Abstract
Background
Hepatitis E virus is a re-emerging pathogen with an increase in human cases that can lead to chronic infection in immunosuppressed patients. Turkey is located between Asia and Europe, 2 regions with distinct epidemiological and clinical features of hepatitis E virus infection. This multicenter cross-sectional study aimed to investigate the prevalence of hepatitis E virus infection in liver and kidney transplant recipients in Turkey and to determine the role of possible transmission factors.
Methods
A total of 485 plasma samples of solid organ recipients were collected from 7 transplantation centers in Turkey. Samples were tested for anti-hepatitis E virus immunoglobin M, immunoglobin G, and hepatitis E virus ribonucleic acid. Water- and food-related risk factors were evaluated by a questionnaire.
Results
Samples of 300 kidney and 185 liver recipients were collected. Hepatitis E virus ribonucleic acid was tested in 472 samples and none were positive. Anti-hepatitis E virus immunoglobin G and immunoglobin M were detected in 84 (17.3%) and 3 (0.6%) patients, respectively. Seropositivity was associated with older age, male gender, being a liver recipient, and being infected with hepatitis B virus and/or hepatitis C virus. None of the patients under the age of 30 were seropositive. Hepatitis E virus immunoglobin G prevalence was higher in the Central East and Southeast Anatolia. Eating raw meat was the only independent variable associated with hepatitis E virus seropositivity.
Conclusion
This is the first prevalence study of hepatitis E virus infection in solid organ recipients in Turkey. Anti-hepatitis E virus immunoglobin G prevalence was 17.3% which was higher than the previously reported rate in blood donors. Seropositivity was significantly higher in liver recipients. Despite the high antibody prevalence, none of the patients were viremic.
Keywords: Hepatitis antibodies, hepatitis E virus, kidney transplantation, liver transplantation, organ transplantation
Main Points
Anti-hepatitis E virus immunoglobin G prevalence was 17.3% in a group of Turkish solid organ transplant recipients. None of the patients were viremic.
Seropositivity was significantly higher in liver vs. kidney recipients.
Hepatitis E virus immunoglobin G prevalence was higher in Central East and Southeast Anatolia.
Introduction
Hepatitis E virus (HEV) belongs to the Hepeviridae family, has a positive-sense, single-stranded ribonucleic acid (RNA) genome, and usually causes self-limiting hepatitis worldwide. In 2005, it was estimated that nearly 20 million HEV infections occur globally with 3 million symptomatic cases, although this estimate requires updating in the light of changing epidemiology of the infection.1,2 Hepatitis E virus became a re-emerging pathogen after the recognition of its zoonotic origin in developed countries, the potential of causing chronic hepatitis in immunosuppressed patients, and the risk of transmission by blood transfusion.3 There are 4 main HEV genotypes infecting humans with distinct differences: genotype 1 (gt1) and 2 (gt2) are endemic/hyperendemic in developing countries, restricted to humans and transmitted by fecal-oral route through contaminated water, whereas genotype 3 (gt3) and 4 (gt4) are observed in developed countries, infect humans, and other mammalian species, transmitted as a zoonotic, food-borne infection.3,4 In recent years, studies showed an increasing incidence of autochthonous HEV infection in some European countries. The number of confirmed cases increased more than 3-fold between 2005 and 2015 according to the surveillance report of the European Centre for Disease Prevention and Control.5,6 This increase is mainly due to gt3 virus, and analysis of the risk factors shows that the consumption of undercooked or raw pork meat is the main route of the transmission. Hepatitis E virus genotypes 3 and 4 can also lead to chronic infection in immunosuppressed patients, which is mainly described in solid organ transplant recipients. A clinical practice guideline has recently been published focusing on HEV infection including chronic hepatitis.2
Turkey is located between HEV hyperendemic Asian countries and Europe, that is, 2 regions with distinct epidemiological and clinical features of hepatitis E infection. No HEV outbreaks have been reported in the country so far. The seroprevalence of HEV increased by age in Turkey and ranged between 0% and 12.4% as reported in a recent systematic review.7 Seroprevalence among 2011 blood donors was 11.5% by Dia.Pro and 12.2% by Wantai kits.8 Although these studies showed that HEV is endemic in Turkey, there is no data regarding viral genotypes and transmission routes. Hepatitis E virus infection is not routinely tested. Studies regarding HEV infection among the immunosuppressed population are also lacking.
