Abstract
This study investigated behavioral indicators of social fear in preschool boys with fragile X syndrome (FXS) with a low degree of autism spectrum disorder (ASD) symptoms (FXS-Low; n = 29), FXS with elevated ASD symptoms (FXS-High; n = 25), idiopathic ASD (iASD; n = 11), and typical development (TD; n = 36). Gaze avoidance, escape behaviors, and facial fear during a stranger approach were coded. Boys with elevated ASD symptoms displayed more avoidant gaze, looking less at the stranger and parent than those with low ASD symptoms across etiologies. The iASD group displayed more facial fear than the other groups. Results suggest etiologically distinct behavioral patterns of social fear in preschoolers with elevated ASD symptoms.
Keywords: Fragile X Syndrome, Autism Spectrum Disorder, Stranger Fear, Anxiety, Temperament
Fragile X Syndrome (FXS) and Idiopathic Autism Spectrum Disorder (iASD)
Fragile X syndrome (FXS) is a severe neurodevelopmental disorder caused by a CGG trinucleotide repeat expansion mutation on the Fragile X Mental Retardation-1 (FMR1) gene on the X chromosome. Fragile X syndrome has an estimated prevalence rate of approximately 1 in 3,700 males and 1 in 6,000 females (Crawford, Acuña, & Sherman, 2001; Hagerman, 2008). Males with FXS typically present with moderate to severe intellectual disability and exhibit distinct cognitive profiles and elevated rates of maladaptive behaviors (Bailey, Raspa, Olmsted, & Holiday, 2008; Cornish, Cole, Longhi, Karmiloff-Smith, & Scerif, 2013; Hall, Lightbody, & Reiss, 2008; Wheeler et al., 2014).
Autism spectrum disorder (ASD) is a neurodevelopmental disorder characterized by deficits in social communication and a pattern of repetitive interests and/or behaviors (American Psychiatric Association [APA], 2013). ASD is quite prevalent, impacting 1 in 68 children (Center for Disease Control and Prevention, 2014), with boys being five times more likely to be diagnosed than girls (Baio, 2014). Although the precise causal mechanisms underlying ASD are unknown, there is clear evidence that both genetic and epigenetic influences and environmental mechanisms interact to confer increased risk (Geschwind, 2011; Gurrieri & Neri, 2009). This etiological complexity challenges efforts to understand and treat the underlying neurobiological mechanisms that cause the core behavioral symptoms of ASD.
Interestingly, 10% of ASD cases are known to be caused by single gene disorders (Abrahams & Geschwind, 2008; Cohen et al 2005), which provide a unique and informative context within which to study the underlying mechanisms and observable symptoms of ASD. Fragile X syndrome is the most common monogenic cause of ASD (Crawford et al., 2002; Hagerman, 2008), accounting for 2–6% of ASD cases (Bailey, Phillips, & Rutter, 1996; Cohen et al., 2005; Dykens & Volkmar, 1997). There is a great deal of symptom overlap between FXS and ASD, and approximately 25–50% of individuals with FXS also meet DSM diagnostic criteria for ASD (Budimirovic et al 2006, Harris et al., 2008; Kaufmann et al., 2004, Rogers et al., 2001), suggesting that shared molecular-genetic and neurobiological mechanisms may contribute to qualitatively similar behavioral characteristics in these two disorders. Because the presence of ASD symptoms in FXS is associated with more severe impairment and poorer functioning, research aimed at delineating the precise nature of ASD symptomatology in FXS is critical (Bailey et al., 2001; Kaufmann et al., 2004; Rogers et al., 2001). This work is challenging, however, as both FXS and idiopathic ASD (iASD; ASD with an unidentified cause) are compounded by multiple comorbidities including intellectual disability, ADHD, and anxiety (Yu & Berry-Kravis, 2014). These comorbid disorders make attempts to elucidate common and unique symptoms in FXS and iASD more difficult. Disentangling the core symptoms of both disorders from their shared and unique comorbidities, particularly early in development, is therefore of critical importance to facilitate early identification and treatment. Cross-syndrome research studies have proven to be an innovative and valuable approach to addressing these important questions (Sacrey, Bennett, & Zwaigenbaum, 2015). The present study is a cross-syndrome comparison of early behavioral risk markers for anxiety in FXS and iASD, aimed at identifying early risk markers that might be shared or distinct between the two disorders, and to determine whether ASD symptomatology in either disorder is associated with early anxiety risk markers.
Anxiety in FXS and iASD
Of the many psychiatric comorbidities that are common to FXS and iASD, anxiety is considered one of the most prevalent and debilitating (Cordeiro et al., 2011; Muris, Steerneman, Merckelbach, Holdrinet, & Meesters, 1998). In FXS, approximately 86% of males with FXS meet diagnostic criteria for one or more anxiety disorders (Cordeiro, Ballinger, Hagerman, & Hessl, 2010) and 70% receive treatment for anxiety symptoms (Bailey et al., 2008). As many as 84% of males with iASD under the age of 18 years also meet diagnostic criteria for at least one anxiety disorder (Muris et al., 1998). Anxiety is associated with poorer academic and social-emotional functioning in both typically-developing and atypical populations, particularly later in development (Cassady & Johnson, 2002; La Greca & Lopez, 1998; Lopata & Thomeer, 2014). Thus, understanding the early manifestations of anxiety in FXS and iASD can improve risk classification and enable early intervention, allowing for the optimization of social-emotional, academic, and adaptive outcomes.
In both FXS and iASD, social anxiety is one of the more frequently diagnosed anxiety disorders (Cordeiro et al., 2011). Social anxiety is characterized by anxiety surrounding contexts that involve interpersonal encounters or social situations, such as speaking or eating in public and expressing thoughts in front of others (APA, 2013; Stein & Stein, 2008). Estimates of the prevalence of social anxiety throughout the lifetime range from 5–12% in the general population (Grant et al., 2005; Ruscio et al., 2008; Stein & Stein, 2008) and 6–8% of youth (Chavira, Stein, Bailey, & Stein, 2004; Stein & Stein, 2008). Individuals with FXS often display many symptoms of social anxiety, including shyness, avoidance of social situations, gaze aversion, difficulty understanding social cues, fearfulness, poor socio-emotional processing, and poor social skills during interpersonal interactions (Cordeiro et al., 2011; Tonnsen, Malone, Hatton, & Roberts, 2013; Williams, Porter, & Langdon, 2014). Cordeiro et al. (2011) reported that 58% of males and females with FXS displayed clinical symptoms of social anxiety. It is estimated that 17% of children with iASD under the age of 18 meet diagnostic criteria for social anxiety disorder (van Steensel, Bögels, & Perrin, 2011). A positive relationship between symptoms of iASD and social anxiety with overall greater social deficits has been documented (Cath, Ran, Smit, van Balkom, & Comijs, 2007).
Though there appears to be a high prevalence of social anxiety in FXS and iASD, challenges arise in identifying anxiety in disorders in which intellectual disability and social-communicative deficits are inherent. For example, many individuals with FXS and iASD have cognitive impairments that reduce their ability to self-report or have insight into the symptoms they are experiencing. Thus, many research studies of children with FXS and iASD have relied primarily on parent report of child anxiety symptoms (Cordeiro et al., 2010; Lopata et al., 2010; Strang et al., 2012; White et al., 2009). Though parent insight is very valuable, there are certain weaknesses associated with this approach. For example, parents may have a limited awareness of the symptoms of anxiety that their children with FXS or iASD are experiencing, particularly if their child is unable to explain their symptoms verbally. Along these lines, Lesniak-Karpiak et al. (2003) found that parents did not indicate elevated levels of social anxiety despite reporting higher levels of social difficulty in females with FXS. These results suggest that parents may be aware of social impairments that their children with FXS or iASD are displaying; however, they may not attribute the difficulties to anxiety.
Another significant challenge in characterizing social anxiety in FXS and iASD is the fact that both FXS and iASD are inherently defined by social-communicative deficits that are also often observed in individuals with social anxiety without FXS or iASD. This phenotypic overlap can make it difficult to dissociate symptoms of social anxiety from symptoms of FXS and iASD. In iASD, social impairments are often categorized under the diagnostic umbrella of iASD rather than that of social anxiety, and this process may not adequately capture all the symptoms present in an individual (White et al., 2009). For example, individuals with ASD may avoid social situations because of a lack of social reciprocity or reduced desire to engage in social interactions with others, which is a core diagnostic symptom associated with ASD (APA, 2013; Kreiser & White, 2014). However, in cases where an individual with ASD has comorbid social anxiety, they may avoid social contexts because of symptoms of anxiety directly related to the interpersonal situations or the fear of being rejected or humiliated in some way. Although the behavior is similar (i.e., social avoidance), the mechanisms may differ for how and why social impairments are manifested. Additionally, the relationship between symptoms of social anxiety and FXS or iASD is likely bidirectional, in that FXS and iASD are characterized by a lack of insight or awareness in social contexts. This lack of awareness may result in social impairments, which may in turn cause or exacerbate social anxiety and lead to increased avoidance of social interactions, thus enhancing the effects of each individual disorder in an additive or multiplicative manner.
