ABSTRACT.
The impact and disruption of infectious disease outbreaks stretch far beyond their direct death toll, as they often overburden health systems, reduce treatment seeking behaviors, and interrupt treatment regimens. This study examines the impact of the 2014–2016 Ebola virus outbreak on tuberculosis (TB) treatment outcomes at the 34 Military Hospital in Freetown, Sierra Leone. We used retrospective data from 1,085 TB patient outcome data registers to build a multinomial logistic regression model to evaluate the change in TB treatment outcomes before and after the Public Health Emergency of International Concern (PHEIC) declaration in August 2014. These results showed that HIV status, patient age, whether patients had active versus latent TB, and the time since the start of the outbreak were significantly associated with TB treatment outcomes. The model showed an increase in probability of unknown and unsuccessful (died or treatment failed) treatment outcomes with each month after the PHEIC declaration, across age groups, TB status, and HIV status.
INTRODUCTION
The 2014–2016 Ebola virus (EVD) outbreak was devastating to West Africa, causing an estimated 28,616 cases and 11,310 deaths as of June 2016, as well as a number of indirect health consequences.1 The outbreak originated in Guinea, where the first recorded case was reported in December 2013.2 Cases continued to spread along the country borders and into Liberia and Sierra Leone, increasing rapidly by May 2014.2 Ebola virus swept through Sierra Leone communities, leading to an estimated 14,124 cases and 3,956 deaths, representing nearly half of all cases in West Africa.1 Although these high case numbers and death rates emphasize the severity of the outbreak, they do not capture the extent of indirect deaths that may have occurred as a result of disrupted health systems for other diseases such as tuberculosis (TB) and HIV.3,4
During the EVD outbreak, Sierra Leone’s health care services were overburdened and understaffed.5 People were reluctant to seek treatment at health centers due to widespread fear of infection and discrimination, closures of health facilities, and the implementation of new curfews, laws, and border closures.4,6 Healthcare workers (HCWs) in Sierra Leone also experienced a disproportionate burden of EVD infections, with 28% of 1,100 total HCWs infected, 72% of whom died.7 Cases among HCWs peaked 3 to 4 weeks before the overall peak of the EVD outbreak, reducing the number of available medical personnel to provide care, and posing an additional challenge to containing the outbreak.7,8 The frequency of nosocomial infections also created fear and public distrust surrounding healthcare facilities.9
The EVD outbreak also impacted treatment-seeking behaviors for other prevalent diseases that require continuous care, such as TB and HIV. Before the EVD outbreak, Sierra Leone experienced the third highest TB incidence in the world, as well as an HIV prevalence of 54,708, which increases the likelihood of latent TB infection (LTBI) reactivation.10–12 In 2012, there was a reported incidence of 674 TB cases per 100,000 people and prevalence of 1,300 TB cases per 100,000 people.13 In 2013 and 2014, Sierra Leone registered an average of 12,000 to 13,000 cases of TB each year, with treatment completion rates estimated at 85% to 87%.13,14
During the EVD outbreak, directly observed therapy short course (DOTS) programs for TB were interrupted, shortages in HIV drugs occurred, and patients undergoing TB and/or HIV treatment were disengaged.7 After the height of the outbreak, issues related to the repurposing of clinical teams and facilities to respond to the outbreak, along with a “no touch” policy, decreased accessibility to DOTS for TB.8 Given the possibility of an estimated reduction in treatment accessibility of 50% due to the EVD outbreak, it is predicted that the deaths attributed to HIV and TB would increase by 9% and 61%, respectively, in Sierra Leone.4 Considering each of these integrated factors impacting TB treatment access during the EVD outbreak, the potential for increases in deaths is substantial.
These numbers become increasingly important in understanding the broader implications of epidemics given the ongoing COVID-19 pandemic, which will likely cause similar diagnostic and treatment interruptions worldwide.15 To better understand the potential impacts that treatment interruptions may have on diseases such as TB, it is vital to use existing information from previous outbreaks. This study uses retrospective TB patient outcome data, paired with HIV status, from a military hospital in Freetown, Sierra Leone, to quantify the impact of the 2014 EVD outbreak on TB treatment outcomes. The study applies a robust modeling framework to understand which patient characteristics influenced TB treatment outcomes during the Ebola epidemic and to determine how the influence of those factors on predicted treatment outcomes changed through time.
METHODS
Study design and setting.
