ABSTRACT
To understand the molecular mechanisms that contribute to the stress responses of the important foodborne pathogen Listeria monocytogenes, we collected 139 strains (meat, n = 25; dairy, n = 10; vegetable, n = 8; seafood, n = 14; mixed food, n = 4; and food processing environments, n = 78), mostly isolated in Ireland, and subjected them to whole-genome sequencing. These strains were compared to 25 Irish clinical isolates and 4 well-studied reference strains. Core genome and pan-genome analysis confirmed a highly clonal and deeply branched population structure. Multilocus sequence typing showed that this collection contained a diverse range of strains from L. monocytogenes lineages I and II. Several groups of isolates with highly similar genome content were traced to single or multiple food business operators, providing evidence of strain persistence or prevalence, respectively. Phenotypic screening assays for tolerance to salt stress and resistance to acid stress revealed variants within several clonal complexes that were phenotypically distinct. Five of these phenotypic outliers were found to carry mutations in the sigB operon, which encodes the stress-inducible sigma factor sigma B. Transcriptional analysis confirmed that three of the strains that carried mutations in sigB, rsbV, or rsbU had reduced SigB activity, as predicted. These strains exhibited increased tolerance to salt stress and displayed decreased resistance to low pH stress. Overall, this study shows that loss-of-function mutations in the sigB operon are comparatively common in field isolates, probably reflecting the cost of the general stress response to reproductive fitness in this pathogen.
IMPORTANCE The bacterial foodborne pathogen Listeria monocytogenes frequently contaminates various categories of food products and is able to cause life-threatening infections when ingested by humans. Thus, it is important to control the growth of this bacterium in food by understanding the mechanisms that allow its proliferation under suboptimal conditions. In this study, intraspecies heterogeneity in stress response was observed across a collection consisting of mainly Irish L. monocytogenes isolates. Through comparisons of genome sequence and phenotypes observed, we identified three strains with impairment of the general stress response regulator SigB. Two of these strains are used widely in food challenge studies for evaluating the growth potential of L. monocytogenes. Given that loss of SigB function is associated with atypical phenotypic properties, the use of these strains in food challenge studies should be re-evaluated.
KEYWORDS: Listeria monocytogenes, phylogeny, food-borne infections, salt stress, acid stress, SigB, σB, RsbV, RsbU, general stress response
INTRODUCTION
Listeria monocytogenes is a Gram-positive facultative anaerobic bacterium that is of concern from a food safety and clinical perspective because it can contaminate ready-to-eat food products and lead to the severe foodborne illness listeriosis. The ability to survive stresses encountered in the food chain aids in the transmission of this pathogen from food to the human host. L. monocytogenes is intrinsically stress tolerant compared to other food borne pathogens, with a remarkable tolerance of low water activity (aw) and low temperatures (1), as well an ability to withstand very high bile concentrations (2). These features can allow this pathogen to survive and grow in some ready-to-eat (RTE) foods. Upon ingestion by consumers, this bacterium can survive the stressful conditions in the gastrointestinal (GI) tract, including the acidic stomach environment and the presence of intestinal bile salts. L. monocytogenes infections often occur in immunocompromised individuals, with mortality rates typically circa 20% (3). A steadily increasing occurrence of listeriosis has been observed in recent years in the European Union as well as in Ireland (4). At present, European Union regulations treat all food isolates as equally virulent, but heterogeneities in stress responses and virulence have been demonstrated among different sublineages of L. monocytogenes (5). However, little is known as to why these isolates differ in terms of their stress response.
L. monocytogenes has a highly clonal and deeply branched population structure with four major genetic lineages identified to date. Lineage I (LI) and lineage II (LII) are most frequently isolated, and they are overrepresented in clinical cases and food samples, respectively (6, 7). These two lineages represent seven of the 13 known serotypes: 1/2b, 3b, 3c, and 4b in LI and 1/2a, 1/2c, and 3a in LII. The majority of listeriosis cases are caused by LI 4b strains, and occasionally some are caused by LI 1/2b and LII 1/2a strains (8). Genomic characterizations, including whole-genome sequencing (WGS) and core genome multilocus sequence typing (cgMLST) identified several prevalent clonal complexes (CCs) of L. monocytogenes from food products and food processing environments, namely, CC121, CC9, CC155, CC8, and CC101 in LII (6, 7). In contrast, despite relatively low occurrences in food products, four CCs (CC1, CC2, CC4, and CC6) appear to be highly associated with clinical cases and are considered hypervirulent CCs (6, 9). Meanwhile, easily accessible WGS can facilitate the identification of persistent strains in food processing facilities and tracking the route of transmission for outbreaks. Stasiewicz et al. reported the persistence and prevalence of L. monocytogenes isolates in the U.S. deli industry by comparing WGS data (10). Pasquali et al. identified L. monocytogenes CC121 and CC14 strains persisting in a meat processing plant (11). Meanwhile, WGS was applied in recent outbreaks caused by L. monocytogenes to pinpoint an epidemiological link between clinical cases and contaminated food products (12–14). It is noteworthy that CC6 strains have emerged as a causative agent in several recent outbreaks and are therefore attracting increased attention (14–16). Furthermore, the wide availability of WGS data has also made it possible to elucidate molecular mechanisms underlying the phenotypic heterogeneity associated with stress response and virulence (6, 17).
While earlier studies showed that food isolates of L. monocytogenes differ in their responses to food formulation- and processing-related stresses, these differences were not linked to their phylogeny (18–21). Several recent works achieved this with the aid of WGS/MLST (reviewed by Bergholz et al. [5]). Osmotic and acid stresses are among the most studied, due to their wide application in food preservation as well as being part of host defensive mechanisms. Serotype 4b (LI) strains were reported to show increased growth under osmotic stress compared to serotype 1/2a (LII) strains (1, 22–24). Horlbog and coworkers (25) have also reported an extended lag phase in LII strains (CC121 and CC9) grown under mild NaCl stress (4%) compared to LI strains (CC6 and CC1). However, no statistically significant difference in growth under salt stress between lineages or serogroups was observed by Hingston et al. (26), possibly due to different experimental conditions and diversities between CCs within each lineage/serogroup. The ability of L. monocytogenes isolates from various origins to survive acidic pH conditions (defined here as “acid resistance”) is largely strain dependent and, in some cases, linked to origin of isolation (18, 21, 25, 27). However, it remains to be determined if acid resistance varies in relation to either lineage or CC. Enhanced growth performance under mildly acidic conditions (defined here as “acid tolerance”) has been reported for serotype 4b strains compared to serotype 1/2a strains (1, 26). Despite variations in stress response among field isolates, genes involved in acid and salt stresses are generally conserved, with stress survival islet 1 (SSI-1) and SSI-2 being notable exceptions (28, 29).
The alternative sigma factor SigB is perhaps the best-studied genetic determinant of stress response in L. monocytogenes. Complex regulatory circuitry is responsible for transducing stress signals into a pathway that controls the interaction of SigB with its anti-sigma factor RsbW, which determines whether the general stress response is activated (30, 31). Stress signals are thought to be sensed by a supermolecular protein complex termed the “stressosome,” which consists of RsbR1, RsbS, RsbT, and four RsbR1 paralogues. Following the detection of a stress signal, RsbT is released from the stressosome and interacts with a phosphatase called RsbU, which in turn dephosphorylates RsbV. RsbV, RsbW, and SigB consist of a partner-switching system in which the anti-sigma factor RsbW has a higher affinity for dephosphorylated RsbV than for SigB. Meanwhile, another negative SigB regulator, the phosphatase RsbX, is thought to dephosphorylate RsbS and RsbR1 in the stressosome to reset the signal-ready ground state (32). The first two genes in the transcription unit of sigB operon, mazEF, encode a toxin/antitoxin system (33). MazEF influence antibiotic stress response at high temperature; however, it remains unclear whether and how this system influences SigB activity (34). When SigB activity is induced, the bacterium increases its resistance to various stresses, e.g., osmolarity, acidity, blue light, bile salt, antimicrobial agents (30, 31, 35). SigB activation is also essential for L. monocytogenes to establish systematic infection as SigB mediates resistance to acid and bile, which is required for survival in the GI tract (36, 37), while also regulating the inlAB operon, encoding surface proteins for attachment and invasion into intestinal epithelial cells (38, 39).
Being such an important regulator, the sigB operon is highly conserved among L. monocytogenes strains (40). However, with over 300 genes identified as SigB dependent in serotype 1/2a reference strains, only 63 genes were classified as members of the core SigB regulon between lineages (41, 42). Interestingly, several recent studies suggested that sigB is under negative selective pressure under laboratory stresses (e.g., salt stress and heat stress) (35, 43–46). Previous analysis through publicly available databases revealed high premature stop codon (PMSC) frequency associated with positive SigB regulators, which further suggested that loss of SigB activity may confer a fitness advantage in some environments (46).
The availability of a large national collection of systematically collected food isolates (47) provided us with an opportunity to study whether and how stress tolerance in L. monocytogenes is influenced by phylogeny. In this study, we performed a phylogenetic and phenotypic comparison of 168 L. monocytogenes strains comprising 114 food and food processing environment isolates from Ireland, 25 strains recommended for food challenge studies by the European Union Reference Laboratory for L. monocytogenes (EURL-Lm), 25 Irish clinical isolates, and 4 laboratory reference strains. We interrogated genotypes between closely related strains to understand the genetic basis of phenotypical heterogeneities. Six strains with sigB operon alleles were investigated for their ability to induce SigB activity.
RESULTS
Phylogenetic characterization of a diverse collection of L. monocytogenes isolates.
WGS were determined for 114 Irish food isolates (47) and 25 EURL-Lm strains in order to determine their phylogenetic relatedness as well as to investigate the molecular bases for their stress response properties. The sizes of the genome assemblies ranged from 2.72 Mbp to 2.99 Mbp (median, 2.85 Mbp). The quality of the genome assemblies was evaluated with Quast, resulting in calculated mean NG50 (defined as the sequence length of the shortest contig at 50% of total assembly length) of 460 kbp. Mean genome sequence coverage was calculated at 113× (ranging from 41× to 256×). Together, these measures suggested that the WGS data were of good quality and suitable for phylogenetic analyses and functional genomics.
These genome assemblies were combined with 25 previously described clinical isolates (48) and 4 frequently used laboratory reference strains (Table S1), and the overall phylogeny was inferred by core genome single nucleotide polymorphism (coreSNP) analysis. A previous study suggested that coreSNP is a reliable method for determining phylogeny of L. monocytogenes (49). Indeed, the phylogeny inference by Parsnp nicely coincided with in silico MLST results; i.e., strains in same ST/CC appeared in the same phylogenetic branches, with strain 1147 as the sole exception (Fig. 1). All strains characterized belonged to the two most predominant lineages of L. monocytogenes (73 and 95 strains in LI and LII, respectively) (Fig. 1). The majority of the LII strains belonged to serogroup IIa (91 of 95), while the LI population consisted of serogroups IIb (n = 25) and IVb (n = 48).
FIG 1.
