Abstract
Background
Gastric cancer is one of the most common cancers with high mortality. In Iran, the high-risk regions include Northern and Northwestern parts. The aim of this study was to assess the operative link on gastritis assessment- and operative link on gastric intestinal metaplasia-based staging in patients with upper gastrointestinal symptoms.
Methods
Totally, 345 patients underwent upper gastrointestinal endoscopy. Also, the status of Helicobacter pylori infection was evaluated using rapid urease test and histological method. Moreover, histological changes were assessed using the Update Sydney System. The operative link on gastritis assessment- and operative link on gastric intestinal metaplasia-based stages of 0-II were considered as low-risk stages and stages III and IV were considered as high-risk stages.
Results
Most of the patients were lower than 60 years (245 patients, 71%), and 71.9% of our patients had H. pylori infection. The frequency of atrophic gastritis and intestinal metaplasia was 44.9% and 25.2%, respectively (P < .001). Eleven patients (73.7%) with gastric adenocarcinoma had a low risk and 2 patients with low-grade dysplasia had a high risk of operative link on gastritis assessment and operative link on gastric intestinal metaplasia. Almost, 62.5% of gastric cancer patients with an intestinal type of gastric adenocarcinoma were at low-risk stages.
Conclusions
Although high stages of operative link on gastritis assessment and operative link on gastric intestinal metaplasia need further follow-up, lower stages of atrophy or intestinal metaplasia also require follow-up. Furthermore, operative link on gastritis assessment method in detecting a greater number of patients who need follow-up is more successful and profitable.
Keywords: Endoscopy, gastritis, Helicobacter pylori, OLGA, OLGIM
Introduction
Gastric cancer is one of the most common cancers with high mortality.1 Its incidence and prevalence are high (989 000 cases per year, 7.8% of all cancers) and is at fourth place after pulmonary, breast, and colorectal cancers.2 In Iran, the high-risk regions include Northern and Northwestern parts, while southern parts have the lowest incidence and other parts have low to moderate risk.3 Based on the study in 5 provinces of Ardabil, Guilan, Mazandaran, Golestan, and Kerman, gastric cancer was the most prevalent cancer in men (22.5%) and the second prevalent cancer in women (9.3%). Moreover, Guilan and Ardabil provinces are high at-risk regions for gastric cancer.4,5 Gastric cancer can be classified as intestinal and diffuse forms with distinct morphologic, epidemiologic, pathologic, and genetic characteristics.6 Intestinal form is associated with environmental and dietary factors plus Helicobacter pylori infection and is mostly seen in regions with a high prevalence of gastric cancer.7 On the other hand, 2 staging systems called operating link for gastritis assessment (OLGA) and operating link for gastric intestinal metaplasia (OLGIM) were introduced by an international group of gastroenterologists and pathologists to evaluate the lesions and predict the risk of cancer progression.8,9
The H. pylori-related gastric atrophy and intestinal metaplasia (IM) are the known risk factors of gastric cancer,10 and it has been expressed that gastritis staging can be a reliable indicator of cancer risk.11 Therefore, we aimed to evaluate the results of OLGA- and OLGIM-based staging systems in patients with gastrointestinal complaints in the Guilan province of Iran as it is an area with a high risk of gastric cancer and a high prevalence of H. pylori infection.
MATERIALS AND Methods
Patients
The sample size of this cross-sectional study was set as 345 by considering P = .56, α = 0.05, d (the minimum absolute size difference) = 0.05. Thus, all patients with age 20-70 years who were referred to the outpatient of Gastrointestinal and Liver Disease Research Center of Razi Hospital affiliated to Guilan University of Medical Sciences due to gastrointestinal symptoms and need endoscopy were included. Patients with pregnancy, upper gastrointestinal (GI) surgery, other non-GI cancers, chronic hepatic, renal, pulmonary failures, unstable hemodynamic, and previous treatment of H. pylori infection were excluded. The protocol of this study was approved by a local ethical committee of Guilan University of Medical Sciences (No. IR.GUMS.REC.1395.270) and was based on the Declaration of Helsinki. Informed consent was obtained from all patients, and all securities were applied to their data.
Endoscopy Procedure and Biopsy Collection
Gastroscopy was performed with Olympus video endoscopes (Olympus Optical Co., Ltd., GIF type V, Hamburg, Germany) in the standard manner. Six biopsy samples were obtained from dyspeptic patients with endoscopy indications based on the Updated Sydney System. Two A1 and A2 samples were collected from the antrum at a 3-cm distance from the pyloric ring in both lesser and greater curvature sides. The A3 biopsy was obtained from incisura angularis. Two C1 and C2 samples were obtained from the corpus part at lesser and greater curvatures of mid-body, 4 cm distal to the gastroesophageal junction (Figure 1). All samples were stored in 10% buffer formalin solution, separately. The sixth sample was taken from the non-oxyntic part of the antrum to evaluate H. pylori infection by rapid urease test (RUT).
Figure 1.
