, Shan T, Nie Y, Ratliff TL, Gavin TP, Kuang S, Pten is necessary for the quiescence and maintenance of adult muscle stem cells, Nat. Commun 8 (1) (2017) 1–13, 10.1038/ncommsl4328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Wang G, Zhu H, Situ C, Han L, Yu Y, Cheung TH, Liu K, Wu Z, pllOα of PI3K is necessary and sufficient for quiescence exit in adult muscle satellite cells, e98239-e98239, EMBO J. 37 (8) (2018), 10.15252/embj.201798239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Zhang P, Liang X, Shan T, Jiang Q, Deng C, Zheng R, Kuang S, mTOR is necessary for proper satellite cell activity and skeletal muscle regeneration, Biochem. Biophys. Res. Commun 463 (2015) 102–108, 10.1016/j.bbrc.2015.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Rion N, Castets P, Lin S, Enderle L, Reinhard JR, Ruegg MA, mTORC2 affects the maintenance of the muscle stem cell pool, 30-30, Skelet. Muscle 9 (1) (2019) 30, 10.1186/s13395-019-0217-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Ganassi M, Badodi S, Wanders K, Zammit PS, Myogenin is an essential regulator of adult myofibre growth and muscle stem cell homeostasis, eLife 9 (2020) 1–23, 10.7554/eLife.60445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Tidball JG, Welc SS, Macrophage-derived IGF-1 is a potent coordinator of myogenesis and inflammation in regenerating muscle, Mo!. Ther. J. Am. Soc.Gene Ther 23 (7) (2015) 1134–1135, 10.1038/mt.2015.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Tonkin J, Temmerman L, Sampson RD, Gallego-Colon E, Barberi L, Bilbao D, Schneider MD, Musarò A, Rosenthal N, Monocyte/macrophage-derived IGF-1 orchestrates murine skeletal muscle regeneration and modulates autocrine polarization, Mol. Ther 23 (7) (2015) 1189–1200, 10.1038/MT.2015.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Hammers DW, Rybalko V, Merscham-Banda M, Hsieh P-L, Suggs LJ, Farrar RP, Anti-inflammatory macrophages improve skeletal muscle recovery from ischemia-reperfusion, J. Appl. Physiol 118 (8) (2015) 1067–1074, 10.1152/japplphysiol.00313.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Rybalko V, Hsieh P-L, Merscham-Banda M, Suggs LJ, Farrar RP, The development of macrophage-mediated cell therapy to improve skeletal muscle function after injury, e0145550-e0145550, PLOS ONE 10 (12) (2015), 0145550, 10.1371/journal.pone.0145550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Grubb A, Joanisse S, Moore DR, Bellamy LM, Mitchell CJ, Phillips SM, Parise G, IGF-1 colocalizes with muscle satellite cells following acute exercise in humans, Appl. Physiol. Nutr. Metab 39 (4) (2014) 514–518, 10.1139/apnm-2013-0430. [DOI] [PubMed] [Google Scholar]
- [48].García-Prat L, Perdiguero E, Alonso S, FoxO maintains a genuine muscle stemcell quiescent state until geriatric age, Nat. Cell Biol 2020 (2020) 1–12, 10.1038/S41556-020-00593-7. [DOI] [PubMed] [Google Scholar]