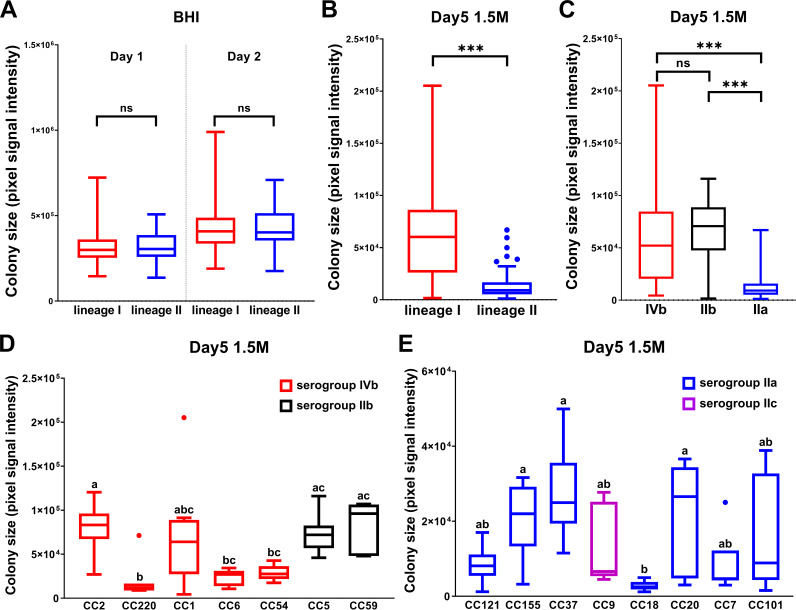
Phylogenetic relatedness and phenotypic heterogeneities in salt tolerance and acid resistance among 168 L. monocytogenes strains. Sources of isolation, identity of strains, and in silico MLST results are presented along with phylogeny inferred by core genome SNP analysis. Phylogenetically indistinguishable strains are highlighted with yellow boxes between the strain names and phylogeny. Salt tolerance, defined as growth performance at different time points at several NaCl concentrations, is shown in the heat map. Acid resistance, defined as survival in pH 2.3, is indicated in binary format (detectable survival in red, nondetectable survival in green) for 7 time points.
The deeply branched population structure revealed by coreSNP analysis was also evident from the pan-genome analysis (Fig. 2). The size of the pan-genome for our strain collection was determined to be 6,878 genes, similar to that reported by other researchers (50–52). Strikingly, considerable numbers of genes were enriched in each lineage (present in >95% strains of one lineage but <5% strains of the other) represented. Two hundred forty-seven genes (Fig. 2, group c) were highly enriched in LI and 234 genes (Fig. 2, group g) were highly enriched in LII in our strain collection. MLST analysis suggested that this strain collection included a good diversity of the major prevalent and virulent CCs. This included strains from CC121, CC8, CC9, CC155, CC37, CC5, and CC101, which are commonly found in the food chain (6, 7), and strains from CC4, CC6, CC2, and CC1 that have been reported to be hypervirulent (6, 9). Indeed, the majority of CC6 and CC1 strains were isolated from Irish clinical cases. However, 7 clinical isolates were from CC121 (n = 2), CC101 (n = 4), and CC37 (n = 1). Additionally, this strain collection also encompassed other CCs that are less frequently isolated (e.g., CC220, CC224, CC379, and CC224) (7). Analysis of known listerial pathogenicity islands revealed that all strains harbored LIPI-I but not LIPI-II. Longer and shorter versions of prfA were found in one ST155 strain (1385) and two CC31 strains (1389 and 1371), respectively, in accordance with previous studies (17, 53). Thirty-two strains, belonging to CC1, CC4, CC54, CC224, CC3, and CC6, carry LIPI-3, while LIPI-4 was found in both CC4 and CC87, as previously reported (6, 54), and also found in CC220.
FIG 2.
Deeply branched and highly clonal population structure of L. monocytogenes field isolates as revealed by pan-genome gene presence/absence analysis. The phylogenetic tree was built with Parsnp. Gene presence is highlighted in color, while absence is left blank. Pan-genomes are divided into eight color groups based on their percent prevalence in the whole collection, LI, and LII, as follows: a, ≥ 95% in whole collection; b, ≥ 95% in LI, between 5 and 95% in LII; c, ≥ 95% in LI, <5% in LII; d, between 5 and 95% in LI, <5% in LII; e, between 5 and 95% in both LI and LII; f, between 5 and 95% in LII, <5% in LI; g, ≥ 95% in LII, <5% in LI; h, ≥ 95% in LII, between 5 and 95% in LI; i, < 5% in both LI and LII. Within each group, genes are ranked by the LI/LII ratio of percent presence, from left to right.
Phylogenetically indistinguishable strains were detected in the collection based on the coreSNP analysis, defined as strains that share fewer than 15 core SNPs identified from CC-specific Parsnp analysis (Fig. 1). In one case, five almost identical ST836 strains (1306, 1304, 1427, 1428, and 1502) were isolated from the same seafood factory over a period of 3 years (June 2013 to February 2015) (47). Interestingly, these strains share a novel cgMLST profile (55), which suggests the possibility that this strain might have specifically evolved to adapt to this niche, and the genetic factors contributing to its persistence warrant further investigation. In another case, 5 of 6 ST7 strains in our collection were phylogenetically indistinguishable. While 4 of these were associated with food production (strains 1003, 1445, 946, and 991), obtained from meat products and food processing environments, strain MQ140029 was isolated from a human listeriosis case. This finding provided evidence for a potential link between an L. monocytogenes strain prevalent in the food chain and a case of listeriosis.
Lineage I CCs are more tolerant to salt stress.
To understand the genetic factors that determine differences in the response to food-related stress among these L. monocytogenes isolates, we surveyed their ability to grow under osmotic stress. An agar plate-based screening method was applied using brain heart infusion (BHI) agar supplemented with NaCl. The ability of these strains to grow on BHI agar without added salt was rather similar (Fig. 3A), whereas tolerance to salt stress (0.8 M to 1.5 M NaCl) varied in our strain collection (Fig. 1). A statistical analysis of the growth performance of strains on plates containing 1.5 M NaCl after 5 days of incubation revealed significant differences in salt tolerance within the collection. In line with previous observations in liquid culture (1, 22–24), LI strains were generally more salt tolerant than LII strains (P < 0.001) (Fig. 3B). Serogroups IIb and IVb in LI showed no statistically significant difference, whereas both were significantly more salt tolerant than serogroup IIa (P < 0.001), which accounts for the majority of LII strains (Fig. 3C). Comparisons between individual CCs were also carried out within each lineage. CCs with at least 5 representative member strains were selected, to avoid potential biases that could be introduced by small population sizes. CC220, CC6, and CC54 were identified as salt-sensitive groups in LI (Fig. 3D), CC121 and CC18 were identified as salt sensitive groups in LII (Fig. 3E). Note that all CCs analyzed from LII were less salt tolerant than those from LI. Among LI CCs analyzed, CC2, CC1, CC5, and CC59 displayed the greatest levels of salt tolerance.
FIG 3.
Heterogeneous salt tolerance displayed by different lineages, serotypes, and CCs of L. monocytogenes field isolates. Growth performance in BHI (A) or BHI plus 1.5 M NaCl (B, C, D, and E) in relation to lineage (A and B), serogroup (C), and CC (D and E) is presented. Statistical significance was determined by Mann-Whitney test for panels A, B, and C (ns, not significant; ***, P < 0.001) and by Kruskal-Wallis for panels D and E, where groups with same letters are not significantly different, as assigned by Dunn’s multiple comparisons (P < 0.05).
Phenotypic characterization of acid tolerance and resistance.
Acid tolerance was found to be rather similar across the strain collection using a growth screen on acidified BHI agar (ranging from pH 4.9 to pH 5.2) (Fig. 4A). However, two strains were identified that grew on nonacidified BHI agar but did not grow on acidified BHI agar, CC8 strain 2294 and CC2 strain 1381. This phenotype was reconfirmed by plating dilution series of these two strains on acidified BHI agar surfaces along with several other strains from CC8 and CC2 (Fig. S1B). Moreover, while strain 2294 showed some growth at the 10−1 dilution, strain 1381 did not show any visible growth, although both grew normally on nonacidified BHI agar (Fig. S1A). A rapid acid survival experiment was designed to semiquantitatively measure acid resistance, defined as the ability of these strains to survive a lethal pH challenge (pH 2.3). The latest time point (from <1 h to 7 h) at which viability was measured during the pH 2.3 challenge was used to indicate acid resistance (Fig. 1). While the acid resistance differed significantly across the whole collection, in most cases strains from the same genetic background (CC) exhibited similar survival phenotypes (Fig. 1). In particular, CC18, CC9, and CC31 (all LII) were extremely sensitive to acid stress with most strains losing viability within the first 60 min of treatment; while several strains in CC7, CC20, CC121, and CC2 (all in LII except CC2 strain 1375 in LI) were extremely resistant to acid stress and maintained viability after 7 h of treatment. Generally, LII strains were more resistant to acid stress than LI strains (P < 0.01) (Fig. 4B). When the acid tolerance profiles were compared to the acid resistance phenotypes, the two acid-intolerant isolates, CC8 strain 2294 and CC2 strain 1381, showed different acid resistance patterns. Strain 2294 (CC8) showed the same resistance to pH 2.3 as two other CC8 strains (inconsistent with acid tolerance profile), while the strain 1381 (CC2) was more sensitive to pH 2.3 than the majority of CC2 strains (consistent with acid tolerance profile).
FIG 4.
Similar acid tolerances but distinct acid resistance profiles among LI and LII strains. When strains were grouped by lineage, LI and LII displayed similar acid tolerance levels (A) but statistically significantly different acid resistance (B). Growth after 4 days of incubation on BHI agar pH 4.9 was determined to represent acid tolerance, while the ability to survive in BHI at pH 2.3 was determined as an indication of acid resistance. Statistical significance was determined by Mann-Whitney test (ns, not significant; **, P < 0.01).
Genome sequence analysis on outlier strains revealed sigB operon alleles.
Of further interest were the strains that exhibited rather different phenotypes despite being in closely related clusters (e.g., strain MQ140025 grew faster under salt stress but was less resistance to pH 2.3 than other CC1 strains; strains 1313, F111-17, 1423, and 2256 were less resistant to pH 2.3 than other CC121 strains) (Fig. 1). We refer to these strains as outliers and sought to investigate the genetic basis for these phenotypes, using the closely related strains in each of the clusters as references to help narrow down the possible genetic causes. These outliers were first compared with closely related strains (same CC) using CC-specific core genome alignments with Parsnp. An analysis was performed on the unique sequence polymorphisms associated with each outlier, compared to the reference genomes from the same CC. From this analysis three unique SNPs were identified within the sigB operon, one in strain 1374 (rsbW-G104R) and two in strain MQ140025 (rsbU-Q317* and rsbX-N77K). Subsequently, an unbiased full-sequence alignment performed on the sigB operon from all 168 strains in the collection revealed 6 strains (3.57%) carrying truncation/substitution/deletions within this operon (Table 1). Importantly, 4 of these 6 strains included the phenotypic outlier strains 1147, 1374, 1388, and MQ140025 (Fig. 1).
TABLE 1.
Overview of phenotypes and genotype in sigB operon allele strainse
Increased (+) and decreased (−) salt tolerance were defined as obvious growth advantages and disadvantages in at least one of the incubation conditions tested (37°C, 30°C, and 23°C) (Fig. S2 to S5); decreased (−) acid resistance was defined as a significantly reduced survival rate or nondetectable survival (Fig. 5).
Genes encoding proteins have an unclear impact on SigB activity.
Genes encoding SigB or proteins have a positive impact on SigB activity.
Genes encoding proteins have a negative impact on SigB activity.
Green and red indicate genes that positively and negatively affect SigB activity, respectively. Orange indicates an uncertain role in regulating SigB. aa, amino acids.
