Abstract
Behavioral reactivity to novel stimuli, which is greater in the adolescent than young adult population, is associated with drug abuse liability, suggesting that the increased addiction vulnerability of adolescents may be related to heightened novel stimulus reactivity and underlying cellular processes. We examined the hypothesis that adolescent animals who exhibit higher novel stimulus reactivity, exhibit greater locomotor activity in response to nicotine than adolescents who exhibit lower novel stimulus reactivity, and that this difference is associated with alterations in CREB expression and activity in the ventral striatum (vStr) and prefrontal cortex (PFC). Adolescents exhibiting high locomotor activity (HLA) in the novel open field developed tolerance to the locomotor depressant effects of nicotine with fewer exposures and at lower doses than adolescents with low locomotor activity (LLA). Further, HLA adolescents exhibited lower CREB activity in the vStr than LLA adolescents and this difference was attenuated by repeated exposure to high, but not low doses of nicotine. Thus, inherent differences in the reactivity to novel stimulation during the adolescent period appear to predict sensitivity to the behavioral and cellular effects of nicotine and may underlie differences in progression to addiction.
Keywords: Adolescent, CREB, Locomotor Activity, Nicotine, Novelty, Ventral Striatum
1. Introduction
Several studies have demonstrated a relationship between various measures of behavior and drug abuse liability, with high responders to novel environments demonstrating more rapid establishment of drug self-administration [1–4] and high novelty-preferring animals exhibiting greater sensitivity to the rewarding effects of abused compounds [5–7]. We recently demonstrated that, relative to young adult rats, adolescent rats exhibit greater behavioral reactivity to novel stimulation and more frequently express behaviors associated with a high sensation-seeking profile [8]. The finding that adolescents exhibit high behavioral reactivity to novel stimuli more frequently than adults suggests that the adolescent population may be more vulnerable to the reinforcing and rewarding effects of drugs of abuse than the adult population. Indeed, human adolescents exhibit elevated sensation-seeking behavior [9] and several studies have reported that adolescent drug users have a heightened risk of progressing to addiction [10–12].
During the adolescent period, tobacco is among the most frequently used of all drugs. Approximately 1 in 4 high school students have been classified as current smokers [13] and every day 3,500 12–17 year olds initiate tobacco use, and an additional 1,000 become regular smokers in the U.S. [14]. Subjective experiences with smoking during adolescence may be a predictor of progression to addiction [15, 16], as individuals who experience more intense feelings when first smoking are more likely to increase their smoking rate and develop stronger nicotine dependence [17–20]. Recent work in humans has shown that initial sensitivity to the rewarding and reinforcing effects of acute nicotine is directly related to novelty-seeking behaviors, with higher sensation-seekers experiencing greater nicotine reward [21–23]. Further, greater sensation-seeking behavior indicates a greater probability of nicotine use during adolescence [23], as well as higher odds of being a current smoker [24]. Thus, the behavioral and neurobiological consequences of nicotine exposure during adolescence among individuals with different sensation-seeking tendencies are of particular significance to understanding the development of addiction in adolescent drug users.
The rewarding and behavioral activating effects of drugs of abuse are thought to be mediated by alterations in the expression and/or activation of cAMP response element binding protein (CREB), a constitutively expressed transcription factor regulated by phosphorylation at serine 133 [25]. Studies have shown that CREB expression throughout the brain and phosphorylation in the nucleus accumbens (NAcc) are necessary to establish a conditioned place preference (CPP) to nicotine [26, 27]. Further, the rewarding and activating effects of cocaine are reduced following the overexpression of CREB in the NAcc, and increased following CREB repression in this brain region [28]. Interestingly, CREB overexpression also decreases behavioral reactivity to environmental stimuli [29] and regional repression enhances active coping behaviors [30, 31]. Thus, the regional expression and/or activity of CREB may represent a mechanism linking behavior with vulnerability to drugs of abuse, including nicotine.
This study was designed to better understand the relationship between adolescent behavior, nicotine-induced locomotion and CREB expression. The primary hypothesis tested was that adolescent animals exhibiting high activity in the novel open field exhibit greater behavioral reactivity to nicotine than adolescent animals exhibiting low activity in the novel open field. Further, it was postulated that this difference is associated with differences in CREB expression and activity in the ventral striatum (vStr) or prefrontal cortex (PFC).
2. Methods
2.1 Subjects
Timed pregnant Sprague-Dawley dams (n=8) were obtained from Harlan Laboratories, Inc. (Prattville, AL). The offspring of these rats were used as subjects for these experiments. The day of birth was defined as postnatal day 0 (PND 0). To assure similar development across litters, all animals were culled to 12 pups per litter (6 males/6 females) on PND 1, and remained housed with their respective dams until PND 21 at which time animals were weaned and housed in groups of 3 in standard polypropylene cages with corncob bedding on the floor. Although culled litters consisted of males and females, to avoid potential changes in behavior introduced by the emergence of the estrous cycle during adolescence, only the male offspring were used for experimentation. All experimental animals were housed at the University of South Florida in a temperature and humidity-controlled vivarium on a 12:12-hr light–dark cycle (7 a.m./7 p.m.). Experiments were conducted during the light phase, and the care and use of animals was in accordance with guidelines set by the Institutional Animal Care and Use Committee and the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
2.2 Novel Open Field Test
Beginning on PND 28, male offspring were handled for 5 minutes, 2 times a day (10 a.m. and 4 p.m.), for a total of 3 days (PND 28–30) to familiarize the animals to experimenter manipulation. Following 3 days of handling, on PND 31, locomotor activity in the novel open field was determined. For adolescent animals, the total distance moved in the novel open field correlated significantly with measures of activity (distance and velocity) when animals were provided a novel object to explore [8]. Thus, total distance moved in the novel open field was utilized as the primary measure.