- [49].Kneppers A, Verdijk L, de Theije C, Corten M, Gielen E, van Loon L, Schols A, Langen R, A novel in vitro model for the assessment of postnatal myonuclear accretion, Skelet. Muscle 8 (1) (2018) 4, 10.1186/s13395-018-0151-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Barton ER, Park S, James JK, Makarewich CA, Philippou A, Eletto D, Lei H,Brisson A, Ostrovsky O, Li Z, Argon Y, Deletion of muscle GRP94 impairs both muscle and body growth by inhibiting local IGF production, FASEB J. 26 (9) (2012) 3691–3702, 10.1096/fj.ll-203026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Pelosi L, Giacinti C, Nardis C, Borsellino G, Rizzuto E, Nicoletti C,Wannenes F, Battistini L, Rosenthal N, Molinaro M, Musar A, Local expression of IGF-1 accelerates muscle regeneration by rapidly modulating inflammatory cytokines and chemokines, FASEB J. 21 (7) (2007) 1393–1402, 10.1096/fj.06-7690com. [DOI] [PubMed] [Google Scholar]
- [52].Park S, Brisson BK, Liu M, Spinazzola JM, Barton ER, Mature IGF-1 excels in promoting functional muscle recovery from disuse atrophy compared with pro-IGF-IA, J. Appl. Physiol 116 (7) (2014) 797–806, 10.1152/japplphysiol.00955.2013 (Bethesda, Md.: 1985). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Joe AWB, Yi L, Natarajan A, Le Grand F, So L, Wang J, Rudnicki MA, Rossi FMV, Muscle injury activates resident fibro/adipogenic progenitors that facilitate myogenesis, Nat. Cell Biol 12 (2) (2010) 153–163, 10.1038/ncb2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Farup J, Madaro L, Puri PL, Mikkelsen UR, Interactions between muscle stem cells, mesenchymal-derived cells and immune cells in muscle homeostasis, regeneration and disease, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Reggio A, Rosina M, Palma A, Cerquone Perpetuini A, Petrilli LL, Gargioli C,Fuoco C, Micarelli E, Giuliani G, Cerretani M, Bresciani A, Sacco F,Castagnoli L, Cesareni G, Adipogenesis of skeletal muscle fibro/adipogenic progenitors is affected by the WNT5a/GSK3/β-catenin axis, Cell Death Differ. 27 (10) (2020) 2921–2941, 10.1038/s41418-020-0551-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Reggio A, Rosina M, Krahmer N, Palma A, Petrilli LL, Maiolatesi G,Massacci G, Salvatori I, Valle C, Testa S, Gargioli C, Fuoco C, Castagnoli L, Cesareni G, Sacco F, Metabolic reprogramming of fibro/adipogenic progenitors facilitates muscle regeneration, Life Sci. Alliance 3 (3) (2020), 10.26508/lsa.202000660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Kostallari E, Baba-Amer Y, Alonso-Marrin S, Ngoh P, Relaix F, Lafuste P, Gherardi RK, Pericytes in the myovascular niche promote post-natal myofiber growth and satellite cell quiescence, Development Camb. 142 (7) (2015) 1242–1253, 10.1242/dev.115386. [DOI] [PubMed] [Google Scholar]
- [58].McKellar DW, Walter LD, Song LT, Mantri M, Wang MFZ, de Vlaminck I, Cosgrove BD, Strength in numbers: Large-scale integration of single-cell transcriptomic data reveals rare, transient muscle progenitor cell states in muscle regeneration, bioRxiv (2020), 2020.12.01.407460-2020.12.01.407460. [Google Scholar]
- [59].Börsch A, Ham DJ, Mittal N, Tintignac LA, Migliavacca E, Feige JN, Riiegg MA, Zavolan M, Molecular and phenotypic analysis of rodent models reveals conserved and species-specific modulators of human sarcopenia, 194-194, Commun. Bioi 4 (1) (2021) 194, 10.1038/s42003-021-01723-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Ascenzi F, Barberi L, Dobrowolny G, Villa Nova Bacurau A, Nicoletti C,Rizzuto E, Rosenthal N, Scicchitano BM, Musarò A, Effects of IGF-1 isoforrns on muscle growth and sarcopenia, Aging Cell 18 (3) (2019) 12954, 10.1111/acel.12954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Matheny RW, Carrigan CT, Abdalla MN, Geddis AV, Leandry LA, Aguilar CA, Hobbs SS, Urso ML, RNA transcript expression of IGF-I/PI3K pathway components in regenerating skeletal muscle is sensitive to initial injury intensity, Growth Horm. IGF Res 32 (2017) 14–21, 10.1016/j.ghir.2016.09.002. [DOI] [PubMed] [Google Scholar]
