Abstract
Purpose:
To systematically review the role of antioxidants in management of patients with thyroid eye disease (TED).
Methods:
A literature search of the electronic databases was performed without restrictions on the date of publication till the end of March 2021, using the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines. Clinical trials, case–control studies, cohorts, case series, case reports, and experimental (including in vitro) studies in the English language were included. The primary outcome in human studies was improvement in severity, activity scores, and/or quality of life scores. There was a decrease in the level of H2O2-dependent oxidative stress, Hyaluronic acid release, reactive oxygen species, cell proliferation, or antifibrotic/antiproliferative actions in the in vitro studies.
Results:
Out of 374 initially screened articles, 157 studies were selected, the full texts of 82 were reviewed, and 14 papers were finally included. There were 4 clinical and 10 in vitro studies from 1993 to 2018. While β-carotene, retinol, Vitamin E, Vitamin C, melatonin, resveratrol, N-acetyl-l-cysteine, and quercetin showed some efficacy in in vitro studies; allopurinol, nicotinamide, pentoxifylline, and selenium (Se) were effective in both clinical and experimental reports. Se was the only recommended antioxidant based on one high-level randomized controlled trial.
Conclusion:
While different antioxidants could potentially be effective in the management of TED, no strong recommendation for any or combination of antioxidants could be made to be implemented in the daily practice.
Keywords: Antioxidants, Selenium, Systematic review, Thyroid eye disease
INTRODUCTION
Thyroid eye disease (TED) is an autoimmune inflammatory disease known as the most common cause of orbital disease worldwide.1 Although it is mostly associated with hyperthyroidism (Graves' disease [GD]), hypothyroidism and euthyroidism are present in some patients.2,3 TED is clinically present in one-third of the patients with different underlying thyroid diseases.3 Clinical presentation of the TED is the same for different underlying thyroid diseases2 and between patients with unilateral versus bilateral orbital involvement.4 While the quality of life (QoL) is impaired in patients with TED,5 medical and surgical treatments lead to improvement of both visual and psychosocial QoL.6,7 To prevent the progression of TED to sight threatening stages with detrimental effect on QoL of patients,8 early diagnosis and treatment of TED among patients with thyroid dysfunction has received added emphasis.9
Oxidative stress is a process which is normally controlled under physiological conditions.10,11 It is believed that any alteration of cell oxidative stability leads to cell damage.12 A rise in several parameters of oxidative stress seems to be involved in the development of some autoimmune and endocrine diseases namely hyperthyroidism and TED.12,13 Therefore, antioxidants have been studied to find out how they might change the effect of oxidative stress in different diseases. Consequently, a few studies13,14,15,16,17 have attempted to investigate the role of antioxidants in autoimmune hyperthyroidism/thyroiditis and TED treatment with inconsistent conclusions on the effect and type of antioxidants in this regard. This study was designed to investigate the efficacy and safety of reported antioxidants in patients with TED.
METHODS
A comprehensive literature search of the EMBASE, PubMed, MEDLINE, Google Scholar, and Scopus electronic databases was performed without restrictions on the date of publication till the end of March 2021, using the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines.18 The research keywords were “Thyroid eye disease OR Thyroid-associated ophthalmopathy OR Thyroid-associated orbitopathy OR Graves' ophthalmopathy OR Graves' orbitopathy,” AND “Antioxidants OR Selenium OR Pentoxifylline OR Vitamin C OR Vitamin E OR Carotenoids OR Glutathione OR Uric acid.”
Included articles were the clinical trials, case–control studies, cohorts, case series, case reports, and experimental (including in vitro) studies published in the English language. Review articles, patents, book chapters, commentaries, editorials, animal studies, and articles with irrelevant content or insufficient information were excluded. Two authors (S.C. and S.A.) independently followed the search strategy, screened the abstracts, then the full-text, and also the quality of evidence of the selected articles, and finally included the articles for the systematic review. Disagreement was resolved by the involved senior author (M.B.K.). The reference list of included papers was checked for further reports and citations of published or unpublished researches, and simultaneously registered trials were screened on three different websites including https://clinicaltrials.gov, https://who.int and https://www.cochranelibrary. com.
Extracted and documented data were the authors' name and year of publication, the design of the study, average age, stage of TED, thyroid function status, type of antioxidants, results, follow-up duration, and side effects.
The primary outcome in human studies was improvement in severity, activity scores, and/or QoL scores. Reduced lid retraction, proptosis, and/or diplopia were the outcome measures in the studies where the primary outcomes had not been reported.
The primary outcome of in vitro studies was a decrease in the level of H2O2-dependent oxidative stress, hyaluronic acid (HA) release, reactive oxygen species (ROS), cell proliferation or antifibrotic/antiproliferative actions.
The risk of bias was evaluated using a rating scheme [Table 1].19 Briefly, the level of evidence was assessed using “hierarchy of evidence pyramid.”
Table 1.
Rating of the level of evidence19 in the studies included in this systematic review
| Level of evidence | Included studies | Description |
|---|---|---|
| Level I | None | Evidence from a systematic review or meta-analysis of all relevant RCTs or evidence-based clinical practice guidelines based on systematic reviews of RCTs or three or more RCTs of good quality that have similar results |
| Level II | Finamor et al.20, Marcocci et al.21 | Evidence obtained from at least one well-designed RCT (e.g. large multi-site RCT) |
| Level III | Balazs et al.22, Bouzas et al.23 | Evidence obtained from well-designed controlled trials without randomization (i.e. quasi-experimental) |
| Level IV | Dehina et al.24, Federige et al.25, Liu et al.26, Olesik et al.27 | Evidence from well-designed case-control or cohort studies |
| Level V | None | Evidence from systematic reviews of descriptive and qualitative studies (meta-synthesis) |
| Level VI | None | Evidence from a single descriptive or qualitative study |
| Level VII | None | Evidence from the opinion of authorities and/or reports of expert committees |
| Level VIII (foundational evidence) | Chang et al.28, Burch et al.29 Hiromatsu et al.3, Lisi et al.31, Yoon et al.32, Tsai et al.33, Kim et al.34, Dottore et al.35, Dottore et al.36, Dottore et al.37, Dottore et al.38, Kim et al.39 | Evidence from animal/laboratory research and in-vitro studies |
RCT: Randomized controlled trial
RESULTS
Out of 386 initially screened articles, 177 studies were selected for more assessment. Reviewing the abstracts led to exclusion of 81 articles. Full-text review was performed on the remaining 96 articles, from which 76 did not have a defined outcome. Finally, 20 papers met the inclusion criteria and were considered in this review [Figure 1]. There were four human studies20,21,22,23 and sixteen in vitro studies24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39 from 1993 to 2021 [Table 2]. Fifteen studies were from Taiwan (2), Canada (1), Greece (1), Brazil (2), Poland (1), the USA (1), Japan (1), Italy (5), South Korea (3), Germany (1), China (1), and one was supervised by the international multicentral European Group on Graves' Orbitopathy (EUGOGO) association.
