Background.
The effect of pregnancy on the course of estimated glomerular filtration rate (eGFR) is unknown in kidney transplant recipients (KTRs).
Methods.
We conducted a nationwide multicenter cohort study in KTRs with pregnancy (>20 wk) after kidney transplantation (KT). Annual eGFRs after KT until death or graft loss and additional eGFRs before each pregnancy were collected according to protocol. Changes in eGFR slope before and after each pregnancy were analyzed by generalized estimating equations multilevel analysis adjusted for transplant vintage.
Results.
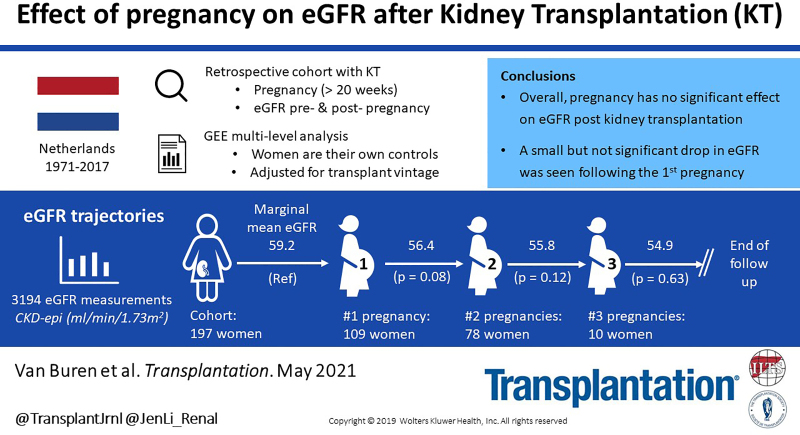
We included 3194 eGFR measurements before and after pregnancy in 109 (55%) KTRs with 1, 78 (40%) with 2, and 10 (5%) with 3 pregnancies after KT. Median follow-up after first delivery post-KT was 14 y (interquartile range, 18 y). Adjusted mean eGFR prepregnancy was 59 mL/min/1.73 m2 (SEM [standard error of the mean] 1.72; 95% confidence interval [CI], 56-63), after the first pregnancy 56 mL/min/1.73 m2 (SEM 1.70; 95% CI, 53-60), after the second pregnancy 56 mL/min/1.73 m2 (SEM 2.19; 95% CI, 51-60), and after the third pregnancy 55 mL/min/1.73 m2 (SEM 8.63; 95% CI, 38–72). Overall eGFR slope after the first, second, and third pregnancies was not significantly worse than prepregnancy (P = 0.28). However, adjusted mean eGFR after the first pregnancy was 2.8 mL/min/1.73 m2 (P = 0.08) lower than prepregnancy.
Conclusions.
The first pregnancy has a small, but insignificant, effect on eGFR slope in KTRs. Midterm hyperfiltration, a marker for renal reserve capacity, was associated with better eGFR and death-censored graft survival. In this KTR cohort with long-term follow-up, no significant effect of pregnancy on kidney function was detected.
INTRODUCTION
Pregnancy after kidney transplantation (KT) is increasingly common. To date, the voluntary Transplant Pregnancy Registry International (Philadelphia, USA) has registered >1100 pregnancies after KT.1 There have been data that indicates pregnancies may lead to higher risk of death-censored graft loss (DCGL) if there is presence of risk factors like creatinine >1.5 mg/dL.2 Nevertheless, the incidence of DCGL was not higher for kidney transplant recipients (KTRs) with a history of pregnancy than for nulliparous KTRs in multiple studies3-12; however, these studies used very heterogenic control groups and did not account for the fact that nulliparous KTRs might have other underlying conditions, such as syndromic disease, which could also influence the choice of not conceiving or could affect the incidence of DCGL.
Besides postpregnancy DCGL, little is known about the effect of pregnancy on the course of graft function in KTRs. Women with gestational hypertension show a decrease instead of the normal physiological increase in estimated glomerular filtration rate (eGFR) during pregnancy13; however, in these women, the temporary decrease in eGFR during pregnancy did not persist or progress after pregnancy.14 This physiological increase in eGFR during pregnancy is also known as midterm hyperfiltration. The absence of midterm hyperfiltration is related to worse pregnancy outcomes in the general population.15 Bramham et al16 described an absence of serum creatinine (SCr) fall during pregnancy in almost 49% of KTR patients; in this study, no relationship with adverse pregnancy outcomes was found. Whether midterm hyperfiltration during pregnancy has an effect on long-term eGFR in the KT population is unknown.
Recently, our meta-analysis among KTRs showed higher SCr from 6 to 24 mo after pregnancy compared with prepregnancy SCr17; however, this increase was not detectable beyond 2 y after pregnancy in several small studies.4,11,18-20 Although reassuring, only 1 larger study addresses the effect of pregnancy on the long-term course of kidney function.11 Therefore, we conducted an evaluation of individual eGFR slopes before and after pregnancy in a large nationwide KTR cohort. Additionally, we identified the most important predictors for eGFR decline and DCGL following pregnancy after KT.
MATERIALS AND METHODS
Study Design
For the collection of data, we used data from the Dutch PARTOUT network (Pregnancy After Renal Transplantation Outcomes). This nationwide network consists of obstetricians and transplant nephrologists from all 8 Dutch transplant centers and an epidemiologist. The study protocol, data management, and data analyses plan were designed within the multidisciplinary team of the PARTOUT network. All women who underwent a KT in the Netherlands since 1971 and became pregnant afterward were included in this data set. Data of KTs as well as pregnancy outcomes were collected by examining the medical and obstetrical charts. Data were collected until December 31, 2017. The PARTOUT study was approved by the Medical Ethics Committee of all transplant centers (MEC-2016-634, 16-021/C, G16.014, 2015-2262).
Selection of Participants
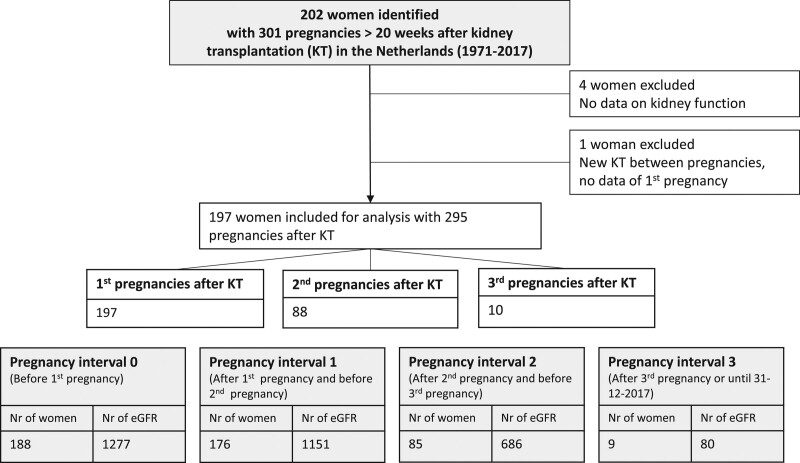
Participants were identified by systemic search in the Dutch Organ Transplant Registry. All patients transplanted in the Netherlands are registered in the Dutch Organ Transplant Registry. We complemented this by questioning nephrologists and gynecologists involved in pregnancy in KTRs of all transplant centers in the Netherlands. Of the 202 women identified with pregnancies after KT, 197 KTRs were included for analysis (Figure 1).
