Abstract
Background
Caregivers and family members of Intensive Care Unit (ICU) survivors can face emotional problems following patient discharge from hospital. We aimed to evaluate the impact of a multi-centre integrated health and social care intervention, on caregiver and family member outcomes.
Methods
This study evaluated the impact of the Intensive Care Syndrome: Promoting Independence and Return to Employment (InS:PIRE) programme across 9 sites in Scotland. InS:PIRE is an integrated health and social care intervention. We compared caregivers who attended this programme with a contemporary control group of ICU caregivers (usual care cohort), who did not attend.
Results
The primary outcome was anxiety measured via the Hospital Anxiety and Depression Scale at 12 months post-hospital discharge. Secondary outcome measures included depression, carer strain and clinical insomnia. A total of 170 caregivers had data available at 12 months for inclusion in this study; 81 caregivers attended the InS:PIRE intervention and completed outcome measures at 12 months post-hospital discharge. In the usual care cohort of caregivers, 89 completed measures. The two cohorts had similar baseline demographics. After adjustment, those caregivers who attended InS:PIRE demonstrated a significant improvement in symptoms of anxiety (OR: 0.42, 95% CI: 0.20–0.89, p = 0.02), carer strain (OR: 0.39; 95% CI: 0.16–0.98 p = 0.04) and clinical insomnia (OR: 0.40; 95% CI: 0.17–0.77 p < 0.001). There was no significant difference in symptoms of depression at 12 months.
Conclusions
This multicentre evaluation has shown that caregivers who attended an integrated health and social care intervention reported improved emotional health and less symptoms of insomnia, 12 months after the delivery of the intervention.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13054-022-04014-z.
Keywords: Critical care, Rehabilitation, Family, Caregivers and mental health
Background
The profile of Intensive Care Unit (ICU) survivorship has been brought into sharp focus due to the COVID-19 pandemic [1, 2]. Following a critical illness, patients can experience clinically important physical, emotional and cognitive symptoms [3–5]. These problems can have major implications for the patient and healthcare system [6, 7].
Less focus has been placed on family members and caregivers of ICU survivors. This vulnerable group often have a challenging trajectory during the critical care recovery period and are known to experience emotional and social problems [8–10]. For example, recent evidence has shown that over two thirds of caregivers may experience symptoms of depression, up to 80% can experience symptoms of anxiety and more than half can experience caregiver strain in the months following critical care discharge [11–13].
To address this pressing issue, we designed a multi-centre health and social care intervention to support caregivers recovery following critical care discharge. Specifically, we sought to understand what impact this intervention had on emotional outcomes and social disruption for the caregiver.
Methods
Study design
Using a multicentre cohort design, we compared caregivers who attended an integrated health and social care intervention aimed at improving outcomes for ICU survivors, with a contemporary, usual care cohort (caregivers who had been exposed to critical care and were currently caring for a critical care survivor) who did not attend the programme. Previous work has described the outcomes of patients who participated in this intervention, alongside a detailed process evaluation [14].
A caregiver was defined as the individual who provided most of the financial, emotional and physical support for the patient, or the individual primarily responsible for caring for the patient on an unpaid basis [15].
We report a cohort study, as per the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines [16].
Approval
Ethical approval was granted by the Northwest (Liverpool Central) Research Ethics Committee (reference number: 17/NM/0199). All participants provided written consent.
Intervention
The intervention is described in accordance with the Template for intervention Description and Replication checklist [17] (Additional file 1: S1).
The Intensive Care Syndrome: Promoting Independence and Return to Employment (InS:PIRE) programme is a complex intervention and has been described previously [18–20]. InS:PIRE is an integrated health and social care intervention aimed at improving outcomes for ICU survivors and their caregivers in the year following hospital discharge.
Briefly, all patients receive individual reviews with different members of the multidisciplinary team (MDT) including: an ICU nurse and doctor; pharmacist; and physiotherapist. These specialists offer a debrief of the ICU stay, an assessment of ongoing problems, goal setting and patient-directed management plans. Clinical Psychology services, alongside occupational therapy, was also available. Peer support was embedded throughout InS:PIRE through the use shared waiting areas and group sessions. Patient and caregiver volunteers further along the recovery trajectory also attended the programme and provided peer support [21].
Several group sessions were set up for caregivers, including the clinical psychological support. Every site involved local community organisations who provided welfare advice including information on relevant government benefits and housing. This support was delivered through a combination of individual appointments, drop-in sessions or group discussions. Support for caregivers from local agencies was also integrated at every site. In contrast with other ICU recovery services which often include a single clinical appointment, InS:PIRE was designed as a recovery programme; patients and caregivers attend each week for five weeks and are followed up for 1 year after initial attendance. During the intervention period, the InS:PIRE service took place in the hospital setting.