Figure 1.
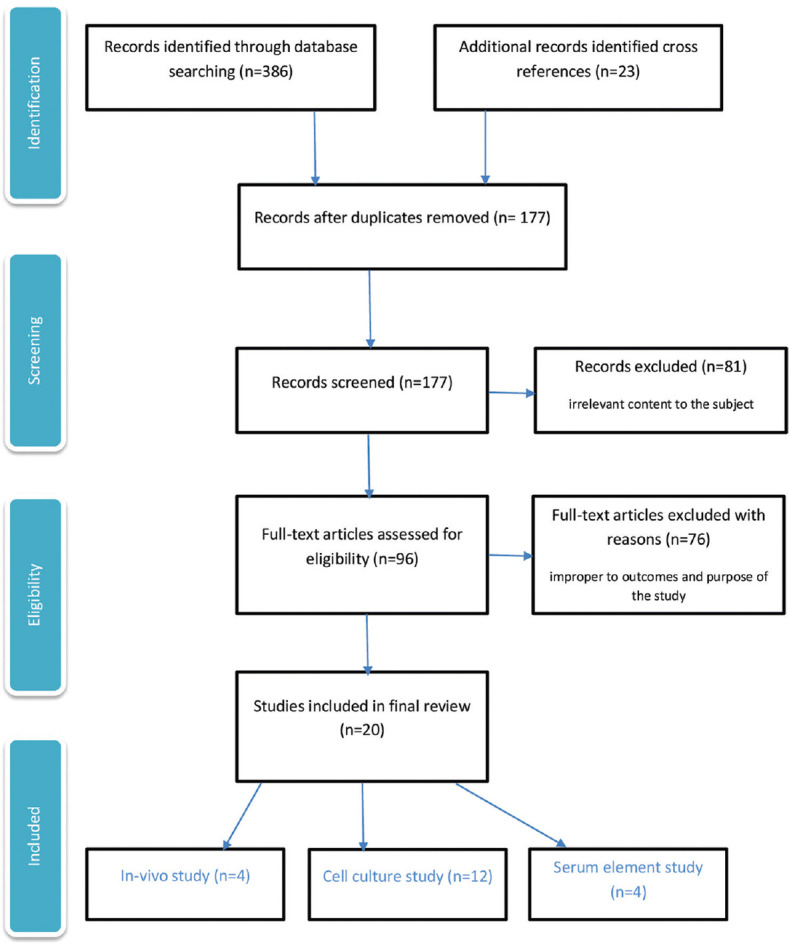
Screening and selection process of the articles on the role of antioxidants in thyroid eye disease
Table 2.
Comparing different studies on the role of antioxidants in thyroid eye disease
| Writer/year | Study design | Population | Mean age (year) | TED grade | Thyroid status | Type of antioxidant treatment | Follow-up | Result | Side effects | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Chang et al., 199328 | Experimental, orbital fibroblast cell culture | Case: 2 Normal: 2 (2 PTM) |
32 | Moderately severe, activity is not specified/with OD | Not mentioned | PTX | NA | Inhibition of GAG release and fibroblast proliferation | NA | |
| 2 | Balazs et al., 199722 | Quasi-experimental, pilot study | Case: 10 | 45.2 | Moderately severe, activity is not specified/with OD | Euthyroid | PTX | 12 weeks | ↓Serum GAG and TNFα, ↓Soft tissue involvement | Moderate and persistent nausea at the begging | |
| 3 | Burch et al., 199729 | Experimental, orbital fibroblast cell culture | Case: 2 Normal: 2 |
Not available | Moderately severe, activity is not specified/with OD | Not mentioned | Allopurinol nicotinamide | NA | ↓Cell proliferation | NA | |
| 4 | Hiromatsu et al., 199830 | Experimental, orbital fibroblast cell culture | Case: 4 Normal: 3 |
Not available | Activity and severity are not specified/with OD | Not mentioned | Nicotinamide | NA | ↓ICAM-1 and HLA-DR expression and | ↓Cell proliferation | NA |
| 5 | Bouzas et al., 200023 | Prospective nonrandomized comparative study | Case: 11 Normal: 11 |
Case: 36.7 Control: 34.8 |
Mild and moderately severe, active | Euthyroid | Allopurinol nicotinamide | 3 months | ↑Total eye score (NOSPECS) ↑Patient satisfaction |
None | |
| 6 | Finamor et al., 200420 | Prospective randomized trial | Case: 9 Normal: 9 |
Case: 41.5 Control: 40 |
All 3 severity stages but inactive | Euthyroid | PTX | 6 months | ↓Proptosis and questionnaire scores | Nausea, abdominal pain | |
| 7 | Marcocci et al. 201121 | Prospective randomized double-blind, placebo-controlled trial | Total: 159 Se: 55 PTX: 52 Placebo: 52 |
Se: 43 PTX: 43.7 Placebo: 44.6 |
Mild GO, active and inactive | Not mentioned | Se, PTX | 12 months | ↑QoL ↓CAS |
Se (none) gastrointestinal with PTX | |
| 8 | Lisi et al., 201131 | Experimental, orbital fibroblast cell culture | Case: 5 Normal: 5 |
Case: 47.4 Control: NA |
Moderately severe, inactive/with OD | Euthyroid | Quercetin | NA | ↓Cell proliferation and HA release | NA | |
| 9 | Yoon et al., 201232 | Experimental, orbital fibroblast cell culture | Case: 13 Normal: 3 |
Case: 46 Control: 56 |
Moderately severe, inactive/with OD | Euthyroid | Quercetin | NA | ↓MMP-2 and MMP-9, fibrotic markers and suppressive effects | NA | |
| 10 | Tsai et al., 201333 | Experimental, orbital fibroblast cell culture | Case: 7 Normal: 5 |
Case: 37.6 Control: 35.2 |
Severity is not specified inactive/with OD | Euthyroid | Vitamin C N-acetyl-l-cysteine |
NA | ↓Cell proliferation and superoxide anion | NA | |
| 11 | Kim et al., 201534 | Experimental, orbital fibroblast cell culture | Case: 6 Normal: 4 |
Not available | Moderately severe, (CAS <4)/with OD | Euthyroid | Resveratrol | NA | ↓Oxidative stress and ROS | NA | |
| 12 | Dehina et al., 201624 | Case-control, serum elements study | Total: 84 Mild: 31 Severe: 53 Active: 62 Inactive: 22 |
Not available (median: 46) | Used NOSPECS (mild, moderately severe, and sight threatening), active and inactive | Hyper: 51 Hypo: 10 Eu: 23 |
Serum Se, SePP | NA | No significant associations and changes | NA | |
| 13 | Dottore et al. 201735 | Experimental, Orbital fibroblast Cell culture | Case: 6 Normal: 6 |
Case: 60.1 Control: 66.1 |
