Abstract
Background
Thyroid storm (TS) complicated by cardiogenic shock is associated with high mortality due to the high incidence of multiple organ failure. It is recommended that TS patients with hepatic failure undergo plasma exchange (PE) and receive optimal anti-hyperthyroid medications. However, the effect of PE on cardiac dysfunction in TS patients has been unclear.
Case summary
A 46-year-old woman was admitted to our hospital for dyspnoea and tachycardia. She was diagnosed with TS pursuant to Graves’ disease complicated by acute decompensated heart failure (ADHF). Cardiac function was remarkably impaired [left ventricular ejection fraction (LVEF) = 15–20%], with rapid atrial fibrillation. Despite the management of both ADHF and hyperthyroidism, cardiogenic shock developed; therefore, veno-arterial extracorporeal membrane oxygenation (VA-ECMO) and intra-aortic balloon pumping (IABP) were initiated. Plasma exchange was performed after severe hepatic failure manifested on Day 2. After the first three PE treatments, cardiac and hepatic function improved immediately but deteriorated the next day. The improvement persisted after the fourth PE, and the patient was weaned from VA-ECMO and IABP on Days 10 and 11, respectively. She was discharged on Day 37, and her cardiac function was still normal 1.5 years later.
Discussion
In hyperthyroidism, severe hepatic dysfunction is more likely to occur in patients with acute ADHF than in those without it. Plasma exchange has the potential to improve not only hepatic but also cardiac dysfunction under optimal antithyroid treatment, especially in patients with TS complicated by severe hepatic dysfunction.
Keywords: Case report, Thyroid storm, Graves’ disease, Cardiogenic shock, Percutaneous mechanical circulatory support, Plasma exchange
Learning points.
Plasma exchange (PE) has the potential to improve cardiac function in patients with thyroid storm, especially in those with hepatic dysfunction.
Simultaneous mechanical circulatory support and PE can be tolerated by critically ill patients, which may help improve haemodynamics and both cardiac and hepatic function over the course of medical treatment for thyrotoxicosis.
Although the optimal medical therapies for post-TS heart failure have not been defined, careful observation after discharge is needed.
Introduction
Thyroid storm (TS) is an endocrinological crisis complicated by heart failure (HF) and multiple organ dysfunction.1 Several reports have described the important role of early plasma exchange (PE) as an effective salvage therapy for lowering thyroid hormone levels in patients with TS complicated by multiple organ failure.2,3 Current guidelines indicate that PE may be a second-line treatment in patients with hepatic failure and disturbance of consciousness or those with resistance to first-line anti-hyperthyroid medical treatments.4,5 However, the effects of PE on both hepatic and cardiac dysfunction in TS patients remain unclear.
Timeline
| Three years before admission: Diagnosed with hyperthyroidism, but received no medical follow-up. |
| Three months before admission: Exertional dyspnoea was observed under extraordinal physical activity (New York Heart Association functional classification II). |
| Day 0: Admitted to our hospital with thyroid storm (TS) complicated by cardiogenic shock. Exhibited extremely low left ventricular ejection fraction (LVEF 15–20%) and atrial fibrillation. |
| Intubated and began treatment with veno-arterial extracorporeal membrane oxygenation (VA-ECMO) and intra-aortic balloon pumping (IABP) to support haemodynamics. |
| Day 2: Reverted to sinus rhythm. First plasma exchange (PE) due to hepatic failure [% prothrombin time (%PT) = 20%]. After PE, cardiac function transiently improved. |
| Day 3: %PT deteriorated from 65 to 38%. Underwent second PE. After PE, cardiac function transiently improved. |
