Abstract
Hyperviscosity syndrome (HVS) recently emerged as a complication of coronavirus disease 2019 (COVID-19) and COVID-19 vaccines. Therefore, the objectives of this critical review are to establish the association between COVID-19 and COVID-19 vaccines with the development of HVS. HVS may develop in various viral infections due to impairment of humoral and cellular immunity with elevation of immunoglobulins. COVID-19 can increase blood viscosity (BV) through modulation of fibrinogen, albumin, lipoproteins, and red blood cell (RBC) indices. HVS can cause cardiovascular and neurological complications in COVID-19 like myocardial infarction (MI) and stroke. HVS with or without abnormal RBCs function in COVID-19 participates in the reduction of tissue oxygenation with the development of cardio-metabolic complications and long COVID-19. Besides, HVS may develop in vaccine recipients with previous COVID-19 due to higher underlying Ig concentrations and rarely without previous COVID-19. Similarly, patients with metabolic syndrome are at the highest risk for propagation of HVS after COVID-19 vaccination. In conclusion, COVID-19 and related vaccines are linked with the development of HVS, mainly in patients with previous COVID-19 and underlying metabolic derangements. The possible mechanism of HVS in COVID-19 and related vaccines is increasing levels of fibrinogen and immunoglobulins. However, dehydration, oxidative stress, and inflammatory reactions are regarded as additional contributing factors in the pathogenesis of HVS in COVID-19. However, this critical review cannot determine the final causal relationship between COVID-19 and related vaccines and the development of HVS. Prospective and retrospective studies are warranted in this field.
Keywords: COVID-19, Hyperviscosity syndrome, COVID-19 vaccination
Introduction
Hyperviscosity syndrome (HVS) is a group of symptoms induced by high blood viscosity (BV) including bleeding, headache, visual disturbances, seizure, vertigo, and coma [1]. HVS is characterized by the triad of mucosal bleeding, visual changes, and neurological deficits [1]. The main cause of HVS is Waldenstrom macroglobulinemia, which is an abnormal proliferation of plasma cells and lymphoplasmacytoid cells [2]. HVS is also caused by polycythemia, leukemia, multiple myeloma, sepsis, and sickle cell anemia [3]. As a result, HVS is caused by an increase in the number of red blood cells (RBCs) or a deformity in RBC shape, as well as an increase in serum proteins [3]. Normal BV is typically between 1.4 and 1.8 centipoise (cp), and symptoms of HVS develop when BV exceeds 4.0 cp [4].
HVS recently emerged as a complication of coronavirus disease 2019 (COVID-19) and COVID-19 vaccines [5]. Therefore, the objectives of this critical review are to determine the association between COVID-19 and/or COVID-19 vaccines with the development of HVS.
Hyperviscosity syndrome and viral infections
HVS can occur in a variety of viral infections, including human immunodeficiency virus type 1 (HIV-1) infections, as a result of impaired humoral and cellular immunity and an increase in immunoglobulin (IgG) [6]. The underlying mechanisms of HVS in patients with HIV-1 are related to the direct activation of B cells by HIV-1, alteration of T cells-mediated B cell regulation, chronic exposure to the antigens of HIV-1 and high IL-6 [6]. However, hyper-activation of B cells with high production of IgG could be the main mechanistic pathway of HVS in HIV-1 infection [7]. According to Jin et al., HVS was linked to the formation of myeloma-associated IgG1paraprotein against HIV-1 p24 antigen in HIV-1 patients [8].
As well, HVS has been demonstrated in patients with acute respiratory viral infections, including influenza complicated by pneumonia [9]. A study involving 232 patients with influenza and acute respiratory viral infections showed significant alterations in the microcirculation, intravascular homeostasis, hypercoagulation, augmentation of fibrinolytic activity, and an increase in BV [9]. Generally, Sloop and colleagues revealed that severe infections increase BV with the development of HVS due to inflammation-induced hypergammaglobulinemia and elevation of acute-phase reactants that increase BV [10]. High BV or HVS fosters aggregation of RBCs with an increasing risk of thrombosis due to augmentation of vascular resistance, which impedes peripheral tissue perfusion [10]. Of note, previous acute infection and chronic bronchitis within two months caused by influenza infection predispose to the risk of acute ischemic stroke, and influenza vaccine did not offered a protection against the development of acute ischemic stroke [11]. This observation suggests that HVS could be a possible risk factor for the development of acute ischemic stroke in patients with influenza infection.
Furthermore, indices of blood viscosity are increased in patients with hepatitis B virus (HBV) infection who are at risk for the development of HVS [12]. A study of 55 patients with HBV infection illustrated that RBCs aggregation index, hematocrit, and whole BV were higher compared with control groups and unrelated to the state of oxidative stress and hemorheology indices [12]. Of interest, HVS is implicated in the pathogenesis of septic shock in parallel with high fibrinogen levels [13]. Van et al. reported that soluble fibrinogen-like protein 2 (sFGL2) is increased in patients with HBV infection [14]. Therefore, high sFGL2 plasma levels could be the potential cause of HVS in HBV infection. These findings indicated that HVS may be developed in various viral infections and contribute to the development of complications.
Hyperviscosity syndrome and immunoinflammatory disorders
It has been reported that HVS is linked with acute inflammatory disorders since BV is sensitive to acute-phase reactants [15]. Therefore, HVS is high in subpopulations with high C-reactive protein and erythrocyte sedimentation rate (ESR) as compared with subpopulations with low CRP and ESR [15]. HVS has been shown to develop in patients with rheumatoid arthritis due to the formation of immunocomplexes which affect RBCs deformability and vascular resistance [15]. HVS in rheumatoid arthritis can be developed with a level of IgG less than in Waldenstrom macroglobulinemia [16]. It developed in patients with rheumatoid arthritis due to the formation of an intermediate complex from the aggregation of Ig, RBCs aggregation, and high fibrinogen levels [17]. However, HVS in rheumatoid arthritis is rare in treated patients, so treating with plasmapheresis and immunosuppressive agents can reduce the risk of development of HVS [17]. Likewise, HVS in rheumatoid arthritis is significantly correlated with high activity of rheumatoid factor [18].
Furthermore, HVS may be the presenting feature in patients with systemic lupus erythematosus (SLE) due to hyper-paraproteinemia and monoclonal gammopathy [19]. Besides, HVS is also developed in IgG4-related disorders, which are systemic fibro-inflammatory disorders characterized by elevation of Ig, including IgG4 [20].