In addition to the measurement and characterization challenges, developmental considerations also complicate research into social anxiety in FXS and iASD. Social anxiety disorder is typically diagnosed in late childhood and adolescence in community samples, with an average age of onset at 8 years of age (Beesdo et al., 2007; Simonoff et al., 2008; Vasa et al., 2013). Despite later diagnosis of social anxiety, symptoms often emerge in early childhood in typical samples and in children with iASD as well. For example, Davis et al. (2011) found that anxiety tends to rise from toddlerhood to childhood, decrease from childhood to young adulthood, and then rise again from young adulthood to older adulthood in individuals with iASD. These developmental trends suggest that there may be specific risk factors for experiencing increased anxiety during toddler years, and the emergence of these factors may be expressed differently depending on the developmental and cognitive level of the child. Specifically, preschool-aged children may have limited abilities to understand and communicate their symptoms and emotions, and thus may display more behavioral symptoms associated with anxiety. Cervantes et al. (2013) demonstrated that toddlers with iASD who displayed more severe anxiety had higher rates of challenging behaviors including increased aggression, stereotypies, and self-injurious behaviors than children with minimal anxiety. Overall, however, there is a paucity of prior work focused on how social anxiety symptoms first present in young children with iASD (Cervantes et al., 2013; Davis et al., 2011; Vasa et al., 2013).
A temperament framework is a valuable approach to study how anxiety disorders emerge and develop over time through the characterization of risk and resiliency factors of later psychopathology (Durbin et al., 2005; Lonigan & Phillips, 2001; Rapee & Coplan, 2010). Temperament has been defined as “individual differences in reactivity and self-regulation” (p. 123) in response to varying environmental contexts (Rothbart, Ahadi, & Evans, 2000). Temperament is biologically based, with traits emerging early in infancy and remaining relatively stable across time highlighting the value of temperament models as particularly instrumental in studies of emerging psychopathology (Clark & Watson, 1999; Durbin, Klein, Hayden, Buckley, & Moerk, 2005; Rothbart, Derryberry, & Hershey, 2000). Three factors of temperament have been identified in early development including characteristics of extraversion/surgency, negative affectivity, and effortful control (Rothbart et al., 2001). Of particular importance, temperament characteristics of negative affectivity (e.g., discomfort, fear, anger/frustration, sadness) have been found to be associated with the later development of anxiety in both atypical and typical populations (Clark, Watson, & Mineka, 1994; Kapula & Garstein, 2016; Lonigan, Philips, & Hooe, 2003; Roberts, Tonnsen, Robinson, McQuillin, & Hatton, 2013)
Given the unique biological and behavioral phenotype associated with FXS, characteristics of temperament have been studied in early childhood in FXS to help distinguish factors of temperament related to learning and behavioral outcomes (Kapula & Garstein, 2016; Roberts et al., 2014). Boys with FXS display greater activity and shyness and less soothability, inhibitory control, and attentional focusing which suggests a distinct temperament profile related to the development of psychopathology (Kapula & Garstein, 2016). Tonnsen and colleagues (2013) studied negative affect and reported that elevated behavioral fear and social shyness in children with FXS may indicate risk for anxiety (Tonnsen, Shinkareva, Deal, Hatton, & Roberts, 2013). Additionally, increased traits of fear and shyness in FXS are associated with poor social approach and withdrawal, which may contribute to increased symptoms of ASD in this population (Roberts et al., 2014). However, it remains unclear whether children with FXS display emergent anxiety symptoms in similar ways as neurotypical children or children with iASD, given a lack of previous cross-syndrome comparisons.
Temperament research in typically developing children has established that fearfulness in response to novel people (i.e., strangers) or novel situations early in life is a reliable index of increased behavioral inhibition, or withdrawal and avoidance, a known risk factor for anxiety (Colennesi, Napoleone, & Bögels, 2014; Rapee, 2014). For example, Brooker and colleagues (2013) examined stranger fear at 6 and 12 months and determined that children with high, stable levels of stranger fear in infancy were more likely to exhibit increased behavioral inhibition at 36 months. Given that behavioral inhibition is tied to anxiety, particularly social anxiety, by adolescence (Rapee, 2014), stranger fear is thought to be an early risk marker for anxiety. Thus, studying stranger fear in young children with FXS and iASD, and examining the relationship between ASD symptoms and stranger fear, can provide critical insight into the early risk markers of social anxiety in these disorders. Such information will enable early identification of anxiety risk factors in both typical and atypical populations, allowing for earlier intervention and improved social and academic outcomes.
The Present Study
The overarching objective of this study is to characterize early behavioral markers of risk for social anxiety in FXS and iASD by utilizing a cross-syndrome temperament framework. Observable fear in response to a stranger is posited to be a robust early risk marker for social anxiety in typically developing children. Thus, the present study examined multiple fear behaviors including facial fear, escape behaviors, and gaze avoidance, during a stranger approach in four groups of preschool-aged boys; a) FXS with low ASD symptoms (FXS-Low); b) FXS with high ASD symptoms (FXS-High); c) idiopathic ASD (iASD); and d) typical development (TD). Based on previous evidence of elevated social anxiety associated with ASD symptomatology, it was hypothesized that early risk markers of social anxiety (i.e., stranger fear) are common in young children with FXS and iASD. Specifically, we predicted that children with higher ASD symptoms would display more behavioral markers of social fear during a stranger approach, with the FXS-High and iASD groups exhibiting the most behavioral fear, followed by the FXS-Low group with the typically-developing control group displaying the least amount of fear.
The secondary objective of the study is to investigate the relationship between ASD symptomology and stranger fear in children with high ASD symptoms (i.e., boys with iASD and FXS-High). We hypothesized that higher ASD symptomatology predisposes young children to elevated levels of social anxiety. Therefore, we anticipated that ASD symptoms would be a significant predictor of stranger fear in those children with elevated ASD symptomatology.
Method
Participants
Participants included 107 male preschoolers between two and five years of age distributed among the following four groups: FXS-Low (n = 31), FXS-High (n = 26), iASD (n = 12), and TD (n = 38). See Table 1 for participant characteristics. Data were collected from participants across two associated studies examining temperament and early development in FXS. The first study is a completed project from the University of North Carolina at Chapel Hill (UNC; PI: Bailey) that focused on preschool-aged males with FXS, accounting for 28 boys with FXS-Low, 17 boys with FXS-High, and 30 TD boys (total n = 75). The second study is an ongoing longitudinal project at the University of South Carolina (USC; PI: Roberts) focused on infant and preschool development in children with FXS. The USC study adds a total of 26 participants that included one boy with FXS-Low, eight boys with FXS-High, six TD boys, and 12 boys with iASD. Both sites had measures of observed stranger fear (Laboratory Temperament Assessment Battery [Lab-TAB]; Goldsmith & Rothbart, 1996), cognitive development (Mullen Scales of Early Learning [MSEL]; Mullen, 1995), and ASD symptoms (Childhood Autism Rating Scale [CARS]; Schopler, Reichler, & Renner, 1988). Data collection and coding procedures were identical across sites; however, only the USC site had ADOS-2 data for a subset of participants given different study aims. Site effects were examined for participant characteristics, and within groups, developmental level and ASD symptomology did not differ within groups across sites (ps > .05). However, given the different age groups targeted by the two studies, chronological age differed between the sites. Typically developing children and children with FXS-High from the UNC study were older than children from the USC study: TD (UNC: M = 3.69, SD = 0.58; USC: M = 2.02, SD = 0.05; t(31) = 15.36, p < .01), FXS-High (UNC: M = 4.89, SD = 0.72; USC: M = 2.12, SD = 0.11; t(18) = 15.42, p < .01).
Table 1.
Participant Characteristics
| TD M (SD) |
FXS-Low M (SD) |
FXS-High M (SD) |
iASD M (SD) |
Post-Hoc Group Differences | |
|---|---|---|---|---|---|
|
|
|||||
| Chronological Age (years) | 3.42 (0.83) | 5.17 (0.89) | 4.00 (1.44) | 4.42 (1.22) | FXS-Low, iASD > TD; FXS-Low > FXS-High |
| CARS Total Score | 15.42 (0.88) | 24.64 (3.13) | 35.14 (3.35) | 35.05 (6.93) | FXS-Low, FXS-High, iASD > TD; FXS-High, iASD > FXS-Low |
| MSEL ELC | 108.22 (15.11) | 51.25 (2.95) | 49.68 (2.61) | 52.91 (5.49) | TD > FXS-Low, FXS-High, iASD; FXS-Low > FXS-High |
| MSEL Nonverbal Composite | 51.21 (9.20) | 20.93 (2.39) | 20.26 (1.30) | 27.77 (13.64) | TD > FXS-Low, FXS-High, iASD |
| CBCL Anxiety Raw Score | 2.26 (1.91) | 3.96 (2.90) | 3.90 (2.51) | 3.56 (2.70) | FXS-Low, FXS-High > TD |
| CBCL Anxiety T-score | 52.00 (3.06) | 55.41 (7.30) | 55.29 (5.95) | 54.89 (5.64) | FXS-Low, FXS-High > TD |
Notes. Values in the same row with different subscripts differ, ps < .05. MSEL Nonverbal Composite = average of t-scores on the Fine Motor and Visual Reception subscales on the Mullen Scales of Early Learning. CARS = Childhood Autism Rating Scale. MSEL ELC = Mullen Sales of Early Learning Early Learning Composite. CBCL = Child Behavior Checklist.
Participants were recruited through a national registry for research, support groups, or advertising through community centers near the universities. Participants were included in our study if English was the primary language spoken in their home and they currently lived with their biological mother. Participants in the TD group were required to perform within the average range on the MSEL. Exclusionary criteria included prematurity (<37 weeks gestation) or any known genetic or neurological condition (e.g., tuberous sclerosis, seizure disorder) that may affect development. When multiple assessments were available for the same participant, the earliest timepoint was used due to the present study’s focus on early development.