Moderately severe, inactive/with OD | Not mentioned | Se | NA | ↓Oxidative stress and cell proliferation | NA | |
| 14 | Dottore et al., 201736 | Experimental, orbital fibroblast cell culture | Case: 6 Normal: 6 |
Case: 60.1 Control: 66.1 |
Severity is not specified inactive/with OD | Not mentioned | Se | NA | ↓Apoptosis, LDH (necrosis), inhibition of oxidative stress (↓GSSG) | NA | |
| 15 | Federige et al., 201725 | Case-control, serum elements study | GD without GO: 19 GD with GO: 21 HT: 14 HT+LT4: 19 Control: 21 |
Case: 52.5 Control: 51 |
GO defined as having proptosis and CAS >1, severity is not specified | Euthyroid | Serum Se, SePP | NA | ↓SePP serum level in GO and HT patients | NA | |
| 16 | Dottore et al., 201837 | Experimental, orbital fibroblast cell culture | Case: 6 Normal: 6 |
Case: 49.1 Control: 62.6a |
Moderately severe, inactive/with OD | Not mentioned | Retinol, β-carotene, Vitamin E | NA | ↓H2O2-dependent oxidative stress, antiproliferative action | NA | |
| 17 | Dottore et al., 201838 | Experimental, orbital fibroblast cell culture | Case: 6 Normal: 6 |
Case: 49.1 Control: 62.6 |
Moderately severe, inactive/with OD | Not mentioned | Vitamin C >N-acetyl-l-cysteine, Melatonin |
NA | ↓H2O2-dependent oxidative stress | NA | |
| 18 | Liu et al., 201826 | Case-control, serum elements study | Newly diagnosed GD: 66 Euthyroid GD: 55 Euthyroid GO: 57 Control: 66 |
Case: 38.06 Control: 42.3 |
Mild-to-moderate GO according to EUGOGO classification, severity is not specified | Euthyroid/subclinical hyper | Serum Se | NA | ↓Se serum level in all cases than control group | NA | |
| 19 | Olesik et al., 202027 | Case-control, serum elements study | TAO: 56 Control: 20 |
Case: Hyper: 53 Eu: 48 Control: Not available |
Active (CAS >3), moderate-to-severe according to EUGOGO classification | Hyper: 34 Eu: 22 |
Serum Vitamin C, Uric acid | NA | Lower Vitamin C, higher uric acid levels in active TAOs than controls | NA | |
| 20 | Kim et al., 202139 | Experimental, orbital fibroblast cell culture | Case: 7 Normal: 5 |
Case: 41.1 Control: Not available |
Inactive (CAS <3), severity is not specified | Euthyroid | Se | NA | Suppression of hyaluronan production, IL1α, and TNFα (all in dose-dependent manner) nInhibition of ROS generation and IL8 |
NA |
TED: Thyroid eye disease, PTX: Pentoxifylline, GD: Graves’ disease, GO: Graves’ orbitopathy, HT: Hashimoto’s thyroiditis, TAO: Thyroid-associated orbitopathy, Se: Selenium, NOSPECS: No sign or symptoms, only signs, soft tissue involvement with symptoms and signs, proptosis, extraocular muscle involvement, corneal involvement, sight loss, CAS: Clinical activity score, EUGOGO: European Group on Graves’ Orbitopathy, SePP: Selenoprotein P , GAG: Glycoseaminoglycan, TNFα: Tumor necrosis factor-α, ICAM-1: Intercellular adhesion molecule 1, HLA: Human leukocyte antigen, QoL: Quality of life, MMP-2: Matrix metalloproteinase-2, ROS: Reactive oxygen species, LDH: Lactate dehydrogenase, GSSG: Glutathione disulfide, IL: Interleukin, NA: Not applicable, H2O2: Hydrogen peroxide, PTM: Pretibial myxedema, LT4: Levothyroxine, OD: Orbital decompression, HA: Hyaluronic acid, HLA-DR: Human leukocyte antigen-DR isotype, Eu: Euthyroid, ↓: Decreased, ↑: Increased
Chang et al.28 investigated the role of PTX (0.1–1,000 mg/L) on cultured fibroblasts in 1993. The cultures were collected from biopsies of extraocular muscles (EOM) from two severe TED patients during orbital decompression surgery and two normal EOM tissues of individuals with strabismus during resection surgery and from the skin of two patients affected with pretibial myxedema. Dose-dependent inhibition of orbital fibroblast proliferation and glycosaminoglycan (GAG) release was reported.