Of interest, CD169 macrophages contribute to the process of bone marrow erythropoiesis and maturation of RBCs. Over-activation of CD169 macrophages may be associated with the development of polycythemia [21]. Thus, depletion of CD169 macrophages reduces bone marrow erythroblasts and prevents erythropoietic recovery from anemia [21]. According to Asano et al., CD169 macrophages control and modulate immunological responses in the circulating fluid by recruiting monocytes and producing chemokines [22]. CD169 macrophages are activated during immunological disorders, tumor growth, and viral infections to produce immunological tolerance and antiviral effects [23–25]. As a result, in immunological diseases, activated CD169 macrophages may increase BV via boosting erythropoiesis.
Indeed, there is a close relationship between HVS and inflammation due to the increase in acute-phase reactant fibrinogen, whose level is correlated with increasing blood viscosity [26]. Gordy et al. revealed that fibrinogen-related proteins are increased during the immune response to various inflammatory stimuli [27]. Fibrinogen and fibrinogen-related proteins play a critical role in neutralizing invading pathogens [28]. In turn, exaggerated immune responses and high levels of fibrinogen-related proteins are associated with the development of HVS.
These observations indicated that high BV or HVS is linked with underlying immunoinflammatory disorders.
Hyperviscosity syndrome and COVID-19
Effects of COVID-19 on blood viscosity
COVID-19 is a pandemic disease caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) leading to worldwide crisis with high morbidity [29]. Till late January 2022, the total number of infected cases reached more than 370 million, with about 5 million confirmed deaths. In general, the clinical presentation of COVID-19 is mild in the majority of cases, though 15% of COVID-19 patients presented with pulmonary and extra-pulmonary manifestations including headache, fever, dry cough, sweating, and fatigue [30, 31]. About 5% of COVID-19 patients may develop severe and critical outcomes due to the development and propagation of acute lung injury (ALI) and acute respiratory distress syndrome (ARDS) that require intensive care unit admission [32].
SARS-CoV-2 exploits different receptor types to enter the affected cells. The angiotensin-converting enzyme 2 (ACE2) is a pioneer one related in the pathogenesis of SARS-CoV-2 infection [33]. This interaction induces downregulation of ACE2, which is necessary for conversion of pro-inflammatory/vasoconstrictor angiotensin II (AngII) to vasodilator/anti-inflammatory Ang1-7 [34]. In severe cases, SARS-CoV-2 infection may result in an exaggerated immune response, hyperinflammation, and hypercytokinemia, as well as a cytokine storm [35, 36]. Therefore, SARS-CoV-2-induced upregulation of AngII may provoke the development of HVS in COVID-19 through the induction of inflammatory changes and vasoconstriction.
It has been shown that SARS-CoV-2 infection is associated with microcirculation failure in hospitalized patients with severe COVID-19 characterized by weak peripheral pulses, cold extremities, and metabolic acidosis [37]. Microcirculation dysfunction/failure has been linked to severe sepsis because of increased RBC aggregation, decreased RBC deformability, and alterations in RBC physiology/morphology [38, 39]. Endothelial dysfunction, coagulation problems, and cytokine storm were observed to contribute to the development of microcirculation failure in septic COVID-19 patients by Colantuoni et al. [40]. A study that included 7 hospitalized COVID-19 patients compared to 7 non-COVID-19 septic patients and 7 healthy control illustrated that RBCs deformability was reduced in both COVID-19 patients and non-COVID-19 septic patients compared to the controls (P < 0.05) [41]. Moreover, RBCs aggregation was higher in COVID-19 patients compared to 7 non-COVID-19 septic patients without significant changes in BV and fibrinogen levels [37]. This small sample study does not give any concrete clues about normal BV and fibrinogen levels in COVID-19. A retrospective study involving 41 COVID-19 patients revealed that estimated BV was higher in COVID-19 patients than in the control group [41].
Enhanced RBCs aggregation with reduction of RBCs deformability in COVID-19 is increased in both stasis and low-shear flow [37] that together with increasing fibrinogen level may increase BV and development of HVS. Of note, acute viral infections are linked with development of HVS due to hypergammaglobulinemia and elevation of acute-phase reactants which might cause thromboembolic disorders and cardiovascular complications [42]. Increasing of BV and development of HVS in COVID-19 could be related to different mechanisms including exaggerated immune response, endothelial dysfunction, hypoxia, coagulation disorders [41].
Similarly, changes in RBCs morphology/function, platelet hyper-reactivity, high ferritin, and P-selectin activity in COVID-19 could contribute in the development of HVS [43]. As well, psychological stress, fever, and dehydration may increase BV and compensatory increment in the release of arginine vasopressin in COVID-19 patients [44]. High arginine vasopressin triggers release of pro-inflammatory cytokines through activation of nuclear factor kappa B (NF-κB) and nod-like receptor pyrin 3 (NLRP3) inflammasome which participate in increasing of BV [44]. Both of NF-κB and NLRP3 inflammasome induce asymmetry of RBCs membrane with reduction of RBCs deformability in normal and sickle RBCs [45, 46]. In addition, NF-κB and NLRP3 inflammasome are highly activated in COVID-19 [47] and could a potential causes for reduction of RBCs deformability in COVID-19.
Of note, CD169 macrophages are involved in the maturation of RBCs and development of polycythemia [21]. CD169 monocytes are expressed in 93.7% of COVID-19 patients and could be of diagnostic benefits [48]. Therefore, SARS-CoV-2-induced CD169 macrophages/monocytes may cause polycythemia and elevation of BV in COVID-19.
Exaggerated immune response and release of pro-inflammatory cytokines mainly IL-6 are linked with development of cytokine storm and multi-organ injury [49]. Panigada et al. observed that IL-6 is regarded as a powerful activator for synthesis of fibrinogen in COVID-19 [50]. Also, dysregulation of RAS with high AngII in COVID-19 may induce expression and synthesis of fibrinogen [35, 51]. Fibrinogen activates RBC membrane integrinαvβ3 receptors resulting in the activation of RBCs aggregation with subsequent development of HVS [50]. As well, hypoalbuminemia is linked with increasing of blood viscosity and development of HVS [52]. Serum albumin is inversely correlated with D-dimer and CRP, and hypoalbuminemia is associated with an increased risk of development of coagulopathy in COVID-19 patients by a reduction in the anticoagulant and antiplatelet effects of albumin [53]. A retrospective study involving 113 COVID-19 patients illustrated that a high fibrinogen/albumin ratio was associated with a high risk of thrombosis, disease severity, and poor clinical outcomes [54]. As a result, the BV is augmented and reaches 4.2 cp. Therefore, hyperfibrinogenemia and hypoalbuminemia may increase BV and participate in the development of HVS and thrombotic events in COVID-19. Notably, severe COVID-19 is associated with the development of arterial and venous thromboembolic events due to direct SARS-CoV-2 cytopathic effects and associated endothelial dysfunction, platelet activation, coagulation activation, and inhibition of the fibrinolytic pathway [55]. Furthermore, downregulation of ACE2 with dysregulation of RAS together with exaggerated pro-inflammatory cytokines may initiate endothelial dysfunction by reduction of prostacyclin and nitrous oxide (NO) [56]. Felicetti et al. recently illustrated that thrombotic events may increase the risk of development of HVS [57]. These observations suggest a mutual interaction between HVS and thrombotic events in COVID-19.
