Abstract
Background
Between September 2017 and June 2019, an outbreak of hepatitis A virus (HAV) occurred in Louisville, Kentucky, resulting in 501 cases and 6 deaths, predominantly among persons who experience homelessness or who use drugs (PEH/PWUD). The critical vaccination threshold (Vc) required to achieve herd immunity in this population is unknown. We investigated Vc and vaccination impact using epidemic modeling.
Methods
To determine which population subgroups had high infection risks, we employed a technique based on comparing the proportion of cases arising before and after the epidemic peak. We developed a dynamic deterministic model of HAV transmission among PEH/PWUD to estimate the basic reproduction number (R0), herd immunity threshold, Vc and the effect of timing of the vaccination intervention on epidemic and economic outcomes.
Results
Of the 501 confirmed or probable cases, 385 (76.8%) were among PEH/PWUD. Among PEH/PWUD and within the general population, homelessness was a significant risk factor for infection in the initial stages of the outbreak (odds ratios for homeless versus not homeless: 2.62; 95% confidence interval (CI): 1.62-4.25 for PEH/PWUD and 2.39; 95% CI: 1.51-3.78 for all detected cases). Our estimate for R0 ranges between 2.85 and 3.54, corresponding to an estimate of 69% (95% CI: 65-72) for herd immunity threshold and 76% (95% CI: 72%-80%) for Vc, assuming a vaccine with 90% efficacy. The observed vaccination program was estimated to have averted 30 hospitalizations (95% CI: 19-43), associated with over US$490,000 (95% CI: $310,000–700,000) in hospitalization cost. Greater impact was observed with earlier and faster vaccination implementation.
Conclusions
Vaccination coverage of at least 77% is likely required to prevent outbreaks of HAV among PEH/PWUD in Louisville, assuming a 90% vaccine efficacy. Proactive hepatitis A vaccination programs among PEH/PWUD will maximize health and economic benefits of these programs and reduce the likelihood of another outbreak.
Keywords: hepatitis A, critical vaccination coverage, persons who experience homelessness or who use drugs, herd immunity, dynamic modeling
1. Introduction
An estimated 1.5 million cases of hepatitis A virus (HAV) infection occur worldwide annually [1]. Hepatitis A is a liver disease, typically characterized by fatigue, nausea, jaundice, stomach pain and appetite loss [2]. HAV is transmitted via the fecal-oral route, through close personal contact with an infected person or by ingesting contaminated food or water [2], [3]. Consequently, individuals living in poor sanitation conditions as well as men who have sex with men (MSM) are at increased infection risk [4], [5]. Hepatitis A vaccines are highly effective, offering up to 95% protection [1], [6].
The United States (US) introduced hepatitis A vaccination into its routine vaccination program in 1996 for children ≥24 months of age in high-burden communities and for adults with increased risk for HAV infection or severe disease from HAV. In 2006, recommendations expanded to include vaccination of all children between 12-24 months of age, regardless of risk category or location. Therefore, although vaccination coverage among adolescents (aged 13-17 years in 2019) is moderate (two-dose coverage: 77.1%) [7], it is substantially lower among adults (aged ≥19 years in 2018) (two-dose coverage: 11.9%) [8]. Additionally, despite being recommended for adults at increased risk such as people who use drugs (PWUD), and (since 2018) people experiencing homelessness or unstable housing (PEH), vaccination coverage among these groups, measured by antibodies to HAV (anti-HAV) seroprevalence, remains low with estimates from 33% to 52% [9], [10], [11].
Since 2016, widespread hepatitis A outbreaks have been reported across various states in the US [5]. Many of these outbreaks have affected MSM populations and persons who experience homelessness or who use drugs (PEH/PWUD) [5], [12], [13]. As of September 24, 2021, 42 223 cases, 25 666 hospitalizations and 385 deaths had been recorded across 36 states due to the outbreaks [5]. In response, state health departments initiated public education and vaccination programs, with the latter mostly targeted at groups at high risk [13]. However, few data are available on the vaccination coverage required to achieve herd immunity among populations at high risk in the US.
To provide evidence-based recommendations for public health outbreak response, we analyze the 2017-2019 HAV outbreak in Louisville, Kentucky. We assess the risks of different population subgroups to HAV infection during the initial outbreak. Further, using a dynamic model of HAV transmission among PEH/PWUD in Louisville, we estimate the basic reproduction number, the critical vaccination threshold and impact of vaccination strategies within this population.
2. Methods
2.1. Data
2.1.1. Surveillance
During the outbreak, surveillance was conducted by the Louisville Metro Department of Public Health and Wellness (LMPHW). Cases were categorized based on the 2012 US Council of State and Territorial Epidemiologists (CSTE) clinical description for hepatitis A [14]. Three case categories were considered: confirmed, probable and suspected. Cases that satisfied the CSTE clinical description and had laboratory confirmation of immunoglobulin M (IgM) anti-HAV were classified as confirmed while cases that satisfied the CSTE clinical description and had an epidemiologic linkage to a person who had laboratory-confirmed hepatitis A were classified as probable. All other cases were classified as suspected.
For each reported case, data were collected on a range of time, demographic and epidemiologic variables. Our study employed a subset of these variables namely, reporting date (year and week), age, sex, housing status (sheltered, unsheltered or unstable housing), illicit drug-use status (yes or no), hospitalization status and mortality. Illicit drug use was defined as stipulated by the US Substance Abuse and Mental Health Services Administration [15]. Persons who reported homelessness, unstable housing or illicit drug use (intravenous or non-intravenous) were classified as PEH/PWUD.
Although data exist on all reported cases (n = 659), our analysis considers only confirmed or probable cases (n = 501) as evidence for HAV infection among suspected cases is weak. Hereafter, confirmed and probable cases are referred to as detected or observed cases. We use incident or true underlying cases to refer to all cases possessing characteristics of a confirmed or probable case, irrespective of reporting status.
This study used deidentified surveillance data and was determined by the Centers for Disease Control and Prevention to constitute research that does not require review by an Institutional Review Board.
2.1.2. Vaccination
In response to the high proportion of cases observed among PEH/PWUD, the LMPHW implemented vaccination programs mainly targeted at PEH/PWUD as well as health and social workers who tend to have regular contact with PEH/PWUD. Vaccines were administered through street outreach and at drug rehabilitation centers, tuberculosis clinics, homeless shelters and correction centers. For each individual vaccinated, data were collected on the date of vaccine administration, age, sex, race, MSM status, housing status and illicit drug use status.
