Abstract
Background
Metabolic dysfunction associated fatty liver disease (MAFLD) is the most common hepatopathy worldwide due to the obesity epidemic and is associated with chronic low-grade inflammation. High-sensitive C-reactive protein (hsCRP) as an inflammatory marker has been used in diagnosing MAFLD. However, the association between hsCRP and the severity of liver steatosis and fibrosis among obese patients with MAFLD remains to be elucidated.
Objective
To explore the correlation of hsCRP with the severity of liver steatosis and fibrosis among Chinese obese patients with MAFLD.
Methods
A total of 393 obese patients with mean BMI 34.8 ± 6.6 kg/m2 were selected and categorized as MAFLD and non-MAFLD groups. Anthropometric data, biochemical indices, and hsCRP were measured. The severity of hepatic steatosis and fibrosis was assessed using FibroScan. Multivariate logistic regression analysis was performed to determine the relationship between hsCRP and the risk of MAFLD and its disease severity.
Results
Patients with MAFLD showed significantly elevated hsCRP levels and were more likely to have severe steatosis and fibrosis compared to those without MAFLD. The proportions of MAFLD, severe steatosis, and severe fibrosis were significantly increased across the hsCRP quartiles (P-trend = 0.004, 0.021, and 0.006, respectively). After multivariable adjustments, the adjusted ORs (AORs) and 95%CI for MAFLD were 1.00 (reference), 1.298 (0.587-2.872), 2.407 (1.002-5.781), and 2.637(1.073-6.482) (Q1-Q4, P-trend = 0.014). Likewise, the AORs (95%CI) for severe steatosis and severe fibrosis were remarkably increased with the increment of serum hsCRP quartiles (P-trend < 0.001, P-trend = 0.021, respectively).
Conclusions
Elevated serum hsCRP levels were associated with increased risk of MAFLD among Chinese obese patients and correlated positively with the severity of liver steatosis and fibrosis, suggesting that hsCRP can be used as a potential biomarker to monitor and predict disease severity among Chinese obese population with MAFLD.
Keywords: high-sensitive C-reactive protein, metabolic dysfunction associated fatty liver disease, hepatic fibrosis, inflammation, obesity
Introduction
Metabolic dysfunction associated fatty liver disease (MAFLD), the consensus-driven proposed nomenclature for non-alcoholic fatty liver disease (NAFLD) (1), has currently reached a worldwide epidemic, affecting approximately 25% of the overall population (2). It is increasing in its prevalence due to the obesity epidemic and thus poses enormous health and economic burden globally (3). It is reported that almost 80% of patients with MAFLD are accompanied by obesity that is believed to carry a higher risk for developing fibrosis and cirrhosis (4). MAFLD encompasses a spectrum of liver conditions ranging from simple steatosis to steatohepatitis, fibrosis, and cirrhosis (5). The pathogenic process of MAFLD is well−thought−out as a systemic metabolic dysfunction state (6) and commonly linked to various metabolic disorders, including fibrosis and cirrhosis (7), liver cancer (8), obesity (9), type 2 diabetes (T2DM) (10, 11), and even cardiovascular disease (12). It is worth noting that the clinical management and consequence of MAFLD patients are influenced strongly by the more aggressive phenotypes. For instance, fibrosis is the major determinant of adverse outcomes in patients with MAFLD, which could ultimately lead to liver cirrhosis, hepatocellular cancer, and death (13). Hence, early and accurate identification of patients with significant fibrosis and its possible pathogenesis among obese patients with MAFLD are crucial for clinicians to make the right therapeutic decisions and predict clinical outcomes.
Although the pathophysiology of MAFLD has been extensively studied, there is still a lot to uncover, especially in obese patients. Previous studies indicate that chronic low-grade inflammation occurs as a consequence of obesity (14) whereas recent insights suggest that it may play a causative role in generating insulin resistance (IR), insulin secretion deficiency, and imbalance of energy homeostasis (15). Of note, obesity-induced low-grade inflammation in the liver has been proposed as a possible contributory mechanism of MAFLD (16). High-sensitive C-reactive protein (hsCRP) as an inflammatory marker has been recently reported to be associated with MAFLD. Kumar et al. (17) reported that hsCRP levels were significantly higher in individuals with NAFLD and positively correlated with the disease severity of NAFLD in north Indian population. Kogiso et al. (18) also revealed that hsCRP was a strong predictor of the disease progression in NAFLD in the general Japanese population. Additionally, Yoneda et al. (19) showed significantly elevated hsCRP levels in patients with advanced fibrosis compared to those with mild fibrosis. Inconsistently, Zimmermann et al. (20) demonstrated a positive association of hsCRP with the degree of steatosis but not with the severity of NAFLD among obese patients in France. Collectively, the correlation between hsCRP and the disease progression of MAFLD might be complex based on different genetic background and study population.
Although hsCRP has been used clinically as an inflammatory marker in the diagnosis of MAFLD, little is known regarding its role in the disease progression of MAFLD among the Chinese obese population. In this work, we investigated the correlation of serum hsCRP with the risk of MAFLD and the severity of hepatic steatosis and fibrosis among the Chinese obese population, aiming to gain novel insights into the development and the disease progression of MAFLD.
Methods
Study Design and Participants
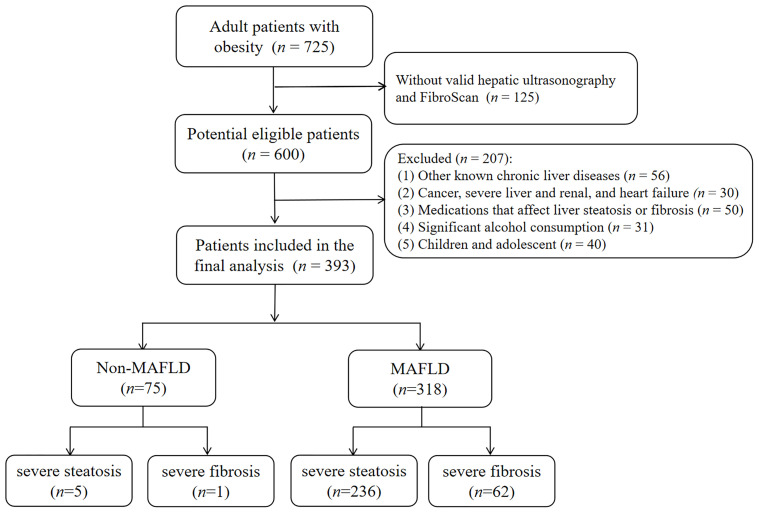
This retrospective cross-sectional study was conducted at the Department of Endocrinology and Metabolism, Shanghai Tenth People’s Hospital in China between January 2017 and October 2021. Initially, 725 adult obese patients who were admitted to our hospital were recruited. As a result, 393 subjects were finally included and divided into a MAFLD group (n = 318) and a non-MAFLD group (n = 75) based on the inclusion and exclusion criteria. The inclusion criteria were as follows (1): aged 18~65 years old (2), body mass index (BMI) ≥ 28 kg/m2 according to the diagnostic criteria for obesity in a Chinese population (21), and (3) underwent the biochemical tests, hepatic ultrasonography, and valid transient elastography (FibroScan) examination. Exclusion criteria included the presence of (1) other known chronic liver diseases, such as chronic hepatitis B or C, autoimmune hepatitis (2), pre-existing cancer, severe renal and liver dysfunction, and congestive heart disease (3), history of hyperthyroidism or hypothyroidism (4), significant alcohol consumption (22), and (5) pregnancy. Patients who were treated using any medication or other therapeutic methods that could influence liver steatosis or fibrosis, and glucolipid metabolism within 6 months prior to this study were also excluded. The flowchart of this study is shown in Figure 1 .
Figure 1.
Flowchart of study enrollment. Of 725 adult obese patients, those who did not meet inclusion criteria (n =207) were excluded. Another 125 patients without valid hepatic ultrasonography and FibroScan were also excluded. As a result, 393 patients were included in the final analysis.