| Day 4: %PT deteriorated from 74 to 51%. Underwent third PE. After PE, cardiac function transiently improved. |
| Day 7: %PT deteriorated to 21%. Underwent fourth PE. After PE, both cardiac and hepatic dysfunction improved simultaneously. |
| Day 10: Weaned from VA-ECMO. |
| Day 11: Weaned from IABP. |
| Day 14: Extubated. |
| Day 37: Discharged from our hospital with LVEF 50–55%. |
| One and a half years: Left ventricular ejection fraction and brain natriuretic peptide level normalized without any signs of heart failure. |
Case presentation
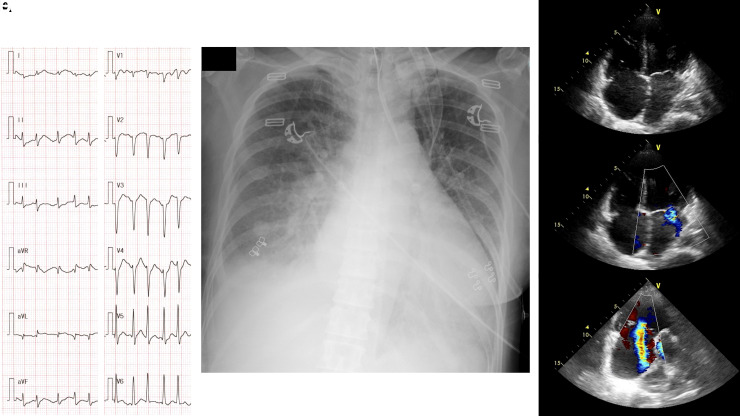
A 46-year-old woman with a 3-month history of exertional dyspnoea was referred to our hospital for dyspnoea at rest. She was diagnosed with hyperthyroidism 3 years before admission but did not receive any medical follow-up. In our emergency room, her body temperature was 38.5°C, her blood pressure was 181/131 mmHg, her pulse was irregular at 198 beats per minutes (the electrocardiogram indicated atrial fibrillation), her respiratory rate was 36 breaths per minute, and her arterial oxygen saturation was 90% on room air. Physical examination revealed exophthalmos, thyromegaly, and pretibial myxoedema. Laboratory data revealed an elevated plasma brain natriuretic peptide (BNP) level (973.9 pg/mL) and prolonged % prothrombin time (%PT, 62%). Hyperthyroidism was confirmed (free T4, >7.7 ng/dL; free T3, 25.5 pg/mL; TSH, <0.01 μIU/mL) and TSH receptor antibody was positive (39.1 U/L). Blood gas analysis revealed metabolic acidosis (pH 7.210, HCO3− 8.7 mmol/L) and an elevated lactate acid level (11.0 mmol/L). Chest X-ray revealed cardiomegaly and pulmonary congestion. Echocardiography revealed a severely reduced left ventricular ejection fraction (LVEF, 15–20%) and severe tricuspid regurgitation (Figure 1, see Supplementary material online, Video S1). Based on these findings, the patient was admitted to our hospital with a diagnosis of TS due to Graves’ disease complicated by cardiogenic shock.
Figure 1.
Electrocardiogram (A), chest X-ray (B), and echocardiography (C–E) on admission. Electrocardiogram revealed atrial fibrillation (heart rate, 198 beats per minute). Chest X-ray revealed cardiomegaly (cardiothoracic ratio 60%) and congestion. Echocardiography indicated deterioration of the left ventricular ejection fraction (15–20%) with bi-atrial enlargement (C), mild mitral regurgitation (D), and severe tricuspid regurgitation (E).
Despite the administration of nitroglycerin and landiolol, an intravenous beta-blocker, her symptoms, and respiratory state deteriorated, leading to intubation. Thiamazole, potassium iodide, and hydrocortisone were initiated to treat hyperthyroidism. However, 9 h after admission, hypotension (80 mmHg) and oliguria developed, with an elevated lactate acid level (13.2 mmol/L). Because of the diagnosis of cardiogenic shock, veno-arterial extracorporeal membrane oxygenation (VA-ECMO) was initiated for oxygenation and biventricular circulatory support, and intra-aortic balloon pumping (IABP) was started for left ventricular unloading during VA-ECMO support.6 After the introduction of VA-ECMO and IABP, right heart catheter examination revealed a decrease in mean pulmonary artery wedge pressure and mean right atrial pressure (from 18 to 14 mmHg and 25 to 6 mmHg, respectively), and an increase in mixed venous oxygen saturation (SvO2) (from 54 to 77%). To assess whether the patient could be weaned from VA-ECMO, the corrected left ventricular ejection time (LVETc) and left ventricular outflow tract velocity-time integral (LVOT-VTI) were evaluated repeatedly.7 Immediately after VA-ECMO initiation, the LVETc and LVOT-VTI on echocardiography were 191 ms and 5.4 cm, respectively. Atrial fibrillation spontaneously reverted to sinus rhythm 30 h after admission (Table 1).