InS:PIRE was co-designed with local patients and caregivers. During this process, patients and caregivers both described the necessity for a specific and deliberate focus on caregivers within any rehabilitation service. This is also consistent with previous evidence is this field [22]. Initial single-centre work showed that supporting caregivers was feasible, safe and acceptable; thus, this support was included in this multi-centre scale up [12, 23]. Caregivers could attend all sessions and were eligible to receive the same support as patients. This only differed with the physiotherapy and pharmacy sessions which were aimed specifically at patient recovery.
Participants
Participants were invited to the programme between 4 and 12 weeks after hospital discharge. Patient inclusion criteria were any patient receiving level three care (multiple organ support and / or invasive respiratory support) or more than seven days of level two care (single organ support or postoperative care). There was no upper age limit for inclusion. Exclusion criteria were any patient who was terminally ill, had suffered a traumatic brain injury or was an in-patient under psychiatric services. Caregiver inclusion criteria were: paired consented patient data had to be available and caregiving responsibilities occurred in an informal, non-paid basis. We limited inclusion in the research to one caregiver per patient; however, multiple caregivers could attend InS:PIRE.
Intervention cohort
Five sites provided the InS:PIRE programme as part of a quality improvement collaborative (intervention cohort) over 2 years. Caregivers in the intervention cohort were consecutively recruited to this study between May 2016 and October 2018 (follow-up completed December 2019). Caregiver outcome measures were completed via a pre-planned 12-month follow-up appointment. Participants were given the opportunity to complete questionnaires at InS:PIRE attendance or via telephone. Fidelity of the intervention was assessed by the number of patients who completed the three ‘core’ interventions: medical/nursing consultation; pharmacy; and physiotherapy review. As such, only the caregivers of patients who completed these core interventions were included in this final analysis.
Usual care cohort
We recruited a contemporary usual care cohort from sites who had no active follow-up or rehabilitation for ICU survivors. Patients in the usual care cohort were recruited from eight hospitals in Scotland between 10 and 16 months post-hospital discharge. Patients were identified by searching the local electronic records system and were recruited by postal survey. Questionnaire packs were sent to eligible patients with pre-paid return envelopes. Alongside these patient packs, we asked the patient’s primary caregiver (or closest family member) to complete a separate pack. Reminder questionnaire packs were sent if the pack was not returned after one month. Participants were also given opportunity to call to discuss issues or recruitment with researchers. The usual care cohort was recruited between July 2017 and March 2020. Ethical approval was in place to continue beyond March 2020; however, the impact of the coronavirus disease 2019 pandemic was unknown; thus, recruitment to this study was closed in an attempt to reduce any confounding effect.
Data collection
We created a short questionnaire in order to collect family member demographics. Data collected included age, relationship to patient and gender.
In-hospital patient demographic and clinical data were obtained from clinical notes and discharge summaries. Patient comorbidity data (including mental health data) were obtained from medical notes and critical care admission records. Critical care length of stay was taken for the highest level of care and during the first critical care admission only.
The Scottish Index of Multiple Deprivation (SIMD) is produced by the Scottish Government as a measure of deprivation, with postcode areas defining data on socio-economic status. This research split the SIMD into five categories to define socio-economic status; quintile one represented the most deprived and quintile five the least [24].
Outcome measures
Hospital Anxiety and Depression Scale
The primary outcome measure in this study was caregiver anxiety. Anxiety and depression were measured using the Hospital Anxiety and Depression Score (HADS) [25]. The HADS questionnaire contains 14 statements relating to mood, with seven statements relating to depression and seven to anxiety. Each statement has four potential options (scored 0–3). The cutoff points widely adopted for HADS are described in Additional file 2: S2. In this analysis, we classified anxiety and depression as a score of 8 or above utilising the score subscales [25]. Appropriate licensing requirements were in place for the use of the HADS.
Carer Strain Index
Alongside the HADS, the Carer Strain Index (CSI) was utilised [26]. The CSI measures strain, including social strain, related to care provision from the caregiver’s perspective. There are elements related to emotional adjustment, social issues, physical and financial strain. Each question is given one point; a score of seven or greater is the generally accepted cut off point for a high level of strain.
The Insomnia Severity Index
The Insomnia Severity Index (ISI) is a seven-item questionnaire which has been validated as a screening tool for clinical insomnia. Participants are asked to rank the severity of their sleep problems on a scale of zero to four and answer four other questions regarding satisfaction with their sleeping patterns [27]. We defined insomnia as a score of 8 and greater from a maximum score of 28 (range 0 to 28).
Statistical analysis
Analyses were undertaken using R (Version 4.0.3). All missing covariates were imputed using categorical and regression trees analysis with the Multivariate Imputation by Chained Equations (MICE) software package. We used 5 imputations and 10 iterations.
Continuous variables were expressed as medians and interquartile range (IQR). The Kruskall–Wallis test was used to compare different sub-groups and the Chi-squared test to analyse categorical variables. Logistic regression was used to understand the impact of the intervention on the main outcomes of this study.