This retrospective study reviewed TB patient outcome data from the 34 Military Hospital in Freetown, the capital of Sierra Leone. The two-tier health care system in Sierra Leone is composed of Peripheral Healthcare Units and secondary care consisting of three referral hospitals, 21 district hospitals, 27 private hospitals, and 45 private clinics, most of which are located in Freetown.16 The 34 Military Hospital is one of three referral hospitals in Freetown, providing service to 200 to 250 patients per day.17 The hospital is located at Wilberforce Barracks and serves 25% of the western section of Freetown, including soldiers, their families, and the general population. It is a teaching and general hospital, with 369 personnel. A study from 2011 to 2014 indicates that the 34 Military Hospital consisted of four departments, a laboratory facility, and staffed 17 permanent military Medical Officers, three specialists, and five consultants at the time of this study.17 As part of a Frontline Field Placement Surveillance Report, data were extracted from Patient Hospital Register TB Records between January 3, 2012, to December 22, 2016, and included in this analysis. Coinfection with HIV was also examined as a variable of interest related to patient outcomes. The declaration of a Public Health Emergency of International Concern (PHEIC) on August 8, 2014, was used as the distinguishing start date of the EVD outbreak throughout the following analyses.16
Pearson’s chi-square tests.
Patient data was grouped into two categories based on the individual’s enrollment date: pre- and post-PHEIC.18 Frequency tables were calculated containing treatment outcomes based on pre- or post-PHEIC status. which were then used to calculate a Pearson’s χ2 test probability of treatment outcomes based on a patient’s pre- or post-PHEIC enrollment status. Separate Pearson’s χ2 tests were run for both active and latent tuberculosis patients. The null hypothesis was no association between pre- or post-PHEIC enrollment status and treatment outcome.
Regression analysis.
To further quantify the impact the EVD outbreak may have had on TB treatment outcomes at 34 Military Hospital, this study used a multinomial logistic regression model (see Supplemental Information).19 For each patient record, we quantified the number of months since the PHEIC declaration on August 8, 2014, at which point 717 cases were confirmed in Sierra Leone.1,18 The variable “months after outbreak” for all patient records with entry dates between January 3, 2012 and August 8, 2014, were set to the value of zero. Treatment outcomes were included in hospital records in the following categories: cured, completed, defaulted, died, failed, lost to follow-up, transferred, and unknown. Because of the limited clarity related to the definitions of the outcome data reported, outcomes were reclassified based on the objectives of this paper and merged into the following groups: “successful” (completed or cured), “unsuccessful” (died or failed), “lost to follow-up” (lost to follow-up, abscond, or default), and “unknown” (unknown or transferred).20
A series of multinomial logistic regressions were performed to evaluate the change in treatment outcomes following the PHEIC declaration. The full model included the following variables: HIV status, sex, age, TB type (active or latent), and the number of months after the outbreak. An interaction term between age and HIV status was also tested under the hypothesis that the effect of HIV status on treatment outcome could vary depending on the patient’s age. All explanatory variables were examined for collinearity before inclusion in the model, using a cutoff correlation of 0.60 to be included. After constructing the full model, the model was tested for any violations of the independence irrelevant alternatives (IIA) assumption of multinomial logistic regression models by conducting a Hausman diagnostic test, following the procedure outlined by Kwak and Clayton-Matthews (2002).19 Under the Hausman diagnostic test, the treatment outcomes were dropped one at a time from the data set, and coefficients were recalculated using the constrained model, with the full set of covariates.19 Because the coefficients of each of the constrained models were statistically identical to the relevant coefficients of the full model, the IIA assumption was satisfied.
The model selection process was conducted using an information-theoretic approach.21 A backward-stepwise process was used, dropping one covariate at a time to determine which model resulted in the lowest Akaike information criteria (AIC) value.21 Covariates were dropped until eliminating any other covariate from the model would result in a higher AIC value, and the resulting model was chosen as our optimal model. Multinomial logistic regressions were calculated using the multinom function in the nnet package in R, version 3.6.1.22,23
FINDINGS
Study group demographics and descriptive data.
After patients without an enrollment date were removed (N = 1), records from 1,085 patients remained (Table 1) that were used for the regression and χ2 analyses. The mean patient age was 35.57 years old (SD = 18.61) with a range of 58 days to 98 years old. Regarding HIV status, 679 patients (62.58%) were HIV negative, 290 patients (26.73%) were HIV-positive, and 116 patients (10.69%) were not tested for HIV. Overall, 377 patients (34.75%) were female, and 708 patients (65.25%) were male. There were 711 patients with successful outcomes, 250 who were lost to follow-up, 89 with unsuccessful outcomes, and 35 with unknown outcomes. Patients were split by TB status, with 553 patients (50.97%) classified as having active TB and 532 patients (49.03%) having latent TB. Of the 553 patients with active TB, 72.33% were successful, 18.44% were lost to follow-up, 6.51% were unsuccessful, 2.71% had unknown outcomes. Of the 532 patients with latent TB, 58.46% of outcomes were successful, 27.82% were lost to follow-up, 3.76% had unknown outcomes, and 9.96% were unsuccessful outcomes.