This multicenter, cross-sectional study aimed to investigate the prevalence of HEV infection in liver and kidney transplant recipients in Turkey and to determine the role of possible transmission factors.
Materials and Methods
Plasma Samples
Seven transplantation centers participated in the study by providing plasma samples from kidney or liver transplant recipients. A total of 485 samples (1 sample per patient) were collected and tested for anti-HEV antibodies and HEV RNA at the Medical Microbiology Department, Dokuz Eylül University Hospital. Samples closest to the study date and collected at least 6 months after the transplantation were selected from the archive being stored at -80°C by each center. In addition, medical records were reviewed, and medical history, demographic, and laboratory data were collected. Water- and food-related risk factors for HEV infection were evaluated by a short questionnaire which included 4 questions about the source of drinking water and habits of consuming game and/or raw meat. Data were collected by the same researcher through phone interview.
The study was reviewed and approved by the Ethics Committee of the Dokuz Eylül University, Faculty of Medicine (Date and number: 03.06.2016-530).
Detection of Anti-hepatitis E Virus Antibodies and Hepatitis E Virus Ribonucleic Acid
All plasma samples were analyzed for anti-HEV IgM and anti-HEV IgG by ELISA (recomWell HEV IgM and IgG, Microgen Diagnostics, Germany) and HEV RNA by real-time RT-PCR (RealStar HEV RT-PCR Kit 2.0, Altona Diagnostics, Germany) according to the manufacturer’s instructions. Viral RNA was extracted by using QIAsymphony DSP Virus/Pathogen Kit, in combination with the QIAsymphony SP (Qiagen, Hilden, Germany) instrument using 500 µL input and 90 µL elution volume. QCMD 2018 HEV RNA panel was used for external quality assessment and correct results were obtained for all panel samples.
Statistical Methods
Statistical analysis of test results and patient data were done by chi-square and t-test using Statistical Package for the Social Sciences (SPSS) 22.0 program. The risk factors were first analyzed one-by-one and then using logistic models with a P-value < .05 considered as significant.
Results
A total of 485 samples were analyzed for anti-HEV antibodies and HEV RNA. The mean and median age of the patients were 42.9 (±16.5) and 45 years, respectively. Among the patents, 314 (64.7%) were men and 171 (35.3%) were women. The study group consisted of 300 (61.9%) kidney and 185 (38.1%) liver transplant recipients. Of the 171 female patients, 53 (31.0%) had liver and 118 (69%) had kidney transplantation, while among the 314 men, 132 (42.0%) were liver and 182 (58.0%) were kidney recipients.
Prevalence of Hepatitis E Virus Ribonucleic Acid and Anti-hepatitis E Virus Immunoglobin M
Hepatitis E virus ribonucleic acid was undetectable in 472 (97.3%) patients. The volume of 13 (2.7%) samples were not sufficient for viral RNA RT-PCR assay.
In 3 patients (0.6%), anti-HEV IgM was repeatedly detectable, while HEV RNA was negative. These patients were followed up by testing a second plasma sample collected 8 to 12 months after the first one. The results of these 3 patients are shown in Table 1. Hepatitis E virus ribonucleic acid was undetectable in all samples.
Table 1.
Results of the Anti-hepatitis E Virus Immunoglobin M Positive Patients
| Patient Number and Gender | Medical History | First Serum Sample | Second Serum Sample | |
|---|---|---|---|---|
| Anti-HEV Antibodies | ALT/AST (IU/mL) | Anti-HEV Antibodies | ||
| Patient 1, male | Liver tx HCC + HCV | IgM (+) IgG (+) | 112/140 | IgM (+) IgG (+) |
| Patient 2, male | Liver tx HCC + HBV | IgM (+) IgG (+) | Normal | IgM (−) IgG (+) |
| Patient 3, female | Liver tx HCC + HCV | IgM (+) IgG (+) | Normal | IgM (−) IgG (−) |
tx, transplantation; HCC, hepatocellular cancer; IgM, immunoglobin M; IgG, immunoglobin G; HEV, hepatitis E virus.