Clinical and Biochemical Measurements
Demographic and anthropometric data, including age, sex, height, body weight, systolic blood pressure (SBP), diastolic blood pressure (DBP), lifestyle factors (smoking status and alcohol consumption), comorbidities, and medical history were directly collected by trained physicians. Morning venous blood was drawn from all the participants after a 12-h overnight fast to measure the levels of alanine transaminase (ALT), aspartate aminotransferase (AST), gamma-glutamyl transferase (γ-GT), total cholesterol (TC), triglycerides (TG), low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), fasting plasma glucose (FPG), and fasting insulin (FINS). Additionally, inflammation markers including interleukin (IL)-6, IL-8, tumor necrosis factor-α (TNF-α), and hsCRP levels were also measured. All the laboratory measurements were conducted in our department by the clinical laboratory using standard methodologies.
Herein, BMI was calculated as weight in kilograms divided by height in meters squared. Insulin resistance was calculated using the homeostasis model assessment of IR (HOMA-IR) as described by Matthews et al. (23): FPG (mmol/L) × FINS (mU/L)/22.5. According to the serum hsCRP levels, participants were divided into four quartiles (Q1, Q2, Q3, and Q4) as follows: Q1, <3.30 mg/L; Q2, 3.30~4.46 mg/L; Q3, 4.46~6.68 mg/L; and Q4, ≥ 6.68 mg/L.
Definition of MAFLD
Patients were diagnosed as MAFLD based on evidence of ultrasonically diagnosed hepatic steatosis in addition to one of the following three criteria, namely overweight/obesity, T2DM, or metabolic dysregulation regardless of alcohol consumption or other concomitant liver diseases, which was proposed by the international expert consensus statement in 2020 (1). Herein, the metabolic dysregulation was defined by the presence of at least two metabolic risk abnormalities found in lean or normal weight patients, including hypertension, dyslipidemia, hyperglycemia, IR, and high CRP levels. Among which, hypertension was considered if their SBP ≥ 130 mmHg or DBP ≥ 85 mmHg or they were taking antihypertensive drugs. The presence of dyslipidemia was ascertained by plasma TG ≥ 1.7mmol/L in the total population or plasma HDL‐C < 1.0 mmol/L for men and < 1.3 mmol/L for women or was taking specific lipid-lowering drugs. T2DM was diagnosed according to the guideline for the prevention and treatment of type 2 diabetes mellitus in China (2020 edition) (24). In addition, plasma CRP > 2 mg/L or HOMA-IR ≥ 2.5 were also considered as MAFLD-associated metabolic abnormalities. As a result, 25 patients were stratified into the MAFLD group only based on ultrasonically diagnosed hepatic steatosis and high hsCRP levels. Patients who did not meet any of the above conditions were referred to as the non-MAFLD group.
Liver Assessment and Subgroups
Hepatic steatosis in this study was determined by hepatic ultrasonography according to the guidelines for prevention and treatment of NAFLD (25). Controlled attenuation parameter (CAP) and liver stiffness measurement (LSM) were obtained from transient elastography (FibroScan®) by physicians trained and certified by the manufacturer. Only examinations with at least 10 valid individual measurements were deemed valid. Furthermore, we estimated the severity of liver steatosis and fibrosis according to results from FibroScan (26). Based on CAP values, liver steatosis was graded as follows: absent: < 302 dB/m, mild to moderate: 302-337 dB/m, and severe: ≥337 dB/m. Based on LSM values, hepatic fibrosis was staged as follows: absent: < 8.2 kPa, mild to moderate: 8.2-13.6 kPa, and severe: ≥13.6 kPa. All the hepatic ultrasonography and transient elastography were performed and evaluated by experienced ultrasonographers who were blinded to the participants’ clinical and biochemical details.
Statistical Analysis
All statistical analyses were performed using SPSS 23.0 software (SPSS Inc., Chicago, IL) and the figures were produced by GraphPad Prism 6.0 project. Continuous variables were reported as means ± standard deviation (SD) or medians (interquartile ranges) based on data distribution and compared by independent Student’s t-test or one-way analysis of variance (ANOVA). Categorical variables were expressed as numbers and percentages and compared by chi-squared test. Non-normally distributed data were analyzed by non-parametric test and logarithmically transformed to normality when appropriate. The bivariate Pearson correlation analysis was carried out to determine the relationship between hsCRP and other related metabolic parameters. Univariable logistic regression analysis was conducted to explore the contribution of hsCRP and other risk factors in MAFLD and its progression. Multivariate logistic regression analysis was used to estimate the adjusted odds ratios (AORs) and 95% confidence intervals (CIs) for MAFLD, severe steatosis, and severe fibrosis in each hsCRP quartile in three regression models (1): for age, gender, and BMI (2); for age, gender, BMI, ALT, AST, and γ-GT; and (3) for TC, TG, HDL-C, LDL-C, LnFINS, LnHOMA-IR in addition to all covariates in (2). A two‐tailed P < 0.05 was considered to be statistically significant.
Results
Clinical Characteristics of Study Participants
In total, 393 obese patients (192 men and 201 women) with mean age 38.9 ± 16.6 years and mean BMI 34.8 ± 6.6 kg/m2 were analyzed in the present study. The prevalence of MAFLD, severe liver steatosis, and liver fibrosis in the cohort was 80.9% (n = 318), 61.3% (n = 241), and 16.0% (n = 63), respectively. Baseline clinical characteristics of the study population stratified by MAFLD status are depicted in Table 1 . Compared to the non-MAFLD group, the MAFLD group tended to be younger (P < 0.001) and had significantly higher BMI, DBP, ALT, AST, γ-GT, TC, TG, LDL-C, LnFINS, and LnHOMA-IR, as well as lower HDL-C levels (all P < 0.05). Concerning inflammatory markers, serum hsCRP levels were remarkably elevated in the MAFLD group (5.9 ± 3.8 mg/L vs. 4.5 ± 2.2 mg/L, P < 0.001) as opposed to their counterparts whereas IL-6, IL-8, and TNF-α levels were unchanged between the two groups. This increased hsCRP level in the MAFLD group is at least partly due to the study design in that serum hsCRP was used as a criterion to stratify MAFLD. Additionally, the overall proportions of hypertension, hyperlipidemia, and T2DM in the MAFLD group were significantly higher than those in the non-MAFLD group (P = 0.004, P = 0.008, P = 0.021, respectively). According to the results from FibroScan, severe and mild to moderate liver steatosis were found in 236 (74.3%) and 82 (25.7%) patients in the MAFLD group, which was significantly higher than those in the non-MAFLD group (all P <0.001). Likely, the percentage of patients with severe and mild to moderate liver fibrosis was remarkably higher in the MAFLD group as opposed to those in the non-MAFLD group (severe: 19.6% vs. 1.3%, P < 0.001; mild-moderate: 46.8% vs. 24.0%, P < 0.001).
Table 1.
Baseline characteristics of the study cohort stratified by MAFLD status.