Since the previous phenotypic screening approaches were semiquantitative measurements of stress tolerance/resistance, the salt tolerance and acid resistance of these 6 strains were re-evaluated by plating dilution series on BHI agar with 1.4 M NaCl (Fig. S2 to S6) and by measuring survival rates during acid challenge assays at pH 2.3 (Fig. 5), respectively. Because these strains are from different genetic backgrounds, they were compared individually with closely related strains (from the same CC). These experiments confirmed that strains 1370rsbV-V65fs, 1388sigB-V15fs, and MQ140025rsbU-Q317*, rsbX-N77K were more tolerant to salt (Fig.S2, S3, S6) but less resistant to acid (Fig. 5). These data are consistent with phenotypes reported to be associated with mutants lacking sigma B (ΔsigB) (43, 56). These data suggested that the sigB operon mutations in these outlier strains (1370rsbV-V65fs, 1388sigB-V15fs, and MQ140025rsbU-Q317*, rsbX-N77K) may be responsible for their unique salt and acid phenotypes by negatively affecting SigB activity. The acid-sensitive phenotype in strain 1147rsbT-del65-68 and the salt-sensitive phenotype in strain 1374rsbW-G104R were also confirmed in these experiments (Fig. 1 and 4; Fig. S5), while strain F2365mazF-I90fs exhibited phenotypes similar to those of other CC1 strains (Fig. 5; Fig. S2). Given the phenotypes associated with ΔsigB (43, 56), it is reasonable to hypothesize that SigB activity is reduced in strain 1147rsbT-del65-68, increased in strain 1374rsbW-G104R, and not influenced in strain F2365mazF-I90fs.
FIG 5.
Strains carrying sigB operon mutations display acid-sensitive phenotypes. Strains 1147rsbT-del65-68, 1370rsbV-V65fs, 1388sigB-V15fs, and MQ140025rsbU-Q317*, rsbX-N77K displayed acid-sensitive phenotypes when individually compared to their closely related field isolates. Abilities to survive lethal pH stress were quantitatively determined by measuring CFU after pH 2.3 exposure for 120 min, 20 min, 40 min, 60 min, and 40 min, respectively, for CC7, CC9, CC3, CC2, and CC1. Data represent three independent experiments, each with three technical repeats. One-way analysis of variance (ANOVA) was performed using the logarithm survival rate within individual CC strains that remained above the detection limit. Tukey’s test was used to point out significant differences between groups (P < 0.05). Strains below the detection limit were excluded from analysis. n.s., not significant.
Strains 1388 and MQ140025 have reduced SigB activity.
To investigate whether SigB activity is influenced by these sigB operon alleles, a previously constructed enhanced green fluorescent protein (EGFP)-based transcriptional reporter assay was employed (57). In this integrative reporter system, the native promoter region of highly σB-dependent gene lmo2230 is fused with egfp (pKSV7-Plmo2230::egfp), and thus, the production of EGFP in reporter strains largely reflects SigB activity. For each sigB allele-carrying strain, another strain from the same CC was included as a control. Reference strain 10403S and its isogenic mutant ΔsigB were tested in reporter assays along with field isolates as a positive control for the EGFP dependency on SigB. Integration of pKSV7-Plmo2230::egfp into the genome was achieved in all strains (Table 2), with the exception of the two from CC9. As SigB is known to be highly activated during stationary phase (58), fluorescent signals were inspected microscopically for 11 reporter strains after incubation in BHI at 37°C with aeration for 18 h (Fig. 6A). As expected, significant reduction of fluorescent cells was observed for ΔsigB::pKSV7-Plmo2230::egfp strains compared with 10403S wild type. Percentages of fluorescent cells in 1388sigB-V15fs::pKSV7-Plmo2230::egfp and MQ140025rsbU-Q317*, rsbX-N77K::pKSV7-Plmo2230::egfp were similar to that observed in ΔsigB::pKSV7-Plmo2230::egfp strains and significantly lower than in their individual controls (Fig. 6B). Very weak fluorescent signals appeared for these three reporter strains, providing strong evidence for loss of SigB activity in these backgrounds (Fig. 6A). Moderately increased levels of fluorescent cells were observed in 1374::pKSV7-Plmo2230::egfp and F2365::pKSV7-Plmo2230::egfp compared to other strains from their individual genetic backgrounds, while no difference found between 1147::pKSV7-Plmo2230::egfp and 10403S::pKSV7-Plmo2230::egfp. Interestingly, although the control strains used for comparison harbored wild-type sigB operons, they activated SigB slightly differently under these conditions (Fig. 6A and B). Overall, these results suggest that during stationary phase, SigB activity is essentially abolished in strains 1388sigB-V15fs and MQ140025rsbU-Q317*, rsbX-N77K, somewhat increased in strains F2365mazF-I90fs and 1374rsbW-G104R, and not affected in strain 1147rsbT-del65-68.
TABLE 2.
EGFP reporter strains and alternative reference strains used in this study
| Strain name | Lab stock | Source or reference |
|---|---|---|
| L. monocytogenes 10403S | COB46 | K. Boor, Cornell University |
| L. monocytogenes 10403S ΔsigB | COB45 | K. Boor, Cornell University |
| L. monocytogenes EGD-e | COB261 | K. Boor, Cornell University |
| L. monocytogenes EGD-e ΔsigB | COB262 | K. Boor, Cornell University |
| L. monocytogenes 10403S::pKSV7-Plmo2230::egfp | COB528 | This study |
| L. monocytogenes 10403S ΔsigB::pKSV7-Plmo2230::egfp | COB530 | This study |
| L. monocytogenes 1147::pKSV7-Plmo2230::egfp | COB1309 | This study |
| L. monocytogenes 1372::pKSV7-Plmo2230::egfp | COB1206 | This study |
| L. monocytogenes 1374::pKSV7-Plmo2230::egfp | COB1207 | This study |
| L. monocytogenes 1413::pKSV7-Plmo2230::egfp | COB1209 | This study |
| L. monocytogenes 1388::pKSV7-Plmo2230::egfp | COB1208 | This study |
| L. monocytogenes F2365::pKSV7-Plmo2230::egfp | COB1210 | This study |
| L. monocytogenes MQ130042::pKSV7-Plmo2230::egfp | COB1211 | This study |
| L. monocytogenes MQ140033::pKSV7-Plmo2230::egfp | COB1213 | This study |
| L. monocytogenes MQ140025::pKSV7-Plmo2230::egfp | COB1212 | This study |
| Escherichia coli TOP10/pKSV7-Plmo2230::egfp | COB516 | 81 |
FIG 6.
Strains 1388sigB-V15fs and MQ140025rsbU-Q317*, rsbX-N77K displayed reduced SigB activity in stationary phase. Representative fluorescent images (A) of pKSV7-Plmo2230::egfp reporter strains grown in BHI to stationary phase (18 h) and quantification of percentage of fluorescent cells relative to bright-field microscopy (B) are presented. Ten fields were randomly selected and analyzed for each of three biological replicates performed for strains tested. Asterisks indicate statistically significant differences determined by paired t test (two-tailed) (*, P < 0.05; **, P < 0.01; ***, P < 0.001; ns, not significant). All images underwent the same manipulation in ImageJ.
The rsbV-V65fs allele produces a small but significant effect on SigB activity during stationary phase.
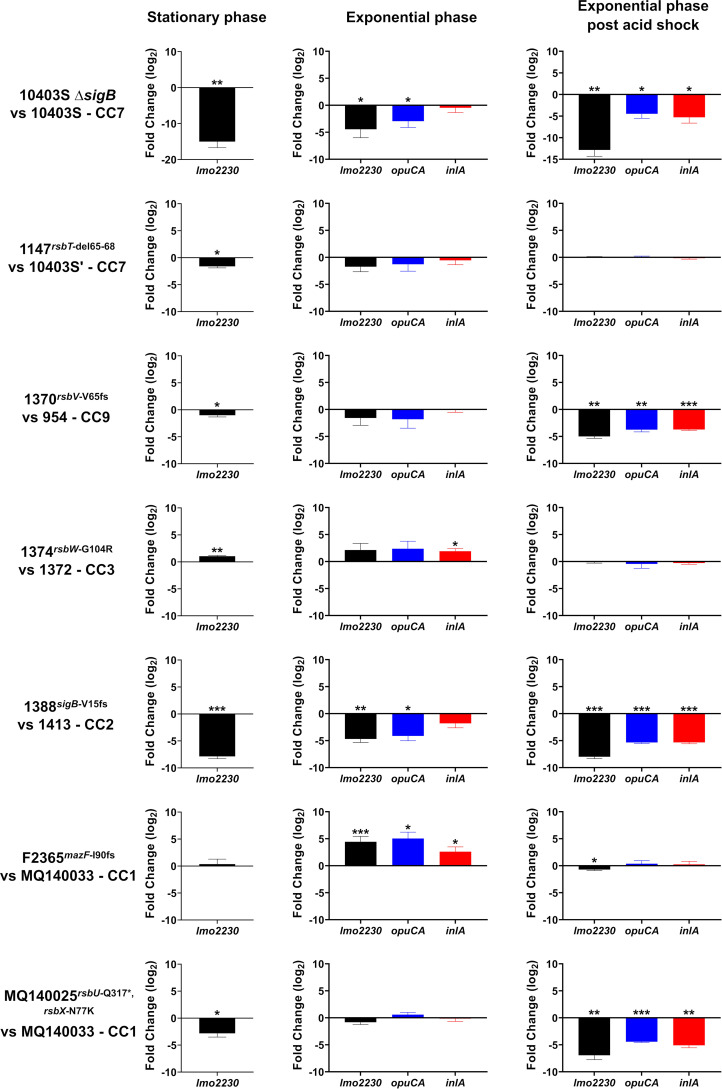
In strain 1370rsbV-V65fs, we anticipated that such significant truncation in the anti-anti-sigma factor RsbV would be detrimental for SigB induction; however, several attempts to transform CC9 strains 1370 and 954 with pKSV7-Plmo2230::egfp failed. Therefore, the transcription of lmo2230 during stationary phase in these strains was measured as a proxy for SigB activity using with RT-qPCR (Fig. 7). For all RT-qPCR analyses, transcription of lmo2230 in each sigB allele relative to a control strain was calculated to evaluate the impacts of these sigB alleles on SigB activity. Significant downregulation of lmo2230 in ΔsigB confirms that this gene is a reliable indicator of SigB activity (Fig. 7). As expected, transcription of lmo2230 in the ΔsigB strain and strains 1388sigB-V15fs and MQ140025rsbU-Q317*, rsbX-N77K were significantly reduced relative to the chosen reference strains. Surprisingly, lmo2230 transcription was minimally (although significantly) reduced in 1370rsbV-V65fs compared to strain 954 (−2.02-fold). lmo2230 transcription was also minimally decreased in strain 1147rsbT-del65-68 (−3.03-fold) and increased in strain 1374rsbW-G104R (2.09-fold). Transcription of lmo2230 in strain F2365mazF-I90fs was not significantly different from that in the control strain. Taken together, these results are consistent with those of the EGFP reporter assay, indicating that rsbV-V65fs allele has a small but significant effect on SigB activity in strain 1370 during stationary phase.
FIG 7.