2.2. Analysis of risks among sub-populations
To determine HAV infection risk associated with PEH/PWUD and non-PEH/PWUD, we utilized a statistical technique proposed by Worby et al. [16], based on relative risks. The method involves comparing the pre- and post-peak incidence for population subgroups of interest to ascertain which group(s) stood a higher risk of infection during the initial outbreak stages.
We defined the epidemic peak as the period within which the maximum number of cases is observed (weeks 32-34 after the index case (April 9-April 29, 2018), similar for PEH/PWUD and non-PEH/PWUD, Figure 2A). We estimated the relative risk (RR), defined for each population subgroup as the ratio of the proportion of detected cases in that subgroup during the pre-peak period versus the corresponding ratio during the post-peak period. Case-detection rate within each subgroup was assumed to be constant over the duration of the outbreak [16], enabling the estimation of the odds ratio (OR) for the incidence of cases in subgroup i versus subgroup j for the pre-peak relative to the post-peak:
| (1) |
Figure 2:
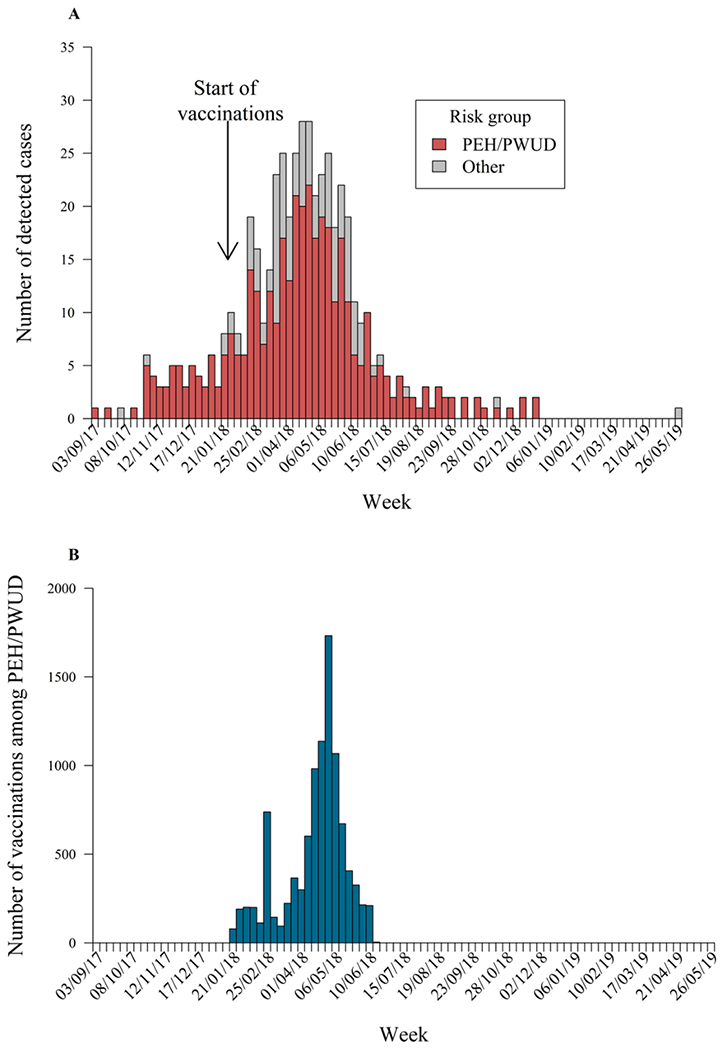
A) Distribution of all detected cases plotted by risk group; colored bars represent cases among persons experiencing homelessness or who use drugs (PEH/PWUD). Week labels correspond to the first day of the week. B) Weekly distribution of vaccines administered to PEH/PWUD in Louisville during the outbreak. Week labels correspond to the first day of the week.
An OR value differing significantly from 1 is indicative of a difference between pre-peak and post-peak incidence rates between the subgroups. Specifically, ORi,j > 1 suggests infection risk for subgroup i during the initial epidemic is higher than for subgroup j. We note that the principles of RR and OR used here are the same as the traditional concepts [17].
Among confirmed or probable cases, we investigated risk by PEH/PWUD status, age group and sex. Focusing exclusively on PEH/PWUD cases, we conducted further risk analysis after classifying cases by housing status, illicit drug use, age and sex.
2.3. Epidemic modelling
2.3.1. Model description
To study HAV outbreak dynamics among PEH/PWUD specifically, we developed a deterministic, compartmental mathematical model for HAV transmission among this population. The population, assumed to be closed and of a fixed size N for the duration of the outbreak, was disaggregated into five mutually exclusive compartments based on their infection status: susceptible (S), latent (L), infectious (I), temporary remission (R) or immune (Z).
The model dynamics are as follows: Susceptible individuals contact infected individuals at an effective rate β, and move to a state of latency for 1/α days on average, after which they become infectious. At a rate of θ(t), individuals are vaccinated. Susceptible individuals enter the immune state at rate ωτθ(t)S(t)/N, the effective number of vaccinations at a given time t. This is the product of the total number of vaccinations θ(t) at t, the fraction of susceptible individuals S(t)/N at t, the first-dose vaccine efficacy τ, and the fraction ω of vaccine doses given to individuals at risk. A proportion, 1 − η, of infectious individuals recover temporarily at an average rate of γ, entering the R state. Individuals in the R state experience a relapse after a period of 1/σ days on average, becoming infectious. Relapse of symptoms may occur in about 10–15% of cases [18], [19] lasting 4–8 weeks, and usually tending to be milder than the initial phase [20]. A schematic showing movements between compartments is presented in Figure 1.
Figure 1:
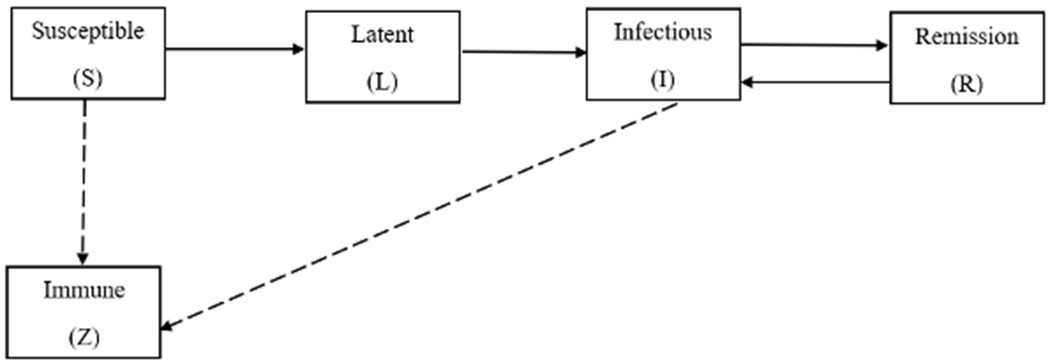
Schematic diagram illustrating the transitions between states for the hepatitis A virus transmission model among persons experiencing homelessness or who use drugs in Louisville, Kentucky. Transition rates between compartments are detailed in the main text. Solid arrows indicate movements for infected individuals while dashed arrows indicate movements for immune individuals.
