Summary
Late presentation for care is a major impediment to prevention and effective treatment of HIV infection. Older individuals are at increased risk for late presentation, represent a growing proportion of all those with late presentation, and may require interventions tailored to their age group. We provide a summary of the worldwide literature published between 2016–21 (reporting data from 1984–2018) quantifying the association of age with delayed presentation. Using the most common definitions of late presentation and older age from these earlier studies, we update this work with data from the International epidemiology Databases to Evaluate AIDS (IeDEA) consortium focusing on data from 2000 to 2019 encompassing 4 continents. Finally, we consider how late presentation among older individuals might be more effectively addressed as electronic medical records become widely adopted.
Introduction
The successful scale-up of effective antiretroviral therapy (ART) has supported the long-term survival of people with HIV infection (PWH). More people are living with HIV than ever before and this population is aging(1–4). Globally, between 2015 and 2020, UNAIDS estimated that the total number of PWH over the age of 50 years increased from 5.4 million to 8.1 million (aidsinfo.unaids.org). In this four-part series co-sponsored by The Lancet HIV and The Lancet Healthy Longevity, we explore pressing issues facing those aging with HIV in the era of ART. In this article, we begin by addressing risk of delayed presentation for ART, subsequent articles consider 1) evidence for and against accentuated biologic ageing compared with people without HIV infection, 2) how health systems might adapt to an ageing population of PWH, and finally 3) the syndemic of stigma particular to those aging with HIV.
In many settings, as the prevalence of HIV among older individuals has grown the number of new infections in this age group has increased. For example, between 2015 and 2019 in the United States, the overall prevalence of PWH increased by 8% and incident infections decreased by 4%(5). In contrast, we saw a 40% increase (289,900 to 407,100) in prevalence and 15% increase (2700 to 3100) in incidence among those 50 years and older – the largest increases of any age group(5). This is likely due to intra-generational and cross generational unprotected sexual activity(6, 7).
Large scale population based statistics on HIV incidence in older age groups are limited outside the United States but some data is available from South Africa. By the end of 2013, 14% (6304/44909) of PWH in care were ≥50 years(8). Among 84,078 patients starting antiretroviral therapy from 2004 to 2013, the proportion of those ≥50 years increased from 6% (290/4999) in 2004 to 10% (961/9657) in 2012–13(8). Another study tested in 2010 and retested in 2015 a cohort of 1,360 individuals aged 40 or more years in 2015 (6). HIV prevalence increased from 21% to 23% corresponding to 33 incident infections (0.49 infections per 100 Person Years); only those 80 or more years of age experienced no new infections(6).
Twelve years ago, we used data from the United States and Canada to compare CD4 cell count and AIDS-defining conditions at presentation for HIV care among those under 50 and those 50 years of age and older(9). Older individuals had lower CD4 counts and a higher prevalence of AIDS-defining conditions at diagnosis, and these gaps between younger and older at presentation persisted over calendar time despite decreases in new diagnoses among both groups(9). Now that an even larger proportion of individuals living with HIV are 50 years and older worldwide, we revisit the relationship between age and delayed presentation for care globally with a review of recent literature, data analyses from the International epidemiology Databases to Evaluate AIDS (IeDEA) network, and a consideration of what might be done to decrease new HIV infections and delayed presentation for care among older individuals.
Review of Recent Literature (2016–2021)
We conducted a structured review of recent literature (see Search Strategy and Table 1). These studies were conducted in North and South America, Europe, Africa, Middle East, Asia, and Australia and include observations from 1984 through 2018. Most studies (n=32) defined late presentation as having a CD4 count of <350 cells/μL or an AIDS diagnosis at or near the time of presentation for care. Although these studies document improvements in recent years, delayed presentation remains a significant global issue in HIV care. In many settings, approximately half of those newly diagnosed with HIV infection have CD4 counts below 350 cells/μL at presentation and the proportion is even higher in lower- and middle-income countries.
Table 1.
Structured Review of Delayed Presentation Publications Last Five Years
| 1st Author | Definition of Late Presenter | New Diagnoses (n) | Late Presenters (n, %) | Years | Location | Age Variable (Yrs) | Odds Ratio | 95% CI | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Gardner | AIDS | 1385 | 422 | 31% | 2009–14 | USA (single site) | 65+ vs. 25–44 | 1.3 | 0.6 | 2.6 |
| Nduaguba | AIDS | 77844 | 30359 | 39% | 1996–2001 | USA (multisite) | 60+ vs<30 | 4.0 | 3.3 | 4.7 |
| Nduaguba | AIDS | 77844 | 30359 | 39% | 2002–2007 | USA (multisite) | 60+ vs<30 | 4.6 | 3.9 | 5.4 |
| Kwobah | CD4<100 | 10533 | 2421 | 23% | 2010–11 | Kenya (multisite) | >24 vs. <19 | 1.6 | 1.0 | 2.6 |
| Kadam | CD4<200 | 659 | 264 | 40% | 2011–15 | India (single site) | continuous variable | na | ||
| Honge | CD4<200 | 3720 | 1810 | 48% | 2005–13 | West Africa (single site) | 50+ vs.<30 | 1.5 | 1.1 | 2.0 |
| Senard | CD4<200 | 186 | 49 | 26% | 2012–13 | France (single site) | continuous variable | 1.1 | 1.0 | 1.1 |
| Taborelli | CD4<200 or AIDS | 16601 | 7720 | 47% | 1999–13 | Italy (multisite) Italians | 50+ vs.35–49 | 1.5 | 1.4 | 1.7 |
| Taborelli | CD4<200 or AIDS | 4152 | 2831 | 68% | 1999–14 | Italy (multisite) Non- Italians | 50+ vs.35–49 | 0.9 | 0.7 | 1.2 |
| Tang | CD4<200 or AIDS | 528234 | 179700 | 34% | 2006–14 | China (multisite) | 55+ vs.15–24 | 2.9 | 2.9 | 3.0 |
| Mohammadi | CD4<350 | 4402 | 2562 | 58% | 1987–2016 | Iran (158 sites) | 50+ vs.<30 | 3.6 | 2.6 | 4.8 |
| Rava | CD4<350 | 14876 | 6635 | 45% | 2004–18 | Spain (46 sites) | 50+ vs. <30 | 2.8 | 2.5 | 3.1 |
| Ribeiro | CD4<350 | 356 | 218 | 59% | 2017 | Brazil (single site) | continuous variable | 1.0 | 1.0 | 1.1 |
| Bath | CD4<350 | 2469 | 1342 | 54% | 2008–14 | England (multisite) | 55+ vs. 16–19 | 3.5 | 1.6 | 7.7 |
| Cuzin | CD4<350 | 1421 | 625 | 44% | 2014–15 | France (10 sites) | >47 vs. <29 | 1.9 | 1.4 | 2.5 |
| MacCarthy | CD4<350 | 1970 | 698 | 61% | 2010 | Brazil (3 sites) | 45+ vs.18–44 | 1.7 | 1.1 | 2.5 |
| Hu | CD4<350 | 519 | 188 | 38% | 2011–14 | China (8 cities) | 40+ vs.18–24 | 3.1 | 1.8 | 5.5 |
| Gullon | CD4<350 | 316 | 158 | 50% | 2007–14 | Spain (single site) | >38 vs. <38 | 2.2 | 1.3 | 3.7 |
| Schafer | CD4<350 | 165 | 105 | 64% | 2009–11 | Germany (single site) | mean age LP 41 vs 32 | na | ||
| Miranda | CD4<350 or AIDS | 907 | 459 | 51% | 1984–2017 | Portugal (single site) | >56 vs. <30 | 2.9 | 1.5 | 5.9 |
| Jablonowska | CD4<350 or AIDS | 1522 | 682 | 45% | 2016–17 | Poland (13 sites) | per decade | 1.5 | 1.4 | 1.7 |
| Robles | CD4<350 or AIDS | 3842 | 2793 | 73% | 2012–17 | Panama (multisite) | >65 vs. 18–24 | 2.9 | 1.7 | 5.0 |
| Palacios-Baena | CD4<350 or AIDS | 205 | 102 | 50% | 2014–18 | Spain (single site) | 32+ vs. <32 | 3.4 | 1.9 | 6.1 |
| Muelas Fernandez | CD4<350 or AIDS | 74 | 33 | 45% | 2013–18 | Spain (single site) | 40+ vs. <40 | 2.6 | 1.0 | 6.9 |
| Karaosmanoglu | CD4<350 or AIDS | 1673 | 826 | 49% | 2003–16 | Turkey (single site) | >50 vs ≤50 | 1.8 | * | * |
| Krueger | CD4<350 or AIDS | 1644585 | Na | na | 2013–16 | USA (multisite) | 45+ vs. 25–44 | 1.7 | * | * |
| Siwak | CD4<350 or AIDS | 3972 | 2288 | 58% | 2000–15 | Poland (14 sites) | 60+ vs.<20 | 5.2 | 1.9 | 14.0 |
| Hu | CD4<350 or AIDS | 45118 | 31673 | 70% | 2012–16 | China (multisite) | >50 vs. 15–30 | 1.5 | 1.4 | 1.6 |
| Zhonghua | CD4<350 or AIDS | 293187 | 200503 | 68% | 2009–17 | China (multisite) | 60+ vs. 18–29 | 2.3 | 2.3 | 2.4 |
| Wilton | CD4<350 or AIDS | 1819 | 1476 | 54% | 1999–2013 | Canada (multisite) | 50+ vs.<30 | 2.8 | 1.9 | 4.1 |
| Lin | CD4<350 or AIDS | 436 | 82 | 19% | 2000–14 | Australia (single site) | mean age LP 45 vs 39 | na | ||
| Rao | CD4<350 or AIDS | 474 | 356 | 75% | 2012–13 | India (single site) | ≤50 vs.≤25 | 4.2 | 1.3 | 13.2 |
| Darcis | CD4<350 or AIDS | 687 | 302 | 44% | 2006–17 | Belgium (single site) | 10 year increments | 1.3 | 1.1 | 1.5 |
| Wojcik-Cichy | CD4<350 or AIDS | 412 | 259 | 63% | 2009–16 | Poland (single site) | 10 year increments | 1.8 | 1.4 | 2.4 |
| Johnson | CD4<350 or AIDS | 401 | 307 | 77% | 2013–16 | Sudan (single site) | 34+ vs. <34 | na | ||
| Jin | CD4<350 or AIDS | 7073 | 2949 | 42% | 2011–15 | China (multisite) | 60+ vs. 0–19 | 2.2 | 1.5 | 3.1 |
| Gesesew | CD4<350 or AIDS | 4900 | 3268 | 67% | 2003–15 | Ethiopia (single site) | 50+ vs. 15–24 | 0.4 | 0.3 | 0.6 |
| Fomundam | CD4<350 or AIDS | 8138 | 4817 | 59% | 2014–15 | South Africa (35 sites) | 50+ vs. <50 | 1.9 | * | * |
| Levy | CD4<350 or AIDS | 356 | 118 | 33% | 2010–15 | Israel (single site) | >50 vs. <50 | 2.4 | 1.1 | 5.0 |
| Raffetti | CD4<350 or AIDS | 19391 | 10471 | 54% | 1985–2013 | Italy (multisite) | 55+ vs.<25 | 7.5 | 6.1 | 9.2 |
| Brannstrom | CD4<350 or AIDS | 575 | 334 | 58% | 2009–12 | Sweden (12 sites) | >50 vs.<30 | 4.0 | 2.1 | 7.6 |
| Kesselring | CD4<500 | 702 | 442 | 63% | 2013–15 | Canada (multisite) | continuous variable | 1.0 | 1.02 | 1.05 |
Older age was variably defined, sometimes as young as “35 years or older”, but older age (usually defined as ≥50-years-old) was consistently associated with delayed presentation. Relative risk (typically measured using adjusted odds ratios but in some cases we calculated unadjusted odds ratios from data provided) for delayed presentation associated with older compared to younger individuals (variably defined as <35 or <20 years) ranged from 1.1–7.4. The most common odds ratios were from 1.5–4.