Strains 1370rsbV-V65fs, 1388sigB-V15fs, and MQ140025rsbU-Q317*, rsbX-N77K are unable to adequately induce SigB activity under stationary phase or mild acid stress. Expression of SigB-dependent genes in sigB allele strains relative to their individual reference strains during stationary phase, exponential phase, and exponential phase after HCl shock was determined by RT-qPCR. Three independent experiments were performed with technical duplicates. Relative expression was calculated with Q-Gene by using expression of 16S as the reference. Asterisks indicate statistically significant differences determined by paired t test (two-tailed) (*, P < 0.05; **, P < 0.01; ***, P < 0.001).
Acid-induced activation of SigB is attenuated in strains 1370, 1388, and MQ140025.
To investigate SigB induction in these sigB allele strains in response to acid stress, we compared the transcription of SigB-dependent genes in these strains during exponential growth phase before and after acid adaptation (HCl, ~pH 5.0) for 15 min by reverse transcription-quantitative PCR (RT-qPCR). Exponential growth in BHI is known to produce low levels of SigB activity, while acid adaptation for 15 min is known to strongly induce SigB activity (59). As acid exposure is also known to induce internalin production (60), transcription of inlA was measured in addition to two SigB-dependent genes, lmo2230 and opuCA. The relative transcript levels of the reporter genes in each of the sigB allele-carrying strains was measured in exponential phase before (Fig. 7) and after (Fig. 7) acid shock. Absence/truncation of SigB in the ΔsigB/1388sigB-V15fs strain resulted in significantly reduced transcription of lmo2230 in exponential phase (Fig. 7). SigB activity in other sigB allele-carrying strains in exponential phase was not influenced, except for an increase detected in F2365mazF-I90fs (Fig. 7). After acid adaptation, lmo2230 transcription was significantly lower in strains 1370rsbV-V65fs, 1388sigB-V15fs, and MQ140025rsbU-Q317*, rsbX-N77K than the control strains, similar to that observed for ΔsigB strains. lmo2230 transcription in other strains carrying sigB alleles was similar to that in the controls (Fig. 7). Interestingly, although lmo2230 transcription in control strains from different CCs differed slightly during exponential phase, they reached a similar level after acid adaptation (data not shown). This observation implies that acid treatment induces SigB activity to the maximal extent even for L. monocytogenes from different genetic backgrounds. Moreover, the transcription patterns of opuCA and inlA mirrored that of lmo2230, suggesting that these two genes are largely SigB dependent under these conditions. Overall, this experiment demonstrates that strains 1370rsbV-V65fs, 1388sigB-V15fs, and MQ140025rsbU-Q317*, rsbX-N77K are not able to properly induce SigB activity upon acid adaptation, thus confirming that these alleles negatively impact SigB activity under stress conditions.
DISCUSSION
The ability of L. monocytogenes to endure external stresses determines its growth potential in food products and virulence potential inside the host. In this study, a heterogeneous phenotypic response to stress was observed across the 168 strains studied. Increased tolerance to salt stress and decreased resistance to acid stress were found to be associated with LI strains when they were compared collectively to LII strains. This discrepancy in salt stress tolerance was previously observed in liquid media (1, 22–24). Our results showed a similar trend on solid medium, potentially suggesting that the enhanced salt tolerance of LI strains could favor growth on solid food surfaces with reduced aw. The acid resistance among field isolates is largely CC dependent (Fig. 1). The observed significant difference between the two lineages could result from the large number of CC121 strains in this collection, a CC that exhibited particularly high acid resistance (Fig. 1). These results were not surprising given the deeply branched population structure.
Large numbers of lineage-specific genes were identified in the pan-genome analysis (Fig. 2). It is, however, plausible that these variations account for differential mechanisms employed by the two lineages. Moreover, coreSNP analysis with Parsnp detected 158,897 SNPs across all strains in our collection, while only 32,248 SNPs and 90,297 SNPs were detected in LI and LII, respectively, when a lineage-specific coreSNP analysis was performed. It is anticipated that a large number of SNPs between two lineages might also influence the function or regulation of stress response mechanisms. LII strains were more frequently isolated from food products while LI strains were more often associated with clinical cases (8). These two lineages may have evolved to adapt to different lifestyles and thus regulate stress response and virulence differently. Likewise, phenotypic differences were observed frequently across phylogenetic groups (i.e., CC and ST) but relatively rarely within phylogenetic groups.
It is possible that these phylogenetic groups have specifically adapted to certain environmental niches. The seafood-associated CC155 strains serve as an excellent example for this; all CC155 strains in this study were isolated from seafood or seafood processing environments, a finding that is consistent with previous studies (61). Although the specific niche adaptations are clear, the molecular mechanisms involved in these adaptations remain poorly understood. While the absence/presence of several genomic pathogenicity islands and the internalin profile largely explain heterogeneities in virulence (6, 53, 62–65), little is known about the genetic determinants contributing to stress response except SSI-1 and SSI-2 (28, 29). The present study provides an impetus to further investigate the genetic factors that underpin the intraspecies heterogeneity in stress responses, especially as these responses are likely to determine the behavior of different strains in the food chain (66).
The identification of closely related strains that display clear phenotypic differences provides an excellent opportunity to uncover novel mechanisms that contribute to stress tolerance. Clinical isolate MQ150008 exhibited a drastically attenuated ability to grow under conditions of osmotic stress compared with strain MQ150007, although only one SNP (a silent mutation in lmo1388) was identified between them from the CC101-specific coreSNP analysis. Whole-genome alignment with Mauve revealed 9 additional polymorphisms and acquisition/possession of a mobile genetic element in MQ150008, including a truncation in the ATP binding subunit of glycine betaine transporter GbuA (E141Efs*11). GbuA is known to contribute to osmoregulation and salt tolerance (67); therefore, this truncation could account for reduced salt tolerance in strain MQ150008. Interestingly, strains 1003 and F111-17 were both more sensitive to acid stress than closely related strains, but Mauve alignments identified no polymorphisms between strains 1003 and 1445 or between strains F111-17 and F114-17 (Fig. 1). It is anticipated that these phenotypical differences are due to either (i) polymorphisms that were not identified by short-read (Illumina) sequencing (e.g., genome rearrangements) or (ii) mutations that arose during routine laboratory cultivation after the WGS analysis was performed (45, 46). To further investigate this, long read (Nanopore) sequencing could be performed on the outlier strains to obtain a complete reference genome sequence and compare these data with the Illumina sequencing results to check for disagreements. In addition, although CC2 strain 1381 did not grow under low-pH conditions, suppressor mutants can be readily isolated that exhibit normal growth as other CC2 strains (data not shown). We are actively investigating several of these outlier/suppressor strains to further understand the stress response mechanisms employed by this pathogen.
Six strains were found carrying sigB operon alleles. To investigate if SigB activity in these strains was affected, we compared the transcription of SigB-dependent genes between these strains and individual control strains (which all carry a wild-type sigB operon). Transcriptional analysis suggested that SigB activity is clearly negatively influenced in strains 1370rsbV-V65fs, 1388sigB-V15fs, and MQ140025rsbU-Q317*, rsbX-N77K. This was expected in strain 1388sigB-V15fs, because a frameshift near the N-terminal of SigB essentially abolishes the sigma factor. In CC9 strain 1370, only ~57% of the amino acid-coding sequence of RsbV is retained, resulting in the absence of key residues (e.g., Arg84/Arg87 and Ile91) involved in the interaction with RsbW (68). This was predicted to reduce the interaction with RsbW and thus reduce the amount of SigB available to participate in transcription. However, in contrast to the clearly attenuated SigB activation during acidification, SigB activity in strain 1370 was only mildly lower than that in the control strain during stationary phase (Fig. 6A). It is noteworthy that SigB activity in CC9 control strain 954 was lower than in other genetic backgrounds (data not shown), so the consequence of rsbV truncation was perhaps partially masked. Two alternative possibilities that could account for the limited effect of the rsbV-V65fs allele on SigB activity are the presence of residual RsbW-sequestering activity in the truncated RsbV protein or the possibility that SigB activation might occur downstream of RsbV through an unknown mechanism, as has been proposed previously by others (57). In strain MQ140025rsbU-Q317*, rsbX-N77K, two sigB operon alleles were identified. The near C-terminal truncation in RsbU results in the loss of a DDFTLIVL motif that is required for the coordination of Mn2+ in the catalytic center of the enzyme (69). This truncated RsbU is therefore likely to be defective in the dephosphorylation of RsbV, which is predicted to reduce SigB activity. Asn77 was not among critical residues reportedly required for RsbX function (69), but it is highly conserved compared with similar proteins in our analysis (>99% conserved by MAFFT alignment on the top 5,000 BLASTp hits from the nonredundant protein database) (data not shown). Therefore, it is possible that this substitution could influence the activity of RsbX, which would likely result in increased SigB activity. Since RsbU acts downstream of the stressosome (the likely dephosphorylation target of RsbX) according to the current model of the SigB activation pathway, we speculate that the RsbU truncation is the principal reason for the negative impact on SigB activity in this strain.
Three strains that carried sigB operon alleles were found to retain similar levels of SigB activity as the control strains, giving insights into genetic changes that can be tolerated without altering signal transduction through the pathway. First, the in-frame deletion in RsbT in strain 1147rsbT-del65-68 does not include the Asn49 residue, which has been shown to be critical for kinase activity (70), and this suggests that residues 65 to 68 are not essential for RsbT function. Second, the RsbW residue altered by substitution in 1374rsbW-G104R was not clearly visible from the recently inspected RsbW crystal structure in Bacillus subtilis (68) and likely does not affect the interaction of this anti-sigma factor with either SigB or RsbV. Finally, the mazF frameshift mutation, predicted to truncate the C-terminal region of the MazF protein, appeared to have little impact on SigB activity. Previously, work on this strain reported only a limited role for SigB in the stress response, although the presence of the mazF mutation was not discussed (71). Others have shown that loss of both mazEF genes from L. monocytogenes influence the expression of genes in the SigB regulon through an unknown mechanism (34). We recently reported that premature stop codons in the mazF gene occur at a high frequency compared to other loci, suggesting that there is some selective pressure for loss of function in field isolates (46), although further work is needed to fully establish its role in the physiology of L. monocytogenes. Given the complex signal transduction pathway for SigB activation, it is possible that these sigB operon alleles might influence SigB activity under conditions that were not tested in the present study. Further investigations into the behavior of these strains over a range of different conditions might give additional insights into the significance of these alleles for the general stress response in L. monocytogenes.
EURL-Lm provides technical guidance for carrying out food challenge and durability tests to food industries to facilitate the assessment of the growth potential of L. monocytogenes in food products (72). Twenty-five strains are recommended by EURL-Lm for these assessments, to prevent an underestimation of the risk of L. monocytogenes exposure. These strains were selected from multiple studies based on their specific growth rates under various stressful conditions (i.e., low aw, low pH, or low temperature) (73). In the present study, these strains were phenotypically and genotypically characterized in comparison with the food and clinical isolates. Interestingly, sigB operon alleles that negatively impact SigB activity were identified in two EURL-Lm reference strains, 1370rsbV-V65fs (strain 12MOB045LM) and 1388sigB-V15fs (strain 12MOB103LM). Strain 1370rsbV-V65fs was recommended for testing meat products under low-temperature, low-pH, and low-aw conditions, while strain 1388sigB-V15fs was recommended for testing fish products under low-pH and low-aw conditions (72, 73). Interestingly, strain 1388sigB-V15fs also exhibited the highest growth rate at low temperature (8°C) among strains tested by EURL-Lm (73). Consistent with this, these two strains exhibited enhanced growth performance under suboptimal conditions (NaCl stress), suggesting that the use of these strains would not lead to an underestimation of the potential growth of L. monocytogenes in foods preserved with salt. Nevertheless, these experiments were performed in rich media under laboratory conditions in the presence of a single stress, while L. monocytogenes simultaneously encounters multiple stresses (e.g., food formulation and competing microflora) in food products. Since it is unknown how SigB contributes to the growth of L. monocytogenes in various food products, it is important to assess growth of multiple strains in food challenge studies, as suggested by EURL-Lm, particularly when strains carrying sigB operon mutations are included.