Table 1.
Parameter of echocardiography
| Day | 1 | 2 | 2 after 1st PE | 3 | 3 after 2nd PE | 4 | 4 after 3rd PE | 5 | 6 | 7 | 7 after 4th PE | 10 before ECMO withdrawal | 37 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| VA-ECMO support (L/min) | 4.1 | 3.6 | 3.7 | 3.9 | 3.7 | 3.8 | 2.3 | 3.9 | 3.8 | 3.7 | 2.2 | 2.0 | |
| Rhythm | Af | SR | SR | SR | SR | SR | SR | SR | SR | SR | SR | SR | SR |
| Heart rate (bpm) | 122 | 94 | 93 | 79 | 92 | 53 | 52 | 46 | 60 | 54 | 57 | 80 | 73 |
| LVDd (mm) | 48 | 47 | 46 | 50 | 51 | 49 | 52 | 49 | 45 | 42 | 51 | 48 | 53 |
| LVDs (mm) | 43 | 42 | 40 | 42 | 41 | 41 | 37 | 40 | 43 | 38 | 44 | 37 | 39 |
| LVEF (%) | 21 | 23 | 27 | 33 | 37 | 35 | 53 | 40 | 12 | 19 | 30 | 46 | 51 |
| LVOT-VTI (cm) | 5.4 | 9.5 | 14.8 | 13.3 | 14.4 | 11.5 | 20 | 11.9 | 4.8 | 5.5 | 13.9 | 15.2 | 22 |
| TAPSE (cm) | 11.0 | 11.1 | 13.4 | 13.7 | 15.5 | 15.5 | 23.4 | 14.3 | 8.69 | 8.76 | 19.3 | 16.8 | 20.3 |
| PAWP (mmHg) | 14 | 16 | 13 | 13 | 16 | 18 | 17 | 13 | 14 | 13 | 13 | 14 | |
| RAP (mmHg) | 6 | 11 | 7 | 7 | 10 | 14 | 13 | 9 | 11 | 10 | 11 | 7 | |
| CI (L/min/m2) | 1.8 | 2.4 | 2.3 | 2.3 | N/A | 1.6 | 3.3 | 1.9 | N/A | N/A | 2.4 | 4.2 |
LVEF was calculated by the Teichholz method. LVEF, LVOT-VTI, and TAPSE improved after plasma exchange and deteriorated the next day. PE, plasma exchange; ECMO, extracorporeal membrane oxygenation; Af, atrial fibrillation; SR, sinus rhythm; LVDd, left ventricular end-diastolic diameter; LVDs, left ventricular end-systolic diameter; LVEF, left ventricular ejection fraction; LVOT-VTI, left ventricular out tract velocity-time integral; TAPSE, tricuspid annular plane systolic excursion; PAWP, pulmonary artery wedge pressure; RAP, right atrial pressure; CI, cardiac index.
