Abstract
Rapid, direct methods are needed to assess active bacterial populations in water and foods. Our objective was to determine the efficiency of bacterial detection by immunomagnetic separation (IMS) and the compatibility of IMS with cyanoditolyl tetrazolium chloride (CTC) incubation to determine respiratory activity, using the pathogen Escherichia coli O157:H7. Counterstaining with a specific fluorescein-conjugated anti-O157 antibody (FAb) following CTC incubation was used to allow confirmation and visualization of bacteria by epifluorescence microscopy. Broth-grown E. coli O157:H7 was used to inoculate fresh ground beef (<17% fat), sterile 0.1% peptone, or water. Inoculated meat was diluted and homogenized in a stomacher and then incubated with paramagnetic beads coated with anti-O157 specific antibody. After IMS, cells with magnetic beads attached were stained with CTC and then an anti-O157 antibody-fluorescein isothiocyanate conjugate and filtered for microscopic enumeration or solid-phase laser cytometry. Enumeration by laser scanning permitted detection of ca. 10 CFU/g of ground beef or <10 CFU/ml of liquid sample. With inoculated meat, the regression results for log-transformed respiring FAb-positive counts of cells recovered on beads versus sorbitol-negative plate counts in the inoculum were as follows: intercept = 1.06, slope = 0.89, and r2 = 0.95 (n = 13). The corresponding results for inoculated peptone were as follows: intercept = 0.67, slope = 0.88, and r2 = 0.98 (n = 24). Recovery of target bacteria on beads by the IMS-CTC-FAb method, compared with recovery by sorbitol MacConkey agar plating, yielded greater numbers (beef, 6.0 times; peptone, 3.0 times; water, 2.4 times). Thus, within 5 to 7 h, the IMS-CTC-FAb method detected greater numbers of E. coli O157 cells than were detected by plating. The results show that the IMS-CTC-FAb technique with enumeration by either fluorescence microscopy or solid-phase laser scanning cytometry gave results that compared favorably with plating following IMS.
In environmental microbiology, the need for rapid methods to detect specific bacteria and confirm their viability or metabolic activity has been widely acknowledged. It is well known that traditional culture methods for detecting indicator and pathogenic bacteria in food and water may underestimate numbers due to sublethal environmental injury, inability of target bacteria to take up nutrient components in the medium, and other physiological factors which reduce culturability (18, 20, 28). Existing cultural methods for examining water and foods generally require enrichment followed by identification of the contaminating bacteria. More sensitive, rapid microbial detection methods are needed for many applications to complement or replace these traditional culture procedures, which require at least 1 day before definitive results are available. In the food industry, the U.S. Department of Agriculture has recently adopted Hazard Analysis and Critical Control Point procedures and enhanced inspection protocols to ensure the safety of meat products. Recently introduced food inspection regulations will require postharvest microbiological assays. In the event of an outbreak, rapid methods can improve the probability of identifying the source and, ultimately, controlling the spread of infections. Other examples include the monitoring of spacecraft water prior to launch (23) and the examination of military environments for possible biological warfare agents. The use of more rapid methods for confirmation, including immunomagnetic separation (IMS) and fluorescent-antibody (FAb) identification, has become more common in the last several years (9, 10, 12, 15, 24, 25, 31, 35).
Since the 1992 outbreak of enterohemorrhagic Escherichia coli (EHEC) infections from the consumption of hamburger in some fast-food restaurants in the northwestern United States (6), other outbreaks of EHEC infections have been attributed to a variety of sources (11), including ground beef (2), alfalfa sprouts (3), apple cider (7), and water (13, 30). EHEC infections caused by several serotypes, as well as O157:H7, have been reported in countries around the world (1, 3, 8, 21, 32, 33).
Traditional culture methods typically lack the sensitivity needed for direct detection of environmental pathogens. Thus, enrichment cultures are used so that low numbers of pathogens can multiply to detectable numbers before confirmatory tests are applied, and there is a considerable time delay from sampling until the results are available. IMS is being used more often for the detection of pathogens in food and other specimens following enrichment culture (9, 25, 32, 33, 35). One objective of the research reported here was to adapt IMS for direct detection of pathogens such as E. coli O157:H7 from food and water without enrichment culture.
FAbs have been used to confirm and enumerate E. coli O157:H7 bacteria by a direct epifluorescent filter technique (31). FAbs have also been used in conjunction with IMS (12, 24) for detection of pathogens, including E. coli O157:H7, in meat and other samples. Without enrichment culture, there is no proven way of determining the viability or metabolic activity of bacteria detected by IMS and FAb staining. We have incorporated incubation with a respiratory fluorochrome, cyanoditolyl tetrazolium chloride (CTC) (22, 27, 29), to determine the respiratory activity of cells captured by IMS. Our hybrid technique includes IMS of target bacteria, incubation with CTC to determine respiratory activity, staining with a direct FAb to identify the captured cells, and enumeration by epifluorescence microscopy or solid-phase laser scanning. To facilitate rapid enumeration and increase sensitivity, we used a laser scanning cytometer (Scan RDI; Chemunex, Inc.). This is based on direct solid-phase laser scanning to detect fluorescently stained bacteria on a membrane filter (19, 34). The system has been validated for routine microbiology quality control analysis of pharmaceutical-grade water (14).
The novel approach reported here for the direct detection of respiring E. coli O157:H7 can be completed in less than an 8-h working day. This is a significant advantage over existing techniques for detecting active EHEC which typically require a 24-h incubation for preenrichment, followed by confirmatory tests (15).