In 1997, the human orbital fibroblast was obtained from two severe TED patients and two normal controls to investigate the effect of allopurinol (1.0 mM) and nicotinamide (10 μM) on cell proliferation induced by xanthine oxidase/hypoxanthine system.29 The proliferation inhibition was significant after preincubation with allopurinol, nicotinamide, and methimazole (0–25 μM), but not with propylthiouracil (10 μM).
The role of nicotinamide was exclusively evaluated on orbital fibroblast to explore cell surface expression of human leukocyte antigen (HLA)-A, B, C antigen, HLA-DR antigen, intercellular adhesion molecule 1 (ICAM-1), CD44, and Fas expression induced by cytokines (interferon-γ [IFNγ] and tumor necrosis factor-α [TNFα]). Tissue harvest was from four TED and three glaucomatous patients. It was observed that nicotinamide did not interfere with induction of HLA-A, B, C, or CD44 expression but demonstrated an inhibitory effect on ICAM-1 and HLA-DR expression as well as the proliferation of orbital fibroblasts.30
To investigate the effect of quercetin on orbital fibroblasts, tissue samples from 5 TED patients and 5 control subjects were exposed to quercetin or its glycosides rutin and quercitrin, and subsequently, apoptosis, necrosis, cell proliferation, HA production, and cell cycle were measured.31 Quercetin inhibited cell proliferation and HA production in both TED patients and control subjects.
Another study assessed the effect of quercetin on fibrotic markers and matrix metalloproteinases (MMP) of cultures from 13 TED patients and 3 normal females aged between 51 and 63.32 Quercetin decreased the secretion of MMP-2 and MMP-9 proteins and inhibited fibrotic markers in the TED group. Its antifibrotic effects occurred through a noncytotoxic process.
In 2013, Tsai et al.33 examined the biphasic effect of H2O2 on cellular proliferation of Graves' orbitopathy (GO) orbital fibroblasts and also protective effect of N-acetyl-l-cysteine (NAC) and Vitamin C against it. Cultures from seven GO patients and five age-matched normal subjects were exposed to various concentrations of H2O2. The peak cellular proliferation was observed at 6.25 μM of H2O2 in GO fibroblasts. Protective effects were reported when GO cells pretreated with NAC (200 μM) and Vitamin C (500 μM) for 1 h and followed by the addition of 6.25 μM H2O2 for 24 h, by reversing enhanced proliferation and increased levels of transforming growth factor, beta 1 (TGF-β1), interleukin-1 β (IL1 β), and superoxide anion.
Kim et al.34 exposed orbital connective tissue of 6 TED patients and 4 controls to assess the effect of resveratrol (30–50 μM) treatment on intracellular ROS levels and the expression of heme oxygenase-1 (HO-1), superoxide dismutase (SOD), catalase, and thioredoxin. It decreased ROS production, adipogenesis, and HO-1 level induced by oxidative stress. Treatment with 50 μM resveratrol also reduced ROS levels during adipogenesis.
Selenium (Se), in the form of selenium-(Methyl) selenocysteine (SeMCys), was examined on primary cultures of orbital fibroblasts from 6 TED patients and 6 control subjects in 2017.35 While SeMCys inhibited proliferation and HA secretion just in TED patients, its effect on various concentrations of H2O2-induced oxidative stress (glutathione disulfide [GSSG]) and cytokines were similar in both groups.
SeMCys was also investigated in another experiment.36 Tissue samples from six GO patients and six normal ones were collected and preincubated for 2 days at 37°C with a medium containing a 10 μM concentration of SeMCys hydrochloride, then treated with a concentration of 50 μM H2O2. Increased GSSG, apoptosis, and lactate dehydrogenase as a measure of necrosis were counteracted by SeMCys with no differences between GO and control fibroblasts.
Tissue samples from 6 TED patients and 6 patients with other conditions lacking fibroadipose tissue were treated with retinol, β-carotene, and Vitamin E at various concentrations.37 β-carotene significantly decreased the raised H2O2-induced proliferation in TED but not the control fibroblasts. Retinol and Vitamin E had no effect. None had a significant effect on HA. Among TNFα, IL1 β, IFNγ, and endogenous cytokines which are involved in the pathogenesis of TED, IL1 β was the only responder to all the three antioxidant substances. Its H2O2-dependent rise significantly reduced in both TED and control fibroblasts.
Antioxidant effects of Vitamin C, NAC, and melatonin on primary cultures of 6 TED patients and 6 normal individuals were evaluated.38 Vitamin C and NAC reduced H2O2-induced proliferation in TED fibroblasts. Melatonin and NAC decreased IFNγ in the TED fibroblasts.
Obtained orbital adipose/connective tissue samples from seven GO patients and five individuals with noninflammatory problems were studied to determine the effect of Se in the form of sodium selenite at various concentrations.39 Hyaluronan, ROS production, and inflammatory cytokines including IL1α, IL1 β, IL6, IL8, and TNFα concentrations were measured and showed that serum Se suppressed hyaluronan production, IL1α, and TNFα in cultured orbital fibroblasts of patients with GO in a dose-dependent manner while suppression intracellular ROS generation and IL8 were not dose-dependent. IL1 β and IL6 were not suppressed by sodium selenite in cultured GO orbital fibroblasts.
