ABSTRACT.
Scrub typhus group (STG), typhus group (TG), and spotted fever group (SFG) rickettsiae are pathogens distributed worldwide and are important causes of febrile illnesses in southeast Asia. The levels of rickettsioses burden and distribution in Thai communities are still unclear. Nonspecific symptoms, limit diagnostic capacity and underdiagnoses contribute to the absence of clarity. The objective of this study was to determine the nationwide IgG seroprevalence of STG, TG, and SFG by ELISA in repository sera from the Royal Thai Army recruits collected during 2007–2008 and 2012 to estimate rickettsiae exposure in young Thai men to better understand rickettsiae exposure distribution in the Thai population. IgG seroprevalence of STG, Orientia tsutsugamushi; TG, Rickettsia typhi; and SFG, R. rickettsii was 12.4%, 6.8%, and 3.3% in 2007–2008 and 31.8%, 4.2%, and 4.5% in 2012, respectively. The STG had the highest seroprevalence of Rickettsia assessed, with the highest regional seroprevalence found in southern Thailand. The STG seroprevalence changed significantly from 2007 to 2008 (P value < 0.05), which corresponds with morbidity rate of scrub typhus from the last decade in Thailand. We were unable to determine the causality for seroprevalence changes between the two periods due to the limitation in sample numbers for intervening years and limited information available for archived specimens. Additional research would be required to determine agency. However, study results do confirm Rickettsia endemicity in Thailand lends weight to reports of increasing STG seroprevalence. It also corroborates the need to raise rickettsial disease awareness and educate the general public in prevention measures.
INTRODUCTION
The order Rickettsiales, the family Rickettsiaceae, includes the genera Rickettsia and Orientia which are further divided into three groups: typhus group (TG), spotted fever group (SFG), and scrub typhus group (STG). They are Gram-negative bacteria that are zoonotic pathogens causing murine typhus, spotted fever group rickettsiosis, and scrub typhus, respectively. These rickettsiae are widely distributed in southeast Asia, including Thailand. The etiologic agent producing scrub typhus is Orientia tsutsugamushi, which is transmitted to humans and animals by the bite of infected larval stage Leptotrombidium mites (chiggers). Murine typhus is caused by Rickettsia typhi with the primary vector being the Oriental rat flea, Xenopsylla cheopis. The pathogen is transmitted to mammals via flea bites and/or infected flea feces exposure in skin abrasions, bite sites, or mucous membranes. Spotted fever group is comprised of many pathogenic agents that cause spotted fevers, typically transmitted to a human or animal host by infected tick bite. Mites (STG) and rodents (TG and SFG) are the principal reservoir hosts of these pathogens and humans are accidental hosts.1–3
Clinical signs of rickettsioses vary from mild, nonspecific flu-like symptoms such as fever, headache, and abdominal pain to life-threatening illnesses. Using disease sequelae for the diagnosis of rickettsial infections is challenging. Differentiation of rickettsial infections from other infectious diseases is often difficult due to similarities in clinical presentation with other regional diseases, for example, the rash produced by dengue infection may be difficult to distinguish from the rash produced by TG. Some clinical signs may be more specific and useful as diagnostic clues such as eschar at the site of STG and SFG infection; however, it’s only present in from 20% to 90% of the cases.4 Rickettsioses have been reported on every continent except for Antarctica, but nonspecific symptoms and lack of laboratory confirmation contributes to underreporting of the disease burden.1–3
Globally, an estimated one million people were infected with STG and more than one billion are at risk. Multiple rickettsial diseases are endemic to southeast Asia where infections with serious or life-threatening complications have been reported from both local populations and from European, North American, or Japanese travelers returning from southeast Asian countries including Burma, Vietnam, Malaysia, and often Thailand.1,2
Indirect immunofluorescence assay (IFA) is the gold standard for serodiagnosis of rickettsioses. However, laboratory IFA performance capabilities are often inadequate as extensive training is required to effectively perform fluorescent microscopic evaluation.1 Enzyme-linked immunosorbent assay (ELISA) was successfully adapted to detect antibodies against O. tsutsugamushi, R. typhi, and R. rickettsii in serum and is a useful assay for surveillance studies in endemic areas.5–8
Rickettsioses in Thailand, especially scrub typhus, has been reported as sporadic outbreaks among the military, hikers, and hill tribe people.9,10 The scrub typhus is a Thai Ministry of Public Health (MOPH) voluntarily notifiable disease, whereas murine typhus and spotted fever group rickettsiosis are not. However, scrub typhus is not on the list of statutorily required notifiable diseases in Thailand and this may cause the underestimation of true incidence. The reported annual incidence of scrub typhus in Thailand ranged from 6.67 to 16.99 per 100,000 people in 2008–2018, with the highest mortality rate of 0.02 per 100,000 people noted in 2012, 2015, and 2017.9 Reports of murine typhus and spotted fever group rickettsiosis were found only in sporadic instances in Thailand.2 Seroprevalence studies of STG, TG, and SFG in the Thai general population are limited. The available studies generally cover small populations and/or are conducted with undifferentiated fever patients.2,10 A nationwide seroprevalence of rickettsioses in Thailand is needed to confirm areas of pathogen distribution.