Only one study that considered the role of age in late presentation concluded that older individuals were at decreased risk of late presentation for care. Gesewew et al. studied 4,900 people presenting for care at a single site in Southwestern Ethiopia and found that, compared to those 15–24 years of age, those 50 years and older were less likely to experience a delayed presentation (HR 0.4; 95% CI 0.3–0.6)(10). Another study conducted in Italy separated Italians from non-Italians and found that, compared to those 35–49 years of age, Italians >50 years of age were at increased risk (HR 1.5; 95% CI 1.4–1.7) but non-Italians 50 years of age were not (HR 0.9; 95% CI 0.7–1.2)(11).
(12)Some of these studies considered whether there had been opportunities for earlier diagnosis and whether these differed by age(12–16). These opportunities were variably defined from as broad as “any prior medical encounter” to very specific as “diagnosis with an AIDS defining condition”. These studies documented more “missed opportunities” among older individuals.
IeDEA Data
To add a more recent and standardized accounting of delayed presentation for HIV care around the world, we present data from the International epidemiology Databases to Evaluate AIDS (IeDEA) Global Consortium. IeDEA harmonizes data on care and treatment of people with HIV from seven international regional data centers including four in Africa, and one each in Asia-Pacific (which includes an Australia sub-cohort), Central/South America (also includes Mexico, Haiti, Honduras), and North America (United States and Canada). Each region contributed aggregated data from adults (≥18 years old) to the Epidemiology and Biostatistics Core of the North American AIDS Cohort Collaboration on Research and Design (NA-ACCORD), the North American region of IeDEA, where the figures presented were created. Cohorts in most regions have an ongoing process of adding all individuals presenting for HIV care, with the exception of cohorts in Southern Africa and the Asia Pacific. In the Southern African IeDEA region, participants enter into observation at ART initiation which may occur after presentation for HIV care; this region did not contribute to data visualizations of those presenting for HIV care. Asia-Pacific data combine two approaches to cohort enrollment – selectively enrolling patients to replace participants who died, were transferred, or were lost to follow-up (including all Australian sub-cohort sites), or enrolling all patients seen at the site. The results presented may not be representative of all persons in HIV care in the specified regions of the world as the IeDEA regional cohorts are observational and do not employ sampling strategies for representativeness. Additional information regarding selection of participants for enrollment into the IeDEA regional cohorts, the adoption of the Treat All guidelines, and the changes in CD4 testing that influence the results presented can be found in Supplement Table 1 (pages 1–3 of the supplement) and a recent global IeDEA study (17).
Three study populations were defined. First, the population of individuals observed to present for HIV care at an IeDEA-contributing clinical care site was restricted to those who did not have prior evidence of an HIV care visit, a history of antiretroviral therapy, or a suppressed HIV viral load. Second, the population of individuals observed to be in HIV care in any calendar year from 2000 to the most recent data available for the region was restricted to those who were receiving ART, had a CD4 or HIV RNA measurement, or had evidence of an HIV care encounter. Third, the study population of individuals presenting for HIV care were further restricted to those who were observed to initiate ART at, or after, presentation for care.
Age was measured from year of birth. Sex was defined as sex at birth. The CD4 count closest to the date of presentation for HIV care measured within +/− 12 months and no more than 7 days after ART start was selected for this analysis. For the CD4 at ART initiation, we used a window of 12 months prior through 7 days post ART start to select the closest measurement for this analysis.
Histograms were created for each region to visualize the age distribution at presentation for care, and in the most recent complete calendar year of data available among those who were in HIV care. A kernel density smoothing bandwidth of 2.0 was used to visualize the age distribution histograms. We quantified the difference between the observed medians and the kernel density median estimate (which is not necessarily equivalent to the observed medians). Animated age distributions that visualize these changing age distributions over the last two decades can be found on the IeDEA YouTube Channel (https://www.youtube.com/channel/UC9cfdwlBl944eQ9AGj1EOkw). The proportion of adults presenting for HIV care was estimated within age groups (<50, 50–64, and 65+ years) among the total presenters for HIV care.
In 2013, the World Health Organization recommended viral load testing (and not CD4 testing) to monitor virologic failure after ART initiation(17–20). In 2018, the President’s Emergency Plan for AIDS Relief (PEPFAR) reduced their support for CD4 testing to prioritize viral load monitoring(19). IeDEA has previously shown a decline in pre-ART CD4 testing after adoption of Treat All policies that is steeper in low- and middle-income countries than in high-income countries(17). Trends in median and interquartile range of CD4 count at presentation for care and at ART initiation were stratified by age at presentation for care (< and ≥50 years) to the calendar year through which complete data were available in each region.
Adults presenting for HIV care who had a CD4 count <350 cells/μL at presentation for care were considered “late presenters.” The proportion of late presenters was estimated within each age category (<50, 50–64, and 65+ years) for those presenting for care in the most recent complete calendar year of data available.
The most recent, complete calendar years of data contributed by each IeDEA region were as follows: North America: 2018; Central and South America and the Caribbean: 2019; Central Africa: 2019; East Africa: 2019; West Africa: 2017; Southern Africa: 2017; Asia-Pacific: 2019 (Australia sub-cohort: 2016).
Age in IeDEA Regions
The proportion of adults in HIV care who are ≥50-years-old is substantial throughout IeDEA regions ranging from a low of 17% in Southern Africa to 50% in North America (Figure 1). The proportions of women and men in care who are ≥50-years-old are similar in North America and the Central and South America and Caribbean regions; however, there is a lower proportion of older women in care (compared to men) in the African and Asia-Pacific (including the Australian sub-cohort) regions.
Figure 1.