- [62].Kosaki N, Takaishi H, Kamekura S, Kimura T, Okada Y, Minqi L, Arnizuka N, Chung UI, Nakamura K, Kawaguchi H, Toyama Y, D’Armiento J, Impaired bone fracture healing in matrix metalloproteinase-13 deficient mice, Biochem. Biophys. Res. Commun 354 (4) (2007) 846–851, 10.1016/j.bbrc.2006.12.234. [DOI] [PubMed] [Google Scholar]
- [63].Sternlicht MD, Werb Z, How matrix metalloproteinases regulate cell behavior, Armu Rev. Cell Dev. Bioi 17 (2001) 463–516, 10.1146/annurev.cellbio.17.1.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Gaffney J, Solomonov I, Zehorai E, Sagi I, Multilevel regulation of matrix metalloproteinases in tissue homeostasis indicates their molecular specificity in vivo, Matrix Bioi. 44-46 (2015) 191–199, 10.1016/j.matbio.2015.01.012. [DOI] [PubMed] [Google Scholar]
- [65].Inada M, Wang Y, Byrne MH, Rahman MU, Miyaura C, Lopez-Otin C, Krane SM, Critical roles for collagenase-3 (Mmp13) in development of growth plate cartilage and in endochondral ossification, Proc. Natl. Acad. Sci. USA 101 (49) (2004) 17192–17197, 10.1073/pnas.0407788101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Gill SE, Parks WC, Metalloproteinases and their inhibitors: regulators of wound healing, Int. J. Biochem Cell Biol 40 (6-7) (2008) 1334–1347, 10.1016/j.biocel.2007.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Laronha H, Caldeira J, Structure and function of human matrix metalloproteinases, Cells 9 (5) (2020), 10.3390/cells9051076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Nagase H, Woessner JF Jr., Matrix metalloproteinases, J. Bioi. Chern 274 (31) (1999) 21491–21494, 10.1074/jbc.274.31.21491. [DOI] [PubMed] [Google Scholar]
- [69].Arnold LH, Butt LE, Prior SH, Read CM, Fields GB, Pickford AR, The interface between catalytic and hemopexin domains in matrix metalloproteinase-1 conceals a collagen binding exosite, J. Bioi. Chern 286 (52) (2011) 45073–45082, 10.1074/jbc.M111.285213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Massova I, Kotra LP, Fridman R, Mobashery S, Matrix metalloproteinases: structures, evolution, and diversification, FASEB J. 12 (12) (1998) 1075–1095. [PubMed] [Google Scholar]
- [71].Gomis-Ruth FX, Maskos K, Betz M, Bergner A, Huber R, Suzuki K, Yoshida N, Nagase H, Brew K, Bourenkov GP, Bartunik H, Bode W, Mechanism of inhibition of the human matrix metalloproteinase stromelysin-1 by TIMP-1, Nature 389 (6646) (1997) 77–81, 10.1038/37995. [DOI] [PubMed] [Google Scholar]
- [72].Chen X, Li Y, Role of matrix metalloproteinases in skeletal muscle, Cell Adhes. Migr 3 (4) (2009) 337–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Bellayr I, Holden K, Mu X, Pan H, Li Y, Matrix metalloproteinase inhibition negatively affects muscle stem cell behavior, Int J. Clin. Exp. Pathol 6 (2) (2013) 124–141. [PMC free article] [PubMed] [Google Scholar]
- [74].Holmbeck K, Bianco P, Caterina J, Yamada S, Kromer M, Kuznetsov SA, Mankani M, Robey PG, Poole AR, Pidoux I, Ward JM, Birkedai-Hansen H, MTl-MMP-deficient mice develop dwarfism, osteopenia, arthritis, and connective tissue disease due to inadequate collagen turnover, Cell 99 (1) (1999) 81–92, 10.1016/s0092-8674(00)80064-1. [DOI] [PubMed] [Google Scholar]