Models were created using domain knowledge and previous evidence in the field. All models were adjusted for: relationship with the patient; caregiver age; caregiver gender; time to follow-up; and socio-economic status (SIMD quintiles). We also adjusted patient level demographics which are known to influence recovery including: hospital length of stay; patient age; and the presence of a mental health comorbidity (pre-existing).
Results
Demographics
Two hundred and six patients attended InS:PIRE across the five participating sites and consented to participation in the study. Of these patients, 136 attended with caregivers who consented to participation in the study at baseline (initial InS:PIRE attendance). Eighty-one (60%) of these caregivers completed outcomes measures at 1 year and were eligible for inclusion in this analysis (intervention cohort).
In the usual care cohort, 452 patients were sent questionnaire packs across the four usual care cohort sites; 115 patients returned the packs for analysis in the study. Of these, 89 (77%) paired caregivers also completed outcome measures at 1 year and were included in the caregiver usual care cohort.
The caregiver intervention and usual care cohort was similar in all baseline demographics. Both cohorts were predominantly female (intervention: 49 (60.5%) vs. usual care: 57 (64%)) and had similar age profiles (intervention:58 (IQR: 48.0–66.3) vs. usual care 58 (IQR: 47.7–69.0) years (Table 1). There was also a similar spread across the socio-economic gradient in both groups. Patient and caregiver demographics are shown in Table 1.
Table 1.
Caregiver demographics, alongside paired patient demographics
| Usual care cohort (n = 89) | Intervention cohort (n = 81) | p value | |
|---|---|---|---|
| Caregiver demographics | |||
| Relationship with patient, n (%) | 0.47 | ||
| Partner or spouse | 66 (74.2) | 54 (66.6) | |
| Child or grandchild | 16 (18) | 13 (16.1) | |
| Parent | 3 (3.3) | 8 (9.9) | |
| Other | 4 (4.5) | 6 (7.4) | |
| Age, years, median (IQR) | 58.0 (47.7–69.0) | 58.0 (48.0–66.3) | 0.88 |
| Gender, male, n (%) | 32 (36.0) | 30 (37.0) | 0.79 |
| Socio-economic status | 0.50 | ||
| SIMD 1 (most deprived) | 25 (28.1) | 20 (24.7) | |
| SIMD 2 | 19 (21.3) | 19 (23.4) | |
| SIMD 3 | 12 (13.5) | 16 (19.8) | |
| SIMD 4 | 12 (13.5) | 7 (8.6) | |
| SIMD 5 (least deprived) | 19 (21.3) | 11 (13.6) | |
| Patient demographics | |||
| Age at ICU admission, median (IQR) | 63.9 (49.7–71.4) | 58.7 (51.1–67.7) | 0.16 |
| Gender, male, number (%) | 50 (56.2) | 41 (50.6) | 0.33 |
| ICU LOS, days, median (IQR) | 4.75 (2.4–9.6) | 11.3 (7.0–19.7) | < 0.01 |
| Hospital LOS, days, median (IQR) | 19.0 (11.2–33.5) | 32.0 (17.0–51.7) | < 0.01 |
| APACHE II, median (IQR) | 19.0 (14.9–25.0) | 19.0 (15.0–26.0) | 0.49 |
| Mental health issues pre-ICU, n (%) | 20 (22.5) | 21 (25.9) | 0.72 |
| Ventilation required, n (%) | 76 (85.4) | 77 (95.1) | 0.12 |
| Two or more comorbidities, n (%) | 42 (47.2) | 34 (42) | 0.34 |
| Surgical admission | 42 (47.2) | 31 (38.3) | 0.17 |
| Time to follow-up, median months (IQR) | 15.0 (13.1–16.5) | 16.0 (14.8–17.5) | < 0.01 |
Missing data information available in Additional file 2: S2
IQR interquartile range, SIMD Scottish Index of Multiple Deprivation, ICU Intensive Care Unit, LOS length of stay, APACHE II Acute Physiology and Chronic Health Evaluation
The dataset was 96.2% complete; a breakdown of the missing variables is shown in Table 2.
Table 2.