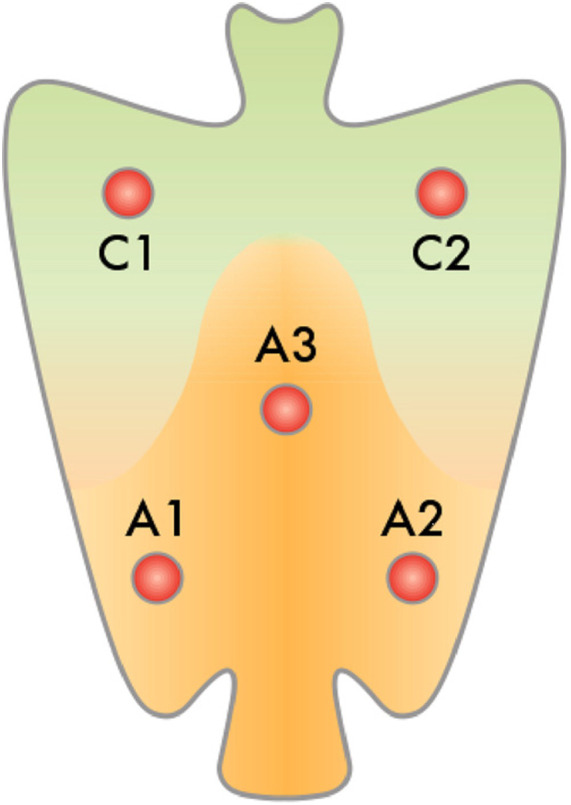
The place of obtained biopsy samples from dyspeptic patients.
Pathology and RUT samples were taken from the subjects. If the RUT was positive, treatment was started immediately. Therefore, a positive RUT was sufficient to begin treatment. But if the RUT was negative, we waited for the pathology response to start treatment.
Evaluations
Formalin-fixed tissues were sectioned and stained with hematoxylin and eosin12 and Giemsa stain to recognize H. pylori density. Biopsy specimens were evaluated and reviewed by 2 blinded expert pathologists for giving a histological diagnosis. Samples were analyzed and compared. Kappa value (overall agreement) between 2 pathologists was 0.96 (95% CI 0.98-0.94). The discrepancy between the pathologists was resolved by consensus or a third pathologist.
Gastric atrophy and IM were scored based on OLGA (Table 1) and OLGIM (Table 2) staging system, respectively. Stages III and IV in both OLGA and OLGIM systems were considered as high-risk stages.
Table 1.
Operative Link on Gastritis Assessment Staging
| Corpus | |||||
|---|---|---|---|---|---|
| Atrophy Score | No | Mild | Moderate | Severe | |
| Antrum | No | Stage 0 | Stage I | Stage II | Stage II |
| Mild | Stage 1 | Stage I | Stage II | Stage III | |
| Moderate | Stage II | Stage II | Stage III | Stage IV | |
| Severe | Sage III | Stage III | Stage IV | Stage IV | |
Table 2.
Operative Link on Gastric Intestinal Metaplasia Staging
| Corpus | |||||
|---|---|---|---|---|---|
| Intestinal Metaplasia Score | No | Mild | Moderate | Severe | |
| Antrum | No | Stage 0 | Stage I | Stage II | Stage II |
| Mild | Stage 1 | Stage I | Stage II | Stage III | |
| Moderate | Stage II | Stage II | Stage III | Stage IV | |
| Severe | Sage III | Stage III | Stage IV | Stage IV | |
Statistical Analysis
Histopathological stages and H. pylori status were the main variables, while age and gender were the secondary variables. All data were expressed as mean and standard deviation for quantitative data and frequency (percentage) for qualitative data and analyzed using statistical package for the social sciences (SPSS) version 16.0. The chi-square and 2 independent sample t-tests were used to find significant differences (P < .05).
Results
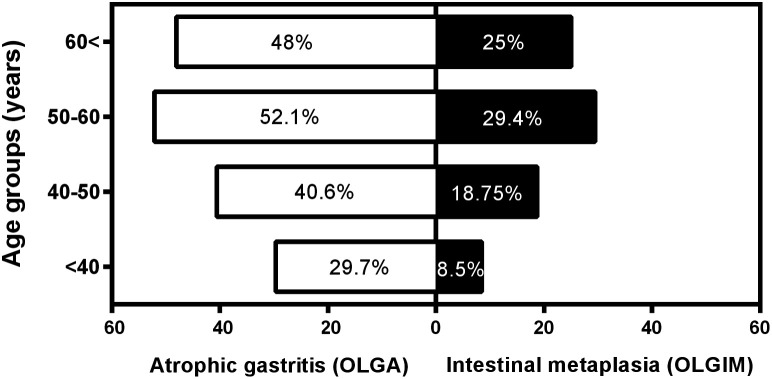
In the present study, 204 women (59.1%) and 141 men (40.9%) were included with a mean age of 53.5 ± 15.2 years, and the most prevalent age range was 50-60 years (102 patients, 29.5%). The prevalence of atrophic gastritis based on OLGA staging and IM based on OLGIM staging was 44.9% and 25.2%, respectively (P = .001). Also, atrophic gastritis and IM were more prevalent in men than women (52.4% vs. 39.7% and 32.6% vs. 20.7%, respectively). The distribution of atrophic gastritis and IM in different age ranges is presented in Figure 2.