FIGURE 1.
Flowchart. (For missing subjects per interval see Figure S1, SDC, http://links.lww.com/TP/C283.) eGFR, estimated glomerular filtration rate; KT, kidney transplantation; Nr, number.
Data Collection
Data collection, entry, and access were organized by the PARTOUT network using Open Clinica open-source software.21 The information required was obtained by thoroughly examining all available medical and obstetrical charts.
Baseline KT data included specifications of the cause of end-stage renal disease, type of KT, immunosuppressive and antihypertensive drug use, and medical history. Rejection was defined as having a biopsy-proven rejection or treatment for rejection by clinical diagnosis.
Furthermore, obstetric outcomes were collected. Preexisting hypertension was defined as systolic blood pressure ≥140 mm Hg, diastolic blood pressure ≥90 mm Hg, or antihypertensive drug use before pregnancy.22 The same definition was used for pregnancy-induced hypertension for KTRs who developed hypertension during pregnancy without having preexisting hypertension. Preeclampsia during pregnancy was not uniformly defined because it was defined by the attending physician at the time of pregnancy. It could not be defined uniformly retrospectively because of the large number of missing proteinuria values. According to guidelines valid at that time, preeclampsia was marked by the presence of pregnancy-induced hypertension, >20 wk of gestation, and proteinuria.23 Midterm hyperfiltration was defined as having >15% decrease of SCr during pregnancy. This was calculated by comparing the lowest SCr between 8 and 20 wk of gestation with prepregnancy SCr.24,25 Proteinuria levels were unavailable for analysis because of missing data.
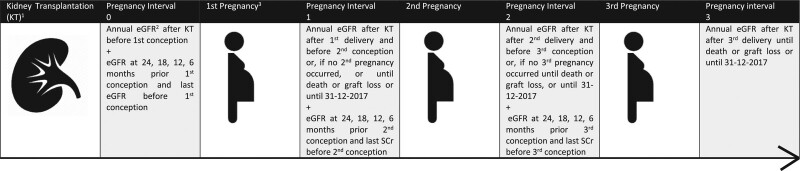
For the longitudinal analysis of kidney function, outpatient clinic SCr levels were collected after 1 y after KT (ie, most recent prepregnancy KT) and every year thereafter until graft loss or death occurred or until the end of follow-up, which was December 31, 2017. Additionally, SCr levels were collected at 5 consecutive time points before conception to ensure a sufficient amount of SCr levels were available before pregnancy. For each measurement, the exact interval after KT (in days) was calculated. A visual overview of the study design is presented in Figure 2. For this longitudinal analysis, SCr levels during pregnancy and within 6 mo after delivery were excluded. Also, SCr measurements before the age of 18 y were excluded (pregnancy before the age of 18 y did not occur). The eGFR was calculated with the chronic kidney disease epidemiology collaboration formula and expressed in mL/min/1.73 m2.26
FIGURE 2.
Schematic overview of the study design. 1—last KT before pregnancy. 2—eGFR calculated with CKD-EPI formula expressed in mL/min/1.73 m2. 3—first pregnancy means first pregnancy after KT. eGFR values during pregnancy within 6 mo after delivery and eGFR values before the age of 18 y were excluded from this analysis. CKD-EPI, chronic kidney disease epidemiology collaboration; eGFR, estimated glomerular filtration rate; KT, kidney transplantation.
Statistical Analysis
Data were analyzed using SPSS, version 25 (SPSS) and Graph Pad Prism version 8.4.1 (Graph Pad Software). Two types of analyses were performed to examine the effect of pregnancy on kidney function after KT.
First, the effect of pregnancy on eGFR was explored by means of generalized estimating equation (GEE) analysis. GEE is an established method for multilevel analysis. GEE is a population average model that captures average trajectories across the overall study population and estimates the marginal associations between the repeated outcome measures and the risk factors.27 Therefore, GEE allows us to analyze the change in eGFR over time with varying numbers of observations per KT. The number of days after KT of each individual measurement was used as the within-subject level and as a continuous covariate (years after KT) in the model. In addition, eGFR measurements were divided into 2 to 4 “pregnancy intervals” depending on the number of pregnancies (Figure 2). Analysis of the effect of pregnancy was adjusted for transplant vintage (years). Pregnancy interval was used as a categorical variable with pregnancy interval 0 as reference category.
Additionally, interaction was examined by adding the interaction term, “pregnancy interval × transplant vintage.” Because of the large number of within-subject levels, defined by time between eGFR measurement and KT, an exchangeable correlation matrix structure GEE analysis was used. This assumes a fixed correlation between eGFR measurements within the same subject.
Furthermore, a sub-GEE analysis was performed to identify other possible predictors for eGFR deterioration after KT. A dichotomous variable, “after first pregnancy,” was created to discriminate eGFRs measured before or after the first pregnancy. In this subanalysis, the variable “after first pregnancy” includes all eGFR measurements after the first pregnancy (pregnancy interval 1), after the second pregnancy (pregnancy interval 2), and after the third pregnancy (pregnancy interval 3). For all prognostic variables, adjustments were made for transplant vintage. A directed acyclical graph was created to identify the most important potential confounders (Figure S1, SDC, http://links.lww.com/TP/C283). Variables tested were age at KT (years), year of KT, year of pregnancy, body mass index, primipara at first pregnancy after KT, living donor KT, preemptive KT, >1 KT before pregnancy, rejection before first pregnancy, transplant-to-conception interval in years, prepregnancy eGFR, prepregnancy hypertension, and calcineurin inhibitor (CNI) use. When a possible predictor turned out to be significant, the interaction term “[significant variable] × after first pregnancy” was added to the model. This additional analysis was performed to test if pregnancy amplifies the negative effect of the specific predictor on eGFR. Furthermore, for multivariate analysis, all significant predictive variables were put together in the GEE model.
Second, we examined the association between possible predictors and DCGL after pregnancy. Kaplan-Meier and Cox proportional hazards regression analyses were performed to calculate hazard ratio (HR) and 95% confidence interval (CI). We tested the same possible predictors as used for the eGFR analysis. In the proportional hazard model, person time was counted from the delivery date of their first pregnancy after KT to graft loss or December 31, 2017. Censoring was applied in cases of death or loss to follow-up.