Participants with FXS (with and without ASD symptoms) had completed previous genetic testing to confirm the presence of the full mutation. The CARS (Schopler et al., 1988) was used to determine subgroups of children with FXS based on high and low ASD features. The Autism Diagnostic Observation Schedule-2 (ADOS-2; Lord et al., 2012) was used to validate the CARS scores in a subsample with available ADOS-2 data. Based on the CARS, 25 boys with FXS (46% of the sample) had a total score of 30 or greater, exceeding the cutoff for ASD symptoms. This subgroup of boys with FXS was categorized as the FXS-High group. The FXS-Low group included those remaining 29 boys with a CARS score of less than 30.
Children included in the iASD group were required to have met standard diagnostic criteria for ASD through a diagnostic evaluation by a qualified community professional (e.g., licensed psychologist; n = 10) or to exceed diagnostic cutoff on the ADOS-2 (n =1), as well as receive a total score greater than 30 on the CARS as part of this study (n =12). Previous community diagnosis was confirmed through review of evaluation reports provided by the parents. Participants with ASD were screened via parent report for personal and family history of genetic syndromes related to ASD (e.g., FXS, tuberous sclerosis).
Procedures
Informed consent was obtained from the parents of all participants included in the study. Individual assessments were conducted either in the participants’ homes or at the university’s research laboratory based on the age, preference, and location of the families. Since a larger battery of behavioral assessments tested other aspects of temperament and development, behavioral tasks during each assessment were administered using a standard order at similar times of day to control for reactivity and carry-over effects. All assessments were conducted by trained post-baccalaureate research assistants, graduate students, or Ph.D.-level examiners.
Measures
Observed stranger fear.
The Stranger Approach epoch from the Laboratory Temperament Assessment Battery (Lab-TAB; Goldsmith & Rothbart, 1996) was used to elicit behavioral indicators of stranger fear from the participants. The Stranger Approach was embedded in a larger series of standardized epochs from the Lab-TAB and followed a non-demanding, engaging task that measured the participants’ attention. Prior to the start of the task, participants were in an affectively neutral state and seated on a caregiver’s lap or seated beside their caregiver on a chair. Per standardized procedures, the “stranger” was a female examiner that wore black sunglasses, a baseball hat, an oversized gray sweatshirt, and a long black skirt. The Stranger Approach epoch has three distinct phases: 1) the approach phase, in which the stranger appeared in the room then slowly walked toward the child; 2) the kneel phase, during which the stranger kneeled for approximately two minutes in front of the child with a neutral affect; and 3) the recovery phase, in which the stranger slowly exits the room. In all, the Stranger Approach epoch lasts approximately 2.5 minutes.
The Stranger Approach epoch was videotaped and the behavioral variables of gaze, escape, and facial fear were later coded from video, using Noldus The Observer XT (version 10.0 software, Noldus Information Technology, Leesburg, VA, USA). For gaze, the amount of time spent looking at the stranger, at the parent, at the second examiner, and away from anyone in the room was coded. Escape behaviors and facial fear were coded for both time exhibited and intensity (e.g., no behavior = 0, highest level of behavior = 3). Escape behaviors reflected head turns and whole body movements (e.g., twisting away). Per Lab-TAB procedures and previous work on social fear (Brooker et al., 2013), facial fear was coded using the Maximally Discriminative Facial Movement Coding System (Izard, 1979), which defines three specific facial regions (eyebrows/forehead, eyes, and mouth) and the intensity of movement associated with fear to the stranger. All variables were coded only if the behavior was readily visible (e.g., facial fear was marked as obscured if more than 50% of the facial region was not visible). Detailed descriptions of how the behavioral variables were coded are provided in Tables 2 and 3.
Table 2.
Facial fear definitions by facial region
| Facial region | Movement |
|---|---|
| Forehead/brow | Entire brow should be raised and drawn together; brows may also look straighter across than usual; faint horizontal furrows may be present in forehead |
| Eyes/nose/cheek | Upper eyelids raise making the eyes appear wider; eye have tense appearance |
| Mouth/lips/chin | Lip corners are drawn straight back; mouth is usually less than wide open |
Notes. Coding schemes were used from the LocoMotor Version 3.1 Laboratory Temperament Assessment Battery (Goldsmith and Rothbart 1996)
Table 3.
Behavioral descriptions for facial fear, escape behavior, and gaze codes
| Behavior | Level | Behavioral description |
|---|---|---|
| Facial fear | 0 | No facial region shows fear |
| 1 | One facial region shows fear/low intensity fear | |
| 2 | Two facial regions show fear or one region shows very distinct facial fear | |
| 3 | Change occurs in all three facial regions; impression of strong facial fear | |
| Escape behavior | 0 | No escape behavior or social referencing |
| 1 | Mild or fleeting escape behavior (e.g. turning away, sinking into chair) | |
| 2 | Moderate escape behavior resulting in significant, but not extreme attempts to get away or resist; Full body movements such as arching back, twisting away, and leaning away are included, as well as hitting, pushing, and slapping | |
| 3 | Vigorous escape behavior, usually involving linked, intense full-body movements like those found in “2” | |
| Gaze | Looking at parent: must be looking at the parent at or above shoulders | |
| Looking away: gaze not directed at the parent, stranger, or examiner | ||
| Looking at stranger: must be looking at the stranger at or above shoulders |
Notes. Coding schemes for facial fear were used from the LocoMotor Version 3.1 Laboratory Temperament Assessment Battery (Goldsmith and Rothbart 1996)
Coding was completed by trained research assistants that established reliability with a master coder across all variables. Reliability standards required that the research assistant obtain 80% agreement with the master coder on three consecutive videos. Ongoing reliability was determined for 20% of videos coded using Cohen’s kappa coefficient (Berry & Mielke, 1988). Reliability was deemed to be excellent for gaze (.83), escape behaviors (.83) and facial fear (.89).
Composite intensity scores were calculated for escape behaviors and facial fear, by multiplying the proportion of time spent exhibiting behaviors of each intensity level by the intensity level, then dividing that number by the total observation time (Gagne et al., 2011). Gaze was not analyzed on an intensity scale but as a percentage of time calculated as the total duration of a focus of gaze (e.g. the stranger) divided by the total observation time.
Because the dependent variables used in statistical analyses were based on either a proportion of time or a composite score derived from durations of time, cases that had a total duration for any behavior that fell outside two standard deviations of the mean (e.g., because of obscured gaze) were removed from analyses to control for variation in the stranger task. A total of six cases (FXS-Low: n = 2, FXS-High: n = 1, iASD: n = 1, and TD: n = 2) were removed from analyses for this reason, resulting in a final sample of 101 participants.
To confirm that behavioral ratings of stranger fear were linked to anxiety symptoms, the Child Behavior Checklist (CBCL/1.5–5; Achenbach & Rescorla, 2001) was used to measure parent-reported anxiety symptoms. The CBCL is often utilized to assess internalizing symptoms, such as anxiety, in preschool-aged children. Pearson correlations were employed to investigate the relationship between Stranger Fear variables and CBCL Anxiety raw score. In the TD group, higher escape behavior composite score was associated with higher CBCL Anxiety raw score, r = .38, p < .05. In the FXS-High group, higher proportion of time spent looking at the parent was associated with higher CBCL Anxiety raw score, r = .60, p < .01. These correlations provide evidence that observable stranger fear is associated with parent-reported anxiety and support the investigation of stranger fear as a precursor to the development of social anxiety.
ASD symptomatology.
The Childhood Autism Rating Scale (CARS; Schopler et al., 1988) is a well-established measure often used as part of the ASD diagnostic process (Rellini, Tortolani, Trillo, Carbone, & Montecchi, 2004). The CARS measures the severity of 15 ASD-related symptoms and behaviors. Scores for each item are rated on 4-point Likert scale ranging from 1 (within normal limits) to 4 (severely abnormal). The total score is calculated by summing the item scores. Total scores of less than 30 are indicative of minimal to no symptoms of ASD, scores of 30–36.5 represent mild to moderate symptoms and severe symptoms of ASD, and scores over 36.5 indicate severe ASD symptomatology. The CARS measures ASD symptom severity in children as young as two years of age. The CARS does not consider the developmental level of the child into the total score; however, despite this limitation, it is considered to be a reliable and valid measure of ASD symptom severity (Falkmer, Andersaon, Falkmer, & Horlin, 2013). The CARS has an internal consistency of .94 and a test-retest stability of a .88 (Schopler et al., 1988).
In the present study, the Autism Diagnostic Observation Schedule, Second Edition (ADOS-2; Lord et al.,2012), a gold standard observational diagnostic measure, was administered to a subset of participants from the USC cohort (n = 20), including 5 children with FXS-Low, 7 children with FXS-High, 1 child with iASD, and 7 TD controls. The ADOS-2 total raw score and the CARS total score had high agreement within these participants, with a correlation of r = .90. Also, results from an independent samples t-test suggest no significant group differences on CARS scores between the FXS-High and the iASD groups; t(33) = 4.52, p = .96.
Cognitive ability.
The Mullen Scales of Early Learning (MSEL; Mullen, 1995) is a developmental measure used to assess cognitive abilities in young children. The MSEL measures abilities in five domains (Gross Motor, Visual Reception, Fine Motor, Expressive Language, and Receptive Language) and calculates an Early Learning Composite (ELC; M = 100, SD = 15) score based on all domains excluding the Gross Motor domain. A nonverbal composite score was calculated by averages the t-scores for the Visual Reception and Fine Motor subscales to obtain a measure of nonverbal cognitive ability. The atypical groups (e.g. FXS-Low, FXS-High, iASD) did not differ significantly on the nonverbal composite score (see Table 1). The MSEL has high test-retest reliability (.70- .80), median split-half internal consistency ranges for each of the scales (0.75- .83), and inter-rater reliabilities (.91-.96) (Mullen, 1995).