By limiting the model to PEH/PWUD, we assumed that all infector-infectee pairs were contained within the risk population. We also assumed homogenous mixing. The model does not account for hepatitis A-related deaths due to the low hepatitis A mortality rate observed among the risk group (0.8 deaths per 100 cases). Also, the model does not include background mortality due to the relatively short epidemic duration (< 2 years) compared to the average lifespan of PEH/PWUD [17]. Lastly, births were not accounted for as no cases were reported among very young children (age range of detected cases was 10–83 years).
2.3.2. Model identifiability
Prior to parameter estimation, we checked for structural identifiability of all model parameters; that is, whether the parameters could be uniquely estimated given the model structure. Identifiability is a necessary condition for accurate parameter estimation in dynamic models [21]. For fixed N, all parameters except the effective contact rate β, the case detection rate κ, the vaccine efficacy τ, and the fraction of vaccines given to individuals at risk ω were identifiable. We reparameterised the model equations (see Supplementary material), so that all parameters were locally structurally identifiable. Due to the reparameterization, the resulting set of model equations (Supplementary equations (S3) and (S4)) represents the dynamics for observed (detected) cases, rather than true (incident) cases. Subscript 1 denotes variables in the reparameterized model. The identifiability analyses were performed in Mathematica and Maple using differential algebra-based methods [22], [23].
Although ω × τ, the effective vaccination coverage, was structurally identifiable, it was not practically identifiable: that is, the observed data did not hold adequate information to estimate it. This situation can arise, for example, if the impact of vaccination was only in the exponential decay portion of the epidemic and was thus harder to detect. Based on vaccination reports, we assumed ω = 1, that is, all people who received vaccines were at risk of hepatitis A infection, and fixed τ, the first-dose efficacy, at a reasonable value as described below.
2.3.3. Model parameterization
Parameters of the reparameterised model are described in Table 1. We fixed all natural history parameters (1/α, 1/γ, 1/σ and 1/η) at values informed by the literature [19], [20]. As no Louisville-specific estimates were available for the proportion of PEH/PWUD immune to hepatitis A at the start of the epidemic (ε), its value was fixed at the midpoint of the range of estimates of anti-HAV seroprevalence within the San Francisco homeless population [10] and within populations of PWUD in Wisconsin [11] and San Diego [9].
Table 1:
Descriptions, values and references for model parameters. For estimated parameters, values are estimates, with 95% confidence intervals in parentheses.
| Description | Symbol | Value | Reference |
|---|---|---|---|
| Effective contact rate at the start of the outbreak | βs | 0.61 (0.54-0.67) | Estimated |
| Effective contact rate later in the outbreak | βl | 0.12 (0.05-0.18) | Estimated |
| Transition midpoint time from βs to βl (in weeks) | t* | 36 (33-39) | Estimated |
| Speed of transition from βs to βl | c | 0.75 (0.24-∞) | Estimated |
| Number of infectious individuals in week 0 | I1(0) | 0.92 (0.63-2.01) | Estimated |
| Duration of latent period (in weeks) | 1/α | 1.57 | [19] |
| Duration of infectious period (in weeks) | 1/γ | 4.64 | [19] |
| Duration of remission period (in weeks) | 1/σ | 4.30 | [20] |
| Probability of experiencing a relapse | 1 −η | 0.11 | [20] |
| Proportion of initially immune individuals | ε | 0.43 | [9], [10], [11] |
| First dose vaccine efficacy (%) | τ | 90 | See Section 3 |
| Fraction of vaccine doses given to at-risk individuals | ω | 1 | Assumed |
| PEH/PWUD population size | N | 69862 | [24], [26], [25], [27] |
Bounds for the population size of PEH/PWUD, N, were estimated by aggregating information from a range of sources [24], [25], [26], [27]; see Supplementary material.
Due to higher rates of comorbidities among PEH/PWUD [28,29], we hypothesized that vaccine protection levels would be lower within this group, since comorbidities tend to decrease an individual’s immune response [30]. We therefore fixed the first dose vaccine efficacy parameter, τ, at 90%, approximately 5% less than that expected in the general population [19].
A single value for the effective contact rate β1 yielded a poor fit to the model (Supplementary Figure S3); thus, we implemented a time-varying β1, which provided a better fit. This was of the form of a sigmoidal function:
| (3) |
where βs is the value of β1 at the start of the outbreak, βl is the value of β1 later in the outbreak, t* is the transition midpoint time between βs and βl, and c is a rate parameter that controls the speed of the transition (c > 0).
2.3.4. Parameter estimation
Using maximum likelihood estimation (MLE) and assuming the case counts were Poisson-distributed, we estimated I1(0), the initial observed number of infected individuals, and the four parameters associated with β1, namely βs, βl, c, and t*. To account for overdispersion, we initially assumed a negative binomial distribution (parameterized with a mean and dispersion parameter k) for the observed case counts; however, the estimate of the dispersion parameter was large (1/k ≈ 0.01) suggesting the data did not significantly differ from a Poisson distribution [31]. Simultaneous 95% profile-likelihood-based confidence intervals (CIs) (with bounds at the 2.5th and 97.5th percentiles) were obtained for all estimated parameters [32]. In the MLE procedure, N was fixed at its mean value, but in estimating the CI for the observed infection trajectory I1(t), uncertainty in N was incorporated. Uncertainty estimates for I1(t) were calculated using a Latin hypercube approximation [33] of the parameter space confidence region.
2.3.5. The reproduction number and critical vaccination threshold
To quantify the epidemic potential, we calculated the basic reproduction number, R0, defined as the expected number of secondary infections caused by a single infected individual in a wholly susceptible population. Using the next generation matrix method [34], we derived an expression for R0 (see Supplementary material):
| (3) |
The herd immunity threshold (ψ), defined as the population proportion that should be immune to ensure decreasing or stable incidence, is given by ψ = 1 − 1/R0 [35]. The critical vaccination threshold (Vc) required to achieve herd immunity is given by [36]
| (5) |
We report maximum likelihood estimates and 95% CIs for R0, ψ and Vc.