RESULTS
Baseline Characteristics
Table 1 shows baseline characteristics of the study participants (n = 197) who had 295 pregnancies during follow-up. Characteristics of the first pregnancies of these women are described in Table 2. Pregnancy outcomes were complicated by preterm birth (<37 wk) in >50% of the pregnancies; mean birthweight was 2281 (±853) g. Of the 99 women who had hypertension before their first pregnancy, we could retrieve hypertensive agents of 87 KTRs during the first trimester of their first pregnancy after transplantation. Seventy percent had 1 antihypertensive agent, 29% had 2 antihypertensive agents, and 1 woman had 3 antihypertensive agents. Gestational hypertension occurred in almost 46% of the women and preeclampsia in 31%. Almost half of the women had midterm hyperfiltration (SCr increase >15%). The differences in baseline characteristics of women transplanted before and after 1990 are highlighted in Tables S1 and S2 (SDC, http://links.lww.com/TP/C283).
TABLE 1.
Baseline characteristics for women
| Total group (n = 197) | ||
|---|---|---|
| n (%)a/mean ± SD | Missing, n (%)a | |
| Follow-up time after first delivery, yb | 14 (18) | 0 |
| Total pregnancies | 295 | |
| 1 pregnancy after KT | 109 (55) | |
| 2 pregnancies after KT | 78 (40) | |
| 3 pregnancies after KT | 10 (5) | |
| Cause of ESRD | 18 (9) | |
| Glomerulonephritis | 77 (39) | |
| Diabetes mellitus | 6 (3) | |
| Autoimmune (SLE/vasculitis) | 8 (4) | |
| Tubulointerstitial | 29 (15) | |
| Cystic kidney disease | 8 (4) | |
| Renal vascular disease (excl. vasculitis) | 7 (4) | |
| Urologic | 7 (4) | |
| Other congenital hereditary | 23 (12) | |
| Other multicystic diseases | 5 (3) | |
| Other | 22 (11) | |
| Maternal death during follow-up | 28 (14) | 0 |
| Time between first delivery and death, yb | 14 (10) | |
| Age at KT, y | 25 (6.1) | 0 |
| Year of KT | 1995 (11.6) | 0 |
| 1971–1989 | 65 (33) | |
| 1990–1999 | 50 (25) | |
| 2000–2009 | 56 (28) | |
| 2010–2015 | 26 (13) | |
| Living donor KT | 83 (42) | 6 (3) |
| Preemptive KT | 36 (18) | 18 (9) |
| >1 KT before pregnancy | 39 (20) | 5 (3) |
| Rejection before pregnancy | 68 (35) | 46 (23) |
| Graft loss during pregnancy | 1 (0.5) | 0 |
| Graft loss after first pregnancy | 42 (24) | 25 (13) |
| Time between first pregnancy and graft loss, yb | 6 (7) | 0 |
| Time between KT and graft loss, yb | 12 (7) | 0 |
aDue to rounding it can be possible that percentages do not reach 100%.
bMedian (IQR).
ESRD, end-stage renal disease; IQR, interquartile range; KT, kidney transplantation (last KT before pregnancy); SLE, systemic lupus erythematosus.
TABLE 2.
Characteristics of all first pregnancies (n = 197)
| Total group (N = 197) | ||
|---|---|---|
| N (%)a/mean ± SD | Missing, N (%)a | |
| KT to conception interval, yb | 4 (6) | 8 (4) |
| 0–2 y | 29 (15) | |
| 2–4 y | 78 (41) | |
| 5–9 y | 49 (25) | |
| 10–24 y | 33 (17) | |
| Prepregnancy eGFR | 62 (±21) | 7 (4) |
| eGFR <45 mL/min/1.73 m2 | 34 (17) | |
| eGFR <30 mL/min/1.73 m2 | 8 (4) | |
| Prepregnancy MAP | 95 (±11) | 38 (19) |
| Prepregnancy hypertension | 99 (50) | 24 (12) |
| CNI before first pregnancy | 97 (49) | 8 (4) |
| Year of pregnancy | 2001 (±10.9) | 0 |
| 1979–1989 | 37 (19) | |
| 1990–1999 | 44 (22) | |
| 2000–2009 | 54 (27) | |
| 2010–2017 | 62 (32) | |
| Prepregnancy BMI | 25 (±4) | 70 (36) |
| Primipara at first pregnancy after KT | 154 (78) | 5 (3) |
| Pregnancy outcomes | ||
| Preterm <37 wk | 102 (52) | 13 (7) |
| Preterm <34 wk | 50 (25) | |
| Birthweight, g | 2281 (± 853) | 13 (7) |
| Low birthweight (<2500 g) | 103 (52) | |
| Very low birthweight (<1500 g) | 30 (15) | |
| Gestational hypertension | 90 (46) | 37 (19) |
| Severe hypertensionc | 30 (15) | 56 (28) |
| Preeclampsia | 60 (31) | 32 (16) |
| % SCr decrease during pregnancy | 17 (±10) | 45 (23) |
| >15% SCr decrease during pregnancy | 90 (46) | |
aDue to rounding, it can be possible that percentages not reach 100%.
bMedian (IQR).
cBlood pressure systolic ≥160 mm Hg or diastolic ≥100 mm Hg.
BMI, body mass index; CNI, calcineurin inhibitors; eGFR, estimated glomerular filtration rate; IQR, interquartile range; KT, kidney transplantation; MAP, mean arterial pressure; SCr, serum creatinine.
Change of Mean eGFR Before and After Pregnancy
Of the 197 KTRs with at least 1 pregnancy, of 9 women (5%), no eGFR was available before pregnancy (pregnancy interval 0), mostly because they got pregnant within 6 mo after KT. Of 17 KTRs (9%), no eGFR was available of pregnancy interval 1, mainly because their second pregnancy soon followed and no eGFR of pregnancy interval 1 could be included. Nevertheless, the follow-up of these women was continued after the second pregnancy in pregnancy interval 2 and, if a third pregnancy occurred, also in pregnancy interval 3. Specified reasons for missing values per subject are described in Table S3 (SDC, http://links.lww.com/TP/C283).