Statistical Analysis Plan
One-way analyses of variance (ANOVAs) were conducted to examine group differences in stranger fear variables, with group entered as the between-subjects fixed factor. Scheffe post-hoc comparisons were employed to examine pairwise group differences for significant models. Multiple regression models were employed to examine the relationship between symptoms of ASD (i.e., CARS scores) on each measure of stranger fear (e.g. gaze behavior, escape behavior, and facial fear) in the FXS-High and iASD groups. After centering the CARS scores and computing the group-by-CARS interaction term, the two predictors (CARS score; group) and the interaction term were entered into a sequential regression model. The two main effects of group and CARS scores were entered into the first model, and the interaction term was added in the second model (Keith, 2014).
Results
Preliminary Analyses
Preliminary analyses revealed that percentage of time gazing at parent and the facial fear composite score were not normally distributed and therefore log and square root transformations were performed, respectively. Levene’s test was employed to test the assumption of homogeneity of variance, and results indicated that the assumption of homogeneity of variance was satisfied for all dependent variables (ps > .05). We also examined the relationship of cognitive ability (MSEL), chronological age and anxiety on the behavioral fear variables both for all groups combined then individually within each group. In the FXS-High group, we found relationships between higher cognitive ability and increased gaze to parent (r = .52, p < .01); higher cognitive ability and more escape behaviors (r = .46, p < .05); elevated scores on the DSM anxiety problem scale of the CBCL and increased gaze to parent (r = .60, p < .01); and older chronological age associated with reduced facial fear (r = −.72, p < .001). In the FXS-Low group, higher cognitive ability was correlated with more gaze to stranger (r = .42, p < .05) and less gaze away (r = −.45, p < .05). Also in the FXS-O group, chronological age was associated with gaze to stranger (r = −.41, p < .05) and facial fear (r = .38, p < 05). In the TD group, results indicated that chronological age was associated with gaze to stranger (r = .43, p < .05), gaze away (r = −.68, p < .001), gaze to parent (r = .57, p = .001), and facial fear (r = −.55, p < .01). Additionally, in the TD group, higher scores on the DSM anxiety problem scale and the anxiety/depression scale of the CBCL were correlated with more escape behaviors (rs > .38, ps < .05). No correlations were significant in the iASD group (rs < +/− .50, ps > .18).
Group Differences in Observed Stranger Fear
Gaze behavior.
Analyses of Variance (ANOVA) were used to examine group differences in gaze behavior. Results revealed group differences for the proportion of time spent looking at the stranger, F(3, 95) = 5.32, p < .01, η2 = .15. Scheffe post-hoc comparisons indicated that the TD group spent a significantly higher proportion of time gazing at the stranger than the FXS-High and iASD groups, ps < .05 (see Figure 1). The FXS-Low, FXS-High, and iASD groups did not differ in the proportion of time spent gazing at the stranger, ps > .05. Table 4 depicts the group means for all stranger fear variables.
Fig 1.
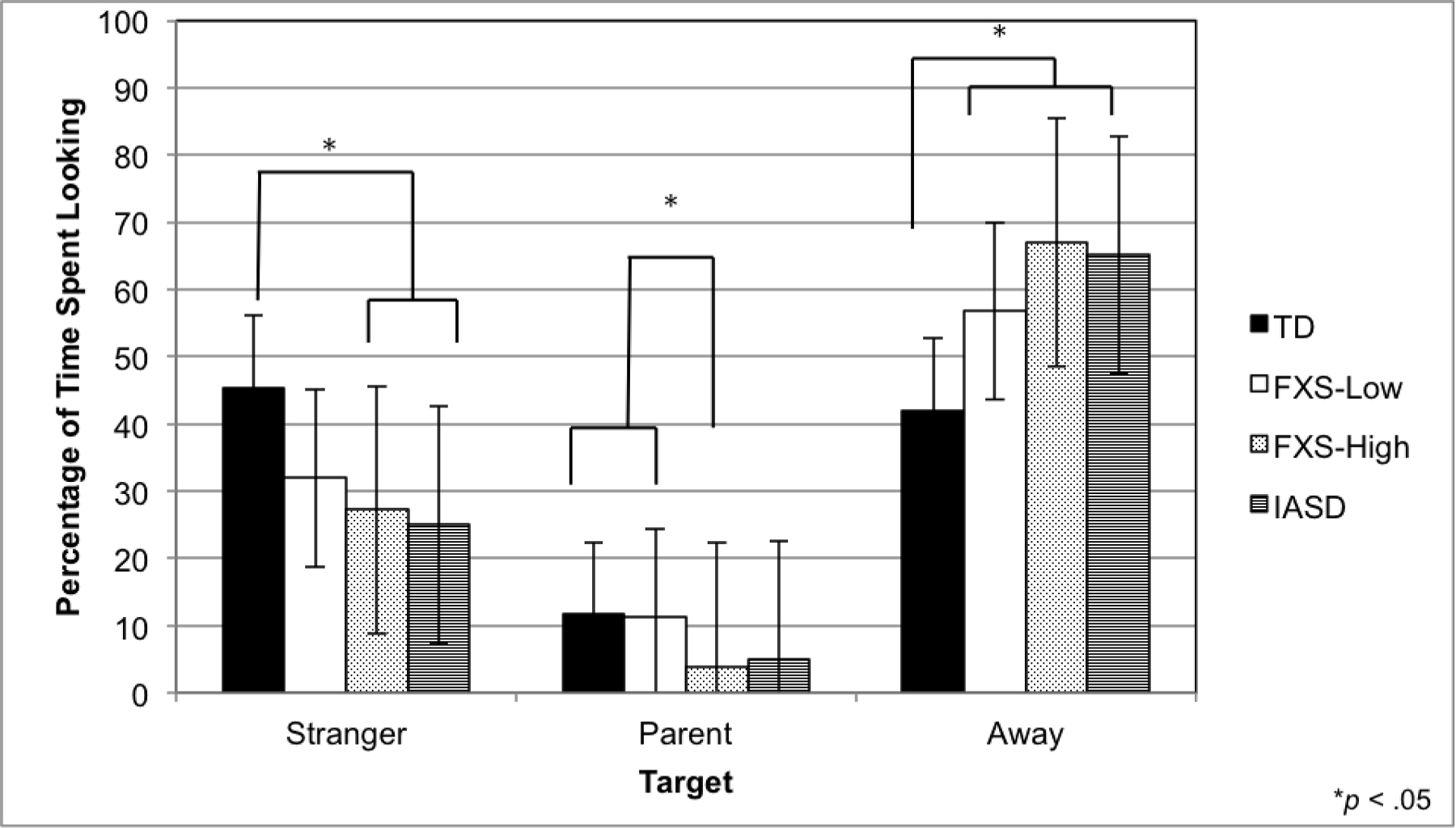
Gaze behavior during social approach. Mean proportion of time spent looking at the stranger, at the parent, and away from the stranger and the parent, are depicted. Error bars indicate standard deviations. Groups with different superscripts are significantly different (p < .05).
Table 4.
Group means for stranger fear variables
| Variable | TD M (SD) |
FXS-Low M (SD) |
FXS-High M (SD) |
iASD M (SD) |
Post-Hoc Group Differences |
|---|---|---|---|---|---|
| Gaze | |||||
| Stranger | 45.40 (19.74) | 31.94 (23.58) | 27.22 (18.29) | 24.96 (13.11) | TD > FXS-High, iASD |
| Away | 41.98 (17.95) | 56.83 (23.41) | 66.99 (19.27) | 65.10 (12.98) | FXS-Low, FXS-High, iASD > TD |
| Parent | 0.82 (0.54) | 0.87 (0.46) | 0.43 (0.45) | 0.61 (0.42) | TD, FXS-Low > FXS-High |
| Escape behavior | 1.22 (0.43) | 1.00 (0.57) | 0.99 (0.54) | 0.97 (0.46) | – |
| Facial fear | 0.32 (0.30) | 0.16 (0.24) | 0.33 (0.43) | 0.68 (0.32) | iASD > TD, FXS-Low, FXS-High |
Notes. Means for gaze behaviors are reported in percentage of time. Means for percentage of time gazing at parent were log transformed. Means for facial fear were square root transformed.
Groups also differed on the percentage of time looking away from the stranger and parent, F(3, 95) = 8.94, p < .001, η2 = .23. Specifically, the TD group spent a smaller proportion of time looking away from people than the FXS-Low, FXS-High, and iASD, groups. The FXS-Low, FXS-High and iASD groups did not differ in the proportion of time gazing away, ps > .05.
The proportion of time spent gazing at parent also differed between groups, F(3, 95) = 4.37, p < .01, η2 = .13. The FXS-High group spent significantly less time looking at their parent than the FXS-Low and the TD groups (see Figure 1). The FXS-High and iASD groups did not differ in the proportion of time spent gazing at the parent, p > .05.
Escape behavior.
An ANOVA was performed to examine group differences in the escape behavior composite score. No significant group differences were observed for escape behaviors, F(3, 100) = 1.56, p > .05 (Figure 2).
Fig 2.