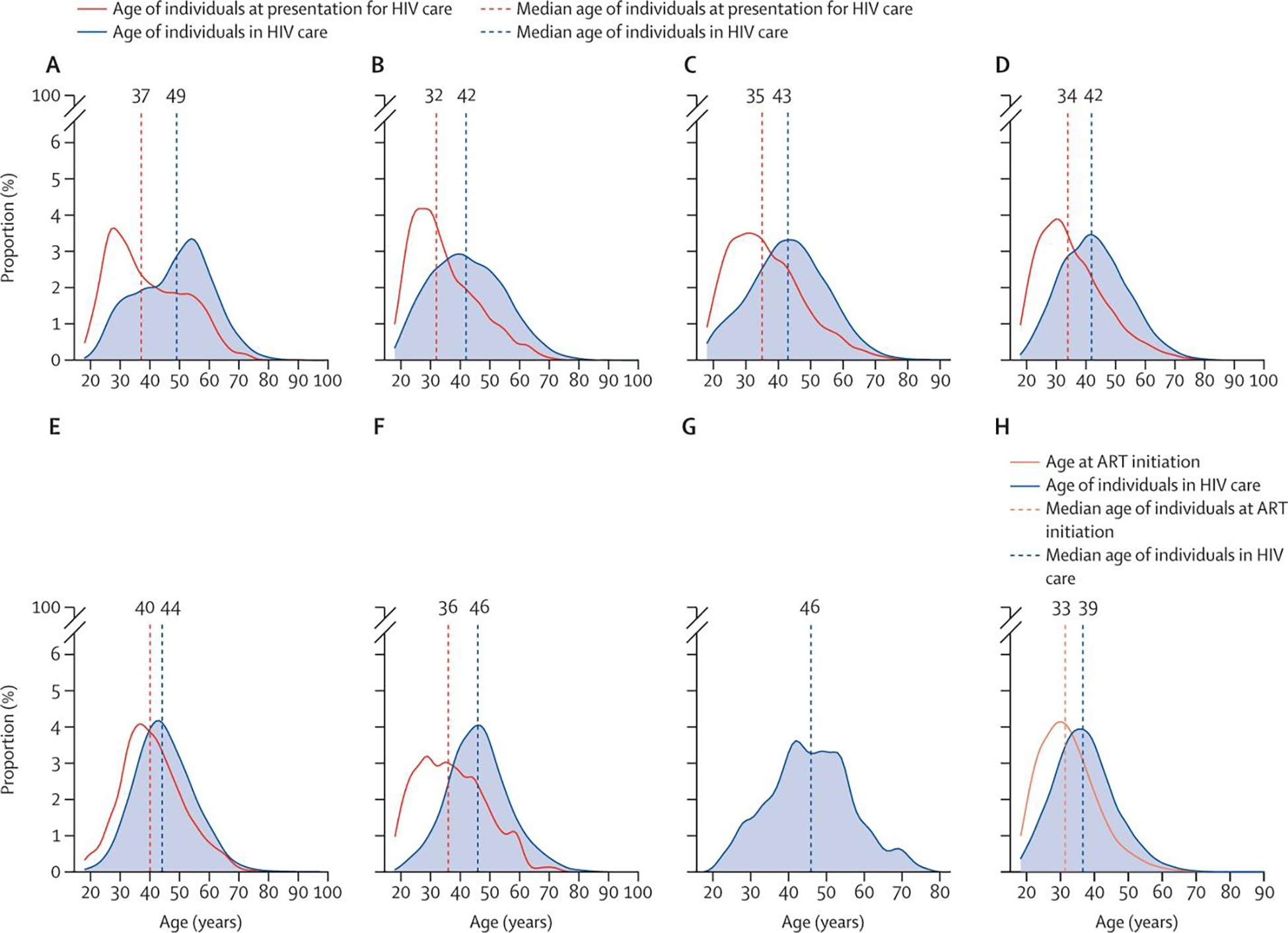
Age at presentation for HIV care and age among all those in HIV care in IeDEA regions (A) North America region (2018). (B) Central America, South America, and the Caribbean region (2019). (C) Central Africa region (2019). (D) East Africa region (2019). (E) West Africa region (2017). (F) Asia-Pacific region (2019). (G) Asia-Pacific region Australia subcohort (2016). (H) Southern Africa region (2017). In the IeDEA Southern Africa regional cohort, participants were observed from ART initiation (not from presentation for HIV care); age at ART initiation is believed to be reflective of age at presentation for HIV care as of 2017 when the Treat All guidelines were adopted in Southern Africa. The age at presentation for HIV care in the Australia subcohort of IeDEA Asia-Pacific in 2016 is not presented because the median age at presentation for HIV care was based on a relatively small subpopulation (<20 participants) of those presenting for HIV care. Participants were recruited to replenish the Australian subcohort in 2016. ART=antiretroviral therapy. IeDEA=International epidemiology Databases to Evaluate AIDS.
A concerning proportion of adults were ≥50-years-old at initial presentation for care: 24% in the North America region; 11% in Central and South America and the Caribbean; 13% in Central Africa; 12% in East Africa; 19% in West Africa;16% in Asia (excluding Australia). The proportion of older adults (≥50-years) initiating ART in Southern Africa was 8% in the Treat All era (Figure 1 and Table 2). Differences in the proportion presenting for care at older ages (≥50-years-old) in women vs. men also varied by region: 32% vs. 22% in the North America region; 16% vs. 10% in Central and South America and the Caribbean; 12% vs. 15% in Central Africa; 10% vs. 15% in East Africa; 17% vs. 26% in West Africa; 15% vs. 16% in Asia (excluding Australia); and 7% vs. 9% at ART initiation in the Treat All era in Southern Africa.
Table 2:
Age at presentation for HIV care or ART initiation, IeDEA regions
| IeDEA Region | Range of the number presenting for care or ART initiation | % <50 at presentation or ART initiation | % 50–64 at presentation or ART initiation | % 65+ at presentation or ART initiation |
|---|---|---|---|---|
|
| ||||
| Presenting for care | ||||
|
| ||||
| North America (2018) | 1,000–1,500 | 76% | 21% | 3% |
| Central and South America & the Caribbean (2019) | 1,000–1,500 | 89% | 10% | 1% |
| Central Africa (2019) | 1,500–2,000 | 87% | 11% | 2% |
| East Africa (2019) | 10,000–10,500 | 88% | 10% | 2% |
| West Africa (2017) | 1,000–1,500 | 81% | 17% | 2% |
| Asia-Pacific (2019) | 500–1,000 | 84% | 14% | 1% |
|
| ||||
| Initiating ART (in the Treat All era) | ||||
|
| ||||
| Southern Africa (2017) | 22,000–22,500 | 93% | 7% | 1% |
Footnotes:
In the IeDEA Southern Africa regional cohort, participants are observed at ART initiation (as opposed to at presentation for HIV care) and then followed forward in time; age at ART initiation is believed to be reflective of age at presentation for HIV care as of 2017 when the “Treat All” guidelines were adopted in Southern Africa.
Estimates of age at presentation for HIV care are not presented for the Australia sub-cohort of the IeDEA Asia-Pacific region. Participants were recruited to replenish the Australian sub-cohort in 2016; the median age at presentation for HIV care is based on a relatively small sub-population (<20 participants) of those presenting for HIV care at participating IeDEA clinics. Presenting estimates in these age groups would involve subgroups of <5, which breaches confidentiality arrangements.
While the differences vary by IeDEA region, in nearly every region, PWH ≥50-years-old are presenting with lower CD4 counts than their younger counterparts (Figure 2). Even more concerning, in many regions (Central and South America and the Caribbean, Central Africa, East Africa, and Asia-Pacific Region), the gaps are widening over time as the average CD4 count at presentation rises in younger adults presenting for care.
Figure 2.