Several parameters associated with locomotor activity were measured under moderate levels of illumination (approximately 20 lux) in a standard sized arena [8]. The arena consisted of a black plastic circular platform 100 cm in diameter, with a perimeter of 314 cm and an area of 7854 cm2, 70 cm above the ground; a white plastic barrier (30 cm high) enclosed the arena. A video camera was suspended directly over the arena and behaviors were recorded automatically based on the center of body mass (defined as crown to rump length/2) of the animals (EthoVision, Noldus Information Technology, Leesburg, VA). Animals were placed in 1 of 4 randomly selected zones in the open field and allowed to explore the novel environment freely for 5 minutes. The parameters measured included: total distance moved (TDM in cm) and velocity during movement (VEL in cm/sec) with a velocity of 1.25cm/sec as a minimum criterion; movement initiation frequency (MIF, the number of starts or stops/5 min); and angular velocity (ANG, degrees of rotation/sec). Following 5 minutes in the novel environment, each rat was removed from the arena and the arena wiped with a 70% ethanol solution to reduce olfactory influences on the behavior of subsequent animals.
Animals were characterized as exhibiting low locomotor activity (LLA) or high locomotor activity (HLA) using a variation of commonly used methods [32–35] (Fig. 1). To ensure distinct subject populations, animals whose activity fell in the middle 20% of the distribution [determined using the equation Mean ± (Standard Deviation * Z10%)], were removed from the experiment. Animals whose activity fell in the bottom 40% of the distribution were classified as LLA and those whose activity fell in the top 40% of the distribution were classified as HLA. Following behavioral characterization, animals were returned to their home cages until experimental manipulation beginning on PND 35.
Fig. 1.
Distribution of total distance moved (TDM) in the novel open field. Animals were handled for 3 days twice per day from postnatal day (PND) 28–30 and assessed for activity in a novel, inescapable open field on PND 31. Animals were classified as exhibiting either low locomotor activity [LLA; 5166 ± 54 cm (mean ± sem), n=26] or high locomotor activity [HLA; 6218 ± 104 cm (mean ± sem), n=26] by removing animals (n=8; dashed circles) in the middle 20% of the activity distribution (5504 to 5724 cm; dashed lines) and classifying the bottom 40% as LLA and the top 40% as HLA. Responses from each animal are shown in the scatter plot.
2.3 Nicotine Treatment and Locomotor Assessment
During the mid-adolescent period (PND 35 to PND 42), animals were randomly assigned to groups and received injections (once daily, s.c.) of either saline (0.9%) or doses of nicotine ranging from 0.14 to 0.56 mg free base/kg. This range was chosen based on studies in the literature [36–38] and used to categorize the effects of nicotine resulting from low, moderate or high doses. Immediately following the first, fourth and eighth injections, animals were placed in the open field and locomotor activity was measured for 5 min.
2.4 Western Blot Analysis
At 18 hours following their final (eighth) nicotine injection, rats were decapitated rapidly and the vStr and PFC isolated, quick frozen on dry ice and stored at −80°C. Whole-cell homogenates were prepared by a combination of Dounce homogenization (glass:glass) and sonication (15 µl per mg wet weight) in homogenization buffer (pH 7.9 at 25°C) containing: 0.5 M sucrose; 10 mM HEPES; 1.5 mM MgCl2; 10 mM KCl; 10% glycerol; 1 mM EDTA; 1 mM NaF; 2 mM sodium orthovanadate; 5 µg/ml leupeptin; 5 µg/ml aprotinin; 5 µg/ml pepstatin; 1 mM PMSF; and 1 mM DTT. The protein concentration of the homogenates was determined using the Bio-Rad DC protein assay. Immunoblot analyses were performed using 25 µg protein. Proteins were separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE; 10% polyacrylamide) and transferred electrophoretically to polyvinylidene fluoride (PVDF) membranes. The membranes were blocked for 1 hour in Tris-buffered saline (TBS) containing 0.05% Tween 20 and 5% non-fat dry milk. Subsequently, primary antibody [CREB (48H2) #9197 or phospho-CREB (Ser133) (87G3) #9198, Cell Signaling Technology, Beverly, MA] was added to the blocking solution and the membranes were incubated overnight at 4°C. Sixteen hours later, the membranes were washed and incubated with secondary antibody [goat anti-rabbit IgG-HRP, Santa Cruz, Santa Cruz, CA] in blocking solution for 1 hour, and signals visualized using enhanced chemiluminescence. After immunodetection for CREB or pCREB, blots were incubated with a primary antibody directed against β-tubulin [H-235, Santa Cruz] as a loading control. Signals were quantified using a densitometer and Un-Scan-It gel digitizing software (Silk Scientific Inc, Orem, Utah) and CREB and pCREB expressed relative to β-tubulin.
2.5 Statistical Analysis
A mixed factorial 2 (HLA or LLA) × 4 (treatment-saline or 3 doses of nicotine) × 4 (assessment day) ANOVA was performed on each behavioral measure to identify main effects and interactions. Post hoc t-tests were performed, where appropriate, to isolate significant differences. For measures of CREB and pCREB, a factorial 2 (HLA or LLA) × 4 (treatment-saline or 3 doses of nicotine) ANOVA was performed with post hoc t-tests where appropriate.
3. Results
3.1 Nicotine-Induced Locomotor Activity in Adolescent Animals
Analysis indicated that the mean TDM of animals receiving 0.14 or 0.21 mg free base nicotine/kg did not differ either during the initial assessment in the novel open field [t(19) = 0.5285, ns] or following nicotine injections on trials one, four or eight following nicotine challenge [One: t(19) = 0.5637, ns; Four: t(19) = 1.103, ns; Eight: t(19) = 1.636, ns]. Therefore, the behavioral data from these animals were combined in a single group termed ‘low dose’ nicotine. There were significant differences in the effects of 0.42 and 0.56 mg free base nicotine/kg on trial one [t(20) = 2.615, p<0.05]; therefore, the effects of these doses were analyzed separately, as ‘moderate dose’ (0.42 mg/kg) and ‘high dose’ (0.56 mg/kg), respectively.