Figure 2.
Percentage of patients with atrophic gastritis based on OLGA staging and intestinal metaplasia based on OLGIM staging in different age categories.
The prevalence of S0 based on OLGA staging in women and men was 60.3% and 47.5%, respectively. Also, the prevalence of S0 based on OLGIM staging in women and men was 79.9% and 67.4%, respectively. Thus, S0 was more prevalent in OLGA and OLGIM. Distribution of the patients by gastritis stage was reported in Table 3.
Table 3.
Distribution of the Patients by Gastritis Stage
| Stage | OLGA Staging | OLGIM Staging | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Demographic profile | S0 | S1 | S2 | S3 | S4 | P | S0 | S1 | S2 | S3 | S4 | P |
| Gender, n (%) | ||||||||||||
| Female | 123 (60.3) | 66 (32.4) | 9 (4.4) | 5 (2.5) | 1 (0.5) | .02 | 163 (79.9) | 34 (16.7) | 2 (1) | 4 (2) | 1 (0.4) | .05 |
| Male | 67 (47.5) | 49 (34.8) | 17 (12.1) | 5 (3.5) | 3 (2.1) | 95 (67.4) | 33 (23.4) | 6 (4.3) | 5 (3.5) | 2 (1.4) | ||
| Age (years), n (%) | ||||||||||||
| <40 | 33 (70.2) | 13 (27.7) | 1 (2.1) | 0 (0) | 0 (0) | .01 | 43 (91.5) | 4 (8.5) | 0 (0) | 0 (0) | 0 (0) | .005 |
| 40-50 | 57 (59.4) | 31 (32.3) | 4 (4.2) | 4 (4.2) | 0 (0) | 78 (81.2) | 12 (12.5) | 2 (2.1) | 4 (4.2) | 0 (0) | ||
| 50-60 | 48 (47.1) | 43 (42.1) | 9 (8.8) | 2 (2) | 0 (0) | 72 (70.6) | 26 (25.5) | 3 (2.9) | 1 (1) | 0 (0) | ||
| >60 | 52 (52) | 28 (28) | 12 (12) | 4 (4) | 4 (4) | 65 (65) | 25 (25) | 3 (3) | 4 (4) | 3 (3) | ||
OLGA, operating link for gastritis assessment; OLGA, operating link for gastric intestinal metaplasia.
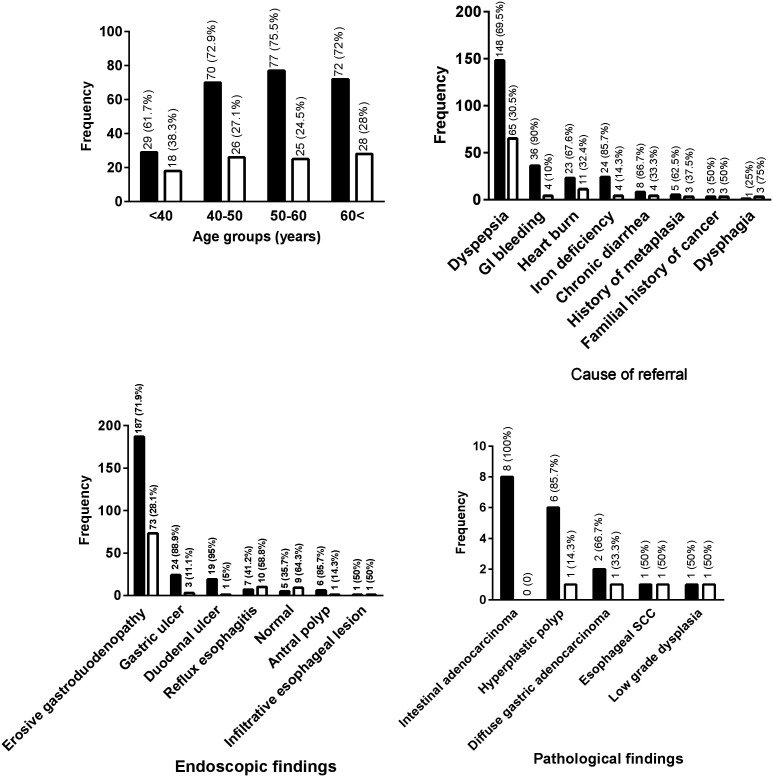
In this study, gastric intestinal adenocarcinoma was more prevalent than other pathological findings.
High-risk stages (III and IV) of OLGA and OLGIM were detected in 4.3% and 4% of patients, respectively (P > .05) while none of them had an age lower than 40 years. The most prevalent causes of referring were dyspepsia (61.8%), gastrointestinal bleeding (11.6%), reflux (9.8%), iron deficiency (8.1%), chronic diarrhea (3.5%), familial history of IM (2.3%), familial history of cancer (1.7%), and dysphagia (1.2%). High-risk stages of OLGA and OLGIM were detected in 50% of patients with familial history of IM, 25% of patients with dysphagia, 7.1% of cases with iron deficiency, and 2.9% of dyspeptic patients.