Table 1.
Demographic information from patients with TB (N = 1,085) at 34 Military Hospital in Freetown, Sierra Leone, from 2012 to 2016
| Demographics | Pre-PHEIC, n (%) | Post-PHEIC, n (%) | Total population, N = 1,085 (%) |
|---|---|---|---|
| Sex | |||
| Female | 239 (62.7) | 142 (37.3) | 381 (35.12) |
| Male | 449 (63.8) | 255 (36.2) | 704 (64.9) |
| Civilian/soldier status | |||
| Civilian | 474 (61.4) | 298 (38.6) | 772 (71.2) |
| Soldier/retired soldier | 214 (68.4) | 99 (31.6) | 313 (28.8) |
| Age* | |||
| 0–17 | 125 (74.9) | 42 (25.1) | 167 (15.4) |
| 18–24 | 68 (61.2) | 45 (39.8) | 113 (10.4) |
| 25–34 | 152 (63.9) | 86 (36.1) | 238 (21.9) |
| 35–44 | 162 (63.3) | 94 (36.7) | 256 (23.6) |
| 45–54 | 94 (63.5) | 54 (36.5) | 148 (13.6) |
| 55–64 | 43 (51.8) | 40 (48.2) | 83 (7.6) |
| 65–74 | 23 (50.0) | 23 (50.0) | 46 (4.2) |
| ≥ 75 | 21 (61.8) | 13 (38.2) | 34 (3.1) |
| HIV coinfection | |||
| Positive | 176 (60.7) | 114 (39.3) | 290 (26.7) |
| Negative | 444 (65.4) | 235 (34.6) | 679 (62.6) |
| Not tested | 68 (58.6) | 48 (41.4) | 116 (10.7) |
| TB type | |||
| Active | 307 (55.5) | 246 (44.5) | 553 (51) |
| Latent | 381 (71.6) | 151 (28.4) | 532 (49) |
PHEIC = Public Health Emergency of International Concern; TB = tuberculosis.
*Percentages do not equal 100% due to rounding.
Pearson’s chi-squared tests.
We conducted a series of three Pearson’s χ2 tests to test the null hypothesis that there was no association between pre- and post-PHEIC status and TB treatment outcome (Table 2). When pre- and post-PHEIC status was determined as before or after August 8, 2014, and all patients were included, the test showed that there was a difference in treatment outcomes between pre- and post-PHEIC status patients (χ2 = 16.25, P = 0.001) (Table 3). Additionally, when pre- and post-PHEIC status was determined including all patients with active TB, the test showed that there was a difference in treatment outcomes between pre- and post-PHEIC status patients (χ2 = 21.04, P < 0.001). However, there was no statistically significant difference between treatment outcomes determined between pre- and post-PHEIC status of patients with latent TB (χ2 = 1.6215, P = 0.66).
Table 2.
Summary of TB treatment outcomes of patients (N = 1,085) at 34 Military Hospital in Freetown, Sierra Leone, from 2012 to 2016
| Outcome | Pre-PHEIC, n (%) | Post-PHEIC, n (%) | Total, n |
|---|---|---|---|
| Cured | 85 (37.1) | 144 (62.9) | 229 |
| Completed | 174 (36.1) | 308 (63.9) | 482 |
| Abscond | 0 (0) | 1 (100) | 1 |
| Default | 47 (28.1) | 120 (71.9) | 167 |
| Lost to follow-up | 0 (0) | 7 (100) | 7 |
| Transferred | 10 (28.6) | 25 (71.4) | 35 |
| Unknown | 45 (60) | 30 (40) | 75 |
| Failed | 2 (100) | 0 (0) | 2 |
| Died | 34 (39.1) | 53 (60.9) | 87 |
PHEIC = Public Health Emergency of International Concern; TB = tuberculosis.
Percentages are reported as percentage of all patients in each category.
Table 3.