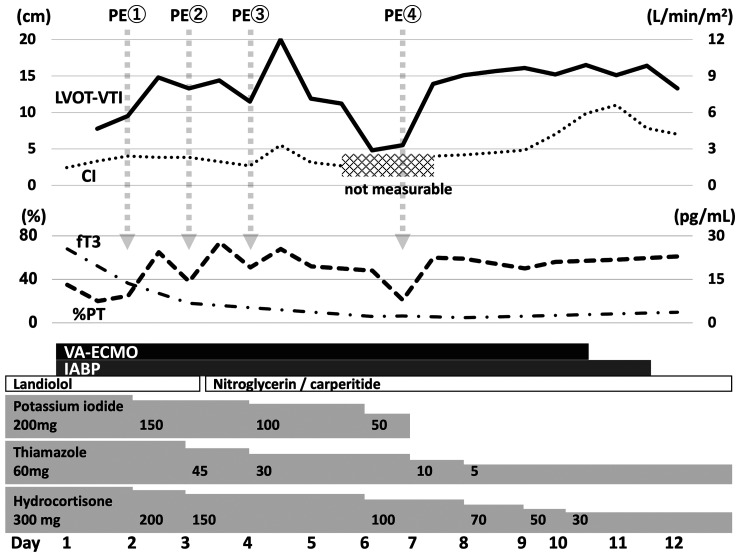
On Day 2, hepatic failure became apparent (total bilirubin, 5.7 mg/dL; %PT, 20%), and plasma exchange (PE) was started. Total bilirubin and PT transiently improved after the first PE but worsened the next day. Consequently, second and third PEs were performed on Days 3 and 4, respectively. Interestingly, before and after each PE, both cardiac and hepatic function improved. In particular, before the third PE, the LVETc, LVOT-VTI, and %PT were 208 ms, 11.5 cm, and 51%, respectively (Figure 2 and Video 1). After the third PE, each parameter immediately improved (LVETc, 222 ms; LVOT-VTI, 20 cm; %PT, 68%), and blood pressure was markedly elevated, leading to a reduction of VA-ECMO flow support from 3.7 to 2.2 L/min (Video 2). However, cardiac and hepatic dysfunction was again obvious by Day 6 (LVETc, 188 ms; LVOT-VTI, 4.8 cm; %PT, 21%; Figure 2 and Video 3); thus, a fourth PE was performed on Day 7. This led to improvements in both cardiac and hepatic function, and cardiac parameters persisted on the next day (LVETc, 232 ms; LVOT-VTI, 13.9 cm; %PT, 60%) (Figure 2 and see Supplementary material online, Video S2). Subsequently, cardiac and hepatic function stabilized without any additional PE treatments, followed by withdrawal of VA-ECMO and IABP on Days 10 and 11, respectively (Figure 2 and see Supplementary material online, Video S3). To maintain VA-ECMO and IABP support, intravenous heparin was administered at an activated partial thromboplastin time that was 1.5–2 times higher than the normal range despite the presence of hepatic dysfunction. Intravenous vasodilators (specifically, nitroglycerin and carperitide), but not any inotropes, were used to optimize the afterload of VA-ECMO because systemic and pulmonary pressures were temporarily elevated by recovery of cardiac function after each PE treatment. No life-threatening ventricular arrhythmia was observed in this patient during hospitalization. Additionally, rapid atrial fibrillation resolved on Day 2 after VA-ECMO administration, and sinus rhythm was maintained thereafter. Hence, intravenous amiodarone was not administered, because amiodarone itself can cause thyrotoxicosis. Left ventricular ejection fraction improved to 50–55% (Figure 3 and see Supplementary material online, Video S4), and the patient was discharged from our hospital on Day 37 under treatment with thiamazole and bisoprolol. The patient has since undergone medical follow-up every 3 months while receiving thiamazole. Bisoprolol was discontinued 6 months after discharge due to the absence of sinus tachycardia and signs of HF. At 1.5 years after discharge, LVEF and plasma BNP levels were normal, without any signs of HF (Figure 3 and see Supplementary material online, Video S5).
Figure 2.
Clinical course after admission. Left ventricular outflow tract velocity-time integral, cardiac index, and triiodothyronine levels are shown as graphs. Plasma exchange was initiated to treat hepatic failure. After plasma exchange, left ventricular outflow tract velocity-time integral and % prothrombin time improved simultaneously. In particular, left ventricular outflow tract velocity-time integral and cardiac index improved after the third and fourth plasma exchanges. PE, plasma exchange; VA-ECMO, veno-arterial extracorporeal membrane oxygenation; IABP, intra-aortic balloon pumping.