Prevalence of Anti-hepatitis E Virus Immunoglobin G
Anti-hepatitis E virus immunoglobin G was detectable in 17.3% (84/485) of the patients. Anti-hepatitis E virus immunoglobin G positivity rate was higher among male compared to female patients (22% vs 8.8%, respectively, P < .001). The mean age of anti-HEV IgG positive patients was significantly higher than negative patients (55.5% vs 40.3%, respectively, P < .001). None of the patients under the age of 30 were seropositive. Increased anti-HEV IgG positivity with age was detected in both male and female groups. In male patients, the highest HEV IgG positivity was in the 51-60 age group (31.3%), in female patients the highest rate (50%) was in the 61-70 group (Figure 1).
Figure 1.
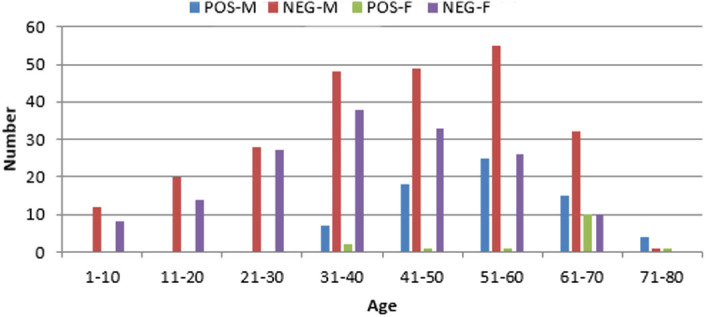
Hepatitis E virus immunoglobin G seropositivity according to age groups of male (M) and female (F) patients. POS, positive; NEG, negative.
Anti-hepatitis E virus immunoglobin G positivity was assessed according to the provinces where patients live. Turkey has 12 regions according to NUTS-1 classification based on socioeconomic factors. The distribution of anti-HEV IgG showed a significantly higher prevalence in the Central East (42.9%) and Southeast Anatolia (31.4%) (Table 2).
Table 2.
Anti-hepatitis E Virus Immunoglobin G Positivity According to Regions of Patients’ Residence Areas
| Region | Tested Samples (n)* | Anti-HEV IgG Positive Samples, n (%) |
|---|---|---|
| Central East Anatolia | 28 | 12 (42.9) |
| Southeast Anatolia Region | 51 | 15 (31.4) |
| Mediterranean Region | 36 | 8 (22.2) |
| Aegean region | 190 | 29 (15.3) |
| West Black Sea Region | 15 | 2 (13.3) |
| İstanbul region | 28 | 3 (10.7) |
| Central Anatolia Region | 39 | 4 (10.3) |
| West Anatolia Region | 66 | 6 (9.1) |
| Total | 453 | 79 (17.4) |
*Four regions with a number of patients lower than 10 and 5 patients living abroad are not shown on the table.
Anti-HEV IgG, anti-hepatitis E virus immunoglobin G.
Hepatitis E Virus Immunoglobin G in Liver Transplant Recipients
Hepatitis E virus immunoglobin G seropositivity was significantly higher among liver transplant recipients (59/185, 31.9%) compared to kidney recipients (25/300, 8.3%) (P < .001). Underlying viral hepatitis as a cause of liver transplantation was a factor related to higher HEV seroprevalence. More than half (53.2%) of the 47 liver transplant patients infected with HBV, HDV or HCV were also positive for HEV IgG, while only 9.1% of 22 patients transplanted due to non-hepatitis reasons had HEV IgG (P < .001).
Relation of Anti-hepatitis E Virus Immunoglobin G Positivity with Risk Factors
Questionnaires on the 4 risk factors for transmission of HEV infection were sent to all 485 patients. Out of which, 241 (49.6%) completed the questionnaire. Anti-hepatitis E virus immunoglobin G seroprevalence was significantly higher in all patients with risky behaviors when factors were analyzed individually (Table 3). However, logistic regression analysis showed a significant relation only with eating raw meat (P = .001).
