ABSTRACT.
Eschar is used to establish the clinical diagnosis of scrub typhus. To identify the prevalence of eschar and its location in scrub typhus, we reviewed globally published peer-reviewed articles. We conducted a systematic literature review, using publications extracted from PubMed from January 1943 to August 31, 2019 with the keywords “scrub typhus” or “tsutsugamushi.” We collected articles associated with eschar prevalence and distribution in scrub typhus infection and reported on the body locations of eschar. A total of 458 articles containing information on the prevalence, geographical distribution, and body distribution of eschar were used for this review. The overall prevalence rate of eschar was 58.0% with the greatest prevalence in East Asia (78.7%) followed by Oceania (52.2%), Southeast Asia (41.4%), and then the lowest in South Asia (32.8%). The frequency of eschar distribution was highest in the inguinal area (28.5%), followed by the anterior area (28.0%), and the lower extremities (22.0%). The upper extremities (8.9%), back (6.6%), and head and neck (6.1%) regions had relatively low frequencies of distribution. We identified the prevalence of eschar differed by geographic locations and anatomical sites on the patient of scrub typhus.
INTRODUCTION
Scrub typhus is a potentially fatal mite-born infectious disease that is endemic mostly to the Tsutsugamushi Triangle.1 More than 1 million new cases are diagnosed every year, and more than 1 billion people are estimated to be at risk to acquire the scrub typhus infection globally.2–5
An eschar, a pathognomonic sign of scrub typhus, is a painless black crust at the site of the chigger bite.6–8 It is a useful diagnostic clue in patients with acute febrile illness in endemic areas for scrub typhus.9 However, its presence in patients varies widely.4,10–18 The exact reasons for this variation have not yet been identified, and comparisons between international eschar prevalence rates have not been made.
Chigger mite travels an average of 11 inches in 5 minutes, and these mites can crawl rapidly anywhere on the human body.19 However, chiggers seem to have a predilection for the region of pressure between clothing and skin such as the beltline, underpants, brazier lines, ankles, and beneath leggings.19,20 Thus, it appears that the site of the mite attachment is dependent on two factors: the availability of a foreign object on which the mites crawl seeking a suitable place and an area of pressure wherein they attach.19,20 It is crucial to comprehensively identify the geographical distribution where eschar frequently occurs for the diagnosis of scrub typhus.6,9,21 Although several articles describing the body distribution of eschar have been published,6,9,21–26 only a limited number of patients were included. Therefore, it is difficult to draw generalizations about the body distribution of eschar. In this study, we investigated the geographical distribution and body distribution of eschar prevalence rate through a systematic review of globally published data.
METHODS
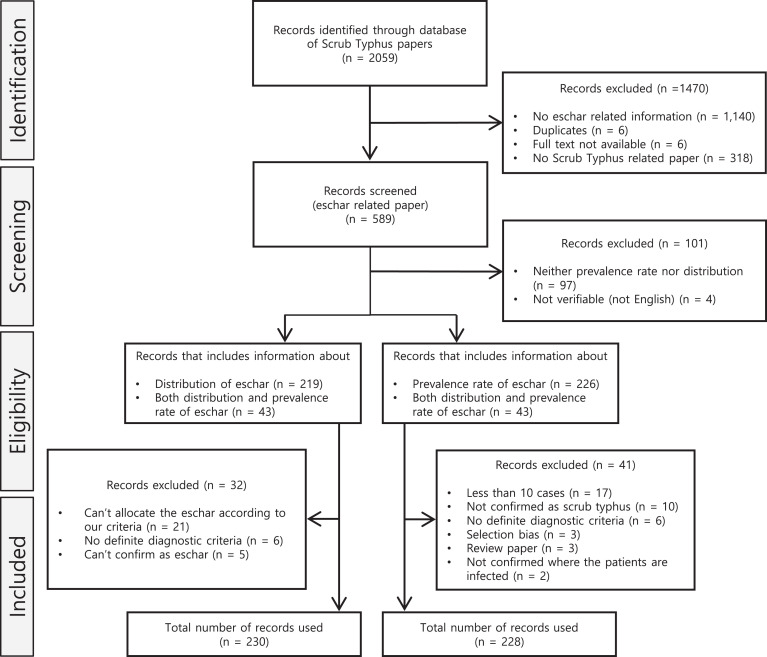
Using the search term “scrub typhus” or “tsutsugamushi,” we searched articles published in PubMed from January 1, 1943 to August 31, 2019 and collected articles associated with eschar prevalence or distribution of scrub typhus infection. The search strategy is shown in Figure 1. All abstracts and full texts were reviewed independently by three authors. For the inclusion and exclusion criteria, we included studies in which scrub typhus was diagnosed based on clinical symptoms and laboratory results (serology), for example, indirect immunofluorescence assay, ELISA, indirect immunoperoxidase assay, polymerase chain reaction, culture, and Weil–Felix test. We excluded the article that includes the number of patients with scrub typhus less than 10. Furthermore, we excluded articles if they did not report on the prevalence rate or distribution of eschar or the full text of the article was not available, or were editorials, reviews, or commentaries without primary data or peer review. In addition, articles with no definite diagnostic criteria or could not classify eschar according to our criteria were excluded. Most of the publications were written in English, but some Japanese and Chinese-language publications were also included. For non-English articles, extraction was done together with native speakers, to ensure all data was extracted as accurately as possible.