Missing values per variable for caregivers (intervention and usual care)
| Variable | Missingness, N (170) (%) |
|---|---|
| Caregiver demographics | |
| Caregiver relationship with patient | 2 (1.2) |
| Caregiver age | 24 (14.1) |
| Caregiver gender | 2 (1.2) |
| Caregiver SIMD | 10 (5.9) |
| Caregiver time to follow-up | 6 (3.5) |
| Hospital anxiety and depression scale (HADS) questions | |
| I feel tense or wound up | 6 (3.5) |
| I still enjoy the things I used to enjoy | 4 (2.4) |
| I get a sort of frightened feeling as if something awful is about to happen | 4 (2.4) |
| I can laugh and see the funny side of things | 3 (1.8) |
| Worrying thoughts go through my mind | 4 (2.4) |
| I feel cheerful | 3 (1.8) |
| I can sit at ease and feel relaxed | 4 (2.4) |
| I feel as if I am slowed down | 4 (2.4) |
| I get a sort of frightened feeling like “butterflies” in the stomach | 5 (2.9) |
| I have lost interest in my appearance | 5 (2.9) |
| I feel restless as if I have to be on the move | 7 (4.1) |
| I look forward with enjoyment to things | 6 (3.5) |
| I get sudden feelings of panic | 5 (2.9) |
| I can enjoy a good book or radio or television programme | 8 (4.7) |
| Carer strain index questions | |
| Sleep is disturbed | 5 (2.9) |
| It is inconvenient | 6 (3.5) |
| It is a physical strain | 7 (4.1) |
| It is confining | 7 (4.1) |
| There have been family adjustments | 5 (2.9) |
| There have been changes in personal plan | 5 (2.9) |
| There have been emotional adjustments | 7 (4.1) |
| Some behaviour is upsetting | 5 (2.9) |
| It is upsetting to find has changed so much from his or her former self | 10 (5.9) |
| There have been work adjustments | 7 (4.1) |
| It is a financial strain | 5 (2.9) |
| Feeling completely overwhelmed | 5 (2.9) |
| There have been other demands on my time | 5 (2.9) |
| Insomnia severity index questions | |
| Difficulty falling asleep | 19 (11.2) |
| Difficulty staying asleep | 20 (11.8) |
| Problem wakening up too early | 31 (18.2) |
| How many nights per week were you bothered by problems sleeping | 4 (2.4) |
| How satisfied or dissatisfied are you with your current sleep pattern | 4 (2.4) |
| To what extent do you consider your sleep problem to interfere with your daily functioning | 3 (1.8) |
| How noticeable to others do you think your sleeping problem is in terms of impairing the quality of your life | 3 (1.8) |
| How worried or distressed are you about your current sleep problem | 3 (1.8) |
SIMD Scottish Index of Multiple Deprivation, ICU Intensive Care Unit, APACHE Acute Physiology and Chronic Health Evaluation
Outcomes
Anxiety and depression
After adjustment, there was a 58% adjusted odds reduction in symptoms of anxiety (measured as a score of 8 or greater on the HADS anxiety scale) in those caregivers who received the InS:PIRE intervention (OR: 0.42, 95% CI: 0.20–0.89, p = 0.02) (Table 3). The number of caregivers who had depression (classified as a score of 8 or greater on the HADS depression scale) was 27% in the usual care cohort versus 22% in the intervention cohort; however, the odds of depressive symptoms at 12 months was not significantly different in those who received the InS:PIRE intervention, (OR: 0.58, 95% CI: 0.26–1.31, p = 0.19) (Table 3).
Table 3.
Summary of the effect of InS:PIRE on study outcomes at 12 months estimated via logistic regression models
| Outcome measure | Adjusted estimate | p value | 95% confidence interval |
|---|---|---|---|
| HADS anxiety | 0.42 | 0.02 | 0.20–0.89 |
| HADS depression | 0.58 | 0.19 | 0.26–1.31 |
| Carer strain | 0.39 | 0.04 | 0.16–0.98 |
| Clinical insomnia | 0.36 | < 0.001 | 0.17–0.77 |
Statistical Signifance was defined as p < 0.05
Carer strain
After adjustment, there was a significant, 61% reduction in the odds of carer strain in those who received the InS:PIRE intervention (OR: 0.39; 95% CI: 0.16–0.98 p = 0.04). The rate of strain (defined as a score equal to or greater than 7) was 30% in the usual care cohort compared to a rate of 19% in the intervention group. There were no significant differences between individual components of the CSI. Considering both groups together, 25% of caregivers indicated that there had been work adjustments during the recovery period and over one quarter (26%) described financial strain.
Insomnia
After adjustment, there was a significant reduction in the odds of clinically important Insomnia, measured via the ISI in those caregivers who received the InS:PIRE intervention by 65% (OR: 0.36; 95% CI: 0.17–0.77 p < 0.001) (Table 3). The incidence of insomnia (defined as an ISI score above 7) was 61% in the usual care cohort, and 38% in the intervention group.
A visual representation of the estimated impact of the InS:PIRE programme on caregiver outcomes at 12 months is shown in Fig. 1. A full description of all outcome model estimates is provided in Additional file 3: S3.
Fig. 1.