MATERIALS AND METHODS
Bacterial strains and culture conditions.
A human isolate of E. coli O157:H7, strain 3A-3299/85, was obtained from R. Wilson, Pennsylvania State University, University Park, Pa. Strain 932 was supplied by the U.S. Environmental Protection Agency in Cincinnati, Ohio.
Unless stated otherwise, dehydrated media were obtained from Difco and chemicals were obtained from Sigma. Culture slopes were subcultured on tryptone lactose yeast extract (TLY) agar and incubated overnight at 35°C. Growth was harvested in 20% glycerol–2% peptone and stored at −85°C. Cultures were streaked on MacConkey agar, sorbitol MacConkey (SMAC) agar, and sheep blood agar plates to confirm purity. Identification was confirmed by using the API 20E system (bioMérieux).
Yeast tryptone medium (4) was inoculated with a frozen stock culture and incubated for 20 h at 35°C and 150 rpm. Cells were collected by microcentrifugation (2,500 rpm for 2 min; DuPont Sorvall MC12V microcentrifuge), resuspended in one half of the volume of 0.1% peptone, and vortexed for 1 min. The suspension was passed through a 5-μm-pore-size nylon mesh filter to remove clumps, vortexed for 1 min, and diluted to ca. 108 CFU/ml by adjusting the optical density to 50 Klett units (Klett-Summerson Photoelectric Colorimeter). The final suspension was diluted to obtain the required numbers of cells for inoculation of liquid or meat samples.
Immunomagnetic beads.
Superparamagnetic beads with a nominal diameter of 0.6 μm (Bangs Laboratories, Inc.) were coated with anti-O157 rabbit serum (Difco). The adsorption procedure was adapted from the sodium phosphate–phosphate-buffered saline (PBS) method (5). The beads (0.1 ml of a 2% suspension) were removed from suspension with a magnet and resuspended in 0.344 ml of phosphate buffer (pH 5.5) containing 0.056 ml of antiserum. The suspension was incubated for 1 h while being mixed at 60 rpm at room temperature. After being coated, the beads were rinsed by removal from the solution with a magnet and resuspension in 0.4 ml of phosphate buffer, rinsed again in 0.1 ml of PBS (pH 7.5), and stored in 0.1 ml of PBS with 3% bovine serum albumin (BSA) at 4°C. Coated beads were refrigerated for up to 1 month before use.
Sample preparation.
Suspensions prepared as described above were used to inoculate liquid suspensions of water, 0.1% peptone, PBS, PBS-BSA, and enzyme-detergent solution. Beef hamburger was freshly ground on request at local supermarkets, transported to the laboratory within 10 min at ambient temperature, and refrigerated for up to 4 h before use. A 1-g sample of ground beef was weighed into a stomacher ’80’ bag (4 by 6 in.; Seward), and 0.1 ml of an O157 suspension (101 to 107 CFU/ml) was added. The meat and suspension were mixed by massaging the bag manually for 5 min. Peptone (20 ml of a 0.1% solution) was added before the bag was sealed with a heated pouch sealer (Kapak Corp.). The meat and peptone were homogenized in a stomacher (Seward) for 2 min. The bag was opened aseptically, and 20 ml of enzyme-detergent solution (26) was added. The bag was resealed and incubated for 20 min at room temperature with mixing at 120 rpm on an orbital shaker. After homogenization for 2 min in the stomacher, the contents were passed through a 25-mm-diameter coarse plastic Swinnex filter screen (Millipore) without filter paper to remove fibrous material. Uninoculated control samples were also tested to confirm the absence of detectable numbers of E. coli O157 bacteria.
IMS.
A sample of liquid or meat homogenate suspension was removed, and 0.01 ml of immunomagnetic beads coated with anti-O157 antibody per ml of sample was added. The vial was incubated at room temperature horizontally at 60 rpm for 1 h. An aliquot was transferred to a sterile vial, and the remaining suspension was retained for plating.
Superparamagnetic particles were removed from the samples by using a magnetic particle concentrator (Dynal) and locating the magnetic concentrator and tube horizontally for 10 min for liquid samples and 30 min for meat suspensions. The supernatant was removed with a long Pasteur pipette, taking care not to disturb the tube contents and retaining the supernatant.
Plating.
Beads were resuspended in the original volume of PBS or 0.1% peptone for plating. The original inoculum, suspensions retained prior to bead removal, resuspended beads, and supernatant removed from the beads were plated on SMAC, TLY, and TLYD (TLY with 0.1% deoxycholate) agar. Plates were incubated for 24 h at 35°C. Sorbitol-negative colonies on SMAC agar were enumerated as E. coli O157:H7, and the results from TLY and TLYD plates were compared to determine injury (28).
CTC incubation and filtration.
For liquid samples, beads were resuspended to the original volume and 1 ml was filtered through a 25-mm-diameter black polycarbonate membrane with a 0.2-μm pore size (Millipore) and incubated for 3 h in the dark at room temperature on an absorbent pad (Millipore) saturated with 0.6 ml of 5 mM CTC (Polysciences) in 0.1% peptone. The filter was transferred to a pad saturated with 0.6 ml of 3.7% formalin and incubated for at least 5 min at room temperature or stored in a refrigerator overnight. Beads removed from a hamburger mixture were either resuspended and incubated on pads as described above or suspended in 0.5 ml of 5 mM CTC and incubated in solution for 3 h.
FAb staining.