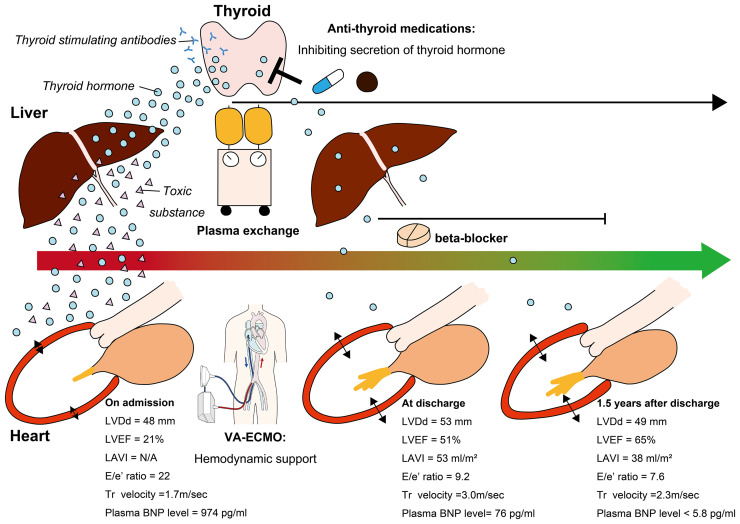
Figure 3.
Treatments, clinical time course, and proposed mechanism of illness resolution. This patient underwent plasma exchange to remove excessive thyroid hormone, cardio-depressants, thyroid-stimulating autoantibodies, and toxic substances generated due to liver dysfunction. Veno-arterial extracorporeal membrane oxygenation was also performed to maintain haemodynamics during the cardio-depressive state. Both procedures were well tolerated. Simultaneously, anti-thyroid medications and a beta-blocker were administered to control secretion of thyroid hormone and prevent it from affecting downstream organs, including the heart. The patient could be weaned from veno-arterial extracorporeal membrane oxygenation following plasma exchange. Close follow-up enabled normalization of cardiac structure and function by optimizing medical therapy for hyperthyroidism and beta-blocker treatment for heart failure. BNP, brain natriuretic peptide; LAVI, left atrial volume index; LVDD, left ventricular end-diastolic dimension size; LVEF, left ventricular ejection fraction; Tr, tricuspid regurgitation; VA-ECMO, veno-arterial extracorporeal membrane oxygenation.
Discussion
Thyroid storm, a manifestation of hyperthyroidism, is associated with substantial morbidity and mortality and requires prompt recognition and treatment.1 Regarding cardiohepatic interaction, hyperthyroidism complicated by HF is associated with severe hepatic dysfunction due to hypoxic liver injury.8 Plasma exchange is an adjunctive treatment for TS patients who experience such liver abnormalities and who are adequately treated with anti-hyperthyroid agents.4,5 However, the effect of PE on the recovery of cardiac dysfunction in TS patients has been unclear.9 A previous report described a patient with TS complicated by cardiogenic shock who could be weaned from mechanical circulatory support after PE treatment, indicating that PE has the potential to improve cardiac function in TS patients, especially those with hepatic dysfunction.2 The causes of cardiac dysfunction associated with thyrotoxicosis are reduced myocardial contractility due to exposure to excess T3,10 volume overload resulting from increased blood volume, and supraventricular ectopic activity followed by the onset of atrial fibrillation and tachycardia-induced cardiomyopathy. Repetitive PE treatment can replace plasma proteins bound to toxins generated due to hepatic dysfunction, as well as those bound to excessive thyroid hormones, catecholamines, and cardio-depressant autoantibodies that arise while receiving anti-hyperthyroid medications.4,11 Simultaneous ECMO and PE can be tolerated by critically ill patients.12 In the present case, these treatments may have helped gradually improve haemodynamics, as well as cardiac and hepatic function, over the course of medical treatment for thyrotoxicosis (Figure 3). Finally, the patient in this case demonstrated recovery from HF with reduced ejection fraction due to TS, and cardiac function was normal 1.5 years after discharge. The ideal medical therapies for HF secondary to TS are not specified in the current ESC guidelines, and close follow-up intervals are needed to optimize medications in post-TS patients who have been recently discharged from the hospital.13
Conclusion
We highlight a patient with TS complicated by cardiogenic shock and severe hepatic dysfunction who required VA-ECMO but who experienced continuous, gradual cardiac improvement by sequential PE, followed by successful weaning from VA-ECMO and rescue from thyrotoxicosis.