| Parameters | Total population | Non-MAFLD | MAFLD | P value |
|---|---|---|---|---|
| n = 393 | n = 75 | n = 318 | ||
| Demographics | ||||
| Gender (male) | 192 (48.8%) | 35 (46.6%) | 157 (49.4%) | 0.674 |
| Age (yr) | 38.9 ± 16.6 | 47.2 ± 18.3 | 36.9 ± 15.6 | <0.001 |
| BMI (kg/m2) | 34.8 ± 6.6 | 31.8 ± 5.4 | 35.4 ± 6.7 | <0.001 |
| SBP (mmHg) | 137.0 ± 17.0 | 135.0 ± 18.0 | 137.0 ± 18.0 | 0.390 |
| DBP (mmHg) | 81.0 ± 14.0 | 76.0 ± 11.0 | 82.0 ± 14.0 | 0.006 |
| Laboratory parameters | ||||
| ALT (U/L) | 46.9 ± 40.3 | 29.6 ± 23.4 | 50.9 ± 42.3 | <0.001 |
| AST (U/L) | 32.5 ± 22.4 | 24.5 ± 14.8 | 34.4 ± 23.5 | <0.001 |
| γ-GT (U/L) | 44.4 ± 37.9 | 29.6 ± 19.4 | 47.4 ± 39.8 | <0.001 |
| TC (mmol/L) | 4.6 ± 0.9 | 4.4 ± 0.9 | 4.7 ± 0.9 | 0.040 |
| TG (mmol/L) | 2.2 ± 1.3 | 1.7 ± 0.8 | 2.3 ± 1.4 | <0.001 |
| HDL-C (mmol/L) | 1.1 ± 0.3 | 1.2 ± 0.6 | 1.0 ± 0.2 | 0.001 |
| LDL-C (mmol/L) | 2.8 ± 0.8 | 2.5 ± 0.8 | 2.8 ± 0.8 | 0.015 |
| FPG (mmol/L) | 7.4 ± 2.5 | 7.4 ± 2.5 | 7.4 ± 2.6 | 0.985 |
| Ln FINS (mU/L) | 3.0 ± 0.7 | 2.7 ± 0.7 | 3.1 ± 0.7 | <0.001 |
| Ln HOMA-IR | 1.9 ± 0.7 | 1.6 ± 0.7 | 1.9 ± 0.7 | <0.001 |
| Inflammatory markers | ||||
| IL-6 (pg/ml) | 5.7 (11.2) | 4.1 (8.1) | 5.8 (11.3) | 0.054 |
| IL-8 (pg/ml) | 106.0 (462.4) | 53.7 (356.6) | 119.0 (474.7) | 0.106 |
| TNF-α (pg/ml) | 12.2 (15.4) | 8.6 (9.3) | 12.9 (15.8) | 0.263 |
| hsCRP (mg/L) | 5.6 ± 3.6 | 4.5 ± 2.2 | 5.9 ± 3.8 | <0.001 |
| Comorbidities, n (%) | ||||
| Hypertension | 253 (65.9) | 39 (52.0) | 214 (67.3) | 0.004 |
| Hyperlipidemia | 376 (95.7) | 69 (89.6) | 307 (97.2) | 0.008 |
| Type 2 diabetes | 370 (75.2) | 45 (64.3) | 225 (77.9) | 0.021 |
| Liver steatosis, n (%) | ||||
| Absent | 64 (16.3) | 64 (85.5) | 0 (0.0) | <0.001 |
| Mild to moderate | 88 (22.4) | 6 (8.0) | 82 (25.7) | <0.001 |
| Severe steatosis | 241 (61.3) | 5 (6.5) | 236 (74.3) | <0.001 |
| Liver fibrosis, n (%) | ||||
| Absent | 163 (41.5) | 56 (74.6) | 107 (33.6) | <0.001 |
| Mild to moderate | 167 (42.5) | 18 (24.0) | 149 (46.8) | <0.001 |
| Severe fibrosis | 63 (16.0) | 1 (1.3) | 62 (19.6) | <0.001 |
Continuous data are presented as means ± standard deviations (SD) or medians (interquartile ranges). Non-normally distributed data were log-transformed before analysis. Categorical variables are presented as percentages (%). BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure; ALT, alanine transaminase; AST, aspartate aminotransferase; γ-GT, gamma-glutamyl transferase; TC, total cholesterol; TG, triglyceride; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; FPG, fasting plasma glucose; FINS, fasting insulin; HOMA-IR, homeostasis model assessment of insulin resistance; hsCRP, high-sensitive C reactive protein; IL-6, interleukin-6; IL-8, interleukin-8; TNF-α, tumor necrosis factor-α. MAFLD vs. Non-MAFLD, P-values < 0.05 were accepted as statistically significant.
Proportions of MAFLD, Severe Steatosis, and Fibrosis Across hsCRP Quartiles
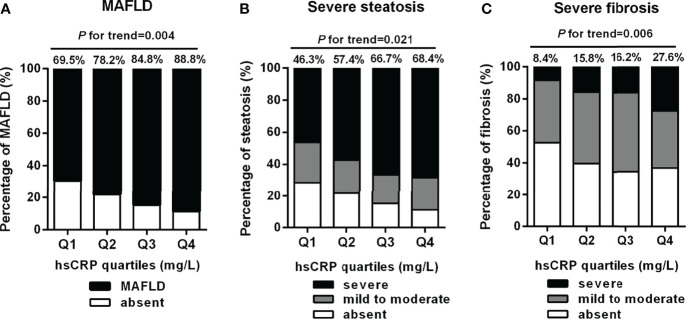
To explore the correlation of serum hsCRP with risk of MAFLD and its progression among obese subjects, we grouped patients into four groups (Q1, Q2, Q3, Q4) based on serum hsCRP levels. As shown in Figure 2 , there was a significantly increasing trend in the percentage of MAFLD, severe steatosis, and severe fibrosis with the increment of serum hsCRP quartiles. The constituent ratio of MAFLD was 69.5% in Q1, 78.2% in Q2, 84.8% in Q3, and 88.8% in Q4, respectively (P-trend = 0.004) ( Figure 2A ). Accordingly, the proportion of severe steatosis was 46.3%, 57.4%, 66.7%, and 68.4% (Q1-Q4, P-trend = 0.021) ( Figure 2B ). Likewise, the proportion of severe fibrosis was significantly increased across the serum hsCRP quartiles (8.4%, 15.8%, 16.2%, and 27.6%, Q1-Q4, P-trend = 0.006) ( Figure 2C ).
Figure 2.
Percentage of MAFLD, severe steatosis, and severe fibrosis across serum hsCRP quartiles in obese patients. Serum hsCRP levels were plotted into four quartiles (Q1, Q2, Q3, and Q4). There was a significantly increasing trend in the percentage of MAFLD (A), severe steatosis (B), and severe fibrosis (C) across serum hsCRP quartiles (P-trend = 0.004, 0.021, and 0.006, respectively). P-values < 0.05 were accepted as statistically significant.
Indicators for MAFLD and Severe Steatosis and Fibrosis
The factors associated with the risk of MAFLD, severe steatosis, and fibrosis in obese patients are presented in Table 2 . Among them, age, BMI, ALT, AST, γ-GT, TC, TG, HDL-C, LDL-C, FPG, LnFINS, and LnHOMA-IR were fitted in quartile categories. The category boundaries of each variable are described in the footnote of Table 2 . The univariate logistic regression analysis demonstrated a significantly higher odds ratios (ORs) for MAFLD in obese patients with elevated BMI (ORs 1.637, 95%CI 1.183-2.266, P = 0.003), γ-GT (ORs 1.500, 95%CI 1.068-2.107, P = 0.019), LnHOMA-IR (ORs 1.195, 95%CI 1.076-1.842, P = 0.009), and hsCRP (ORs 1.203, 95%CI 1.065-2.358, P = 0.003). In addition, the ORs for severe steatosis were significantly increased among obese participants with women (ORs 2.093, 95%CI 1.222-2.583, P < 0.001), higher BMI (ORs 1.824, 95%CI 1.405-2.367, P < 0.001), γ-GT (ORs 1.342, 95%CI 1.018-1.768, P = 0.037), LDL-C (ORs 1.426, 95%CI 1.040-1.955, P = 0.027), LnHOMA-IR (ORs 1.140, 95%CI 1.060-1.338, P = 0.030), and hsCRP (ORs 1.159, 95%CI 1.065-2.262, P = 0.001). Similarly, there was a remarkably elevated risk for severe fibrosis among obese patients with higher BMI (ORs 1.488, 95%CI 1.044-2.119, P = 0.028), TC (ORs 1.567, 95%CI 1.126-1.986, P = 0.044), TG (ORs 1.582, 95%CI 1.128-2.220, P = 0.008), LnHOMA-IR (ORs 1.178, 95%CI 1.073-1.346, P = 0.010), and hsCRP (ORs 1.163, 95%CI 1.078-1.254, P < 0.001). Conversely, age (ORs 0.678, 95%CI 0.468-0.981, P = 0.039) was inversely associated with severe fibrosis.
Table 2.