The filters incubated on a pad saturated with CTC solution were transferred onto a drop (0.025 ml) of PBS in a petri dish. A drop (0.025 ml) of fluorescein isothiocyanate (FITC)-conjugated anti-E. coli O157 antibody (Kirkegaard and Perry Laboratories), diluted 1:100 in PBS, was spread over the membrane filter. A pad saturated with water was added to the lid of the dish, which was incubated at room temperature for 20 min. The filter was transferred to a filter apparatus and rinsed three times with 1 ml of PBS.
Beads incubated in CTC solution were removed by IMS, resuspended in 0.5 ml of FITC conjugate diluted 1:100, and incubated for 20 min. The beads were removed from FITC solution by IMS, reconstituted to the original volume with peptone, filtered through a polycarbonate filter, and rinsed three times with 1 ml of PBS. The inoculum suspension was also incubated with CTC and FITC, stained, and enumerated for comparison with the numbers of cells removed by IMS.
Epifluorescence examination.
A pad containing calcium buffer (pH 9.0) with 2% diazabicyclo[2.2.2]octane (DABCO; Sigma) added to retard photobleaching was mounted on a 1-in. circular magnet attached to a slide. The filter was placed on the pad, an additional drop of buffer was added, and a coverslip was placed on top. The sample was examined by using a 100× oil immersion objective with epifluorescence filters appropriate for FITC and CTC (22).
Solid-phase laser cytometer enumeration.
For the enumeration of membrane filters with less than 103 cells on them, a Scan RDI rapid solid-phase laser scanning system (Chemunex) was used. Each membrane filter was loaded onto a stainless steel carrier on top of a cellulose acetate membrane (Millipore) saturated with 0.1 ml of buffer. The carrier was placed in the instrument, and the entire filter surface was scanned within about 3 min. The carrier with the filter was transferred to a microscope (Nikon) with a computer-controlled stage (Prion). Under the control of the Scan RDI software, each object identified as a bacterial cell was examined to confirm that it was stained with the anti-O157 antibody-FITC conjugate. The presence of CTC-formazan crystals was also recorded.
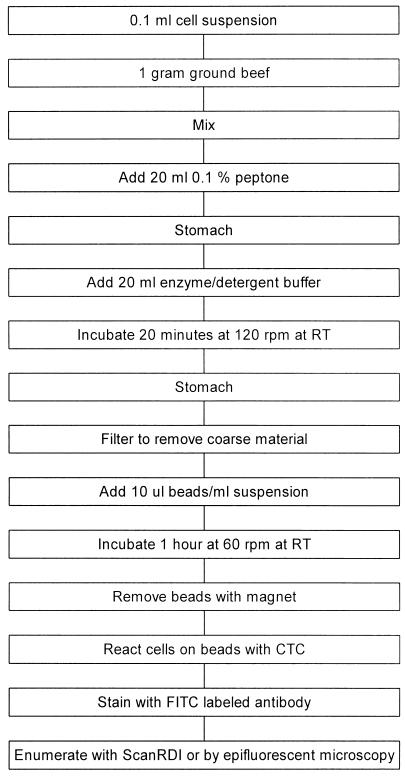
The IMS-CTC-FAb method is summarized in Fig. 1.
FIG. 1.
Flow chart of the steps involved in performing the IMS-CTC-FAb ground beef assay. RT, room temperature.
Micrography.
E. coli O157:H7 cells inoculated into ground beef and enumerated by the IMS-CTC-FAb procedure were photographed by using a Nikon Optiphot microscope with B2A epifluorescence filters, a Nikon N70 camera, and Fujichrome Provia 400 professional 35-mm slide film. Internegatives were made from slides and printed in monochrome on Panalure Select paper (Kodak).
For electron micrographs, E. coli O157:H7 cells were extracted from peptone by IMS, filtered through a polycarbonate membrane, and fixed with 2.5% glutaraldehyde. The filters were examined by using a scanning electron microscope (JEOL), and micrographs were taken on monochrome film (Polaroid) in the Image and Chemical Analysis Laboratory at Montana State University.
Data analysis.
The recovery of added cells was determined for each experiment by comparing the number of E. coli O157 bacteria detected with the number added to the samples. Variability was calculated and reported as the standard error of the mean. Results for replicated experiments were analyzed and plotted by using SigmaPlot version 4.0 for Windows (SPSS, Inc.) to determine and display the linear regressions.
RESULTS
IMS.
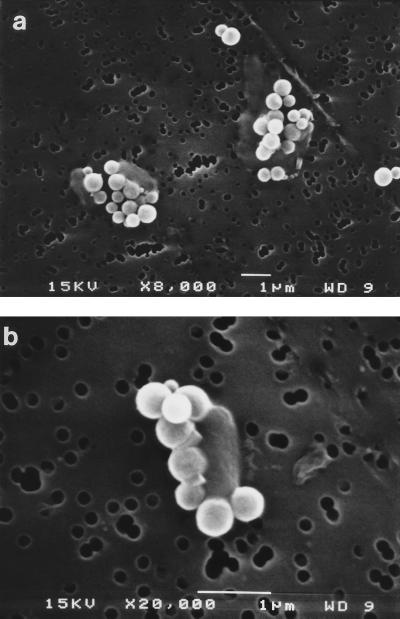
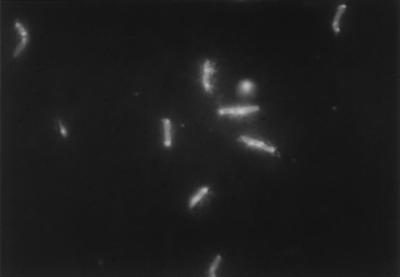
The superparamagnetic beads used in these experiments were smaller than bacteria (0.6 μm in diameter) and were coated by incubation with commercial polyclonal rabbit O157-specific antiserum. Electron microscopy showed that many beads attached to each target E. coli O157:H7 cell (Fig. 2). It is probable that this binding of multiple beads per cell and the resultant strong magnetic force during IMS contributed significantly to the extremely high rates of recovery of target bacteria reported below. In addition, epifluorescence microphotography indicated that some target cells had a halo around them (Fig. 3), which probably represents the numerous attached beads seen in Fig. 2.