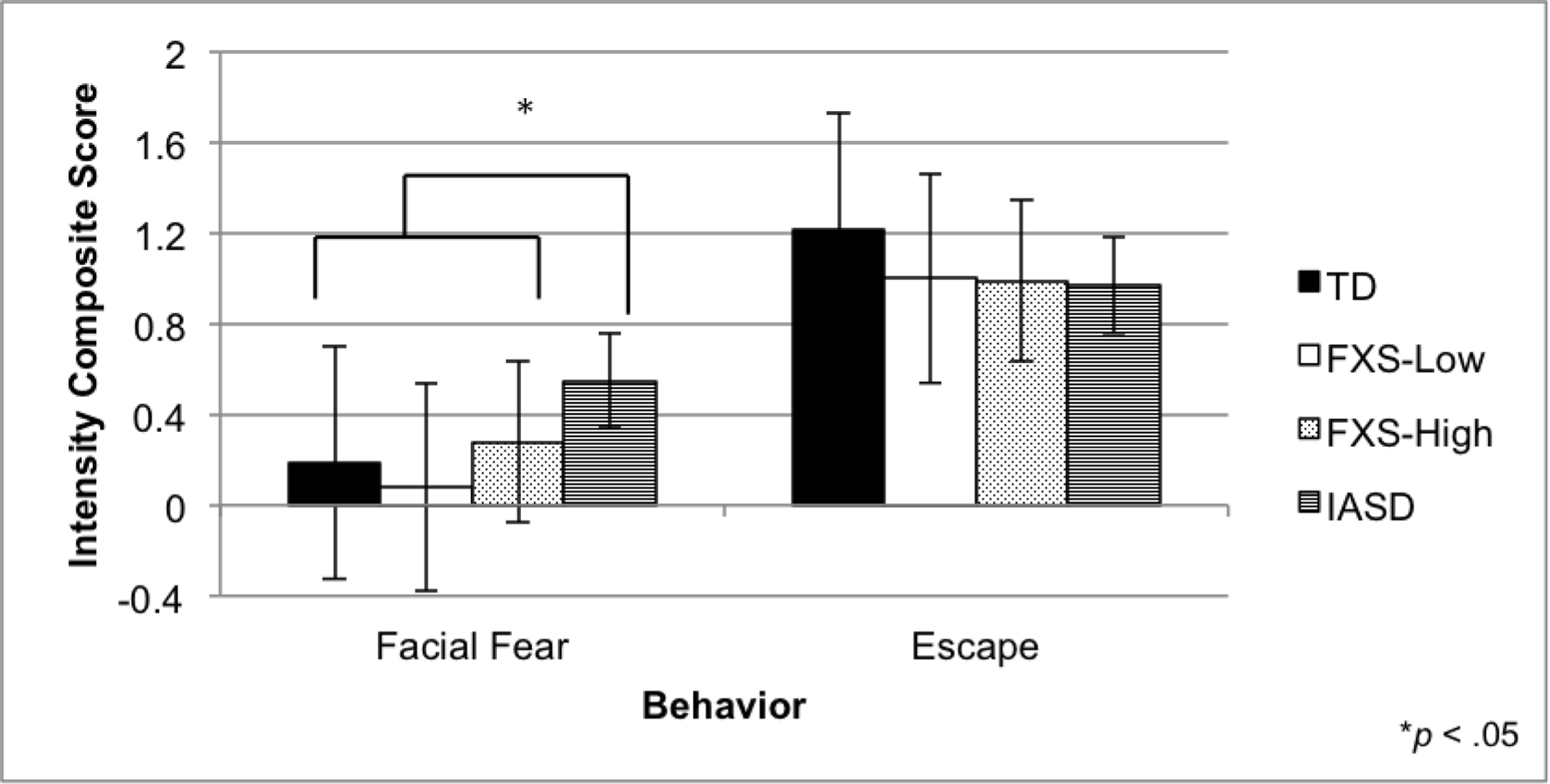
Escape behavior and social fear during social approach. Mean intensity composite score and standard deviations are depicted. Groups with different superscripts are significantly different (p < .05).
Facial fear.
The ANOVA examining the facial fear composite score revealed significant group differences, F(3, 94) = 6.60, p < .001, η2 = .18. Specifically, the iASD group displayed more facial fear than the TD, FXS-High, and FXS-Low groups, ps < .05 (Figure 2). The FXS-Low and FXS-High did not differ on the facial fear composite score.
ASD Symptomatology as a Predictor of Stranger Fear
Gaze behavior.
Results indicated no significant group effect on gaze behaviors of looking at the stranger, looking away, and looking at the parent, suggesting that the FXS-High and the iASD groups are similar. There was not a significant main effect of the combined groups CARS scores and gaze behaviors of looking at the stranger, away, and at the parent. The interactions between group and ASD symptoms for all gaze behaviors were not statistically significant. Table 5 lists results of regression models for gaze behaviors.
Table 5.
Summary of regression analysis for predictors of gaze behavior
| Variable | Strangera | Awayb | Parentc |
|---|---|---|---|
| β | β | β | |
| Model 1 | |||
| Group | 0.08 | 0.06 | −0.22 |
| CARS | −0.20 | 0.15 | −0.03 |
| Model 2 | |||
| Group | 0.08 | 0.06 | −0.22 |
| CARS | −0.18 | 0.08 | 0.17 |
| Group × CARS | −0.02 | 0.12 | −0.33 |
Notes.
R 2 for Model 1 = 0.04, R 2 Δ = 0.00, Total R 2 = 0.04
R 2 for Model 1 = 0.03, R 2 Δ = 0.01, Total R 2 = 0.04
R 2 for Model 1 = 0.05, R 2 Δ = 0.07, Total R 2 = 0.12
Escape behavior.
Results indicated that there were no main effects of group or CARS scores on behavioral measures of escape behaviors during the Stranger Approach. Additionally, the interaction between group and CARS scores for escape behaviors was not statistically significant. Table 6 lists these results.
Table 6.
Summary of hierarchical regression analysis for predictors of escape behavior and facial fear
| Variable | Escape Behaviora | Facial Fearb |
|---|---|---|
| β | β | |
| Model 1 | ||
| Group | −0.05 | −0.35* |
| CARS | 0.11 | 0.30 |
| Model 2 | ||
| Group | −0.05 | −0.35* |
| CARS | 0.11 | 0.18 |
| Group × CARS | 0.01 | 0.20 |
Notes.
R 2 for Model 1 = 0.02, R 2 Δ = 0.00, Total R 2 = 0.02
R 2 for Model 1 = 0.21, R 2 Δ = 0.02, Total R 2 = 0.23
p < 0.05
Facial fear.
Results indicated that there were statistically significant differences between the FXS-High and the iASD group on the facial fear composite score (b = −.320, SEb = .145, β = −.351, p =.036). The iASD group (M = .678, SD = .318) displayed significantly more facial fear than the FXS-High group (M = .328, SD = .430). Although the CARS score did not explain facial fear as a main effect, there was a positive trend observed (b = .017 SEb = .019, β = .182, p =.066), suggesting that higher CARS are associated with more facial fear during the Stranger Approach across both groups. The interaction between group and ASD symptoms for facial fear was not statistically significant (see Table 6).
Discussion
Despite the high prevalence and impact of anxiety in FXS and iASD, little is understood about how anxiety features emerge and develop in either disorder. Moreover, no study has examined behavioral fear as it relates to ASD traits in children with FXS or compared patterns of behavioral fear in FXS to those in iASD. The bulk of work examining anxiety in children with iASD has focused on high-functioning older children and adolescents, with no published studies focused on preschool-aged children with iASD and comorbid intellectual disability. Because stranger fear is known to be related to social anxiety later in life in otherwise typically developing children (Brooker et al., 2013; Kagan & Snidman, 1999), it may serve as a promising early risk marker for social anxiety in clinical populations as well. The early detection of specific anxiety risk markers is important given that differential diagnosis and targeted treatments can have a substantive positive impact on later anxiety (Rapee et al., 2002).
Behavioral Indicators of Stranger Fear across Atypical and Normative Development
In this study, we examined group differences across multiple behavioral indicators of stranger fear, as well as the relationship between stranger fear and ASD symptom severity using a cross-syndrome approach. Our results suggest that children across all three disorder groups showed a similar pattern of reduced gaze to the stranger and elevated gaze away from social targets that distinguished them from typical controls. However, reduced gaze to the parent appears unique to the groups with elevated ASD traits as the FXS-High group displayed less gaze to their parent than both FXS-Low and TD groups, and the FXS-High and iASD did not differ in the proportion of time gazing at the parent. These results suggest that gaze avoidance to novel social partners and preference to gaze at non-social stimuli may represent a general response associated with developmental delay or elevated social anxiety whereas reduced gaze to parents is unique to those with elevated ASD symptomology and may indicate aloofness rather than anxiety. This interpretation is partially supported by the strong correlation of reduced gaze to parents to lower anxiety in the FXS-High group.
Hypervigilance to both feared and preferred stimuli has been studied across both healthy and anxious children. A two-stage model of attention bias in anxiety suggests that anxious children initially orient their attention towards threat but soon direct their attention away from fearful stimuli or engage in avoidant behaviors (Mogg, Bradley, de Bono, & Painter, 1997). This model is consistent with our findings of reduced gaze to the stranger in our atypical groups, and suggests that avoidant behaviors (e.g., looking away) may be related to attention bias away from threat (e.g., the stranger). Attention bias towards threat may also be developmentally normative in younger children as some studies have found that typically developing children display a greater bias towards threat, which they overcome over time (Kindt et al., 2003). Shechner and colleagues (2012) also highlight the role of attention bias to rewards, which provides support for our findings of increased gaze to parents in the FXS-Low and TD groups as a mechanism not only to avoid threat, but also to gain comfort and reassurance from a caregiver in response to a novel stranger.