Results from Pearson’s chi-square tests, including expected and observed proportions for each treatment outcome, before and after the PHEIC declaration on August 8, 2014
| Treatment outcome | ||||
|---|---|---|---|---|
| Time | Successful | Lost to follow-up | Unknown | Unsuccessful |
| Expected proportions, all patients* (χ2 = 16.25, P = 0.001)* | ||||
| Pre-PHEIC | 0.24 | 0.06 | 0.04 | 0.03 |
| Post-PHEIC | 0.42 | 0.10 | 0.06 | 0.05 |
| Observed proportions, all patients* | ||||
| Pre-PHEIC | 0.24 | 0.04 | 0.05 | 0.03 |
| Post-PHEIC | 0.42 | 0.12 | 0.05 | 0.05 |
| Expected proportions, active TB patients (χ2 = 21.038, P < 0.001)* | ||||
| Pre-PHEIC | 0.32 | 0.05 | 0.05 | 0.03 |
| Post-PHEIC | 0.40 | 0.06 | 0.06 | 0.04 |
| Observed proportions, active TB patients | ||||
| Pre-PHEIC | 0.32 | 0.03 | 0.07 | 0.03 |
| Post-PHEIC | 0.41 | 0.08 | 0.03 | 0.03 |
| Expected proportions, latent TB patients (χ2 = 1.6215, P = 0.6545) | ||||
| Pre-PHEIC | 0.17 | 0.06 | 0.03 | 0.03 |
| Post-PHEIC | 0.42 | 0.16 | 0.07 | 0.07 |
| Observed proportions, latent TB patients | ||||
| Pre-PHEIC | 0.16 | 0.06 | 0.03 | 0.03 |
| Post-PHEIC | 0.43 | 0.16 | 0.07 | 0.07 |
PHEIC = Public Health Emergency of International Concern; TB = tuberculosis.
Indicates significant differences (P < 0.05) in treatment outcomes between groups.
Regression analyses.
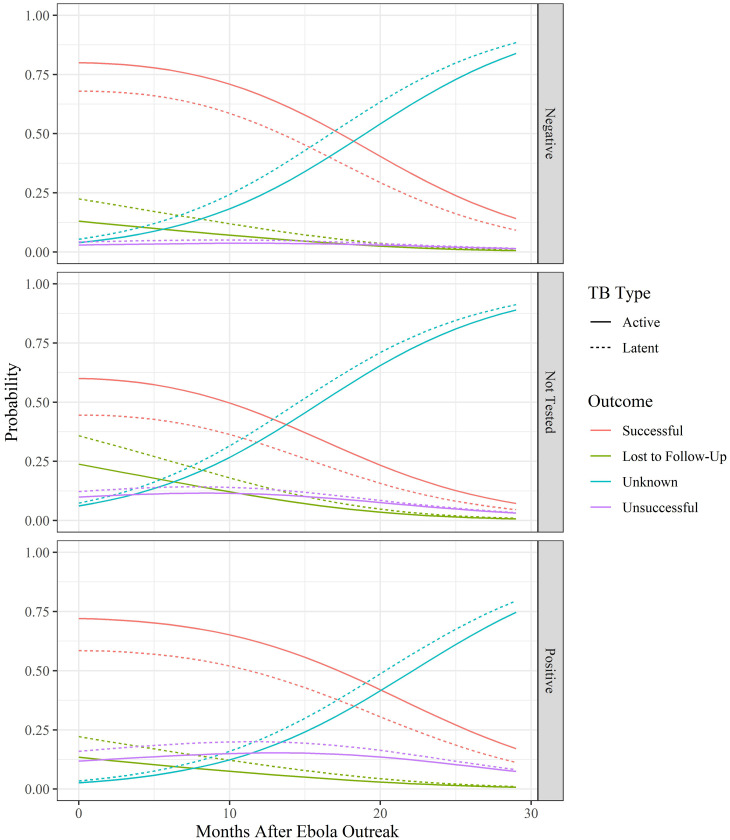
A series of multinomial logistic regressions were calculated to test the probabilities of treatment outcomes based on patient characteristics and the number of months since the EVD outbreak. The model selection process showed that the optimal model included TB type, the number of months after the EVD outbreak, HIV status, age, and an interaction between age and HIV status (Supplemental Table 1). Calculation of the Akaike weight showed that this model had a 67% chance of being the optimal model for the data, and no other model fell within a ΔAIC of 2 points of the optimal model. Full reporting of odds ratios and 95% confidence intervals (CIs) calculated from the coefficients of the optimal model can be found in Table 4. Overall, regression results showed that, regardless of HIV status, as the number of months since the EVD outbreak increased, the probability of a successful treatment outcome occurring declined whereas the probability of an unknown or unsuccessful outcome increased (Figure 1). The odds of an unknown outcome and unsuccessful outcome were 1.179 (95% CI: 1.157–1.203) and 1.034 (95% CI: 1.007–1.0623) more likely than a successful outcome, respectively, for each progressive month after the PHEIC was declared (Table 4). The probability of the treatment outcome of lost to follow-up or unsuccessful declined as the number of months since the EVD outbreak also increased, regardless of HIV status.