Moreover, SARS-CoV-2 may directly affect RBCs morphology through binding of membrane CD147 receptor and Band3 protein on the RBCs [58, 59]. These changes hamper functional capacity for oxygen transport by RBCs leading to development of tissue hypoxia [59]. Besides, Foy and colleagues revealed that RBC distribution width and other indices were severely affected in SARS-CoV-2 infection and linked with COVID-19 severity and poor clinical outcomes [60]. In addition, extreme hypoxia and acidosis induce alteration in RBCs morphology [61]. These observations suggest that direct SARS-CoV-2-induced RBCs dysmorphology and associated metabolic acidosis and hypoxia may induce progression of HVS in COVID-19.
Furthermore, lipoproteins can affect blood viscosity since low-density lipoprotein (LDL) is positively correlated, while high-density lipoprotein (HDL) is negatively correlated with BV [62]. HDL is necessary for RBCs morphology and deformability; thus, reduction of HDL may reduce RBCs life span by increasing osmotic fragility and reduction of RBCs deformability [63]. In COVID-19, there is a noteworthy alteration in lipoprotein serum levels, and low HDL levels are associated with COVID-19 severity [64, 65]. Therefore, reduction of HDL in SARS-CoV-2 infection can increase BV with the development of HVS in COVID-19.
Moreover, SARS-CoV-2 infection-induced oxidative stress may trigger elevation of BV [66]. It has been reported that high oxidative stress can induce abnormal hemorheological changes with a reduction of RBCs deformability and the induction of thrombotic changes [67]. In COVID-19, severe oxidative stress triggers endothelial dysfunction and thromboembolic complications [66]. Thus, alterations in RBC fragility and deformability together with endothelial dysfunction by SARS-CoV-2 infection-induced oxidative stress could cause HVS in COVID-19.
Interestingly, RBCs morphology and functions are affected in COVID-19 with the development of abnormal erythrocrine function [68]. Development of abnormal RBCs may contribute to the progression of endothelial dysfunction and vascular injury by increasing oxidative stress [69]. RBCs from COVID-19 patients induce expression and upregulation of endothelial arginase with the production of reactive oxygen species (ROS), reduction of endothelial NO and development of endothelial dysfunction [69]. Therefore, SARS-CoV-2 infection-induced oxidative stress could in part be mediated by the development of abnormal RBCs in COVID-19.
Moreover, COVID-19 is commonly associated with psychological stress and sympathetic outflow [70]. Of interest, psychological stress increases circulating AngII as well, AngII promotes psychological stress through augmentation of sympathetic activation [71]. Likewise, AngII receptor blockers attenuate stress pressor in young adults [71]. Thus, COVID-19-induced psychological stress may augment the dysregulated RAS by increasing AngII with subsequent development of HVS.
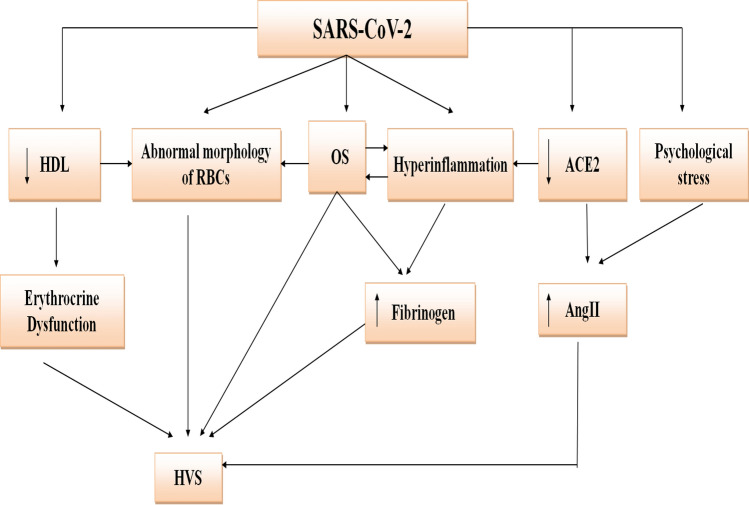
Taken together, COVID-19 can increase BV through modulation of fibrinogen, albumin, lipoproteins, and RBC indices (Fig. 1).
Fig. 1.
Proposed mechanism of hyperviscosity syndrome in COVID-19: SARS-CoV-2 through induction of the downregulation of angiotensin-converting enzyme 2 (ACE2), psychological stress, hyperinflammation, oxidative stress (OS), abnormal morphology of red blood cells (RBCs), and reduction of high density lipoprotein (HDL). These changes increase fibrinogen, angiotensin II (AngII), and the induction of erythrocrine dysfunction with the subsequent development of hyperviscosity syndrome (HSV)
Complications of hyperviscosity in COVID-19
COVID-19 HVS has been linked with various cardiovascular and neurological complications such as myocardial infarction (MI) and stroke [72, 73]. The incidence of MI in COVID-19 has increased by up to 5% [74]. That could be due to the development of HVS. In addition, increasing of RBCs aggregation and SARS-CoV-2 infection-induced endothelial dysfunction and immunothrombosis may elevate BV in COVID-19 [75]. These changes increase the risk of the development of MI in surviving COVID-19 patients due to the development of coronary microangiopathy [76].
HVS in COVID-19 causes poor tissue perfusion, peripheral vascular resistance, and thrombosis [77]. Low-shear areas are susceptible to thrombosis due to reduction in dispersion of clotting factors and attenuation of shear-induced release of antithrombotic molecules like NO and prostacyclin [77]. Remarkably, most of the COVID-19 patients with BV greater than 3.5cp had coagulation disorders [78]. Herein, there is a close relationship between HVS and thrombotic events in COVID-19. Maier and coworkers reported 15 critical COVID-19 with thrombotic complications. All patients had a BV greater than 3.5cp (the normal range is 1.4–1.8 cp) as tested by a traditional capillary viscometer. The high BV was correlated with thrombotic events (r = 0.84, P < 0.01) [78]. Further, a case series reported by Truong et al. showed symptoms of HVS were more evident in COVID-19 patients with BV greater than 4.2 cp [75]. These findings suggest that higher BV is linked with more severe HVS in COVID-19.
Furthermore, HVS may lead to complications like acute kidney injury, glucose intolerance, skeletal muscle ischemia, and myocardial necrosis [79]. As well, HVS leads to pulmonary hypoperfusion and the development of ventilation-perfusion mismatch. These changes cause silent hypoxemia with the propagation of high pulmonary vascular resistance [80].