These findings expand upon previous work that indicates gaze patterns of children with FXS are highly specific to the presence of ASD features. In our earlier work, we reported avoidant gaze behavior in a naturalistic social approach paradigm lasting 3 to 4 hours, with school-aged children with FXS-Low and FXS-High both displaying poor eye contact during the initial introduction to a novel person (Roberts et al., 2007). However, individuals with FXS-Low improved their eye contact over the course of the assessment period, whereas individuals with FXS-High displayed avoidant eye contact across the entire assessment. The current study extends these findings by suggesting that gaze behaviors are distinct between FXS-Low and FXS-High groups in a preschool-aged sample and during a rather brief social fear experiment. Our current findings of gaze differences in iASD also correspond with previous studies in which preschoolers with iASD looked less frequently towards a stranger, referenced their mother less often, and spent greater amounts of time avoiding social initiation by looking away than TD children during a social approach task (Pisula, 2004).
Distinguishing Behavioral Traits of Stranger Fear in FXS-High and iASD
When facial fear and escape behaviors were examined, only children with iASD differed from the control group on facial fear, and no group differences were observed for escape behaviors. These findings suggest that while gaze indices of fear may be effective behavioral risk markers in FXS-High and iASD, atypical facial fear may not manifest in children with FXS, and escape behaviors may not help discriminate stranger fear in any of the developmentally delayed children. These findings contradict a previous study by Hall et al. (2006), which reported that males with FXS are more prone to display problem behaviors in social situations that include the escape behaviors of face-hiding, fidgeting, refusal, eye-rubbing, leaving the chair, and hand biting. However, important methodological differences exist between these two studies, with the Hall et al. (2006) study focusing on older adolescents and using different paradigms to elicit social anxiety (e.g. a face-to-face interview, a singing task, a silent reading task, and an oral reading task). The difference in ages between the two studies is of particular importance, and suggests that social fear exhibited through facial fear and escape behaviors may not be present in younger children with FXS or that there may be more behavioral variability in these younger children as symptoms of anxiety or fear are beginning to emerge. Providing further support that some fear behaviors may be particularly salient at specific points in development, our findings also differ from previous evidence that during infancy, children with FXS display elevated facial fear in response to a stranger (Tonnsen et al., 2013). Thus, stranger fear may be developmentally sensitive and reflect variable behavioral trajectories of escape behaviors and facial fear over time that is dependent on age.
Taken together, these results suggest that although individuals with FXS-Low and FXS-High presumably share similar genetic profiles with FMR1 gene dysfunction, ASD symptoms may predispose a subset of children with FXS to atypical responses to novel social stimuli. Our results and others contribute to an emerging profile of initial gaze avoidance that may be universal in males with FXS independent of the presence of ASD features while persistent gaze avoidance, including less gaze toward a parent, is unique to those with elevated ASD features. This implies that anxiety may be mechanistically associated with the initial gaze avoidance in those without ASD who are aware of but leery of novel social interactions. In contrast, males with FXS and elevated ASD symptoms may display pervasive and persisting gaze avoidance due to the presence of ASD features. This information contributes to the ongoing debate regarding the shared and unique associations of ASD in FXS versus non-syndromic ASD, as these two groups were identical in their gaze profile regardless of ASD etiology. They were, however, distinct in facial fear with iASD displaying elevated facial fear compared to those with FXS-High. These patterns are consistent with literature that has documented individuals with iASD have trouble recognizing and regulating their own facial expressions due to dysfunction of the amygdala (Baron-Cohen et al., 2000).
Future Directions
To our knowledge, no study to date has investigated early social fear in young children with FXS and iASD using behavioral observations. Therefore, this study contributes to the literature by providing an increased understanding to how anxiety emerges in FXS (with and without ASD) in comparison to normative development and to iASD using behavioral indicators of fear in response to a stranger approach. Some limitations should be considered, however, and we consider this study as an important “first step” in this line of work with future studies able to more definitely address some of the complexities we have identified. First, the cross-sectional nature of the study precludes any conclusions to be drawn about the dynamic development of social fear over time. Longitudinal studies would allow for trends to be seen over time, providing critical insight into how social fear emerges and changes across development and how cognitive level and age might impact the results. Second, the participants included in our sample were only male, which may limit generalization to females with FXS. Because females with FXS are often less impacted than males with FXS, behavioral symptoms of social anxiety may be expressed differently in males versus females (Bennetto et al., 2001). The interplay of how sex influences the emergence of stranger fear in FXS should be investigated to better understand the mechanisms of anxiety in FXS. Finally, our sample sizes, particularly for the iASD group, were small precluding confidence in analyses of multiple predictors.
Additionally, although there was high agreement between the CARS and ADOS-2 in the present study, we were limited in using CARS data to categorize ASD symptomatology in our groups. Clinical best estimate diagnosis using the ADOS-2 is considered the gold-standard for diagnosing iASD, and differs from the CARS in providing specific behavioral presses to measure ASD symptomology rather than relying on naturalistic observation during a period of time (Lord et al., 2000). The ADOS-2 takes the child’s developmental level and language ability into consideration to guide what module or behavioral presses to deliver. The CARS does not factor a child’s developmental level into the total score and is a limitation particularly when comparing children with developmental delay to typically developing children. Despite these limitations in using the CARS, we report a very strong correlation (.90) between the CARS and the ADOS-2 for the subset who had both these measures. Also, a further limitation of this study was the discrepancy in age and developmental level of the participants in the atypical and typical groups. Although these differences were accounted for in the analyses, consideration of developmental differences between chronological age and cognitive ability across the groups should be examined in future studies. Finally, given the genetic and physiological etiologies involved in the phenotypes of iASD and FXS, a biobehavioral model to study anxiety using biomarkers, such as cortisol or heart activity, and behavioral outcomes might be informative. The use of biomarkers provides information in children and individuals with lower cognitive ability, and thus impaired ability to self-report, by reflecting various states of arousal.
Conclusions and Implications
Employing a cross-syndrome approach, we report etiologically distinct patterns of stranger fear and ASD symptom severity in preschool boys with FXS contrasted to boys with iASD and typical controls. We employed a direct observation of stranger fear to counter limitations inherent in parent reports and self-reports of anxiety in young children with intellectual impairments. This approach revealed a nuanced set of results that highlight the complexities of this work. For example, the lack of group differences in escape behavior suggests that this variable may not be a sensitive behavioral indicator of anxiety in young children. Also, the relationship between the CBCL and the behavioral stranger fear indices suggests selective associations that vary by index and group with no consistent relationships emerging. This suggests that the CBCL and behavioral stranger fear indices are fundamentally measuring different constructs. These results are not surprising given that the CBCL is a parent report of broad indicators of anxiety, including social and non-social fear, while the stranger fear indices are highly specific to interactions with a novel social partner. However, the lack of correspondence between these two anxiety measures reflects challenges to measurement of complex traits, like anxiety, in young children. Likewise, the inconsistent relationship of age and developmental level to the stranger fear variables across groups highlights the importance and challenge to including individual differences in valid measurement of anxiety in clinical populations. Findings like these highlight the challenges and potential pitfalls of pursuit of a “gold standard” measure of anxiety against which to validate other measures. For example, is the CBCL or the stranger fear paradigm the more “valid” measure of anxiety in our study? We would argue that each measure has validity within its intended application and both are important to capture the different facets of anxiety. However, this creates a measurement conundrum that underscores the importance of characterizing anxiety through implementation of multiple dimensions rather than a blunt assessment of anxiety captured through a single index. Thus, this study serves as a model of the complexities and challenges inherent in developmental disorders research, with findings reported as preliminary and tentative until replicated and extended by future work.
Our findings that children with FXS spent less time looking at a novel stranger compared to typically developing children during an initial social approach contributes to our understanding of behavioral features of social anxiety in FXS. Roberts et al. (2007) demonstrated a difference in gaze patterns over time that distinguished children with FXS with high and low ASD symptomology suggesting that eye contact in children with FXS and low ASD symptomology was poor initially but improved over an assessment session. Collectively, these results have implications for clinical practice and may help aid in the differential diagnosis of social anxiety and ASD in FXS. For example, children with FXS may be “slow to warm up” and may need time to adapt to new novel contexts and people more so than most children with neurodevelopmental disorders. In this case, social behaviors, including eye contact, would improve over time as the child became comfortable with his or her new setting and would distinguish traits of aloofness and lack of reciprocity observed in ASD. Future studies should consider studying multiple observations or periods over time that measure temporal aspects social fear. Therefore, studying observation periods by examining initial and end states may reflect different patterns of behavior responses to stressful or fearful conditions in children with FXS compared to children with ASD or that are typically developing and further inform clinical assessment and treatment practice.
Acknowledgments:
The authors are thankful to the families who participated in this research. This project was funded by the National Institute of Mental Health (R01MH107573; R01MH0901194; PI: Roberts) and the National Institute of Child Health and Human Development (P30-HD003110–35S1; PI: Bailey).