Odds ratios (ORs) of multiple variables for MAFLD, severe steatosis, and severe fibrosis.
| Variables | MAFLD | Severe steatosis | Severe fibrosis | ||||||
|---|---|---|---|---|---|---|---|---|---|
| ORs | 95%CI | P value | ORs | 95%CI | P value | ORs | 95%CI | P value | |
| Gender | 1.532 | 0.788, 2.980 | 0.208 | 2.093 | 1.222, 2.583 | 0.007 | 1.195 | 0.597, 2.394 | 0.615 |
| Age quartiles | 0.951 | 0.680, 1.329 | 0.767 | 0.983 | 0.748, 1.292 | 0.903 | 0.678 | 0.468, 0.981 | 0.039 |
| BMI quartiles | 1.637 | 1.183, 2.266 | 0.003 | 1.824 | 1.405, 2.367 | <0.001 | 1.488 | 1.044, 2.119 | 0.028 |
| CRP | 1.203 | 1.065, 2.358 | 0.003 | 1.159 | 1.065, 1.262 | 0.001 | 1.163 | 1.078, 1.254 | <0.001 |
| ALT quartiles | 1.417 | 0.924, 2.173 | 0.110 | 1.294 | 0.914, 1.831 | 0.146 | 0.995 | 0.633, 1.565 | 0.984 |
| AST quartiles | 0.811 | 0.541, 1.214 | 0.308 | 0.758 | 0.544, 1.055 | 0.101 | 0.906 | 0.596, 1.379 | 0.646 |
| γ-GT quartiles | 1.500 | 1.068, 2.107 | 0.019 | 1.342 | 1.018, 1.768 | 0.037 | 1.219 | 0.833, 1.785 | 0.308 |
| TC quartiles | 0.807 | 0.537, 1.214 | 0.304 | 0.805 | 0.568, 1.143 | 0.225 | 1.567 | 1.126, 1.986 | 0.044 |
| TG quartiles | 1.119 | 0.813, 1.541 | 0.489 | 0.971 | 0.748, 1.261 | 0.825 | 1.582 | 1.128, 2.220 | 0.008 |
| HDL-C quartiles | 0.859 | 0.642, 1.147 | 0.303 | 1.006 | 0.794, 1.274 | 0.961 | 1.176 | 0.860, 1.606 | 0.310 |
| LDL-C quartiles | 1.305 | 0.902, 1.888 | 0.158 | 1.426 | 1.040, 1.955 | 0.027 | 1.565 | 0.949, 2.582 | 0.080 |
| FPG quartiles | 1.025 | 0.752, 1.397 | 0.875 | 0.993 | 0.773, 1.277 | 0.959 | 0.827 | 0.599, 1.142 | 0.250 |
| LnFINS quartiles | 0.911 | 0.588, 1.412 | 0.678 | 1.032 | 0.720, 1.479 | 0.863 | 1.406 | 0.891, 2.218 | 0.143 |
| LnHOMAIR quartiles | 1.532 | 0.788, 2.980 | 0.208 | 2.093 | 1.222, 2.583 | 0.007 | 1.195 | 0.597, 2.394 | 0.615 |
ORs were determined by univariable logistic regression analysis. Age was plotted in quartiles with value set at < 26, 26-35, 35-54, and ≧54 years old. BMI was plotted in quartiles with levels set at < 29.1, 29.1-33.3, 33.3-38.5, and ≧38.5kg/m2. ALT was plotted in quartiles with levels set at <20.6, 20.6-33.9, 33.9-63.9, and ≧63.9 U/L. AST was plotted in quartiles with levels set at <17.7, 17.7-25.9, 25.9-39.5, and ≧39.5 U/L. γ-GT was plotted in quartiles with levels set at < 23.1, 23.1-35.8, 35.8-55.3, and ≧55.3 U/L. TC was plotted in quartiles with levels set at < 4.04, 4.04-4.61, 4.61-5.30, and ≧5.30 mmol/L. TG was plotted in quartiles with levels set at <1.3, 1.3-1.8, 1.80-2.80, and ≧2.80 mmol/L. HDL-C was plotted in quartiles with levels set at <0.89, 0.89-1.04, 1.04-1.19, and ≧1.19 mmol/L. LDL-C was plotted in quartiles with levels set at <2.1, 2.1-2.7, 2.7-3.4, and ≧3.4 mmol/L. FPG was plotted in quartiles with levels set at <5.6, 5.6-7.0, 7.0-8.2, and ≧8.2 mmol/L. LnFINS was plotted in quartiles with levels set at < 2.5, 2.5-3.1, 3.1-3.6, and ≧3.6 mU/L. LnHOMA-IR was plotted in quartiles with levels set at < 1.4, 1.4-1.9, 1.9-2.4, and ≧2.4. P-values < 0.05 were accepted as statistically significant.
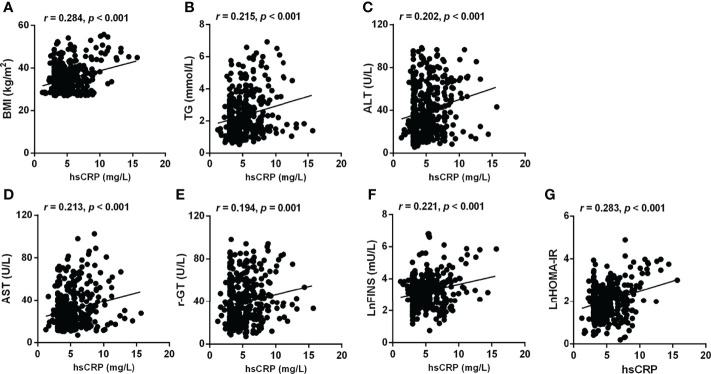
In the bivariate correlation analysis, serum hsCRP was positively associated with BMI (r = 0.284, P < 0.001), TG (r = 0.215, P < 0.001), ALT (r = 0.202, P < 0.001), AST (r = 0.213, P < 0.001), γ-GT (r = 0.194, P = 0.001), LnFINS (r = 0.221, P < 0.001), and LnHOMA-IR (r = 0.283, P < 0.001) ( Figure 3 ). However, no significant correlation was observed between hsCRP and age, TC, HDL-C, LDL-C, and FPG.
Figure 3.
Correlations between hsCRP and other metabolic risk factors related to MAFLD, steatosis, and fibrosis. Serum hsCRP levels were positively associated with BMI (A), TG (B), ALT (C), AST (D), γ-GT (E), LnFINS (F), and LnHOMA-IR (G). Non-normally distributed data were log-transformed before analysis. P-values < 0.05 were accepted as statistically significant.
Relationship Between hsCRP, MAFLD, and the Severity of Liver Steatosis and Fibrosis
Binary logistic regression analysis was applied to further delineate the relationship between hsCRP and MAFLD and its disease progression based on hsCRP serum quartiles. In the univariate logistic regression analysis, the unadjusted ORs (95%CI) for MAFLD were significantly higher in Q3 (ORs 2.461, 95%CI 1.220-4.964, P = 0.012) and Q4 (ORs 3.475, 95%CI 1.618-7.462, P = 0.001) than that in Q1 (as reference) ( Figure 4A ). A similar result was found in the correlation between hsCRP and severe steatosis ( Figure 4B ). Furthermore, there was a dramatically elevated risk for severe fibrosis in Q4 (ORs 3.335, 95%CI 1.409-7.895, P = 0.006) compared to Q1 ( Figure 4C ). After adjusting for potential confounders, the adjusted ORs (AORs) and 95%CI for MAFLD, severe steatosis, and severe fibrosis remained increased across the hsCRP quartiles in three models (age, gender, and BMI involved in Model 1; age, gender, BMI, ALT, AST, and γ-GT involved in Model 2; age, gender, BMI, ALT, AST, γ-GT, TC, TG, HDL-C, LDL-C, LnFINS, and LnHOMA-IR involved in Model 3) ( Table 3 ). The P for trend for MAFLD was 0.001 in Model 1, 0.006 in Model 2, and 0.014 in Model 3. Also, the P for trend for severe steatosis (all P-trend < 0.001) and fibrosis (all P-trend < 0.05) was significant in all three models.
Figure 4.