Indeed, HVS is linked with the development of post-COVID-19 syndrome (long COVID-19), which is the persistence of symptoms like dyspnea, fatigue, cognitive dysfunction, and headache following recovery from COVID-19 [81]. Long-term COVID-19 is associated with immunosuppression and cardio-pulmonary fibrosis due to upregulation of transforming growth factor beta (TGF-β) [82]. Prolonged inflammatory changes and high blood viscosity in patients with long COVID-19 can reduce tissue perfusion and cellular metabolism [83]. As mentioned above, prolonged abnormal RBCs function following COVID-19 may cause tissue hypoxia and subnormal cell metabolism with accentuation of long COVID-19 [69].
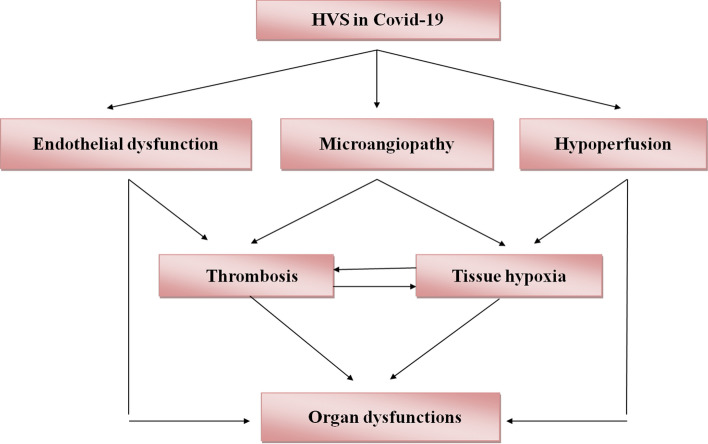
Taken together, HVS with or without abnormal RBCs function in COVID-19 participates in reduction of tissue oxygenation with the development of cardio-metabolic complications and long COVID-19 (Fig. 2).
Fig. 2.
Complications of hyperviscosity in COVID-19: Hyperviscosity (HVS) in COVID-19 induces the development of endothelial dysfunction, microangiopathy, and hypoperfusion with the development of thrombosis and tissue hypoxia, which ultimately cause organ dysfunction
Hyperviscosity and COVID-19 vaccination
COVID-19 vaccine was developed on the 8th of April 2020 to control the spread of SARS-CoV-2 infection and limit morbidity and mortality caused by COVID-19 [84]. Following COVID-19 vaccination, some reports showed that the BV was increased because of the induction of Ig [85]. HVS may develop after COVID-19 vaccination, leading to immunopathological changes [86]. HVS is correlated with the concentration of Ig, though the lowest normal Ig concentrations are 545 mg/dl, while the lowest BV is 1.5 cp [86]. The BV will be 2.6 cp when Ig concentrations reach 6160 mg/dl [85]. Of note, symptoms of HVS are developed when BV exceeds 4.0 cp [4].
Surprisingly, HVS can develop in vaccine recipients who have previously received COVID-19 due to higher underlying Ig concentrations, and only rarely in those who have never received COVID-19 [85]. Thus, screening for previous COVID-19 is essential before induction of COVID-19 vaccination to prevent the development of HVS and related hemorheological adverse effects. Alongside, use of contraceptives may augment the risk of development of HVS after COVID-19 vaccination [87]. Therefore, we suggest taking the risk into consideration for patients taking contraceptives at the time of COVID-19 vaccination.
Different studies revealed that metabolic alterations in patients with metabolic syndrome increase BV and the risk for development of HVS [88]. Metabolic syndrome is linked with systemic inflammation and oxidative stress which affect the microcirculation by increasing of BV due to reduction of RBCs deformability [89]. Therefore, patients with metabolic syndrome are at the highest risk for propagation of HVS after COVID-19 vaccination. Joob and Wiwanitkit confirmed that COVID-19 vaccination increases the risk of development of HVS in patients with metabolic syndrome [90]. The BV is increased by 2.7 times in healthy subjects and by 2.99 in patients with metabolic syndrome following COVID-19 vaccination [91]. This increment in BV did not reach the state of HVS in both healthy subjects and patients with metabolic syndrome, which might be due to the validity of the method in the assessment of blood viscosity [91].
Generally, BV in healthy COVID-19 vaccine recipients is increased by 2.4 cp [92]. However, COVID-19 vaccine-induced HVS is common in patients with metabolic syndrome due to high underlying metabolic disorders which increase BV [93]. Sookaromdee et al. proposed that underlying chronic liver diseases with high bilirubin levels may cause HVS after COVID-19 vaccination since hyperbilirubinemia is linked with the development of HVS [93]. Patients with underlying metabolic disorders have a higher chance of developing HVS following COVID-19 vaccination. Thus, close monitoring of blood viscosity in COVID-19 vaccine recipients is necessary to prevent post-vaccine complications [94].
Interestingly, oxidative stress can induce a reduction in RBCs deformability with a subsequent elevation of BV [95]. In obesity, high oxidative stress and fibrinogen together with prolonged low-grade inflammation are linked with the development of HVS [96, 97]. Therefore, depending on these findings, obese patients are at high risk for the development of HVS after COVID-19 vaccination. Pivonello and colleagues suggested that the immune response in obese patients against the COVID-19 vaccine is low due to impaired reactivity of T and B cells [98]. Therefore, a delay in immune response may reduce Ig concentrations following COVID-19 vaccination, and this may affect the development of HVS in obesity. Of note, the immune response in obese patients was low following the influenza vaccine [99]. These findings are premature to draw a final association between COVID-19 vaccination and the risk of HVS, and thus, prospective and retrospective studies are warranted in this regard.
The present review had many limitations, including the rarity of prospective studies that evaluate BV in COVID-19 at the time of admission and discharge. Also, most studies were speculative in their explanation of HVS in COVID-19 and COVID-19 vaccination. Despite these limitations, the present critical review revealed that HVS is an important mechanistic pathway in the development of complications in COVID-19 and related vaccines.
Conclusions
COVID-19 and related vaccines are linked with the development of HVS mainly in patients with previous COVID-19 and underlying metabolic derangements. The possible mechanism of HVS in COVID-19 and related vaccines is increasing levels of fibrinogen and immunoglobulins. Dehydration, oxidative stress, and inflammatory reactions are regarded as additional contributing factors in the pathogenesis of HVS in COVID-19. However, this critical review cannot determine the final causal relationship between COVID-19 and related vaccines and the development of HVS. Prospective and retrospective studies are warranted in this field.
Authors' contribution
All authors contributed to the study conception and design. Data collection and analysis were performed by HMA-k, AIA-G. The first draft of the manuscript was written by HMA-k, AIA-G, MME-B, and GEB and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Funding
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Data availability
All data generated or analyzed during this study are included in this published article.
Declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Consent for publication
This article does not contain any studies with human or animal subjects.
Ethics approval and consent to participate
This article does not contain any studies with human or animal subjects.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Hayder M. Al-kuraishy, Email: Hayderm36@yahoo.com
Ali I. Al-Gareeb, Email: Dr.alialgareeb78@yahoo.com
Maisra M. El-Bouseary, Email: maysra_mohamed@pharm.tanta.edu.eg
Fatma I. Sonbol, Email: fatma.sonbol@pharm.tanta.edu.eg
Gaber El-Saber Batiha, Email: gaberbatiha@gmail.com.
References
- 1.Rogers AP, Estes M. Hyperviscosity syndrome. StatPearls[internet]; 2021Jul21.
- 2.Owen RG, McCarthy H, Rule S, d’Sa S, et al. Acalabrutinib monotherapy in patients with Waldenströmmacroglobulinemia: a single-arm, multicentre, phase 2 study. Lancet Haematol. 2020;7(2):e112–e121. doi: 10.1016/S2352-3026(19)30210-8. [DOI] [PubMed] [Google Scholar]
- 3.Stone MJ, Bogen SA. Evidence-based focused review of management of hyperviscosity syndrome. Blood. 2012;119(10):2205–2208. doi: 10.1182/blood-2011-04-347690. [DOI] [PubMed] [Google Scholar]
- 4.Bekelman J, Jackson N, Donehower R. Oncologic emergencies. 2nd ed. Philadelphia: Saunders Elsevier; 2006.
- 5.Joob B, Wiwanitkit V. Expected viscosityafter COVID-19 vaccination, hyperviscosity and previous COVID-19. Clin ApplThromb Hemost. 2021 doi: 10.1177/10760296211020833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Garderet L, Fabiani B, Lacombe K, Girard PM, Fléjou JF, Gorin NC. Hyperviscosity syndrome in an HIV-1—positive patient. Am J Med. 2004;117(11):891–893. doi: 10.1016/j.amjmed.2004.09.011. [DOI] [PubMed] [Google Scholar]
- 7.De Milito A, Nilsson A, Titanji K, et al. Mechanisms of hypergammaglobulinemia and impaired antigen-specific humoral immunity in HIV-1 infection. Blood. 2004;103(6):2180–2186. doi: 10.1182/blood-2003-07-2375. [DOI] [PubMed] [Google Scholar]
- 8.Jin DK, Nowakowski M, Kramer M, Essex DW. Hyperviscosity syndrome secondary to a myeloma-associated IgG1κparaprotein strongly reactive against the HIV-1 p24 gag antigen. AmJ Hematol. 2000;64(3):210–213. doi: 10.1002/1096-8652(200007)64:3<210::aid-ajh13>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 9.Bogomolov BP, Deviatkin AV. Microcirculation and hemostasis in influenza and acute viral respiratory infections complicated with pneumonia. Ter Arkh. 2002;74(3):44–48. [PubMed] [Google Scholar]
- 10.Sloop GD, De Mast Q, Pop G, Weidman JJ, St Cyr JA. The role of blood viscosity in infectious diseases. Cureus. 2020;12(2):e7090. doi: 10.7759/cureus.7090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Piñol-Ripoll G, De La Puerta I, Santos S, Purroy F, Mostacero E. Chronic bronchitis and acute infections as new risk factors for ischemic stroke and the lack of protection offered by the influenza vaccination. CerebrovascDis. 2008;26(4):339–347. doi: 10.1159/000151636. [DOI] [PubMed] [Google Scholar]
- 12.Yang Y, Wang K, Han LY, Li XH, Wang HM. Hemorheologic changes in patients with chronic hepatitis B.Zhonghua Shi Yan He Lin Chuang Bing Du Xue Za Zhi. 2005;19(1):61–3. [PubMed]
- 13.Richardson SG, Matthews KB, Cruickshank JK, Geddes AM, Stuart J. Coagulation activation and hyperviscosity in infection. BrJ Haematol. 1979;42(3):469–480. doi: 10.1111/j.1365-2141.1979.tb01155.x. [DOI] [PubMed] [Google Scholar]
- 14.Van Tong H, Van Ba N, Hoan NX, et al. Soluble fibrinogen-like protein 2 levels in patients with hepatitis B virus-related liver diseases. BMC Infect Dis. 2018;18(1):553. doi: 10.1186/s12879-018-3473-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nwose EU. Whole blood viscosity assessment issues IV: Prevalence in acute phase inflammation. North Am J Med Sci. 2010;2(8):353–358. doi: 10.4297/najms.2010.2353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Miller JB, Baer AN. Hyperviscosity syndrome in rheumatoid arthritis. J Rheumatol. 2021;48(5):788–789. doi: 10.3899/jrheum.200591. [DOI] [PubMed] [Google Scholar]
- 17.Gustine JN, Meid K, Dubeau T, et al. Serum IgM level as predictor of symptomatic hyperviscosity in patients with Waldenströmmacroglobulinaemia. Br J Haematol. 2017;177(5):717–725. doi: 10.1111/bjh.14743. [DOI] [PubMed] [Google Scholar]
- 18.Lokhandwala PM, Shabihkhani M, Ness PM, Bloch EM. Therapeutic plasma exchange for hyperviscosity syndrome secondary to high rheumatoid factor. Transfus ApherSci. 2018;57(2):225–227. doi: 10.1016/j.transci.2018.02.004. [DOI] [PubMed] [Google Scholar]
- 19.CorriganIII FE, Leventhal AR, Khan S, Rao S, Christopher-Stine L, Schulman SP. A rare cause of cardiac ischemia: systemic lupus erythematosus presenting as the hyperviscosity syndrome. Ann Intern Med. 2010;153(6):422–424. doi: 10.7326/0003-4819-153-6-201009210-00023. [DOI] [PubMed] [Google Scholar]
- 20.Chen LY, Wong PC, Noda S, Collins DR, Sreenivasan GM, Coupland RC. Polyclonal hyperviscosity syndrome in IgG4-related disease and associated conditions. Clin Case Rep. 2015;3(4):217–226. doi: 10.1002/ccr3.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chow A, Huggins M, Ahmed J, et al. CD169+ macrophages provide a niche promoting erythropoiesis under homeostasis and stress. Nat Med. 2013;19(4):429–436. doi: 10.1038/nm.3057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Asano K, Kikuchi K, Tanaka M. CD169 macrophages regulate immune responses toward particulate materials in the circulating fluid. J Biochem. 2018;164(2):77–85. doi: 10.1093/jb/mvy050. [DOI] [PubMed] [Google Scholar]