Figure 1.
Flow diagram for literature search and study selection for scrub typhus.
We categorized the locations as East Asia, Southeast Asia, South Asia, and Oceania. We divided the nations according to the United Nations Statistics Division classification based on geographical region and composition.27 In that classification, East Asia includes China, Japan, Korea, and Taiwan. Southeast Asia includes Laos, Malaysia, Myanmar, Philippines, Thailand, and Vietnam. South Asia includes Bhutan, India, Nepal, and Sri Lanka. Oceania includes Australia, New Guinea, and Micronesia, which are islands on the South Pacific Ocean. We defined the country as “Undefined” if a location of a specific country was not indicated, such as “Africa” or “Vietnam and Cambodia.”
We divided the human body into six parts: head/neck (H/N), anterior, back, upper extremities, lower extremities, and inguinal area. H/N included the head, face, and neck up the clavicle. The anterior region included the anterior part of the upper body: axilla and flank. The back region included the posterior part of the upper body: waist and lumbar regions. When the location of eschar was mentioned as “trunk” without any distinction whether it was anterior or posterior, the location was excluded. The upper extremities included the shoulders. Lower extremities included the area below the pelvis. Inguinal areas were defined as areas below the umbilicus to areas above the pelvis.
RESULTS
We selected articles containing any information about eschar in patients with scrub typhus. A total of 2,059 articles were screened, and the full texts of relevant articles were reviewed. We excluded 1,470 articles: 1,140 had no related information about eschar, 6 were duplicates, another 6 had no full text available while 318 articles were not scrub typhus related (Figure 1). Out of the 589 remaining articles, 101 were further excluded; 97 articles contained information on neither the prevalence rate nor the distribution of eschar, and 4 articles were not in English and could not be verified. We then divided the remaining articles into two subgroups: 1) articles including information about the distribution of eschar, and 2) articles including information about the prevalence rate of eschar. Finally, after further eligibility screening, 230 articles were included in group 1, while 228 articles were included in group 2.
The presence of eschar was reported in 19,535 out of 33,664 patients. The overall prevalence rate of eschar was 58.0% (Table 1). The greatest prevalence was in East Asia, which was 78.7%, followed by Oceania 52.2%, Southeast Asia 41.4%, and then the lowest in South Asia 32.8% (Table 1).
Table 1.