Se and selenoprotein P (SePP) concentrations were assessed against clinical activity score (CAS) and severity status (based on NOSPECS [No sign or symptoms, only signs, soft tissue involvement with symptoms and signs, proptosis, extraocular muscle involvement, corneal involvement, sight loss]) as well as concentrations of thyroid stimulating hormone (TSH) receptor autoantibody (TRAB) or IGF1 receptor (IGF1R-aAB) autoantibodies in 31 mild and 53 moderately severe TED patients.24 Se level did not significantly correlate with thyroid hormone concentrations, activity, and the severity of TED. Se or SePP concentrations were significantly different among the patients with mild versus moderately severe and active versus inactive TED. A significant inverse correlation was reported between serum Se and TRAB. This was not significant for the SePP. There were also no connections between IGF1R-aAB levels and serum SePP/serum Se concentrations.
In 2017, Federige et al.25 studied and compared Se and SePP values in patients with GD with and without GO, Hashimoto's thyroiditis (HT) patients and in control individuals. Although serum Se levels were similar among all groups, SePP serum was lower in GO and HT patients compared to the control group.
Serum trace elements were assessed in four groups of newly diagnosed GD patients, GD and GO patients in euthyroid status or subclinical thyroidism after treatment, and normal controls in a population in northeast China.26 Among evaluated elements, serum Se levels in three first groups were significantly lower than those in normal individuals and serum copper levels were significantly low only in the GO group than those in normal ones.
The effect of thyroid hormone abnormalities on selected antioxidant parameters were investigated in GD patients with thyroid-associated orbitopathy (TAO) from both hyperthyroid and euthyroid patient categories.27 The blood was collected, and the sera were obtained after centrifugation. Enzymatic and nonenzymatic components of the antioxidant system were assessed, including the activity of glutathione peroxidase (GPx), SOD, and paraoxonase 1, as well as the total oxidant status expressed as the ferric reducing ability of plasma. The levels of Vitamin C, uric acid, and lipid peroxidation products (conjugated dienes [CD] and malondialdehyde [MDA]) were examined as well. While in hyperthyroid patients all values were significantly different from those in control ones, in euthyroid patients, only the activity of GPx was significantly higher than in controls, and other values nonsignificantly changed compared with the control group.
A pilot study of 10 patients described the beneficial effects of PTX therapy in euthyroid moderately-severe TED in 1997.22 PTX (200 mg/day) was administered intravenously for 10 days and continued at 1800 mg/day orally for the first 4 weeks and then 1200 mg/day for the rest of treatment. Any improvement in total eye score of NOSPECS was defined as response. Serum GAG, TNFα, anti-TSH-receptor, anti-eye-muscle, anti-thyroglobulin, and anti-thyroid peroxidase antibodies were also documented sequentially. Soft tissue inflammation significantly improved in 80% of cases (8/10) with less significant improvement in proptosis and extraocular myopathy. Although moderate and persistent nausea was reported by two cases at the beginning of therapy, it did not interrupt treatment and responders continued oral PTX 1200 mg/day to maintain initial clinical benefit after 12 weeks.
Allopurinol and nicotinamide (300 mg/day, oral, 3 months) were compared with a placebo in a prospective nonrandomized study in 2000.23 Treatment improved NOSPECS total eye score from 4.3 ± 1.9 to 2.0 ± 1.0 (P = 0.0001) and self-reported satisfaction in 22 newly diagnosed mild or moderately-severe TED patients.
Finamore et al.20 compared an oral PTX (1200 mg/day) in 9 TED patients with placebo (9 TED patients) in a crossover (6-month) study. Exophthalmometry and health-related QoL questionnaire were the outcomes which were measured at baseline, 3, and 6 months after the treatment. Both showed significant improvement in the treatment group compared with placebo group. Gastrointestinal symptoms were, however, notable. The questionnaire scores were 5.5 and 5 (P = 0.01) at baseline and 6th months in the treatment group, respectively. A significant (P < 0.05) improvement of proptosis was also reported in the treatment group. Neither of the criteria changed significantly in the placebo group within two-time intervals of the crossover study.
In a randomized clinical trial, Se, PTX, and placebo were compared in 159 patients with mild TED.21 They were assigned to three groups receiving sodium selenite (100 μg twice daily), PTX (600 mg twice daily), or placebo (twice daily) orally for 6 months and were followed for the next 6 months without treatment. Significant (P < 0.001) improvements in QoL (GO-QoL), CAS, soft tissue involvement and eyelid retraction with a less progression in TED severity (P = 0.01) were observed in the Se as compared with the placebo group. PTX group, however, did not show significant improvement as compared with the placebo group. While skin and gastrointestinal side effects were observed in the PTX group, no adverse effect was in the Se and placebo groups.
The result of hierarchical evaluation for included studies has been detailed in Table 1. Among all, the experiments of Finamor et al.20 and Marcocci et al.21 are in level 2, while the ones for Balazs et al.22 and Bouzas et al.23 are in level 3. According to defined criteria, the studies by Dehina et al.,24 Federige et al.,25 Liu et al.,26 and Olesik et al.27 stand at level 4, and the rest lie at the lowest part of the pyramid.
DISCUSSION
Impaired balance between production of oxidative stress and consumption of antioxidant defenses (inactivation or excessive usage) leads to oxidative damage of biological membranes and molecules, which can be measured by either direct estimation of the ROS or indirect methods including detection of the resulting damage to biomolecules (DNA/RNA damage, lipid peroxidation, and protein oxidation/nitration) and antioxidant levels.40 Increased oxidative stress and decreased scavenging ability of the cells have been recognized to be involved in the pathogenesis of autoimmune disorders particularly GD and TED.12 Using a variety of techniques to induce oxidative stress while applying diverse measuring systems (ROS production, MDA, SOD activity, LPO, 8-OHdG, GSH, and GSH/GSSG ratio), certain studies have found a significant imbalance of prooxidant/antioxidants status in TED versus normal orbital fibroblasts.29,41,42,43,44,45 This imbalance accounts for proliferation of orbital fibroblasts, synthesis of autoantibodies, breakdown of preadipocytes into adipocytes, secretion of endogenous cytokines (TNFα, IL1 β and IFNγ), and increased production and secretion of GAGs in TED patients which consequently lead to fibroadipose tissue expansion and infiltration of EOM.14
The aim of this systematic review was to provide the clinicians with essential information about potential role of antioxidant agents in the management of TED. Some of the studies have compared the efficacy of more than one type of antioxidants21,23,27,29,33,37,38 in a single experiment.