Data about low and high-risk stages on OLGA and OLGIM-based staging in patients with different endoscopic and pathologic findings are presented in Table 4. As seen, high-risk stages were not detected in patients with normal endoscopic findings, duodenal ulcer, reflux, and antral polyp. Although significant differences were seen in the patients with duodenal ulcer, gastric ulcer, and reflux based on OLGA staging (P = .03), no significant differences were detected between high- and low-risk stages in neither OLGA nor OLGIM staging in different categories of endoscopic findings (P > .05). There were no significant associations between low- and high-risk stages of OLGA and OLGIM with the status of H. pylori infection (P = .3 and P = .5, respectively). However, higher stages of OLGA and OLGIM were rare in patients with negative H. pylori infection and were 3.1% and 2.1%, respectively.
Table 4.
Frequency (Percentage) of Low- and High-Risk Patients Based on OLGA and OLGIM Staging in Different Categories of Endoscopic Findings
|
Parameters (n) |
OLGA Staging | OLGIM Staging | ||
|---|---|---|---|---|
| Low Risk | High Risk | Low Risk | High Risk | |
| Endoscopic findings n (%) | ||||
| Erosive gastrodeodenopathy (n = 259) | 250 (96.5) | 9 (3.5) | 252 (97.9) | 8 (3.1) |
| Gastric ulcer (n = 27) | 24 (88.9) | 3 (11.1) | 24 (88.9) | 3 (11.1) |
| Duodenal ulcer (n = 20) | 20 (100) | 0 (0) | 20 (100) | 0 (0) |
| Reflux esophagitis (n = 17) | 17 (100) | 0 (0) | 17 (100) | 0 (0) |
| Antral polyp (n = 7) | 7 (100) | 0 (0) | 7 (100) | 0 (0) |
| Esophageal infiltrative lesion (n = 2) | 1 (50) | 1 (50) | 1 (50) | 1 (50) |
| Pathological findings, n (%) | ||||
| Gastric intestinal adenocarcinoma (n = 8) | 8 (100) | 0 (0) | 8 (100) | 0 (0) |
| Hyperplastic polyp (n = 7) | 7 (100) | 0 (0) | 7 (100) | 0 (0) |
| Gastric diffuse adenocarcinoma (n = 3) | 3 (100) | 0 (0) | 3 (100) | 0 (0) |
| Esophageal SCC (n = 2) | 1 (50) | 1 (50) | 1 (50) | 1 (50) |
| Low-grade dysplasia (n = 2) | 0 (0) | 2 (100) | 0 (0) | 2 (100) |
| Helicobacter pylori status n (%) | ||||
| Positive (n = 248) | 237 (95.6) | 11 (4.4) | 238 (96) | 10 (4) |
| Negative (n = 97) | 94 (96.9) | 3 (3.1) | 95 (97.9) | 2 (2.1) |
SCC, squamous cell carcinoma; OLGA, operating link for gastritis assessment; OLGA, operating link for gastric intestinal metaplasia.
About the status of H. pylori infection, there was a significant association between male gender and positive infection when 78.8% of men and 67.2% of women were positive (P = .01). Distribution of H. pylori infection based on age, the cause of referral, endoscopic and pathological findings are presented in Figure 3. As seen, a significant association was seen between lower age and negativity of H. pylori infection (P = .003). Also, in a positive significant relationship, all patients who suffered from gastric intestinal-type adenocarcinoma had H. pylori infection.
Figure 3.
The frequency of patients with and without H. Pylori infection based on age, the cause of referral, endoscopic and pathological findings. Black bar, positive infection; White bar, negative infection.
Discussion
In the present study, the results of OLGA and OLGIM-based staging systems in the patients with gastrointestinal complaints in an area with a high risk of gastric cancer and a high prevalence of H. pylori infection were evaluated. We found a likeness between the 2 staging methods in higher stages in addition to the high frequency of H. pylori infection. Also, most of the patients with certain cancers had lower stages in both staging methods which confirmed this note that patients with all stages of OLGA and OLGIM need follow-up.