MATERIALS AND METHODS
This retrospective seroprevalence of rickettsioses was conducted utilizing repository serum specimens obtained from young Thai men entering the Royal Thai Army (RTA) during 2007–2008 (N = 7,760) and 2012 (N = 6,627). We combined samples from 2007 and 2008 because of limited samples in some provinces. The recruitment criteria and sample size calculations were previously described.11 Briefly, the RTA repository sera were collected from recruits after entry to the RTA as part of their HIV screening program. These repository serum specimens, obtained under informed consent with permission for future studies, have been used in the previous studies to monitor the prevalence of exposure to pathogens causing hepatitis E, measles, and leptospirosis among young adults in Thailand.11–13
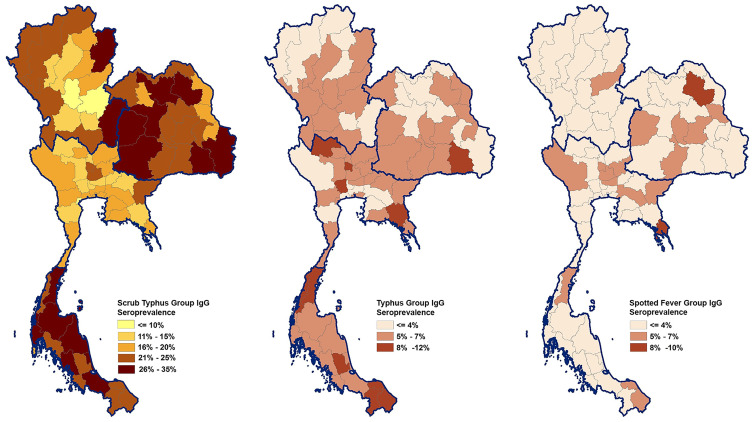
The IgG antibody against STG, TG, and SFG was measured by ELISA, which was used in seroprevalence studies described earlier.6,7 The antigens used for detection were combinations of recombinant 56-kDa proteins from the Kato, Gillian, and chimeric 1 proteins (prepared by shuffling the epitopes in the variable domain 1 of the Karp 56-kDa protein sequence with the TA763 sequence) of O. tsutsugamushi for STG, fragment AN and fragment K from the OmpB gene product of R. typhi for TG, and fragment X and fragment Y from the OmpA gene product of R. rickettsii for SFG.8,14 Positive and negative controls (Scimedx Corp) were included in all ELISA assay plates. A positive cut-off value was set at two standard deviations above the mean optical density of negative control sera.5,8 Associations between demographic characteristics and seroprevalence of STG, O. tsutsugamushi; TG, R. typhi; and SFG, R. rickettsii were tested with a Pearson’s χ2 two-tailed test. Combined seroprevalence of samples at both time points, 2007–2008 and 2012 were used to create spatial distribution maps to illustrate STG, TG, and SFG seroprevalence across Thailand. Spatial distribution maps, Figure 1, were generated using ArcView 10.4 (ESRI, Redlands, CA). Seroprevalence data from all provinces were used to create spatial distribution maps represented by the combined cohorts from two time periods with the completeness of the dataset. Statistical analyses of STG, TG, and SFG seroprevalence in Thailand were tested using SPSS version 26 (SPSS, Chicago, IL); a P value < 0.05 was considered statistically significant.
Figure 1.
Choropleth maps of average rickettsiae seroprevalence in young Thai men, 2007–2012. * Prevalence is stratified by color and location determined by reported residence province during the 2 years prior until Royal Thai Army enlistment. Thailand shapefile in the public domain. ** Region designation follows Department of Provincial Administration, Ministry of Interior. This figure appears in color at www.ajtmh.org.
This study was approved by the Institutional Review Board, RTA Medical Department, Bangkok, Thailand, and was approved as an exempt protocol by the Human Subjects Protection Branch, Walter Reed Army Institute of Research, Silver Spring, MD.