Eschar prevalence rates in the countries classified according to region
| Region | Country | Patients with eschar (n) | Patients with scrub typhus (n) | Rate (%) |
|---|---|---|---|---|
| East Asia | China | 1,799 | 2,086 | 86.2 |
| Japan | 590 | 672 | 87.8 | |
| Korea | 9,997 | 12,831 | 77.9 | |
| Taiwan | 1,051 | 1,495 | 70.3 | |
| Sub total | 13,437 | 17,084 | 78.7 | |
| Southeast Asia | Laos | 111 | 340 | 32.6 |
| Malaysia | 38 | 209 | 18.2 | |
| Myanmar | 95 | 159 | 59.7 | |
| Philippine | 120 | 276 | 43.5 | |
| Thailand | 981 | 2,621 | 37.4 | |
| Vietnam | 379 | 556 | 68.2 | |
| Sub total | 1,724 | 4,161 | 41.4 | |
| South Asia | Bhutan | 6 | 12 | 50.0 |
| India | 3,362 | 10,098 | 33.3 | |
| Nepal | 33 | 502 | 6.6 | |
| Sri Lanka | 178 | 283 | 62.9 | |
| Sub total | 3,579 | 10,895 | 32.8 | |
| Oceania | Australia | 65 | 128 | 50.8 |
| New Guinea | 730 | 1,381 | 52.9 | |
| Micronesia | 0 | 15 | 0.0 | |
| Sub total | 795 | 1,524 | 52.2 | |
| Overall | 19,535 | 33,664 | 58.0 |
In East Asia, we identified South Korea had the highest number of scrub typhus patients, whereas Japan had the highest prevalence of eschar (87.8%). In Southeast Asia, Vietnam had the highest (68.2%) and Malaysia had the lowest prevalence (18.2%). In South Asia, Sri Lanka had the highest (62.9%) and Nepal had the lowest prevalence (6.6%). The eschar prevalence rate among countries was similar in Oceania outside of Micronesia (50.8–52.9%).
We identified the frequency of eschar distribution was the highest in the inguinal area (28.5%) and the anterior area (28.0%), followed by that in the lower extremities (22.0%) (Table 2). The upper extremities (8.9%), back (6.6%), and H/N (6.1%) regions had relatively low frequencies of distribution.
Table 2.
Eschar distribution rate on patient diagnosed with scrub typhus across the different countries
| Region | Country | H & N | Upper Ext. | Lower Ext. | Anterior | Back | Inguinal area | Total |
|---|---|---|---|---|---|---|---|---|
| East Asia | China | 22 (4.4) | 30 (6.0) | 26 (5.2) | 145 (29.0) | 13 (2.6) | 264 (52.8) | 500 (7.9) |
| Hong Kong | 1 (2.6) | 5 (12.8) | 10 (25.6) | 7 (17.9) | 2 (5.1) | 14 (35.9) | 39 (0.6) | |
| Japan | 37 (6.9) | 99 (18.4) | 128 (23.8) | 147 (27.3) | 41 (7.6) | 86 (16.0) | 538 (8.5) | |
| Korea | 193 (6.1) | 299 (9.5) | 676 (21.5) | 855 (27.2) | 265 (8.4) | 857 (27.2) | 3,145 (49.6) | |
| Taiwan | 24 (12.8) | 24 (12.8) | 11 (5.9) | 69 (36.7) | 19 (10.1) | 41 (21.8) | 188 (3.0) | |
| Sub total | 277 (6.3) | 457 (10.4) | 851 (19.3) | 1,223 (27.7) | 340 (7.7) | 1,262 (28.6) | 4,410 (69.5) | |
| Southeast Asia | Burma | 1 (7.1) | 1 (7.1) | 0 (0.0) | 5 (35.7) | 1 (7.1) | 6 (42.9) | 14 (0.2) |
| Laos | 1 (7.7) | 2 (15.4) | 0 (0.0) | 6 (46.2) | 2 (15.4) | 2 (15.4) | 13 (0.2) | |
| Malaysia | 2 (10.5) | 3 (15.8) | 1 (5.3) | 9 (47.4) | 0 (0.0) | 4 (21.1) | 19 (0.3) | |
| Singapore | 0 (0.0) | 0 (0.0) | 1 (50.0) | 0 (0.0) | 0 (0.0) | 1 (50.0) | 2 (0.03) | |
| Thailand | 6 (6.9) | 7 (8.0) | 11 (12.6) | 25 (28.7) | 9 (10.3) | 29 (33.3) | 87 (1.4) | |
| Vietnam | 1 (2.6) | 0 (0.0) | 21 (55.3) | 12 (31.6) | 0 (0.0) | 4 (10.5) | 38 (0.6) | |
| Sub total | 11 (6.4) | 13 (7.5) | 34 (19.7) | 57 (32.9) | 12 (6.9) | 46 (26.6) | 173 (2.7) | |
| South Asia | India | 71 (9.2) | 37 (4.8) | 58 (7.5) | 326 (42.2) | 38 (4.9) | 242 (31.3) | 772 (12.2) |
| Nepal | 0 (0.0) | 1 (50.0) | 0 (0.0) | 0 (0.0) | 1 (50.0) | 0 (0.0) | 2 (0.03) | |
| Sri Lanka | 1 (16.7) | 0 (0.0) | 0 (0.0) | 4 (66.7) | 0 (0.0) | 1 (16.7) | 6 (0.1) | |