2.3.6. Impact of vaccination program and timing
We assessed the impact of the observed vaccination program conducted among PEH/PWUD by comparing simulations with vaccination during the outbreak to a scenario with no vaccination. In particular, we assessed impact on the number of detected cases prevented, hospitalizations prevented, and the amount (in US$) saved in hospitalization costs. To estimate the number of hospitalizations based on the number of detected cases, we assumed the observed hospitalization rate among detected PEH/PWUD cases in Louisville. We also assumed hospitalization cost per hepatitis A case was US$16 232, based on a national estimate from the 2017 Healthcare Cost and Utilization Project National Inpatient Sample [37].
To assess the potential benefits had vaccination efforts been accelerated, we analyzed two vaccination scenarios with earlier program initiation week (3 and 11 weeks earlier than the observed vaccination start date, corresponding to weeks 10 and 18 of the outbreak), with total vaccination counts constant across each scenario. Lastly, we modified the earliest initiation scenario (week 10) such that the weekly vaccination rate was double the observed up to the observed total number of vaccinations. The potential impact of these programs was compared to a scenario with no vaccination.
2.3.7. Sensitivity analysis
We performed a variance-based global sensitivity analysis [38], [39] to assess the sensitivity of the model’s output (quantified by the total number of detected cases) to changes in model parameters. The analysis considered all natural history parameters, ε, N and all estimated parameters and involved varying parameters by ±20% of their respective values as given in Table 1. Details are in the Supplementary Material.
2.3.8. Sensitivity analysis
All analyses were performed in R version 4.0.5 [49]. Code for reproducing the results in this paper is freely available on GitHub: emmanuelle-dankwa/HAV-outbreak-Louisville (github.com).
3. Results
Between September 2017 and June 2019, there were 501 detected HAV cases in Louisville, among whom 385 (76.8%) were PEH/PWUD. The weekly detected cases by risk group are shown in Figure 2A. Counts of detected cases, hospitalizations and deaths by year of detection, sex, age group and risk factor are presented in Table 2. The observed hospitalization rate among PEH/PWUD in Louisville (66.2%) was slightly higher than the national rate of 59%, calculated from nationally reported outbreak data up to June 28, 2019 [5].
Table 2:
Summary of detected cases by year, sex, age group, housing status and illicit drug use.
| Variable | Number of cases (%) | Number of hospitalizations (%) | Number of deaths (%) |
|---|---|---|---|
| Year | |||
| 2017 | 42 (8.4) | 32 (9.7) | 0 (0) |
| 2018 | 458 (91.4) | 299 (90.3) | 6 (100) |
| 2019 | 1 (0.2) | 0 (0) | 0 (0) |
| Sex | |||
| Male | 332 (66.3) | 223 (67.4) | 4 (66.7) |
| Female | 169 (33.7) | 108 (32.6) | 2 (33.3) |
| Age (in years) | |||
| 10-19 | 5 (1.0) | 0 (0) | 0 (0) |
| 20-29 | 95 (19.0) | 59 (17.8) | 0 (0) |
| 30-39 | 185 (36.9) | 119 (36.0) | 0 (0) |
| 40-49 | 120 (24.0) | 85 (25.7) | 2 (33.3) |
| 50-59 | 65 (13.0) | 44 (13.3) | 3 (50.0) |
| 60-69 | 23 (4.5) | 18 (5.4) | 0 (0) |
| 70+ | 8 (1.6) | 6 (1.8) | 1 (16.7) |
| Housing status | |||
| Homeless | 128 (25.5) | 80 (24.2) | 1 (16.7) |
| Not homeless | 358 (71.5) | 244 (73.7) | 5 (83.3) |
| Unknown | 15 (3.0) | 7 (2.1) | 0 (0) |
| Illicit drug use, intravenous | |||
| Yes | 276 (55.1) | 192 (58.0) | 3 (50.0) |
| No | 134 (26.7) | 89 (26.9) | 2 (33.3) |
| Unknown | 91 (18.2) | 50 (15.1) | 1 (16.7) |
| Illicit drug use, non-intravenous | |||
| Yes | 189 (37.7) | 129 (39.0) | 2 (33.3) |
| No | 146 (29.2) | 107 (32.3) | 2 (33.3) |
| Unknown | 166 (33.1) | 95 (28.7) | 2 (33.3) |
| PEH/PWUD a | |||
| Yes | 385 (76.8) | 255 (77.0) | 3 (50.0) |
| No | 116 (23.2) | 76 (23.0) | 3 (50.0) |
| Total | 501 (100) | 331 (100) | 6 (100) |
PEH/PWUD: Persons experiencing homelessness or who use drugs.
3.1. Analysis of risks among subpopulations
Among all cases, there was a significant difference between pre-peak versus post-peak incidence rates for PEH versus non-PEH (OR: 2.39; 95% CI: 1.51-3.78; Supplementary Table S1). No significant difference was found between pre-peak versus post-peak incidence rates for PEH/PWUD versus non-PEH/PWUD (OR: 1.01; 95% CI: 0.64-1.59), or by illicit drug use, sex and age groups (Supplementary Tables S1 and S2).
Among PEH/PWUD cases, the estimated RR was significant for individuals who experience homelessness (RR: 1.92; 95% CI: 1.37-2.69). The OR for the homeless group versus the housed group for the pre-peak versus post-peak period was 2.62 (95% CI: 1.62-4.25). No significant differences were observed by sex, age group and illicit drug use among PEH/PWUD (Table 3).
Table 3:
Relative risk (RR) and odds ratio (OR) estimates for detected cases among persons experiencing homelessness or who use drugs (n=385) by risk status (homelessness and illicit drug use), sex and age group (in years). For each variable level, total case counts as well as case counts by period (pre-peak, peak and post-peak) are presented. RR and OR used here are as defined in Worby et al. [16].
| Variable | Number of cases | RRa (95% CI) | ORb (95% CI) | |||
|---|---|---|---|---|---|---|
|
| ||||||
| Pre-peak | Peak | Post-peak | Total | |||
| Homelessness | ||||||
| Yes | 70 | 22 | 36 | 128 | 1.92 (1.37–2.69) | 2.62 (1.62–4.25) |
| No | 92 | 41 | 124 | 257 | 0.73 (0.63–0.86) | |
| Illicit drug use | ||||||
| Yes | 155 | 56 | 155 | 366 | 0.99 (0.95–1.03) | 0.71 (0.22–2.30) |
| No | 7 | 7 | 5 | 19 | 1.38 (0.45–4.27) | |
| Sex | ||||||
| Male | 102 | 43 | 112 | 257 | 0.90 (0.77–1.05) | 0.73 (0.46–1.16) |
| Female | 60 | 20 | 48 | 128 | 1.23 (0.91–1.68) | |
| Age group (years) | ||||||
| 10-19 | 2 | 0 | 0 | 2 | - | |
| 20-29 | 40 | 9 | 32 | 81 | 1.23 (0.82–1.86) | Refer to Table S3c |
| 30-39 | 72 | 25 | 63 | 160 | 1.13 (0.87–1.46) | |
| 40-49 | 33 | 16 | 42 | 91 | 0.78 (0.52–1.16) | |
| 50-59 | 14 | 10 | 16 | 40 | 0.86 (0.44–1.71) | |
| 60-69 | 1 | 2 | 6 | 9 | - | |
| 70+ | 0 | 1 | 1 | 2 | - | |
| Period totals | 162 | 63 | 160 | 385 | ||
Estimates are not computed for groups with fewer than ten cases in total.