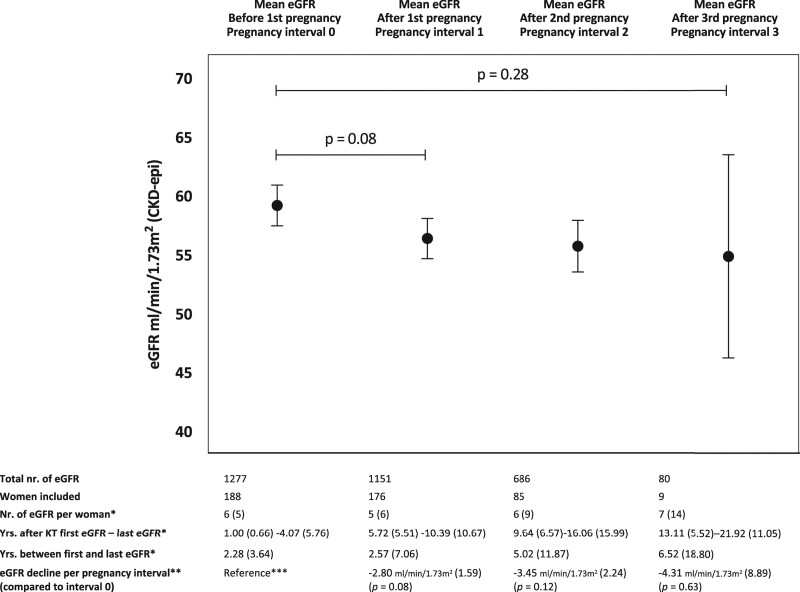
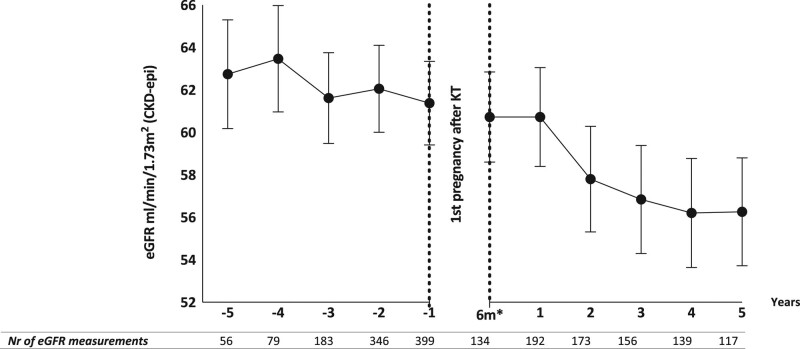
In our study population of 197 KTRs, the overall effect of transplant vintage on eGFR slope was –0.58 mL/min/1.73 m2 per year (SEM [standard error of the mean] 0.13; 95% CI, –0.84 to –0.31; P < 0.001). Overall mean eGFR after the first, second, and third pregnancies was not significantly worse than prepregnancy (P = 0.28). Adjusted mean eGFR decline in pregnancy interval 1 was –2.80 mL/min/1.73 m2 (SEM 1.59; 95% CI, –5.92 to 0.33; P = 0.08) over a median of 2.57 y (IQR, 7.06). During pregnancy interval 2, mean eGFR decline was –3.45 mL/min/1.73 m2 (SEM 2.24; 95% CI, –7.84 to 0.94; P = 0.12) over a median of 5.02 y (IQR, 11.87). During pregnancy interval 3, mean eGFR decline was –4.31 mL/min/1.73 m2 (SEM 8.89; 95% CI, –21.73 to 13.11; P = 0.63) over a median of 6.52 y (IQR, 18.80). Adjusted mean eGFRs per pregnancy interval are illustrated in Figure 3. Pregnancy interval 3 (ie, eGFR measurements after third pregnancy after KT) had a wide CI due to a small number of KTRs included. As expected, time between the first and last eGFR measurements was longer after second and third pregnancies as shown in Figure 3. The same analysis was also performed with KTRs who only had 1 pregnancy after KT. In this analysis, mean eGFR after pregnancy was significantly lower (P = 0.02) (Figure S2, SDC, http://links.lww.com/TP/C283).
FIGURE 3.
Adjusted mean eGFR before and after pregnancies after KT (GEE) (n = 197). *Median (IQR), **mean (SEM), and ***annual eGFR decline after KT in this population: mean 0.58 mL/min/1.73 m2 (SEM 0.13). In this model, “years after KT” was used as a continuous covariate and “pregnancy interval” as a categorical factor. Error bars illustrate SD. eGFRs during pregnancy and within 6 mo after delivery were excluded. CKD-epi, chronic kidney disease epidemiology collaboration; eGFR, estimated glomerular filtration rate; GEE, generalized estimated equations—a multilevel method; KT, kidney transplantation (last KT before pregnancy); SEM, standard error of the mean; subject level, subject ID; within-subject level, days after KT.
To test if pregnancy causes a faster decline of eGFR, the interaction term pregnancy interval × transplant vintage was added to the model. The interaction term pregnancy interval × transplant vintage was not significant for pregnancy interval 1 (β = –0.29, P = 0.29), pregnancy interval 2 (β = –0.55, P = 0.08), and pregnancy interval 3 (β = –0.46, P = 0.39). No additional effect of pregnancy on eGFR slope was observed.
Figure 4 illustrates the estimated marginal means per year of eGFR before and after first pregnancy adjusted for transplant vintage. To calculate the marginal means per year before and after first pregnancy, an additional GEE model was constructed. For this GEE model, a dichotomous variable, “after first pregnancy,” was created (ie, before or after first pregnancy). “After first pregnancy” implies all eGFR measurements after the first, second, and third pregnancies. The variables “after first pregnancy” and “years after first pregnancy” (after rounding visit dates into whole years) were added to the model as categorical factors.
FIGURE 4.
Adjusted mean eGFR before and after first pregnancy after KT (GEE). In this model, “years after KT” was used as a continuous covariate and “pregnancy interval” and “years after pregnancy” as categorical factors. Error bars illustrate SD. eGFRs during pregnancy and within 6 mo after delivery were excluded. *6 mo after first delivery after KT. For this analysis, all eGFR measurements after first pregnancy were included, as well as eGFR measurements after second (pregnancy interval 2) and third pregnancies (pregnancy interval 3). CKD-epi, chronic kidney disease epidemiology collaboration; eGFR, estimated glomerular filtration rate; GEE, generalized estimated equations—a multilevel method; KT, kidney transplantation; subject level, subject ID; within-subject level, days after KT.
Other Predictors That Affect eGFR After KT
To determine which other predictors might have an effect on eGFR after KT, we performed a GEE analysis with possible predictors of deterioration of eGFR (Table 3). For this analysis, all eGFR measurements after the first pregnancy were included, as well as eGFR measurements after the second (pregnancy interval 2) and third pregnancies (pregnancy interval 3). All variables were analyzed with adjustment for transplant vintage.
TABLE 3.