RESULTS AND DISCUSSION
The majority of the study population was 21 years of age, unmarried, had completed junior high school, and resided in rural areas (Supplemental Table 1). The overall seroprevalence of STG, O. tsutsugamushi increased in 2012 over 2007–2008 by 31.8% (95% CI = 30.6–32.9%) compared with 12.4% (95% CI = 11.7–13.2%) (Table 1). Regional STG seroprevalence ranged from 9.2–17.7% in 2007–2008 to 26.3–38.5% in 2012, confirming the endemicity of STG throughout Thailand. No significant differences were observed among age groups in STG, TG, and SFG seroprevalence in 2007–2008 and 2012 cohorts. Seroprevalence of TG, R. typhi decreased from 6.8% (95% CI = 6.3–7.4%) in 2007–2008 to 4.2% (95% CI = 3.7–4.7%) in 2012, but seroprevalence of SFG, R. rickettsii increased from 3.3% (95% CI = 2.9–3.7%) in 2007–2008 to 4.5% (95% CI = 4.0–5.0%) in 2012. The TG and SFG exposures were detected in every region of Thailand, but differences in the distributions are shown in Table 1.
Table 1.
Univariate analysis results of demographic variables associated with STG, TG, and SFG IgG seroprevalence in young Thai men, 2007–2008 and 2012
| IgG seroprevalence, % (95% CI*) | ||||||
|---|---|---|---|---|---|---|
| STG | TG | SFG | ||||
| Demographic characteristics | 2007–2008 | 2012 | 2007–2008 | 2012 | 2007–2008 | 2012 |
| Total | 12.4 (11.7–13.2) | 31.8 (30.6–32.9) | 6.8 (6.3–7.4) | 4.2 (3.7–4.7) | 3.3 (2.9–3.7) | 4.5 (4.0–5.0) |
| Marital status | † | |||||
| Single | 12.5 (11.6–13.3) | 31.3 (30.0–32.5) | 6.9 (6.3–7.5) | 4.1 (3.5–4.6) | 3.3 (2.9–3.8) | 4.2 (3.6–4.7) |
| Married | 13.3 (11.5–15.0) | 33.8 (31.2–36.3) | 6.6 (5.3–7.8) | 4.9 (3.7–6.0) | 3.5 (2.6–4.4) | 5.6 (4.4–6.8) |
| Education level | † | † | ||||
| Primary school and less | 15.6 (14.0–17.1) | 36.1 (33.5–38.6) | 7.6 (6.5–8.7) | 4.5 (3.4–5.6) | 2.7 (2.0–3.4) | 4.8 (3.7–6.0) |
| Middle school | 12.7 (11.4–14.0) | 31.3 (29.5–33.1) | 7.0 (6.0–7.9) | 4.6 (3.8–5.4) | 3.3 (2.6–4.0) | 5.1 (4.3–6.0) |
| Senior high school and vocational | 10.7 (9.3–12.1) | 30.9 (28.7–33.0) | 6.4 (5.3–7.5) | 3.8 (2.9–4.7) | 3.8 (2.9–4.6) | 3.9 (3.0–4.8) |
| Diploma: high vocational or Bachelor’s degree | 8.9 (7.1–10.6) | 28.6 (25.8–31.5) | 5.8 (4.4–7.3) | 3.6 (2.5–4.8) | 4.0 (2.8–5.1) | 3.2 (2.1–4.3) |
| Residential area | † | † | † | † | ||
| Urban | 10.4 (9.2–11.6) | 29.1 (27.5–30.8) | 5.8 (4.9–6.8) | 4.0 (3.3–4.7) | 3.1 (2.4–3.8) | 3.7 (3.1–4.4) |
| Rural | 13.3 (12.3–14.4) | 34.0 (32.4–35.5) | 7.3 (6.5–8.1) | 4.4 (3.8–5.1) | 3.6 (3.0–4.2) | 5.1 (4.4–5.8) |
| Region of residence | † | † | † | † | ||
| Central | 9.2 (8.1–10.3) | 26.3 (24.4–28.1) | 6.7 (5.7–7.7) | 4.3 (3.4–5.2) | 3.6 (2.9–4.3) | 4.5 (3.6–5.4) |
| North | 11.4 (9.9–12.9) | 30.1 (27.5–32.8) | 5.6 (4.5–6.7) | 3.9 (2.8–5.0) | 3.2 (2.3–4.0) | 3.7 (2.6–4.8) |
| Northeast | 13.9 (12.4–15.4) | 34.7 (32.8–36.6) | 6.4 (5.4–7.5) | 4.2 (3.4–5.0) | 2.6 (1.9–3.3) | 5.6 (4.6–6.5) |
| South | 17.7 (15.7–19.7) | 38.5 (35.4–41.6) | 9.2 (7.7–10.7) | 5.0 (3.6–6.3) | 4.1 (3.1–5.1) | 2.5 (1.5–3.5) |
SFG = spotted fever group; STG = scrub typhus group; TG = typhus group. No significant differences were found among age groups in STG, TG, and SFG seroprevalence in 2007–2008 and 2012 cohorts.