First rows for all variables (except age group) used as reference for OR.
3.2. Transmission model results
The model fit well to observed data (Figure 3A), with Table 1 presenting parameter estimates. The R0 estimate was 3.24 (95% CI: 2.85–3.54), corresponding to estimates of 69% (95% CI: 65-72) for herd immunity threshold and 76% (95% CI: 72-80) for critical vaccination threshold, assuming a vaccine with 90% efficacy. The results also suggest that the transmission began to decrease rapidly and substantially from mid-April 2018 (Supplementary Figure S2), driving the end of the outbreak.
Figure 3:
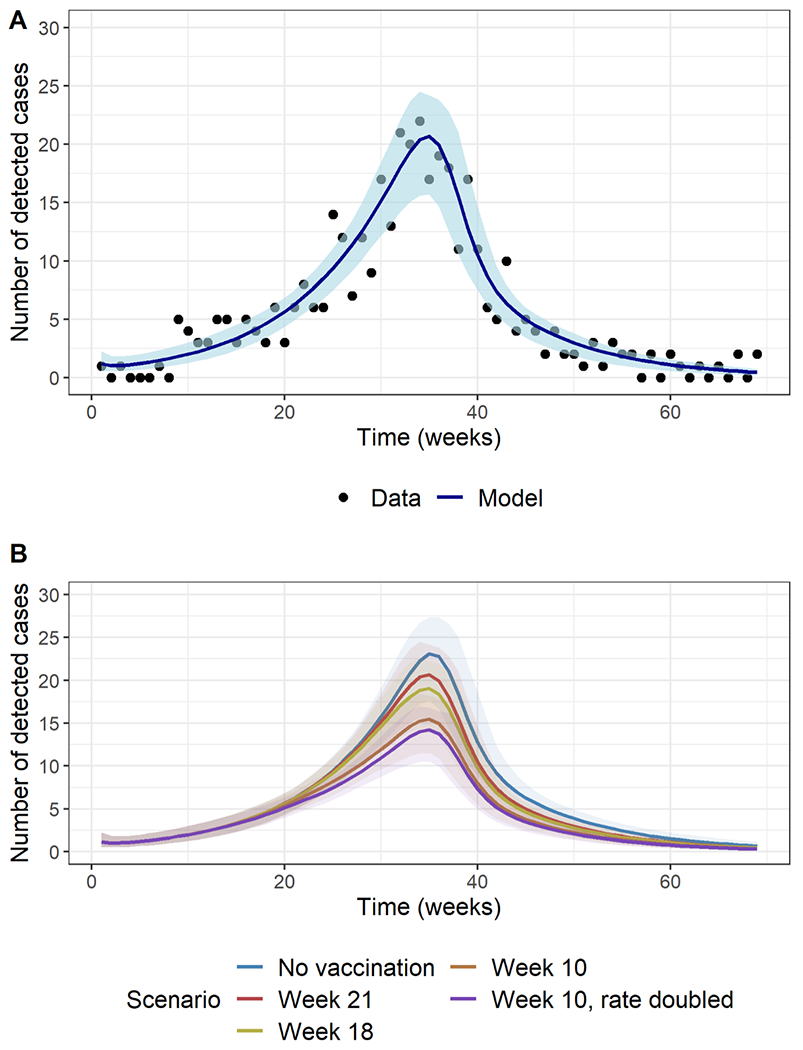
A) Model fit (blue line) to weekly case counts of detected cases of hepatitis A among persons experiencing homelessness or who use drugs in Louisville (black dots). Shaded area is the 95% confidence region for the model estimates. B) Model estimates for the weekly number of detected cases under the various vaccination scenarios: no vaccination (blue line), vaccination initiation in week 21 as observed (reddish brown line), week 18 (green line), and week 10 with the observed coverage (brown line) and double the observed coverage (magenta line). 95% confidence regions for all scenarios are shaded in corresponding colors.
The weekly vaccinations provided to PEH/PWUD are shown in Figure 2B. The vaccination program commenced 21 weeks after the index case and reached 9 999 PEH/PWUD over a 22-week period. We estimated this vaccination program averted 30 hospitalizations (95% CI: 19–43) and US$490 000 (95% CI: US$310 000–US$700 000) in hospitalization costs. The impacts across all measures increase with earlier and faster vaccination interventions, with 99 more detected cases and 66 more hospitalizations averted if vaccinations had been initiated in week 10 of the outbreak, at double the observed coverage, compared to week 21 (Table 4). The differences in the epidemic trajectories under the different vaccination scenarios become prominent only after week 20 and are most evident at the peak (Figure 3B).
Table 4:
Comparison of vaccination scenarios by week of initiation and administration rate, using key impact measures. Values recorded are model estimates and 95% CIs (in parentheses).
|
|
||||
|---|---|---|---|---|
| Vaccination scenarios by week of initiation | ||||
|
| ||||
| Measure | Week 21 | Week 18 | Week 10 | Week 10, rate doubled |
| Number of detected cases prevented | 46 (29–65) | 65 (43–90) | 121 (86–160) | 145 (104–188) |
| Number of hospitalizations prevented | 30 (19–43) | 43 (28–59) | 80 (57–106) | 96 (69–124) |
| Amount (in millions of US$) saved in hospitalization cost | 0.49 (0.31–0.70) | 0.69 (0.46–0.96) | 1.30 (0.92–1.72) | 1.55 (1.12–2.02) |
The sensitivity analysis showed the initial effective contact rate (βs), the fraction of immune individuals (ε) and the duration of infectiousness (1/γ) as the most influential parameters in driving the variation in the total number of detected cases y1 (Supplementary Figure S4). A scatterplot of y1 against these three parameters (Supplementary Figure S5) revealed the following trends: larger mean values of y1 correspond to small values for ε and large values for βs and 1/γ, while smaller mean values of y1 correspond to large values for ε and small values for βs and 1/γ.