Another SigB-negative strain (strain MQ140025rsbU-Q317*, rsbX-N77K) was isolated from a patient with listeriosis (48). It is well established that SigB is critical for GI tract survival and entry into the epithelial cell layer but dispensable for systematic infection (38, 42). Although it is not possible to determine which of the two alleles in this strain arose first (the rsbU-Q317* or the rsbX-N77K strain) or in what circumstances they arose, it is interesting to speculate that they may have arisen during the course of the infection. If the N77K substitution negatively affects RsbX function (which remains to be established), this would likely have a positive effect on SigB activity, since RsbX is believed to function as a phosphatase that resets the sensing-ready state of the stressosome after the stress response has been deployed (32, 74). A strain with increased SigB activity might have a survival advantage in the early stages of an infection, as it would likely have increased resistance to low pH (32) and therefore be more likely to survive the passage through the stomach. Internalin expression is also positively controlled by SigB (75), so the rsbX allele might also confer increased ability to invade epithelial cells in the GI tract. During the latter stages of the infection, the growth disadvantage that is associated with increased SigB activity might provide a selective pressure for a mutation that reduces SigB activity (43, 46), which could potentially explain the emergence of the rsbU allele in this strain (which appears dominant based on the data presented herein). While this scenario is purely speculative, there is ample evidence that clinical isolates of pathogenic bacteria do acquire mutations during the course of infections (76, 77). Further time-resolved genome sequencing of L. monocytogenes isolates during the course of an infection may shed further light on this phenomenon, potentially giving insights into the nature of the selective pressures encountered within the host.
The general stress response regulator, SigB, is required for surviving various challenging environmental stresses and is highly conserved across different lineages of L. monocytogenes and among Listeria species (40). However, we have previously reported that multiple passages under mildly stressful laboratory conditions impose negative selective pressure on SigB activity (31, 46). Camargo et al. (78) identified several Brazilian food isolates carrying truncations in sigB operon genes, while Asakura et al. (44) reported that several strains recovered from mice infection have reduced SigB activity. In this study, six strains carrying sigB operon alleles were identified, with three being shown to be defective in SigB activation. These results are in line with our previous observation that high PMSC (truncated by >10%) rates were associated with sigB operon genes that positively regulate SigB activity (46). These observations indicate that SigB activity is under negative selective pressure in some circumstances. Indeed, we have observed growth advantages under several mildly stressful conditions associated with ΔsigB, i.e., salt stress, blue light stress, heat stress, and isoleucine depletion (31, 35, 43, 46, 79). The exact mechanisms for these growth advantages were not clear, except that the reduced expression of SigB-dependent ncRNA rli47 partially explained the growth advantage under isoleucine depletion condition (79, 80). The prevalence of sigB operon mutations in field isolates of L. monocytogenes further highlights the burden on cellular resources that stem from deploying the general stress response, notwithstanding the benefits that this response confers in extreme environments that threaten its survival.
MATERIALS AND METHODS
Strains and general growth conditions.
One hundred thirty-nine L. monocytogenes strains isolated from clinical cases and food processing environments in Ireland were chosen for this study to compare with 25 strains recommended by the European Union Reference Laboratory of Listeria monocytogenes and 4 reference strains with complete genome sequences available (Table S1). Stocks of all strains were stored at −80°C with 7% dimethyl sulfoxide (DMSO). To prepare overnight cultures, stocks were streaked on BHI agar plates; then, single colonies were selected to inoculate fresh cultures, followed by incubation for 18 h at 37°C in the dark with agitation. For phenotypic assays, overnight cultures were grown in 24-well plates containing 0.4 mL medium in each well; for transcription assays and plasmid integration, overnight cultures were grown in 50-mL centrifuge tubes with 5 mL medium. For integration of pKSV7-Plmo2230::egfp, a protocol was adapted from the work of Utratna et al. (81). Briefly, electrocompetent cells were prepared and transformed with pKSV7-Plmo2230::egfp according to Monk et al. (82). Transformants were selected by incubating for 48 h on BHI agar with 10 μg mL−1 chloramphenicol. Plasmids in transformants were integrated by 2 or 3 passages of incubation at 40°C to avoid positive selection for mutations that undermine SigB activity (46). Site-specific integration was confirmed by PCR (81).
WGS.
Genomic DNA for 115 Irish food isolates and 25 EURL-Lm reference strains were extracted with DNeasy blood and tissue kit (Qiagen). Overnight cultures of L. monocytogenes isolates were resuspended in 180 μL enzymatic lysis buffer (20 mM Tris-HCl, 2 mM EDTA, 1.2% Triton X-100, and lysozyme 20 mg mL−1) for 30 min incubation at 37°C. Subsequently, 25 μL proteinase K and 200 μL buffer AL were added separately and further incubated for 30 min at 56°C. Procedures were carried out according to the manufacturer’s instructions (Qiagen DNeasy Blood and Tissue Kit). The resulting genomic DNA was sent for Illumina sequencing with MicrobesNG.
Comparative genomics.
Genome assembly was performed with trimmed paired-end raw sequencing reads by SKESA (83). Sequencing coverage was calculated by mapping raw sequencing reads to each genome assembly (84) and then dividing total mapped bases by genome size. N50 and NG50 were calculated by Quast (N50: mean, 46 kb; median, 47 kb; NG50: mean, 46 kb; median, 46 kb). Genome sequences for 25 clinical isolates (48) as well as reference strains EGD-e, 10403S, 6179, and F2365 were extracted from the NCBI database. Core genome phylogeny was inferred by Parsnp using EGD-e as the reference sequence. In silico MLST was performed with mlst (85) and cross checked with the Center for Genomic Epidemiology’s online tool (http://www.genomicepidemiology.org/). Serogroups were determined by checking the presence/absence of marker PCR fragments using BLASTn (86). PROKKA 1.14.6 (87) was used to annotate all genome sequences according to published genome annotation of EGD-e, using the “–proteins” flag. Pan-genome gene presence/absence analysis was carried out with Roary default settings (88). Several categories of genes were identified based on their percent presence in the whole collection or two genetic lineages and plotted against coreSNP phylogeny. For intra-CC genome comparison, a publicly available complete chromosome sequence for each CC, if available, was used as the reference genome in Parsnp. The resulting list of SNPs detected, stored in .vcf files, was annotated with a piece of bio-python script. The gene presence/absence matrix was also interpreted to check for unique gene presence/absence. The unique gene presence/absence in phenotypical outlier strains was examined because protein truncations caused by indels lead to misannotation as one or two shorter coding sequences (CDSs). Draft genome sequences were visualized with Geneious 10.2.6.
Characterization of growth under salt/acid stress.
For growth screening under salt stress or low-pH stress, unmodified BHI agar, BHI agar supplemented with NaCl (0.8 M, 1.0 M, 1.2 M, 1.3 M, 1.4 M, and 1.5 M), and BHI agar acidified by 5 M HCl (pH 5.2, pH 5.1, pH 5.0, and pH 4.9) were prepared. All agar plates were air dried in a fume hood to the same standard. Overnight culture was diluted in phosphate-buffered saline (PBS) by a factor of 3 × 106, and 5 μL was spotted on the agar surface so that individual colonies could be observed after incubation. Three independent experiments were carried out, each with three technical repeats, and the order of the strain was shuffled for each experiment to minimize the impact of differential dehydration across the agar plates during prolonged incubation. Then, agar plates were incubated in the dark at 37°C in plastic containers.
Pictures of plates were taken every day for up to 8 days with a scanner (Canon 9000F MarkII). Image processing performed with ImageJ 1.52a. For each condition at each time point, all images captured were first cropped and stacked and then montaged to generate a picture matrix with nine replicates of each strain in rows and 168 strains in columns. This montage was converted to “8-bit” and then transferred to result (using “transfer to result” function in ImageJ) to facilitate analysis. The result from each pixel was considered the raw signal intensity, and signals detected from each strain across different replicates were applied with a threshold (65 to 100) to erase background. These signals were normalized using total colony numbers, which were obtained with the “find particle” function. The normalized signal intensities were interpreted as an indication of growth performance. The images from replicates of each strain were also cropped and montaged in descending order of normalized signal intensity for visual examination and confirmation. To compare several groups of strains with close genetic backgrounds, dilution series of overnight cultures were spotted on top of agar plates with NaCl supplement or acidification by HCl. Images were taken with a scanner, and pictures were visually examined to compare differences in tolerance to salt or acid stress.
Characterization of survival at pH 2.3.
Since there was some strain-to-strain variation in the growth behavior in BHI, stationary-phase cultures, grown for 24 h at 37°C, were used to assay acid resistance. Two hundred microliters of overnight culture from each well in 24-well plates was transferred to 96-well plates so that a 96-well replicator could be used to transfer approximately 1.5 μL culture from each well to another 96-well plate filled with 200 μL BHI pH 2.3 (acidified with 5 M HCl) per well for acid challenge. Plates were incubated at 37°C with agitation during challenge and samples were taken every 60 min for 7 h. A 96-well plate replicator was used to take samples by dipping into the challenge medium and then allowing them to stand on BHI agar plates for 2 to 3 s. The 96-well replicator was sterilized before each operation with 70% ethanol and subsequent flaming. BHI plates were incubated for 2 days before results were interpreted. Experiments were carried out using three biological replicates and three technical replicates. For each time point, strains were considered viable only if colonies were detected on plates from at least two technical replicates and at least two biological replicates. To compare several groups of strains with close genetic backgrounds, 100 μL overnight culture was diluted into 900 μL BHI (pH 2.3) in microcentrifuge tubes and incubated at 37°C. Acid resistance was quantitatively compared by calculating the percentage survival using CFU counts from overnight culture and after acid exposure for the specified times (CC7, 120 min; CC9, 20 min; CC3, 40 min; CC2, 60 min; and CC1, 40 min).
Fluorescent-reporter assay.
Overnight cultures of reporter strains were grown in BHI with 10 μg mL−1 chloramphenicol. Methods for sample preparation and image analysis were adapted from those of Utratna et al. (81) with minor modifications. Cultures were fixed in an equal volume of ice-cold ethanol-methanol (1:1 [vol/vol]), stored at −20°C for 10 min, and then resuspended (10,000 × g for 10 min at 4°C) in ice-cold PBS. All samples were adjusted to an optical density at 600 nm (OD600) of 1.0 for microscopic analysis (Nikon Eclipse E600). For each sample, 10 random fields were selected for fluorescent signal capture using a B-2A filter in comparison with bright-field images. All image processing was performed in ImageJ. A threshold (0, 2) was first applied to the resulting fluorescent images, followed by “Convert to Mast,” “Make Binary,” and “Watershed” steps. Finally, results generated by “Analyze Particles” (size = 5-Infinity show=Outlines summarize) were considered an estimation of fluorescent cells. Cell numbers obtained in bright-field microscopy were estimated by applying “Invert” and “Subtract Background” steps and then following the protocol for fluorescent image processing. Fluorescence levels were quantified and compared by calculating the percentage of particles. Three independent experiments were carried out for each strain. Statistical differences between strains in each pair compared were determined with two-tailed paired Student's t test. For all images presented for visualization in Fig. 6A, all fluorescent images were converted to “green” and adjusted for brightness/contrast (minimum: 11; maximum: 31).