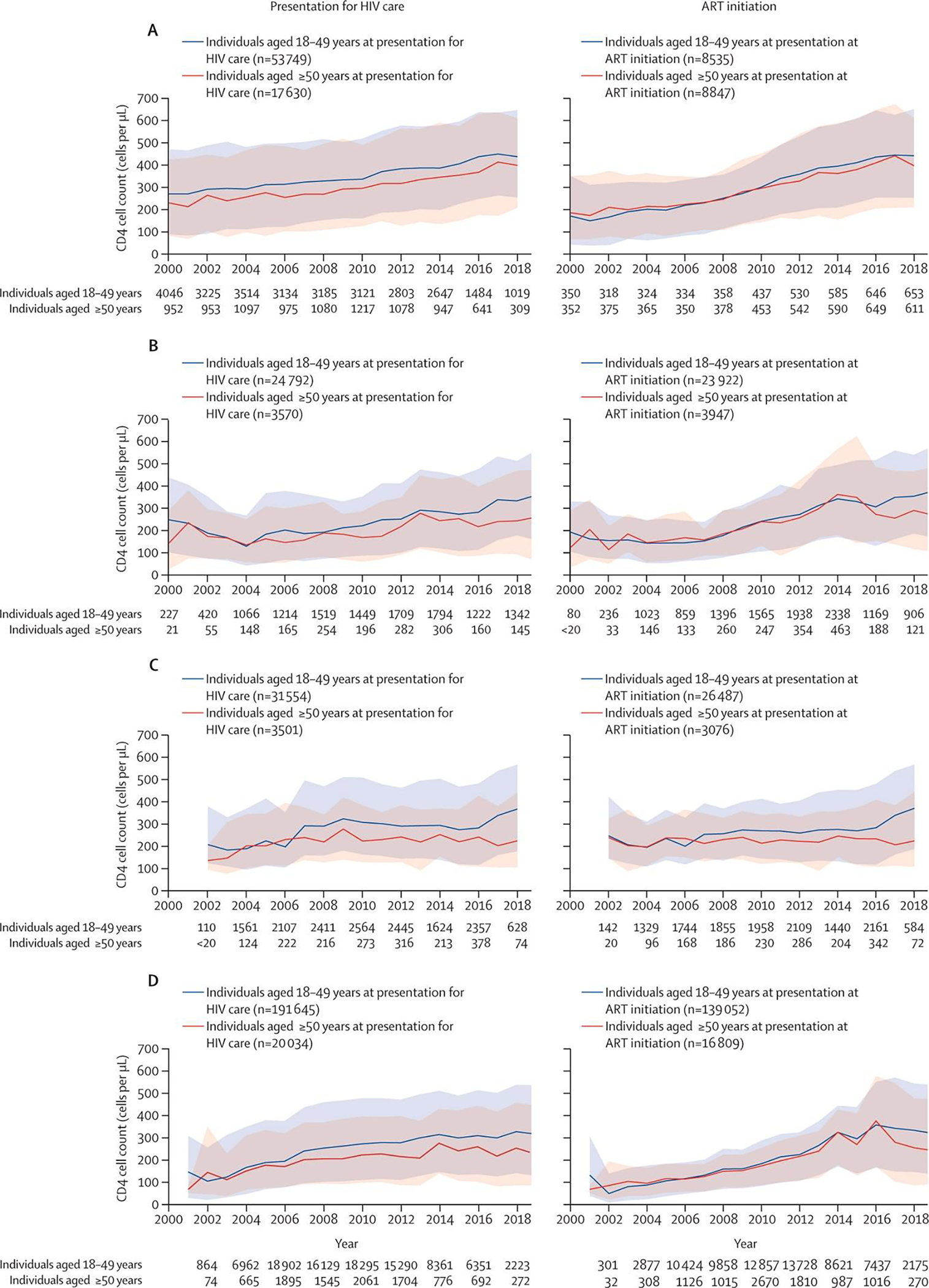
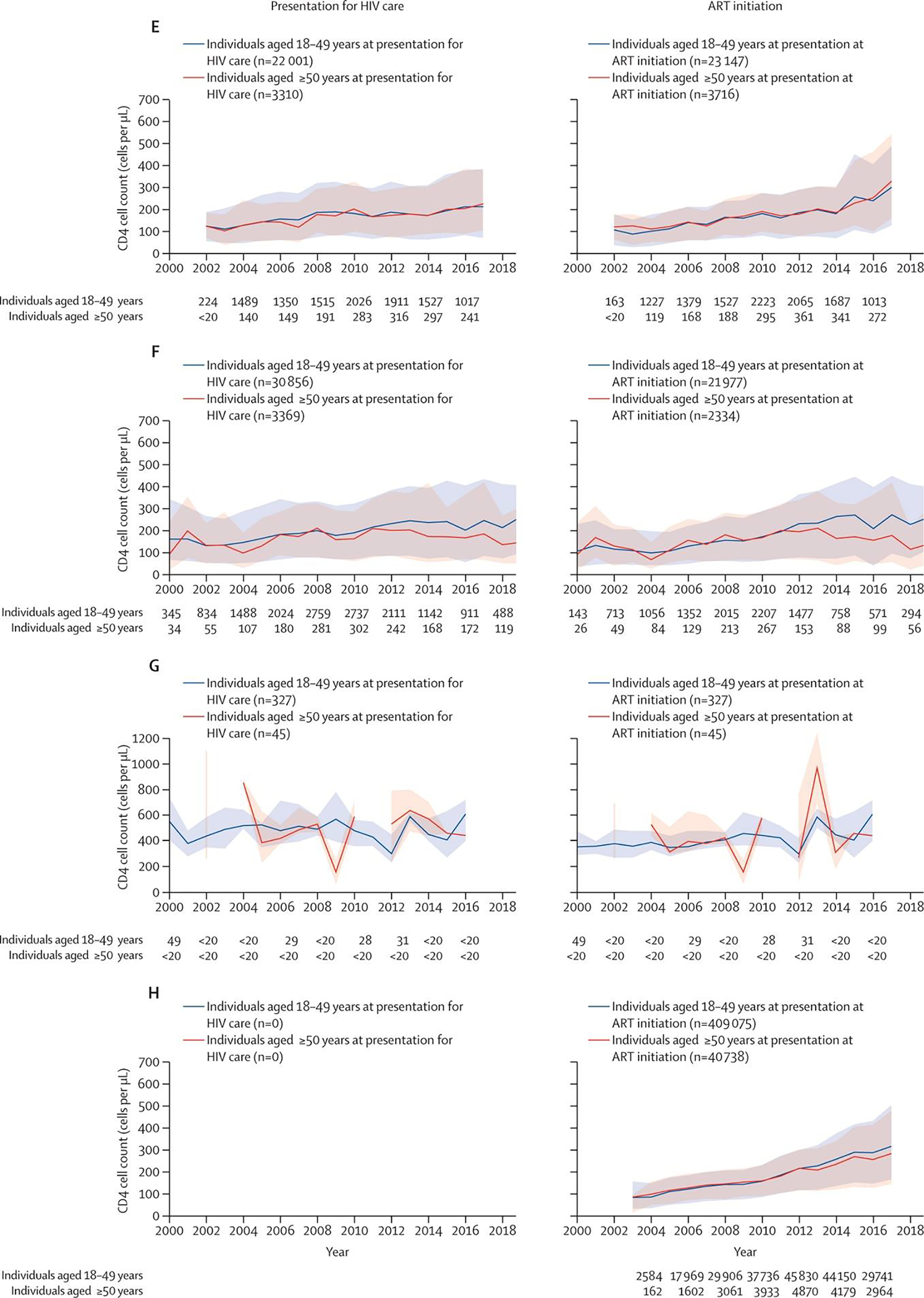
Median CD4 cell count per year by age group at presentation for HIV care and at ART initiation in IeDEA regions (A) North America region (2018). (B) Central America, South America, and the Caribbean region (2019). (C) Central Africa region (2018). (D) East Africa region (2019). (E) West Africa region (2017). (F) Asia-Pacific region (2019). (G) Asia-Pacific region Australia subcohort (2016). (H) Southern Africa region (2017). The total number of people contributing to the estimates in each year, by age (18–49 years and ≥50 years) are noted for the calendar years labelled on the x axis. The plots for Central Africa end in 2018 due to the decrease in CD4 cell count measured in 2019 (the last complete calendar year of data available from the Central Africa region). Estimates of CD4 cell count at presentation for HIV care are not available for Southern Africa. The Southern Africa regional cohort observes participants starting from ART initiation; age at ART initiation is believed to be reflective of age at presentation for HIV care as of 2017, when the Treat All guidelines were adopted in Southern Africa. In the Australia subcohort of the IeDEA Asia-Pacific region, participants were recruited into the clinical cohort to replenish the cohort; the median age at presentation for HIV care was based on a subpopulation (<20 participants) of those presenting for HIV care at participating IeDEA clinics between 2000 and 2016. Breaks in the line representing CD4 cell count at ART initiation among those older than 50 years old shows that no individuals older than 50 years initiated ART in that year. The y axis for CD4 count is different for the Australian subcohort compared with the other regions. ART=antiretroviral therapy. IeDEA=International epidemiology Databases to Evaluate AIDS.
Finally, recent IeDEA data (Table 3) support findings from the structured review of the literature (Table 1). Compared to those less than 50 years of age, those ≥50-years-old are substantially more likely to experience late presentation for care. In most regions, the majority of those ≥50-years-old present late to care.
Table 3:
Late presentation (CD4 <350 cells/μL) for HIV care, by age, in the most recent complete calendar year of data available, IeDEA regions
| IeDEA Region | Range of the number of late Presenters (CD4<350 cells/μL) | % of <50 years old who were late presenters | % of 50-to-64-year-olds who were late presenters | % of 65+ years-old who were late presenters |
|---|---|---|---|---|
|
| ||||
| Presenting for HIV care | ||||
| North America (2018) | 500–1,000 | 38% | 42% | 47% |
| Central and South America & the Caribbean (2019) | 1–500 | 49% | 61% | 60% |
| Central Africa (2019) | 1–500 | 52% | 57% | 25% |
| East Africa (2019) | 1,500–2,000 | 54% | 67% | 50% |
| West Africa (2017) | 500–1,000 | 63% | 62% | 64% |
| Asia-Pacific (2019) | 1–500 | 69% | 81% | 75% |
| Initiating ART (in the Treat All era) | ||||
| Southern Africa (2017) | 4,500–5,000 | 55% | 62% | 50% |
Footnotes:
Estimates of CD4 at presentation for HIV care is not available for Southern Africa. In the IeDEA Southern Africa regional cohort, participants are observed at ART initiation (as opposed to at presentation for HIV care) and then followed forward in time; age at ART initiation is believed to be reflective of age at presentation for HIV care as of 2017 when the “Treat All” guidelines were adopted in Southern Africa. Estimates of CD4 at presentation for HIV care are not presented for the Australia sub-cohort of the IeDEA Asia-Pacific region. Participants were recruited to replenish the sub-cohort in 2016; the median age at presentation for HIV care is based on a relatively small sub-population (<20 participants) of those presenting for HIV care at participating clinics. Presenting estimates would involve subgroups of <5, which breaches confidentiality arrangements.
Discussion
Increasingly, older people are presenting for HIV care. Some of these individuals were recently infected, but a disproportionate number of them have experienced a substantial delay in diagnosis. While it is known that CD4 counts decline with age among uninfected individuals(21), these disparities in CD4 count at presentation are unlikely to be explained by the biology of aging alone. This is especially true since the gap appears to be widening in much of the world as the CD4 count at presentation is increasing at a faster rate among younger adults who are often targeted for test-and-treat strategies. Further, a natural decline in CD4 counts and the phenomenon of accentuated aging with HIV (paper 2 in series “Biologic Ageing in PWH) only underscores the need for earlier diagnosis and treatment for older individuals.