4. Discussion
This study analyzed a recent HAV outbreak in Louisville, Kentucky, with a focus on outbreak dynamics among PEH/PWUD, who constituted the majority (76.8%) of all detected cases.
Within the general population and among PEH/PWUD, we found homelessness to be an influential risk factor for HAV infection during the initial outbreak (Table 3, Supplementary Table S1), underscoring the increased risk of hepatitis A transmission among PEH. Our results point to the importance of proactive targeting of PEH in vaccination campaigns, and to the importance of housing and sanitation programs for those living in unstable housing situations in mitigating HAV infection risk. While drug use has been suggested as a risk factor for hepatitis A transmission [2], it was not significant in our analysis, either in the overall population or among PEH/PWUD (Table 3, Supplementary Table S1); however, there was a high rate of non-response for drug use (33.1%, Table 2), which may result in an underestimate of the true difference in infection risk between PWUD versus non-PWUD in the initial outbreak. Further work is needed to understand whether drug use on its own is an indicator of high risk of hepatitis A infection.
Our results suggest no significant difference in infection risk by sex and age group among PEH/PWUD (Table 3) and in the general population (Supplementary Table S1).
We found a high herd immunity threshold (~69%; 95% CI: 65-72) likely required to prevent HAV outbreaks among PEH/PWUD in Louisville, comparable to corresponding estimates from an MSM population in Australia (~65%) [40]. Assuming a vaccine with 90% efficacy, our model yielded a critical vaccine threshold of 76% (95% CI: 72-80). Our 95% CI for R0 (2.85–3.54) is higher than an estimated range for R0 of HAV derived for the general US population in the pre-vaccine era (R0: 1.11-1.55 [41]). We expect this to be the case, as the risk profile of PEH/PWUD is generally higher than that of the general population. In particular, PEH are likely to have more effective contacts providing opportunities for transmission due to reduced access to and use of sanitation and hygiene facilities. This elevated risk of transmission within this population results in a higher R0.
We found the implemented vaccination program prevented many cases, but even more could have been prevented if initiated earlier and implemented at a faster rate (Table 4, Figure 3B), corroborating published evidence on vaccination as a key intervention strategy to mitigate the spread of HAV both in the US and elsewhere [3], [41], [42], [43], [44], [45]. It is possible that the vaccinations in Louisville also mitigated the spread of HAV neighbouring counties, as there are indications of a ring effect in spread, with Louisville as the center ([46], Figure 8).
It can be challenging to initiate vaccination programs early on in an outbreak, given the considerable amount of time required to obtain the relevant information (e.g., pathogen specimens, risk factors) needed to arrange logistics and funding. To navigate these time constraints, practitioners may benefit from using information from earlier outbreaks. In the current context, this could have been, for instance, risk factor information from surveillance data on earlier HAV outbreaks in other states.
Although data collected at vaccination clinics suggest that all the vaccinations included here were given to PEH/PWUD, making our assumption ω=1 reasonable, it is not clear that all PEH/PWUD were necessarily at risk. If ω were substantially below 1, then our estimates of the impact of the alternate vaccination scenarios may be overestimates. More work is needed to understand how risk indicators such as homelessness and drug use translate to actual risk.
We found that beginning mid-April 2018, the effective contact rate β1 rapidly and substantially decreased to an average of about 20% of its initial value at the start of the outbreak (Table 1, Supplementary Figure S2). Dynamically, this change was distinct from the susceptible burnout that generally drives the end up of epidemics and distinct from the impact of vaccination. This time-varying effective contact rate might be driven by seasonality, which would be consistent with the findings of Brouwer et al. [45] where a model with seasonal transmission yielded a better fit to the data than a model with no seasonal pattern. In general, the evidence for the role of seasonality in HAV transmission is mixed (c.f. [19], [47]), although it may be stronger for certain groups, such as PEH. Aside from seasonality, changes in behaviour, possibly influenced by education and media reporting, may have resulted in the observed change in the effective transmission rate.
The infinite upper bound in the 95% confidence interval for c, the transition midpoint time between the initial and latter values of β1 (Table 1), suggests the data could be modelled with the effective contact rate as a piecewise function; that is, allowing an abrupt change in rates (e.g., [40]) over a one-week period, although this may well be less realistic.
The sensitivity analysis showed that the initial effective contact, the fraction of initially immune individuals and the duration of the infectious period, in that order, contributed the most to the variation in the number of detected cases (Supplementary Figure S4). As expected, we found that smaller effective contact rates, shorter durations of infectiousness and larger fractions of initially immune individuals yielded fewer detected cases, given our model assumptions (Supplementary Figure S5). Two recommendations for control are in order: 1) improving early case detection through increased surveillance to decrease the period of exposure of an infectious individual hence decreasing transmission risk, and 2) increasing the rate of vaccination of individuals at risk to ensure a larger fraction of immune individuals and consequently, a smaller chance of take-off in the event of a future outbreak.
Given the substantial overlap between the populations of PEH and PWUD in Louisville (about 73.5% of individuals who experience homelessness in Louisville also use drugs) [24] and the lack of data on mixing between these groups, it was not possible to model transmission within the PEH and PWUD populations separately. The dynamics of HAV transmission within and between these groups is a worthwhile subject for future investigations, potentially providing useful insights for outbreak control within each subpopulation.
Like all modelling studies, ours contains limitations, mainly due to parameter uncertainty. First, there was appreciable uncertainty associated with N, the population size estimate of PEH/PWUD at risk for HAV. It was particularly challenging to estimate the total population size at risk – in particular, those who are at risk among persons who use drugs. Our variance-based sensitivity analysis indicated that our findings were not overly sensitive to uncertainty in population size, but we acknowledge that these estimates are prone to bias. Our analysis assumed all PWUD in Louisville were at risk, but this number may have overestimated the number truly at risk if not all who use drugs are at high risk for HAV infection. On the other hand, drug use is highly stigmatized and is often under-reported and therefore this estimate could possibly have underestimated the number at risk. As such it is difficult to assess implications for our analysis, and better at-risk population size estimates would help inform the public health response and related modeling. Second, no local data were available on baseline immunity against HAV among PEH/PWUD, so studies among similar populations in other locations were used. Additional data collection would improve understanding of existing immunity among local PEH/PWUD populations. Third, we neglect MSM status or risk in our analysis due to substantial missing data in relation to this risk factor. If a proportion of male PEH/PWUD were at an additional risk through sex with men, this risk was missing from our analysis. Studies examining populations with overlapping multiple risks (such as MSM who are homeless and/or who use drugs) are warranted. Fourth, it is probable the assumption of a constant rate of case-detection throughout the outbreak, does not hold completely, likely due to under-reporting among PEH/PWUD. Cases among PEH in particular may go unreported due to multiple factors including inability to afford healthcare costs and fear of hostility by service providers [48]. Reporting rates are likely to have been lower during the pre-peak period, compared to the post-peak period, due to the educational campaign introduced later in the outbreak. Thus, the OR for PEH/PWUD versus non-PEH/PWUD may be an underestimation of the true corresponding values.