Because the effects of differing doses of nicotine on locomotor activity exhibited by adolescent animals have not been well described, the dose-response effects of nicotine on adolescent locomotor activity was examined in the open field following one, four and eight days of nicotine administration. A mixed factorial ANOVA revealed a significant interaction of nicotine dose and days of exposure on the open field activity of animals, F(9,132) = 16.312, p<0.05. Animals exhibited a significant dose-dependent depression of locomotor activity following the initial nicotine injection when compared to saline-injected animals (Fig. 2, One) [Low Dose t(29) = 3.216, p<0.05; Moderate Dose t(19) = 4.905, p<0.05; High Dose t(19) = 6.442, p<0.05]. The high dose of nicotine reduced activity by 60%, an effect that was significantly greater than the suppressive effects of the low [t(30) = 4.243, p<0.05] or moderate doses of nicotine [t(20) = 2.615, p<0.05]. Following the fourth nicotine exposure, the low dose produced a significant increase in locomotion when compared to saline-injected animals (Fig. 2, Four) [t(29) = 3.149, p<0.05], whereas the moderate and high doses of nicotine had no effect, indicating that animals adapted to the depressant properties of nicotine within four exposures and that stimulant effects of nicotine were apparent only following a low dose. Following the eighth exposure to nicotine, both the low and moderate doses produced significant increases in locomotor activity compared to saline-injected animals (Fig. 2, Eight) [Low Dose t(29) = 4.244, p<0.05; Moderate Dose t(19) = 4.548, p<0.05]; activity following the high dose of nicotine did not differ significantly from that of saline-injected animals. Thus, as the dose of nicotine increased, more exposures were required to observe nicotine’s stimulant properties.
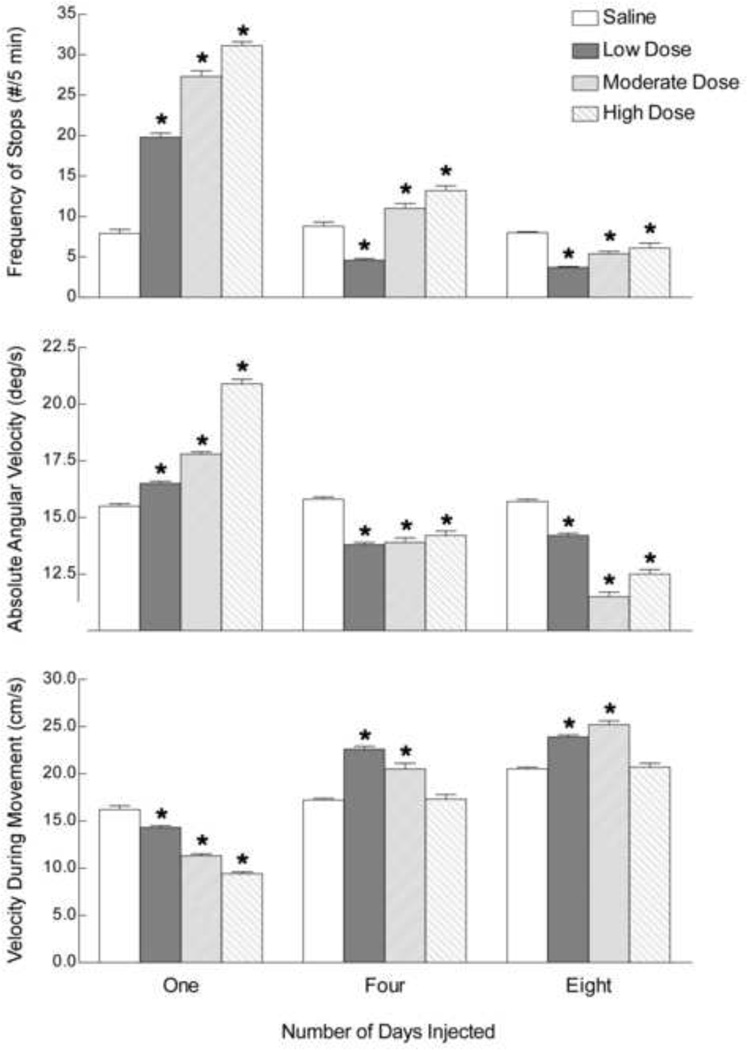
Fig. 2.
The effect of nicotine administration on the total distance moved (TDM) by adolescent rats. Animals (n=52) received injections (s.c.) of either saline (n=10) or a low (0.14 or 0.21 mg free base/kg; n=21), moderate (0.42 mg free base/kg; n=11) or high (0.56 mg free base/kg; n=10) dose of nicotine once a day for eight days from PND 35–42. Immediately following injection on PND 35 [One], 38 [Four] and 42 [Eight], TDM was assessed for a total of 5 min. Bars represent mean + sem. * Means differ significantly, p<0.05.
To determine whether the locomotor suppressing and activating effects of nicotine were related to stereotypic-like behavior, the MIF, ANG and VEL of each animal were compared for each dose of nicotine following injections one, four and eight (Fig. 3). There were significant interactions between nicotine dose and days of exposure for MIF [F(6,147)=429, p<0.05], VEL [F(6,147)=66.08, p<0.05] and ANG [F(6,147)=50.9, p<0.05]. Following the first injection, MIF and ANG increased [MIF: low dose t(4)=19.21, p<0.05; moderate dose t(4)=27.54, p<0.05; high dose t(4)=32.9, p<0.05. ANG: low dose t(4)=5.1, p<0.05; moderate dose t(4)=10.3, p<0.05; high dose t(4)=24.2, p<0.05], and VEL decreased [VEL: low dose t(4)=4.2, p<0.05; moderate dose t(4)=9.5, p<0.05; high dose t(4)=13.2, p<0.05] in a dose-dependent manner. These results indicate that stereotypic-like behavior (pauses in horizontal movement and increased rotational behavior]) and decreased linear activity (VEL) both contributed to the observed locomotor suppressing effects of nicotine.
Fig. 3.
The effect of nicotine administration on the movement initiation frequency (MIF), angular velocity (ANG) and velocity during movement (VEL) of adolescent rats. Animals (n=52) received injections of either saline or nicotine as in Fig. 2, and behaviors (MIF, ANG and VEL) assessed immediately for a total of 5 min following injection on PND 35 [One], 38 [Four] and 42 [Eight]. * Means differ significantly from saline-injected animals, p<0.05.