- [75].Mackey AL, Heinemeier KM, Koskinen SO, Kjaer M, Dynamic adaptation of tendon and muscle connective tissue to mechanical loading, Connect Tissue Res. 49 (3) (2008) 165–168, 10.1080/03008200802151672. [DOI] [PubMed] [Google Scholar]
- [76].Zhang Q, Joshi SK, Lovett DH, Zhang B, Bodine S, Kim H, Liu X, Matrix metalloproteinase-2 plays a critical role in overload induced skeletal muscle hypertrophy, Muscles Ligaments Tendons J. 4 (3) (2014) 362–370. [PMC free article] [PubMed] [Google Scholar]
- [77].Hadler-Olsen E, Solli AI, Hafstad A, Winberg JO, Uhlin-Hansen L, Intracellular MMP-2 activity in skeletal muscle is associated with type II fibers, J. Cell Physiol 230 (1) (2015) 160–169, 10.1002/jcp.24694. [DOI] [PubMed] [Google Scholar]
- [78].Sun S, Henriksen K, Karsdal MA, Armbrecht G, Belavy DL, Felsenberg D, Rittweger J, Wang Y, Zheng Q, Nedergaard AF, Measurement of a MMP-2 degraded Titin fragment in serum reflects changes in muscle turnover induced by atrophy, Exp. Gerontal 58 (2014) 83–89, 10.1016/j.exger.2014.07.016. [DOI] [PubMed] [Google Scholar]
- [79].Shiba N, Miyazaki D, Yoshizawa T, Fukushima K, Shiba Y, Inaba Y, Imamura M, Takeda S, Koike K, Nakamura A, Differential roles of MMP-9 in early and late stages of dystrophic muscles in a mouse model of Duchenne muscular dystrophy, Biochim. Biophys. Acta 1852 (10 Pt A) (2015) 2170–2182, 10.1016/j.bbadis.2015.07.008. [DOI] [PubMed] [Google Scholar]
- [80].Ogura Y, Tajrishi MM, Sato S, Hindi SM, Kumar A, Therapeutic potential of matrix metalloproteinases in Duchenne muscular dystrophy, Front Cell Dev. Bioi 2 (2014) 11, 10.3389/fcell.2014.00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Hindi SM, Shin J, Ogura Y, Li H, Kumar A, Matrix metalloproteinase-9 inhibition improves proliferation and engraftroent of myogenic cells in dystrophic muscle of mdx mice, PLoS One 8 (8) (2013) 72121, 10.1371/joumal.pone.0072121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Li H, Mittal A, Makonchuk DY, Bhatnagar S, Kumar A, Matrix metalloproteinase-9 inhibition ameliorates pathogenesis and improves skeletal muscle regeneration in muscular dystrophy, Hum. Mol. Genet 18 (14) (2009) 2584–2598, 10.1093/hmg/ddpl91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Dahiya S, Bhatnagar S, Hindi SM, Jiang C, Paul PK, Kuang S, Kumar A, Elevated levels of active matrix metalloproteinase-9 cause hypertrophy in skeletal muscle of normal and dystrophin-deficient mdx mice, Hum. Mol. Genet 20 (22) (2011) 4345–4359, 10.1093/hmg/ddr362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Murach KA, Vechetti IJ, Van Pelt DW, Crow SE, Dungan CM, Figueiredo VC, Kosmac K, Fu X, Richards CI, Fry CS, McCarthy JJ, Peterson CA, Fusion-independent satellite cell communication to muscle fibers during load-induced hypertrophy, 9-9, Funct. (Oxf., Engl. ) 1 (1) (2020) 009, 10.1093/function/zqaa009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Leeman MF, Curran S, Murray GI, The structure, regulation, and function of human matrix metalloproteinase-13, Grit. Rev. Biochem Mol. Bioi 37 (3) (2002) 149–166, 10.1080/10409230290771483. [DOI] [PubMed] [Google Scholar]