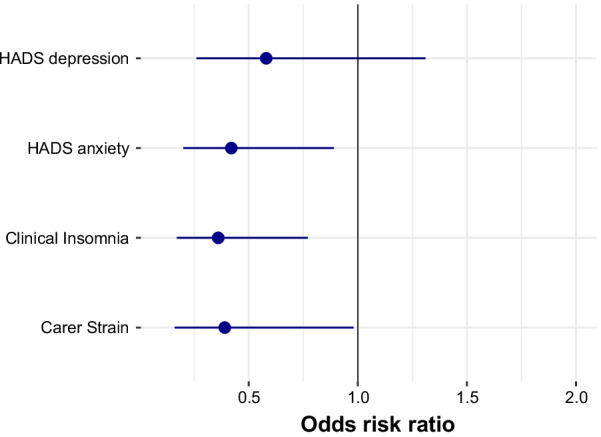
Coefficients estimates demonstrating the impact of the InS:PIRE programme on caregiver outcomes at 1 year. Odds ratio of risk of screening positive for each outcome measure at 1 year in the InS:PIRE intervention cohort compared to the usual care cohort. Estimate with 95% confidence interval. Anxiety and depression: measured by Hospital Anxiety and Depression Scale (HADS), positive screening if score ≥ 8/21 for each component score; Carer strain: measured by Carer Strain Index (CSI) with positive screening defined as a score of ≥ 7/13; Insomnia: measured using the Insomnia Severity Index (ISI) with positive screening defined as a score of ≥ 8/28
Discussion
This evaluation of a multicentre integrated health and social care intervention demonstrated a positive impact on the emotional health of ICU survivor caregivers at 12 months. As far as we can establish, this is the first multicentre intervention internationally to report benefit for caregivers during the post-ICU recovery phase.
The InS:PIRE intervention was designed as a peer supported, multi-disciplinary programme for both patients and caregivers [19]. Previous research has described multiple benefits of peer support for both patients and caregivers in the post-ICU setting such as reduced anxiety, improved external validation of progress and support with expectation management [28, 29]. A further mechanism which we hypothesise has driven improvements, is the intentional focus on caregiver outcomes, rather than caregiver outcomes being viewed as a biproduct of patient treatment. Intentional use of separate caregiver education sessions alongside the integration of local community services which focused on caregiver needs was available at every site. However, it is important to recognise that targeted treatments for both patients and caregivers are likely to have had a bidirectional effect across the family unit. That is, a patient knowing a caregiver is receiving the support they require will potentially have a positive effect on their recovery, and likewise a caregiver seeing the patient receiving the necessary support to recover will likely reduce anxiety and mental health problems.
Carer strain also significantly reduced for those caregivers who attended the programme. The Carer Strain Index is a tool which examines all the global aspects of caring responsibilities (Additional file 1: S1). This includes domains such as return to employment, physical and financial strain [26]. Caregivers had access to social, financial and welfare advice for the duration of the programme; this may well have been the driver for the reduction in carer strain seen. This finding is consistent with work from primary care in the UK; improving the social and financial situation of people, by co-locating social and welfare services in health centres resulted in improvements in mental health [30]. Moreover, recent evidence has demonstrated a direct correlation between emotional and social outcomes in ICU survivors [31]. Future interventions aimed at addressing outcomes in this group must ensure that all aspects of an individual’s wellbeing are considered.
Although there were less symptoms of depression in the InS:PIRE cohort, the intervention did not significantly improve symptoms of depression for caregivers at 12 months. Previous literature has demonstrated that symptoms such as depression improve over the first year of recovery for most critical care survivors [32]. We hypothesise that caregivers have a different recovery trajectory to patients and thus may require targeted interventions at different time periods. There is limited evidence describing the trajectories of symptoms beyond 2 months for caregivers following exposure to critical care illness [33]. Given the large volume of informal, unfunded care provided by this caregiver group in the months following hospital discharge, it is crucial that health services offer adequate support to this cohort. More research is urgently required in this area, in order to fully understand how best to support this vulnerable group, in relation to symptoms of depression.
Limitations
Study limitations are notable. Firstly, we do not know if the caregivers included in this study had pre-existing mental health problems. As such, we are unable to account for an important baseline characteristic in our analysis. Secondly, this was not a randomised control trial. While there was substantial overlap in baseline characteristics between the intervention and usual care cohorts, they were not randomly assigned to either usual care or the intervention; as such, this limits the ability to draw casual inference from the results. Moreover, we experienced attrition over the course of the 12 months, common with other studies in the field, which may have also influenced the reported outcomes [34]. We also do not have data on caregivers who attended the InS:PIRE intervention but did not consent to recruitment in this study. Their experience of the programme may be distinctly different from those represented in this analysis.
It could be argued that the patients and caregivers who attended the InS:PIRE programme were more engaged and motivated about improving their health, thus, accounting for the improved outcomes seen in the intervention cohort. However, there was diversity in the cohort across the socio-economic gradient. Moreover, 40% of patients in the intervention cohort had multi-comorbidities and over a quarter had pre-existing mental health issues. This would suggest that the intervention arm did include patients with significant chronic issues, who often have challenging trajectories following critical illness [35].
The method of data collection varied across the two cohorts (in person or telephone vs. postal completion). This could have influenced how the outcomes were recorded and reported for each cohort. Finally, we only have data at one time point for the control cohort. As such we cannot fully understand the symptom trajectory for caregivers, which limits the interpretability of some of the results.