Effect of predictors on eGFR slope after KT (univariate analysis, GEE)
| β coefficient | SEM | P a | |
|---|---|---|---|
| Glomerulonephritis | 1.01 | 3.13 | 0.75 |
| Age at KT, y | –0.61 | 0.23 | 0.01 |
| Age at first delivery, y | –0.01 | 0.29 | 0.98 |
| Year of KT ≥1990 | –10.90 | 3.16 | <0.01 |
| BMI before first pregnancy | 0.15 | 0.52 | 0.77 |
| Primipara at first pregnancy after KT | 7.94 | 3.75 | 0.03 |
| Living KT | –4.35 | 2.94 | 0.20 |
| Preemptive KT | –0.82 | 3.59 | 0.98 |
| >1 KT before pregnancy | 2.44 | 3.27 | 0.45 |
| Rejection before first pregnancy | –7.01 | 4.09 | 0.046 |
| KT to first conception interval, y | 1.15 | 0.31 | <0.01 |
| <2b | Ref. | – | – |
| 2–4 | 5.33 | 3.98 | 0.18 |
| 5–9 | 7.95 | 4.93 | 0.11 |
| 10–24 | 16.77 | 4.92 | <0.01 |
| Prepregnancy eGFR (first pregnancy) | 0.82 | 0.05 | <0.01 |
| Prepregnancy eGFR <45 mL/min/1.73 m2 (first pregnancy) | –27.94 | 2.28 | <0.01 |
| Prepregnancy MAP (first pregnancy) | –0.43 | 0.15 | <0.01 |
| Prepregnancy hypertension (first pregnancy) | –9.10 | 3.01 | <0.01 |
| CNI before first pregnancy | –9.49 | 2.90 | <0.01 |
In the model, transplant vintage (years) was used as a continuous covariate. All variables above were added 1 by 1 to the model. For this analysis, a dichotomous variable “after first pregnancy” was created (before or after pregnancy). After pregnancy means all eGFR measurements after first pregnancy (pregnancy interval 1), after second pregnancy (pregnancy interval 2), and after third pregnancy (pregnancy interval 3). eGFRs during pregnancy and within 6 mo after delivery were excluded. Bold variables are significant (P < 0.05).
aFor all significant variables, the interaction with “after first pregnancy” was added to the model. Only prepregnancy eGFR × after first pregnancy was significant (β = –0.120, SEM 0.06, P = 0.048); in all the other variables, the interaction term was not significant.
bUsed as a reference category.
BMI, body mass index; CNI, calcineurin inhibitors; eGFR, estimated glomerular filtration rate; GEE, generalized estimated equations—a multilevel method; KT, kidney transplantation (last KT before pregnancy); SEM, standard error of the mean; subject level, subject ID; within-subject level, days after KT.
First, time-related variables were tested. Women who were transplanted and pregnant before 1990 had significantly better posttransplant eGFR than women who were transplanted and pregnant more recently (P < 0.01). Also, KT at a younger age was related to better eGFR after KT. This effect was no longer significant after exclusion of women who received a transplant before the age of 18 y (P = 0.11). Women conceiving with a transplant-to-conception interval of >10 y had a higher posttransplant eGFR; however, when excluding the group with a transplant-to-conception interval >10 y, no significant effect of transplant-to-conception interval on eGFR was observed. Adjusted posttransplant eGFR was higher in women who had not been pregnant before KT. Rejection, prepregnancy hypertension, mean arterial pressure, and CNI use had a significantly negative effect on posttransplant eGFR.
After identifying these predictors for worse eGFR after KT, the additive effect of pregnancy on eGFR was tested. Therefore, the interaction term “[significant variable] × after first pregnancy” was added to the univariate model. This interaction term was only significant for prepregnancy eGFR (β = –0.120, SEM 0.06, P = 0.048), concluding that lower prepregnancy eGFR causes worse eGFR after pregnancy. There was no interaction with other variables affecting posttransplant eGFR. Therefore, pregnancy does not seem to amplify the negative effect of these predictors on eGFR decline after KT.
Finally, when all significant variables were put together (except year of KT and year of first delivery after KT) in a multivariate GEE model, transplant vintage, rejection before first pregnancy, prepregnancy eGFR, and transplant-to-conception interval were independent risk factors for accelerated eGFR decline after KT (Table 4).
TABLE 4.
Effect of predictors on eGFR slope after KT (multivariate analysis, GEE)
| β coefficient | SEM | P | |
|---|---|---|---|
| After first pregnancy | –2.90 | 1.83 | 0.11 |
| Year of KT ≥1990 | –1.54 | 1.86 | 0.41 |
| Transplant vintage, y | –0.72 | 0.17 | <0.01 |
| Age at KT | 0.16 | 0.20 | 0.41 |
| Primipara | 2.30 | 2.09 | 0.27 |
| Rejection before first pregnancy | –4.12 | 1.59 | 0.01 |
| KT to first conception interval, y | 0.84 | 0.26 | <0.01 |
| Prepregnancy eGFR (first pregnancy) | 0.81 | 0.04 | <0.01 |
| Prepregnancy hypertension (first pregnancy) | –1.20 | 1.94 | 0.54 |
| CNI before first pregnancy | 0.47 | 1.54 | 0.76 |
For this analysis, a dichotomous variable “after first pregnancy” was created (before or after pregnancy). After pregnancy means all eGFR measurements after first pregnancy (pregnancy interval 1), after second pregnancy (pregnancy interval 2), and after third pregnancy (pregnancy interval 3). eGFRs during pregnancy and within 6 mo after delivery were excluded. Bold variables are significant (P < 0.05).
CNI, calcineurin inhibitor; eGFR, estimated glomerular filtration rate; GEE, generalized estimated equations; KT, kidney transplantation; SEM, standard error of the mean.
Additionally, univariate analysis of the effect of pregnancy outcomes on eGFR after pregnancy was performed (Table 5). For this analysis, eGFR measurements after second and third pregnancies were excluded. This analysis was also adjusted for transplant vintage and pregnancy interval. Midterm hyperfiltration was related to better eGFR after pregnancy (P = 0.04), whereas low birthweight tended to be related to worse eGFR after the first pregnancy (P = 0.06). When these outcomes were added to the multivariate model, none of them were identified as independent predictors for worse eGFR after pregnancy (Table S4, SDC, http://links.lww.com/TP/C283).
TABLE 5.
Effect of first pregnancy outcomes after KT on eGFR slope (univariate analysis, GEE)
| β coefficient | SEM | P b | |
|---|---|---|---|
| Preterm birth <37 wk | –4.74 | 2.65 | 0.74 |
| Low birthweight <2500 g | –5.12 | 2.75 | 0.06 |
| Very low birthweight <1500 g | –2.04 | 4.09 | 0.62 |
| Gestational hypertension | 3.95 | 2.84 | 0.16 |
| Severe hypertensiona | –4.31 | 3.75 | 0.25 |
| Preeclampsia | –0.97 | 2.72 | 0.72 |
| >15% SCr decrease during pregnancy | 5.87 | 2.81 | 0.04 |
In the model, transplant vintage (years) was used as a continuous covariate. All variables above were added 1 by 1 to the model. Bold variables are significant (P < 0.05).
aBlood pressure systolic ≥160 mm Hg or diastolic ≥100 mm Hg. eGFRs during pregnancy and within 6 mo after delivery were excluded. For this analysis, eGFR measurements after second and third pregnancies were excluded.
bFor all significant variables, the interaction with pregnancy interval was added to the model; in none of the cases the interaction term was significant.