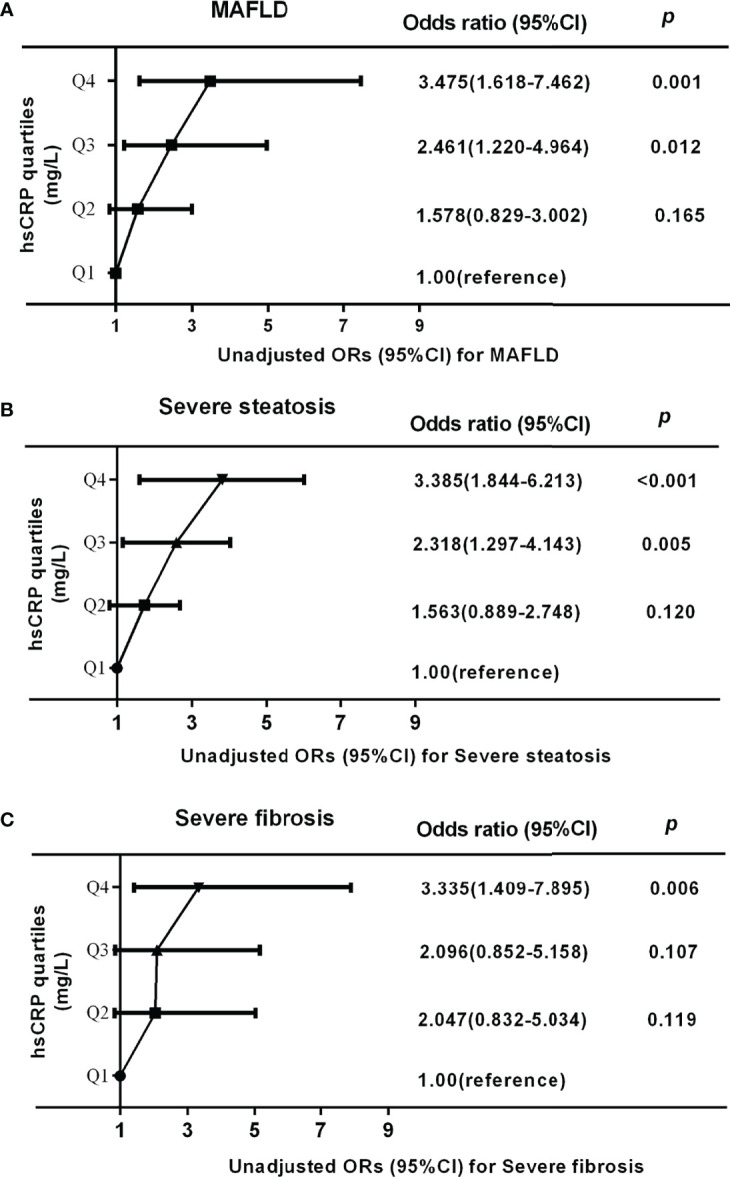
Unadjusted odds ratios (ORs) and 95% confidence intervals (CI) for MAFLD, severe steatosis, and severe fibrosis in obese patients according to serum hsCRP quartiles: results of binary logistic regression analysis. Serum hsCRP levels were plotted into four quartiles (Q1, Q2, Q3, and Q4). (A, B) The ORs (95%CI) for MAFLD and severe steatosis in Q3 and Q4 quartile were significantly higher than that in Q1 quartile (all P-trend < 0.001). (C) The ORs (95%CI) for severe fibrosis in Q4 quartile were significantly higher than that in Q1 quartile (P-trend = 0.007). P-values < 0.05 were accepted as statistically significant.
Table 3.
Adjusted ORs and 95%CI for MAFLD, severe steatosis, and severe fibrosis based on hsCRP quartiles: results of binary logistic regression analysis.
| hsCRP quartiles (mg/L) | For MAFLD | For severe steatosis | For severe fibrosis | ||||||
|---|---|---|---|---|---|---|---|---|---|
| β | Adjusted OR (95%CI) | P | β | Adjusted OR (95%CI) | P | β | Adjusted OR (95%CI) | P | |
| Model 1 | |||||||||
| Q1 (<3.30) | Ref | 0.014 | Ref | 0.001 | 0.079 | ||||
| Q2 (3.30-4.66) | 0.402 | 1.494 (0.755-2.957) | 0.249 | 0.354 | 1.424 (0.778-2.607) | 0.252 | 0.566 | 1.761 (0.690-4.495) | 0.237 |
| Q3 (4.66-6.68) | 0.786 | 2.195 (1.053-4.576) | 0.036 | 0.774 | 2.169 (1.170-4.018) | 0.014 | 0.578 | 1.782 ().701-4.528) | 0.225 |
| Q4 (≥6.68) | 1.241 | 3.460 (1.556-7.697) | 0.002 | 1.303 | 3.681 (1.914-7.076) | <0.001 | 1.169 | 3.218 (1.297-7.983) | 0.012 |
| P for trend | 0.001 | <0.001 | 0.014 | ||||||
| Model 2 | |||||||||
| Q1 (<3.30) | Ref | 0.050 | Ref | 0.001 | Ref | 0.131 | |||
| Q2 (3.30-4.66) | 0.213 | 1.238 (0.596-2.569) | 0.567 | 0.387 | 1.473 (0.789-2.752) | 0.224 | 0.647 | 1.910 (0.738-4.941) | 0.182 |
| Q3 (4.66-6.68) | 0.805 | 2.237 (1.008-4.965) | 0.048 | 0.816 | 2.261 (1.199-4.262) | 0.012 | 0.608 | 1.837 (0.718-4.697) | 0.204 |
| Q4 (≥6.68) | 1.046 | 2.847 (1.203-6.735) | 0.017 | 1.340 | 3.818 (1.946-7.490) | <0.001 | 1.109 | 3.030 (1.203-7.633) | 0.019 |
| P for trend | 0.006 | <0.001 | 0.026 | ||||||
| Model 3 | |||||||||
| Q1 (<3.30) | Ref | 0.093 | Ref | 0.001 | 0.119 | ||||
| Q2 (3.30-4.66) | 0.261 | 1.298 (0.587-2.872) | 0.520 | 0.273 | 1.314 (0.677-2.550) | 0.420 | 0.830 | 2.293 (0.818-6.422) | 0.114 |
| Q3 (4.66-6.68) | 0.878 | 2.407 (1.002-5.781) | 0.049 | 0.838 | 2.312 (1.170-4.569) | 0.016 | 0.441 | 1.554 (0.547-4.414) | 0.408 |
| Q4 (≥6.68) | 0.970 | 2.637 (1.073-6.482) | 0.035 | 1.300 | 3.670 (1.832-7.353) | <0.001 | 1.127 | 3.086 (1.154-8.249) | 0.025 |
| P for trend | 0.014 | <0.001 | 0.021 | ||||||
Serum hsCRP levels were plotted into four quartiles (Q1, Q2, Q3, and Q4). Model 1: age, gender, and BMI were selected. Model 2: age, gender, BMI, ALT, AST, and γ-GT were selected. Model 3: age, gender, BMI, ALT, AST, γ-GT, TC, TG, HDL-C, LDL-C, LnFINS, and LnHOMA-IR were selected. P-values <0.05 were accepted as statistically significant.
Discussion
In the present study, serum hsCRP levels were strongly correlated with the risk of MAFLD and the severity of hepatic steatosis and fibrosis among the Chinese obese population. These results remained significant even after adjusting potential confounding factors, indicating that hsCRP may be an independent risk factor regarding the progression of MAFLD into more aggressive phenotypes. These associations may be mediated by substantial IR and inflammation status in addition to excess weight, dyslipidemia, and increased liver enzymes. Hence, our findings provide a new insight into the correlation between serum hsCRP levels and the occurrence of MAFLD, as well as the severity of liver steatosis and fibrosis. It raises the possibility that hsCRP can be used as a potential biomarker for the disease severity in MAFLD among the Chinese obese population or, at least, identify therapeutic approaches to modulate inflammation in the context of obesity and MAFLD.
MAFLD, formerly named NAFLD, is a new definition of liver disease associated with known metabolic dysfunction (1), and is commonly associated with various metabolic disorders including obesity, IR, T2DM, dyslipidemia, elevated liver enzymes, and inflammatory markers (11, 27–30). It has also been reported to identify individuals with liver steatosis and significant fibrosis better than NAFLD (7). In agreement with this, the current study showed that patients with MAFLD had significantly higher BMI, ALT, AST, γ-GT, TC, TG, LDL-C, LnFINS, and LnHOMA-IR, as well as lower HDL-C levels compared to those without MAFLD. In addition, the proportions of hypertension, hyperlipidemia, T2DM, and severe hepatic steatosis and fibrosis were remarkably increased in the MAFLD group instead of the non-MAFLD group. Based on these complications accompanied by MAFLD, it is extremely crucial for us to determine key factors and potential mechanisms of MAFLD and identify individuals with severe liver steatosis and fibrosis, aiming to provide clinical evidence for prevention and treatment within a similar population.