In conclusion, we find that hepatitis A vaccination programs will need to achieve vaccination coverage of at least 77% among PEH/PWUD in Louisville, based on a vaccine efficacy of 90%, in order to prevent HAV outbreaks among this population. Proactive hepatitis A vaccination for PEH/PWUD can maximize health and economic benefits.
Supplementary Material
Acknowledgments.
The authors thank the staff of the Louisville Metro Department of Public Health and Wellness for the collection and organization of the study data. E.A.D is grateful to Ben Lambert for useful discussions on optimization. The authors are also grateful to two anonymous reviewers for their helpful comments on an earlier version of the manuscript.
Funding:
This work was supported by the US Centers for Disease Control and Prevention National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention (Epidemiologic and Economic Modeling Agreement number NU38PS004650). NKM was additionally supported by the National Institutes of Health NIAID and NIDA (grant number R01AI147490), and the San Diego Center for AIDS Research (SD CFAR), an NIH-funded program (P30 AI036214), which is supported by the following NIH Institutes and Centers: NIAID, NCI, NHLBI, NIA, NICHD, NIDA, NIDCR, NIDDK, NIGMS, NIMH, NIMHD, FIC, and OAR. CAD was supported by the MRC (Centre funding) and NIHR (funded by Vaccine Efficacy Evaluation for Priority Emerging Diseases: PR-OD-1017-20007 and HPRU in Emerging and Zoonotic Infections: NIHR20090). AFB was supported by the National Science Foundation (grant DMS1853032) and the National Institutes of Health (grant U01GM110712).
Footnotes
Publisher's Disclaimer: Disclaimer: The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention or the authors’ affiliated institutions.
References
- [1].World Health Organization. Immunization, vaccines and biologicals 2015. https://www.who.int/immunization/diseases/hepatitisA/en/.
- [2].Nelson NP, Weng MK, Hofmeister MG, Moore KL, Doshani M, Kamili S, et al. Prevention of Hepatitis A Virus Infection in the United States: Recommendations of the Advisory Committee on Immunization Practices, 2020. MMWR Recommendations and and Reports 2020;69:1–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Lemon SM, Ott JJ, Van Damme P, Shouval D. Type A viral hepatitis: a summary and update on the molecular virology, epidemiology, pathogenesis and prevention. J Hepatol 2018;68:167–84. [DOI] [PubMed] [Google Scholar]
- [4].World Health Organization. Hepatitis A fact sheet 2020. https://www.who.int/news-room/fact-sheets/detail/hepatitis-a.
- [5].Centers for Disease Control and Prevention. Widespread outbreaks of hepatitis A across the United States 2020. https://www.cdc.gov/hepatitis/outbreaks/2017March-HepatitisA.htm.
- [6].Demicheli V, Tiberti D. The effectiveness and safety hepatitis A vaccine: a systematic review. Vaccine 2003;21:2242–5. [DOI] [PubMed] [Google Scholar]
- [7].Elam-Evans LD, Yankey D, Singleton JA, Sterrett N, Markowitz LE, Williams CL, et al. National, regional, state, and selected local area vaccination coverage among adolescents aged 13–17 years—United States, 2019. Morbidity and Mortality Weekly Report 2020;69:1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Lu P-J, Hung M-C, Srivastav A, Grohskopf LA, Kobayashi M, Harris AM, et al. Surveillance of Vaccination Coverage Among Adult Populations—United States, 2018. MMWR Surveillance Summaries 2021;70:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Collier MG, Drobeniuc J, Cuevas-Mota J, Garfein RS, Kamili S, Teshale EH. Hepatitis A and B among young persons who inject drugs—vaccination, past, and present infection. Vaccine 2015;33:2808–12. [DOI] [PubMed] [Google Scholar]
- [10].Hennessey KA, Bangsberg DR, Weinbaum C, Hahn JA. Hepatitis A seroprevalence and risk factors among homeless adults in San Francisco: should homelessness be included in the risk-based strategy for vaccination? Public Health Rep 2009;124:813–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Koekpe R, Sill D, Akhtar W, Mitchell K, Guilfoyle S, Westergaard R. Hepatitis A and Hepatitis B Vaccination Coverage Among Persons Who Inject Drugs and Have Evidence of Hepatitis C Infection. Public Health Rep 2019;134:641–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Wooten DA. Forgotten but not gone: learning from the hepatitis A outbreak and public health response in San Diego. Top Antivir Med 2019;26:117. [PMC free article] [PubMed] [Google Scholar]
- [13].Foster M, Ramachandran S, Myatt K, Donovan D, Bohm S, Fiedler J, et al. Hepatitis A virus outbreaks associated with drug use and homelessness—California, Kentucky, Michigan, and Utah, 2017. MMWR Morb Mortal Wkly Rep 2018;67:1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Centers for Disease Control and Prevention, National Notifiable Diseases Surveillance System. Hepatitis A, acute. 2012 case definition 2012. https://wwwn.cdc.gov/nndss/conditions/hepatitis-a-acute/case-definition/2012/.