Following the fourth injection of a low dose of nicotine, MIF and ANG decreased [MIF: t(4)=6.8, p<0.05. ANG: t(4)=10.2,p<0.05], and VEL increased[VEL: t(4)=11.93,p<0.05] relative to saline-injected animals. Following four injections of the moderate dose of nicotine, MIF and VEL were higher [MIF: t(4)=3.1,p<0.05. VEL: t(4)=6.4,p<0.05], and ANG was lower [ANG: t(4)=8.5,p<0.05] than for saline-injected animals. Following four injections of the high dose of nicotine, MIF and ANG were lower than for saline-injected animals [MIF: t(4)=6.3,p<0.05. ANG: t(4)=7.2,p<0.05]; VEL did not differ. These results demonstrate the development of tolerance to the stereotypic-like effects of nicotine administration.
Following the eighth injection, all doses of nicotine reduced MIF and ANG [MIF: low dose t(4)=3.9, p<0.05; moderate dose t(4)=3.7, p<0.05; high dose t(4)=2.7, p<0.05. ANG: low dose t(4)=7.6, p<0.05; moderate dose t(4)=18.8, p<0.05; high dose t(4)=14.3, p<0.05], while the low and moderate doses of nicotine increased VEL [VEL: low dose t(4)=7.5, p<0.05; moderate dose t(4)=9.1, p<0.05] when compared to saline.
To ascertain whether the effects of nicotine on locomotor activity were related to the initial characterization of the animals as LLA or HLA, nicotine-induced changes in locomotor activity were assessed for these groups. To minimize effects attributable to differences in baseline activity, the effect of nicotine administration on locomotor activity was expressed relative to the novel open field activity for each animal (Fig. 4). A mixed factorial ANOVA indicated that LLA and HLA animals exhibited different patterns of response to nicotine administration across days of injection [F(9,132) = 3.253, p<0.05].
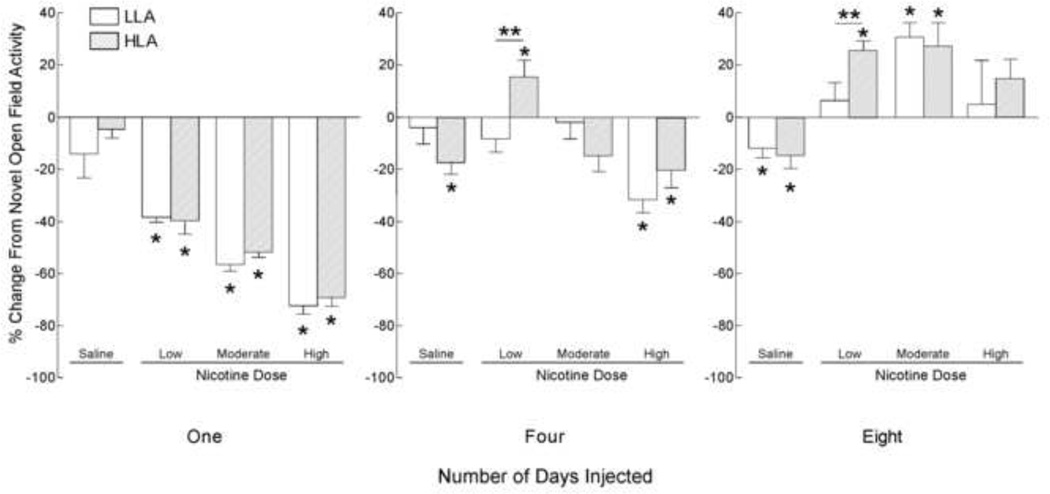
Fig. 4.
The effect of nicotine on the total distance moved (TDM) by low locomotor activity (LLA) and high locomotor activity (HLA) adolescent rats. Animals (n=52) were classified as LLA or HLA as in Fig. 1, received injections of either saline or nicotine, and behavior was assessed as in Fig. 2. Changes in activity during the first 5 min following nicotine administration are expressed relative to drug naïve activity in the novel open field [(Trial Activity – Initial Activity)/ Initial Activity]. Bars represent mean + sem. * Means differ significantly from baseline (0), p<0.05; ** Means differ significantly, p<0.05.
Following the first injection (Fig. 4, left), the activity of LLA and HLA adolescents injected with saline did not differ significantly from their corresponding novel open field activity. Further, nicotine decreased activity in a dose-dependent manner by a similar extent in both LLA and HLA animals (Low Dose [LLA: 30%; t(4) = 5.63, p < 0.05; HLA: 27%; t(4) = 3.03, p < 0.05]; Moderate Dose [LLA: −55%; t(4) = 22.1, p < 0.05; HLA: −50%; t(4) = 26.36, p < 0.05]; or High Dose [LLA: −71%; t(4) = 23.31, p < 0.05; HLA: −67%; t(4) = 19.33, p < 0.05]).
Following the fourth injection (Fig. 4, middle), LLA and HLA rats differed in their responses to both saline and nicotine. Following saline injection, LLA animals did not exhibit any change from novel open field activity, while HLA rats exhibited a significant decrease in activity [−17%; t(4) = 3.75, p < 0.05]. The low dose of nicotine did not alter the activity of LLA animals, while it increased activity significantly in HLA animals [+15%; t(4) = 2.44, p < 0.05]. There were no significant changes in locomotor activity in either the LLA or HLA groups following four injections of the moderate dose of nicotine, while four injections of the high dose suppressed activity in both LLA [−31%; t(4) = 6.22, p < 0.05] and HLA [−19%; t(4) = 2.97, p < 0.05] adolescent animals.
Following the eighth injection of saline (Fig. 4, right), LLA and HLA adolescents did not differ and exhibited significant decreases in locomotor activity. Following eight injections of a low dose of nicotine, only HLA adolescents exhibited a significant increase in locomotor activity [+25%; t(4) = 6.60, p < 0.05]. Following eight injections of a moderate dose of nicotine, both LLA [30%; t(4) = 5.63, p < 0.05] and HLA [27%; t(4) = 3.03, p < 0.05] adolescents exhibited significant increases in locomotor activity. The high dose of nicotine did not alter activity in either LLA or HLA animals.