- 23.Liu Y, Xia Y, Qiu CH. Functions of CD169 positive macrophages in human diseases (Review) Biomed Rep. 2021;14(2):26. doi: 10.3892/br.2020.1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hetta HF, Mekky MA, Zahran AM, et al. Regulatory B cells and their cytokine profile in HCV-related hepatocellular carcinoma: association with regulatory T cells and disease progression. Vaccines. 2020;8(3):380. doi: 10.3390/vaccines8030380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hetta HF, Mwafey IM, Batiha GE, et al. CD19+ CD24hi CD38hi regulatory B cells and memory B cells in periodontitis: association with pro-inflammatory and anti-inflammatory cytokines. Vaccines. 2020;8(2):340. doi: 10.3390/vaccines8020340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pop GAM, Hop WJ, Moraru L, et al. Blood electrical impedance closely matches whole blood viscosity as parameter of hemorheology and inflammation. Appl Rheol. 2003;13(6):305–312. doi: 10.1515/arh-2003-0020. [DOI] [Google Scholar]
- 27.Gordy MA, Pila EA, Hanington PC. The role of fibrinogen-related proteins in the gastropod immune response. Fish Shellfish Immunol. 2015;46(1):39–49. doi: 10.1016/j.fsi.2015.03.005. [DOI] [PubMed] [Google Scholar]
- 28.Påhlman LI, Mörgelin M, Kasetty G, Olin AI, Schmidtchen A, Herwald H. Antimicrobial activity of fibrinogen and fibrinogen-derived peptides–a novel link between coagulation and innate immunity. Thromb Haemost. 2013;109(5):930–939. doi: 10.1160/TH12-10-0739. [DOI] [PubMed] [Google Scholar]
- 29.Al-Kuraishy HM, Al-Gareeb AI, Alzahrani KJ, Cruz-Martins N, Batiha GE. The potential role of neopterin in Covid-19: a new perspective. Mol Cell Biochem. 2021;476(11):4161–4166. doi: 10.1007/s11010-021-04232-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Al-Kuraishy HM, Al-Gareeb AI, Alzahrani KJ, Alexiou A, Batiha GE. Niclosamide for Covid-19: bridging the gap. MolBiolRep. 2021;48(12):8195–8202. doi: 10.1007/s11033-021-06770-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Al-Kuraishy HM, Al-Gareeb AI, Almulaiky YQ, Cruz-Martins N, El-Saber Batiha GE. Role of leukotriene pathway and montelukast in pulmonary and extrapulmonary manifestations of Covid-19: the enigmatic entity. EurJ Pharmacol. 2021;904:174196. doi: 10.1016/j.ejphar.2021.174196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Al-Kuraishy HM, Al-Gareeb AI, Alkazmi L, Alexiou A, Batiha GE. Levamisoletherapy in COVID-19. Viral Immunol. 2021;34(10):722–725. doi: 10.1089/vim.2021.0042. [DOI] [PubMed] [Google Scholar]
- 33.Al-Kuraishy HM, Al-Gareeb AI, Alqarni M, Cruz-Martins N, El-Saber BG. Pleiotropic effects of tetracyclines in the management of COVID-19: emergingperspectives. Front Pharmacol. 2021;12:642822. doi: 10.3389/fphar.2021.642822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Batiha GE, Shaheen HM, Al-Kuraishy HM, et al. Possible mechanistic insights into iron homeostasis role of the action of 4-aminoquinolines (chloroquine/hydroxychloroquine) on COVID-19 (SARS-CoV-2) infection. Eur Rev Med Pharmacol Sci. 2021;25(23):7565–7584. doi: 10.26355/eurrev_202112_27456. [DOI] [PubMed] [Google Scholar]
- 35.Al-Kuraishy HM, Al-Gareeb AI, El-Saber Batiha GE. The possible role of ursolic acid in Covid-19: a real game changer. Clin Nutr ESPEN. 2022;47:414–417. doi: 10.1016/j.clnesp.2021.12.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Al-Kuraishy HM, Al-Gareeb AI, Faidah H, Al-Maiahy TJ, Cruz-Martins N, Batiha GE. The looming effects of estrogen in Covid-19: a rocky rollout. Front Nutr. 2021;8:649128. doi: 10.3389/fnut.2021.649128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Renoux C, Fort R, Nader E, et al. Impact of COVID-19 on red blood cell rheology. Br J Haematol. 2021;192(4):e108–e111. doi: 10.1111/bjh.17306. [DOI] [PubMed] [Google Scholar]
- 38.Lehr HA, Bittinger F, Kirkpatrick CJ. Microcirculatory dysfunction in sepsis: A pathogenetic basis for therapy? J Pathol. 2000;190(3):373–386. doi: 10.1002/(SICI)1096-9896(200002)190:3<373::AID-PATH593>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 39.Lundy DJ, Trzeciak S. Microcirculatory dysfunction in sepsis. Crit Care Nurs Clin North Am. 2011;23(1):67–77. doi: 10.1016/j.ccell.2010.12.004. [DOI] [PubMed] [Google Scholar]
- 40.Colantuoni A, Martini R, Caprari P, et al. COVID-19 sepsis and microcirculation dysfunction. Front Physiol. 2020;26(11):747. doi: 10.3389/fphys.2020.00747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Joob B, Wiwanitkit V. Blood viscosity of COVID-19 patient: a preliminary report. Am J Blood Res. 2021;11(1):93–95. [PMC free article] [PubMed] [Google Scholar]
- 42.Klein SK, Slim EJ, De Kruif MD, et al. Is chronic HIV infection associated with venous thrombotic disease? A systematic review. Neth J Med. 2005;63(4):129–136. [PubMed] [Google Scholar]
- 43.Venter C, Bezuidenhout JA, Laubscher GJ, et al. Erythrocyte, platelet, serum ferritin, and p-selectin pathophysiology implicated in severe hypercoagulation and vascular complications in COVID-19. Int J Mol Sci. 2020;21(21):8234. doi: 10.3390/ijms21218234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Al-Kuraishy HM, Al-Gareeb AI, Qusti S, Alshammari EM, Atanu FO, Batiha GE. Arginine vasopressin and pathophysiology of COVID-19: an innovative perspective. Biomed Pharmacother. 2021;143:112193. doi: 10.1016/j.biopha.2021.112193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Alamri HS, Alsughayyir J, Akiel M, et al. Stimulation of calcium influx and CK1α by NF-κB antagonist [6]-gingerol reprograms red blood cell longevity. J Food Biochem. 2021;45(1):e13545. doi: 10.1111/jfbc.13545. [DOI] [PubMed] [Google Scholar]
- 46.Nader E, Romana M, Connes P. The red blood cell—inflammation vicious circle in sickle cell disease. Front Immunol. 2020;11:454. doi: 10.3389/fimmu.2020.00454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Batiha GE, Al-Gareeb DAI, Qusti S, et al. Common NLRP3 inflammasome inhibitors and Covid-19: divide and Conquer. Sci Afr. 2021 doi: 10.1016/j.sciaf.2021.e01084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bedin AS, Makinson A, Picot MC, et al. Monocyte CD169 expression as a biomarker in the early diagnosis of coronavirus disease 2019. J Infect Dis. 2021;223(4):562–567. doi: 10.1093/infdis/jiaa724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Al-Kuraishy HM, Al-Gareeb AI, Al-Hussaniy HA, Al-Harcan NAH, Alexiou A, Batiha GE. Neutrophil extracellular traps (NETs) and Covid-19: a new frontiers for therapeutic modality. IntImmunopharmacol. 2022;104:108516. doi: 10.1016/j.intimp.2021.108516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Panigada M, Bottino N, Tagliabue P, et al. Hypercoagulability of COVID-19 patients in intensive care unit: a report of thromboelastography findings and other parameters of hemostasis. J Thromb Haemost. 2020;18(7):1738–1742. doi: 10.1111/jth.14850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ozkan S, Cakmak F, Konukoglu D, et al. Efficacy of serumangiotensin IIlevels in prognosis of patients with coronavirus Disease 2019. Crit Care Med. 2021 doi: 10.1097/CCM.0000000000004967. [DOI] [PubMed] [Google Scholar]