We are concerned that a troubling cycle may be developing. The world’s population is experiencing increased life expectancy in general, increasing the absolute number of older individuals(22). With increased life expectancy, older individuals are continuing to enjoy sexual activity(7, 24, 25)(7, 23, 24) which may be both intra and cross generational(6, 7). Many older individuals also continue to use alcohol and other substances(25, 26). Substance use, age-associated erectile dysfunction, and women being beyond child-bearing age all contribute to inconsistent use of condoms(27, 28), increasing opportunities for HIV transmission. This is concerning because we know that older PWH have delayed presentation for HIV treatment compared with younger PWH, prolonging the period in which they may expose others to infection. Delayed presentation also decreases their ability to benefit from early ART initiation(8, 29). Increased HIV incidence among older individuals further increases prevalence and the cycle continues. It is time to tailor language and mediums of communication to reach older individuals more effectively with HIV prevention, diagnosis, and treatment interventions.
We need to implement interventions specifically targeting older individuals. Many of these interventions require health system if not national government involvement (Paper 3 in series, “How health systems can adapt to an ageing population of PWH”). No single intervention will fix this problem. Each country and health system will need to consider which of these interventions are most cost effective in their setting:
Expansion of universal HIV screening
HIV self-testing
Routine clinical discussion of sexual health and substance use
Improved recognition and response to HIV indicator conditions
Use of electronic decision support to prompt and facilitate HIV testing
Discussion of pros/cons of PrEP among older adults at-risk for HIV
We discuss each of these in turn recognizing that their feasibility will need to be determined based upon local resource constraints.
Expansion of universal HIV screening
Universal screening has the advantage that it does not require identification of risk and compliance can be easily assessed. Cost-effectiveness studies, using a QALY threshold of $50,000, suggest that screening is justified in any population with a threshold of ≥0.1% undiagnosed HIV prevalence (30–32). Recent work that considered more effective and durable antiretroviral therapy, adoption of test and treat strategies, and a $100,000 QALY standard found routine testing to be cost-effective at diagnostic rates ≥0.01% (33). This threshold is met (or surpassed) among those ≥65-years-old in many settings. For example, in South Africa the prevalence of HIV in those 50 and more years (7.1%) easily justifies universal screening(34), yet only 54% of those 50 and more years old reported ever testing for HIV compared to 78% of those 25–49 years of age (34). Further, the cost of HIV screening continues to decrease which could lower the threshold for universal screening in the future. Yet, (36)United States Centers for Disease Control and Prevention guidelines for one-time universal screening remain restricted to those between 13 and 64 years(35).
(31–33)(34)It is time to remove age restrictions on universal screening. When screening regardless of age was implemented in the United States Veterans Health Administration in 2009, new HIV diagnoses were established in 0.14% of 210,957 tested from 2009–12 compared to 0.46% of 89,652 tested from 2006–9 under risk-based testing(36). Overall, those ≥65-years-old did not cross the threshold (65–74 years: 0.07% (95% CI 0.02 – 0.09%) and those ≥75 years: 0.02% (95% CI 0.01 – 0.03%))(36). However, corresponding with societal inequities, some populations are at greater risk than others; there are circumstances where universal screening of those ≥65-years-old is justified. The investigators found that rates of new diagnoses among Black patients aged 65–74 and ≥75 years were 0.16% (95% CI 0.07–0.24%) and 0.09% (0.00–0.19%), respectively. Ten years ago, based on a 0.1% diagnostic threshold, universal screening would have been justified among Black veterans in care and came close to being justified among all veterans in care aged 65–74 years(36, 37). What we would see now if the study was repeated is not known. It is time to find out.
(36)There are special reasons to shift away from risk-based testing for older individuals. By making HIV testing the default, it would be less stigmatizing(38). In many countries, older individuals are not viewed by health care providers, nor do they see themselves, as “at risk”. They may also be concerned that their privacy will not be protected making them less likely to request testing or to present where testing is provided(38). Further, while all sexual minorities face challenges in having frank discussions of risk behavior with their providers, older sexual minorities face the combined stigma of age and sexual minority status(39). Finally, prior studies have convincingly demonstrated the value of “normalizing” HIV testing(40) possibly by including HIV testing as part of an array of tests for common age-associated illnesses.
HIV Self Testing
Nearly 40% of new HIV infections are transmitted by people who don’t know that they are infected in the United States and proportions may be higher in countries where testing is less accessible(35). However, stigma, fear of isolation from friends and family, and poor HIV health literacy is particularly strong among older people with HIV(38) (Paper 4 “Aging as a PWH” in the series). Further, older individuals are more likely to have established linkages to care for other chronic conditions (Paper 3 How health systems might adapt to an ageing population of PWH). While these pre-existing conditions may make it more likely that physicians will misattribute signs of HIV infection it may also mean that linkage to care is less challenging for older individuals.
Making self-testing more readily available might be particularly helpful for older individuals by empowering them to first learn their diagnosis and then choose where to seek care(41). This is particularly true for older individuals who are concerned about privacy and/or are sexual minorities(38, 42). Research has begun to identify ideal characteristics of HIV self-tests(43) and, in Agincourt, South Africa, home testing is already available (38). Similarly, expanding point-of-care accessibility for testing in resource constrained settings makes sense, so long as a clear linkage to care is possible(44).
Routine clinical discussion of sexual health and substance use
Guidelines recommend annual testing for anyone with active risk behaviors (35), but providers are often unaware of ongoing substance use or risky sex among their older patients and rarely ask (45, 46). They are particularly uncomfortable discussing risky sexual behaviors with older people who are sexual minorities(39). One study characterized primary care physician’s response to HIV testing among older patients as, “unnecessary and laughable.” Quoting one provider as saying, “older patients are mostly monogamous, so they are low risk, hence low priority…”(47).
Yet older individuals continue to be sexually active, some with multiple intra and cross generational partners(7) and many continue to use alcohol and other substances with multiple implications for their health and well-being including their risk of HIV infection(23, 26, 48). As lifespan has extended, so has sexual healthspan and ongoing sexual activity into older age(48, 49). In South Africa this is particularly true for men who report continuing to have sex with their wives and with younger unmarried women(7). Further, the cohort of individuals currently aging in upper- and middle-income countries commonly used alcohol and other substances in earlier decades of life and many continue to use these substances as they age, especially alcohol, tobacco, marijuana, and cocaine(26). Injection drug use also occurs but is less common than non-injection use among older individuals.
Providers may feel inhibited about discussing sex with their older patients, but HIV risk is only one of many reasons why providers should ask older patients about their sexual health(23, 24, 48, 49). Older men and women experience challenges to continuing sexual activity including erectile dysfunction for men and vaginal dryness for women, both of which are addressable problems. Erectile dysfunction may make use of a condom difficult if not impossible(27, 28). Further, most welcome discussion of their sexuality with their providers but prefer that the provider raise the issue(23, 48, 49). This provides a nonthreatening and non-stigmatizing means of asking about sexual risk behaviors and HIV status of their partners as well.
Similarly, there are compelling reasons why providers should also ask older patients about alcohol(25) and other substance use. Unhealthy alcohol use is increasingly common among older individuals(26) and has critically important health implications including risk of cancer(50), liver disease(25), metabolic disease(51), interaction with prescription medications(52), risk of falls and fractures(53), and cognitive decline(54). Non-injection drug use including alcohol use increases disinhibition and leads to risky behaviors including sex with multiple partners and unprotected intercourse(55, 56). When disinhibition is combined with erectile dysfunction and a perceived lack of concern regarding pregnancy, condoms are rarely employed. Individuals in New York City using heroin or cocaine were equally likely to test positive for HIV infection whether their use was via injection or other means(57). Along with multiple sexual partners and injection drug use, non-injection drug use, including unhealthy alcohol use, should be considered a risk for HIV infection.
Improved recognition and response to HIV indicator conditions
One approach to earlier detection and treatment of HIV infection has been the use of indicator conditions(58–61). The underlying premise is that certain conditions should be considered indications for HIV testing, regardless of disclosed risk behaviors. These conditions fall into three general categories: indicators of risk behaviors that may be undisclosed, indications of early symptomatic HIV disease, and possible indicators of advanced HIV disease. Identified indicators of undisclosed risk behaviors include viral hepatitis and any sexually transmitted infections. Indicators of possible early symptomatic HIV disease include persistent flu like symptoms, a single episode of bacterial pneumonia, herpes zoster, lymphocytopenia, thrombocytopenia and cervical or vulvar dysplasia (CIN2+ or VIN2+). Indicators of possibly advanced HIV disease include cervical cancer, unexplained neuropathy, weight loss, or dementia—while these should always trigger HIV testing, they often occur ten years after initial infection. Tuberculosis also indicates advanced disease but may occur much earlier.
Unfortunately, indicator conditions that might trigger HIV testing among younger individuals may be attributed to other causes in older individuals. Ten years ago, we conducted a study using the US national Veterans Administration data demonstrating that veterans already in care prior to their HIV diagnosis were no more likely to be diagnosed early in the course of their disease as those newly entering VA care(62). Further, only a minority of these patients had an indicator condition prior to their diagnosis. Recently there has been renewed interest in the use of triggers and these studies have confirmed and extended our findings. These studies underscore that trigger conditions are more common among older individuals, but less commonly prompt HIV testing in this age group(58–61).