3.2 CREB Expression and Activity Following Repeated Nicotine Exposure
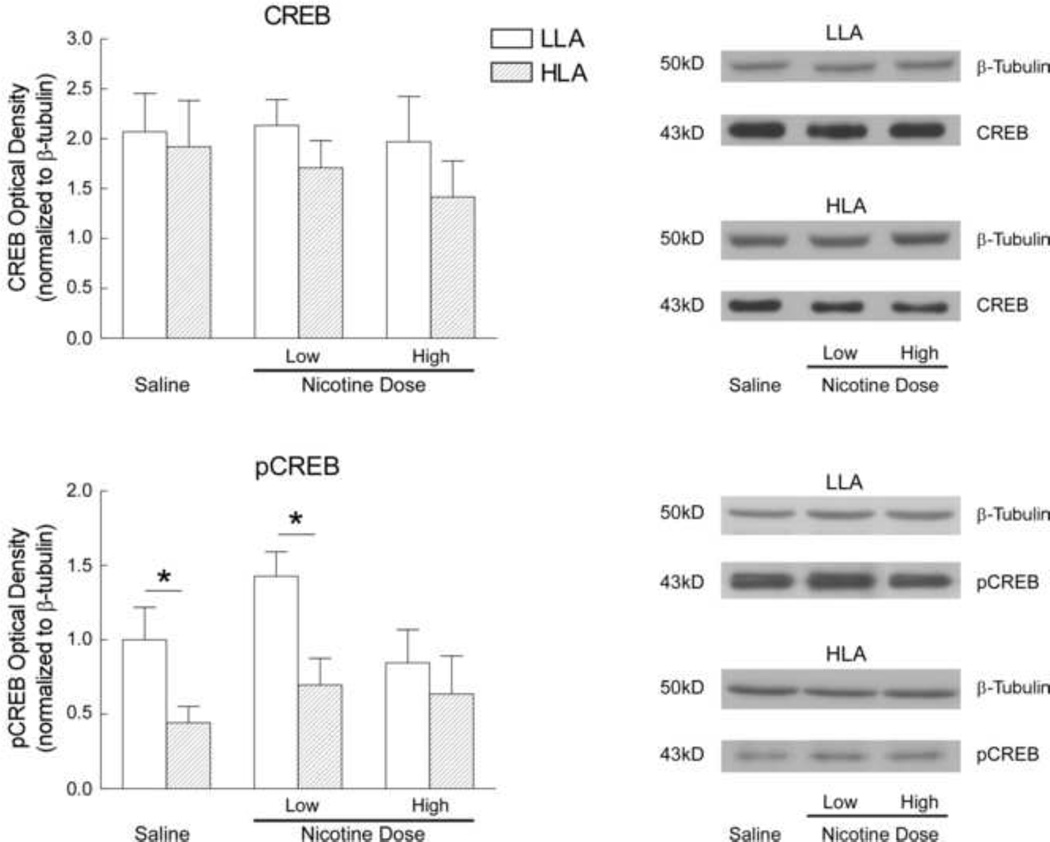
To determine whether activity changes in LLA and HLA animals following eight injections of nicotine were associated with differences in CREB expression or activity in the vStr or PFC, adolescent animals were sacrificed 18 hours following their eighth injection of saline or nicotine and assessment of locomotor activity. In the vStr, CREB expression by HLA animals tended to be lower than LLA animals following eight days of exposure to nicotine, but these decreases were not significant (Fig. 5, top panel). In contrast, nicotine had a differential effect on pCREB in the vStr depending on activity in the novel open field (Fig. 5, bottom panel), [F(1,52) = 8.549, p < 0.05]. pCREB in the vStr of saline-injected HLA animals was 56% less than that measured in similarly treated LLA animals [t(12) = 2.279, p < 0.05]. Because CREB did not differ following saline, this difference in pCREB suggests that LLA adolescents have greater CREB activity in the vStr than their HLA counterparts. Following repeated exposure to a low dose of nicotine, pCREB in the vStr of HLA animals was 51% of that measured in LLA animals [t(12) = 2.986, p < 0.05], while following eight days of high dose nicotine exposure, pCREB did not differ significantly between LLA and HLA animals. Repeated exposure to the high dose of nicotine led to significantly lower pCREB in the vStr than following repeated exposure to the low dose of nicotine [t(20) = 2.109, p<0.05]. No significant differences in CREB or pCREB were observed in the PFC of LLA and HLA adolescents following saline or nicotine exposure (data not shown), indicating selective effects in the vStr.
Fig. 5.
The effect of nicotine administration on CREB and pCREB in the ventral striatum (vStr) of low locomotor activity (LLA) and high locomotor activity (HLA) adolescent rats. Animals (n=36) were classified as LLA (n=18) or HLA (n=18) as in Fig. 1 and received injections of either saline (n=12), low (n=12) or high (n=12) dose nicotine as in Fig. 2. On PND 43, 18 hours following the final nicotine injection, animals were sacrificed and the vStr dissected for western blot analysis. Bars represent optical density (mean + sem) for CREB and pCREB; data are normalized to the optical density for β-tubulin of each sample. *Means differ significantly, p<0.05.
4. Discussion
LLA and HLA adolescents exhibit differences in sensitivity to the locomotor depressant and activating effect of nicotine and these differences are paralleled by differences in pCREB activity in the vStr following repeated nicotine exposure. Acute nicotine exposure during adolescence produces an immediate, dose-related depression of locomotor activity, with both LLA and HLA animals exhibiting similar decreases relative to their novel open field activity. Thus, findings that acute nicotine challenge reduces activity in adults [36, 37, 39] can be extended to the adolescent population. Furthermore, results demonstrate that these depressant effects of acute nicotine are independent of behavioral reactivity to novel stimulation.
The locomotor depressant effect of nicotine attenuates with repeated exposure. However, the rate at which tolerance develops depends upon both dose and initial activity in the novel open field, with HLA adolescent animals developing tolerance and progressing to sensitization at lower doses than LLA animals. Tolerance to the depressant effects of nicotine has been documented in adult animals [36, 37] and can occur within 3 administrations of nicotine. This effect appears to be the result of the central action of nicotine [36, 37, 39–42] and not the result of repeated experience with the experimental situation [36, 43]. In the case of adolescent animals, tolerance to nicotine-induced depression was apparent by the fourth injection of the lowest doses of nicotine (0.14–0.21 mg free base/kg) for both LLA and HLA animals, with HLA animals exhibiting behavioral activation. Multiple studies with adult animals have demonstrated the development of locomotor sensitization following repeated nicotine exposure [36–38, 40], but in the present study increases in locomotor activity following four injections were observed only after the lowest doses of nicotine and only in HLA adolescents. These data indicate that LLA adolescents do not adapt as readily to repeated low doses of nicotine as HLA adolescents.