FIG. 2.
Electron micrographs of immunomagnetic beads attached to E. coli O157:H7 cells. Original magnifications: a, ×8,000; b, ×20,000. Cells appear as faint rods with five or more white beads attached. The pores in the polycarbonate nuclear etched membrane can be seen in the background. WD, working distance.
FIG. 3.
Photograph of E. coli O157:H7 cells after IMS, CTC incubation, and the FAb reaction. The cells were stained with FITC-conjugated O157-specific antibody and bright spots of CTC-formazan, which indicates respiratory activity.
The IMS procedure resulted in both cell injury and recovery, depending on the suspension medium. Initial suspensions made from broth cultures were moderately injured, as determined by their differential growth on TLYD and TLY agar media. With water suspensions, before IMS, 46.8% ± 7.8% (n = 16) of the bacteria were injured, compared with 40.0% ± 16.6% (n = 6) with 0.1% peptone. After IMS, comparable injury values for the target bacteria isolated with beads were 82.5% ± 3.0% for water and 53.8% ± 13.2% for peptone suspensions. Similarly, 30.3% ± 8.4% (n = 11) of the cells inoculated into hamburger and 54.4% ± 17.4% (n = 7) of the cells recovered by IMS were injured. In all cases, the IMS procedure contributed to membrane injury (17), although the effect was less pronounced in peptone suspensions and hamburger than in water suspensions.
Recovery of E. coli O157:H7.
For all three sample types, more respiring, FAb-positive cells were detected than were enumerated by SMAC plating of the beads after IMS (Table 1). The extremely large and highly variable recovery ratio for the water suspensions was observed mainly because of two instances with small inocula (ca. 100 CFU/ml) when the Scan RDI results for CTC-positive, FAb-positive cells captured by immunomagnetic beads were more than 40 times higher than the corresponding plate counts of the captured cells. If these two results were omitted from the analysis, the mean recovery ratio was 3.18 ± 0.55 (n = 7), which is more comparable to the results for meat and peptone suspensions.
TABLE 1.
Recovery of E. coli O157:H7 from ground beef, peptone, and watera
| Comparison | Mean recovery ratiob ± SEM (no. of samples)
|
||
|---|---|---|---|
| Ground beef | Peptone | Water | |
| CTC+, FITC+ cellsc on beads vs CTC+, FITC+ cells in inoculum | 4.89 ± 3.09 (9) | 1.24 ± 0.22 (8) | 7.12 ± 4.50 (11) |
| FITC+ cellsd on beads vs FITC+ cells in inoculum | 4.51 ± 2.34 (10) | 0.84 ± 0.20 (11) | 0.90 ± 0.11 (17) |
| CTC+, FITC+ cells on beads vs PCe of inoculum | 6.04 ± 3.15 (13) | 3.03 ± 0.70 (11) | 2.39 ± 0.70 (10) |
| PC of cells on beads vs PC of inoculum | 1.63 ± 0.29 (23) | 1.26 ± 0.45 (13) | 0.42 ± 0.06 (15) |
| CTC+, FITC+ cells on beads vs PC of cells on beads | 3.79 ± 0.64 (13) | 5.89 ± 3.42 (9) | 12.41 ± 6.13 (9) |
Samples were inoculated with 100 to 106 target bacteria per ml.
E. coli O157:H7 recovered by IMS versus number in inoculum or on beads.
Respiring, FAb-positive E. coli O157:H7.
FAb-positive E. coli O157:H7.
PC, plate count on SMAC agar.
Comparison of the numbers of E. coli O157:H7 bacteria detected by the IMS-CTC-FAb technique with the SMAC plate counts of the inoculum in ground beef shows that the novel method was 6.0 times more productive (Table 1). With artificially contaminated peptone and water, the new method was 3.0 and 2.4 times more productive, respectively. The samples had been inoculated with 100 to 106 CFU of E. coli O157:H7 per g or per ml. E. coli O157:H7 cells were not detected in uninoculated control samples of hamburger (data not shown). Microscopic analyses gave no evidence of clumped cells in the inoculum, which suggested that the apparent increase in cell numbers during the enzyme-detergent treatment, IMS, CTC incubation, and FAb reaction was not due to the dispersion of clumped bacteria. As the increase was observed when using the microscopic methods, as well as when using agar plating, it was attributed to growth of bacteria from the time of sample preparation through to fixation prior to FAb staining. However, the numbers of FAb-positive cells recovered by IMS compared with those in the inoculum did not increase for the peptone or water suspensions, although there was a 4.5-times increase with ground beef samples (Table 1).
The relative numbers of CTC-reducing, FAb-positive target cells observed after capture on beads, compared with those in the inoculum (Table 1), also indicated that more than four times the numbers in the inoculum were detected in hamburger, approximately equal numbers were detected in peptone, and lower numbers were detected when the cells were suspended in sterile water. The lower result for the water suspensions was caused by the failure of many cells to reduce CTC. The results for FAb-positive cells recovered on beads compared to those in the inoculum provide further evidence for growth of E. coli O157:H7 in ground beef preparations but not in peptone or water.