- [15].U.S. Department of Health and Human Services, Substance Abuse and Mental Health Services Administration, Center for Behavioral Health Statistics and Quality. Key substance use and mental health indicators in the United States: results from the 2016 national survey on drug use and health. Rockville, MD: Center for Behavioral Health Statistics and Quality, Substance Abuse and Mental Health Services Administration: 2017. [Google Scholar]
- [16].Worby CJ, Kenyon C, Lynfield R, Lipsitch M, Goldstein E. Examining the role of different age groups, and of vaccination during the 2012 Minnesota pertussis outbreak. Sci Rep 2015;5:13182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Sedgwick P Relative risks versus odds ratios. BMJ 2014;348. [Google Scholar]
- [18].Schiff ER. Atypical clinical manifestations of hepatitis A. Vaccine 1992;10:S18–20. [DOI] [PubMed] [Google Scholar]
- [19].Centers for Disease Control and Prevention, Wodi AP, Hamborsky J, others. Epidemiology and prevention of vaccine-preventable diseases. 14th ed. Washington, D.C.: Public Health Foundation; 2021. [Google Scholar]
- [20].Glikson M, Galun E, Oren R, Tur-Kaspa R, Shouval D. Relapsing hepatitis A. Review of 14 cases and literature survey. Medicine (Baltimore) 1992;71:14–23. [DOI] [PubMed] [Google Scholar]
- [21].Cobelli C, Distefano JJ 3rd. Parameter and structural identifiability concepts and ambiguities: a critical review and analysis. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology 1980;239:R7–24. [DOI] [PubMed] [Google Scholar]
- [22].Meshkat N, Eisenberg M, DiStefano III JJ. An algorithm for finding globally identifiable parameter combinations of nonlinear ODE models using Gröbner Bases. Mathematical Biosciences 2009;222:61–72. [DOI] [PubMed] [Google Scholar]
- [23].Hong H, Ovchinnikov A, Pogudin G, Yap C. SIAN: software for structural identifiability analysis of ODE models. Bioinformatics 2019;35:2873–4. [DOI] [PubMed] [Google Scholar]
- [24].Buchino S, Fosl C, Haynes L, Kinahan K, Omer L, Zero D. Solving street homelessness in Louisville, KY: improving the climate of care for individuals experiencing homelessness. 2019. Louisville: University of Louisville. [Google Scholar]
- [25].Coalition for the Homeless. 2017 homeless census 2017. https://www.louhomeless.org/.
- [26].United States Census Bureau, Population Division. Subcounty population estimates: April 1, 2010 to July 1, 2018 2019. https://data.census.gov/.
- [27].U.S. Department of Health and Human Services, Substance Abuse and Mental Health Services Administration. 2017-2018 national survey on drug use and health: model-based prevalence estimates (50 States and the District of Columbia) 2019. https://datafiles.samhsa.gov/.
- [28].Fazel S, Geddes JR, Kushel M. The health of homeless people in high-income countries: descriptive epidemiology, health consequences, and clinical and policy recommendations. Lancet 2014;384:1529–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Walker ER, Pratt LA, Schoenborn CA, Druss BG. Excess mortality among people who report lifetime use of illegal drugs in the United States: A 20-year follow-up of a nationally representative survey. Drug and Alcohol Dependence 2017;171:31–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Zimmermann P, Curtis N. Factors that influence the immune response to vaccination. Clin Microbiol Rev 2019;32:e00084–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Lloyd-Smith JO. Maximum likelihood estimation of the negative binomial dispersion parameter for highly overdispersed data, with applications to infectious diseases. PloS One 2007;2:e180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Raue A, Kreutz C, Maiwald T, Bachmann J, Schilling M, Klingmüller U, et al. Structural and practical identifiability analysis of partially observed dynamical models by exploiting the profile likelihood. Bioinformatics 2009;25:1923–9. [DOI] [PubMed] [Google Scholar]
- [33].McKay MD, Beckman RJ, Conover WJ. Comparison of three methods for selecting values of input variables in the analysis of output from a computer code. Technometrics 1979;21:239–45. [Google Scholar]
- [34].Heffernan JM, Smith RJ, Wahl LM. Perspectives on the basic reproductive ratio. J R Soc Interface 2005;2:281–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Dietz K. Transmission and control of arbovirus diseases. Epidemiology 1975;104:104–21. [Google Scholar]
- [36].Fine P, Eames K, Heymann DL. “Herd immunity”: a rough guide. Clin Infect Dis 2011;52:911–6. [DOI] [PubMed] [Google Scholar]
- [37].Hofmeister MG, Yin S, Aslam MV, Teshale EH, Spradling PR. Hepatitis A hospitalization costs, United States, 2017. Emerg Infect Dis 2020;26:1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Sobol IM. Sensitivity analysis for non-linear mathematical models. Mathematical Modelling and Computational Experiment 1993;1:407–14. [Google Scholar]
- [39].Saltelli A, Ratto M, Andres T, Campolongo F, Cariboni J, Gatelli D, et al. Global sensitivity analysis: the primer. John Wiley & Sons; 2008. [Google Scholar]
- [40].Zhang X-S, Charlett A Bayesian modelling of a hepatitis A outbreak in men who have sex with men in Sydney, Australia, 1991/1992. Epidemiology & Infection 2019;147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Van Effelterre TP, Zink TK, Hoet BJ, Hausdorff WP, Rosenthal P. A mathematical model of hepatitis a transmission in the United States indicates value of universal childhood immunization. Clinical Infectious Diseases 2006;43:158–64. [DOI] [PubMed] [Google Scholar]
- [42].Fiore AE, Wasley A, Bell BP. Prevention of hepatitis A through active or passive immunization: recommendations of the Advisory Committee on Immunization Practices (ACIP). Morbidity and Mortality Weekly Report: Recommendations and Reports 2006;55:1-CE. [PubMed] [Google Scholar]
- [43].McMahon BJ, Beller M, Williams J, Schloss M, Tanttila H, Bulkow L. A program to control an outbreak of hepatitis A in Alaska by using an inactivated hepatitis A vaccine. Archives of Pediatrics & Adolescent Medicine 1996;150:733–9. [DOI] [PubMed] [Google Scholar]
- [44].Chodick G, Heymann A, Ashkenazi S, Kokia E, Shalev V. Long-term trends in hepatitis A incidence following the inclusion of Hepatitis A vaccine in the routine nationwide immunization program. J Viral Hepat 2008;15:62–5. [DOI] [PubMed] [Google Scholar]
- [45].Brouwer AF, Zelner JL, Eisenberg MC, Kimmins L, Ladisky M, Collins J, et al. The Impact of Vaccination Efforts on the Spatiotemporal Patterns of the Hepatitis A Outbreak in Michigan, 2016–2018. Epidemiology 2020;31:628–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Kentucky Department for Public Health. Acute Hepatitis A Outbreak Weekly Report Weeks 51 and 52, 2019, December 15, 2019 – December 28, 2019. Kentucky Department for Public Health; 2019. [Google Scholar]
- [47].Fares A Seasonality of hepatitis: a review update. Journal of Family Medicine and Primary Care 2015;4:96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Institute of Medicine (US) Committee on Health Care for Homeless. Homelessness, health, and human needs. Washington (DC): National Academies Press (US); 1988. [Google Scholar]
- [49].R Core Team, R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL https://www.R-project.org/. 2021 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