| Sub total | 72 (9.2) | 38 (4.9) | 58 (7.4) | 330 (42.3) | 39 (5.0) | 243 (31.2) | 780 (12.3) | |
| Oceania | Australia | 3 (5.0) | 3 (5.0) | 9 (15.0) | 21 (35.0) | 1 (1.7) | 23 (38.3) | 60 (0.9) |
| New Guinea | 19 (2.2) | 49 (5.8) | 420 (49.6) | 119 (14.1) | 26 (3.1) | 213 (25.2) | 846 (13.3) | |
| Sub total | 22 (2.4) | 52 (5.7) | 429 (47.4) | 140 (15.5) | 27 (3.0) | 236 (26.0) | 906 (14.3) | |
| The other | Undefined | 3 (4.0) | 2 (2.7) | 23 (30.7) | 24 (32.0) | 3 (4.0) | 20 (26.7) | 75 (1.2) |
| Overall | 385 (6.1) | 562 (8.9) | 1,395 (22.0) | 1,774 (28.0) | 421 (6.6) | 1,807 (28.5) | 6,344 |
H & N = head and neck; Ext = extremity. Undefined means that cannot be specified as one country, like “Africa” or “Vietnam and Cambodia.”
By the different regions, we identified the eschar distribution was higher in the lower extremities in Oceania (47.4%) but lower (7.4%) in South Asia than in other regions (Table 2). There was no noticeable difference between the regional eschar distribution and total eschar distribution.
DISCUSSION
In this study, the overall eschar prevalence rate was 58.0%. There were distinct differences by region, of which the highest was in East Asia (78.7%), whereas the lowest was in South Asia (32.8%). The eschar location was the highest in the inguinal area (28.5%) and anterior (28.0%), and the lowest in the H/N (6.1%).
The primary eschar is the initial site of rickettsial invasion and bites by an infected mite.4,6,10 Identification of the eschar helps diagnose scrub typhus.6,7 However, the eschar lesions often do not present an itching sensation and pain. Therefore, identification of the lesion becomes difficult. In addition, an eschar is similar to a scab formed after trauma, and its size may be small, which also hinders the detection of eschar in many cases.3 In addition, the prevalence of eschar varies from country to country, which means the effectiveness of using eschar to diagnose scrub typhus also varies from country to country.
In this study, we identified the differences in eschar prevalence across different regions and countries. Skin color in different regions may affect the different identification rates of the eschar, and immunity levels in different areas of the patients could also attribute to the prevalence. Furthermore, the different genotypes of Orientia tsutsugamushi which includes Gilliam, Karp, and Kato,28 and different types of mites acting as vectors for O. tsutsugamushi may affect our findings.4,12–17,26,29 We identified a high prevalence rate of eschar in the far eastern countries such as South Korea, Japan, and China, and a low prevalence in South Asian countries such as India and Nepal.15–17,26,30–32 This finding suggests that in the countries where there is a high prevalence of eschar, identification of eschar lesions may be important for the diagnosis of scrub typhus. However, in countries with a low prevalence of eschar, absence of eschar cannot be used to exclude the diagnosis of scrub typhus. We identified the anterior chest walls including the axillar and inguinal areas were the most commonly reported areas for eschar (28.0% and 28.5%, respectively), followed by lower extremities (22.0%). This is similar to the findings of some previous studies.6,23–25
Having the knowledge of the eschar distribution is useful to clinicians for the early identification and treatment of patients with scrub typhus. However, there is a lack of study to systematically assess the prevalence rate and distribution of eschar in patients with scrub typhus.