Early screening, diagnosis, and treatment of gastric cancer in high-risk populations is an effective preventive method to decline related mortality.13 The risk factors of gastric cancer are family history, type III incomplete IM, and the extent of preneoplastic changes.14 A Korean study showed that the extent and presence of IM can increase the risk of gastric cancer, and each stage of OLGIM and III-IV OLGA can be an independent risk factor for gastric cancer.15 A multicenter study carried out in Spanish revealed that incomplete IM was a risk factor for gastric cancer.16 In an Italian cohort study, family history increases the risk of gastric cancer.17
It has been reported that the extent and place of mucosal atrophy and IM which are evaluated by OLGA and OLGIM staging methods are related to the risk of gastric cancer.18,19 Also, chronic gastritis along with H. pylori infection is a vital and important step in the oncogenic process of gastric cancer. It is confirmed as evidence-based that atrophic gastritis is a primary risk factor for the intestinal type of gastric cancer.20 However, there is some controversy about the impacts of these 2 staging methods. Operative link on gastritis assessment and OLGIM methods diagnosed atrophy and IM in 44.9% and 25.2% of our patients. This difference is mostly due to the more diagnostic efficacy of OLGA in the early stages (S1) of gastritis which cannot be detected by OLGIM. Rugge and collaborators21 reported that gastritis OLGA staging consistently carries the same unfavorable prognostic message as types II and III IM.21 In a population-based screening of early cancers, the prevalence of precancerous gastric lesions in an area in Shandong province of China was evaluated. Among 3433 patients with age of 35-64 years, chronic atrophic gastritis and IM were seen in 98% and 33%of patients, respectively,22 which is higher than our results about atrophic gastritis but near to our found value about IM. In another study conducted in the Netherlands to analyze the gastric cancer risk in patients with premalignant gastric lesions, 24% of patients were diagnosed with atrophic gastritis, while 67% of their population had IM23 which is more similar to our results than China study. In an Italian study performed on 93 patients with dyspepsia in an area with a high risk of gastric cancer, 89.2% were low stages of OLGA and 10.8% were stage III or IV of OLGA18 which are approximately similar to our findings of 95.7% and 4.3%, respectively. Furthermore, the associations between endoscopic gastric atrophy stages III and IV OLGA gastritis and extensive IM with pathologic characteristics were evaluated by Quach and coworkers in a cross-sectional study of 280 patients with functional dyspepsia. They found that stage III and IV OLGA and extensive IM were categorized in patients with moderate to severe endoscopic gastric atrophy, and thus assessing the severity of gastric atrophy by endoscopy could help to monitor and prevent gastric cancer.24 In addition, it has been reported that OLGA high-stage gastritis was associated with gastric dysplasia and was mostly diagnosed in patients with moderate to severe gastric atrophy.25
H. pylori is a Gram-negative bacterium which causes gastritis and peptic ulcer and is also considered as a risk factor for gastric adenocarcinoma.26 The prevalence of H. pylori infection in Iranian population was reported between 13% in Birjand27 and 82% in Shiraz28 with the overall infection rate estimated as 54%.29 In the Northern province of Iran, this prevalence rate is higher and reported as 78% of males and 82% of females in Babol,30 89% in Ardabil,31,32 and 69% in Tehran.33 In the present study, we found H. pylori infection in 78.8% of males and 67.2% of females (overall rate 72%) and the lowest prevalence was in patients with age lower than 40 years (61.7%) which are approximately similar to previous reports.28,30,33 In a study from Korea which aimed to evaluate the distribution of OLGA and OLGIM staging by age and H. pylori infection status, the overall rate of H. pylori infection was reported as 59%. Moreover, older age groups had a significantly higher proportion of stage III and IV of OLGA and OLGIM stages, and old age and H. pylori infection were independent risk factors for both high-risk OLGA and OLGIM stages. Additionally, high-risk OLGA and OLGIM stages were rare in the H. pylori-negative group and age lower than 40 years.34 We found no high-risk stages of OLGA and OLGIM in patients with ages lower than 40 years, and similar to their study, the prevalence of high stages was increased by age in our study. H. pylori infection and higher age are independent risk factors for gastric cancer, and based on our findings of the uncommon existence of stages III and IV of OLGA and OLGIM in the age lower than 40 years, therefore, it seems that treatment of H. pylori infection at the age of 40 years can decrease the risk of gastric cancer.
We found 20 cases of duodenal ulcer that all of them had low stages of OLGA and OLGIM. In 27 cases with gastric ulcers, 24 cases had low stages of OLGA and OLGIM and the other 3 cases with high stages also had gastric intestinal adenocarcinoma. These findings are similar to those found by Rugge et al18 who reported that all patients with duodenal ulcer had stage 0 OLGA, while 2 cases of low-grade gastric intraepithelial neoplasia were seen and both were associated with stage III OLGA.18 Fassan and collaborators35 also found that all cases with IM which lead to dysplasia had higher stages of OLGA in the primary endoscopic evaluation.35
Capelle and coworkers36 reported that from 20 cases of gastric cancer among 125 patients followed up for 6 years, 10 cases had intestinal-type gastric cancers (5 cases with high-risk stages of OLGA and OLGIM) and other 10 cases had diffuse gastric cancer (1 case was classified in stage III-IV of both OLGA and OLGIM, whereas 9 cases were classified in stage 0-II of both OLGA and OLGIM).36 Similarly, in our study, 11 cases of gastric adenocarcinoma were found, and among them, 8 patients had an intestinal-type and 3 others had the diffuse type of cancer, and totally, 27.3% had stage III or IV of both OLGA and OLGIM staging methods. Conspicuously, molecular survey to have a better and certain result is recommended.37,38
The patients with extensive IM and/or extensive atrophy family history of gastric cancer2, incomplete IM3, autoimmune gastritis, or persistent H. pylori infection should be followed by endoscopy surveillance every 3 years. For patients with atrophic gastritis or IM with mild to moderate atrophy only in the antrum, or no IM, surveillance is not needed. If atrophy or IM in both antrum and corpus was accompanied by a first-degree family history of gastric cancer, endoscopic surveillance should be performed every 1-2 years.14
Our suggestion is the patients aged above 40 years should follow with endoscopy with each stage of OLGA and OLGIM.