Footnotes
Statement of Human Rights:
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
References
- Abrahams BS, & Geschwind DH (2008). Advances in autism genetics: on the threshold of a new neurobiology. Nature Reviews Genetics, 9(5), 341–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Achenbach TM, & Rescorla LA (2001). Manual for the ASEBA School-Age Forms & Profiles Burlington, VT: University of Vermont, Research Center for Children, Youth, & Families. [Google Scholar]
- American Psychiatric Association. (2013). DSM 5 American Psychiatric Association. [Google Scholar]
- Bailey DB Jr, Hatton DD, Skinner M, & Mesibov G (2001). Autistic behavior, FMR1 protein, and developmental trajectories in young males with fragile X syndrome. Journal of autism and developmental disorders, 31(2), 165–174. [DOI] [PubMed] [Google Scholar]
- Bailey A, Phillips W, & Rutter M (1996). Autism: towards an integration of clinical, genetic, neuropsychological, and neurobiological perspectives. Journal of Child Psychology and Psychiatry, 37(1), 89–126. [DOI] [PubMed] [Google Scholar]
- Bailey DB, Raspa M, Olmsted M, & Holiday DB (2008). Co‐occurring conditions associated with FMR1 gene variations: Findings from a national parent survey. American journal of medical genetics part A, 146(16), 2060–2069. [DOI] [PubMed] [Google Scholar]
- Baio J (2012). Prevalence of Autism Spectrum Disorders: Autism and Developmental Disabilities Monitoring Network, 14 Sites, United States, 2008. Morbidity and Mortality Weekly Report. Surveillance Summaries Volume 61, Number 3. Centers for Disease Control and Prevention. [PubMed] [Google Scholar]
- Baron-Cohen S, Ring HA, Bullmore ET, Wheelwright S, Ashwin C, & Williams SCR (2000). The amygdala theory of autism. Neuroscience & Biobehavioral Reviews, 24(3), 355–364. [DOI] [PubMed] [Google Scholar]
- Beesdo K, Bittner A, Pine DS, Stein MB, Höfler M, Lieb R, & Wittchen HU (2007). Incidence of social anxiety disorder and the consistent risk for secondary depression in the first three decades of life. Archives of general psychiatry, 64(8), 903–912. [DOI] [PubMed] [Google Scholar]
- Berry KJ, & Mielke PW (1988). A generalization of Cohen’s kappa agreement measure to interval measurement and multiple raters. Educational and Psychological Measurement, 48(4), 921–933. [Google Scholar]
- Bellini S (2004). Social skill deficits and anxiety in high-functioning adolescents with autism spectrum disorders. Focus on Autism and Other Developmental Disabilities, 19(2), 78–86. [Google Scholar]
- Bennetto L, Taylor AK, Pennington BF, Porter D, & Hagerman RJ (2001). Profile of cognitive functioning in women with the fragile X mutation. Neuropsychology, 15(2), 290. [PubMed] [Google Scholar]
- Brooker RJ, Buss KA, Lemery‐Chalfant K, Aksan N, Davidson RJ, & Goldsmith HH (2013). The development of stranger fear in infancy and toddlerhood: Normative development, individual differences, antecedents, and outcomes. Developmental science, 16(6), 864–878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budimirovic DB, Bukelis I, Cox C, Gray RM, Tierney E, & Kaufmann WE (2006). Autism spectrum disorder in Fragile X syndrome: differential contribution of adaptive socialization and social withdrawal. American journal of medical genetics Part A, 140(17), 1814–1826. [DOI] [PubMed] [Google Scholar]
- Cassady JC, & Johnson RE (2002). Cognitive test anxiety and academic performance. Contemporary educational psychology, 27(2), 270–295. [Google Scholar]
- Cath DC, Ran N, Smit JH, van Balkom AJ, & Comijs HC (2007). Symptom overlap between autism spectrum disorder, generalized social anxiety disorder and obsessive-compulsive disorder in adults: A preliminary case-controlled study. Psychopathology, 41(2), 101–110. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. (2014). Prevalence of autism spectrum disorders among children aged 8 years: Autism and developmental disabilities monitoring network. MMWR Surveillance Summaries, 63(2), 1–22. [PubMed] [Google Scholar]
- Cervantes P, Matson JL, Tureck K, & Adams HL (2013). The relationship of comorbid anxiety symptom severity and challenging behaviors in infants and toddlers with autism spectrum disorder. Research in Autism Spectrum Disorders, 7(12), 1528–1534. [Google Scholar]
- Chavira DA, Stein MB, Bailey K, & Stein MT (2004). Child anxiety in primary care: Prevalent but untreated. Depression and anxiety, 20(4), 155–164. [DOI] [PubMed] [Google Scholar]
- Clark LA, & Watson D (1999). Temperament: A new paradigm for trait psychology. Handbook of personality: Theory and research, 2, 399–423. [Google Scholar]
- Clark LA, Watson D, & Mineka S (1994). Temperament, personality, and the mood and anxiety disorders. Journal of abnormal psychology,103(1), 103. [PubMed] [Google Scholar]
- Cohen D, Pichard N, Tordjman S, Baumann C, Burglen L, Excoffier E, ... & Héron D (2005). Specific genetic disorders and autism: clinical contribution towards their identification. Journal of autism and developmental disorders, 35(1), 103–116. [DOI] [PubMed] [Google Scholar]
- Colonnesi C, Napoleone E, & Bögels SM (2014). Positive and negative expressions of shyness in toddlers: are they related to anxiety in the same way. Journal of personality and social psychology, 106(4), 624. [DOI] [PubMed] [Google Scholar]
- Cordeiro L, Ballinger E, Hagerman R, & Hessl D (2010). Clinical assessment of DSM-IV anxiety disorders in fragile X syndrome: prevalence and characterization. Journal of Neurodevelopmental Disorders, 3(1), 57–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornish K, Cole V, Longhi E, Karmiloff-Smith A, & Scerif G (2013). Do behavioural inattention and hyperactivity exacerbate cognitive difficulties associated with autistic symptoms? Longitudinal profiles in fragile X syndrome. International Journal of Developmental Disabilities, 59(2), 80–94. [Google Scholar]
- Crawford DC, Acuña JM, & Sherman SL (2001). FMR1 and the fragile X syndrome: human genome epidemiology review. Genetics in Medicine, 3(5), 359–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford DC, Meadows KL, Newman JL, Taft LF, Scott E, Leslie M, ... & Sherman SL (2002). Prevalence of the fragile X syndrome in African‐Americans. American journal of medical genetics, 110(3), 226–233. [DOI] [PubMed] [Google Scholar]
- Hagerman PJ (2008). The fragile X prevalence paradox. Journal of medical genetics, 45(8), 498–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis III TE, Hess JA, Moree BN, Fodstad JC, Dempsey T, Jenkins WS, & Matson JL (2011). Anxiety symptoms across the lifespan in people diagnosed with Autistic Disorder. Research in Autism Spectrum Disorders, 5(1), 112–118. [Google Scholar]
- Durbin CE, Klein DN, Hayden EP, Buckley ME, & Moerk KC (2005). Temperamental emotionality in preschoolers and parental mood disorders. Journal of Abnormal Psychology, 114(1), 28. [DOI] [PubMed] [Google Scholar]
- Dykens EM, & Volkmar FR (1997). Medical conditions associated with autism. Handbook of autism and pervasive developmental disorders, 2, 388–407. [Google Scholar]
- Geschwind DH (2011). Genetics of autism spectrum disorders. Trends in cognitive sciences, 15(9), 409–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldsmith H, & Rothbart MK (1996). Prelocomotor and locomotor Laboratory Temperament Assessment Battery (Lab-TAB; version 3.0, Technical Manual) Madison: University of Wisconsin, Department of Psychology. [Google Scholar]
- Grant BF, Hasin DS, Blanco C, Stinson FS, Chou SP, Goldstein RB, ... & Huang B (2005). The epidemiology of social anxiety disorder in the United States: results from the National Epidemiologic Survey on Alcohol and Related Conditions. The Journal of clinical psychiatry, 66(11), 1351–1361. [DOI] [PubMed] [Google Scholar]
- Gurrieri F, & Neri G (2009). Defective oxytocin function: a clue to understanding the cause of autism? BMC medicine, 7(1), 63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagerman PJ (2008). The fragile X prevalence paradox. Journal of medical genetics, 45(8), 498–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall S, DeBernardis M, & Reiss A (2006). Social escape behaviors in children with fragile X syndrome. Journal of Autism and Developmental Disorders, 36(7), 935–947. Chicago. [DOI] [PubMed] [Google Scholar]
- Hall SS, Lightbody AA, & Reiss AL (2008). Compulsive, self-injurious, and autistic behavior in children and adolescents with fragile X syndrome. American Journal on Mental Retardation, 113(1), 44–53. [DOI] [PubMed] [Google Scholar]
- Harris SW, Hessl D, Goodlin-Jones B, Ferranti J, Bacalman S, Barbato I, ... & Hagerman RJ (2008). Autism profiles of males with fragile X syndrome. Journal Information, 113(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izard CE, מיה ויס, & Weiss M (1979). Maximally discriminative facial movement coding system University of Delaware, instructional resources Center. [Google Scholar]
- Kagan J, & Snidman N (1999). Early childhood predictors of adult anxiety disorders. Biological psychiatry, 46(11), 1536–1541. [DOI] [PubMed] [Google Scholar]
- Kapalu L, & Gartstein MA (2016). Boys with fragile X syndrome: investigating temperament in early childhood. Journal of Intellectual Disability Research, 60(9), 891–900. [DOI] [PubMed] [Google Scholar]
- Kaufmann WE, Cortell R, Kau AS, Bukelis I, Tierney E, Gray RM, ... & Stanard, P. (2004). Autism spectrum disorder in fragile X syndrome: communication, social interaction, and specific behaviors. American Journal of Medical Genetics Part A, 129(3), 225–234. [DOI] [PubMed] [Google Scholar]