Lead author biography

Toshiaki Suzuki graduated from Hamamatsu University School of Medicine in Japan in 2012. From April 2020 to March 2022, he worked as a clinical fellow at the cardiovascular care unit of the National Cerebral and Cardiovascular Center. Currently, he is working as a interventinal cardiologist at the Seirei Hamamatsu hospital, Hamamatsu, Japan. His research interest is cardiac intensive care and percutaneous coronary intervention.
Supplementary material
Supplementary material is available at European Heart Journal – Case Reports online.
Supplementary Material
Acknowledgements
We thank Drs Tetsuya Arisato and Fumiki Yoshihara for determining the indication of serial plasma exchanges, Mr Kodai Hirashima for providing care for mechanical circulatory supports (e.g. VA-ECMO and IABP) and plasma exchanges, Dr Tamiko Tamanaha for providing full medical treatments of hyperthyroidism, and all members of the medical staff in our cardiovascular care unit.
Slide sets: A fully edited slide set detailing this case and suitable for local presentation is available online as Supplementary data.
Consent: The authors confirm that the patient gave written consent for the submission and publication of this case report, including images and associated text, in line with COPE guidance.
Conflict of interest: Y.A. has also received a research support from Terumo. The remaining authors have disclosed that they do not have any conflicts of interest.
Funding: This case report was supported in part by a Grants-in-Aid for Scientific Research (KAKENHI grant 21K08044 to Y.A.).
References
- 1.Angell TE, Lechner MG, Nguyen CT, Salvato VL, Nicoloff JT, LoPresti JS. Clinical features and hospital outcomes in thyroid storm: a retrospective cohort study. J Clin Endocrinol Metab 2015;100:451–459. [DOI] [PubMed] [Google Scholar]
- 2.Koh H, Kaushik M, Loh JK, Chng CL. Plasma exchange and early thyroidectomy in thyroid storm requiring extracorporeal membrane oxygenation. Endocrinol Diabetes Metab Case Rep 2019;2019:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sasaki K, Yoshida A, Nakata Y, Mizote I, Sakata Y, Komuro I. A case of thyroid storm with multiple organ failure effectively treated with plasma exchange. Intern Med 2011;50:2801–2805. [DOI] [PubMed] [Google Scholar]
- 4.Padmanabhan A, Connelly-Smith L, Aqui N, Balogun RA, Klingel R, Meyer E, Pham HP, Schneiderman J, Witt V, Wu Y, Zantek ND, Dunbar NM. Guidelines on the use of therapeutic apheresis in clinical practice – evidence-based approach from the writing committee of the American society for apheresis: the eighth special issue. J Clin Apher 2019;34:171–354. [DOI] [PubMed] [Google Scholar]
- 5.Satoh T, Isozaki O, Suzuki A, Wakino S, Iburi T, Tsuboi K, Kanamoto N, Otani H, Furukawa Y, Teramukai S, Akamizu T. 2016 Guidelines for the management of thyroid storm from The Japan Thyroid Association and Japan Endocrine Society (first edition). Endocr J 2016;63:1025–1064. [DOI] [PubMed] [Google Scholar]