GEE, generalized estimated equations–a multilevel method; KT, kidney transplantation (last KT before pregnancy); SCr, serum creatinine; subject level, subject ID; within-subject level, days after KT; SEM, standard error of the mean.
Kaplan-Meier and Cox Regression
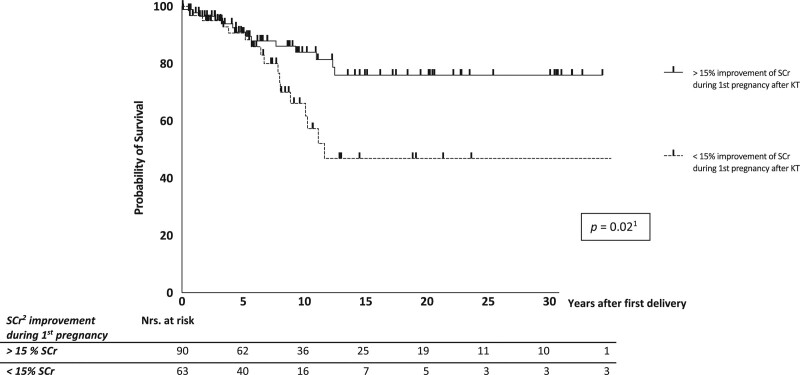
Kaplan-Meier and Cox regression analyses were performed to evaluate graft survival and risk factors (Figure 5). Overall, approximately 10% of the women lost their graft within 5 y after delivery and 20% within 10 y after first delivery. Cox regression analysis was performed to identify risk factors for DCGL. Women with a prepregnancy eGFR <45 mL/min/1.73 m2 had shorter graft survival (HR, 0.48; 95% CI, 0.24-0.94; P = 0.03). No difference in DCGL was observed between women with eGFR values between 45 and 60 mL/min/1.73 m2 and eGFR values >60 mL/min/1.73 m2. Furthermore, the transplant-to-conception interval had no significant effect on DCGL (HR, 0.94; 95% CI, 0.86-1.02; P = 0.14). Figure 5 shows that women with midterm hyperfiltration during their first pregnancy after KT had better graft survival than women without midterm hyperfiltration (HR, 2.31; 95% CI, 1.13-4.72; P = 0.02). Prepregnancy mean arterial pressure and prepregnancy hypertension were not related to DCGL; however, low birthweight was related to an increased risk of DCGL.
FIGURE 5.
Graft survival after first delivery after kidney transplantation (censored for death). 1Mulivariate Cox regression (hazard ratio, 2.31; 95% confidence interval, 1.13-4.72). 2SCr, serum creatinine. KT, kidney transplantation.
DISCUSSION
In this study, we report on longitudinal data of kidney function after pregnancy in women following KT. To our knowledge, no previous reports have been published on eGFR slope after pregnancy in KTRs with proper multilevel analysis, which allows women to be their own control group. For our analysis, we used a large, unique, and unselected retrospective data set from the nationwide Dutch PARTOUT study. We identified 4 important findings. First, in general, pregnancies after KT have no significant effect on eGFR, and pregnancy did not accelerate the eGFR slope. Second, pregnancy does not amplify the negative effect of significant univariate predictors of worse eGFR (eg, rejection, hypertension, CNI use) after KT. Third, multivariate GEE analysis showed that transplant vintage, rejection before first pregnancy, prepregnancy eGFR, and transplant-to-conception interval are predictors for worse eGFR after KT and not pregnancy itself. Finally, eGFR and graft survival after the first delivery were significantly better for women with midterm hyperfiltration (>15% SCr decrease) during the first pregnancy.
We found that eGFR decline after the first pregnancy was not statistically significant (P = 0.08). The almost significant decline in eGFR after first pregnancy can be explained by the fact that most women in this subgroup only had 1 pregnancy after KT. It is likely that if complications occurred during this pregnancy or if their kidney function decreased, these women decided not to become pregnant again. Furthermore, 10 KTRs were pregnant again very soon after their first delivery; therefore, no eGFRs of these KTRs could be included in pregnancy interval 1. Although pregnancy causes a nonsignificant slight drop in an adjusted mean eGFR of approximately 3 mL/min/1.73 m2, it is questionable if such a slight drop is clinically significant. These findings are in line with our previous meta-analysis.17 Furthermore, it is reassuring that pregnancy does not seem to have an effect on eGFR after second and third pregnancies; of course, in a selected “best KTR” group, pregnancy did not have any additional effect on eGFR slope.
Prepregnancy eGFR was a strong predictor for better eGFR after pregnancy. Although previous studies are hardly comparable with our study, because of heterogeneity in SCr cutoff values in these studies, this result is in line with the findings of most of these studies9,20,28-34; however, 3 studies did not find a negative effect of prepregnancy SCr on long-term graft function.30,35,36 This discrepancy might be due to the fact that these studies were underpowered. Moreover, their follow-up after pregnancy consisted of a 1-y SCr measurement instead of the long-term follow-up that took place in our study.17 Hypertension is a known risk factor for eGFR decline in the chronic kidney disease population.37 In this study, it was only a significant risk factor in the univariate analysis.
The relationship between transplant-to-conception interval and graft function after pregnancy was reported earlier by 5 individual studies. These studies report on different periods of transplant-to-conception interval (as a continuous variable, transplant-to-conception interval <1 y, transplant-to-conception interval <2 y, transplant-to-conception interval >5 y).7,8,32,33,38 No negative relationship was found between SCr 1 y after pregnancy and the transplant-to-conception interval.8,33,38 We also found no effect of the transplant-to-conception interval on mean eGFR for women with a transplant-to-conception interval <10 y; however, a transplant-to-conception interval >10 y resulted in significantly better mean eGFR than women who got pregnant at shorter times after KT. This can be because women who were transplanted at childhood selectively received a donor kidney of very good quality, and only good kidneys have long enough graft survival until fertile age is reached. After the exclusion of KTRs transplanted in childhood, the relationship between age and time of KT and mean eGFR after pregnancy was no longer significant. No relationship between DCGL and transplant-to-conception interval was found in our study; this is in contrast with the study by Rose et al.39 Therefore, outcomes of our study give no grounds to change the “timing of pregnancy” advice of the American Society of Transplantation guidelines of >1 y after KT.2
Surprisingly, known predictors for better graft survival in the general KT population, such as preemptive KT and KT with a living kidney, were not associated with better eGFR or better graft survival after pregnancy.40,41 This may have been due to the fact that in the past, KT with a living kidney was not the standard of care and most of these women were transplanted with a deceased donor. Moreover, only women with excellent kidney function were “allowed” to get pregnant, so the best of the deceased donor KTs are overrepresented in our data set. This era effect was also described in an earlier study.10
Both mean eGFR and graft survival after pregnancy were better in the group with >15% decrease in SCr during the first pregnancy. This shows that the functional reserve capacity of the KT can be an important sign of the quality of the graft. As expected, graft survival was better when prepregnancy eGFR was better, according to a study performed earlier in the general KT population.42
This study has several limitations. One limitation is that the study is retrospective; therefore, not all data could be obtained, and residual confounding cannot be excluded. Unfortunately, data on proteinuria and immune status, such as HLA antibodies and HLA mismatches, were insufficient for analysis.43 For measurement of kidney function during pregnancy, the golden standard is 24-h urine creatinine clearance; unfortunately, we did not have those measurements available.44 Although being retrospective in nature, our study allowed proper analyses of eGFR in an unselected, large cohort of KTRs with pregnancy. This is the first study that compares eGFRs prepregnancy and postpregnancy by multilevel analysis, correcting for missing values and correcting for time in the model. Also, the nationwide composition of our cohort provides strong external validity.