- 52.Buyan N, Akçaboy M, Göktaş T, et al. Effects of whole blood viscosity and plasma NOx on cardiac function and cerebral blood flow in children with chronic kidney disease. Turk J Med Sci. 2017;47(5):1482–1491. doi: 10.3906/sag-1609-33. [DOI] [PubMed] [Google Scholar]
- 53.Violi F, Ceccarelli G, Cangemi R, et al. Hypoalbuminemia, coagulopathy, and vascular disease in COVID-19. Circ Res. 2020;127(3):400–401. doi: 10.1161/CIRCRESAHA.120.317173. [DOI] [PubMed] [Google Scholar]
- 54.Bi X, Su Z, Yan H, et al. Prediction of severe illness due to COVID-19 based on an analysis of initial Fibrinogen to Albumin ratio and Platelet count. Platelets. 2020;31(5):674–679. doi: 10.1080/09537104.2020.1760230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ali MAM, Spinler SA. COVID-19 and thrombosis: from bench to bedside. Trends Cardiovasc Med. 2021;31(3):143–160. doi: 10.1016/j.tcm.2020.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Varga Z, Flammer AJ, Steiger P, et al. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020;395(10234):1417–1418. doi: 10.1016/S0140-6736(20)30937-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Felicetti L, Femminella M, Reali G. A molecular communications system for live detection of hyperviscosity syndrome. IEEE Trans Nanobioscience. 2020;19(3):410–421. doi: 10.1109/TNB.2020.2984880. [DOI] [PubMed] [Google Scholar]
- 58.Ulrich H, Pillat MM. CD147 as a target for COVID-19 treatment: suggested effects of azithromycin and stem cell engagement. Stem Cell Rev Rep. 2020;16(3):434–440. doi: 10.1007/s12015-020-09976-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cosic I, Cosic D, Loncarevic I. RRM prediction of erythrocyte Band3 protein as alternative receptor for SARS-CoV-2 virus. Appl Sci. 2020;10(11):4053. doi: 10.3390/app10114053. [DOI] [Google Scholar]
- 60.Foy BH, Carlson JCT, Reinertsen E, et al. Association of red blood cell distribution width with mortality risk in hospitalized adults with SARS-CoV-2 infection. JAMA Netw Open. 2020 doi: 10.1001/jamanetworkopen.2020.22058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kavanagh BD, Coffey BE, Needham D, Hochmuth RM, Dewhirst MW. The effect of flunarizine on erythrocyte suspension viscosity under conditions of extreme hypoxia, low pH, and lactate treatment. BrJ Cancer. 1993;67(4):734–741. doi: 10.1038/bjc.1993.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sloop GD, Garber DW. The effects of low-density lipoprotein and high-density lipoprotein on blood viscosity correlate with their association with risk of atherosclerosis in humans. Clin Sci (Lond) 1997;92(5):473–479. doi: 10.1042/cs0920473. [DOI] [PubMed] [Google Scholar]
- 63.Meurs I, Hoekstra M, van Wanrooij EJ, et al. HDL cholesterol levels are an important factor for determining the lifespan of erythrocytes. Exp Hematol. 2005;33(11):1309–1319. doi: 10.1016/j.exphem.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 64.Tanaka S, De Tymowski C, Assadi M, et al. Lipoprotein concentrations over time in the intensive care unit COVID-19 patients: results from the ApoCOVID study. PLoS ONE. 2020;15(9):e0239573. doi: 10.1371/journal.pone.0239573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hu X, Chen D, Wu L, He G, Ye W. Declined serum high density lipoprotein cholesterol is associated with the severity of COVID-19 infection. Clin Chim Acta. 2020;510:105–110. doi: 10.1016/j.cca.2020.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chang R, Mamun A, Dominic A, Le NT. SARS-CoV-2 mediated endothelial dysfunction: the potential role of chronic oxidative stress. Front Physiol. 2020;11:1752. doi: 10.3389/fphys.2020.605908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Becatti M, Marcucci R, Gori AM, et al. Erythrocyte oxidative stress is associated with cell deformability in patients with retinal vein occlusion. J Thromb Haemost. 2016;14(11):2287–2297. doi: 10.1111/jth.13482. [DOI] [PubMed] [Google Scholar]
- 68.Mortaz E, Malkmohammad M, Jamaati H, et al. Silent hypoxia: higherNO in red blood cells of COVID-19 patients. BMC PulmMed. 2020;20(1):269. doi: 10.1186/s12890-020-01310-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mahdi A, Collado A, Tengbom J, et al. Erythrocytes induce vascular dysfunction in COVID-19. Eur Heart J. 2021 doi: 10.1093/eurheartj/ehab724.3364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Fu W, Wang C, Zou L, et al. Psychological health, sleep quality, and coping styles to stress facing the COVID-19 in Wuhan, China. Transl Psychiatry. 2020;10(1):225. doi: 10.1038/s41398-020-00913-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Jeong JH, Hanevold C, Harris RA, et al. Angiotensin II receptor blocker attenuates stress pressor response in young adult African Americans. J Clin Hypertens (Greenwich) 2019;21(8):1191–1199. doi: 10.1111/jch.13625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tekin Tak B, Ekizler FA, Cay S, et al. Relationship between apical thrombus formation and blood viscosity in acute anterior myocardial infarction patients. Biomark Med. 2020;14(3):201–210. doi: 10.2217/bmm-2019-0483. [DOI] [PubMed] [Google Scholar]