- 6.Russo JJ, Aleksova N, Pitcher I, Couture E, Parlow S, Faraz M, Visintini S, Simard T, Di Santo P, Mathew R, So DY, Takeda K, Garan AR, Karmpaliotis D, Takayama H, Kirtane AJ, Hibbert B. Left ventricular unloading during extracorporeal membrane oxygenation in patients with cardiogenic shock. J Am Coll Cardiol 2019;73:654–662. [DOI] [PubMed] [Google Scholar]
- 7.Sawada K, Kawakami S, Murata S, Nishimura K, Tahara Y, Hosoda H, Nakashima T, Kataoka Y, Asaumi Y, Noguchi T, Sugimachi M, Fujita T, Kobayashi J, Yasuda S. Predicting parameters for successful weaning from veno-arterial extracorporeal membrane oxygenation in cardiogenic shock. ESC Heart Fail 2021;8:471–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fong TL, McHutchison JG, Reynolds TB. Hyperthyroidism and hepatic dysfunction. A case series analysis. J Clin Gastroenterol 1992;14:240–244. [DOI] [PubMed] [Google Scholar]
- 9.Ligtenberg J, Tulleken J, Zijlstra J. Plasmapheresis in thyrotoxicosis. Ann Intern Med 1999;131:71–72. [DOI] [PubMed] [Google Scholar]
- 10.Yu YH, Bilezikian JP. Tachycardia-induced cardiomyopathy secondary to thyrotoxicosis: a young man with previously unrecognized Graves’ disease. Thyroid 2000;10:923–927. [DOI] [PubMed] [Google Scholar]
- 11.Patel PA, Hernandez AF. Targeting anti-beta-1-adrenergic receptor antibodies for dilated cardiomyopathy. Eur J Heart Fail 2013;15:724–729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dyer M, Neal MD, Rollins-Raval MA, Raval JS. Simultaneous extracorporeal membrane oxygenation and therapeutic plasma exchange procedures are tolerable in both pediatric and adult patients. Transfusion 2014;54:1158–1165. [DOI] [PubMed] [Google Scholar]
- 13.McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Bohm M, Burri H, Butler J, Čelutkienė J, Chioncel O, Cleland JGF, Coats AJS, Crespo-Leiro MG, Farmakis D, Gilard M, Heymans S, Hoes AW, Jaarsma T, Jankowska EA, Lainscak M, Lam CSP, Lyon AR, McMurray JJV, Mebazaa A, Mindham R, Muneretto C, Francesco Piepoli M, Price S, Rosano GMC, Ruschitzka F, Kathrine Skibelund A, de Boer RA, Christian Schulze P, Abdelhamid M, Aboyans V, Adamopoulos S, Anker SD, Arbelo E, Asteggiano R, Bauersachs J, Bayes-Genis A, Borger MA, Budts W, Cikes M, Damman K, Delgado V, Dendale P, Dilaveris P, Drexel H, Ezekowitz J, Falk V, Fauchier L, Filippatos G, Fraser A, Frey N, Gale CP, Gustafsson F, Harris J, Iung B, Janssens S, Jessup M, Konradi A, Kotecha D, Lambrinou E, Lancellotti P, Landmesser U, Leclercq C, Lewis BS, Leyva F, Linhart A, Løchen M-L, Lund LH, Mancini D, Masip J, Milicic D, Mueller C, Nef H, Nielsen J-C, Neubeck L, Noutsias M, Petersen SE, Sonia Petronio A, Ponikowski P, Prescott E, Rakisheva A, Richter DJ, Schlyakhto E, Seferovic P, Senni M, Sitges M, Sousa-Uva M, Tocchetti CG, Touyz RM, Tschoepe C, Waltenberger J, Adamo M, Baumbach A, Böhm M, Burri H, Čelutkienė J, Chioncel O, Cleland JGF, Coats AJS, Crespo-Leiro MG, Farmakis D, Gardner RS, Gilard M, Heymans S, Hoes AW, Jaarsma T, Jankowska EA, Lainscak M, Lam CSP, Lyon AR, McMurray JJV, Mebazaa A, Mindham R, Muneretto C, Piepoli MF, Price S, Rosano GMC, Ruschitzka F, Skibelund AK. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J 2021;42:3599–3726. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.