With respect to SMAC plate counts, slightly higher numbers of target E. coli cells were detected by IMS with inoculated hamburger or peptone and lower numbers were detected with water suspensions (Table 1). This result suggests that the conditions in the meat homogenate and peptone suspensions were more favorable to detection by plate counting than those in the water suspensions. This may have been related to the additional cell injury which was observed in the water suspensions compared to the preparations in peptone.
Regression comparison with plating.
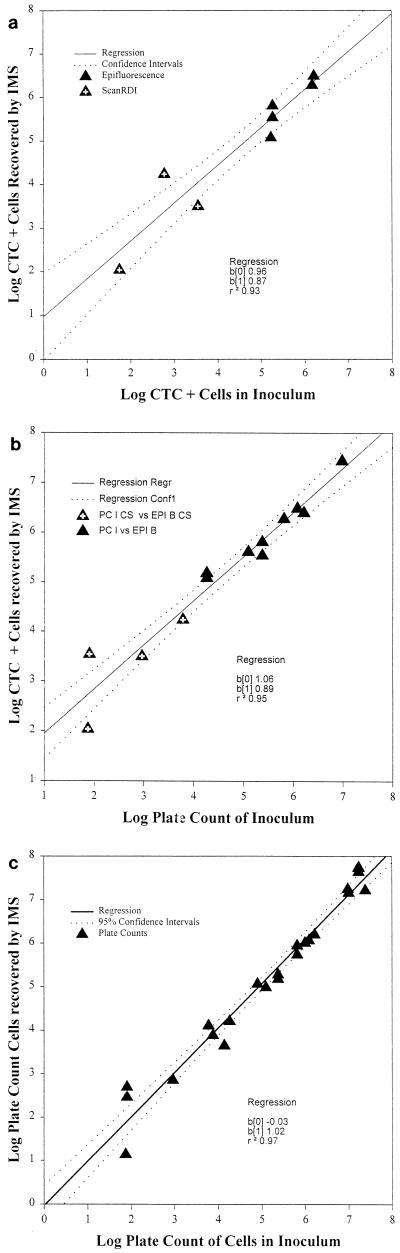
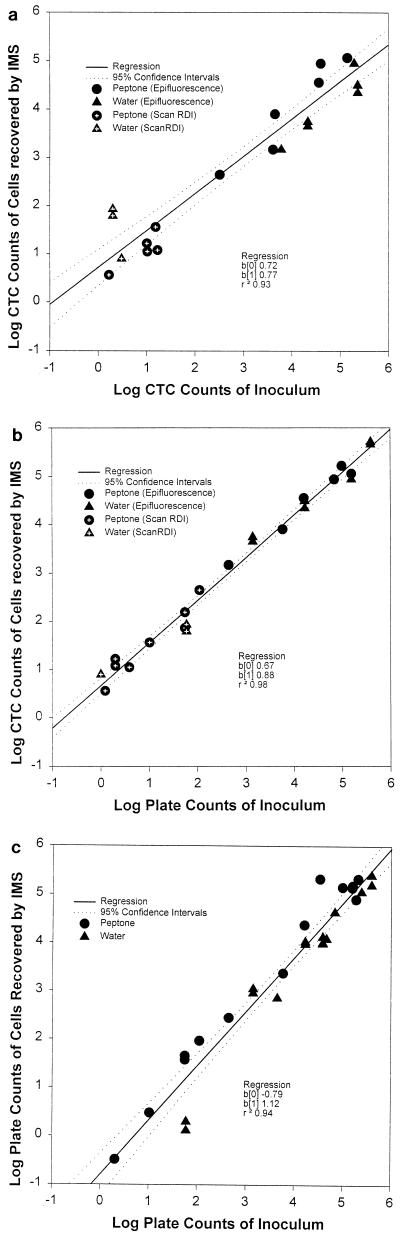
The majority of regression coefficients exceeded 0.93 (Tables 2 and 3). Plots show that most of the data points fell within the 95% confidence intervals of the regression (Fig. 4 and 5). For the smaller inocula (<104/g or ml), the counts were performed by using the Scan RDI system, and when these results were combined with the manual microscope counts for the larger inocula, the data appeared to be linear. This suggested that the Scan RDI results are comparable to direct microscope counts, although the Scan RDI system permits detection to a much lower level, i.e., <10 cells/g of hamburger and ca. 1 cell/ml in suspension.
TABLE 2.
Results for ground beef inoculated with 100 to 106 E. coli O157:H7 bacteria per ga
| Comparison | n | Regression result
|
||
|---|---|---|---|---|
| b0 | b1 | r2 | ||
| CTC+, FITC+ cells on beads vs CTC+, FITC+ cells in inoculum | 9 | 0.96 | 0.87 | 0.93 |
| FITC+ cells on beads vs FITC+ cells in inoculum | 10 | 1.28 | 0.82 | 0.96 |
| CTC+, FITC+ cells on beads vs PCb of inoculum | 13 | 1.06 | 0.89 | 0.95 |
| PC of cells on beads vs PC of inoculum | 23 | −0.03 | 1.02 | 0.97 |
| CTC+, FITC+ cells on beads vs PC of cells on beads | 13 | 0.93 | 0.91 | 0.99 |
Comparisons and descriptions are the same as for Table 1.
PC, plate count.
TABLE 3.