Increases in movement initiation frequency and angular velocity following acute nicotine exposure support the idea that the doses of nicotine used produced stereotypy. Stereotypical behaviors in the rat including rearing, grooming and sniffing have been demonstrated following nicotine administration [44, 45], and one would expect that an increase in stereotypy would interfere with horizontal movement, thus, decreasing total distance moved. Although these behaviors may contribute to the locomotor depression observed following the first injection of nicotine, they cannot be totally responsible because these behaviors were accompanied by decreased velocity during movement.
It is interesting that the relationships observed between novel open field activity and nicotine-induced locomotion exist despite several days of adolescent development between measurements. Four days separate the assessment of novel open field locomotor activity and the initial assessment of nicotine-induced locomotion. Depending on the definition of adolescence in the rat [PND 21–59 [46, 47]; PND 28–42 [48]], this period represents between 10% and 25% of the adolescent developmental window and bridges the periods of early and mid-adolescence. This suggests that relationships between novel open field activity and nicotine-induced locomotion may be unaffected by adolescent development.
In the present study, LLA and HLA adolescents did not exhibit differences in CREB expression, but did exhibit differences in CREB activity with pCREB levels in the vStr from HLA adolescents approximately half that of their LLA counterparts. This difference in saline-injected rats may reflect the drug-naive (basal) state of CREB activation in the vStr of LLA and HLA animals, and could potentially contribute to differences in both behavior in the novel open field and behavior following repeated nicotine administration. Locomotor activity and pCREB levels appear to be inversely related after eight days of low dose nicotine administration with locomotor activity increasing in HLA rats more than two times as much as in LLA rats (Fig. 4) and pCREB levels higher in the latter (Fig. 5). This finding would support the idea that high CREB activity in the vStr mediates decreased stimulus reactivity [27]. Interestingly, neither low nor high doses of nicotine altered pCREB levels in vStr from HLA rats, whereas eight days of low dose nicotine increased pCREB levels significantly in vStr from LLA rats and high doses had no effect. Thus, pCREB levels exhibited by LLA rats following eight days of high dose nicotine resembled those of HLA rats, a time when both groups exhibited similar behavioral responses to nicotine administration. Because alterations in CREB activity in the vStr produce alterations in sensitivity to subsequent stimulation, these results suggest that activity differences in the open field may reflect differences in CREB phosphorylation in the vStr (high pCREB = low locomotor activity) and that differences in both locomotor activity and vStr pCREB can be abolished by repeated exposure to a high dose, but not a low dose, of nicotine.
It is important to note that vStr pCREB was measured 18 hours after the final nicotine injection, and thus, results may reflect consequences of nicotine withdrawal. Several studies have reported changes in pCREB in vStr following nicotine withdrawal with equivocal results [27, 49–51]. These studies all used different injection paradigms, doses and periods of withdrawal. Thus, findings suggest that all of these parameters must be taken into account when assessing the effects of nicotine exposure on pCREB.
Taken together these results indicate that differences in sensitivity to novel stimulation during the adolescent period are related to and may predict sensitivity to the behavioral and cellular effects of nicotine exposure and that these differences may underlie differences in sensitivity to reward. Further research examining the mechanisms mediating differences in sensation-seeking and the long term consequences of adolescent nicotine exposure on cellular changes and reward sensitivity are warranted.
Highlights.
Tolerance to the depressant effects of nicotine depends upon nicotine dose
Tolerance to the depressant effects of nicotine depends upon activity in the novel open field
Only animals high in novel open field activity exhibit sensitization to nicotine
pCREB activity in the vStr is predictive of the behavioral response to nicotine exposure
Acknowledgments
Supported by: NIH Grant AA016449 and the State of Florida
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Dellu F, Piazza PV, Mayo W, Le Moal M, Simon H. Novelty-seeking in rats--biobehavioral characteristics and possible relationship with the sensation-seeking trait in man. Neuropsychobiology. 1996;34:136–145. doi: 10.1159/000119305. [DOI] [PubMed] [Google Scholar]
- 2.Klebaur JE, Bevins RA, Segar TM, Bardo MT. Individual differences in behavioral responses to novelty and amphetamine self-administration in male and female rats. Behav Pharmacol. 2001;12:267–275. doi: 10.1097/00008877-200107000-00005. [DOI] [PubMed] [Google Scholar]
- 3.Nadal R, Armario A, Janak PH. Positive relationship between activity in a novel environment and operant ethanol self-administration in rats. Psychopharmacology (Berl) 2002;162:333–338. doi: 10.1007/s00213-002-1091-5. [DOI] [PubMed] [Google Scholar]
- 4.Piazza PV, Deminiere JM, Le Moal M, Simon H. Factors that predict individual vulnerability to amphetamine self-administration. Science. 1989;245:1511–1513. doi: 10.1126/science.2781295. [DOI] [PubMed] [Google Scholar]