CI = confidence interval.
Bold values and shaded in gray = χ2 test statistically significant (Pearson’s χ2, two-sided, P < 0.05).
Results show much higher STG seroprevalence, up to 38.5%, compared with TG and SFG, which was less than 10%. This is supported by previous studies that indicated STG was the main pathogen of human rickettsiosis circulating in Thailand. The higher STG seroprevalence corresponds with vector densities reported in a rodent-borne disease vector survey in Thailand, with 98% of the collected ectoparasites being chigger-mites. Of these, 46% were the STG vector mite Leptotrombidium, and less than 1% were tick and flea vectors associated with TG and SFG.15,16
The TG seroprevalence was found to be associated with defined Thai regions in 2007–2008, but not 2012; however, the south region was found to have the highest TG seroprevalence for both time periods. The SFG seroprevalence had no significant association with any region in 2007–2008, but did show significant association with lowest seroprevalence in the south region and highest in northeast region in 2012. Study results show that exposure of TG and SFG in recruits occurred in all regions of the country; however, low seroprevalence makes it difficult to identify risk factors for exposure to these two pathogens.
We analyzed subject occupation groups with each Rickettsia pathogen exposure from samples collected in 2012 (no occupation data was available for the samples collected in 2007–2008). The seroprevalence of STG, O. tsutsugamushi and SFG, R. Rickettsia had a positive association with agricultural-related occupations, with SFG also having a positive association with business occupations that included business owners, merchants, and company/government employees (Supplemental Table 2). The STG and SFG exposure relationship with agriculture is likely due to a higher probability for vector/pathogen exposure. No association was found with TG for occupations. That includes data indicating significantly higher TG seroprevalence demographic associations found in rural areas in 2007–2008 but no association with rural areas was found for the 2012 data. This may support previous studies that TG exposure was more common in urban areas.2
The results of multivariate analysis on significant demographic factors are shown in Table 2. Primary school only graduates were associated with higher STG seropositivity in northeast and south regions for year 2007–2008 and 2012 cohorts. This elevated seropositivity association was true for middle school graduates living in rural areas but for only the 2007–2008 cohort. Living in rural areas of the south region was associated with TG seropositivity in the 2007–2008 cohort. Spotted fever group seropositivity in the 2012 cohort was associated with living in rural, Central and northeast regions. These results indicate that Rickettsia exposure is higher in the general population in rural areas and regional distribution of STG and TG were different than for SFG.
Table 2.
Adjusted ORs* of rickettsiae seroprevalence from multivariable analysis
| Variable | ORs | 95% CI† | P value |
|---|---|---|---|
| Scrub typhus group 2007–2008 | |||
| Diploma, high vocational or Bachelor’s degree | |||
| Senior high school and vocational | 1.18 | 0.90–1.57 | 0.24 |
| Middle school | 1.53 | 1.18–1.98 | < 0.01 |
| Primary school and less | 1.83 | 1.41–2.38 | < 0.01 |
| Living in rural area | 1.18 | 1.00–1.40 | 0.04 |
| Central | |||
| North | 1.13 | 0.90–1.43 | 0.29 |
| Northeast | 1.55 | 1.26–1.90 | < 0.01 |
| South | 1.98 | 1.60–2.45 | < 0.01 |
| Scrub typhus group 2012 | |||
| Diploma: high vocational or Bachelor’s degree | |||
| Senior high school and vocational | 1.06 | 0.88–1.28 | 0.54 |
| Middle school | 1.08 | 0.89–1.31 | 0.42 |
| Primary school and less | 1.32 | 1.07–1.62 | 0.01 |
| Living in rural area | 1.10 | 0.99–1.24 | 0.08 |
| Central | |||
| North | 1.16 | 0.98–1.37 | 0.08 |
| Northeast | 1.37 | 1.19–1.58 | < 0.01 |
| South | 1.59 | 1.34–1.87 | < 0.01 |
| Student | |||
| Farming, Fishing, Herdsman | 1.17 | 0.96–1.43 | 0.12 |
| Labor, General hire | 1.07 | 0.87–1.32 | 0.51 |
| Trades, Technician | 1.02 | 0.81–1.29 | 0.88 |
| Business, Company, Government employee | 1.01 | 0.84–1.21 | 0.94 |
| Typhus group 2007–2008 | |||
| Living in rural area | 1.31 | 1.06–1.62 | 0.01 |
| Central | |||
| North | 0.81 | 0.60–1.08 | 0.15 |
| Northeast | 0.78 | 0.60–1.03 | 0.08 |
| South | 1.40 | 1.08–1.82 | 0.01 |
| Spotted fever groups 2012 | |||
| Married status | 1.31 | 1.00–1.73 | 0.05 |
| Living in rural area | 1.30 | 1.01–1.68 | 0.04 |
| South | |||
| Central | 1.92 | 1.23–2.99 | < 0.01 |
| North | 1.50 | 0.92–2.44 | 0.11 |
| Northeast | 2.12 | 1.40–3.22 | < 0.01 |
| Student | |||
| Farming, Fishing, Herdsman | 1.52 | 1.00–2.32 | 0.05 |
| Labor, General hire | 1.20 | 0.77–1.89 | 0.42 |
| Trades, Technician | 0.95 | 0.54–1.65 | 0.84 |
| Business, Company, Government employee | 1.51 | 1.01–2.27 | 0.05 |
OR = odds ratio.