In conclusion, to the best of our knowledge, this is the largest study analyzing the effect of pregnancy in KTRs on eGFR slope to date. The outcomes of our study demonstrate that pregnancy causes a small and nonsignificant decline in adjusted mean eGFR after the first pregnancy but does not accelerate eGFR slope after the first or subsequent pregnancies. Furthermore, pregnancy does not amplify the negative effect of known risk factors on eGFR after KT. Midterm hyperfiltration might be a marker for favorable graft outcomes after pregnancy. The absence of midterm hyperfiltration as a marker of renal reserve might be considered as a risk factor for long-term graft loss in addition to traditional risk factors.
ACKNOWLEDGMENTS
The authors wish to acknowledge the services of the other members of the PARTOUT network: R. van der Molen, O.W.H. van der Heijden, F.E. van Reekum, F.J. Bemelman, W. Ganzevoort, S.A. Nurmohammed, M. de Boer, J.H. Eijsink, A.P.J. de Vries, M. Sueters, W. Visser, M.H.L. Christiaans, M.E.A. Spaanderman, M. Groenewout, L. van Laar, N. Paauw, B. Reijtenbagh, A. Schaeffers, A. Schellekens, A. Slob, L. Koenjer, and J.R. Meinderts. The authors gives special thanks to J. Rischen-Vos. The PARTOUT network is related to the European Rare Kidney Disease Reference Network (ERK-NET); A.T. Lely is a member of ERK-NET.
Supplementary Material
Footnotes
A full list of members of the PARTOUT working group is included under Acknowledgments.
J.v.d.W and A.T.L. contributed equally.
M.v.B. and M.G. participated in research design and performance, data analysis and interpretation, and writing of the article. H.G. participated in research design, data analysis, and interpretation. H.v.H. participated in research design, data interpretation, and review of the article. M.d.J. participated in data interpretation and review of the article. M.B. participated in writing and review of the article. B.Z. participated in review of the article. J.v.W. and T.L. participated in research design and performance, data interpretation, and writing of the article.
The authors declare no conflicts of interest.
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Supplemental Visual Abstract; http://links.lww.com/TP/C284.
Supplemental digital content (SDC) is available for this article. Direct URL citations appear in the printed text, and links to the digital files are provided in the HTML text of this article on the journal’s Web site (www.transplantjournal.com).
REFERENCES
- 1.Moritz MJ, Constantinescu S, Coscia LA, et al. ; Transplant Pregnancy Registry International. 2017 Annual Report. Philadelphia, PA, Gift of Life Institute; 2018. [Google Scholar]
- 2.McKay DB, Josephson MA, Armenti VT, et al. ; Women’s Health Committee of the American Society of Transplantation. Reproduction and transplantation: report on the AST Consensus Conference on Reproductive Issues and Transplantation. Am J Transplant. 2005;5:1592–1599. [DOI] [PubMed] [Google Scholar]
- 3.First MR, Combs CA, Weiskittel P, et al. Lack of effect of pregnancy on renal allograft survival or function. Transplantation. 1995;59:472–476. [PubMed] [Google Scholar]
- 4.Sturgiss SN, Davison JM. Effect of pregnancy on the long-term function of renal allografts: an update. Am J Kidney Dis. 1995;26:54–56. [DOI] [PubMed] [Google Scholar]
- 5.Fischer T, Neumayer HH, Fischer R, et al. Effect of pregnancy on long-term kidney function in renal transplant recipients treated with cyclosporine and with azathioprine. Am J Transplant. 2005;5:2732–2739. [DOI] [PubMed] [Google Scholar]
- 6.Pour-Reza-Gholi F, Nafar M, Farrokhi F, et al. Pregnancy in kidney transplant recipients. Transplant Proc. 2005;37:3090–3092. [DOI] [PubMed] [Google Scholar]
- 7.Rahamimov R, Ben-Haroush A, Wittenberg C, et al. Pregnancy in renal transplant recipients: long-term effect on patient and graft survival. A single-center experience. Transplantation. 2006;81:660–664. [DOI] [PubMed] [Google Scholar]
- 8.Kashanizadeh N, Nemati E, Sharifi-Bonab M, et al. Impact of pregnancy on the outcome of kidney transplantation. Transplant Proc. 2007;39:1136–1138. [DOI] [PubMed] [Google Scholar]
- 9.Kim HW, Seok HJ, Kim TH, et al. The experience of pregnancy after renal transplantation: pregnancies even within postoperative 1 year may be tolerable. Transplantation. 2008;85:1412–1419. [DOI] [PubMed] [Google Scholar]
- 10.Levidiotis V, Chang S, McDonald S. Pregnancy and maternal outcomes among kidney transplant recipients. J Am Soc Nephrol. 2009;20:2433–2440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stoumpos S, McNeill SH, Gorrie M, et al. Obstetric and long-term kidney outcomes in renal transplant recipients: a 40-yr single-center study. Clin Transplant. 2016;30:673–681. [DOI] [PubMed] [Google Scholar]
- 12.Svetitsky S, Baruch R, Schwartz IF, et al. Long-term effects of pregnancy on renal graft function in women after kidney transplantation compared with matched controls. Transplant Proc. 2018;50:1461–1465. [DOI] [PubMed] [Google Scholar]
- 13.Lopes van Balen VA, van Gansewinkel TAG, de Haas S, et al. Maternal kidney function during pregnancy: systematic review and meta-analysis. Ultrasound Obstet Gynecol. 2019;54:297–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Paauw ND, van der Graaf AM, Bozoglan R, et al. Kidney function after a hypertensive disorder of pregnancy: a longitudinal study. Am J Kidney Dis. 2018;71:619–626. [DOI] [PubMed] [Google Scholar]