- 5.Klebaur JE, Bardo MT. Individual differences in novelty seeking on the playground maze predict amphetamine conditioned place preference. Pharmacol Biochem Behav. 1999;63:131–136. doi: 10.1016/s0091-3057(98)00258-5. [DOI] [PubMed] [Google Scholar]
- 6.Zheng X, Ke X, Tan B, Luo X, Xu W, Yang X, et al. Susceptibility to morphine place conditioning: relationship with stress-induced locomotion and novelty-seeking behavior in juvenile and adult rats. Pharmacol Biochem Behav. 2003;75:929–935. doi: 10.1016/s0091-3057(03)00172-2. [DOI] [PubMed] [Google Scholar]
- 7.Zheng XG, Tan BP, Luo XJ, Xu W, Yang XY, Sui N. Novelty-seeking behavior and stress-induced locomotion in rats of juvenile period differentially related to morphine place conditioning in their adulthood. Behav Processes. 2004;65:15–23. doi: 10.1016/s0376-6357(03)00151-7. [DOI] [PubMed] [Google Scholar]
- 8.Philpot RM, Wecker L. Dependence of adolescent novelty-seeking behavior on response phenotype and effects of apparatus scaling. Behav Neurosci. 2008;122:861–875. doi: 10.1037/0735-7044.122.4.861. [DOI] [PubMed] [Google Scholar]
- 9.Crawford AM, Pentz MA, Chou CP, Li C, Dwyer JH. Parallel developmental trajectories of sensation seeking and regular substance use in adolescents. Psychol Addict Behav. 2003;17:179–192. doi: 10.1037/0893-164X.17.3.179. [DOI] [PubMed] [Google Scholar]
- 10.Anthony JC, Petronis KR. Early-onset drug use and risk of later drug problems. Drug Alcohol Depend. 1995;40:9–15. doi: 10.1016/0376-8716(95)01194-3. [DOI] [PubMed] [Google Scholar]
- 11.Kandel DB, Yamaguchi K, Chen K. Stages of progression in drug involvement from adolescence to adulthood: further evidence for the gateway theory. J Stud Alcohol. 1992;53:447–457. doi: 10.15288/jsa.1992.53.447. [DOI] [PubMed] [Google Scholar]
- 12.Taioli E, Wynder EL. Effect of the age at which smoking begins on frequency of smoking in adulthood. N Engl J Med. 1991;325:968–969. doi: 10.1056/NEJM199109263251318. [DOI] [PubMed] [Google Scholar]
- 13.CDC. Youth risk behavior surveillance--United States, 2009. Morbidity and Mortality Weekly Report: Center for Disease Control. 2010 [PubMed] [Google Scholar]
- 14.CDC. Youth risk behavior surveillance - United States, 2007. Morbidity and Mortality Weekly Report: Center for Disease Control. 2008 [PubMed] [Google Scholar]
- 15.DiFranza JR, Savageau JA, Fletcher K, Ockene JK, Rigotti NA, McNeill AD, et al. Recollections and repercussions of the first inhaled cigarette. Addict Behav. 2004;29:261–272. doi: 10.1016/j.addbeh.2003.08.002. [DOI] [PubMed] [Google Scholar]
- 16.Pomerleau OF, Hariharan M, Pomerleau CS, Cameron OG, Guthrie SK. Differences between smokers and never-smokers in sensitivity to nicotine: a preliminary report. Addiction. 1993;88:113–118. doi: 10.1111/j.1360-0443.1993.tb02768.x. [DOI] [PubMed] [Google Scholar]
- 17.Pomerleau OF. Individual differences in sensitivity to nicotine: implications for genetic research on nicotine dependence. Behav Genet. 1995;25:161–177. doi: 10.1007/BF02196925. [DOI] [PubMed] [Google Scholar]
- 18.Audrain-McGovern J, Al Koudsi N, Rodriguez D, Wileyto EP, Shields PG, Tyndale RF. The role of CYP2A6 in the emergence of nicotine dependence in adolescents. Pediatrics. 2007;119:e264–e274. doi: 10.1542/peds.2006-1583. [DOI] [PubMed] [Google Scholar]
- 19.Difranza JR. Hooked from the first cigarette. J Fam Pract. 2007;56:1017–1022. [PubMed] [Google Scholar]
- 20.Urban R, Sutfin E. Do early smoking experiences count in development of smoking?: temporal stability and predictive validity of an early smoking experience questionnaire in adolescents. Nicotine Tob Res. 2010;12:1265–1269. doi: 10.1093/ntr/ntq178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Perkins KA, Gerlach D, Broge M, Grobe JE, Wilson A. Greater sensitivity to subjective effects of nicotine in nonsmokers high in sensation seeking. Exp Clin Psychopharmacol. 2000;8:462–471. doi: 10.1037//1064-1297.8.4.462. [DOI] [PubMed] [Google Scholar]
- 22.Perkins KA, Lerman C, Coddington SB, Jetton C, Karelitz JL, Scott JA, et al. Initial nicotine sensitivity in humans as a function of impulsivity. Psychopharmacology (Berl) 2008;200:529–544. doi: 10.1007/s00213-008-1231-7. [DOI] [PubMed] [Google Scholar]
- 23.Martin CA, Kelly TH, Rayens MK, Brogli BR, Brenzel A, Smith WJ, et al. Sensation seeking, puberty, and nicotine, alcohol, and marijuana use in adolescence. J Am Acad Child Adolesc Psychiatry. 2002;41:1495–1502. doi: 10.1097/00004583-200212000-00022. [DOI] [PubMed] [Google Scholar]
- 24.Spillane NS, Smith GT, Kahler CW. Impulsivity-like traits and smoking behavior in college students. Addict Behav. 2010;35:700–705. doi: 10.1016/j.addbeh.2010.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lonze BE, Ginty DD. Function and regulation of CREB family transcription factors in the nervous system. Neuron. 2002;35:605–623. doi: 10.1016/s0896-6273(02)00828-0. [DOI] [PubMed] [Google Scholar]
- 26.Walters CL, Cleck JN, Kuo YC, Blendy JA. Mu-opioid receptor and CREB activation are required for nicotine reward. Neuron. 2005;46:933–943. doi: 10.1016/j.neuron.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 27.Brunzell DH, Mineur YS, Neve RL, Picciotto MR. Nucleus accumbens CREB activity is necessary for nicotine conditioned place preference. Neuropsychopharmacology. 2009;34:1993–2001. doi: 10.1038/npp.2009.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Carlezon WA, Jr, Thome J, Olson VG, Lane-Ladd SB, Brodkin ES, Hiroi N, et al. Regulation of cocaine reward by CREB. Science. 1998;282:2272–2275. doi: 10.1126/science.282.5397.2272. [DOI] [PubMed] [Google Scholar]