Table 3.
Anti-hepatitis E Virus Immunoglobin G Seropositivity According to Risk Factors
| Risk Factor | Patients with Risk, n (%) | Patients Without Risk, n (%) | P | ||
|---|---|---|---|---|---|
| Number | Anti-HEV IgG (+) | Number | Anti-HEV IgG (+) | ||
| Contact with livestock | 66 | 19 (28.8%) | 175 | 24 (13.7%) | .006 |
| Contact with game animals/meat | 42 | 15 (35.7%) | 199 | 27 (13.6%) | .001 |
| Use of well/artesian water | 69 | 18 (26%) | 172 | 23 (13.3%) | .024 |
| Eating raw meat | 61 | 18 (29.5%) | 180 | 21 (11.6%) | .002 |
Anti-HEV IgG, anti-hepatitis E virus immunoglobin G.
Discussion
This is the first study assessing the prevalence of HEV infection in solid organ transplant (SOT) recipients in Turkey. Anti-hepatitis E virus immunoglobin G was positive in 17.3% of the patients which is significantly higher than the seroprevalence of 12.2% detected in blood donors in Turkey (P = .018).8 Hepatitis E virus immunoglobin G prevalence in this study is also higher than the results of other Turkish studies reporting the rate between 0% and 12.4% in healthy population.7
Seven centers from 6 different provinces participated in this study. In İzmir, samples were collected from 2 different university hospitals showing similar seroprevalence results (16.7% and 18.7%, P > .05). A population-based study also done in İzmir in 2015 by stratified random sampling method reported an anti-HEV IgG prevalence of 6.6%.9 The seroprevalence detected in SOT recipients compared to the results of this study representing the normal population in Izmir showed a significantly higher prevalence of HEV infection (P < .00001).
Anti-hepatitis E virus immunoglobin G positivity differed among the regions of Turkey, showing a higher prevalence in the Central East and Southeast Anatolia with rates exceeding 30%, in line with findings of other studies including blood donors.7,8 The frequencies of other hepatitis viruses (HAV, HBV, HDV, and HCV) also show a similar increase from west to east of Turkey.10 This could be partially related to socioeconomic conditions such as problems in reaching healthy drinking/utility water and/or eating habits. HEV genotypes 1 and 2 known to infect humans only and transmitted mainly by contaminated water whereas 3 and 4 are zoonotic and infect humans mainly by consumption of under/uncooked pork products or shellfish grown in contaminated waters.2,5 Almost half of the patients of this study completed a questionnaire related to their food and water intakes that could play a role in the transmission of HEV infection. The consumption of raw meat was the only significant risk factor related to seropositivity by the multivariate analyses. Although this finding suggests the zoonotic transmission, it needs confirmation with viral genotyping which was not possible in the study since none of the patients were HEV RNA positive. In Turkey, consumption of pork is quite unusual due to religious and cultural factors. However, one of the largest outbreaks of human trichinellosis caused by Trichinella britovi occurred in Izmir, Turkey, related to the consumption of raw meatballs made of beef and pork.11 Therefore, patients may be consciously or unknowingly consuming pork or other types of meat that could transmit HEV.