β-carotene, retinol, Vitamin E, Vitamin C, melatonin, resveratrol, NAC, uric acid, and quercetin were solely evaluated in in vitro environment, and no experiments were found to discuss their clinical results. While quercetin,31,32 Vitamin C,27,33,38 and NAC33,38 were found to be effective in more than one study, the efficacy of other antioxidants has been reported in just one in vitro study. Therefore, further in vitro and more importantly human studies is suggested before drawing any conclusion on their effect in TED management.
Allopurinol, nicotinamide, PTX, and Se, on the other hand, have been studied both in vitro and clinical. Allopurinol and nicotinamide have shown both proliferation inhibition in in vitro and improved patient satisfaction and total eye score in clinical studies. Exposed orbital fibroblast cultures to PTX demonstrated inhibition of GAG release and fibroblast proliferation.28 PTX treatment also led to a marked improvement in total eye score of 80% of TED patients (after 12 weeks),22 and progressive improvement of proptosis and QoL questionnaire response.20 However, no significant difference in overall ophthalmic outcome at 6-month was observed between PTX and placebo in another study.21
Decreased serum Se level was reported in all groups of patients regardless of TED presentation,26,46 and SePP status was lower in GO and HT patients than in normal controls.25 Serum Se and SePP concentrations were not different between mild versus moderately severe as well as active versus inactive TED patients.24 However, this study was unable to draw a strong conclusion about Se supplementation as it was not a longitudinal study and did not include a control group of healthy subjects or GD patients without TED.
SeMCys suppressed fibroblast proliferation, HA secretion, apoptosis, and necrosis in TED orbital fibroblasts in the Dottore et al.'s in vitro studies.35,36 While sodium selenite form of Se inhibited ROS production and inflammatory cytokines (except IL1 β and IL6) in a laboratory experiment,39 it decreased eye involvement, improved QoL, and slowed TED progression in comparison to PTX and placebo in an randomized controlled trial (RCT).21 Consequently, European Thyroid Association/EUGOGO released a guideline in 2016,47 in which they recommended a 6-month use of antioxidants, mainly Se for mild TED in order to prevent its progression to advance stages and improve ocular manifestations and QoL.
Although there are some published reviews on the role of antioxidants in patients with TED, to the best of our knowledge, this is the first systematic review on this topic. While it is clear that antioxidants play an important role in the management of TED, no strong recommendation for any or combination of antioxidants could be made to be implemented in the daily practice on the grounds that all the reviewed studies had used different methods, stage of disease, patient selection, and randomization in this regard. Therefore, further well-designed RCTs are required to especially compare different single or combined antioxidants in different severity grades of TED.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Scott IU, Siatkowski MR. Thyroid eye disease. Semin Ophthalmol. 1999;14:52–61. doi: 10.3109/08820539909056064. [DOI] [PubMed] [Google Scholar]
- 2.Kashkouli MB, Pakdel F, Kiavash V, Heidari I, Heirati A, Jam S. Hyperthyroid vs hypothyroid eye disease: The same severity and activity. Eye (Lond) 2011;25:1442–6. doi: 10.1038/eye.2011.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kashkouli MB, Jam S, Sabzvari D, Ketabi N, Azarinia S, SeyedAlinaghi S, et al. Thyroid-associated ophthalmopathy in Iranian patients. Acta Med Iran. 2011;49:612–8. [PubMed] [Google Scholar]
- 4.Kashkouli MB, Kaghazkanani R, Heidari I, Ketabi N, Jam S, Azarnia S, et al. Bilateral versus unilateral thyroid eye disease. Indian J Ophthalmol. 2011;59:363–6. doi: 10.4103/0301-4738.83612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bahmani-Kashkouli M, Pakdel F, Astaraki A, Hashemi M, Honarbakhsh Y, Mirarmandehi B, et al. Quality of life in patients with thyroid eye disease. J Ophthalmic Vis Res. 2009;4:164–8. [PMC free article] [PubMed] [Google Scholar]
- 6.Kashkouli MB, Heidari I, Pakdel F, Jam S, Honarbakhsh Y, Mirarmandehi B. Change in quality of life after medical and surgical treatment of graves' ophthalmopathy. Middle East Afr J Ophthalmol. 2011;18:42–7. doi: 10.4103/0974-9233.75884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kashkouli MB, Karimi N, Aghamirsalim M, Abtahi MB, Nojomi M, Shahrad-Bejestani H, et al. Measurement properties of the persian translated version of graves orbitopathy quality of life questionnaire: A validation study. Ophthalmic Epidemiol. 2017;24:3–10. doi: 10.1080/09286586.2016.1255974. [DOI] [PubMed] [Google Scholar]
- 8.Stan MN, Garrity JA, Bahn RS. The evaluation and treatment of graves ophthalmopathy. Med Clin North Am. 2012;96:311–28. doi: 10.1016/j.mcna.2012.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.K-Jafari A, Sadeghi-Tari A, Minaee-Noshahr N, Ameri A, Anvari F, Ali-Mahmoudi A, et al. Ocular movement disorders and extraocular muscle involvement in Iranian Graves' ophthalmopathy patients. Binocul Vis Strabismus Q. 2010;25:217–30. [PubMed] [Google Scholar]