Conclusions
This multicentre evaluation has shown that caregivers who attended the InS:PIRE programme reported improved emotional health and less symptoms of insomnia 12 months after hospital discharge, in comparison with a contemporary control group. More work is required to understand how the recovery trajectory for this group interacts with the patient recovery trajectory, to ensure services fully support all needs in this group.
Supplementary Information
Additional file 1: S1. The template for intervention description and replication checklist (S1).
Additional file 2: S2. Details of Hospital Anxiety and Depression Scale alongside common cut-off points.
Additional file 3: S3. Logistic regression model outcomes.
Acknowledgements
The authorship team would like to thank the patients who took the time to participate in this study.
Abbreviations
- APACHE
Acute Physiology and Chronic Health Evaluation
- CI
Confidence interval
- CSI
Carer Strain Index
- HADS
Hospital Anxiety and Depression Scale
- ICU
Intensive Care Unit
- InSPIRE
Intensive Care Syndrome: Promoting Independence and Return to Employment
- ISI
Insomnia Severity Index
- IQR
Interquartile range
- LOS
Length of stay
- MICE
Multiple Imputation by Chained Equations
- OR
Odds ratio
- SIMD
Scottish Index of Multiple Deprivation
- STROBE
Strengthening the Reporting of Observational Studies in Epidemiology
Author contributions
JM and TQ took part in conceptualisation of the study; JM, PH and MS conducted the analysis (formal analysis); JM, MS, PH, TJI contributed to the interpretation of the findings (validation). All authors critically revised the paper for intellectual content and approved the final version of the manuscript. All authors read and approved the final manuscript.
Funding
The project was funded by an award from the Health Foundation (173544) and a THIS. Institute (University of Cambridge) Fellowship 307748-01/PD-2019-02-16.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
All participants provided written informed consent. Ethical approval was granted by the Northwest (Liverpool Central) Research Ethics Committee (reference number: 17/NM/0199).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Joanne McPeake, Email: Joanne.mcpeake@glasgow.ac.uk.
Philip Henderson, Email: Philip.Henderson@ggc.scot.nhs.uk.
Pamela MacTavish, Email: Pamela.mactavish@ggc.scot.nhs.uk.
Helen Devine, Email: helen.devine@ggc.scot.nhs.uk.
Malcolm Daniel, Email: malcolm.daniel@ggc.scot.nhs.uk.
Phil Lucie, Email: lucie@lanarkshire.scot.nhs.uk.
Lynn Bollan, Email: lynn.bollan@lanarkshire.scot.nhs.uk.
Lucy Hogg, Email: lucy.hogg1@nhs.scot.
Mike MacMahon, Email: michael.macmahon@nhs.scot.
Sharon Mulhern, Email: sharon.mulhern@ggc.scot.nhs.uk.
Pauline Murray, Email: pauline.murray@aapct.scot.nhs.uk.
Laura O’Neill, Email: Laura.O'Neill@lanarkshire.scot.nhs.uk.
Laura Strachan, Email: laura.strachan8@nhs.scot.
Theodore J. Iwashyna, Email: tiwashyn@med.umich.edu
Martin Shaw, Email: martin.shaw@ggc.scot.nhs.uk.
Tara Quasim, Email: tara.quasim@ggc.scot.nhs.uk.
References
- 1.Lone NI, McPeake J, Stewart NI, Blayney MC, Seem RC, Donaldson L, Glass E, Haddow C, Hall R, Martin C, et al. Influence of socioeconomic deprivation on interventions and outcomes for patients admitted with COVID-19 to critical care units in Scotland: a national cohort study. Lancet Reg Health Eur. 2021;1:100005. doi: 10.1016/j.lanepe.2020.100005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hosey MM, Needham DM. Survivorship after COVID-19 ICU stay. Nat Rev Dis Primers. 2020;6(1):60. doi: 10.1038/s41572-020-0201-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Haines KJ, Hibbert E, McPeake J, Anderson BJ, Bienvenu OJ, Andrews A, Brummel NE, Ferrante LE, Hopkins RO, Hough CL. Prediction models for physical, cognitive, and mental health impairments after critical illness: a systematic review and critical appraisal. Crit Care Med. 2020;48(12):1871. doi: 10.1097/CCM.0000000000004659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wade DM, Howell DC, Weinman JA, Hardy RJ, Mythen MG, Brewin CR, Borja-Boluda S, Matejowsky CF, Raine RA. Investigating risk factors for psychological morbidity three months after intensive care: a prospective cohort study. Crit Care. 2012;16(5):R192. doi: 10.1186/cc11677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Iwashyna TJ, Ely EW, Smith DM, Langa KM. Long-term cognitive impairment and functional disability among survivors of severe sepsis. JAMA. 2010;304(16):1787–1794. doi: 10.1001/jama.2010.1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cheung AM, Tansey CM, Tomlinson G, Diaz-Granados N, Matté A, Barr A, Mehta S, Mazer CD, Guest CB, Stewart TE. Two-year outcomes, health care use, and costs of survivors of acute respiratory distress syndrome. Am J Respir Crit Care Med. 2006;174(5):538–544. doi: 10.1164/rccm.200505-693OC. [DOI] [PubMed] [Google Scholar]