Table 4.
Odds ratios and 95% confidence intervals (CIs) of optimal multinomial logistic regression model, using successful as reference treatment outcome, HIV-negative status as reference covariate for HIV status, and active TB as reference covariate for Type TB
| Covariate | Successful | Lost to follow-up (95% CI) | Unknown (95% CI) | Unsuccessful (95% CI) |
|---|---|---|---|---|
| Intercept | – | 0.087 (0.052–0.144)* | 0.03 (0.017–0.055)* | 0.008 (0.003–0.02)* |
| Months after outbreak | – | 0.953 (0.922–0.984)* | 1.179 (1.157–1.203)* | 1.034 (1.007–1.062)* |
| Age | – | 1.018 (1.007–1.029)* | 1.014 (1.002–1.026)* | 1.044 (1.026–1.063)* |
| HIV negative | – | – | – | – |
| HIV not tested | – | 1.175 (0.442–3.125) | 3.291 (1.179–9.184)* | 5.095 (1.103–23.534)* |
| HIV-positive | – | 3.852 (1.262–11.756)* | 1.48 (0.414–5.29) | 11.942 (2.969–48.028)* |
| Active TB | – | – | – | – |
| Latent TB | – | 2.029 (1.421–2.898)* | 1.612 (1.112–2.338)* | 1.662 (1.057–2.615)* |
| Age × HIV negative | – | – | – | – |
| Age × HIV not tested | – | 1.021 (0.998–1.044) | 0.987 (0.96–1.015) | 0.996 (0.966–1.028) |
| Age × HIV positive | – | 0.967 (0.938–0.996)* | 0.981 (0.95–1.012) | 0.973 (0.943–1.003) |
Represents a statistically significant odds ratio (P < 0.05) indicating an association between the covariate and outcome, using a 95% CI.
Figure 1.
Predicted tuberculosis (TB) outcomes as Ebola outbreak progresses. Predicted probabilities based on optimal multinomial logistic regression model of the influence of HIV status, TB type, patient age, and time since the EVD outbreak on TB treatment outcome for patients in 34 Military Hospital in Freetown, Sierra Leone, from 2012 to 2016. This figure appears in color at www.ajtmh.org.
HIV status had a significant impact on TB treatment outcomes. Overall, HIV-positive patients had a slightly lower probability of successful treatment and a slightly higher probability of an unsuccessful treatment than HIV negative patients throughout the study period. Patients that had unknown HIV status had the lowest probability of a successful outcome and the highest probability of an unknown outcome (Figure 1). Using HIV-negative status and successful outcomes as reference categories, HIV-positive patients were more likely to be lost to follow-up or have an unsuccessful treatment outcome (Table 4).
TB status also had a significant impact on treatment outcomes. Regardless of HIV status or the number of months after the EVD outbreak, patients with active TB had a higher probability of a successful treatment outcome and a lower probability of an unsuccessful or unknown outcome or being lost to follow-up. Odds ratios for TB status showed that the odds of a patient with latent TB being lost to follow-up were 2.029 times higher (95% CI: 1.421–2.898) than the odds of a patient with active TB being lost to follow-up. Similarly, the odds of a patient with latent TB having an unknown treatment status or an unsuccessful treatment were 1.612 (95% CI: 1.112–2.338) and 1.662 (95% CI: 1.057–2.615) times higher than active TB patients, respectively.
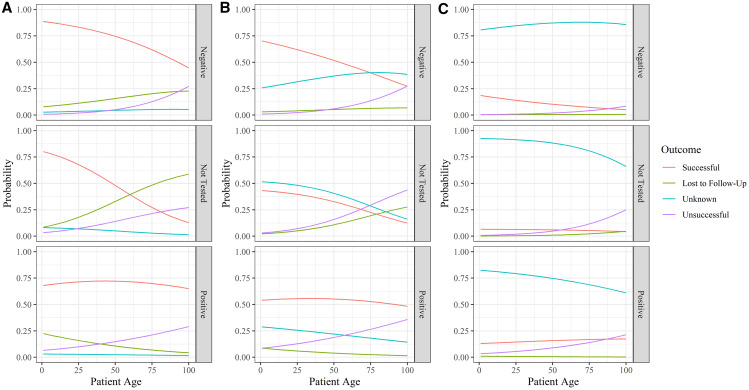
Age of the patient and the interaction between age and HIV status were also included in the optimal model. In general, as patient age increased the probability of a successful outcome decreased (Figure 2). Before the EVD outbreak, as age increased for HIV-positive patients, the probability of an unsuccessful treatment outcome increased, the probability of being lost to follow-up decreased, and the probability of successful or unknown outcomes stayed nearly the same (Figure 2). Alternatively, as age increased in patients who were not tested or tested negative for HIV, the probability of being lost to follow-up increased, and the probability of successful outcomes decreased. Although these same age-related trends occurred after the EVD outbreak, as time passed, the probability of an unknown outcome increased and the probability of a successful outcome decreased, regardless of HIV status or age.