- 73.Lee CH, Jung KH, Cho DJ, Jeong SK. Effect of warfarin versus aspirin on blood viscosity in cardioembolic stroke with atrial fibrillation: a prospective clinical trial. BMC Neurol. 2019;19(1):82. doi: 10.1186/s12883-019-1315-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lala A, Johnson KW, Januzzi JL, et al. Prevalence and impact of myocardial injury in patients hospitalized with COVID-19 infection. J Am Coll Cardiol. 2020;76(5):533–546. doi: 10.1016/j.jacc.2020.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Truong AD, Auld SC, Barker NA, et al. Therapeutic plasma exchange for COVID-19-associated hyperviscosity. Transfusion. 2021;61(4):1029–1034. doi: 10.1111/trf.16218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Song WC, FitzGerald GA. COVID-19, microangiopathy, hemostatic activation, and complement. J Clin Invest. 2020;130(8):3950–3953. doi: 10.1172/JCI140183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sloop GD, Pop GA, Weidman JJ, Cyr JA. The detrimental role of elevated blood viscosity in patients with COVID-19. Cardiol Ther. 2021;8(1):976–980. [Google Scholar]
- 78.Maier CL, Truong AD, Auld SC, Polly DM, Tanksley CL, Duncan A. COVID-19-associated hyperviscosity: A link between inflammation and thrombophilia? Lancet. 2020;395(10239):1758–1759. doi: 10.1016/S0140-6736(20)31209-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lenz C, Rebel A, Waschke KF, Koehler RC, Frietsch T. Blood viscosity modulates tissue perfusion–sometimes and somewhere. Transfus Altern Transfus Med. 2008;9(4):265–272. doi: 10.1111/j.1778-428X.2007.00080.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zubieta-Calleja G, Zubieta-DeUrioste N. Pneumolysis and ”silent hypoxemia” in COVID-19. Indian J Clin Biochem. 2021;36(1):112–116. doi: 10.1007/s12291-020-00935-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wijeratne T, Crewther S, Post C. Post-COVID 19 Neurological Syndrome (PCNS); a novel syndrome with challenges for the global neurology community. J NeurolSci. 2020;419:117179. doi: 10.1016/j.jns.2020.117179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Oronsky B, Larson C, Hammond TC, et al. A review of persistent post-COVID syndrome (PPCS) Clin Rev Allergy Immunol. 2021 doi: 10.1007/s12016-021-08848-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Novak P. Post COVID-19 syndrome associated with orthostatic cerebral hypoperfusion syndrome, small fiber neuropathy and benefit of immunotherapy: a case report. eNeurol Sci. 2020;21:100276. doi: 10.1016/j.ensci.2020.100276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Le Thanh T, Andreadakis Z, Kumar A, et al. The COVID-19 vaccine development landscape. Nat Rev Drug Discov. 2020;19(5):305–306. doi: 10.1038/d41573-020-00073-5. [DOI] [PubMed] [Google Scholar]
- 85.Khon K, Mungmungpuntipantip R, Wiwanitkit V. Post COVID-19 vaccination, increased blood viscosity and impact on laboratory investigation results. Sri Lanka J Child Health. 2021;50(4):747. doi: 10.4038/sljch.v50i4.9908. [DOI] [Google Scholar]
- 86.Dalakas MC. High-dose intravenous immunoglobulin and serum viscosity: risk of precipitating thromboembolic events. Neurology. 1994;44(2):223–226. doi: 10.1212/wnl.44.2.223. [DOI] [PubMed] [Google Scholar]
- 87.Yasri S, Wiwanitkit V. COVID-19 vaccine, contraceptive, viscosity and safety margin change. Vaccine. 2021;1:0–92. [Google Scholar]
- 88.Irace C, Scavelli F, Carallo C, Serra R, Gnasso A. Plasma and blood viscosity in metabolic syndrome. Nutr Metab Cardiovasc Dis. 2009;19(7):476–480. doi: 10.1016/j.numecd.2008.11.005. [DOI] [PubMed] [Google Scholar]
- 89.Gyawali P, Richards RS, Nwose EU, Bwititi PT. Whole-blood viscosity and metabolic syndrome. Clin Lipidol. 2012;7(6):709–719. doi: 10.2217/clp.12.65. [DOI] [Google Scholar]
- 90.Joob B, Wiwanitkit V. Change of blood viscosity after COVID-19 vaccination: estimation for persons with underlying metabolic syndrome. IntJ Physiol Pathophysiol Pharmacol. 2021;13(5):148–151. [PMC free article] [PubMed] [Google Scholar]
- 91.Long DS, Smith ML, Pries AR, Ley K, Damiano ER. Microviscometry reveals reduced blood viscosity and altered shear rate and shear stress profiles in microvessels after hemodilution. Proc Natl Acad Sci U S A. 2004;101(27):10060–10065. doi: 10.1073/pnas.0402937101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sharma O, Sultan AA, Ding H, Triggle CR. A review of the progress and challenges of developing a vaccine for COVID-19. Front Immunol. 2020;11:585354. doi: 10.3389/fimmu.2020.585354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Sookaromdee P, Wiwanitkit V. COVID-19 vaccine, immune thrombotic thrombocytopenia, jaundice, hyperviscosity: concern on cases with underlying liver problem. Ann Hepatol. 2021;24:100525. doi: 10.1016/j.aohep.2021.100525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.D’Onofrio L, Coraggio L, Zurru A, et al. Short-term safety profile of Sars-Cov2 vaccination on glucose control: continuous glucose monitoring data in people with autoimmune diabetes. Diabetes Res Clin Pract. 2021;179:109022. doi: 10.1016/j.diabres.2021.109022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Nwose EU, Richards RS, Bwititi P, Butkowski E. Serum bilirubin and lipoprotein-a: how are these associated with whole blood viscosity? Redox Rep. 2012;17(1):8–13. doi: 10.1179/1351000211Y.0000000017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Furukawa S, Fujita T, Shimabukuro M, et al. Increased oxidative stress in obesity and its impact on metabolic syndrome. J Clin Invest. 2004;114(12):1752–1761. doi: 10.1172/JCI21625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Solá E, Vayá A, Simó M, et al. Fibrinogen, plasma viscosity and blood viscosity in obesity. Relationship with insulin resistance. Clin Hemorheol Microcirc. 2007;37(4):309–318. [PubMed] [Google Scholar]
- 98.Pivonello C, Negri M, Pivonello R, Colao A. How may obesity-induced oxidative stress affect the outcome of COVID-19 accines? Lesson Learn Infect Stresses. 2021;1(2):119–122. [Google Scholar]
- 99.Painter SD, Ovsyannikova IG, Poland GA. The weight of obesity on the human immune response to vaccination. Vaccine. 2015;33(36):4422–4429. doi: 10.1016/j.vaccine.2015.06.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.