- 7.McPeake J, Mikkelsen ME, Quasim T, Hibbert E, Cannon P, Shaw M, Ankori J, Iwashyna TJ, Haines KJ. Return to employment following critical illness and its association with psychosocial outcomes: a systematic review and meta-analysis. Ann Am Thorac Soc. 2019;16:1304–1311. doi: 10.1513/AnnalsATS.201903-248OC. [DOI] [PubMed] [Google Scholar]
- 8.Cox CE, Docherty SL, Brandon DH, Whaley C, Attix DK, Clay AS, Dore DV, Hough CL, White DB, Tulsky JA, et al. Surviving critical illness: acute respiratory distress syndrome as experienced by patients and their caregivers. Crit Care Med. 2009;37(10):2702–2708. doi: 10.1097/CCM.0b013e3181b6f64a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Haines KJ, Denehy L, Skinner EH, Warrillow S, Berney S. Psychosocial outcomes in informal caregivers of the critically ill: a systematic review. Crit Care Med. 2015;43(5):1112–1120. doi: 10.1097/CCM.0000000000000865. [DOI] [PubMed] [Google Scholar]
- 10.Azoulay E, Pochard F, Kentish-Barnes N, Chevret S, Aboab J, Adrie C, Annane D, Bleichner G, Bollaert PE, Darmon M. Risk of post-traumatic stress symptoms in family members of intensive care unit patients. Am J Respir Crit Care Med. 2005;171(9):987–994. doi: 10.1164/rccm.200409-1295OC. [DOI] [PubMed] [Google Scholar]
- 11.Cameron JI, Chu LM, Matte A, Tomlinson G, Chan L, Thomas C, Friedrich JO, Mehta S, Lamontagne F, Levasseur M, et al. One-year outcomes in caregivers of critically ill patients. N Engl J Med. 2016;374(19):1831–1841. doi: 10.1056/NEJMoa1511160. [DOI] [PubMed] [Google Scholar]
- 12.McPeake J, Devine H, MacTavish P, Fleming L, Crawford R, Struthers R, Kinsella J, Daniel M, Shaw M, Quasim T. Caregiver strain following critical care discharge: an exploratory evaluation. J Crit Care. 2016;35:180–184. doi: 10.1016/j.jcrc.2016.05.023. [DOI] [PubMed] [Google Scholar]
- 13.Johnson CC, Suchyta MR, Darowski ES, Collar EM, Kiehl AL, Van J, Jackson JC, Hopkins RO. Psychological sequelae in family caregivers of critically iii intensive care unit patients. A systematic review. Ann Am Thorac Soc. 2019;16(7):894–909. doi: 10.1513/AnnalsATS.201808-540SR. [DOI] [PubMed] [Google Scholar]
- 14.Henderson P, Quasim T, Shaw M, et al. Evaluation of a heath and social care programme to improve outcome following critical illness: a multicentre study. Thorax. 2022 doi: 10.1136/thoraxjnl-2021-218428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Choi J, Donahoe MP, Zullo TG, Hoffman LA. Caregivers of the chronically critically ill after discharge from the intensive care unit: six months' experience. Am J Crit Care. 2011;20(1):12–23. doi: 10.4037/ajcc2011243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. J Clin Epidemiol. 2008;61(4):344–349. doi: 10.1016/j.jclinepi.2007.11.008. [DOI] [PubMed] [Google Scholar]
- 17.Hoffmann TC, Glasziou PP, Boutron I, Milne R, Perera R, Moher D, Altman DG, Barbour V, Macdonald H, Johnston M, et al. Better reporting of interventions: template for intervention description and replication (TIDieR) checklist and guide. BMJ. 2014;348:g1687. doi: 10.1136/bmj.g1687. [DOI] [PubMed] [Google Scholar]
- 18.McPeake JM, Henderson P, Darroch G, Iwashyna TJ, MacTavish P, Robinson C, Quasim T. Social and economic problems of ICU survivors identified by a structured social welfare consultation. Crit Care Lond Engl. 2019;23(1):153. doi: 10.1186/s13054-019-2442-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McPeake J, Iwashyna TJ, Devine H, MacTavish P, Quasim TJT. Peer support to improve recovery following critical care discharge: a case-based discussion. Thorax. 2017;72(9):856–858. doi: 10.1136/thoraxjnl-2016-209661. [DOI] [PubMed] [Google Scholar]
- 20.MacTavish P, Quasim T, Purdie C, Ball M, Barker L, Connelly S, Devine H, Henderson P, Hogg LA, Kishore R. Medication-related problems in intensive care unit survivors: learning from a multicenter program. Ann Am Thorac Soc. 2020;17(10):1326–1329. doi: 10.1513/AnnalsATS.202005-444RL. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Robinson C, Hibbert E, Bastin AJ, Meyer J, Montgomery-Yates A, Quasim T, Slack A, Mikkelsen ME, Iwashyna TJ, Haines KJ. An international study exploring the experience of survivors of critical illness as volunteers within ICU recovery services. Crit Care Explor. 2020;2(11):e0273. doi: 10.1097/CCE.0000000000000273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McPeake J, Boehm LM, Hibbert E, Bakhru RN, Bastin AJ, Butcher BW, Eaton TL, Harris W, Hope AA, Jackson J. Key components of ICU recovery programs: what did patients report provided benefit? Crit Care Explor. 2020;2(4):e0088. doi: 10.1097/CCE.0000000000000088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McPeake J, Shaw M, Iwashyna TJ, Daniel M, Devine H, Jarvie L, Kinsella J, MacTavish P, Quasim T. Intensive care syndrome: promoting independence and return to employment (InS: PIRE). Early evaluation of a complex intervention. PLoS ONE. 2017;12(11):e0188028. doi: 10.1371/journal.pone.0188028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Government S. Scottish index of multiple deprivation. In: Scottish Government Edinburgh; 2016.