High viral IgG prevalence at eastern regions may also be related to a higher prevalence of chronic HBV and/or HCV infections since having a pre-existing liver disease is a risk factor for HEV infection.12-14 European studies show that there are differences with regard to HEV infection rates between countries.2 Hyperendemic regions are also reported in southwest France, central Italy and west-central Poland where seroprevalence rates are reaching up to 50%.15-17
In this study, older age, male gender, liver transplantation, and being infected with other viral hepatitis viruses were factors significantly related to HEV IgG positivity. Although viral genotyping was not possible since there were no viremic patients, studies in Europe and several other developed countries emphasized that autochthonous HEV gt 3 infections are more common in the elderly male population and persons with coexisting diseases.4,5 Anti-hepatitis E virus immunoglobin G positivity was significantly higher in liver transplant recipients (31.9%) compared to kidney recipients in the study group. This feature has also been found in Europe and the United States where the seroprevalence has been reported to be between 3% and 28% in liver transplant recipients.14,18
Acute hepatitis E virus infection in SOT recipients can become chronic in 50%-60% of patients and rapidly progress to cirrhosis under immunosuppressive treatment.19 In England, HEV viremia was found to be between 0% and 3.2% in transplant recipients.18 In a nationwide screening of liver transplant recipients in Japan, IgG positivity was 2.9%, and chronic HEV infection was reported in 2 patients (0.12%).20 Despite the high prevalence of IgG positivity, none of the samples were HEV RNA positive in our study. One of the reasons for the absence of viremia in this group may be infection occurring before the transplantation. This possibility could not be tested since the samples of the pre-transplant period were not available and the prevalence of antibodies before and after transplantation could not be compared. Another factor could be the HEV genotype since chronic HEV infection has been reported only for gt 3 and 4 whereas gt 1 and 2, which are dominant in developing countries of Asia and Africa, are not known to cause chronic infection.14,21 The lack of information about HEV genotypes in Turkey is a major shortcoming in the interpretation of the available data. To our knowledge, there is only 1 publication reporting a genotype 3 HEV infection in a Scottish traveler who returned from Turkey.22
Anti-hepatitis E virus immunoglobin M antibody response in humans is generally used as a marker of acute infection that follows a conventional course by disappearing within 3 months, whereas isolated anti-HEV IgG indicates a prior infection. Anti-hepatitis E virus assays differ regarding their sensitivities and specificities.23,24 In addition, antibody production in immunosuppressed patients could be problematic therefore detection of viral RNA is the preferred method for diagnosing HEV infection in such groups.24,25 There were 3 anti-HEV IgM positive patients with undetectable HEV RNA in this study. They are followed up by testing a second sample collected 8-12 months after the first one. In patient 1, both anti-HEV IgM and IgG antibodies were persistent in the follow-up sample, suggesting prolonged IgM positivity after the acute infection.26 Patient 2 was accepted as recently infected with HEV based on clearance of specific IgM and sustained IgG positivity in the follow-up sample. In patient 3, both antibodies were negative in the follow-up sample, suggesting either a false IgM reaction in the first sample or deficiency in IgG production due to immunosuppression.
Hepatitis E virus can be transmitted by blood transfusion which is a significant risk factor in liver transplant recipients.27 However a recent, large-scale, nationwide study of HEV prevalence in Turkish blood donors found that anti-HEV IgG prevalence was 12.2%, while none of the donors was HEV RNA positive.8 Therefore, the risk of virus transmission from blood donors seems a very low probability in Turkey.
In summary, in this study, SOT recipients had a higher HEV seroprevalence compared to the data of blood donors in Turkey. Seropositivity was associated with liver transplantation, advancing age, being male, having a chronic hepatitis virus infection, living in eastern and southeastern regions. In spite of high seroprevalence rate, none of the patients were viremic, therefore viral genotyping could not be performed. Some characteristics of seropositive patients, such as age and gender, were similar to gt 3 infected populations while the absence of chronic infection suggested gt 1 or 2. These assumptions require further studies regarding viral genotypes and routes of HEV transmission in Turkey. Although not routinely tested, HEV infection should be considered in the differential diagnosis, and serologic and molecular tests should be provided for the diagnosis of HEV infection in SOT transplant patients, particularly for liver recipients in Turkey.
Funding Statement
This study was funded by TUBITAK (The Scientific and Technological Research Council of Turkey) under grant number 216S904 and Dokuz Eylül University Scientific Research Coordination Unit (project number: 2017.KB.SAG.035).
Footnotes
Ethics Committee Approval: The study was approved by the medical ethics committee of Dokuz Eylül University, Faculty of Medicine.
Informed Consent: Written informed consent was obtained from the patients who agreed to take part in the study.
Peer-review: Externally peer-reviewed.
Author Contributions: Consept - A.A.S., S.Ö.; Design - S.Ö., A.A.S.; Supervision - A.A.S.; Resources - S.Ö.; A.A.S.; Materials - S.Ö., B.O., G.B., A.Z., S.A., D.Ç., S.G.; Data Collection and/or Processing -S.Ö., B.O., G.B., A.Z., S.A., D.Ç., S.G., M.A.; Analysis and/or Interpretation - S.Ö, A.A.S., M.A.; Literature Search - S.Ö., A.A.S.; Writing Manuscript - S.Ö., A.A.S.; Critical Review - S.Ö., A.A.S., B.O., G.B., A.Z., S.A., D.Ç., S.G., M.A.