Figure 2.
Probability of tuberculosis (TB) outcomes by age, compared across 15-month intervals. Multinomial logistic regression of TB outcomes by HIV status after the Ebola virus (EVD) outbreak by patient age (0–100 years), at time (A) 0 months, (B) 15 months after outbreak, and (C) 30 months after outbreak for patients in 34 Military Hospital in Freetown, Sierra Leone, from 2012 to 2016. This figure appears in color at www.ajtmh.org.
DISCUSSION
Overall, this retrospective analysis of data from the 34 Military Hospital in Freetown, Sierra Leone, on TB treatment outcomes before and after the 2014 EBV outbreak showed that there were significant differences in treatment outcomes before and after the EVD outbreak. Regression results showed that treatment outcomes were influenced by the length of time passed since the EVD outbreak declaration, HIV and TB status, and patient demographics. The declines in the probability of an outcome being successful, unsuccessful, or lost to follow-up as the EVD outbreak continued was likely due to the vast increase in the number of cases with unknown outcomes. This supports the hypothesis that patients who were previously being seen for TB infections and/or TB/HIV coinfections may have stopped visiting the hospital either because of fear of infection with EVD and/or the discontinuation of treatment programs. Ansumana et al. (2017) similarly described widespread avoidance of healthcare in Sierra Leone during the Ebola epidemic, where current HIV and TB patients discharged themselves and prospective patients did not seek treatment at all.3 A similar study examining HIV during the EVD epidemic described the effects of the Ebola epidemic on HIV treatment of soldiers in Sierra Leone and found that there was a much greater risk of patients being lost to follow-up, defaulting, and going without treatment.24 A study done by Parpia et al. (2016) modeled the effects of treatment coverage reduction caused by the Ebola epidemic in Sierra Leone on deaths related to HIV, TB, and malaria predicted that a 50% reduction in treatment coverage reported for Sierra Leone would cause more than 2,500 indirect deaths.4 A 65% reduction in treatment coverage was predicted to cause equal numbers of direct deaths from Ebola and indirect deaths from HIV, TB, and malaria.4 Taken in this context, the vast increase in unknown treatment outcomes shown in this study not only highlights the interruption in access to healthcare, but it also suggests the possibility that many of those unknown treatment outcomes were unsuccessful TB outcomes as the Ebola epidemic continued. This study also highlights the need for improved efforts to track patients during future treatment interruptions.
The results presented here conflict with those reported by Bah et al. (2017), who reported an increase in the number of successful treatment outcomes of TB patients during the Ebola epidemic in the Bombali District of Sierra Leone.2 These increased successful outcomes were attributed to greater accessibility to healthcare as vast resources were deployed in Sierra Leone.2 Additionally, this study used a different threshold for the beginning of the Ebola period, beginning the EVD outbreak on June 1, 2014, versus August 8, 2014, used in this analysis.2,18 Alternatively, these results were based on a smaller sample size for treatment outcome comparisons (n = 226) and used only χ2 tests for comparisons.2 Our results suggest that other factors influenced treatment outcome, particularly age and HIV status.2 Therefore, differences in demographics between this study and that done by Bah et al. (2017), or the fact that they were not factored into their analysis, may explain the difference in treatment outcome results.
Ortuno-Gutierrez et al. (2016) also reported different results within Guinea, in line with those reported by Bah et al. (2017), where contingency planning and increased health support resulted in slightly higher TB treatment success rates.25 The regression results showed that HIV status, age, and TB status had an influence on TB treatment outcomes. Overall, HIV-positive patients had lower probabilities of successful outcomes and higher probabilities of unsuccessful treatment outcomes in comparison to HIV negative patients. These results are in line with results reported by Tweya et al. (2013) and Kliiman and Altraja (2009), who reported that HIV coinfection was associated with poorer treatment outcomes.26,27
Older patients in this study consistently had higher probabilities of poor treatment outcomes compared with younger patients, which is not surprising given the well-described negative effect of age on TB treatment outcomes described in other studies.28 Patients with active tuberculosis had higher probabilities of successful treatment outcomes than patients with latent tuberculosis. This difference in treatment outcomes is likely due to the nature of active TB, where patients who are experiencing symptoms are more likely to seek treatment for TB and therefore more likely to have a successful outcome.29
Several studies have evaluated the indirect impacts of the EVD outbreak on healthcare services for diseases that require regular care, such as TB, HIV, and malaria.3,4,7 It is also well understood that sporadic interruptions to TB and HIV treatment can increase the risk of poor treatment outcomes, opportunistic infections, and death by any cause.30 The timing of this impact has also been studied, and evidence of decreased testing and treatment of TB and HIV, as well as poor treatment outcomes among those with TB and HIV, correlated with the peak of the EVD outbreak.31
Limitations.