CI = confidence interval. Bold values = multivariable analysis test statistically significant, P < 0.05.
The marital status of young adults in our cohort may be associated with their education level and occupation. As shown in Supplemental Table 1, only 20% of our cohort were married and, not surprisingly, we found only 4.6% of them were students compared with 18.8% in single groups. Almost 48% of the married group worked in agriculture and labor. Regarding education level, 25.9% of the married group had senior high school and vocational diplomas or high vocational or Bachelor’s degrees but 45.7% of the single group had equivalent education. Financial demands on young couples may force an occupational choice where salary is available immediately and it doesn’t require the expenditures incumbent in further education. However, we did not find a significant association between marital status and seroprevalence of STG, O. tsutsugamushi and TG, R. typhi, only with SFG, R. Rickettsia. Spotted fever group has by far the lowest seroprevalence of the three pathogens evaluated and the reason for the increase with marital status is not possible to explain with our current data set. Further epidemiological evaluations are needed to answer the question.
There is no significant association between residential (urban/rural) and education level in cohort year 2007–2008 but it is significant in cohort year 2012. In the 2012 cohort comparison between residential areas, individuals whose maximum educational attainment was primary school constituted 19% of the urban and 21% of the sampled rural populations, individuals completing their education in middle school were equal at 38%, those completing their education in senior high school equal at 27%, and individuals graduating with Bachelor’s degree diplomas were 16% for urban and 14% rural. From the results, it would be difficult to link the small differences in education levels with influencing location of residence. However, 42% of primary school only graduates and 38% of middle school only graduates (low levels of education) who live in rural areas were working in agriculture while 20% of primary school only graduates and 16% of middle school only graduates living in urban areas were working in agriculture. Even though education levels may be similar in urban and rural areas, the availability of agriculturally related occupations for those with lower levels of education were likely higher in rural areas and very well may result in higher risk of Rickettsia exposure in individuals that live in rural areas.
Current study constraints when compared with previous rickettsiae seroprevalence studies conducted in Thailand lie in differences in study design, population investigated, and method of antibody detection. Previous studies primarily involved acute febrile illness patients with much smaller populations.2 Previous studies have used IFA and commercial ELISA kits that produced a wide range of Rickettsia seroprevalence.2 However, our seroprevalence study took a prospective versus retrospective look at pathogen circulation and exposure among Thai populations from all provinces by detecting the presence of IgG in serum samples. A choropleth map for Rickettsia seroprevalence by residential province is shown in Figure 1.
With the data limitations of this study, we are unable to explain the increase of STG and decrease of TG seroprevalence in Thailand during this time period. However, a previous study describing the increase of scrub typhus incidence in Thailand, reported positive correlation between STG incidence and residence population size, temperature, rainfall, and habitat complexity with large cover of open forested habitat.15 However, additional investigations need to be accomplished to ascertain whether this increase is a trend or only normal annual variation in scrub typhus incidence. Even though our results show a significant TG seroprevalence decrease in 2012, the TG seroprevalence is low at 6.8% to 4.2% and that coupled with the limited Thai MOPH case report data makes it difficult to explain the results. Further murine typhus epidemiology study is needed.