Acknowledgments: We thank Filiz Akan for her excellent technical support in the laboratory.
Conflict of Interest: The authors have no conflict of interest to declare.
References
- 1.Rein DB, Stevens GA, Theaker J, Wittenborn JS, Wiersma ST. The global burden of hepatitis E virus genotypes 1 and 2 in 2005. Hepatology. 2012;55(4):988–997.. 10.1002/hep.25505) [DOI] [PubMed] [Google Scholar]
- 2.European Association for the Study of the Liver. EASL clinical practice guidelines on hepatitis E virus infection. J Hepatol. 2018;68(6):1256–1271.. 10.1016/j.jhep.2018.03.005) [DOI] [PubMed] [Google Scholar]
- 3.Sooryanarain H, Meng XJ. Hepatitis E virus: reasons for emergence in humans. Curr Opin Virol. 2019;34:10–17.. 10.1016/j.coviro.2018.11.006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kamar N, Dalton HR, Abravanel F, Izopet J. Hepatitis E virus infection. Clin Microbiol Rev. 2014;27(1):116–138.. 10.1128/CMR.00057-13) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.European Centre for Disease Prevention and Control (ECDC). Hepatitis E in the EU/EEA, 2005–2015. Stockholm: ECDC; 2017. Available at: https://www.ecdc.europa.eu/en/publications-data/hepatitis-e-eueea-2005-2015. [Google Scholar]
- 6.Adlhoch C, Avellon A, Baylis SA, et al. Hepatitis E virus: assessment of the epidemiological situation in humans in Europe, 2014/15. J Clin Virol. 2016;82:9–16.. 10.1016/j.jcv.2016.06.010) [DOI] [PubMed] [Google Scholar]
- 7.Leblebicioglu H, Ozaras R. Hepatitis E virus infection in Turkey: a systematic review. Ann Clin Microbiol Antimicrob. 2018;17(1):17. 10.1186/s12941-018-0269-6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yaşar O, Karatayli E, Cengiz G, et al. HEV seroprevalence in blood donors in Turkey by two commercial total anti-HEV Ab ELISA kits. J Med Virol. 2019;91(12):2174–2181.. 10.1002/jmv.25567) [DOI] [PubMed] [Google Scholar]
- 9.Kalfaoğlu H, Zeytinoglu A. Hepatitis A and E Seroprevalance in Izmir Province (in Turkish). [Medical Microbiology Fellowship Thesis]. Izmir, Turkey: Ege; University Medical Faculty; 2015. [Google Scholar]
- 10.Viral Hepatitis Prevention Board (VHPB). Burden and Prevention of Viral Hepatitis in Turkey. 2010. Available at: http://www.vhpb.org/vhpb-newsletter, Accessed June 7, 2020. [Google Scholar]
- 11.Akkoc N, Kuruuzum Z, Akar S, et al. A large-scale outbreak of trichinellosis caused by Trichinella britovi in Turkey. Zoonoses Public Health. 2009;56(2):65–70.. 10.1111/j.1863-2378.2008.01158.x) [DOI] [PubMed] [Google Scholar]
- 12.Atiq M, Shire NJ, Barrett A, Rouster SD, Sherman KE, Shata MT. Hepatitis E virus antibodies in patients with chronic liver disease. Emerg Infect Dis. 2009;15(3):479–481.. 10.3201/eid1503.080740) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bayram A, Eksi F, Mehli M, Sözen E. Prevalence of hepatitis E virus antibodies in patients with chronic hepatitis B and chronic hepatitis C. Intervirology. 2007;50(4):281–286.. 10.1159/000103916) [DOI] [PubMed] [Google Scholar]
- 14.Behrendt P, Steinmann E, Manns MP, Wedemeyer H. The impact of hepatitis E in the liver transplant setting. J Hepatol. 2014;61(6):1418–1429.. 10.1016/j.jhep.2014.08.047) [DOI] [PubMed] [Google Scholar]