- 25.Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67(6):361–370. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- 26.Robinson BC. Validation of a caregiver strain index. J Gerontol. 1983;38(3):344–348. doi: 10.1093/geronj/38.3.344. [DOI] [PubMed] [Google Scholar]
- 27.Bastien CH, Vallières A, Morin CM. Validation of the Insomnia Severity Index as an outcome measure for insomnia research. Sleep Med. 2001;2(4):297–307. doi: 10.1016/S1389-9457(00)00065-4. [DOI] [PubMed] [Google Scholar]
- 28.Haines KJ, Beesley SJ, Hopkins RO, McPeake J, Quasim T, Ritchie K, Iwashyna TJ. Peer support in critical care: a systematic review. Crit Care Med. 2018;46(9):1522–1531. doi: 10.1097/CCM.0000000000003293. [DOI] [PubMed] [Google Scholar]
- 29.Hope AA, Johnson A, McPeake J, Felt H, Sevin CM, Mikkelsen ME, Iwashyna TJ, Lassen-Greene C, Haines KJ, Agarwal S. Establishing a peer support program for survivors of COVID-19: a report from the Critical and Acute Illness Recovery Organization. Am J Crit Care. 2021;30(2):150–154. doi: 10.4037/ajcc2021675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Woodhead C, Khondoker M, Lomas R, Raine R. Impact of co-located welfare advice in healthcare settings: prospective quasi-experimental controlled study. Br J Psychiatry. 2017;211(6):388–395. doi: 10.1192/bjp.bp.117.202713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McPeake J, Iwashyna TJ, Henderson P, et al. Long term outcomes following critical care hospital admission: a prospective cohort study of UK Biobank participants. Lancet Reg Europe. 2021;6:100121. [DOI] [PMC free article] [PubMed]
- 32.Rabiee A, Nikayin S, Hashem MD, Huang M, Dinglas VD, Bienvenu OJ, Turnbull AE, Needham DM. Depressive symptoms after critical illness: a systematic review and meta-analysis. Crit Care Med. 2016;44(9):1744–1753. doi: 10.1097/CCM.0000000000001811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Choi J, Sherwood PR, Schulz R, Ren D, Donahoe MP, Given B, Hoffman LA. Patterns of depressive symptoms in caregivers of mechanically ventilated critically ill adults from ICU admission to two months post-ICU discharge: a pilot study. Crit Care Med. 2012;40(5):1546. doi: 10.1097/CCM.0b013e3182451c58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cox CE, Hough CL, Carson SS, White DB, Kahn JM, Olsen MK, Jones DM, Somers TJ, Kelleher SA, Porter LS. Effects of a telephone- and web-based coping skills training program compared with an education program for survivors of critical illness and their family members. A randomized clinical trial. Am J Respir Crit Care Med. 2018;197(1):66–78. doi: 10.1164/rccm.201704-0720OC. [DOI] [PubMed] [Google Scholar]
- 35.Griffith DM, Salisbury LG, Lee RJ, Lone N, Merriweather JL, Walsh TS. Determinants of health-related quality of life after ICU: importance of patient demographics, previous comorbidity, and severity of illness. Crit Care Med. 2018;46:594–601. doi: 10.1097/CCM.0000000000002952. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: S1. The template for intervention description and replication checklist (S1).
Additional file 2: S2. Details of Hospital Anxiety and Depression Scale alongside common cut-off points.
Additional file 3: S3. Logistic regression model outcomes.
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.