Use of electronic decision support to prompt and facilitate HIV testing
There is a practical problem with all the HIV testing strategies we have discussed. All these strategies require individuals who are not focused on HIV or its treatment to consider the possibility of HIV infection, obtain the test, and act on the results.
For many primary care and specialty providers in higher-income countries throughout North America, Europe, and Australia/New Zealand, few things are further from their clinical focus. Even in countries with higher HIV prevalence and greater general awareness, providers may not consider testing older individuals who they deem to be at lower risk. In this context, 20 years of experience with a fully paperless, national, electronic medical record in the US Veterans Healthcare System may offer important insights(47, 63–66). Electronic health record (EHR) clinical reminders may help overcome documented failures of one-time universal screening, and risk based and indicator condition testing.
When effectively implemented and maintained, universal screening facilitates more timely diagnoses of HIV infection. In August 2009, The US Veterans Health Administration (VA) revised its HIV testing policies to promote voluntary routine one-time testing of all adults regardless of age and to streamline testing procedures through a clinical reminder. Streamlining eventually included a transition from requiring written informed consent to verbal consent. These changes tripled the lifetime HIV testing prevalence within the national VA(36).
A multimodal HIV testing intervention was also launched with site-specific study teams consisting of an infectious disease specialist, a primary care team leader, and other stakeholders. The intervention included an electronic clinical reminder, a multifaceted provider activation program, social marketing to providers and patients, regular informal conversations with providers, and quarterly feedback on rates of testing.(47) The proportion of newly diagnosed persons ≥60-years-old increased from 7.5% to 15.3% (p=0.10) and the proportion of patients with CD4 counts <200 cells/μL (well below the more commonly used “late presenter” threshold of <350 cells/ μL) decreased from 43% to 29% (p=0.04). A facility that implemented only the electronic reminder linked to a test order achieved the same improvement in testing as the facility with the full multimodal intervention suggesting that this was the element most critical to success(67). Similarly, clinical prompts could also improve adherence to risk based and indicator condition testing.
For resource limited settings, innovative approaches using solar power(68), cloud-based systems(69), and mobile phone applications(70) for data entry have been developed to support EHRs in the context of intermittent, or non-existent, electricity. These have been successfully applied in Kenya(71), India(72), and other low to middle-income countries(73). They have already demonstrated effectiveness at improving the timing of ART in Kenya(69).
Discussion of pros/cons of PrEP among older adults at risk for HIV
Among those at substantial risk of HIV infection, a frank discussion of the pros/cons of pre-exposure prophylaxis (PrEP), tailored to this age group, is indicated. Importantly, based on studies focused on HIV and non-HIV medications, older individuals are more capable of achieving excellent medication adherence than younger individuals(74). On the other hand, addition of two antiretrovirals (a fixed dose, single-tablet combination of tenofovir (300 mg) and emtricitabine (200 mg)) to a medication regimen that may already cross the line into polypharmacy (≥5 chronic medications) (75) and increased risks of hospitalization and mortality(75) has its downside. Polypharmacy is a growing problem among older individuals(76) and the long term safety of these medications in individuals 65 years of age or older is largely unknown(77).
Tenofovir is associated with nephrotoxicity and is contraindicated for those with a creatinine clearance less than 60 mL/min(78, 79). Tenofovir is also associated with bone loss and may contribute to osteoporosis (78, 79), a particular concern among older individuals, especially women. A careful consideration of what other medications the individual is taking and whether these toxicities might exacerbate those of the other medications is indicated(77).
Further, before initiating PrEP, patients must be tested for HIV since PrEP is not an effective treatment for HIV infection and can lead to viral resistance. While receiving PrEP, patients should be monitored every 3 months for declining renal function, sexually transmitted infections, and HIV infection. All this may seem like too much additional effort to patients who may only have sexual intercourse or use injection drugs intermittently(80).
Momentum is building for “on-demand” PrEP(79, 81, 82). The IPERGAY (Intervention Preventive de l’Exposition aux Risques avec et pour les Gays) randomized MSM to receive pericoital PrEP—two pills between 2 and 24 hours before anal intercourse and one pill daily for two days following sex but not more than 7 pills in a single week. This might substantially curtail concerns about toxicity. While this may be an appealing solution, further work is clearly needed.
Conclusion
Although older individuals more commonly present for HIV care late and have more contact with the healthcare system, few studies have focused on factors associated with late presentation specifically among older individuals. This is important because older age is independently associated with risk for indicator conditions possibly rendering them less informative for detection of undiagnosed HIV infection. As the population of older adults with HIV continues to grow, in-depth studies are needed to inform guidelines for HIV testing and determine how best to implement more wide-spread testing and earlier diagnosis and treatment in this growing age group.
Supplementary Material
Key Messages.
Late presentation for HIV care is a major impediment to prevention and effective treatment of HIV infection.
A growing proportion of adults presenting for HIV care are ≥50-years-old and nearly half of them have delayed presentation.
In many regions of the world, the age associated gap in CD4 count at presentation is widening as the average CD4 count at presentation rises for younger adults.
Few studies have focused on factors associated with late presentation specifically for older individuals.
Early diagnosis and treatment of HIV for older individuals is particularly challenging because early signs and symptoms may be attributed to diseases of aging and because neither these individuals nor their care providers perceive them to be at risk for HIV.
If the widening age associated CD4 gap is to be addressed, interventions will need to be explicitly targeted to older individuals.
Acknowledgements
The International Epidemiology Databases to Evaluate AIDS (IeDEA) is supported by the U.S. National Institutes of Health’s National Institute of Allergy and Infectious Diseases, the Eunice Kennedy Shriver National Institute of Child Health and Human Development, the National Cancer Institute, the National Institute of Mental Health, the National Institute on Drug Abuse, the National Heart, Lung, and Blood Institute, the National Institute on Alcohol Abuse and Alcoholism, the National Institute of Diabetes and Digestive and Kidney Diseases, the Fogarty International Center, and the National Library of Medicine: AsiaPacific, U01AI069907; CCASAnet, U01AI069923; Central Africa, U01AI096299; East Africa, U01AI069911; NA-ACCORD, U01AI069918; Southern Africa, U01AI069924; West Africa, U01AI069919. Informatics resources are supported by the Harmonist project, R24AI24872. This work is solely the responsibility of the authors and does not necessarily represent the official views of any of the institutions mentioned above.
Footnotes
Declaration of Interests
ACJ has no conflict of interest.
KNA reports consulting fees paid to her by the All of Us Research Study (NIH) and TrioHealth.
Search strategy and selection criteria
References for this Review were identified through a search of PubMed on 5/19/2021 using the search terms (“late presentation” or “delayed diagnosis”) and “HIV” restricted to manuscripts published at least in part in English in the last 5 years. This yielded 371 citations. We required that the manuscript be original research, include an adult population (age>15 years), define late presentation, and adequately characterize the sample evaluated including sample size, region and calendar period from which the sample was drawn, and the proportion or number of late presenters. A review of titles and abstracts eliminated all but 74 manuscripts. When these were further restricted to manuscripts reporting the association of age with late presentation the number reduced to 40.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
NA-ACCORD:
Amy C Justice, Matthew B Goetz, Keri N Althoff, Cameron N Stewart, Brenna C Hogan, and Elizabeth Humes
CCASANet:
Paula M Luz and Jessica L Castilho
Central Africa:
East Africa:
Beverly Musick and Constantin Yiannoutsos
West Africa:
Karen Malateste and Antoine Jaquet
Southern Africa:
Asia Pacific:
References
- 1.Tavoschi L, Gomes Dias J, Pharris A. New HIV diagnoses among adults aged 50 years or older in 31 European countries, 2004–15: an analysis of surveillance data. The lancet HIV. 2017;4(11):e514–e21. [DOI] [PubMed] [Google Scholar]