The STG seroprevalence increased in 2012 compared with 2007–2008. Case reports of scrub typhus in Thailand increased dramatically during that period (Supplemental Figure 1). Samples collected in the south region displayed the highest STG seroprevalence; however, the morbidity rate reported over the past decade were higher in the north region indicating that scrub typhus may be underreported and/or underdiagnosed in southern areas of Thailand (Table 1, Supplemental Figure 1). Living in or transiting areas for work or recreation with more suitable rodent habitat such as grasslands, forest, vegetable fields, and hilly areas have been reported as elevating risk for rickettsioses transmission, especially for STG.9 The Annual Epidemiology Surveillance Report of Thailand stated that the most at risk occupations for scrub typhus include agriculturist, military, border patrol police, forest officers, hunter-gatherers, and individuals living in forest areas.9 This corresponds with our results that higher STG IgG seropositivity was found in individuals residing in rural areas and working in agriculture (Table 1, Supplemental Table 2).
We found significant differences in STG seroprevalence among four regions of Thailand, where the south exhibited the highest seroprevalence and the Central exhibited the lowest during both time periods evaluated (Table 1). The study distribution of human STG positive seroprevalence data between regions did not correspond with a previous rodent-borne disease/vector survey in Thailand,16 although comparison with rodent seroprevalence is an inexact relationship at best and study scales were different. The STG was reported as the principal pathogen found in rodents trapped in Thailand, with highest positive rodents found in north (39%), followed by northeast (28%), south (17%), and central (8%).16 The lowest STG seropositivity rates in rodents were found in the Central region, agreeing with our seroprevalence results in humans even though the regions with highest seropositivity for rodents and humans were different in the two studies. The reason may be due to other transmission factors, such as differing intensities of human activity in infested areas. Regional transmission variation in humans depends on several factors, including presence of STG vector and reservoir, suitable climate and environmental conditions, and human activities.1,2
Higher seroprevalence in the south region may be related to rainfall and activity. The south region has the highest average rainfall days per year. Tropical weather with high temperature and humidity as found in the south region of Thailand is an optimal climate for mite activity.17 South regional terrain is predominated by forest, with rubber and oil-palm cultivation areas (refers to land use data “under fruit trees and tree crops” category in Supplemental Figure 2)18; these areas supply plentiful food for wild rodents and leaf litter on the ground are a good habitat for chiggers. People who work or live around these areas may be at increased risk for STG exposure, as previously reported in Malaysia, a bordering country with the south region of Thailand with similar climate and environmental conditions.19 The differences in land use data in each region are shown in Supplemental Figure 2.18 The association analysis between STG seroprevalence and land use data is limited in this study due to data resolution.
In Thailand, the incidence rates of scrub typhus were higher in male than females, with a case ratio range from 1.4:1 to 1.5:1; peaking in working age populations between 25 and 44 years old.9 In this study, samples from separate male recruit populations at ages 18–30 years old were collected and tested. Due to narrow age and gender representation, our nationwide seroprevalence results for STG, TG, and SFG reflects Rickettsia exposure in only the young Thai male adult populations and the corresponding geographic distribution throughout the country.
Until vaccines are developed for the Rickettsia, education programs detailing methods to reduce risk, especially in areas with elevated seroprevalence, need to be initiated. Prevention of disease transmission by utilizing clothing or uniform treatment and using insect repellent when engaged in outdoor activities in high-risk areas is also needed. Local medical staff need to be familiar with diagnostic clinical manifestations of rickettsial diseases, such as acute febrile illness, malaise, headache and cough, and an eschar.20 However, prompt availability of laboratory diagnostics is important for confirmation and rapid treatment as there are a broad range of general symptoms that are common in several infectious diseases which makes clinical diagnosis based on symptoms difficult. Proper diagnosis allows for antibiotic selection and treatment. The STG and SFG infection especially can be life-threatening without treatment.1,2 Providing disease awareness information to the general population helps facilitate treatment in a timely manner.