- 15.Mansuy JM, Gallian P, Dimeglio C, et al. A nationwide survey of hepatitis E viral infection in French blood donors. Hepatology. 2016;63(4):1145–1154.. 10.1002/hep.28436) [DOI] [PubMed] [Google Scholar]
- 16.Lucarelli C, Spada E, Taliani G, et al. High prevalence of anti-hepatitis E virus antibodies among blood donors in central Italy, February to March 2014. Euro Surveill. 2016;21(30). 10.2807/1560-7917.ES.2016.21.30.30299) [DOI] [PubMed] [Google Scholar]
- 17.Bura M, Łagiedo M, Michalak M, Sikora J, Mozer-Lisewska I. Hepatitis E virus IgG seroprevalence in HIV patients and blood donors, west-central Poland. Int J Infect Dis. 2017;61:20–22.. 10.1016/j.ijid.2017.05.014) [DOI] [PubMed] [Google Scholar]
- 18.McPherson S, Elsharkawy A, Ankcorn M, et al. Summary of British Transplantation Society UK guidelines for hepatitis E and solid organ transplantation. Transplantation. 2018;102(1):15–20.. 10.1097/TP.0000000000001908) [DOI] [PubMed] [Google Scholar]
- 19.Legrand-Abravanel F, Kamar N, Sandres-Saune K, et al. Characteristics of autochthonous hepatitis E virus infection in solid-organ transplant recipients in France. J Infect Dis. 2010;202(6):835–844.. 10.1086/655899) [DOI] [PubMed] [Google Scholar]
- 20.Inagaki Y, Oshiro Y, Tanaka T, et al. A nationwide survey of hepatitis E virus infection and chronic hepatitis E in liver transplant recipients in Japan. EBiomedicine. 2015;2(11):1607–1612.. 10.1016/j.ebiom.2015.09.030) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kamar N, Garrouste C, Haagsma EB, et al. Factors associated with chronic hepatitis in patients with hepatitis E virus infection who have received solid organ transplants. Gastroenterology. 2011;140(5):1481–1489.. 10.1053/j.gastro.2011.02.050) [DOI] [PubMed] [Google Scholar]
- 22.Ramalingam S, Smith D, Wellington L, et al. Autochthonous hepatitis E in Scotland. J Clin Virol. 2013;58(4):619–623.. 10.1016/j.jcv.2013.10.002) [DOI] [PubMed] [Google Scholar]
- 23.Pas SD, Streefkerk RHRA, Pronk M, et al. Diagnostic performance of selected commercial HEV IgM and IgG ELISAs for immunocompromised and immunocompetent patients. J Clin Virol. 2013;58(4):629–634.. 10.1016/j.jcv.2013.10.010) [DOI] [PubMed] [Google Scholar]
- 24.Vollmer T, Diekmann J, Eberhardt M, Knabbe C, Dreier J. Monitoring of anti-hepatitis E virus antibody seroconversion in asymptomatically infected blood donors: systematic comparison of nine commercial anti-HEV IgM and IgG assays. Viruses. 2016;8(8):232. 10.3390/v8080232) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Haagsma EB, van den Berg AP, Porte RJ, et al. Chronic hepatitis E virus infection in liver transplant recipients. Liver Transpl. 2008;14(4):547–553.. 10.1002/lt.21480) [DOI] [PubMed] [Google Scholar]
- 26.Riveiro‐Barciela M, Rando‐Segura A, Barreira‐Díaz A, et al. Unexpected long‐lasting anti‐HEV IgM positivity: is HEV antigen a better serological marker for hepatitis E infection diagnosis? J Viral Hepat. 2020;27(7):747–753.. 10.1111/jvh.13285) [DOI] [PubMed] [Google Scholar]
- 27.Vercouter AS, Van Houtte F, Verhoye L, et al. Hepatitis E virus prevalence in Flemish blood donors. J Viral Hepat. 2019;26(10):1218–1223.. 10.1111/jvh.13161) [DOI] [PubMed] [Google Scholar]