- 15.Park S, Lee SM, Park JS, et al. Midterm eGFR and adverse pregnancy outcomes: the clinical significance of gestational hyperfiltration. Clin J Am Soc Nephrol. 2017;12:1048–1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bramham K, Nelson-Piercy C, Gao H, et al. Pregnancy in renal transplant recipients: a UK national cohort study. Clin J Am Soc Nephrol. 2013;8:290–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.van Buren MC, Schellekens A, Groenhof TKJ, et al. Long-term graft survival and graft function following pregnancy in kidney transplant recipients: a systematic review and meta-analysis. Transplantation. 2020;104:1675–1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Başaran O, Emiroğlu R, Seçme S, et al. Pregnancy and renal transplantation. Transplant Proc. 2004;36:122–124. [DOI] [PubMed] [Google Scholar]
- 19.Little MA, Abraham KA, Kavanagh J, et al. Pregnancy in Irish renal transplant recipients in the cyclosporine era. Ir J Med Sci. 2000;169:19–21. [DOI] [PubMed] [Google Scholar]
- 20.Thompson BC, Kingdon EJ, Tuck SM, et al. Pregnancy in renal transplant recipients: the Royal Free Hospital experience. QJM. 2003;96:837–844. [DOI] [PubMed] [Google Scholar]
- 21.Open Clinica. Version 3.1. OpenClinica LLC. Available at www.OpenClinica.com. Accessed December 31, 2017. [Google Scholar]
- 22.Webster K, Fishburn S, Maresh M, et al. ; Guideline Committee. Diagnosis and management of hypertension in pregnancy: summary of updated NICE guidance. BMJ. 2019;366:l5119. [DOI] [PubMed] [Google Scholar]
- 23.Davey DA, MacGillivray I. The classification and definition of the hypertensive disorders of pregnancy. Am J Obstet Gynecol. 1988;158:892–898. [DOI] [PubMed] [Google Scholar]
- 24.Lopes van Balen VA, Spaan JJ, Ghossein C, et al. Early pregnancy circulatory adaptation and recurrent hypertensive disease: an explorative study. Reprod Sci. 2013;20:1069–1074. [DOI] [PubMed] [Google Scholar]
- 25.Sturgiss SN, Dunlop W, Davison JM. Renal haemodynamics and tubular function in human pregnancy. Baillieres Clin Obstet Gynaecol. 1994;8:209–234. [DOI] [PubMed] [Google Scholar]
- 26.Levey AS, Stevens LA. Estimating GFR using the CKD Epidemiology Collaboration (CKD-EPI) creatinine equation: more accurate GFR estimates, lower CKD prevalence estimates, and better risk predictions. Am J Kidney Dis. 2010;55:622–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shou H, Hsu JY, Xie D, et al. ; Chronic Renal Insufficiency Cohort (CRIC) Study Investigators. Analytic considerations for repeated measures of eGFR in cohort studies of CKD. Clin J Am Soc Nephrol. 2017;12:1357–1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Queipo-Zaragoza JA, Vera-Donoso CD, Soldevila A, et al. Impact of pregnancy on kidney transplant. Transplant Proc. 2003;35:866–867. [DOI] [PubMed] [Google Scholar]
- 29.Kato M, Hattori R, Kinukawa T, et al. Correlation between treated hypertension in prepregnancy and transplanted kidney function deterioration during pregnancy even if within pregnancy permission criteria. Transplant Proc. 2012;44:635–637. [DOI] [PubMed] [Google Scholar]
- 30.Rocha A, Cardoso A, Malheiro J, et al. Pregnancy after kidney transplantation: graft, mother, and newborn complications. Transplant Proc. 2013;45:1088–1091. [DOI] [PubMed] [Google Scholar]
- 31.O’Reilly B, Compton F, Ogg C, et al. Renal function following pregnancy in renal transplant recipients. J Obstet Gynaecol. 2001;21:12–16. [DOI] [PubMed] [Google Scholar]
- 32.Keitel E, Bruno RM, Duarte M, et al. Pregnancy outcome after renal transplantation. Transplant Proc. 2004;36:870–871. [DOI] [PubMed] [Google Scholar]
- 33.Aivazoglou L, Sass N, Silva HT, Jr, et al. Pregnancy after renal transplantation: an evaluation of the graft function. Eur J Obstet Gynecol Reprod Biol. 2011;155:129–131. [DOI] [PubMed] [Google Scholar]
- 34.Alfi AY, Al-essawy MA, Al-lakany M, et al. Successful pregnancies post renal transplantation. Saudi J Kidney Dis Transpl. 2008;19:746–750. [PubMed] [Google Scholar]
- 35.Hooi LS, Rozina G, Shaariah MY, et al. Pregnancy in patients with renal transplants in Malaysia. Med J Malaysia. 2003;58:27–36. [PubMed] [Google Scholar]
- 36.Vannevel V, Claes K, Baud D, et al. Preeclampsia and long-term renal function in women who underwent kidney transplantation. Obstet Gynecol. 2018;131:57–62. [DOI] [PubMed] [Google Scholar]
- 37.Ku E, Lee BJ, Wei J, et al. Hypertension in CKD: core curriculum 2019. Am J Kidney Dis. 2019;74:120–131. [DOI] [PubMed] [Google Scholar]
- 38.Chittka D, Hutchinson JA. Pregnancy after renal transplantation. Transplantation. 2017;101:675–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rose C, Gill J, Zalunardo N, et al. Timing of pregnancy after kidney transplantation and risk of allograft failure. Am J Transplant. 2016;16:2360–2367. [DOI] [PubMed] [Google Scholar]
- 40.Arze Aimaretti L, Arze S. Preemptive renal transplantation—the best treatment option for terminal chronic renal failure. Transplant Proc. 2016;48:609–611. [DOI] [PubMed] [Google Scholar]
- 41.Terasaki PI, Cecka JM, Gjertson DW, et al. High survival rates of kidney transplants from spousal and living unrelated donors. N Engl J Med. 1995;333:333–336. [DOI] [PubMed] [Google Scholar]
- 42.Kasiske BL, Israni AK, Snyder JJ, et al. ; Patient Outcomes in Renal Transplantation (PORT) Investigators. The relationship between kidney function and long-term graft survival after kidney transplant. Am J Kidney Dis. 2011;57:466–475. [DOI] [PubMed] [Google Scholar]
- 43.Zhang R. Donor-specific antibodies in kidney transplant recipients. Clin J Am Soc Nephrol. 2018;13:182–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ahmed SB, Bentley-Lewis R, Hollenberg NK, et al. A comparison of prediction equations for estimating glomerular filtration rate in pregnancy. Hypertens Pregnancy. 2009;28:243–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.