Obesity-induced chronic low-grade inflammation has been documented to play a fundamental role in the pathogenesis of MAFLD (31). As one of the inflammatory markers, hsCRP has been previously recommended to help diagnose NAFLD or NASH. Yeniova et al. (32) demonstrated that hsCRP levels were significantly higher in patients with NAFLD compared to their counterparts. In one case-control study among Asian Indians in North India, higher hsCRP levels were correlated with increased risk of NAFLD (33). In another case-control study including 32 cases with biopsy-proven NAFLD and 34 non-obese control subjects, serum hsCRP levels were found to be higher in the NASH group compared to its control group among NAFLD cases, and considerably increased in higher fibrosis stage (34). Consistently, Yoneda et al. (19) revealed marked elevated hsCRP levels in cases with NASH than in those with simple steatosis. Also, elevated hsCRP levels were correlated with increased risk for advanced fibrosis in the same study, suggesting that serum hsCRP may be a useful non-invasive marker that not only differentiates between NASH and simple steatosis in NAFLD patients, but also predicts the severity of hepatic fibrosis in cases with NASH. In disagreement with this, one study including 627 obese adults showed a positive correlation of hsCRP with degree of steatosis but not with severity of NAFLD (20). In contrast with NAFLD, MAFLD is a multisystem metabolic disease with higher proportions of metabolic comorbidities, supporting that MAFLD is more practical for identifying patients with fatty liver disease with high risk of disease progression (35). However, there is scarce evidence regarding the association between hsCRP and the risk of MAFLD especially among Chinese obese patients. In the present study, we demonstrated that serum hsCRP levels were significantly higher in individuals with MAFLD than in those without MAFLD among Chinese obese patients. In addition, the proportion of MAFLD was significantly increased with the increment of serum hsCRP quartiles. Furthermore, we observed a significantly increased risk for MAFLD across the hsCRP quartiles even after adjusting for potential confounding factors (age, gender, BMI, ALT, AST, γ-GT, TC, TG, HDL-C, LDL-C, LnFINS, and LnHOMA-IR). These results indicate that serum hsCRP might be an independent risk factor of MAFLD among Chinese obese patients.
Accumulating evidence has validated that hepatic fibrosis is the major adverse outcome in patients with MAFLD (13). Although serum hsCRP has been confirmed previously to have significant impact on the NAFLD pathophysiology and can be used clinically as an inflammatory marker in diagnosing MAFLD in lean or normal weight patients, extremely limited studies were done regarding the influence of serum hsCRP on the disease progression of MAFLD. It is worth noting that CAP and LSM obtained from transient elastography (FibroScan®) have been recommended as useful tools to detect both hepatic steatosis and fibrosis in MAFLD (26). In accordance with literature consensus (36), we assessed the severity of liver steatosis and fibrosis in MAFLD using FibroScan and investigated their correlation with serum hsCRP level. The results showed that the proportions of severe steatosis and fibrosis were increased significantly across serum hsCRP quartiles. Moreover, the binary logistic regression analysis demonstrated remarkably increased ORs (95%CI) for severe steatosis and fibrosis with the rise in serum hsCRP levels. This result remained statistically significant after adjustment for cardiometabolic risk factors (age, gender, BMI, ALT, AST, γ-GT, TC, TG, HDL-C, LDL-C, LnFINS, and LnHOMA-IR), which are widely held MAFLD determining factors. These findings implied that elevated serum hsCRP could be a potential biomarker to monitor the disease progression of MAFLD among Chinese obese patients. However, the previous evidence produced conflicting results. Some study reported that hsCRP levels were positively correlated with the grade of steatosis among Asian Indians (17), whereas another study demonstrated no association between hsCRP and the severity of steatosis and fibrosis in obese women (37). Another study showed that hsCRP was positively associated with degree of steatosis but not the severity of fibrosis in obese adults from France and Belgium (20). These discrepancies might be explained by differences in the race, dietary structure, and environment, as well as the genetic makeup of the study population which have been validated to be important predictors in the development of MAFLD (32).
It is well demonstrated that various metabolic risk factors related to MAFLD are associated with a systemic inflammatory response. Several studies have proven significant association between hsCRP and MAFLD risk factors. Frohlich et al. (38) reported that hsCRP was positively associated with BMI, TC, TG, and FPG but negatively associated with HDL-C levels. Timpson et al. (39) also demonstrated marked association between hsCRP and BMI, SBP, WHR, HDL-C, and TG, as well as HOMA-IR in British women. Moreover, higher hsCRP levels were found to be correlated with elevated ALT and AST in addition to higher BMI, FPG, TG, and LDL-C (30). Indeed, our findings confirmed that serum hsCRP levels were positively correlated with BMI, TG, ALT, AST, γ-GT, LnFINS, and LnHOMA-IR. Besides, the univariate logistic regression in our study showed that levels of hsCRP, BMI, γ-GT, and LnHOMA-IR were positively associated with the risk of MAFLD. Furthermore, hsCRP, BMI, γ-GT, LDL-C, and LnHOMA-IR were found to be significantly correlated with the risk of severe steatosis whereas hsCRP, BMI, TC, TG, and LnHOMA-IR were correlated with the risk of severe fibrosis. After adjusting these significant variables, these correlations remain significant. These findings raised the possibility that systemic inflammation is commonly accompanied by MAFLD and serum hsCRP could be an important clinical feature of MAFLD and its disease severity in addition to excess weight, dyslipidemia, elevated liver enzymes, and increased IR.
The underlying mechanism linking hsCRP to MAFLD may include the following points. One possibility might be that the liver is the main target organ of glucolipid metabolism regulation and is a key site of metabolic homeostasis and inflammation in obesity (40). Obesity causes a marked increase in the hepatic recruitment of macrophage, accompanied by the local production of inflammatory chemokines and cytokines that can induce IR in hepatocytes and ultimately promote hepatic steatosis (41). Furthermore, Koyama et al. (42) reviewed the close association among obesity, hepatic inflammation, and the development of NAFLD, which can progress to nonalcoholic steatohepatitis, fibrosis, and eventually cirrhosis due to sequential activation of inflammatory cascade (10). Another explanation is that overwhelming evidence suggests that IR is the main mediator linking inflammation to MAFLD. By inhibiting the key inflammatory signaling pathways including NF-κB and JNK pathways (43), as well as various proinflammatory signaling molecules and cytokines, the connection between obesity and IR can be blocked, finally causing significant tissue damage such as liver steatosis and fibrosis (44, 45). Due to that the roles of hsCRP in the MAFLD disease progression have been poorly understood, further investigation needs to be performed in large-scale populations.
There are some limitations to our work that must be acknowledged. First, our cross-sectional study might not reflect the causal relationship between serum hsCRP, MAFLD, and the severity of hepatic steatosis and fibrosis among Chinese obese patients. Second, the severity of hepatic steatosis and fibrosis in our study was assessed using noninvasive methods but not liver biopsy, which is well known to be the gold standard for the diagnosis of MAFLD. The reason is that these non-invasive techniques have been validated to be accurate and widely available in the general population (26). Third, other unmeasured confounding variables may exist. Finally, newly diagnosed MAFLD has not been widely applied in the real world. Despite these caveats, our findings provide a novel insight regarding the correlation of hsCRP with MAFLD among Chinese obese participants and raise the possibility that hsCRP might be used as a potential biomarker to monitor the disease severity in MAFLD. Future studies with many patients whose liver assessment is determined by non-invasive methods and MAFLD is diagnosed based on novel diagnostic criteria are warranted to further validate the findings from this study and identify their underlying mechanism.
Conclusion
Serum hsCRP is an independent risk factor of MAFLD and positively associated with the severity of hepatic steatosis and fibrosis among the Chinese obese population even after adjusting for multiple confounding factors. These associations may be attributed to inflammation status and IR in addition to excess weight, dyslipidemia, and elevated liver enzymes. Further studies should focus on delving into the possible mechanism and its clinical significance.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by the Research Ethics Review Committee of Shanghai Tenth People’s Hospital, School of Medicine, Tongji University, Shanghai, China. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
ZC and HD contributed to the first drafting of the manuscript. ZC, HD, MH, QC, and YH contributed to the data collection, statistical analysis, and interpretation of the data. QC revised this manuscript critically for important intellectual content. QS and BL contributed to the conception and design of this study, and critical revision of the manuscript, as well as approved the final version of the submitted manuscript. All authors read and approved the final manuscript.