- 10.Kehrer JP. Free radicals as mediators of tissue injury and disease. Crit Rev Toxicol. 1993;23:21–48. doi: 10.3109/10408449309104073. [DOI] [PubMed] [Google Scholar]
- 11.Harman D. Aging and oxidative stress. J Int Fed Clin Chem. 1998;10:24–7. [PubMed] [Google Scholar]
- 12.Halliwell B, Gutteridge JM. Free Radicals in Biology and Medicine. 4th ed. New York: Oxford University Press; 2007. Cellular responses to oxidative stress: Adaptation, damage, repair, senescence and death; pp. 187–267. [Google Scholar]
- 13.Marcocci C, Leo M, Altea MA. Oxidative stress in graves' disease. Eur Thyroid J. 2012;1:80–7. doi: 10.1159/000337976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Strianese D. Update on Graves disease: Advances in treatment of mild, moderate and severe thyroid eye disease. Curr Opin Ophthalmol. 2017;28:505–13. doi: 10.1097/ICU.0000000000000402. [DOI] [PubMed] [Google Scholar]
- 15.Kashkouli MB, Aghamirsalim M, Karimi N, Shahrzad S. Autoimmune hyperthyroidism and thyroid eye disease: What is the role of pro-oxidants and antioxidants? Exp Rev Ophthalmol. 2015;10:135–43. [Google Scholar]
- 16.Winther KH, Bonnema SJ, Hegedüs L. Is selenium supplementation in autoimmune thyroid diseases justified? Curr Opin Endocrinol Diabetes Obes. 2017;24:348–55. doi: 10.1097/MED.0000000000000356. [DOI] [PubMed] [Google Scholar]
- 17.Lanzolla G, Marcocci C, Marinò M. Antioxidant therapy in graves' orbitopathy. Front Endocrinol (Lausanne) 2020;11:608733. doi: 10.3389/fendo.2020.608733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moher D, Liberati A, Tetzlaff J, Altman DG PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Ann Intern Med. 2009;151:264–9, W64. doi: 10.7326/0003-4819-151-4-200908180-00135. [DOI] [PubMed] [Google Scholar]
- 19.Ackley BJ, Ladwig GB, Swan BA, Tucker SJ. St Louis, MO: Mosby Elsevier; 2008. Evidence-Based Nursing Care Guidelines: Medical-Surgical Interventions; p. 7. [Google Scholar]
- 20.Finamor FE, Martins JR, Nakanami D, Paiva ER, Manso PG, Furlanetto RP. Pentoxifylline (PTX): An alternative treatment in Graves ophthalmopathy (inactive phase): Assessment by a disease specific quality of life questionnaire and by exophthalmometry in a prospective randomized trial. Eur J Ophthalmol. 2004;14:277–83. [PubMed] [Google Scholar]
- 21.Marcocci C, Kahaly GJ, Krassas GE, Bartalena L, Prummel M, Stahl M, et al. Selenium and the course of mild Graves' orbitopathy. N Engl J Med. 2011;364:1920–31. doi: 10.1056/NEJMoa1012985. [DOI] [PubMed] [Google Scholar]
- 22.Balazs C, Kiss E, Vamos A, Molnar I, Farid NR. Beneficial effect of pentoxifylline on Thyroid Associated Ophthalmopathy (TAO): A pilot study. J Clin Endocrinol Metab. 1997;82:1999–2002. doi: 10.1210/jcem.82.6.9995. [DOI] [PubMed] [Google Scholar]
- 23.Bouzas EA, Karadimas P, Mastorakos G, Koutras DA. Antioxidant agents in the treatment of Graves' ophthalmopathy. Am J Ophthalmol. 2000;129:618–22. doi: 10.1016/s0002-9394(00)00359-7. [DOI] [PubMed] [Google Scholar]
- 24.Dehina N, Hofmann PJ, Behrends T, Eckstein A, Schomburg L. Lack of association between selenium status and disease severity and activity in patients with graves' ophthalmopathy. Eur Thyroid J. 2016;5:57–64. doi: 10.1159/000442440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Federige MA, Romaldini JH, Miklos AB, Koike MK, Takei K, Portes ES. Serum selenium and selenoprotein-P levels in autoimmune thyroid diseases patients in a select center: A transversal study. Arch Endocrinol Metab. 2017;61:600–7. doi: 10.1590/2359-3997000000309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu Y, Liu S, Mao J, Piao S, Qin J, Peng S, et al. Serum trace elements profile in graves' disease patients with or without orbitopathy in Northeast China. Biomed Res Int. 2018;2018:3029379. doi: 10.1155/2018/3029379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Londzin-Olesik M, Kos-Kudła B, Nowak A, Wielkoszyński T, Nowak M. The effect of thyroid hormone status on selected antioxidant parameters in patients with Graves' disease and active thyroid-associated orbitopathy. Endokrynol Pol. 2020;71:418–24. doi: 10.5603/EP.a2020.0049. [DOI] [PubMed] [Google Scholar]
- 28.Chang CC, Chang TC, Kao SC, Kuo YF, Chien LF. Pentoxifylline inhibits the proliferation and glycosaminoglycan synthesis of cultured fibroblasts derived from patients with Graves' ophthalmopathy and pretibial myxoedema. Acta Endocrinol (Copenh) 1993;129:322–7. doi: 10.1530/acta.0.1290322. [DOI] [PubMed] [Google Scholar]
- 29.Burch HB, Lahiri S, Bahn RS, Barnes S. Superoxide radical production stimulates retroocular fibroblast proliferation in Graves' ophthalmopathy. Exp Eye Res. 1997;65:311–6. doi: 10.1006/exer.1997.0353. [DOI] [PubMed] [Google Scholar]