This study was limited in that it assumed a constant level of impact on TB outcomes throughout the EVD outbreak after the PHEIC declaration, without accounting for fluctuations in disease incidence and societal disruptions in the region. This simplification, not accounting for changes in rate of new infections over time in the model, simplifies the model to an extent. Furthermore, by using a military hospital, this study captures both military and civilian populations, which may not be representative of the general population of Freetown. Finally, this study is exposed to many of the general weaknesses inherent in examining retrospective clinical data, such as missing patient data, potential misrepresentation of the general population due to a limited sample, limited measures of other risk factors that may be important to treatment outcomes, and potential for misclassification of diagnoses. Another limitation of this retrospective dataset was a lack of clearly defined diagnosis criteria for active and latent TB and no specification of drug types used for treatment.
Conclusions.
TB treatment outcomes were shown to be influenced by the length of time that had passed since the EVD outbreak declaration, patient age, whether patients had active or latent TB, and their HIV infection status. Most notably was the change in the proportion of patients with treatment outcome categorized as successful, unsuccessful, or lost to follow-up shifting to unknown as the outbreak progressed. These results identify patients who are most at risk for unsuccessful TB treatment outcomes based on the factors indicated earlier during different points in the timeline after a disturbance. Because 34 Military Hospital is currently the main referral hospital for Sierra Leone’s severe COVID-19 cases, further studies on the impact shifting resources, interruptions to care, and decreased treatment seeking on TB outcomes could further inform our understanding of whether these disturbances are uniform in their impact on TB treatment outcomes.
Supplemental Material
Note: Supplemental materials appear at www.ajtmh.org.
REFERENCES
- 1. WHO , 2016. WHO Situation Report: Ebola Virus Disease. Geneva, Switzerland: World Health Organization. Available at: https://apps.who.int/iris/bitstream/handle/10665/208883/ebolasitrep_10Jun2016_eng.pdf;jsessionid=56E8AD39CA7BCF6B762FDE9D0E851240?sequence=1. Accessed June 14, 2020. [Google Scholar]
- 2. Bah OM et al. 2017. The influence of the Ebola outbreak on presumptive and active tuberculosis in Bombali District, Sierra Leone. Public Health Action 7: S3–S9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ansumana R et al. 2017. Impact of infectious disease epidemics on tuberculosis diagnostic, management, and prevention services: experiences and lessons from the 2014–2015 Ebola virus disease outbreak in West Africa. Int J Infect Dis 56: 101–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Parpia AS Ndeffo-Mbah ML Wenzel NS Galvani AP , 2016. Effects of response to 2014–2015 Ebola outbreak on deaths from malaria, HIV/AIDS, and tuberculosis, West Africa. Emerg Infect Dis 22: 433–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sylvester Squire J Hann K Denisiuk O Kamara M Tamang D Zachariah R , 2017. The Ebola outbreak and staffing in public health facilities in rural Sierra Leone: who is left to do the job? Public Health Action 7: S47–S54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Briand S et al. 2014. The international Ebola emergency. N Engl J Med 371: 1180–1183. [DOI] [PubMed] [Google Scholar]
- 7. Elston JWT et al. 2016. Impact of the Ebola outbreak on health systems and population health in Sierra Leone. J Public Health 38: 673–678. [DOI] [PubMed] [Google Scholar]
- 8. Edelstein M Angelides P Heymann DL , 2015. Ebola: the challenging road to recovery. Lancet 385: 2234–2235. [DOI] [PubMed] [Google Scholar]
- 9. Wilhelm JA Helleringer S , 2019. Utilization of non-Ebola health care services during Ebola outbreaks: a systematic review and meta-analysis. J Glob Health 9: 010406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. WHO , 2012. World Health Organization (WHO) Estimates of Tuberculosis Incidence by Country, 2012. London, United Kingdom: TB Section, Centre for Infectious Disease Surveillance and Control, Public Health England. Available at: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/332777/TB_Worldwide_by_Country_2012.pdf. Accessed July 12, 2020. [Google Scholar]