Transcriptional analysis by RT-qPCR.
For transcriptional analysis during stationary phase, samples were directly taken from overnight cultures. For expressional analysis during exponential phase or exponential phase after acid adaptation, sample preparation was done as described by Guerreiro et al. (59). Briefly, overnight cultures were inoculated into 5 mL fresh BHI medium in 50-mL centrifuge tubes in duplicates to achieve an OD600 of 0.05. Cultures were incubated at 37°C in the dark for ~3 h (~3.5 h for strains F2365 and 1413) with agitation until mid-exponential phase (OD600, ~0.4), at which point 30 μL of HCl 5 M was applied to one tube for acidification (pH 5). After further incubation for 15 min, both HCl-adapted and unadapted samples were harvested. For RNA preparation, 1 mL of culture was mixed with 5 mL RNAlater (Sigma), incubated at room temperature for 5 min, resuspended (8,000 × g for 5 min at 4°C) in 700 μL RLT buffer (Qiagen RNeasy minikit), and transferred to lysis matrix B tubes (Fisher Scientific) for mechanical lysis (FastPrep, twice for 40 s at 6 m s−1). The remainder of the RNA preparation followed the manufacturer’s instructions (Qiagen RNeasy Minikit). The resulting RNA samples were treated with DNase (Invitrogen Turbo) to avoid contamination with genomic DNA. Reverse transcription was performed using Superscript III (Invitrogen) with a random primer mix (Invitrogen). Resulting reaction mixture cDNA was diluted and used as the template for qPCR (Qiagen QuantiTect SYBR). All primers used in qPCR experiments were designed to anneal to the consensus region of all strains tested (Table 3). qPCR was carried out with a Roche LightCycler 480 system as described elsewhere (46). Primer efficiencies were determined by testing serial dilutions of cDNA samples (89). Three biological samples were prepared for RT-qPCR analysis, and technical duplicates were performed for each sample tested. Transcription of all genes was calculated with Q-Gene (90) with 16S as the reference gene. Log2 scale-normalized expression of each gene between tested strains and their individual reference strains was tested for statistical significance with two-tailed paired Student's t test.
TABLE 3.
Primers used in this study
| Primer | Sequence (5′-3′) |
|---|---|
| 16S_RT_F | TGGGGAGCAAACAGGATTAG |
| 16S_RT_R | TAAGGTTCTTCGCGTTGCTT |
| lmo2230_RT_F | TGGGCGAAAAGACTTTCACT |
| lmo2230_RT_R | TGGAAATTTTGGTGCAGTTTCA |
| lmo1428_RT_F | TTATCGGTCCAAGTGGTTGT |
| lmo1428_RT_R | CGAATCGTCATATGTGGCATC |
| rli47_RT_F | TTACGGGCGCTCATTTATTC |
| rli47_RT_R | AACATTCGGAAAACGGTGAG |
| inlA_RT_F | CGATTAGTGATATTAGTGCGCTTTC |
| inlA_RT_R | GGTTGTTAGTAGCGATAAGACTTTC |
| lmo2229-F | GCCTTGTCGCCATCTTTG |
| egfp-R | GGCCGTTTACATCTCCATC |
Data availability.
All genome assemblies were deposited in NCBI under BioProject accession numbers PRJNA808240, PRJNA788387, and PRJNA796187.
ACKNOWLEDGMENTS
We are grateful to colleagues at NUI Galway, especially Pilib Ó’Broin, for helpful discussions and advice during the early stages of this project.
This project received funding from the Irish Department of Agriculture, Food and the Marine (17/F/244 and 11/F/008).
Footnotes
Supplemental material is available online only.
Contributor Information
Conor O’Byrne, Email: conor.obyrne@nuigalway.ie.
Edward G. Dudley, The Pennsylvania State University
REFERENCES
- 1.van der Veen S, Moezelaar R, Abee T, Wells-Bennik MHJ. 2008. The growth limits of a large number of Listeria monocytogenes strains at combinations of stresses show serotype- and niche-specific traits. J Appl Microbiol 105:1246–1258. 10.1111/j.1365-2672.2008.03873.x. [DOI] [PubMed] [Google Scholar]
- 2.Begley M, Gahan CGM, Hill C. 2002. Bile stress response in Listeria monocytogenes LO28: adaptation, cross-protection, and identification of genetic loci involved in bile resistance. Appl Environ Microbiol 68:6005–6012. 10.1128/AEM.68.12.6005-6012.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ricci A, Allende A, Bolton D, Chemaly M, Davies R, Fernández Escámez PS, Girones R, Herman L, Koutsoumanis K, Nørrung B, Robertson L, Ru G, Sanaa M, Simmons M, Skandamis P, Snary E, Speybroeck N, Ter Kuile B, Threlfall J, Wahlström H, Takkinen J, Wagner M, Arcella D, Da Silva Felicio MT, Georgiadis M, Messens W, Lindqvist R, EFSA Panel on Biological Hazards (BIOHAZ). 2018. Listeria monocytogenes contamination of ready‐to‐eat foods and the risk for human health in the EU. EFSA J 16:e05134. 10.2903/j.efsa.2018.5134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.European Food Safety Authority, European Centre for Disease Prevention and Control. 2021. The European Union One Health 2019 zoonoses report. EFSA J 19:e06406. 10.2903/j.efsa.2021.6406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bergholz TM, Shah MK, Burall LS, Rakic-Martinez M, Datta AR. 2018. Genomic and phenotypic diversity of Listeria monocytogenes clonal complexes associated with human listeriosis. Appl Microbiol Biotechnol 102:3475–3485. 10.1007/s00253-018-8852-5. [DOI] [PubMed] [Google Scholar]
- 6.Maury MM, Tsai Y-H, Charlier C, Touchon M, Chenal-Francisque V, Leclercq A, Criscuolo A, Gaultier C, Roussel S, Brisabois A, Disson O, Rocha EPC, Brisse S, Lecuit M. 2016. Uncovering Listeria monocytogenes hypervirulence by harnessing its biodiversity. Nat Genet 48:308–313. 10.1038/ng.3501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Painset A, Björkman JT, Kiil K, Guillier L, Mariet J-F, Félix B, Amar C, Rotariu O, Roussel S, Perez-Reche F, Brisse S, Moura A, Lecuit M, Forbes K, Strachan N, Grant K, Møller-Nielsen E, Dallman TJ. 2019. LiSEQ – whole-genome sequencing of a cross-sectional survey of Listeria monocytogenes in ready-to-eat foods and human clinical cases in Europe. Microb Genom 5:e000257. 10.1099/mgen.0.000257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McLauchlin J, Mitchell RT, Smerdon WJ, Jewell K. 2004. Listeria monocytogenes and listeriosis: a review of hazard characterisation for use in microbiological risk assessment of foods. Int J Food Microbiol 92:15–33. 10.1016/S0168-1605(03)00326-X. [DOI] [PubMed] [Google Scholar]
- 9.Lee S, Chen Y, Gorski L, Ward TJ, Osborne J, Kathariou S. 2018. Listeria monocytogenes source distribution analysis indicates regional heterogeneity and ecological niche preference among serotype 4b clones. mBio 9:e00396-18. 10.1128/mBio.00396-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stasiewicz MJ, Oliver HF, Wiedmann M, den Bakker HC. 2015. Whole-genome sequencing allows for improved identification of persistent Listeria monocytogenes in food-associated environments. Appl Environ Microbiol 81:6024–6037. 10.1128/AEM.01049-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pasquali F, Palma F, Guillier L, Lucchi A, De Cesare A, Manfreda G. 2018. Listeria monocytogenes sequence types 121 and 14 repeatedly isolated within one year of sampling in a rabbit meat processing plant: persistence and ecophysiology. Front Microbiol 9:596. 10.3389/fmicb.2018.00596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Smith AM, Tau NP, Smouse SL, Allam M, Ismail A, Ramalwa NR, Disenyeng B, Ngomane M, Thomas J. 2019. Outbreak of Listeria monocytogenes in South Africa, 2017–2018: laboratory activities and experiences associated with whole-genome sequencing analysis of isolates. Foodborne Pathog Dis 16:524–530. 10.1089/fpd.2018.2586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Halbedel S, Wilking H, Holzer A, Kleta S, Fischer MA, Lüth S, Pietzka A, Huhulescu S, Lachmann R, Krings A, Ruppitsch W, Leclercq A, Kamphausen R, Meincke M, Wagner-Wiening C, Contzen M, Kraemer IB, Al Dahouk S, Allerberger F, Stark K, Flieger A. 2020. Large nationwide outbreak of invasive listeriosis associated with blood sausage, Germany, 2018–2019. Emerg Infect Dis 26:1456–1464. 10.3201/eid2607.200225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nüesch-Inderbinen M, Bloemberg GV, Müller A, Stevens MJA, Cernela N, Kollöffel B, Stephan R. 2021. Listeriosis caused by persistence of Listeria monocytogenes serotype 4b sequence type 6 in cheese production environment. Emerg Infect Dis 27:284–288. 10.3201/eid2701.203266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.European Food Safety Authority, European Centre for Disease Prevention and Control. 2018. Multi‐country outbreak of Listeria monocytogenes serogroup IV B, multi‐locus sequence type 6, infections linked to frozen corn and possibly to other frozen vegetables–first update. EFSA Supporting Publications 15:1448E. https://www.efsa.europa.eu/en/supporting/pub/en-1448. [Google Scholar]
- 16.Thomas J, Govender N, McCarthy KM, Erasmus LK, Doyle TJ, Allam M, Ismail A, Ramalwa N, Sekwadi P, Ntshoe G, Shonhiwa A, Essel V, Tau N, Smouse S, Ngomane HM, Disenyeng B, Page NA, Govender NP, Duse AG, Stewart R, Thomas T, Mahoney D, Tourdjman M, Disson O, Thouvenot P, Maury MM, Leclercq A, Lecuit M, Smith AM, Blumberg LH. 2020. Outbreak of listeriosis in South Africa associated with processed meat. N Engl J Med 382:632–643. 10.1056/NEJMoa1907462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wagner E, Zaiser A, Leitner R, Quijada NM, Pracser N, Pietzka A, Ruppitsch W, Schmitz-Esser S, Wagner M, Rychli K. 2020. Virulence characterization and comparative genomics of Listeria monocytogenes sequence type 155 strains. BMC Genomics 21:847. 10.1186/s12864-020-07263-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dykes GA, Moorhead SM. 2000. Survival of osmotic and acid stress by Listeria monocytogenes strains of clinical or meat origin. Int J Food Microbiol 56:161–166. 10.1016/S0168-1605(99)00205-6. [DOI] [PubMed] [Google Scholar]
- 19.Faleiro ML, Andrew PW, Power D. 2003. Stress response of Listeria monocytogenes isolated from cheese and other foods. Int J Food Microbiol 84:207–216. 10.1016/s0168-1605(02)00422-1. [DOI] [PubMed] [Google Scholar]
- 20.Adrião A, Vieira M, Fernandes I, Barbosa M, Sol M, Tenreiro RP, Chambel L, Barata B, Zilhao I, Shama G, Perni S, Jordan SJ, Andrew PW, Faleiro ML. 2008. Marked intra-strain variation in response of Listeria monocytogenes dairy isolates to acid or salt stress and the effect of acid or salt adaptation on adherence to abiotic surfaces. Int J Food Microbiol 123:142–150. 10.1016/j.ijfoodmicro.2007.12.016. [DOI] [PubMed] [Google Scholar]