- [86].Lei H, Leong D, Smith LR, Barton ER, Matrix metalloproteinase 13 is a new contributor to skeletal muscle regeneration and critical for myoblast migration, Am. J. Physiol. Cell Physiol 305 (5) (2013) C529–C538, 10.1152/ajpcell.00051.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Wu N, Opalenik S, Liu J, Jansen ED, Giro MG, Davidson JM, Real-time visualization of MMP-13 promoter activity in transgenic mice, Matrix Bioi. 21 (2) (2002) 149–161, 10.1016/s0945-053x(01)00192-5. [DOI] [PubMed] [Google Scholar]
- [88].Freije JM, Diez-Itza I, Balbin M, Sanchez LM, Blasco R, Tolivia J, Lopez-Otin C, Molecular cloning and expression of collagenase-3, a novel human matrix metalloproteinase produced by breast carcinomas, J. Bioi. Chern 269 (24) (1994) 16766–16773. [PubMed] [Google Scholar]
- [89].Souza LB, Maziero C, Lazzarin MC, Quintana HT, Tome TC, Baptista VIA,de Oliveira A, Presence of metalloproteinases 2 and 9 and 8-OHdG in the fibrotic process in skeletal muscle of Mdx mice, Acta Histochem. 122 (1) (2020), 151458, 10.1016/j.acthis.2019.151458. [DOI] [PubMed] [Google Scholar]
- [90].Alameddine HS, Morgan JE, Matrix metalloproteinases and tissue inhibitor of metalloproteinases in inflammation and fibrosis of skeletal muscles, J. Neuromuscul. Dis 3 (2016) 455–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Bedair H, Liu TT, Kaar JL, Badlani S, Russell AJ, Li Y, Huard J, Matrix metalloproteinase-1 therapy improves muscle healing (1985), J. Appl. Physiol 102 (6) (2007) 2338–2345, 10.1152/japplphysiol.00670.2006. [DOI] [PubMed] [Google Scholar]
- [92].Pan H, Vojnits K, Liu TT, Meng F, Yang L, Wang Y, Huard J, Cox CS, Lally KP, Li Y, MMPl gene expression enhances myoblast migration and engraftrnent following implanting into mdx/SCID mice, Cell Adhes. Migr 9 (4) (2015) 283–292, 10.4161/19336918.2014.983799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Hasty KA, Pourmotabbed TF, Goldberg GI, Thompson JP, Spinella DG, Stevens RM, Mainardi CL, Human neutrophil collagenase. A distinct gene product with homology to other matrix metalloproteinases, J. Bioi. Chem 265 (20) (1990)11421–11424. [PubMed] [Google Scholar]
- [94].Herman MP, Sukhova GK, Libby P, Gerdes N, Tang N, Horton DB, Kilbride M, Breitbart RE, Chun M, Schonbeck U, Expression of neutrophil collagenase (matrix metalloproteinase-8) in human atheroma: a novel collagenolytic pathway suggested by transcriptional profiling, Circulation 104 (16) (2001) 1899–1904, 10.1161/hc4101.097419. [DOI] [PubMed] [Google Scholar]
- [95].Hasty KA, Jeffrey JJ, Hibbs MS, Welgus HG, The collagen substrate specificity of human neutrophil collagenase, J. Bioi. Chem 262 (21) (1987) 10048–10052. [PubMed] [Google Scholar]
- [96].Deshmukh AS, Murgia M, Nagaraj N, Treebak JT, Cox J, Mann M, Deep proteomics of mouse skeletal muscle enables quantitation of protein isoforms, metabolic pathways, and transcription factors, Mol. Cell Proteom 14 (4) (2015) 841–853, 10.1074/mcp.Mll4.044222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Lund DK, Mouly V, Comelison DD, MMP-14 is necessary but not sufficient for invasion of three-dimensional collagen by human muscle satellite cells, Am. J. Physiol. Cell Physiol 307 (2) (2014) C140–C149, 10.1152/ajpcell.00032.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].L Jones J, Gockerman A, Busby WH Jr., Camacho-Hubner C, Clemmons DR, Extracellular matrix contains insulin-like growth factor binding protein-5: potentiation of the effects of IGF-1, J. Cell Bioi 121 (3) (1993) 679–687, 10.1083/jcb.121.3.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Beattie J, Kreiner M, Allan GJ, Flint DJ, Domingues D, van der Walle CF, IGFBP-3 and IGFBP-5 associate with the cell binding domain (CBD) of fibronectin, Biochem. Biophys. Res. Commun 381 (4) (2009) 572–576, 10.1016/j.bbrc.2009.02.088. [DOI] [PubMed] [Google Scholar]