The study has limitations. One shortcoming is lack of a robust validation of anti-SFG and anti-TG antibody ELISAs. Although previous data has demonstrated highly concordant results of the anti-SFG and anti-TG ELISAs (91.7% and 84% matched results, respectively) versus the IFA but only a small number of samples were included (N = 24 for SFG and N = 25 for TG).7 Larger number of samples and comparison to the IFA in other publications is needed to generate data integrity and reproducibility for ELISA validation. For STG antibody detection, performance of the r56 recombinant-antigen ELISA testing with 350 blinded sera samples provided high sensitivity (91.2%), specificity (96.2%), and accuracy (94.6%) that were equivalent to the IFA used as the gold standard for the diagnosis of scrub typhus.6
Serological evidence obtained in this study indicates that rickettsioses are widespread in all regions of Thailand. The IgG seroprevalence data of years 2007–2008 and 2012 reflects the change of pathogen exposure over the two intervals. Particularly, overall seroprevalence of STG, O. tsutsugamushi was much elevated in 2012 and this was represented in all demographic variables. This suggests that study cohorts could more frequently come in to contact with factors influencing risk for STG exposure, for example, human behaviors, ecological environment, household hygiene, high prevalence of the etiologic agent, and increasing populations of chigger hosts. In contrast, moderately low seroprevalence of TG, R. typhi and SFG, R. Rickettsia was observed. By comparison between the two intervals (2007–2008 and 2012), opposite trends for these two Rickettsia groups were shown with the declined anti-TG IgG antibodies and the elevated anti-SFG IgG antibodies which similar trends consistently distributed in all demographic variables.
The findings reported here reveal the lowest prevalence (2.5%) of antibodies to SFG, R. Rickettsia in south region where this area ranked the highest for STG and TG seroprevalence (38.5% and 5%, respectively) in 2012. It is likely that environmental differences within the regions have a strong influence on distribution of these pathogens and their host reservoirs.
It was noted that limited demographic data is one of the shortcomings. We are unable to describe the change in seroprevalence patterns between the two intervals. Nevertheless, the outcome of this study illustrates the dynamic distribution of STG, TG, and SFG and risk factors associated with demographic variables during a particular period of time. Although, this data presents antibody prevalence over 10 years ago, our cohorts do represent all provinces of Thailand, which reflects the nationwide seroprevalence. Furthermore our data could represent the overall baseline of seroprevalence against rickettsiae in healthy and young Thai population. Persistence of anti-rickettsial IgG antibodies last for years after an infection; however, it is inconclusive to determine whether the elicited antibodies are from sub-clinical infections or previous exposure to the pathogens.21 In this regard, healthy individuals with high rickettsial antibody titers may have potential for sub-clinical infection. Hence, seroprevalence baseline in the normal population is a key information to raise concerns of hygiene awareness and living style.
This serological survey could also provide foundation data to support current serological investigations and disease surveillance. This aids to inform monitoring of the change in serologic patterns, pathogen existence, and the estimate of disease burden. However, caution should be used when comparing our serological data with other seroprevalence studies due to the different research factors, for example, study design, population, sample size, and serological method. Prospective epidemiology and ecology studies concerning rickettsial infections are valuable for understanding the scope of the problem when implementing countermeasures and constructing an intervention plan of current rickettsial diseases in Thailand.
CONCLUSION
In summary, we confirm the endemicity of rickettsioses, especially STG, throughout Thailand with seroprevalence as high as 38.5% in some provinces. Although TG and SFG seroprevalence was less than 10%, our results indicated a wide distribution of these pathogens. The STG was found to be more often associated with individuals with less than a secondary education level, living in rural areas, and working in agriculture. Our study noted elevated seroprevalence of STG in the south region of Thailand, indicating that this disease is possibly underreported in that area. Interestingly, other studies indicated a lower STG seroprevalence in rodents in the south region.16 A suitable environment, such as forest and palm oil cultivation areas, coupled with human activity, are likely key factors for STG transmission to humans in southern Thailand. Further STG, TG, and SFG epidemiology studies and methods to prevent transmission, including vaccine development and personal protective measures against vectors, are needed to improve disease control and prevention plans.
Supplemental Material
ACKNOWLEDGMENTS
We thank the Royal Thai Army (RTA) Medical Department for collection of and access to RTA recruit specimens. We are grateful for rickettsial antigens support provided by Dr. Chien-Chung Chao, Research Chemist, in the Viral and Rickettsial Diseases Department, Infectious Diseases Directorate, Navy Medical Research Center, Silver Spring, MD. We are also grateful to Ms. Nucharee Thongsen for specimen processing, Ms. Somporn Krasaesub for statistical analysis, and Ms. Tippa Wongstitwilairoong for creating a Rickettsia IgG seroprevalence choropleth map of Thailand.
Note: Supplemental tables and figures appear at www.ajtmh.org.