Several and controversial studies have shown that the high stages of OLGA and OLGIM gastric carcinoma classification are important, but there are no meta-analytic studies on the importance of LGA and OLGIM system accuracy.18,36 It is evident that the OLGA and OLGIM classifications have significant clinical value in screening for gastric carcinoma and precancerous lesions. However, follow-up intervals of people with precancerous lesions are discussed. Patients with extensive IM and/or extensive atrophy should be followed by endoscopy surveillance. But according to the new recommendation in Japanese and Chinese populations, for patients with none/very mild/mild gastritis, surveillance should be performed every 3 years, for patients with moderate atrophic gastritis, every 2 years, and for patients with extensive IM and/or extensive atrophy, should be performed every 1 year.39,40
Limitations
Despite our findings, this study has 2 important limitations. First, we only evaluated the patients who were referred to the hospital which can interfere with our outcomes. Second, other uncommon gastritis such as autoimmune gastritis, which is uncommon, was not evaluated.
Conclusion
Although with an increase of OLGA and OLGIM-based stages, the risk of a gastric intestinal type of cancer is increased, just using OLGA and OLGIM-based staging is not completely applicable in the regions with a high prevalence of precancerous and cancerous gastric lesions. Indeed, we found that all of the patients who suffered from gastric intestinal-type cancer had low-risk stages of both OLGA and OLGIM staging methods. Therefore, patients with all degrees of gastric atrophy or IM not the only high-risk stage of OLGA and OLGIM need to follow-up according to the guidelines of the European Society for Gastrointestinal Endoscopy.
Funding Statement
The authors declared that this study has received no financial support.
Footnotes
Ethics Committee Approval: The protocol of this study was approved by a local ethical committee of Guilan University of Medical Sciences (No. IR.GUMS.REC.1395.270) and was based on the Declaration of Helsinki.
Informed Consent: Informed consent was obtained from all patients, and all securities were applied to their data.
Peer Review: Externally peer-reviewed.
Author Contributions: Consept – F.M-GH., F.J.; Design – F.J., S.Y.; Supervision – F.M-GH., F.J.; Resources – M.SM., F.J.; Materials – F.M-GH., F.J.; Data Collection and/or Processing – S.Y., A.D.; Analysis and/or Interpretation – M.SM., M.S.; Literature Search – M.S., F.J.; Writing Manuscript – F.M-GH., S.Y.; Critical Review – F.M-GH., F.J.
Acknowledgments: The authors wish to thank all staff of Gastrointestinal and Liver Diseases Research Center (GLDRC) and personnel of Endoscopy ward of Razi Hospital affiliated to the Guilan University of Medical Sciences for their kind help in all steps of this study.
Conflict of Interest: The authors have no conflict of interest to declare.
References
- 1. . Mansour-Ghanaei F, Joukar F, Baghaei SM, et al. Gastric precancerous lesions in first degree relatives of patients with known gastric cancer: a cross-sectional prospective study in Guilan Province, north of Iran. Asian Pac J Cancer Prev. 2012;13(5):1779 1782. 10.7314/apjcp.2012.13.5.1779) [DOI] [PubMed] [Google Scholar]
- 2. . Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127(12):2893 2917. 10.1002/ijc.25516) [DOI] [PubMed] [Google Scholar]
- . . Malekzadeh R, Derakhshan MH, Malekzadeh Z. Gastric cancer in Iran: epidemiology and risk factors. Arch Iran Med. 2009;12(6):576 583. [PubMed] [Google Scholar]
- 4. . Fallah M. Cancer Incidence in Five Provinces of Iran: Ardebil, Gilan, Mazandaran, Golestan and Kerman, 1996-2000. Finland: University of Tampere; 2007. [Google Scholar]
- 5. . Mansour-Ghanaei F, Sokhanvar H, Joukar F, et al. Endoscopic findings in a mass screening program for gastric cancer in a high risk region - Guilan province of Iran. Asian Pac J Cancer Prev. 2012;13(4):1407 1412. 10.7314/apjcp.2012.13.4.1407) [DOI] [PubMed] [Google Scholar]
- 6. . Shah MA, Khanin R, Tang L, et al. Molecular classification of gastric cancer: a new paradigm. Clin Cancer Res. 2011;17(9):2693 2701. 10.1158/1078-0432.CCR-10-2203) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. . Lee EL, Feldman M. Gastritis and gastropathies. In: Feldman M, Friedman LS, Brandt LJ.eds. Sleisenger and Fordtran’s Gastrointestinal and Liver Disease: Pathophysiology, Diagnosis, Management, Expert Consult Premium Edition-Enhanced Online Features. 1. USA: Elsevier Health Sciences; 2010:845 860. [Google Scholar]