- Keith TZ (2014). Multiple Regression and Beyond: An Introduction to Multiple Regression and Structural Equation Modeling Routledge. [Google Scholar]
- Kindt M, Bögels S, & Morren M (2003). Processing bias in children with separation anxiety disorder, social phobia and generalised anxiety disorder. Behaviour Change, 20(03), 143–150. [Google Scholar]
- Kreiser NL, & White SW (2014). Assessment of Social Anxiety in Children and Adolescents With Autism Spectrum Disorder. Clinical Psychology: Science and Practice, 21(1), 18–31. [Google Scholar]
- La Greca AM, & Lopez N (1998). Social anxiety among adolescents: Linkages with peer relations and friendships. Journal of abnormal child psychology, 26(2), 83–94. [DOI] [PubMed] [Google Scholar]
- Lesniak-Karpiak K, Mazzocco MM, & Ross JL (2003). Behavioral assessment of social anxiety in females with Turner or fragile X syndrome. Journal of Autism and Developmental Disorders, 33(1), 55–67. [DOI] [PubMed] [Google Scholar]
- Lonigan CJ, & Phillips BM (2001). Temperamental influences on the development of anxiety disorders [Google Scholar]
- Lonigan CJ, Phillips BM, & Hooe ES (2003). Relations of positive and negative affectivity to anxiety and depression in children: evidence from a latent variable longitudinal study. Journal of consulting and clinical psychology, 71(3), 465. [DOI] [PubMed] [Google Scholar]
- Lopata C, & Thomeer ML (2014). Autism and anxiety in school. In Handbook of autism and anxiety (pp. 201–214). Springer International Publishing. [Google Scholar]
- Lopata C, Toomey JA, Fox JD, Volker MA, Chow SY, Thomeer ML, et al. (2010). Anxiety and Depression in Children with HFASDs: Symptom Levels and Source Differences. Journal of Abnormal Child Psychology, 38, 765–776. [DOI] [PubMed] [Google Scholar]
- Lord C, Risi S, Lambrecht L, Cook EH Jr, Leventhal BL, DiLavore PC, ... & Rutter M (2000). The Autism Diagnostic Observation Schedule—Generic: A standard measure of social and communication deficits associated with the spectrum of autism. Journal of autism and developmental disorders, 30(3), 205–223. [PubMed] [Google Scholar]
- Lord C, Rutter M, DiLavore P, Risi S, Gotham K, & Bishop S (2012). Autism Diagnostic Observation Schedule–2nd edition (ADOS-2) Los Angeles, CA: Western Psychological Corporation. [Google Scholar]
- Mogg K, Bradley BP, De Bono J, & Painter M (1997). Time course of attentional bias for threat information in non-clinical anxiety. Behaviour Research and Therapy, 35(4), 297–303. [DOI] [PubMed] [Google Scholar]
- Mullen EM (1995). Mullen scales of early learning Circle Pines: American Guidance Services. [Google Scholar]
- Muris P, Steerneman P, Merckelbach H, Holdrinet I, & Meesters C (1998). Comorbid anxiety symptoms in children with pervasive developmental disorders. Journal of anxiety disorders, 12(4), 387–393. [DOI] [PubMed] [Google Scholar]
- Pisula E (2004). Response of children with autism to a brief separation from the mother. Polish Psychological Bulletin, 35(2), 109–115. [Google Scholar]
- Rapee RM (2002). The development and modification of temperamental risk for anxiety disorders: prevention of a lifetime of anxiety?. Biological Psychiatry, 52(10), 947–957. [DOI] [PubMed] [Google Scholar]
- Rapee RM, & Coplan RJ (2010). Conceptual relations between anxiety disorder and fearful temperament. New directions for child and adolescent development, 2010(127), 17–31. [DOI] [PubMed] [Google Scholar]
- Rapee RM (2014). Preschool environment and temperament as predictors of social and nonsocial anxiety disorders in middle adolescence. Journal of the American Academy of Child & Adolescent Psychiatry, 53(3), 320–328. [DOI] [PubMed] [Google Scholar]
- Rellini E, Tortolani D, Trillo S, Carbone S, & Montecchi F (2004). Childhood Autism Rating Scale (CARS) and Autism Behavior Checklist (ABC) correspondence and conflicts with DSM-IV criteria in diagnosis of autism. Journal of autism and developmental disorders, 34(6), 703–708. [DOI] [PubMed] [Google Scholar]
- Roberts JE, Clarke MA, Alcorn K, Carter JC, Long AC, & Kaufmann WE (2009). Autistic behavior in boys with fragile X syndrome: social approach and HPA-axis dysfunction. Journal of Neurodevelopmental Disorders, 1(4), 283–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts JE, Tonnsen BL, Robinson M, McQuillin SD, & Hatton DD (2014). Temperament factor structure in fragile X syndrome: The Children’s Behavior Questionnaire. Research in developmental disabilities,35(2), 563–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts JE, Weisenfeld LAH, Hatton DD, Heath M, & Kaufmann WE (2007). Social approach and autistic behavior in children with fragile X syndrome. Journal of autism and developmental disorders, 37(9), 1748–1760. [DOI] [PubMed] [Google Scholar]
- Rogers SJ, Wehner EA, & Hagerman R (2001). The behavioral phenotype in fragile X: symptoms of autism in very young children with fragile X syndrome, idiopathic autism, and other developmental disorders. Journal of developmental & behavioral pediatrics, 22(6), 409–417. [DOI] [PubMed] [Google Scholar]
- Rothbart MK, Ahadi SA, & Evans DE (2000). Temperament and personality: origins and outcomes. Journal of personality and social psychology, 78(1), 122. [DOI] [PubMed] [Google Scholar]
- Rothbart MK, Ahadi SA, Hershey KL, & Fisher P (2001). Investigations of temperament at three to seven years: The Children’s Behavior Questionnaire. Child development, 72(5), 1394–1408. [DOI] [PubMed] [Google Scholar]
- Rothbart MK, Derryberry D, & Hershey K (2000). Stability of temperament in childhood: Laboratory infant assessment to parent report at seven years. Temperament and personality development across the life span, 85–119. [Google Scholar]
- Ruscio AM, Brown TA, Chiu WT, Sareen J, Stein MB, & Kessler RC (2008). Social fears and social phobia in the USA: results from the National Comorbidity Survey [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacrey LAR, Bennett JA, & Zwaigenbaum L (2015). Early infant development and intervention for autism spectrum disorder. Journal of child neurology, 30(14), 1921–1929. [DOI] [PubMed] [Google Scholar]
- Shechner T, Britton JC, Pérez‐Edgar K, Bar‐Haim Y, Ernst M, Fox NA, ... & Pine DS (2012). Attention biases, anxiety, and development: toward or away from threats or rewards?. Depression and anxiety, 29(4), 282–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schopler E, Reichler RJ, & Renner BR (1988). The childhood autism rating scale (CARS) Los Angeles, CA: Western Psychological Services. [Google Scholar]
- Simonoff E, Pickles A, Charman T, Chandler S, Loucas T, & Baird G (2008). Psychiatric disorders in children with autism spectrum disorders: prevalence, comorbidity, and associated factors in a population-derived sample. Journal of the American Academy of Child & Adolescent Psychiatry, 47(8), 921–929. [DOI] [PubMed] [Google Scholar]
- Stein MB, & Stein DJ (2008). Social anxiety disorder. The Lancet, 371(9618), 1115–1125. [DOI] [PubMed] [Google Scholar]
- Strang JF, Kenworthy L, Daniolos P, Case L, Wills MC, Martin A, & Wallace GL (2012). Depression and anxiety symptoms in children and adolescents with autism spectrum disorders without intellectual disability. Research in Autism Spectrum Disorders, 6(1), 406–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonnsen BL, Cornish KM, Wheeler AC, & Roberts JE (2014). Maternal predictors of anxiety risk in young males with fragile X. American Journal of Medical Genetics Part B: Neuropsychiatric Genetics, 165(5), 399–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonnsen BL, Malone PS, Hatton DD, & Roberts JE (2013). Early negative affect predicts anxiety, not autism, in preschool boys with fragile X syndrome. Journal of abnormal child psychology, 41(2), 267–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonnsen BL, Shinkareva SV, Deal SC, Hatton DD, & Roberts JE (2013). Biobehavioral indicators of social fear in young children with fragile X syndrome. American journal on intellectual and developmental disabilities, 118(6), 447–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Steensel FJ, Bögels SM, & Perrin S (2011). Anxiety disorders in children and adolescents with autistic spectrum disorders: A meta-analysis. Clinical child and family psychology review, 14(3), 302–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasa RA, Kalb L, Mazurek M, Kanne S, Freedman B, Keefer A, ... & Murray D (2013). Age-related differences in the prevalence and correlates of anxiety in youth with autism spectrum disorders. Research in Autism Spectrum Disorders, 7(11), 1358–1369. [Google Scholar]
- Wheeler A, Raspa M, Bann C, Bishop E, Hessl D, Sacco P, & Bailey DB (2014). Anxiety, attention problems, hyperactivity, and the Aberrant Behavior Checklist in fragile X syndrome. American Journal of Medical Genetics Part A, 164(1), 141–155. [DOI] [PubMed] [Google Scholar]
- White SW, Oswald D, Ollendick T, & Scahill L (2009). Anxiety in children and adolescents with autism spectrum disorders. Clinical psychology review, 29(3), 216–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams TA, Porter MA, & Langdon R (2014). Social Approach and Emotion Recognition in Fragile X Syndrome. American Journal on Intellectual and Developmental Disabilities, 119(2), 133–150. [DOI] [PubMed] [Google Scholar]
- Yu TW & Berry-Kravis E (2014). Autism and fragile x syndrome. Seminars in Neurology, 34(3), 258–265. [DOI] [PubMed] [Google Scholar]