REFERENCES
- 1. Abdad MY Abou Abdallah R Fournier PE Stenos J Vasoo S , 2018. A concise review of the epidemiology and diagnostics of rickettsioses: Rickettsia and Orientia spp. J Clin Microbiol 56: e01728–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Low VL Tan TK Khoo JJ Lim FS AbuBakar S , 2020. An overview of rickettsiae in Southeast Asia: vector-animal-human interface. Acta Trop 202: 105282. [DOI] [PubMed] [Google Scholar]
- 3. Paris DH Shelite TR Day NP Walker DH , 2013. Unresolved problems related to scrub typhus: a seriously neglected life-threatening disease. Am J Trop Med Hyg 89: 301–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Aung AK Spelman DW Murray RJ Graves S , 2014. Rickettsial infections in Southeast Asia: implications for local populace and febrile returned travelers. Am J Trop Med Hyg 91: 451–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Suwanabun N Chouriyagune C Eamsila C Watcharapichat P Dasch GA Howard RS Kelly DJ , 1997. Evaluation of an enzyme-linked immunosorbent assay in Thai scrub typhus patients. Am J Trop Med Hyg 56: 38–43. [DOI] [PubMed] [Google Scholar]
- 6. Coleman RE et al. 2002. Comparative evaluation of selected diagnostic assays for the detection of IgG and IgM antibody to Orientia tsutsugamushi in Thailand. Am J Trop Med Hyg 67: 497–503. [DOI] [PubMed] [Google Scholar]
- 7. Forshey BM et al. 2010. Epidemiology of spotted fever group and typhus group rickettsial infection in the Amazon basin of Peru. Am J Trop Med Hyg 82: 683–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chao CC Zhang Z Wang H Alkhalil A Ching WM , 2008. Serological reactivity and biochemical characterization of methylated and unmethylated forms of a recombinant protein fragment derived from outer membrane protein B of Rickettsia typhi. Clin Vaccine Immunol 15: 684–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bureau of Epidemiology, Ministry of Public Health, Thailand, 2001–2018 Annual Epidemiology Surveillance Report. Available at: https://apps.doe.moph.go.th/boeeng/annual.php. Accessed September 10, 2020.
- 10. Leelarasamee A Chupaprawan C Chenchittikul M Udompanthurat S , 2004. Etiologies of acute undifferentiated febrile illness in Thailand. J Med Assoc Thai 87: 464–472. [PubMed] [Google Scholar]
- 11. Gonwong S Chuenchitra T Khantapura P Islam D Mason CJ , 2016. Measles susceptibility in young Thai men suggests need for young adult measles vaccination: a cross sectional study. BMC Public Health 16: 309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gonwong S Chuenchitra T Khantapura P Islam D Sirisopana N Mason CJ , 2014. Pork consumption and seroprevalence of hepatitis E virus, Thailand, 2007–2008. Emerg Infect Dis 20: 1531–1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gonwong S Chuenchitra T Khantapura P Islam D Ruamsap N Swierczewski BE Mason CJ , 2017. Nationwide seroprevalence of leptospirosis among young Thai men, 2007–2008. Am J Trop Med Hyg 97: 1682–1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chao CC Huber ES Porter TB Zhang Z Ching WM , 2011. Analysis of the cross-reactivity of various 56 kDa recombinant protein antigens with serum samples collected after Orientia tsutsugamushi infection by ELISA. Am J Trop Med Hyg 84: 967–972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wangrangsimakul T Elliott I Nedsuwan S Kumlert R Hinjoy S Chaisiri K Day NPJ Morand S , 2020. The estimated burden of scrub typhus in Thailand from national surveillance data (2003–2018). PLoS Negl Trop Dis 14: e0008233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lerdthusnee K et al. 2008. Surveys of rodent-borne disease in Thailand with a focus on scrub typhus assessment. Integr Zool 3: 267–273. [DOI] [PubMed] [Google Scholar]
- 17. Xu G Walker DH Jupiter D Melby PC Arcari CM , 2017. A review of the global epidemiology of scrub typhus. PLoS Negl Trop Dis 11: e0006062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. National Statistical Office, 2020 The Statistics of Land Used Database of Thailand Report. Available at: http://service.nso.go.th/nso/web/statseries/statseries14.html. Accessed September 4, 2020.
- 19. Hoe KB , 2008. Environmental change, development and vector-borne disease: Malaysia’s experience with filariasis, scrub typhus and dengue. Environ Dev Sustain 10: 209–217. [Google Scholar]
- 20. Rajapakse S Weeratunga P Sivayoganathan S Fernando SD , 2017. Clinical manifestations of scrub typhus. Trans R Soc Trop Med Hyg 111: 43–54. [DOI] [PubMed] [Google Scholar]
- 21. Biggs HM et al. 2016. Diagnosis and management of tickborne rickettsial diseases: Rocky Mountain Spotted Fever and other spotted fever group rickettsioses, ehrlichioses, and anaplasmosis—United States. MMWR Recomm Rep 65: 1–44. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.