LIMITATIONS
This study has several limitations. First, publication bias could affect the prevalence rate of eschar. For example, the Republic of Palau is an endemic country for scrub typhus, Micronesia.33 However, the published article related to our subject of interest could not have been identified. Second, articles included in our review varied by different demographics of the patients such as age, sex and comorbidities, and genotype of the pathogen which were not possible to be included in the analysis. Further study is warranted to identify the different prevalence of eschar and body distribution by age, gender and comorbidities as well as the genotype. Third, the method of diagnosis reported in the reviewed articles varied by country and region.
CONCLUSION
In conclusion, the overall eschar prevalence was 58.0%, the highest in East Asia (78.7%) and the lowest in South Asia (32.8%). The anterior chest walls including axillar and inguinal areas were the most common areas of eschar occurrence, whereas the H/N and back areas were the least affected. For early diagnosis of scrub typhus, clinicians should investigate the commonly reported eschar location areas with respect to the region of the patient.
REFERENCES
- 1. Walker DH , 2016. Scrub typhus—scientific neglect, ever-widening impact. N Engl J Med 375: 913–915. [DOI] [PubMed] [Google Scholar]
- 2. Paris DH Shelite TR Day NP Walker DH , 2013. Unresolved problems related to scrub typhus: a seriously neglected life-threatening disease. Am J Trop Med Hyg 89: 301–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Watt G Parola P , 2003. Scrub typhus and tropical rickettsioses. Curr Opin Infect Dis 16: 429–436. [DOI] [PubMed] [Google Scholar]
- 4. Rajapakse S Weeratunga P Sivayoganathan S Fernando SD , 2017. Clinical manifestations of scrub typhus. Trans R Soc Trop Med Hyg 111: 43–54. [DOI] [PubMed] [Google Scholar]
- 5. Kelly DJ Fuerst PA Ching WM Richards AL , 2009. Scrub typhus: the geographic distribution of phenotypic and genotypic variants of Orientia tsutsugamushi. Clin Infect Dis 48 (Suppl 3): S203–S230. [DOI] [PubMed] [Google Scholar]
- 6. Kim DM et al. 2007. Distribution of eschars on the body of scrub typhus patients: a prospective study. Am J Trop Med Hyg 76: 806–809. [PubMed] [Google Scholar]
- 7. Lee CS Hwang JH , 2015. Images in clinical medicine. Scrub typhus. N Engl J Med 373: 2455. [DOI] [PubMed] [Google Scholar]
- 8. Parola P et al. 2013. Update on tick-borne rickettsioses around the world: a geographic approach. Clin Microbiol Rev 26: 657–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kundavaram AP Jonathan AJ Nathaniel SD Varghese GM , 2013. Eschar in scrub typhus: a valuable clue to the diagnosis. J Postgrad Med 59: 177–178. [DOI] [PubMed] [Google Scholar]
- 10. Xu G Walker DH Jupiter D Melby PC Arcari CM , 2017. A review of the global epidemiology of scrub typhus. PLoS Negl Trop Dis 11: e0006062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Rajapakse S Rodrigo C Fernando D , 2012. Scrub typhus: pathophysiology, clinical manifestations and prognosis. Asian Pac J Trop Med 5: 261–264. [DOI] [PubMed] [Google Scholar]
- 12. Hamaguchi S et al. 2015. Clinical and epidemiological characteristics of scrub typhus and murine typhus among hospitalized patients with acute undifferentiated fever in northern Vietnam. Am J Trop Med Hyg 92: 972–978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kim DM et al. 2011. Differences in clinical features according to boryoung and karp genotypes of Orientia tsutsugamushi. PLOS ONE 6: e22731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Weng SC Lee HC Chen JJ Cheng YJ Chi H Lin CY , 2017. Eschar: a stepping stone to scrub typhus. J Pediatr 181: 320–320.e1. [DOI] [PubMed] [Google Scholar]
- 15. Ogawa M et al. 2002. Scrub typhus in Japan: epidemiology and clinical features of cases reported in 1998. Am J Trop Med Hyg 67: 162–165. [DOI] [PubMed] [Google Scholar]