- 21.Werbrouck H, Vermeulen A, Van Coillie E, Messens W, Herman L, Devlieghere F, Uyttendaele M. 2009. Influence of acid stress on survival, expression of virulence genes and invasion capacity into Caco-2 cells of Listeria monocytogenes strains of different origins. Int J Food Microbiol 134:140–146. 10.1016/j.ijfoodmicro.2009.03.022. [DOI] [PubMed] [Google Scholar]
- 22.Bergholz TM, den Bakker HC, Fortes ED, Boor KJ, Wiedmann M. 2010. Salt stress phenotypes in Listeria monocytogenes vary by genetic lineage and temperature. Foodborne Pathog Dis 7:1537–1549. 10.1089/fpd.2010.0624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ribeiro VB, Destro MT. 2014. Listeria monocytogenes serotype 1/2b and 4b isolates from human clinical cases and foods show differences in tolerance to refrigeration and salt stress. J Food Protection 77:1519–1526. 10.4315/0362-028X.JFP-13-548. [DOI] [PubMed] [Google Scholar]
- 24.Aalto-Araneda M, Pöntinen A, Pesonen M, Corander J, Markkula A, Tasara T, Stephan R, Korkeala H. 2020. Strain variability of Listeria monocytogenes under NaCl stress elucidated by a high-throughput microbial growth data assembly and analysis protocol. Appl Environ Microbiol 86:e02378-19. 10.1128/AEM.02378-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Horlbog JA, Kent D, Stephan R, Guldimann C. 2018. Surviving host - and food relevant stresses: phenotype of L. monocytogenes strains isolated from food and clinical sources. Sci Rep 8:12931. 10.1038/s41598-018-30723-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hingston P, Chen J, Dhillon BK, Laing C, Bertelli C, Gannon V, Tasara T, Allen K, Brinkman FSL, Truelstrup Hansen L, Wang S. 2017. Genotypes associated with Listeria monocytogenes isolates displaying impaired or enhanced tolerances to cold, salt, acid, or desiccation stress. Front Microbiol 8:369. 10.3389/fmicb.2017.00369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lundén J, Tolvanen R, Korkeala H. 2008. Acid and heat tolerance of persistent and nonpersistent Listeria monocytogenes food plant strains: acid and heat tolerance of L. monocytogenes. Lett Appl Microbiol 46:276–280. 10.1111/j.1472-765X.2007.02305.x. [DOI] [PubMed] [Google Scholar]
- 28.Ryan S, Begley M, Hill C, Gahan CGM. 2010. A five-gene stress survival islet (SSI-1) that contributes to the growth of Listeria monocytogenes in suboptimal conditions: stress survival islet in L. monocytogenes. J Appl Microbiol 109:984–995. 10.1111/j.1365-2672.2010.04726.x. [DOI] [PubMed] [Google Scholar]
- 29.Harter E, Wagner EM, Zaiser A, Halecker S, Wagner M, Rychli K. 2017. Stress survival islet 2, predominantly present in Listeria monocytogenes strains of sequence type 121, is involved in the alkaline and oxidative stress responses. Appl Environ Microbiol 83:e00827-17. 10.1128/AEM.00827-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dorey A, Marinho C, Piveteau P, O'Byrne C. 2019. Role and regulation of the stress activated sigma factor sigma B (σB) in the saprophytic and host-associated life stages of Listeria monocytogenes. Adv Appl Microbiol 106:1–48. 10.1016/bs.aambs.2018.11.001. [DOI] [PubMed] [Google Scholar]
- 31.Guerreiro DN, Arcari T, O’Byrne CP. 2020. The σB-mediated general stress response of Listeria monocytogenes: life and death decision making in a pathogen. Front Microbiol 11:1505. 10.3389/fmicb.2020.01505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Oliveira AH, Tiensuu T, Guerreiro DN, Tükenmez H, Dessaux C, García-del Portillo F, O’Byrne C, Johansson J. 2022. Listeria monocytogenes requires the RsbX protein to prevent SigB-activation under non-stressed conditions. J Bacteriol 204:e00486-21. 10.1128/JB.00486-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wurtzel O, Sesto N, Mellin JR, Karunker I, Edelheit S, Bécavin C, Archambaud C, Cossart P, Sorek R. 2012. Comparative transcriptomics of pathogenic and non‐pathogenic Listeria species. Mol Syst Biol 8:583. 10.1038/msb.2012.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Curtis T, Takeuchi I, Gram L, Knudsen G. 2017. The influence of the toxin/antitoxin mazEF on growth and survival of Listeria monocytogenes under stress. Toxins 9:31. 10.3390/toxins9010031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.O'Donoghue B, NicAogáin K, Bennett C, Conneely A, Tiensuu T, Johansson J, O'Byrne C. 2016. Blue-light inhibition of Listeria monocytogenes growth is mediated by reactive oxygen species and is influenced by σB and the blue-light sensor Lmo0799. Appl Environ Microbiol 82:4017–4027. 10.1128/AEM.00685-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gahan CGM, Hill C. 2005. Gastrointestinal phase of Listeria monocytogenes infection. J Appl Microbiol 98:1345–1353. 10.1111/j.1365-2672.2005.02559.x. [DOI] [PubMed] [Google Scholar]
- 37.O’Byrne CP, Karatzas KAG. 2008. The role of sigma B (σB) in the stress adaptations of Listeria monocytogenes: overlaps between stress adaptation and virulence. Adv Appl Microbiol 65:115–140. 10.1016/S0065-2164(08)00605-9. [DOI] [PubMed] [Google Scholar]
- 38.Garner MR, Njaa BL, Wiedmann M, Boor KJ. 2006. Sigma B contributes to Listeria monocytogenes gastrointestinal infection but not to systemic spread in the guinea pig infection model. Infect Immun 74:876–886. 10.1128/IAI.74.2.876-886.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Toledo-Arana A, Dussurget O, Nikitas G, Sesto N, Guet-Revillet H, Balestrino D, Loh E, Gripenland J, Tiensuu T, Vaitkevicius K, Barthelemy M, Vergassola M, Nahori M-A, Soubigou G, Régnault B, Coppée J-Y, Lecuit M, Johansson J, Cossart P. 2009. The Listeria transcriptional landscape from saprophytism to virulence. Nature 459:950–956. 10.1038/nature08080. [DOI] [PubMed] [Google Scholar]
- 40.Liao J, Wiedmann M, Kovac J. 2017. Genetic stability and evolution of the sigB allele, used for Listeria sensu stricto subtyping and phylogenetic inference. Appl Environ Microbiol 83:e00306-17. 10.1128/AEM.00306-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Moorhead SM, Dykes GA. 2003. The role of the sigB gene in the general stress response of Listeria monocytogenes varies between a strain of serotype 1/2a and a strain of serotype 4c. Curr Microbiol 46:461–466. 10.1007/s00284-002-3867-6. [DOI] [PubMed] [Google Scholar]
- 42.Oliver HF, Orsi RH, Wiedmann M, Boor KJ. 2010. Listeria monocytogenes σB has a small core regulon and a conserved role in virulence but makes differential contributions to stress tolerance across a diverse collection of strains. Appl Environ Microbiol 76:4216–4232. 10.1128/AEM.00031-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Abram F, Starr E, Karatzas KAG, Matlawska-Wasowska K, Boyd A, Wiedmann M, Boor KJ, Connally D, O'Byrne CP. 2008. Identification of components of the sigma B regulon in Listeria monocytogenes that contribute to acid and salt tolerance. Appl Environ Microbiol 74:6848–6858. 10.1128/AEM.00442-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Asakura H, Kawamoto K, Okada Y, Kasuga F, Makino S, Yamamoto S, Igimi S. 2012. Intrahost passage alters SigB-dependent acid resistance and host cell-associated kinetics of Listeria monocytogenes. Infect Genet Evol 12:94–101. 10.1016/j.meegid.2011.10.014. [DOI] [PubMed] [Google Scholar]
- 45.Quereda JJ, Pucciarelli MG, Botello-Morte L, Calvo E, Carvalho F, Bouchier C, Vieira A, Mariscotti JF, Chakraborty T, Cossart P, Hain T, Cabanes D, García-del Portillo F. 2013. Occurrence of mutations impairing sigma factor B (SigB) function upon inactivation of Listeria monocytogenes genes encoding surface proteins. Microbiology (Reading) 159:1328–1339. 10.1099/mic.0.067744-0. [DOI] [PubMed] [Google Scholar]
- 46.Guerreiro DN, Wu J, Dessaux C, Oliveira AH, Tiensuu T, Gudynaite D, Marinho CM, Boyd A, García-del Portillo F, Johansson J, O’Byrne CP. 2020. Mild stress conditions during laboratory culture promote the proliferation of mutations that negatively affect sigma B activity in Listeria monocytogenes. J Bacteriol 202:e00751-19. 10.1128/JB.00751-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Leong D, NicAogáin K, Luque-Sastre L, McManamon O, Hunt K, Alvarez-Ordóñez A, Scollard J, Schmalenberger A, Fanning S, O'Byrne C, Jordan K. 2017. A 3-year multi-food study of the presence and persistence of Listeria monocytogenes in 54 small food businesses in Ireland. Int J Food Microbiol 249:18–26. 10.1016/j.ijfoodmicro.2017.02.015. [DOI] [PubMed] [Google Scholar]
- 48.Hilliard A, Leong D, O’Callaghan A, Culligan E, Morgan C, DeLappe N, Hill C, Jordan K, Cormican M, Gahan C. 2018. Genomic characterization of Listeria monocytogenes isolates associated with clinical listeriosis and the food production environment in Ireland. Genes 9:171. 10.3390/genes9030171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Henri C, Leekitcharoenphon P, Carleton HA, Radomski N, Kaas RS, Mariet J-F, Felten A, Aarestrup FM, Gerner Smidt P, Roussel S, Guillier L, Mistou M-Y, Hendriksen RS. 2017. An assessment of different genomic approaches for inferring phylogeny of Listeria monocytogenes. Front Microbiol 8:2351. 10.3389/fmicb.2017.02351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.den Bakker HC, Desjardins CA, Griggs AD, Peters JE, Zeng Q, Young SK, Kodira CD, Yandava C, Hepburn TA, Haas BJ, Birren BW, Wiedmann M. 2013. Evolutionary cynamics of the accessory genome of Listeria monocytogenes. PLoS One 8:e67511. 10.1371/journal.pone.0067511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kuenne C, Billion A, Mraheil MA, Strittmatter A, Daniel R, Goesmann A, Barbuddhe S, Hain T, Chakraborty T. 2013. Reassessment of the Listeria monocytogenes pan-genome reveals dynamic integration hotspots and mobile genetic elements as major components of the accessory genome. BMC Genomics 14:47. 10.1186/1471-2164-14-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pirone-Davies C, Chen Y, Pightling A, Ryan G, Wang Y, Yao K, Hoffmann M, Allard MW. 2018. Genes significantly associated with lineage II food isolates of Listeria monocytogenes. BMC Genomics 19:708. 10.1186/s12864-018-5074-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Maury MM, Chenal-Francisque V, Bracq-Dieye H, Han L, Leclercq A, Vales G, Moura A, Gouin E, Scortti M, Disson O, Vázquez-Boland JA, Lecuit M. 2017. Spontaneous loss of virulence in natural populations of Listeria monocytogenes. Infect Immun 85:e00541-17. 10.1128/IAI.00541-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Li X-P, Wang S-F, Hou P-B, Liu J, Du P, Bai L, Fanning S, Zhang H-N, Chen Y-Z, Zhang Y-K, Kang D-M. 2020. Nosocomial cross-infection of hypervirulent Listeria monocytogenes sequence type 87 in China. Ann Transl Med 8:603–603. 10.21037/atm-19-2743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Myintzaw P, Pennone V, McAuliffe O, Begley M, Callanan M. 2022. Correlation of organic acid tolerance and genotypic characteristics of Listeria monocytogenes food and clinical isolates. Food Microbiol 104:104004. 10.1016/j.fm.2022.104004. [DOI] [PubMed] [Google Scholar]