Funding
This work was financially supported by grants from the National Key R&D Program of China (No. 2018YFC1314100), Climbing Talent Program of the 10th People's Hospital affiliated to Tongji University (040120050), Clinical research funds for shanghai municipal health commission (202040170). the Shanghai Committee of Science and Technology of China (Nos. 18411951803 and 17DZ1910603), National Natural Science Foundation of China (No. 81970677 and 82170861), and the Shanghai Pujiang Program (Nos. 2019PJD040 and 2018PJD038).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We would like to thank all the staff from the Department of Endocrinology and Metabolism, Shanghai Tenth People’s Hospital in China, and give our sincere appreciation to the reviewers for their helpful comments on this article.
References
- 1. Eslam M, Newsome PN, Sarin SK, Anstee QM, Targher G, Romero-Gomez M, et al. A New Definition for Metabolic Dysfunction-Associated Fatty Liver Disease: An International Expert Consensus Statement. J Hepatol (2020) 73(1):202–9. doi: 10.1016/j.jhep.2020.07.045 [DOI] [PubMed] [Google Scholar]
- 2. Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global Epidemiology of Nonalcoholic Fatty Liver Disease-Meta-Analytic Assessment of Prevalence, Incidence, and Outcomes. Hepatology (2016) 64(1):73–84. doi: 10.1002/hep.28431 [DOI] [PubMed] [Google Scholar]
- 3. Younossi Z, Anstee QM, Marietti M, Hardy T, Henry L, Eslam M, et al. Global Burden of NAFLD and NASH: Trends, Predictions, Risk Factors and Prevention. Nat Rev Gastroenterol Hepatol (2018) 15(1):11–20. doi: 10.1038/nrgastro.2017.109 [DOI] [PubMed] [Google Scholar]
- 4. Milic S, Lulic D, Stimac D. Non-Alcoholic Fatty Liver Disease and Obesity: Biochemical, Metabolic and Clinical Presentations. World J Gastroenterol (2014) 20(28):9330–7. doi: 10.3748/wjg.v20.i28.9330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chalasani N, Younossi Z, Lavine JE, Charlton M, Cusi K, Rinella M, et al. The Diagnosis and Management of Nonalcoholic Fatty Liver Disease: Practice Guidance From the American Association for the Study of Liver Diseases. Hepatology (2018) 67(1):328–57. doi: 10.1002/hep.29367 [DOI] [PubMed] [Google Scholar]
- 6. Cariou B, Byrne CD, Loomba R, Sanyal AJ. Nonalcoholic Fatty Liver Disease as a Metabolic Disease in Humans: A Literature Review. Diabetes Obes Metab (2021) 23(5):1069–83. doi: 10.1111/dom.14322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Yamamura S, Eslam M, Kawaguchi T, Tsutsumi T, Nakano D, Yoshinaga S, et al. MAFLD Identifies Patients With Significant Hepatic Fibrosis Better Than NAFLD. Liver Int (2020) 40(12):3018–30. doi: 10.1111/liv.14675 [DOI] [PubMed] [Google Scholar]
- 8. Chen VL, Yeh ML, Yang JD, Leong J, Huang DQ, Toyoda H, et al. Effects of Cirrhosis and Diagnosis Scenario in Metabolic-Associated Fatty Liver Disease-Related Hepatocellular Carcinoma. Hepatol Commun (2021) 5(1):122–32. doi: 10.1002/hep4.1606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Li L, Liu DW, Yan HY, Wang ZY, Zhao SH, Wang B. Obesity is an Independent Risk Factor for Non-Alcoholic Fatty Liver Disease: Evidence From a Meta-Analysis of 21 Cohort Studies. Obes Rev (2016) 17(6):510–9. doi: 10.1111/obr.12407 [DOI] [PubMed] [Google Scholar]
- 10. Mantovani A, Byrne CD, Bonora E, Targher G. Nonalcoholic Fatty Liver Disease and Risk of Incident Type 2 Diabetes: A Meta-Analysis. Diabetes Care (2018) 41(2):372–82. doi: 10.2337/dc17-1902 [DOI] [PubMed] [Google Scholar]
- 11. Ballestri S, Zona S, Targher G, Romagnoli D, Baldelli E, Nascimbeni F, et al. Nonalcoholic Fatty Liver Disease is Associated With an Almost Twofold Increased Risk of Incident Type 2 Diabetes and Metabolic Syndrome. Evidence From a Systematic Review and Meta-Analysis. J Gastroenterol Hepatol (2016) 31(5):936–44. doi: 10.1111/jgh.13264 [DOI] [PubMed] [Google Scholar]
- 12. Tana C, Ballestri S, Ricci F, Di Vincenzo A, Ticinesi A, Gallina S, et al. Cardiovascular Risk in Non-Alcoholic Fatty Liver Disease: Mechanisms and Therapeutic Implications. Int J Environ Res Public Health (2019) 16(17):1–19.. doi: 10.3390/ijerph16173104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Vilar-Gomez E, Calzadilla-Bertot L, Wai-Sun Wong V, Castellanos M, Aller-de la Fuente R, Metwally M, et al. Fibrosis Severity as a Determinant of Cause-Specific Mortality in Patients With Advanced Nonalcoholic Fatty Liver Disease: A Multi-National Cohort Study. Gastroenterology (2018) 155(2):443–57.e17. doi: 10.1053/j.gastro.2018.04.034 [DOI] [PubMed] [Google Scholar]
- 14. Esser N, Legrand-Poels S, Piette J, Scheen AJ, Paquot N. Inflammation as a Link Between Obesity, Metabolic Syndrome and Type 2 Diabetes. Diabetes Res Clin Pract (2014) 105(2):141–50. doi: 10.1016/j.diabres.2014.04.006 [DOI] [PubMed] [Google Scholar]
- 15. Amin MN, Hussain MS, Sarwar MS, Rahman Moghal MM, Das A, Hossain MZ, et al. How the Association Between Obesity and Inflammation may Lead to Insulin Resistance and Cancer. Diabetes Metab Syndr (2019) 13(2):1213–24. doi: 10.1016/j.dsx.2019.01.041 [DOI] [PubMed] [Google Scholar]
- 16. Katsarou A, Moustakas II, Pyrina I, Lembessis P, Koutsilieris M, Chatzigeorgiou A. Metabolic Inflammation as an Instigator of Fibrosis During Non-Alcoholic Fatty Liver Disease. World J Gastroenterol (2020) 26(17):1993–2011. doi: 10.3748/wjg.v26.i17.1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kumar R, Porwal YC, Dev N, Kumar P, Chakravarthy S, Kumawat A. Association of High-Sensitivity C-Reactive Protein (Hs-CRP) With Non-Alcoholic Fatty Liver Disease (NAFLD) in Asian Indians: A Cross-Sectional Study. J Family Med Prim Care (2020) 9(1):390–4. doi: 10.4103/jfmpc.jfmpc_887_19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kogiso T, Moriyoshi Y, Shimizu S, Nagahara H, Shiratori K. High-Sensitivity C-Reactive Protein as a Serum Predictor of Nonalcoholic Fatty Liver Disease Based on the Akaike Information Criterion Scoring System in the General Japanese Population. J Gastroenterol (2009) 44(4):313–21. doi: 10.1007/s00535-009-0002-5 [DOI] [PubMed] [Google Scholar]
- 19. Yoneda M, Mawatari H, Fujita K, Iida H, Yonemitsu K, Kato S, et al. High-Sensitivity C-Reactive Protein is an Independent Clinical Feature of Nonalcoholic Steatohepatitis (NASH) and Also of the Severity of Fibrosis in NASH. J Gastroenterol (2007) 42(7):573–82. doi: 10.1007/s00535-007-2060-x [DOI] [PubMed] [Google Scholar]
- 20. Zimmermann E, Anty R, Tordjman J, Verrijken A, Gual P, Tran A, et al. C-Reactive Protein Levels in Relation to Various Features of Non-Alcoholic Fatty Liver Disease Among Obese Patients. J Hepatol (2011) 55(3):660–5. doi: 10.1016/j.jhep.2010.12.017 [DOI] [PubMed] [Google Scholar]