- 8. . Efrati C, Cannaviello C, Mangogna LM, et al. The staging of gastritis with the OLGA system in the Italian setting: histological features and gastric cancer risk. Gastroenterology. 2017;152(5):S473. 10.1016/S0016-5085(17)31774-2) [DOI] [Google Scholar]
- 9. . Kim YI, Kook MC, Cho SJ, et al. Tu1279 comparisons of OLGA and OLGIM stages according to different biopsy sites at gastric antrum and corpus. Gastroenterology. 2016;150(4):S863. 10.1016/S0016-5085(16)32907-9) [DOI] [Google Scholar]
- 10. . Guarner J, Mohar A, Parsonnet J, Halperin D. The association of Helicobacter pylori with gastric cancer and preneoplastic gastric lesions in Chiapas, Mexico. Cancer. 1993;71(2):297 301. [DOI] [PubMed] [Google Scholar]
- 11. . Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61(2):69 90. 10.3322/caac.20107) [DOI] [PubMed] [Google Scholar]
- 12. . Koohi-Hosseinabadi O, Moini M, Safarpoor A, Derakhshanfar A, Sepehrimanesh M. Effects of dietary Thymus vulgaris extract alone or with atorvastatin on the liver, kidney, heart, and brain histopathological features in diabetic and hyperlipidemic male rats. Comp Clin Pathol. 2015;24(6):1311 1315. 10.1007/s00580-015-2070-7) [DOI] [Google Scholar]
- 13. . Wang X, Lu B, Meng L, Fan Y, Zhang S, Li M. The correlation between histological gastritis staging - ‘OLGA/OLGIM’ and serum pepsinogen test in assessment of gastric atrophy/intestinal metaplasia in China. Scand J Gastroenterol. 2017;52(8):822 827. 10.1080/00365521.2017.1315739) [DOI] [PubMed] [Google Scholar]
- 14. . Pimentel-Nunes P, Libânio D, Marcos-Pinto R, et al. Management of epithelial precancerous conditions and lesions in the stomach (maps II): European Society of gastrointestinal endoscopy (ESGE), European Helicobacter and microbiota Study Group (EHMSG), European Society of pathology (ESP), and Sociedade Portuguesa de Endoscopia Digestiva (SPED) guideline update 2019. Endoscopy. 2019;51(4):365 388. 10.1055/a-0859-1883) [DOI] [PubMed] [Google Scholar]
- 15. . Cho SJ, Choi IJ, Kook MC, et al. Staging of intestinal‐ and diffuse‐type gastric cancers with the OLGA and OLGIM staging systems. Aliment Pharmacol Ther. 2013;38(10):1292 1302. 10.1111/apt.12515) [DOI] [PubMed] [Google Scholar]
- 16. . González CA, Sanz-Anquela JM, Companioni O, et al. Incomplete type of intestinal metaplasia has the highest risk to progress to gastric cancer: results of the Spanish follow-up multicenter study. J Gastroenterol Hepatol. 2016;31(5):953 958. 10.1111/jgh.13249) [DOI] [PubMed] [Google Scholar]
- 17. . Lahner E, Esposito G, Pilozzi E, et al. Occurrence of gastric cancer and carcinoids in atrophic gastritis during prospective long-term follow up. Scand J Gastroenterol. 2015;50(7):856 865. 10.3109/00365521.2015.1010570) [DOI] [PubMed] [Google Scholar]
- 18. . Rugge M, De Boni M, Pennelli G, et al. Gastritis OLGA‐staging and gastric cancer risk: a twelve‐year clinico‐pathological follow‐up study. Aliment Pharmacol Ther. 2010;31(10):1104 1111. 10.1111/j.1365-2036.2010.04277.x) [DOI] [PubMed] [Google Scholar]
- 19. . Rugge M, Fassan M, Pizzi M, et al. Operative link for gastritis assessment vs operative link on intestinal metaplasia assessment. World J Gastroenterol. 2011;17(41):4596 4601. 10.3748/wjg.v17.i41.4596) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. . Khatoon J, Rai RP, Prasad KN. Role of Helicobacter pylori in gastric cancer: updates. World J Gastrointest Oncol. 2016;8(2):147-158. 10.4251/wjgo.v8.i2.147) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. . Rugge M, Fassan M, Pizzi M, Pennelli G, Nitti D, Farinati F. Operative Link for Gastritis Assessment gastritis staging incorporates intestinal metaplasia subtyping. Hum Pathol. 2011;42(10):1539 1544. 10.1016/j.humpath.2010.12.017) [DOI] [PubMed] [Google Scholar]
- 22. . You WC, Blot WJ, Li JY, et al. Precancerous gastric lesions in a population at high risk of stomach cancer. Cancer Res. 1993;53(6):1317 1321. [PubMed] [Google Scholar]
- 23. . de Vries AC, van Grieken NC, Looman CW, et al. Gastric cancer risk in patients with premalignant gastric lesions: a nationwide cohort study in the Netherlands. Gastroenterology. 2008;134(4):945 952. 10.1053/j.gastro.2008.01.071) [DOI] [PubMed] [Google Scholar]