- 56.Ferreira A, Sue D, O'Byrne CP, Boor KJ. 2003. Role of Listeria monocytogenes σB in survival of lethal acidic conditions and in the acquired acid tolerance response. Appl Environ Microbiol 69:2692–2698. 10.1128/AEM.69.5.2692-2698.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Utratna M, Cosgrave E, Baustian C, Ceredig RH, O'Byrne CP. 2014. Effects of growth phase and temperature on σB activity within a Listeria monocytogenes population: evidence for RsbV-independent activation of σB at refrigeration temperatures. Biomed Res Int 2014:641647. 10.1155/2014/641647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Utratna M, Shaw I, Starr E, O'Byrne CP. 2011. Rapid, transient, and proportional activation of σB in response to osmotic stress in Listeria monocytogenes. Appl Environ Microbiol 77:7841–7845. 10.1128/AEM.05732-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Guerreiro DN, Pucciarelli MG, Tiensuu T, Gudynaite D, Boyd A, Johansson J, García-del Portillo F, O'Byrne CP. 2021. Acid stress signals are integrated into the σB-dependent general stress response pathway via the stressosome in the food-borne pathogen Listeria monocytogenes. PLoS Pathog 18(3):e1010213. 10.1371/journal.ppat.1010213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Neuhaus K, Satorhelyi P, Schauer K, Scherer S, Fuchs TM. 2013. Acid shock of Listeria monocytogenes at low environmental temperatures induces prfA, epithelial cell invasion, and lethality towards Caenorhabditis elegans. BMC Genomics 14:285. 10.1186/1471-2164-14-285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Palma F, Brauge T, Radomski N, Mallet L, Felten A, Mistou M-Y, Brisabois A, Guillier L, Midelet-Bourdin G. 2020. Dynamics of mobile genetic elements of Listeria monocytogenes persisting in ready-to-eat seafood processing plants in France. BMC Genomics 21:130. 10.1186/s12864-020-6544-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Orsi RH, Ripoll DR, Yeung M, Nightingale KK, Wiedmann M. 2007. Recombination and positive selection contribute to evolution of Listeria monocytogenes inlA. Microbiology (Reading) 153:2666–2678. 10.1099/mic.0.2007/007310-0. [DOI] [PubMed] [Google Scholar]
- 63.Cotter PD, Draper LA, Lawton EM, Daly KM, Groeger DS, Casey PG, Ross RP, Hill C. 2008. Listeriolysin S, a novel peptide haemolysin associated with a subset of lineage I Listeria monocytogenes. PLoS Pathog 4:e1000144. 10.1371/journal.ppat.1000144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Manuel CS, Van Stelten A, Wiedmann M, Nightingale KK, Orsi RH. 2015. Prevalence and distribution of Listeria monocytogenes inlA alleles prone to phase variation and inlA alleles with premature stop codon mutations among human, food, animal, and environmental isolates. Appl Environ Microbiol 81:8339–8345. 10.1128/AEM.02752-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yin Y, Yao H, Doijad S, Kong S, Shen Y, Cai X, Tan W, Wang Y, Feng Y, Ling Z, Wang G, Hu Y, Lian K, Sun X, Liu Y, Wang C, Jiao K, Liu G, Song R, Chen X, Pan Z, Loessner MJ, Chakraborty T, Jiao X. 2019. A hybrid sub-lineage of Listeria monocytogenes comprising hypervirulent isolates. Nat Commun 10:4283. 10.1038/s41467-019-12072-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.NicAogáin K, O'Byrne CP. 2016. The role of stress and stress adaptations in determining the fate of the bacterial pathogen Listeria monocytogenes in the food chain. Front Microbiol 7:1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wemekamp-Kamphuis HH, Wouters JA, Sleator RD, Gahan CGM, Hill C, Abee T. 2002. Multiple deletions of the osmolyte transporters BetL, Gbu, and OpuC of Listeria monocytogenes affect virulence and growth at high osmolarity. Appl Environ Microbiol 68:4710–4716. 10.1128/AEM.68.10.4710-4716.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Pathak D, Jin KS, Tandukar S, Kim JH, Kwon E, Kim DY. 2020. Structural insights into the regulation of SigB activity by RsbV and RsbW. IUCrJ 7:737–747. 10.1107/S2052252520007617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Teh A-H, Makino M, Hoshino T, Baba S, Shimizu N, Yamamoto M, Kumasaka T. 2015. Structure of the RsbX phosphatase involved in the general stress response of Bacillus subtilis. Acta Crystallogr D Biol Crystallogr 71:1392–1399. 10.1107/S1399004715007166. [DOI] [PubMed] [Google Scholar]
- 70.Dessaux C, Pucciarelli MG, Guerreiro DN, O’Byrne CP, García-del Portillo F. 2021. Activation of the Listeria monocytogenes stressosome in the intracellular eukaryotic environment. Appl Environ Microbiol 87:e00397-21. 10.1128/AEM.00397-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Oliver HF, Orsi RH, Wiedmann M, Boor KJ. 2013. σB plays a limited role in the ability of Listeria monocytogenes strain F2365 to survive oxidative and acid stress and in its virulence characteristics. J Food Prot 76:2079–2086. 10.4315/0362-028X.JFP-12-542. [DOI] [PubMed] [Google Scholar]
- 72.Bergis H, Bonanno L, Asséré A, Lombard B. 2021. EURL Lm TECHNICAL GUIDANCE DOCUMENT on challenge tests and durability studies for assessing shelf-life of ready-to-eat foods related to Listeria monocytogenes. https://ec.europa.eu/food/system/files/2021-07/biosafety_fh_mc_tech-guide-doc_listeria-in-rte-foods_en_0.pdf.
- 73.Guillier L, Lardeux A-L, Michelon D, Ng P. 2013. Development of a set of Listeria monocytogenes strains for conducting challenge tests. French Agency for Food, Environmental and Occupational Health Safety, Maisons-Alfort, France. [Google Scholar]
- 74.Eymann C, Schulz S, Gronau K, Becher D, Hecker M, Price CW. 2011. In vivo phosphorylation patterns of key stressosome proteins define a second feedback loop that limits activation of Bacillus subtilis σB: new feedback loop limiting activation of B. subtilis σB. Mol Microbiol 80:798–810. 10.1111/j.1365-2958.2011.07609.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kazmierczak MJ, Mithoe SC, Boor KJ, Wiedmann M. 2003. Listeria monocytogenes σB regulates stress response and virulence functions. J Bacteriol 185:5722–5734. 10.1128/JB.185.19.5722-5734.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Muchaamba F, Eshwar AK, von Ah U, Stevens MJA, Tasara T. 2020. Evolution of Listeria monocytogenes during a persistent human prosthetic hip joint infection. Front Microbiol 11:1726. 10.3389/fmicb.2020.01726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.La Rosa R, Rossi E, Feist AM, Johansen HK, Molin S. 2021. Compensatory evolution of Pseudomonas aeruginosa’s slow growth phenotype suggests mechanisms of adaptation in cystic fibrosis. Nat Commun 12:3186. 10.1038/s41467-021-23451-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Camargo AC, Moura A, Avillan J, Herman N, McFarland AP, Sreevatsan S, Call DR, Woodward JJ, Lecuit M, Nero LA. 2019. Whole‐genome sequencing reveals Listeria monocytogenes diversity and allows identification of long‐term persistent strains in Brazil. Environ Microbiol 21:4478–4487. 10.1111/1462-2920.14726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Marinho CM, Dos Santos PT, Kallipolitis BH, Johansson J, Ignatov D, Guerreiro DN, Piveteau P, O'Byrne CP. 2019. The σB-dependent regulatory sRNA Rli47 represses isoleucine biosynthesis in Listeria monocytogenes through a direct interaction with the ilvA transcript. RNA Biol 16:1424–1437. 10.1080/15476286.2019.1632776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Abram F, Arcari T, Guerreiro D, O'Byrne CP. 2021. Evolutionary trade-offs between growth and survival: the delicate balance between reproductive success and longevity in bacteria. Adv Microb Physiol 79:133–162. 10.1016/bs.ampbs.2021.07.002. [DOI] [PubMed] [Google Scholar]
- 81.Utratna M, Cosgrave E, Baustian C, Ceredig R, O'Byrne C. 2012. Development and optimization of an EGFP-based reporter for measuring the general stress response in Listeria monocytogenes. Bioeng Bugs 3:93–103. 10.4161/bbug.19476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Monk IR, Gahan CGM, Hill C. 2008. Tools for functional postgenomic analysis of Listeria monocytogenes. Appl Environ Microbiol 74:3921–3934. 10.1128/AEM.00314-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Souvorov A, Agarwala R, Lipman DJ. 2018. SKESA: strategic k-mer extension for scrupulous assemblies. Genome Biol 19:153. 10.1186/s13059-018-1540-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Danecek P, Bonfield JK, Liddle J, Marshall J, Ohan V, Pollard MO, Whitwham A, Keane T, McCarthy SA, Davies RM, Li H. 2021. Twelve years of SAMtools and BCFtools. GigaScience 10:giab008. 10.1093/gigascience/giab008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Jolley KA, Maiden MC. 2010. BIGSdb: scalable analysis of bacterial genome variation at the population level. BMC Bioinformatics 11:595. 10.1186/1471-2105-11-595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Doumith M, Buchrieser C, Glaser P, Jacquet C, Martin P. 2004. Differentiation of the major Listeria monocytogenes serovars by multiplex PCR. J Clin Microbiol 42:3819–3822. 10.1128/JCM.42.8.3819-3822.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Seemann T. 2014. Prokka: rapid prokaryotic genome annotation. Bioinformatics 30:2068–2069. 10.1093/bioinformatics/btu153. [DOI] [PubMed] [Google Scholar]
- 88.Page AJ, Cummins CA, Hunt M, Wong VK, Reuter S, Holden MTG, Fookes M, Falush D, Keane JA, Parkhill J. 2015. Roary: rapid large-scale prokaryote pan genome analysis. Bioinformatics 31:3691–3693. 10.1093/bioinformatics/btv421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Pfaffl MW. 2001. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res 29:45e–445. 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Simon P. 2003. Q-Gene: processing quantitative real-time RT-PCR data. Bioinformatics 19:1439–1440. 10.1093/bioinformatics/btg157. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 and Fig. S1 to S6. Download aem.00051-22-s0001.pdf, PDF file, 2.0 MB (2.1MB, pdf)
Data Availability Statement
All genome assemblies were deposited in NCBI under BioProject accession numbers PRJNA808240, PRJNA788387, and PRJNA796187.