- 2.Autenrieth CS, Beck EJ, Stelzle D, Mallouris C, Mahy M, Ghys P. Global and regional trends of people living with HIV aged 50 and over: Estimates and projections for 2000–2020. PLoS One. 2018;13(11):e0207005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Puhr R, Kumarasamy N, Ly PS, Ng OT, Van Nguyen K, Merati TP, et al. HIV and Aging: Demographic Change in the Asia-Pacific Region. J Acquir Immune Defic Syndr. 2017;74(5):e146–e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Caro-Vega Y, Belaunzarán-Zamudio PF, Crabtree-Ramírez B, Shepherd BE, Mejia F, Giganti MJ, et al. Trends in proportion of older HIV-infected people in care in Latin America and the Caribbean: a growing challenge. Epidemiol Infect. 2018;146(10):1308–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Prevention CfDCa. Estimated HIV incidence and prevalence in the United States, 2015–2019. Atlanta, Georgia: Cetners for Disease Control and Prevention; 2021. [Google Scholar]
- 6.Gómez-Olivé FX, Houle B, Rosenberg M, Kabudula C, Mojola S, Rohr JK, et al. Brief Report: HIV Incidence Among Older Adults in a Rural South African Setting: 2010–2015. J Acquir Immune Defic Syndr. 2020;85(1):18–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Houle B, Mojola SA, Angotti N, Schatz E, Gómez-Olivé FX, Clark SJ, et al. Sexual behavior and HIV risk across the life course in rural South Africa: trends and comparisons. AIDS Care. 2018;30(11):1435–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cornell M, Johnson LF, Schomaker M, Tanser F, Maskew M, Wood R, et al. Age in antiretroviral therapy programmes in South Africa: a retrospective, multicentre, observational cohort study. The lancet HIV. 2015;2(9):e368–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Althoff KN, Gebo KA, Gange SJ, Klein MB, Brooks JT, Hogg RS, et al. CD4 count at presentation for HIV care in the United States and Canada: are those over 50 years more likely to have a delayed presentation? AIDS Res Ther. 2010;7:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gesesew HA, Ward P, Woldemichael K, Mwanri L. Late presentation for HIV care in Southwest Ethiopia in 2003–2015: prevalence, trend, outcomes and risk factors. BMC infectious diseases. 2018;18(1):59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Taborelli M, Virdone S, Camoni L, Regine V, Zucchetto A, Frova L, et al. The persistent problem of late HIV diagnosis in people with AIDS: a population-based study in Italy, 1999–2013. Public Health. 2017;142:39–45. [DOI] [PubMed] [Google Scholar]
- 12.Meléndez J, Reinhardt SW, O’Halloran JA, Spec A, Alonzo Cordon A, Powderly WG, et al. Late Presentation and Missed Opportunities for HIV Diagnosis in Guatemala. AIDS and behavior. 2019;23(4):920–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.O’Connell S, Enkelmann J, Sadlier C, Bergin C. Late HIV presentation - missed opportunities and factors associated with a changing pattern over time. Int J STD AIDS. 2017;28(8):814–21. [DOI] [PubMed] [Google Scholar]
- 14.Levy I, Maor Y, Mahroum N, Olmer L, Wieder A, Litchevski V, et al. Missed opportunities for earlier diagnosis of HIV in patients who presented with advanced HIV disease: a retrospective cohort study. BMJ open. 2016;6(11):e012721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gullón A, Verdejo J, de Miguel R, Gómez A, Sanz J. Factors associated with late diagnosis of HIV infection and missed opportunities for earlier testing. AIDS Care. 2016;28(10):1296–300. [DOI] [PubMed] [Google Scholar]
- 16.Lin YD, Garner SE, Lau JSY, Korman TM, Woolley IJ. Prevalence of HIV indicator conditions in late presenting patients with HIV: a missed opportunity for diagnosis? Qjm. 2019;112(1):17–21. [DOI] [PubMed] [Google Scholar]
- 17.Brazier E, Tymejczyk O, Zaniewski E, Egger M, Wools-Kaloustian K, Yiannoutsos CT, et al. Effects of national adoption of Treat-All guidelines on pre-ART CD4 testing and viral load monitoring after ART initiation: A regression discontinuity analysis. Clin Infect Dis. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nash D, Robertson M. How to Evolve the Response to the Global HIV Epidemic With New Metrics and Targets Based on Pre-Treatment CD4 Counts. Curr HIV/AIDS Rep. 2019;16(4):304–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Foundation HJKF. The U.S. Government Engagement in Global Health: a Primer. 2019. [Google Scholar]
- 20.Organization. WH. Consolidated Guidelines on the Use of Antiretroviral Drugs for Treating and Preventing HIV Infection. Recommendations for a Public Health Approach. Geneva, Switzerland: World Health Organization; 2013. [PubMed] [Google Scholar]
- 21.Maini MK, Gilson RJ, Chavda N, Gill S, Fakoya A, Ross EJ, et al. Reference ranges and sources of variability of CD4 counts in HIV-seronegative women and men. GenitourinMed. 1996;72(1):27–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.United Nations doEaSA, Population Division( 2019). World Population Ageing 2019: Highlights. New York: United Nations; 2019. [Google Scholar]
- 23.Lindau ST, Gavrilova N. Sex, health, and years of sexually active life gained due to good health: evidence from two US population based cross sectional surveys of ageing. Bmj. 2010;340:c810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nicolosi A, Buvat J, Glasser DB, Hartmann U, Laumann EO, Gingell C. Sexual behaviour, sexual dysfunctions and related help seeking patterns in middle-aged and elderly Europeans: the global study of sexual attitudes and behaviors. World J Urol. 2006;24(4):423–8. [DOI] [PubMed] [Google Scholar]
- 25.Alcohol use and burden for 195 countries and territories, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kuerbis A Substance Use among Older Adults: An Update on Prevalence, Etiology, Assessment, and Intervention. Gerontology. 2020;66(3):249–58. [DOI] [PubMed] [Google Scholar]
- 27.Ehrenstein V, Horton NJ, Samet JH. Inconsistent condom use among HIV-infected patients with alcohol problems. Drug Alcohol Depend. 2004;73(2):159–66. [DOI] [PubMed] [Google Scholar]
- 28.Schick V, Herbenick D, Reece M, Sanders SA, Dodge B, Middlestadt SE, et al. Sexual behaviors, condom use, and sexual health of Americans over 50: implications for sexual health promotion for older adults. The journal of sexual medicine. 2010;7 Suppl 5:315–29. [DOI] [PubMed] [Google Scholar]
- 29.Shamu T, Chimbetete C, Egger M, Mudzviti T. Treatment outcomes in HIV infected patients older than 50 years attending an HIV clinic in Harare, Zimbabwe: A cohort study. PLOS ONE. 2021;16(6):e0253000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bert F, Gualano MR, Biancone P, Brescia V, Camussi E, Martorana M, et al. Cost-effectiveness of HIV screening in high-income countries: A systematic review. Health Policy. 2018;122(5):533–47. [DOI] [PubMed] [Google Scholar]
- 31.Paltiel AD, Weinstein MC, Kimmel AD, Seage GR III, Losina E, Zhang H, et al. Expanded screening for HIV in the United States--an analysis of cost-effectiveness. NEnglJMed. 2005;352(6):586–95. [DOI] [PubMed] [Google Scholar]
- 32.Sanders GD, Bayoumi AM, Holodniy M, Owens DK. Cost-effectiveness of HIV screening in patients older than 55 years of age. Ann Intern Med. 2008;148(12):889–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lucas A, Armbruster B. The cost-effectiveness of expanded HIV screening in the United States. AIDS. 2013;27(5):795–801. [DOI] [PubMed] [Google Scholar]
- 34.Schatz E, Knight L. “I was referred from the other side”: Gender and HIV testing among older South Africans living with HIV. PLoS One. 2018;13(4):e0196158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Branson BM, Handsfield HH, Lampe MA, Janssen RS, Taylor AW, Lyss SB, et al. Revised recommendations for HIV testing of adults, adolescents, and pregnant women in health-care settings. MMWR Recomm Rep. 2006;55(Rr-14):1–17; quiz CE1–4. [PubMed] [Google Scholar]
- 36.Goetz MB, Hoang T, Kan VL, Rimland D, Rodriguez-Barradas MC, Asch SM. Rates and Predictors of Newly Diagnosed HIV Infection Among Veterans Receiving Routine Once-Per-Lifetime HIV Testing in the Veterans Health Administration. J Acquir Immune Defic Syndr. 2015;69(5):544–50. [DOI] [PubMed] [Google Scholar]
- 37.Owens DK, Sundaram V, Lazzeroni LC, Douglass LR, Sanders GD, Taylor K, et al. Prevalence of HIV infection among inpatients and outpatients in Department of Veterans Affairs health care systems: implications for screening programs for HIV. Am J Public Health. 2007;97(12):2173–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schatz E, Houle B, Mojola SA, Angotti N, Williams J. How to “Live a Good Life”: Aging and HIV Testing in Rural South Africa. J Aging Health. 2019;31(4):709–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Flatt JD, Cicero EC, Kittle KR, Brennan-Ing M. Recommendations for Advancing Research with Sexual and Gender Minority Older Adults. J Gerontol B Psychol Sci Soc Sci. 2021. [DOI] [PubMed] [Google Scholar]
- 40.Bokhour BG, Solomon JL, Knapp H, Asch SM, Gifford AL. Barriers and facilitators to routine HIV testing in VA primary care. J Gen Intern Med. 2009;24(10):1109–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Orne-Gliemann J, Zuma T, Chikovore J, Gillespie N, Grant M, Iwuji C, et al. Community perceptions of repeat HIV-testing: experiences of the ANRS 12249 Treatment as Prevention trial in rural South Africa. AIDS Care. 2016;28 Suppl 3:14–23. [DOI] [PubMed] [Google Scholar]