- 16. Silpapojakul K Chupuppakarn S Yuthasompob S Varachit B Chaipak D Borkerd T Silpapojakul K , 1991. Scrub and murine typhus in children with obscure fever in the tropics. Pediatr Infect Dis J 10: 200–203. [DOI] [PubMed] [Google Scholar]
- 17. Jamil M Lyngrah KG Lyngdoh M Hussain M , 2014. Clinical manifestations and complications of scrub typhus: a hospital based study from north eastern India. J Assoc Physicians India 62: 19–23. [PubMed] [Google Scholar]
- 18. Zhao M Wang T Yuan X Du W Lin M Shen Y , 2016. Comparison of minocycline and azithromycin for the treatment of mild scrub typhus in northern China. Int J Antimicrob Agents 48: 317–320. [DOI] [PubMed] [Google Scholar]
- 19. Irons EM Armstrong HE , 1947. Scrub typhus in Dutch New Guinea. Ann Intern Med 26: 201–220. [DOI] [PubMed] [Google Scholar]
- 20. Traub R Wisseman CL Jr , 1974. The ecology of chigger-borne rickettsiosis (scrub typhus). J Med Entomol 11: 237–303. [DOI] [PubMed] [Google Scholar]
- 21. Thipmontree W Tantibhedhyangkul W Silpasakorn S Wongsawat E Waywa D Suputtamongkol Y , 2016. Scrub typhus in northeastern Thailand: eschar distribution, abnormal electrocardiographic findings, and predictors of fatal outcome. Am J Trop Med Hyg 95: 769–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wang YC Chen PC Lee KF Wu YC Chiu CH , 2013. Scrub typhus cases in a teaching hospital in Penghu, Taiwan, 2006–2010. Vector Borne Zoonotic Dis 13: 154–159. [DOI] [PubMed] [Google Scholar]
- 23. Rose W Rajan RJ Punnen A Ghosh U , 2016. Distribution of eschar in pediatric scrub typhus. J Trop Pediatr 62: 415–420. [DOI] [PubMed] [Google Scholar]
- 24. Park JH Kim SJ Youn SK Park K Gwack J , 2014. Epidemiology of scrub typhus and the eschars patterns in South Korea from 2008 to 2012. Jpn J Infect Dis 67: 458–463. [DOI] [PubMed] [Google Scholar]
- 25. Munegowda KC Nanda S Varma M Bairy I Vidyasagar S , 2014. A prospective study on distribution of eschar in patients suspected of scrub typhus. Trop Doct 44: 160–162. [DOI] [PubMed] [Google Scholar]
- 26. Le Viet N Laroche M Thi Pham HL Viet NL Mediannikov O Raoult D Parola P , 2017. Use of eschar swabbing for the molecular diagnosis and genotyping of Orientia tsutsugamushi causing scrub typhus in Quang Nam Province, Vietnam. PLoS Negl Trop Dis 11: e0005397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Division UNS , 2021. Available at: https://unstats.un.org/unsd/classifications/. Accessed December 21, 2021.
- 28. Kelly DJ Fuerst PA Richards AL , 2019. Origins, importance and genetic stability of the prototype strains Gilliam, Karp and Kato of Orientia tsutsugamushi. Trop Med Infect Dis 4: 75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Taylor AJ Paris DH Newton PN , 2015. A systematic review of mortality from untreated scrub typhus (Orientia tsutsugamushi). PLoS Negl Trop Dis 9: e0003971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sirisanthana V Puthanakit T Sirisanthana T , 2003. Epidemiologic, clinical and laboratory features of scrub typhus in thirty Thai children. Pediatr Infect Dis J 22: 341–345. [DOI] [PubMed] [Google Scholar]
- 31. Gautam R Parajuli K Sherchand JB , 2019. Epidemiology, risk factors and seasonal variation of scrub typhus fever in Central Nepal. Trop Med Infect Dis 4: 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Dorji K Phuentshok Y Zangpo T Dorjee S Dorjee C Jolly P Morris R Marquetoux N McKenzie J , 2019. Clinical and epidemiological patterns of scrub typhus, an emerging disease in Bhutan. Trop Med Infect Dis 4: 56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Durand AM Kuartei S Togamae I Sengebau M Demma L Nicholson W O’Leary M , 2004. Scrub typhus in the Republic of Palau, Micronesia. Emerg Infect Dis 10: 1838–1840. [DOI] [PMC free article] [PubMed] [Google Scholar]