Combined IMS-CTC-FAb results for samples of sterile water or 0.1% peptone inoculated with 100 to 106 CFU of E. coli O157:H7 per mla
| Comparison | n | Regression result
|
||
|---|---|---|---|---|
| b0 | b1 | r2 | ||
| CTC+, FITC+ cells on beads vs CTC+, FITC+ cells in inoculum | 22 | 0.72 | 0.77 | 0.93 |
| FITC+ cells on beads vs FITC+ cells in inoculum | 31 | −0.10 | 0.99 | 0.98 |
| CTC+, FITC+ cells on beads vs PCb of inoculum | 24 | 0.67 | 0.88 | 0.98 |
| PC of cells on beads vs PC of inoculum | 29 | −0.79 | 1.12 | 0.94 |
| CTC+, FITC+ cells on beads vs PC of cells on beads | 20 | 1.32 | 0.76 | 0.96 |
Comparisons are the same as for Table 1.
PC, plate count.
FIG. 4.
Linear regression plots of enumeration results for artificially inoculated hamburger. (a) Respiring O157 cells (CTC positive, FAb positive) enumerated by IMS versus the number used as the inoculum. (b) Respiring O157 cells enumerated by IMS versus SMAC plate counts (PC) of inoculated cells. (c) SMAC plate counts of cells enumerated by IMS versus SMAC plate counts of inoculated cells. The solid line represents the linear regression, and the dotted lines indicate the 95% confidence interval.
FIG. 5.
Linear regression plots of composite enumeration results for artificially inoculated peptone (0.1%) and water. (a) Respiring O157 cells (CTC positive, FAb positive) enumerated by IMS versus the number used as the inoculum. (b) Respiring O157 cells enumerated by IMS versus SMAC plate counts of inoculated cells. (c) SMAC plate counts of cells enumerated by IMS versus SMAC plate counts of inoculated cells. The solid line represents the linear regression, and the dotted lines indicate the 95% confidence interval.
DISCUSSION
For current direct microscope techniques which utilize membrane filtration, we find that the lower limits of detection are ca. 104 cells/g of sample, using our IMS technique followed by CTC incubation and FITC-labeled antibody staining. Others have reported detection limits as low as 16 cells/g for manual microscopy following filtration and FAb staining (31). However, we have been unable to repeat those results because of the inherent difficulty in filtering the ground beef suspensions by that method (data not shown). With >104 cells/g of hamburger, using a 1-ml sample after IMS, it is necessary to count at least 20 microscope fields to obtain a statistically reliable count of at least 300 cells.
With smaller numbers of target bacteria, the lower detection limit of microscopy could be reduced by increasing the number of fields examined, but this would result in a significant increase in the time taken for enumeration and also in the stress on the operator. Alternatively, multiple filters could be compared and enumerated, which would also increase the time input. Application of improved concentration techniques in addition to IMS could also be employed to increase sensitivity. The Scan RDI system obviates the need for increased microscopy time by first enumerating and locating each particle detected and then permitting the operator to examine and validate each particle counted as a bacterial cell. It is also possible to record the presence of CTC-formazan in each cell, as a measure of respiratory activity, when the particles are being validated microscopically after Scan RDI analysis.
Most current techniques that use IMS for the detection of food-borne pathogens incorporate an incubation to enrich the target organism (e.g., see references 9, 10, 12, 24, 25, and 35). The direct detection of E. coli O157 has been demonstrated in samples of water, animal feces, and soil (21) by using commercially prepared immunomagnetic beads (Dynal). The investigators processed samples for IMS in PBS containing 0.1% BSA and 0.1% Tween 20 for extraction and plated captured cells on SMAC agar containing 4-methylumbelliferyl-β-d-glucuronide. Using laboratory cultures, we have demonstrated that 77 to 121% of the inoculum was recovered on the beads with inocula of <10 to 105 CFU/ml. The high recoveries were attributed to cell division, possibly as a result of completion of replication, during the IMS procedure. It is possible, but less likely, that injured target cells within the inoculum which were not able to reduce CTC or react with the FAb recovered during the preparation procedures so that they were observed as respiring, FAb-positive cells after the recovery and incubation steps.
The data presented here suggest that cell numbers increased during IMS, particularly in samples of ground beef. These differences were observed with both agar plating and FAb procedures, which show that the organisms detected react strongly with the confirmatory anti-O157 FAb. This FAb was obtained commercially (Kirkegaard and Perry Laboratories) and had been extensively adsorbed by using a non-O157:H7 E. coli serotype. Reactions with serotypes O55:K59:NM and O124:H30 were demonstrated only at high antibody protein concentrations. E. coli O157:H7 bacteria were not detected in control samples of uninoculated hamburger. Thus, it is unlikely that the increases in cell numbers during IMS resulted from reactions with nontarget bacteria.
The IMS-CTC-FAb procedure described here represents a prototype for the development of techniques for the detection of other pathogenic bacteria in environmental samples. We have demonstrated the detection of Salmonella typhimurium by the CTC-FAb method (22), and the use of a suitable capture antibody for coating beads would be expected to function for that organism. It is also possible to utilize alternative fluorochromes, other than CTC, to assess a variety of physiological functions in combination with specific FAb methods for identification (16). Techniques such as these will have applications both in the timely analysis of food and water samples and in ecological studies (17, 21). With more reliable, rapid procedures such as these, which do not require cultivation of environmentally stressed bacteria, better approaches to the control of water- and food-borne bacterial pathogens will be attainable.
ACKNOWLEDGMENTS
We thank John Lisle for reviewing the manuscript, in addition to Scott Burnett and Patricia Kraft for technical assistance. Rob Bargatze and John Jutila, Montana ImmunoTech, Inc., contributed helpful discussions and advice. Nancy Equall, Image and Chemical Analysis Laboratory at Montana State University, assisted with electron microscopy. Martin Rollefson (Flying Photo, Bozeman, Mont.) and Rick Harrison (Rikshots, Bozeman, Mont.) processed and printed the photographs.