- 30.Hiromatsu Y, Yang D, Miyake I, Koga M, Kameo J, Sato M, et al. Nicotinamide decreases cytokine-induced activation of orbital fibroblasts from patients with thyroid-associated ophthalmopathy. J Clin Endocrinol Metab. 1998;83:121–4. doi: 10.1210/jcem.83.1.4478. [DOI] [PubMed] [Google Scholar]
- 31.Lisi S, Botta R, Lemmi M, Sellari-Franceschini S, Altea MA, Sisti E, et al. Quercetin decreases proliferation of orbital fibroblasts and their release of hyaluronic acid. J Endocrinol Invest. 2011;34:521–7. doi: 10.3275/7321. [DOI] [PubMed] [Google Scholar]
- 32.Yoon JS, Chae MK, Jang SY, Lee SY, Lee EJ. Antifibrotic effects of quercetin in primary orbital fibroblasts and orbital fat tissue cultures of Graves' orbitopathy. Invest Ophthalmol Vis Sci. 2012;53:5921–9. doi: 10.1167/iovs.12-9646. [DOI] [PubMed] [Google Scholar]
- 33.Tsai CC, Wu SB, Kao SC, Kau HC, Lee FL, Wei YH. The protective effect of antioxidants on orbital fibroblasts from patients with Graves' ophthalmopathy in response to oxidative stress. Mol Vis. 2013;19:927–34. [PMC free article] [PubMed] [Google Scholar]
- 34.Kim CY, Lee HJ, Chae MK, Byun JW, Lee EJ, Yoon JS. Therapeutic effect of resveratrol on oxidative stress in graves' orbitopathy orbital fibroblasts. Invest Ophthalmol Vis Sci. 2015;56:6352–61. doi: 10.1167/iovs.15-16870. [DOI] [PubMed] [Google Scholar]
- 35.Rotondo Dottore G, Leo M, Casini G, Latrofa F, Cestari L, Sellari-Franceschini S, et al. Antioxidant actions of selenium in orbital fibroblasts: A basis for the effects of selenium in graves' orbitopathy. Thyroid. 2017;27:271–8. doi: 10.1089/thy.2016.0397. [DOI] [PubMed] [Google Scholar]
- 36.Rotondo Dottore G, Chiarini R, De Gregorio M, Leo M, Casini G, Cestari L, et al. Selenium rescues orbital fibroblasts from cell death induced by hydrogen peroxide: Another molecular basis for the effects of selenium in graves' orbitopathy. Endocrine. 2017;58:386–9. doi: 10.1007/s12020-016-1226-9. [DOI] [PubMed] [Google Scholar]
- 37.Dottore GR, Ionni I, Menconi F, Casini G, Franceschini SS, Nardi M, et al. Antioxidant effects of β-carotene, but not of retinol and vitamin E, in orbital fibroblasts from patients with Graves' orbitopathy (GO) J Endocrinol Invest. 2018;41:815–20. doi: 10.1007/s40618-017-0809-5. [DOI] [PubMed] [Google Scholar]
- 38.Dottore GR, Ionni I, Menconi F, Casini G, Franceschini SS, Nardi M, et al. Action of three bioavailable antioxidants in orbital fibroblasts from patients with Graves' orbitopathy (GO): A new frontier for GO treatment? J Endocrinol Invest. 2018;41:193–201. doi: 10.1007/s40618-017-0718-7. [DOI] [PubMed] [Google Scholar]
- 39.Kim BY, Jang SY, Choi DH, Jung CH, Mok JO, Kim CH. Anti-inflammatory and antioxidant effects of selenium on orbital fibroblasts of patients with graves ophthalmopathy. Ophthalmic Plast Reconstr Surg. 2021;37:476–81. doi: 10.1097/IOP.0000000000001931. [DOI] [PubMed] [Google Scholar]
- 40.Katerji M, Filippova M, Duerksen-Hughes P. Approaches and methods to measure oxidative stress in clinical samples: Research applications in the cancer field. Oxid Med Cell Longev. 2019;2019:1279250. doi: 10.1155/2019/1279250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lu R, Wang P, Wartofsky L, Sutton BD, Zweier JL, Bahn RS, et al. Oxygen free radicals in interleukin-1beta-induced glycosaminoglycan production by retro-ocular fibroblasts from normal subjects and Graves' ophthalmopathy patients. Thyroid. 1999;9:297–303. doi: 10.1089/thy.1999.9.297. [DOI] [PubMed] [Google Scholar]
- 42.Hondur A, Konuk O, Dincel AS, Bilgihan A, Unal M, Hasanreisoglu B. Oxidative stress and antioxidant activity in orbital fibroadipose tissue in Graves' ophthalmopathy. Curr Eye Res. 2008;33:421–7. doi: 10.1080/02713680802123532. [DOI] [PubMed] [Google Scholar]
- 43.Tsai CC, Wu SB, Cheng CY, Kao SC, Kau HC, Chiou SH, et al. Increased oxidative DNA damage, lipid peroxidation, and reactive oxygen species in cultured orbital fibroblasts from patients with Graves' ophthalmopathy: Evidence that oxidative stress has a role in this disorder. Eye (Lond) 2010;24:1520–5. doi: 10.1038/eye.2010.31. [DOI] [PubMed] [Google Scholar]
- 44.Tsai CC, Wu SB, Cheng CY, Kao SC, Kau HC, Lee SM, et al. Increased response to oxidative stress challenge in Graves' ophthalmopathy orbital fibroblasts. Mol Vis. 2011;17:2782–8. [PMC free article] [PubMed] [Google Scholar]
- 45.Tsai CC, Cheng CY, Liu CY, Kao SC, Kau HC, Hsu WM, et al. Oxidative stress in patients with Graves' ophthalmopathy: Relationship between oxidative DNA damage and clinical evolution. Eye (Lond) 2009;23:1725–30. doi: 10.1038/eye.2008.310. [DOI] [PubMed] [Google Scholar]
- 46.Khong JJ, Goldstein RF, Sanders KM, Schneider H, Pope J, Burdon KP, et al. Serum selenium status in Graves' disease with and without orbitopathy: A case-control study. Clin Endocrinol (Oxf) 2014;80:905–10. doi: 10.1111/cen.12392. [DOI] [PubMed] [Google Scholar]
- 47.Bartalena L, Baldeschi L, Boboridis K, Eckstein A, Kahaly GJ, Marcocci C, et al. The 2016 European Thyroid Association/European Group on Graves' Orbitopathy Guidelines for the Management of Graves' Orbitopathy. Eur Thyroid J. 2016;5:9–26. doi: 10.1159/000443828. [DOI] [PMC free article] [PubMed] [Google Scholar]