- 29.Barrot M, Olivier JD, Perrotti LI, DiLeone RJ, Berton O, Eisch AJ, et al. CREB activity in the nucleus accumbens shell controls gating of behavioral responses to emotional stimuli. Proc Natl Acad Sci U S A. 2002;99:11435–11440. doi: 10.1073/pnas.172091899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Newton SS, Thome J, Wallace TL, Shirayama Y, Schlesinger L, Sakai N, et al. Inhibition of cAMP response element-binding protein or dynorphin in the nucleus accumbens produces an antidepressant-like effect. J Neurosci. 2002;22:10883–10890. doi: 10.1523/JNEUROSCI.22-24-10883.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pliakas AM, Carlson RR, Neve RL, Konradi C, Nestler EJ, Carlezon WA., Jr Altered responsiveness to cocaine and increased immobility in the forced swim test associated with elevated cAMP response element-binding protein expression in nucleus accumbens. J Neurosci. 2001;21:7397–7403. doi: 10.1523/JNEUROSCI.21-18-07397.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Calvo N, Cecchi M, Kabbaj M, Watson SJ, Akil H. Differential effects of social defeat in rats with high and low locomotor response to novelty. Neuroscience. 183:81–89. doi: 10.1016/j.neuroscience.2011.03.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jama A, Cecchi M, Calvo N, Watson SJ, Akil H. Inter-individual differences in novelty-seeking behavior in rats predict differential responses to desipramine in the forced swim test. Psychopharmacology (Berl) 2008;198:333–340. doi: 10.1007/s00213-008-1126-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gancarz AM, San George MA, Ashrafioun L, Richards JB. Locomotor activity in a novel environment predicts both responding for a visual stimulus and self-administration of a low dose of methamphetamine in rats. Behav Processes. 86:295–304. doi: 10.1016/j.beproc.2010.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Blanchard MM, Mendelsohn D, Stamp JA. The HR/LR model: Further evidence as an animal model of sensation seeking. Neurosci Biobehav Rev. 2009;33:1145–1154. doi: 10.1016/j.neubiorev.2009.05.009. [DOI] [PubMed] [Google Scholar]
- 36.Morrison CF, Stephenson JA. The occurrence of tolerance to a central depressant effect of nicotine. Br J Pharmacol. 1972;46:151–156. doi: 10.1111/j.1476-5381.1972.tb06857.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Domino EF. Nicotine induced behavioral locomotor sensitization. Prog Neuropsychopharmacol Biol Psychiatry. 2001;25:59–71. doi: 10.1016/s0278-5846(00)00148-2. [DOI] [PubMed] [Google Scholar]
- 38.Clarke PB, Kumar R. Characterization of the locomotor stimulant action of nicotine in tolerant rats. Br J Pharmacol. 1983;80:587–594. doi: 10.1111/j.1476-5381.1983.tb10733.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Clarke PB, Kumar R. The effects of nicotine on locomotor activity in non-tolerant and tolerant rats. Br J Pharmacol. 1983;78:329–337. doi: 10.1111/j.1476-5381.1983.tb09398.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ksir C, Hakan R, Hall DP, Jr, Kellar KJ. Exposure to nicotine enhances the behavioral stimulant effect of nicotine and increases binding of [3H]acetylcholine to nicotinic receptors. Neuropharmacology. 1985;24:527–531. doi: 10.1016/0028-3908(85)90058-9. [DOI] [PubMed] [Google Scholar]
- 41.Morrison CF, Lee PN. A comparison of the effects of nicotine and physostigmine on a measure of activity in the rat. Psychopharmacologia. 1968;13:210–221. doi: 10.1007/BF00401401. [DOI] [PubMed] [Google Scholar]
- 42.Morrison CF, Goodyear JM, Sellers CM. Antagonism by antimuscarinic and ganglion-blocking drugs of some of the behavioural effects of nicotine. Psychopharmacologia. 1969;15:341–350. doi: 10.1007/BF00403709. [DOI] [PubMed] [Google Scholar]
- 43.Hendry JS, Rosecrans JA. The development of pharmacological tolerance to the effect of nicotine on schedule-controlled responding in mice. Psychopharmacology (Berl) 1982;77:339–343. doi: 10.1007/BF00432767. [DOI] [PubMed] [Google Scholar]
- 44.Iwamoto ET. An assessment of the spontaneous activity of rats administered morphine, phencyclidine, or nicotine using automated and observational methods. Psychopharmacology (Berl) 1984;84:374–382. doi: 10.1007/BF00555216. [DOI] [PubMed] [Google Scholar]
- 45.Booze RM, Welch MA, Wood ML, Billings KA, Apple SR, Mactutus CF. Behavioral sensitization following repeated intravenous nicotine administration: gender differences and gonadal hormones. Pharmacol Biochem Behav. 1999;64:827–839. doi: 10.1016/s0091-3057(99)00169-0. [DOI] [PubMed] [Google Scholar]
- 46.Laviola G, Macri S, Morley-Fletcher S, Adriani W. Risk-taking behavior in adolescent mice: psychobiological determinants and early epigenetic influence. Neurosci Biobehav Rev. 2003;27:19–31. doi: 10.1016/s0149-7634(03)00006-x. [DOI] [PubMed] [Google Scholar]
- 47.Tirelli E, Laviola G, Adriani W. Ontogenesis of behavioral sensitization and conditioned place preference induced by psychostimulants in laboratory rodents. Neurosci Biobehav Rev. 2003;27:163–178. doi: 10.1016/s0149-7634(03)00018-6. [DOI] [PubMed] [Google Scholar]
- 48.Spear LP. The adolescent brain and age-related behavioral manifestations. Neurosci Biobehav Rev. 2000;24:417–463. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- 49.Pluzarev O, Pandey SC. Modulation of CREB expression and phosphorylation in the rat nucleus accumbens during nicotine exposure and withdrawal. J Neurosci Res. 2004;77:884–891. doi: 10.1002/jnr.20216. [DOI] [PubMed] [Google Scholar]
- 50.Kivinummi T, Kaste K, Rantamaki T, Castren E, Ahtee L. Alterations in BDNF and phospho-CREB levels following chronic oral nicotine treatment and its withdrawal in dopaminergic brain areas of mice. Neurosci Lett. 491:108–112. doi: 10.1016/j.neulet.2011.01.015. [DOI] [PubMed] [Google Scholar]
- 51.Brunzell DH, Russell DS, Picciotto MR. In vivo nicotine treatment regulates mesocorticolimbic CREB and ERK signaling in C57Bl/6J mice. J Neurochem. 2003;84:1431–1441. doi: 10.1046/j.1471-4159.2003.01640.x. [DOI] [PubMed] [Google Scholar]