- 24. . Quach DT, Le HM, Hiyama T, Nguyen OT, Nguyen TS, Uemura N. Relationship between endoscopic and histologic gastric atrophy and intestinal metaplasia. Helicobacter. 2013;18(2):151 157. 10.1111/hel.12027) [DOI] [PubMed] [Google Scholar]
- 25. . Quach DT, Le HM, Nguyen OT, Nguyen TS, Uemura N. The severity of endoscopic gastric atrophy could help to predict Operative Link on Gastritis Assessment gastritis stage. J Gastroenterol Hepatol. 2011;26(2):281 285. 10.1111/j.1440-1746.2010.06474.x) [DOI] [PubMed] [Google Scholar]
- 26. . Salehi Z, Miri M, Aminian K, Mansour-Ghanaei F. Helicobacter pylori infection and colorectal cancer in Guilan province of Iran. Ann Biol Res. 2011;2:32 39. [Google Scholar]
- 27. . Namakin K. Prevalence of Helicobacter pylori infection in asymptomatic children in Birjand, Eastern Iran. Int J Pediatr. 2014;2:55 63. 10.22038/IJP.2014.3438) [DOI] [Google Scholar]
- 28. . Alborzi A, Soltani J, Pourabbas B, et al. Prevalence of Helicobacter pylori infection in children (south of Iran). Diagn Microbiol Infect Dis. 2006;54(4):259 261. 10.1016/j.diagmicrobio.2005.10.012) [DOI] [PubMed] [Google Scholar]
- 29. . Moosazadeh M, Lankarani KB, Afshari M. Meta-analysis of the prevalence of Helicobacter pylori infection among children and adults of Iran. Int J Prev Med. 2016;7:48. 10.4103/2008-7802.177893) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 0. . Ghadimi R, Taheri H, Suzuki S, et al. Host and environmental factors for gastric cancer in Babol, the Caspian Sea Coast, Iran. Eur J Cancer Prev. 2007;16(3):192 195. 10.1097/01.cej.0000220639.61717.67) [DOI] [PubMed] [Google Scholar]
- 1. . Malekzadeh R, Sotoudeh M, Derakhshan MH, et al. Prevalence of gastric precancerous lesions in Ardabil, a high incidence province for gastric adenocarcinoma in the northwest of Iran. J Clin Pathol. 2004;57(1):37 42. 10.1136/jcp.57.1.37) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. . Sotoudeh M, Derakhshan MH, Abedi-Ardakani B, et al. Critical role of Helicobacter pylori in the pattern of gastritis and carditis in residents of an area with high prevalence of gastric cardia cancer. Dig Dis Sci. 2008;53(1):27 33. 10.1007/s10620-007-9817-1) [DOI] [PubMed] [Google Scholar]
- 3. . Nouraie M, Latifi‐Navid S, Rezvan H, et al. Childhood hygienic practice and family education status determine the prevalence of Helicobacter pylori infection in Iran. Helicobacter. 2009;14(1):40 46. 10.1111/j.1523-5378.2009.00657.x) [DOI] [PubMed] [Google Scholar]
- 4. . Nam JH, Choi IJ, Kook MC, et al. OLGA and OLGIM stage distribution according to age and Helicobacter pylori status in the Korean population. Helicobacter. 2014;19(2):81 89. 10.1111/hel.12112) [DOI] [PubMed] [Google Scholar]
- 5. . Fassan M, Pizzi M, Farinati F, et al. Lesions indefinite for intraepithelial neoplasia and OLGA staging for gastric atrophy. Am J Clin Pathol. 2012;137(5):727 732. 10.1309/AJCPEU41HTGXSJDQ) [DOI] [PubMed] [Google Scholar]
- 6. . Capelle LG, de Vries AC, Haringsma J, et al. The staging of gastritis with the OLGA system by using intestinal metaplasia as an accurate alternative for atrophic gastritis. Gastrointest Endosc. 2010;71(7):1150 1158. 10.1016/j.gie.2009.12.029) [DOI] [PubMed] [Google Scholar]
- 7. . Samadani AA, Norollahi SE, Rashidy-Pour A, et al. Cancer signaling pathways with a therapeutic approach: an overview in epigenetic regulations of cancer stem cells. Biomed Pharmacother. 2018;108:590 599. 10.1016/j.biopha.2018.09.048) [DOI] [PubMed] [Google Scholar]
- 8. . Samadani AA, Noroollahi SE, Mansour-Ghanaei F, Rashidy-Pour A, Joukar F, Bandegi AR. Fluctuations of epigenetic regulations in human gastric adenocarcinoma: how does it affect? Biomed Pharmacother. 2019;109:144 156. 10.1016/j.biopha.2018.10.094) [DOI] [PubMed] [Google Scholar]
- 9. . Shin WG, Kim HU, Song HJ, et al. Surveillance strategy of atrophic gastritis and intestinal metaplasia in a country with a high prevalence of gastric cancer. Dig Dis Sci. 2012;57(3):746 752. 10.1007/s10620-011-1919-0) [DOI] [PubMed] [Google Scholar]
- 40. . Fang JY, Du YQ, Liu WZ, et al. Chinese consensus on chronic gastritis (2017, Shanghai). J Dig Dis. 2018;19(4):182 203. 10.1111/1751-2980.12593) [DOI] [PubMed] [Google Scholar]