Funding from the Program for the Development of Applied Biotechnology at Montana State University, the National Institutes of Health, Environmental Health Sciences, phase I Small Business Innovative Research program, and from the NASA Life Sciences program is gratefully acknowledged. This material is based on work supported, in part, by the U.S. Army Research Office under contract G-DAAH0496-01-0442.
REFERENCES
- 1.Allerberger F, Rossboth D, Dierich M P, Aleksic S, Schmidt H, Karch H. Prevalence and clinical manifestations of Shiga toxin-producing Escherichia coli infections in Austrian children. Eur J Clin Microbiol Infect Dis. 1996;15:545–550. doi: 10.1007/BF01709361. [DOI] [PubMed] [Google Scholar]
- 2.Anonymous. Escherichia coli O157:H7 infections associated with eating a nationally distributed commercial brand of frozen ground beef patties and burger—Colorado, 1997. Morbid Mortal Weekly Rep. 1997;46:777–778. [PubMed] [Google Scholar]
- 3.Anonymous. Outbreaks of Escherichia coli O157:H7 infection associated with eating alfalfa sprouts—Michigan and Virginia, June–July 1997. JAMA. 1997;278:809–810. [PubMed] [Google Scholar]
- 4.Atlas R M. Alphabetical listing of media. In: Parks L C, editor. Handbook of microbiological media. Boca Raton, Fla: CRC Press, Inc.; 1993. p. 1015. [Google Scholar]
- 5.Bangs Laboratories. Adsorption protocols. Technical note 13a. Carmel, Ind: Bangs Laboratories, Inc.; 1996. p. 7. [Google Scholar]
- 6.Bell B, Goldoft M, Griffin P, Davis M, Gordon K, Tarr P, Bartleson C, Lewis J, Barrett T, Wells J, Baron R, Kobayashi J. A multistate outbreak of Escherichia coli O157:H7-associated bloody diarrhea and hemolytic uremic syndrome from hamburgers: the Washington experience. JAMA. 1994;272:1349–1353. [PubMed] [Google Scholar]
- 7.Besser R E, Lett S M, Weber J T, Doyle M P, Barrett T J, Wells J G, Griffin P M. An outbreak of diarrhea and hemolytic uremic syndrome from E. coli O157:H7 in fresh-pressed apple cider. JAMA. 1993;269:2217–2220. [PubMed] [Google Scholar]
- 8.Bettelheim K A. Identification of enterohaemorrhagic Escherichia coli by means of their production of enterohaemolysin. J Appl Bacteriol. 1995;79:178–180. doi: 10.1111/j.1365-2672.1995.tb00932.x. [DOI] [PubMed] [Google Scholar]
- 9.Chapman P A, Cerdan Malo A T, Siddons C A, Harkin M. Use of commercial enzyme immunoassays and immunomagnetic separation systems for detecting Escherichia coli O157 in bovine fecal samples. Appl Environ Microbiol. 1997;63:2549–2553. doi: 10.1128/aem.63.7.2549-2553.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chapman P A, Wright D J, Siddons C A. A comparison of immunomagnetic separation and direct culture for the isolation of verocytotoxin-producing Escherichia coli O157 from bovine faeces. J Med Microbiol. 1994;40:424–427. doi: 10.1099/00222615-40-6-424. [DOI] [PubMed] [Google Scholar]
- 11.Doyle M P, Zhao T, Meng J, Zhao S. Escherichia coli O157:H7. In: Doyle M P, Beuchat L R, Montville T J, editors. Food microbiology fundamentals and frontiers. Washington, D.C: American Society for Microbiology; 1997. pp. 171–191. [Google Scholar]
- 12.Fratamico P M, Schultz F J, Buchanan R L. Rapid isolation of Escherichia coli O157:H7 from enrichment cultures of foods using an immunomagnetic separation method. Food Microbiol. 1992;9:105–113. [Google Scholar]
- 13.Geldreich E E, Fox K R, Goodrich J A, Rice E W, Clark R M, Swerdlow D L. Searching for a water supply connection in the Cabool, Missouri disease outbreak of Escherichia coli O157:H7. Water Res. 1992;26:1127–1137. [Google Scholar]
- 14.Guyomard S. Validation of a scanning laser system for microbiological quality control (QC) analysis. Pharm Technol Eur. 1997;9:50–52. [Google Scholar]
- 15.Hawkins E W, Orme L. Rapid testing methodology for Escherichia coli O157:H7 using commercially available products. Proc Am Soc Anim Sci West Sect. 1995;46:281–283. [Google Scholar]
- 16.Lisle J T, Broadaway S C, Prescott A M, Pyle B H, Fricker C R, McFeters G A. Effects of starvation on physiological activity and chlorine disinfection resistance in Escherichia coli O157:H7. Appl Environ Microbiol. 1998;64:4658–4662. doi: 10.1128/aem.64.12.4658-4662.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lisle, J. T., B. H. Pyle, S. C. Broadaway, and G. A. McFeters. Assessment of disinfection effects on various aspects of physiological activity in Escherichia coli O157:H7 with cellular analyses. Submitted for publication.