- 11. Corbett EL et al. 2003. The growing burden of tuberculosis: global trends and interactions with the HIV epidemic. Arch Intern Med 163: 1009–1021. [DOI] [PubMed] [Google Scholar]
- 12. UNAIDS , 2015. Sierra Leone National AIDS Response Progress Report 2015. Geneva, Switzerland: UNAIDS. Available at: https://www.unaids.org/sites/default/files/country/documents/SLE_narrative_report_2015.pdf. Accessed October 1, 2020. [Google Scholar]
- 13. WHO , 2013. Global Tuberculosis Report 2013. Geneva, Switzerland: World Health Organization. Available at: https://apps.who.int/iris/bitstream/handle/10665/91355/9789241564656_eng.pdf?sequence=1. Accessed June 14, 2020 [Google Scholar]
- 14. WHO , 2014. Global Tuberculosis Report 2014. Geneva, Switzerland: World Health Organization. Available at: https://www.who.int/tb/publications/global_report/gtbr14_main_text.pdf. Accessed October 1, 2020. [Google Scholar]
- 15. Cilloni L et al. 2020. The potential impact of the COVID-19 pandemic on tuberculosis: a modelling analysis. EClinicalMedicine 28: 100603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Robinson C , 2019. Primary health care and family medicine in Sierra Leone. Afr J Prim Health Care Fam Med 11: e1–e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Nabieu PF et al. 2016. Lower limb amputation at the 34 Military Hospital in Freetown, Sierra Leone: causes and indications. Sierra Leone J Biomed Res 8: 9–17. [Google Scholar]
- 18. WHO , 2014. Statement on the 1st Meeting of the IHR Emergency Committee on the 2014 Ebola Outbreak in West Africa. Geneva, Switzerland: World Health Organization. Available at: https://www.who.int/mediacentre/news/statements/2014/ebola-20140808/en/. Accessed June 18, 2020. [Google Scholar]
- 19. Kwak C Clayton-Matthews A , 2002. Multinomial logistic regression. Nurs Res 51: 404–410. [DOI] [PubMed] [Google Scholar]
- 20. WHO , 2014. Guidance for National Tuberculosis Programmes on the Management of Tuberculosis in Children, 2nd edition. Geneva, Switzerland: World Health Organization. Available at: https://www.ncbi.nlm.nih.gov/books/NBK214446/. Accessed November 11, 2019. [PubMed] [Google Scholar]
- 21. Burnham KP Anderson DR , 2002. Model Selection and Multimodel Inference: A Practical Information-Theoretic Approach, 2nd edition. New York, NY: Springer-Verlag. [Google Scholar]
- 22. Venables B Ripley B , 2002. Modern Applied Statistics with S. New York, NY: Springer Science+Business. [Google Scholar]
- 23. R Core Team , 2019. R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing. Available at: http://www.R-project.org/. [Google Scholar]
- 24. Nagel E Blackowicz MJ Sahr F Jarrett OD , 2019. Impact of the Ebola epidemic on clinical outcomes of HIV-infected soldiers and their dependents in Sierra Leone. Int J STD AIDS 30: 106–112. [DOI] [PubMed] [Google Scholar]
- 25. Ortuno-Gutierrez N et al. 2016. Upholding tuberculosis services during the 2014 Ebola storm: an encouraging experience from Conakry, Guinea. PLoS One 11: e0157296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Tweya H et al. 2013. Comparison of treatment outcomes of new smear-positive pulmonary tuberculosis patients by HIV and antiretroviral status in a TB/HIV Clinic, Malawi. PLoS One 8: e56248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kliiman K Altraja A , 2009. Predictors of poor treatment outcome in multi- and extensively drug-resistant pulmonary TB. Eur Respir J 33: 1085–1094. [DOI] [PubMed] [Google Scholar]
- 28. Negin J Abimbola S Marais BJ , 2015. Tuberculosis among older adults—time to take notice. Int J Infect Dis 32: 135–137. [DOI] [PubMed] [Google Scholar]
- 29. Blumberg HM Ernst JD , 2016. The challenge of latent TB infection. JAMA 316: 931–933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Podewils LJ Gler MTS Quelapio MI Chen MP , 2013. Patterns of treatment interruption among patients with multidrug-resistant TB (MDR TB) and association with interim and final treatment outcomes. PLoS One 8: e70064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Konwloh PK et al. 2017. Influence of Ebola on tuberculosis case finding and treatment outcomes in Liberia. Public Health Action 7: S62–S69. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.