- 42.Johnson MC, Chung R, Leung SJ, Edelstein Z, Yuan Y, Flavin SM. Combating Stigma Through HIV Self-Testing: New York State’s HIV Home Test Giveaway Program for Sexual Minorities. J Public Health Manag Pract. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Knight L, Makusha T, Lim J, Peck R, Taegtmeyer M, van Rooyen H. “I think it is right”: a qualitative exploration of the acceptability and desired future use of oral swab and finger-prick HIV self-tests by lay users in KwaZulu-Natal, South Africa. BMC research notes. 2017;10(1):486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Manoto SL, Lugongolo M, Govender U, Mthunzi-Kufa P. Point of Care Diagnostics for HIV in Resource Limited Settings: An Overview. Medicina (Kaunas). 2018;54(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dookeran NM, Burgess JF Jr., Bowman CC, Goetz MB, Asch SM, Gifford AL. HIV screening among substance-abusing veterans in care. Journal of substance abuse treatment. 2009;37(3):286–91. [DOI] [PubMed] [Google Scholar]
- 46.Inelmen EM, Sergi G, De Rui M, Manzato E. Enhancing awareness to mitigate the risk of HIV/AIDS in older adults. Aging Clin Exp Res. 2014;26(6):665–9. [DOI] [PubMed] [Google Scholar]
- 47.Bokhour BG, Saifu H, Goetz MB, Fix GM, Burgess J, Fletcher MD, et al. The role of evidence and context for implementing a multimodal intervention to increase HIV testing. Implement Sci. 2015;10:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lindau ST, Schumm LP, Laumann EO, Levinson W, O’Muircheartaigh CA, Waite LJ. A study of sexuality and health among older adults in the United States. N Engl J Med. 2007;357(8):762–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ni Lochlainn M, Kenny RA. Sexual activity and aging. J Am Med Dir Assoc. 2013;14(8):565–72. [DOI] [PubMed] [Google Scholar]
- 50.Rumgay H SK, Charvat H, et al. Global burden of cancer in 2020 attributable to alcohol consumption: a population-based study. Lancet Oncol. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fan AZ, Russell M, Naimi T, Li Y, Liao Y, Jiles R, et al. Patterns of alcohol consumption and the metabolic syndrome. JClinEndocrinolMetab. 2008;93(10):3833–8. [DOI] [PubMed] [Google Scholar]
- 52.Lindsey WT, Stewart D, Childress D. Drug interactions between common illicit drugs and prescription therapies. Am J Drug Alcohol Abuse. 2012;38(4):334–43. [DOI] [PubMed] [Google Scholar]
- 53.Womack JA, Murphy TE, Rentsch CT, Tate JP, Bathulapalli H, Smith AC, et al. Polypharmacy, hazardous alcohol and illicit substance use and serious falls among PLWH and uninfected comparators. J Acquir Immune Defic Syndr. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Krivanek TJ, Gale SA, McFeeley BM, Nicastri CM, Daffner KR. Promoting Successful Cognitive Aging: A Ten-Year Update. J Alzheimers Dis. 2021;81(3):871–920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cook RL, McGinnis KA, Kraemer KL, Gordon AJ, Conigliaro J, Maisto SA, et al. Intoxication before intercourse and risky sexual behavior in male veterans with and without human immunodeficiency virus infection. Med Care. 2006;44(8 Suppl 2):S31–S6. [DOI] [PubMed] [Google Scholar]
- 56.Cook RL, McGinnis KA, Samet JH, Fiellin DA, Rodriquez-Barradas MC, Kraemer KL, et al. Erectile dysfunction drug receipt, risky sexual behavior and sexually transmitted diseases in HIV-infected and HIV-uninfected men. J Gen Intern Med. 2010;25(2):115–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Des Jarlais DC, Arasteh K, Perlis T, Hagan H, Abdul-Quader A, Heckathorn DD, et al. Convergence of HIV seroprevalence among injecting and non-injecting drug users in New York City. Aids. 2007;21(2):231–5. [DOI] [PubMed] [Google Scholar]
- 58.Basoulis D, Kostaki EG, Paraskevis D, Hatzakis A, Psichogiou M. Tracking missed opportunities for an early HIV diagnosis in a population of people living with HIV with known time of infection. Sexually transmitted infections. 2021. [DOI] [PubMed] [Google Scholar]
- 59.Raben D, Sullivan AK, Mocroft A, Kutsyna G, Hadžiosmanović V, Vassilenko A, et al. Improving the evidence for indicator condition guided HIV testing in Europe: Results from the HIDES II Study - 2012 – 2015. PLoS One. 2019;14(8):e0220108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lhopitallier L, Moulin E, Hugli O, Cavassini M, Darling KEA. Missed opportunities for HIV testing among patients newly presenting for HIV care at a Swiss university hospital: a retrospective analysis. BMJ open. 2018;8(6):e019806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bogers SJ, Hulstein SH, Schim van der Loeff MF, de Bree GJ, Reiss P, van Bergen J, et al. Current evidence on the adoption of indicator condition guided testing for HIV in western countries: A systematic review and meta-analysis. EClinicalMedicine. 2021;35:100877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gandhi NR, Skanderson M, Gordon KS, Concato J, Justice AC. Delayed presentation for human immunodeficiency virus (HIV) care among veterans: a problem of access or screening? Med Care. 2007;45(11):1105–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Goetz MB, Hoang T, Knapp H, Burgess J, Fletcher MD, Gifford AL, et al. Central implementation strategies outperform local ones in improving HIV testing in Veterans Healthcare Administration facilities. J Gen Intern Med. 2013;28(10):1311–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gidwani R, Goetz MB, Kominski G, Asch S, Mattocks K, Samet JH, et al. A budget impact analysis of rapid human immunodeficiency virus screening in Veterans Administration emergency departments. J Emerg Med. 2012;42(6):719–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Goetz MB, Hoang T, Knapp H, Henry SR, Anaya H, Chou AF, et al. Exportability of an intervention to increase HIV testing in the Veterans Health Administration. Jt Comm J Qual Patient Saf. 2011;37(12):553–9. [DOI] [PubMed] [Google Scholar]
- 66.Goetz MB, Hoang T, Henry SR, Knapp H, Anaya HD, Gifford AL, et al. Evaluation of the sustainability of an intervention to increase HIV testing. J Gen Intern Med. 2009;24(12):1275–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Goetz MB, Rimland D. Effect of expanded HIV testing programs on the status of newly diagnosed HIV-infected patients in two Veterans Health Administration facilities: 1999–2009. J Acquir Immune Defic Syndr. 2011;57(2):e23–5. [DOI] [PubMed] [Google Scholar]
- 68.Tierney WM, Rotich JK, Smith FE, Bii J, Einterz RM, Hannan TJ. Crossing the “digital divide” implementing an electronic medical record system in a rural Kenyan health center to support clinical care and research. Proc AMIA Symp. 2002;:792–5.:792–5. [PMC free article] [PubMed] [Google Scholar]
- 69.Haskew J, Rø G, Turner K, Kimanga D, Sirengo M, Sharif S. Implementation of a Cloud-Based Electronic Medical Record to Reduce Gaps in the HIV Treatment Continuum in Rural Kenya. PLoS One. 2015;10(8):e0135361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rotich JK, Hannan TJ, Smith FE, Bii J, Odero WW, Vu N, et al. Installing and implementing a computer-based patient record system in sub-Saharan Africa: the Mosoriot Medical Record System. JAmMedInformAssoc. 2003;10(4):295–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Oyugi B, Makunja S, Kabuti W, Nyongesa C, Schömburg M, Kibe V, et al. Improving the management of hypertension and diabetes: An implementation evaluation of an electronic medical record system in Nairobi County, Kenya. Int J Med Inform. 2020;141:104220. [DOI] [PubMed] [Google Scholar]
- 72.Patel SA, Sharma H, Mohan S, Weber MB, Jindal D, Jarhyan P, et al. The Integrated Tracking, Referral, and Electronic Decision Support, and Care Coordination (I-TREC) program: scalable strategies for the management of hypertension and diabetes within the government healthcare system of India. BMC Health Serv Res. 2020;20(1):1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Dainton C, Chu CH. A review of electronic medical record keeping on mobile medical service trips in austere settings. Int J Med Inform. 2017;98:33–40. [DOI] [PubMed] [Google Scholar]
- 74.G CI, G. BM Drug therapy of the aged: The problem of compliance and the roles of physicians and pharmacists. J AmGeriatr Soc. 1984;32:301–7. [DOI] [PubMed] [Google Scholar]
- 75.Edelman EJ, Rentsch CT, Justice AC. Polypharmacy in HIV: recent insights and future directions. Curr Opin HIV AIDS. 2020;15(2):126–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wastesson JW, Morin L, Tan ECK, Johnell K. An update on the clinical consequences of polypharmacy in older adults: a narrative review. Expert Opin Drug Saf. 2018;17(12):1185–96. [DOI] [PubMed] [Google Scholar]
- 77.Franconi I, Guaraldi G. Pre-exposure Prophylaxis for HIV Infection in the Older Patient: What can be Recommended? Drugs Aging. 2018;35(6):485–91. [DOI] [PubMed] [Google Scholar]
- 78.Liegeon G, Antoni G, Pialoux G, Capitant C, Cotte L, Charreau I, et al. Changes in kidney function among men having sex with men starting on demand tenofovir disoproxil fumarate - emtricitabine for HIV pre-exposure prophylaxis. Journal of the International AIDS Society. 2020;23(2):e25420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Saberi P, Scott HM. On-Demand Oral Pre-exposure Prophylaxis with Tenofovir/Emtricitabine: What Every Clinician Needs to Know. J Gen Intern Med. 2020;35(4):1285–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Beymer MR, Holloway IW, Pulsipher C, Landovitz RJ. Current and Future PrEP Medications and Modalities: On-demand, Injectables, and Topicals. Curr HIV/AIDS Rep. 2019;16(4):349–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Rutstein SE, Smith DK, Dalal S, Baggaley RC, Cohen MS. Initiation, discontinuation, and restarting HIV pre-exposure prophylaxis: ongoing implementation strategies. The lancet HIV. 2020;7(10):e721–e30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Siguier M, Mera R, Pialoux G, Ohayon M, Cotte L, Valin N, et al. First year of pre-exposure prophylaxis implementation in France with daily or on-demand tenofovir disoproxil fumarate/emtricitabine. The Journal of antimicrobial chemotherapy. 2019;74(9):2752–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