- 18.McFeters G A. Enumeration, occurrence, and significance of injured indicator bacteria in drinking water. In: McFeters G A, editor. Drinking water microbiology: progress and recent developments. New York, N.Y: Springer-Verlag; 1990. pp. 478–492. [Google Scholar]
- 19.Mignon-Godefroy K, Guillet J-G, Butor C. Solid phase cytometry for detection of rare events. Cytometry. 1997;27:336–344. [PubMed] [Google Scholar]
- 20.Mossell D A A, Corry J E L, Struikj C B, Baird R M. Essentials of the microbiology of foods: a textbook for advanced studies. Chichester, England: John Wiley & Sons; 1995. pp. 96–106. [Google Scholar]
- 21.Porter J, Mobbs K, Hart C A, Saunders J R, Pickup R W, Edwards C. Detection, distribution and probable fate of Escherichia coli O157 from asymptomatic cattle on a dairy farm. J Appl Microbiol. 1997;83:297–306. doi: 10.1046/j.1365-2672.1997.00230.x. [DOI] [PubMed] [Google Scholar]
- 22.Pyle B H, Broadaway S C, McFeters G A. A rapid, direct method for enumerating respiring enterohemorrhagic Escherichia coli O157:H7 in water. Appl Environ Microbiol. 1995;61:2614–2619. doi: 10.1128/aem.61.7.2614-2619.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pyle B H, Lisle J T, Broadaway S C, McFeters G A. Rapid detection of bacteria in spacecraft water systems. SAE Technical Paper Series 972421. Warrendale, Pa: Society for Automotive Engineers; 1997. [Google Scholar]
- 24.Restaino L, Castillo H J, Stewart D, Tortorello M L. Antibody-direct epifluorescent filter technique and immunomagnetic separation for 10-h screening and 24-h confirmation of Escherichia coli O157:H7 in beef. J Food Prot. 1996;59:1072–1075. doi: 10.4315/0362-028X-59.10.1072. [DOI] [PubMed] [Google Scholar]
- 25.Restaino L, Frampton E W, Irbe R M, Allison D R K. A 5-h screening and 24-h confirmation procedure for detecting Escherichia coli O157:H7 in beef using direct epifluorescent microscopy and immunomagnetic separation. Lett Appl Microbiol. 1997;24:401–404. doi: 10.1046/j.1472-765x.1997.00141.x. [DOI] [PubMed] [Google Scholar]
- 26.Rodrigues-Szulc U M, Ventoura G, Macker B J, Payne M J. Rapid physicochemical detachment, separation and concentration of bacteria from beef surfaces. J Appl Bacteriol. 1996;80:673–681. doi: 10.1111/j.1365-2672.1996.tb03273.x. [DOI] [PubMed] [Google Scholar]
- 27.Rodriguez G G, Phipps D, Ishiguro K, Ridgway H F. Use of a fluorescent redox probe for direct visualization of actively respiring bacteria. Appl Environ Microbiol. 1992;58:1801–1808. doi: 10.1128/aem.58.6.1801-1808.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Smith J J, Howington J P, McFeters G A. Survival, physiological response, and recovery of enteric bacteria exposed to a polar marine environment. Appl Environ Microbiol. 1994;60:2977–2984. doi: 10.1128/aem.60.8.2977-2984.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Smith J J, McFeters G A. Mechanisms of INT (2-(4-iodophenyl)-3-(4-nitrophenyl)-5-phenyl tetrazolium chloride) and CTC (5-cyano-2,3-ditolyl tetrazolium chloride) reduction in Escherichia coli K-12. J Microbiol Methods. 1997;29:161–175. doi: 10.1111/j.1365-2672.1996.tb03212.x. [DOI] [PubMed] [Google Scholar]
- 30.Swerdlow D L, Woodruff B A, Brady R C, Griffin P M, Tippen S, Donnell H D, Jr, Geldreich E, Payne B J, Meyer A, Jr, Well J G, Greene K D, Bright M, Bean N H, Blake P A. A waterborne outbreak in Missouri of Escherichia coli O157:H7 associated with bloody diarrhea and death. Ann Intern Med. 1992;117:812–819. doi: 10.7326/0003-4819-117-10-812. [DOI] [PubMed] [Google Scholar]
- 31.Tortorello M L, Stewart D S. Antibody-direct epifluorescent filter technique for rapid, direct enumeration of Escherichia coli O157:H7 in beef. Appl Environ Microbiol. 1994;60:3553–3559. doi: 10.1128/aem.60.10.3553-3559.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vernozy-Rozand C. Detection of Escherichia coli O157:H7 and other verocytotoxin-producing E. coli (VTEC) in food. J Appl Microbiol. 1997;82:537–551. doi: 10.1111/j.1365-2672.1997.tb03584.x. [DOI] [PubMed] [Google Scholar]
- 33.Vernozy-Rozand C, Mazuy C, Ray-Gueniot S, Boutrand-Loeï S, Meyrand A, Richard Y. Detection of Escherichia coli O157 in French food samples using an immunomagnetic separation method and the VIDASTME. coli O157. Lett Appl Microbiol. 1997;25:442–446. doi: 10.1111/j.1472-765x.1997.tb00014.x. [DOI] [PubMed] [Google Scholar]
- 34.Wallner G, Tillmann D, Haberer K, Cornet P, Drocourt J-L. The ChemScan system: a new method for rapid microbiological testing of water. Eur J Parenteral Sci. 1997;2:89–92. [Google Scholar]
- 35.Wright D J, Chapman P A, Siddons C A. Immunomagnetic separation as a sensitive method for isolating Escherichia coli O157 from food samples. Epidemiol Infect. 1994;113:31–39. doi: 10.1017/s0950268800051438. [DOI] [PMC free article] [PubMed] [Google Scholar]