- 21. Zhu C, Cui R, Gao M, Rampersad S, You H, Sheng C, et al. The Associations of Serum Uric Acid With Obesity-Related Acanthosis Nigricans and Related Metabolic Indices. Int J Endocrinol (2017) 2017:5438157. doi: 10.1155/2017/5438157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Chalasani N, Younossi Z, Lavine JE, Diehl AM, Brunt EM, Cusi K, et al. The Diagnosis and Management of Non-Alcoholic Fatty Liver Disease: Practice Guideline by the American Association for the Study of Liver Diseases, American College of Gastroenterology, and the American Gastroenterological Association. Hepatology (2012) 55(6):2005–23. doi: 10.1002/hep.25762 [DOI] [PubMed] [Google Scholar]
- 23. Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis Model Assessment: Insulin Resistance and Beta-Cell Function From Fasting Plasma Glucose and Insulin Concentrations in Man. Diabetologia (1985) 28(7):412–9. doi: 10.1007/BF00280883 [DOI] [PubMed] [Google Scholar]
- 24. Society CD . Guideline for the Prevention and Treatment of Type 2 Diabetes Mellitus in China:2020 Edition. Zhonghua Tang Niao Bing Za Zhi (2021) 13(4):315–409. doi: 10.3760/cma.j.cn311282-20210304-00142 [DOI] [Google Scholar]
- 25. Fatty Liver Expert Committee CMDA . Guidelines of Prevention and Treatment for Nonalcoholic Fatty Liver Disease: A 2018 Update. Zhonghua Gan Zang Bing Za Zhi (2018) 26(3):195–203. doi: 10.3760/cma.j.issn.1007-3418.2018.03.008 [DOI] [PubMed] [Google Scholar]
- 26. Eddowes PJ, Sasso M, Allison M, Tsochatzis E, Anstee QM, Sheridan D, et al. Accuracy of FibroScan Controlled Attenuation Parameter and Liver Stiffness Measurement in Assessing Steatosis and Fibrosis in Patients With Nonalcoholic Fatty Liver Disease. Gastroenterology (2019) 156(6):1717–30. doi: 10.1053/j.gastro.2019.01.042 [DOI] [PubMed] [Google Scholar]
- 27. Chen YL, Li H, Li S, Xu Z, Tian S, Wu J, et al. Prevalence of and Risk Factors for Metabolic Associated Fatty Liver Disease in an Urban Population in China: A Cross-Sectional Comparative Study. BMC Gastroenterol (2021) 21(1):212. doi: 10.1186/s12876-021-01782-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lee H, Lee YH, Kim SU, Kim HC. Metabolic Dysfunction-Associated Fatty Liver Disease and Incident Cardiovascular Disease Risk: A Nationwide Cohort Study. Clin Gastroenterol Hepatol (2021) 19(10):2138–47.e10. doi: 10.1016/j.cgh.2020.12.022 [DOI] [PubMed] [Google Scholar]
- 29. Kim D, Konyn P, Sandhu KK, Dennis BB, Cheung AC, Ahmed A. Metabolic Dysfunction-Associated Fatty Liver Disease is Associated With Increased All-Cause Mortality in the United States. J Hepatol (2021) 75(6):1284–91. doi: 10.1016/j.jhep.2021.07.035 [DOI] [PubMed] [Google Scholar]
- 30. Zhang X, Li R, Chen Y, Dai Y, Chen L, Qin L, et al. The Role of Thyroid Hormones and Autoantibodies in Metabolic Dysfunction Associated Fatty Liver Disease: TgAb May Be a Potential Protective Factor. Front Endocrinol (Lausanne) (2020) 11:598836. doi: 10.3389/fendo.2020.598836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Rodriguez-Hernandez H, Simental-Mendia LE, Rodriguez-Ramirez G, Reyes-Romero MA. Obesity and Inflammation: Epidemiology, Risk Factors, and Markers of Inflammation. Int J Endocrinol (2013) 2013:678159. doi: 10.1155/2013/678159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Yeniova AO, Kucukazman M, Ata N, Dal K, Kefeli A, Basyigit S, et al. High-Sensitivity C-Reactive Protein is a Strong Predictor of Non-Alcoholic Fatty Liver Disease. Hepatogastroenterology (2014) 61(130):422–5. doi: 10.5754/hge13916 [DOI] [PubMed] [Google Scholar]
- 33. Nigam P, Bhatt SP, Misra A, Vaidya M, Dasgupta J, Chadha DS. Non-Alcoholic Fatty Liver Disease Is Closely Associated With Sub-Clinical Inflammation: A Case-Control Study on Asian Indians in North India. PLoS One (2013) 8(1):e49286. doi: 10.1371/journal.pone.0049286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Maleki I, Rastgar A, Hosseini V, Taghvaei T, Rafiei A, Barzin M, et al. High Sensitive CRP and Pentraxine 3 as Noninvasive Biomarkers of Nonalcoholic Fatty Liver Disease. Eur Rev Med Pharmacol Sci (2014) 18(11):1583–90. [PubMed] [Google Scholar]
- 35. Lin S, Huang J, Wang M, Kumar R, Liu Y, Liu S, et al. Comparison of MAFLD and NAFLD Diagnostic Criteria in Real World. Liver Int (2020) 40(9):2082–9. doi: 10.1111/liv.14548 [DOI] [PubMed] [Google Scholar]
- 36. Lombardi R, Airaghi L, Targher G, Serviddio G, Maffi G, Mantovani A, et al. Liver Fibrosis by FibroScan(R) Independently of Established Cardiovascular Risk Parameters Associates With Macrovascular and Microvascular Complications in Patients With Type 2 Diabetes. Liver Int (2020) 40(2):347–54. doi: 10.1111/liv.14274 [DOI] [PubMed] [Google Scholar]
- 37. Baltieri L, Chaim EA, Chaim FDM, Utrini MP, Gestic MA, Cazzo E. Correlation Between Nonalcoholic Fatty Liver Disease Features and Levels of Adipokines and Inflammatory Cytokines Among Morbidly Obese Individuals. Arq Gastroenterol (2018) 55(3):247–51. doi: 10.1590/s0004-2803.201800000-62 [DOI] [PubMed] [Google Scholar]
- 38. Frohlich M, Imhof A, Berg G, Hutchinson WL, Pepys MB, Boeing H, et al. Association Between C-Reactive Protein and Features of the Metabolic Syndrome: A Population-Based Study. Diabetes Care (2000) 23(12):1835–9. doi: 10.2337/diacare.23.12.1835 [DOI] [PubMed] [Google Scholar]
- 39. Timpson NJ, Lawlor DA, Harbord RM, Gaunt TR, Day IN, Palmer LJ, et al. C-Reactive Protein and its Role in Metabolic Syndrome: Mendelian Randomisation Study. Lancet (2005) 366(9501):1954–9. doi: 10.1016/S0140-6736(05)67786-0 [DOI] [PubMed] [Google Scholar]
- 40. Jones JG. Hepatic Glucose and Lipid Metabolism. Diabetologia (2016) 59(6):1098–103. doi: 10.1007/s00125-016-3940-5 [DOI] [PubMed] [Google Scholar]
- 41. Obstfeld AE, Sugaru E, Thearle M, Francisco AM, Gayet C, Ginsberg HN, et al. C-C Chemokine Receptor 2 (CCR2) Regulates the Hepatic Recruitment of Myeloid Cells That Promote Obesity-Induced Hepatic Steatosis. Diabetes (2010) 59(4):916–25. doi: 10.2337/db09-1403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Koyama Y, Brenner DA. Liver Inflammation and Fibrosis. J Clin Invest (2017) 127(1):55–64. doi: 10.1172/JCI88881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Solinas G, Karin M. JNK1 and IKKbeta: Molecular Links Between Obesity and Metabolic Dysfunction. FASEB J (2010) 24(8):2596–611. doi: 10.1096/fj.09-151340 [DOI] [PubMed] [Google Scholar]
- 44. Vandanmagsar B, Youm YH, Ravussin A, Galgani JE, Stadler K, Mynatt RL, et al. The NLRP3 Inflammasome Instigates Obesity-Induced Inflammation and Insulin Resistance. Nat Med (2011) 17(2):179–88. doi: 10.1038/nm.2279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Li P, Oh DY, Bandyopadhyay G, Lagakos WS, Talukdar S, Osborn O, et al. LTB4 Promotes Insulin Resistance in Obese Mice by Acting on Macrophages, Hepatocytes and Myocytes. Nat Med (2015) 21(3):239–47. doi: 10.1038/nm.3800 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.