- [100].Fowlkes JL, Thrailkill KM, Serra DM, Suzuki K, Nagase H, Matrix metalloproteinases as insulin-like growth factor binding protein-degrading proteinases, Prog. Growth Factor Res 6 (2-4) (1995) 255–263, 10.1016/0955-2235(95)00017-8. [DOI] [PubMed] [Google Scholar]
- [101].Prudova A, auf dem Keller U, Butler GS, Overall CM, Multiplex N-terminome analysis of MMP-2 and MMP-9 substrate degradomes by iTRAQ-TAILS quantitative proteomics, Mol. Cell Proteom 9 (5) (2010) 894–911, 10.1074/mcp.M000050-MCP201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Zhou R, Diehl D, Hoeflich A, Lahm H, Wolf E, IGF-binding protein-4: biochemical characteristics and functional consequences, J. Endocrinol 178 (2) (2003) 177–193, 10.1677/joe.0.1780177. [DOI] [PubMed] [Google Scholar]
- [103].Han JK, Kim HL, Jeon KH, Choi YE, Lee HS, Kwon YW, Jang JJ, Cho HJ,Kang FJ, Oh BH, Park YB, Kim HS, Peroxisome proliferator-activated receptor-delta activates endothelial progenitor cells to induce angio-myogenesis through matrix metallo-proteinase-9-mediated insulin-like growth factor-1 paracrine networks, Eur. Heart J 34 (23) (2013) 1755–1765, 10.1093/eurheartj/ehr365. [DOI] [PubMed] [Google Scholar]
- [104].Risinger GM Jr., Hunt TS, Updike DL, Bullen EC, Howard EW, Matrix metalloproteinase-2 expression by vascular smooth muscle cells is mediated by both stimulatory and inhibitory signals in response to growth factors, J. Bioi. Chem 281 (36) (2006) 25915–25925, 10.1074/jbc.M513513200. [DOI] [PubMed] [Google Scholar]
- [105].Gallego-Colon E, Sampson RD, Sattler S, Schneider MD, Rosenthal N, Tonkin J, Cardiac-restricted IGF-lEa overexpression reduces the early accumulation of inflammatory myeloid cells and mediates expression of extracellular matrix remodelling genes after myocardial infarction, Mediat. lnflamm 2015 (2015), 484357, 10.1155/2015/484357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Alcazar CA, Hu C, Rando TA, Huang NF, Nakayama KH, Transplantation of insulin-like growth factor-1 laden scaffolds combined with exercise promotes neuroregeneration and angiogenesis in a preclinical muscle injury model, Biomater. Sci 8 (19) (2020) 5376–5389, 10.1039/d0bm00990c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Lee H, Ju YM, Kim I, Elsangeedy E, Lee JH, Yoo JJ, Atala A, Lee SJ, A novel decellularized skeletal muscle-derived ECM scaffolding system for in situ muscle regeneration, Methods 171 (2020) 77–85, 10.1016/j.ymeth.2019.06.027. [DOI] [PubMed] [Google Scholar]
- [108].Fields GB, The rebirth of matrix metalloproteinase inhibitors: moving beyond the dogma, Cells 8 (9) (2019), 10.3390/cells8090984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Miyazaki D, Nakamura A, Fukushima K, Yoshida K, Takeda S, Ikeda S, Matrix metalloproteinase-2 ablation in dystrophin-deficient mdx muscles reduces angiogenesis resulting in impaired growth of regenerated muscle fibers, Hum. Mol. Genet 20 (9) (2011) 1787–1799, 10.1093/hmg/ddr062. [DOI] [PubMed] [Google Scholar]