Abstract
A small-volume sentinel chamber was developed to assess the effects of environmental stresses on survival of sucrose-Percoll-purified Cryptosporidium parvum oocysts in soil and animal wastes. Chambers were tested for their ability to equilibrate with external chemical and moisture conditions. Sentinel oocysts were then exposed to stresses of the external environment that affected their viability (potential infectivity), as indicated by results of a dye permeability assay. Preliminary laboratory experiments indicated that temperatures between 35 and 50°C and decreases in soil water potential (−0.003 to −3.20 MPa) increased oocyst inactivation rates. The effects of two common animal waste management practices on oocyst survival were investigated on three dairy farms in Delaware County, N.Y., within the New York City watershed: (i) piling wastes from dairy youngstock (including neonatal calves) and (ii) spreading wastes as a soil amendment on an agricultural field. Sentinel containers filled with air-dried and sieved (2-mm mesh) youngstock waste or field soil were wetted and inoculated with 2 million oocysts in an aqueous suspension and then placed in waste piles on two different farms and in soil within a cropped field on one farm. Controls consisted of purified oocysts in either phosphate-buffered saline or distilled water contained in sealed microcentrifuge tubes. Two microdata loggers recorded the ambient temperature at each field site. Sentinel experiments were conducted during the fall and winter (1996 to 1997) and winter (1998). Sentinel containers and controls were removed at 2- to 4-week intervals, and oocysts were extracted and tested by the dye permeability assay. The proportions of potentially infective oocysts exposed to the soil and waste pile material decreased more rapidly than their counterpart controls exposed to buffer or water, indicating that factors other than temperature affected oocyst inactivation in the waste piles and soil. The effect of soil freeze-thaw cycles was evident in the large proportion of empty sentinel oocysts. The potentially infective sentinel oocysts were reduced to <1% while the proportions in controls did not decrease below 50% potentially infective during the first field experiment. Microscopic observations of empty oocyst fragments indicated that abrasive effects of soil particles were a factor in oocyst inactivation. A similar pattern was observed in a second field experiment at the same site.
Several large-scale outbreaks of cryptosporidiosis caused by contaminated drinking water supplies (19, 23) have increased concern about sources of Cryptosporidium parvum oocysts in watersheds, especially regarding options for preventing raw water contamination. As discussed by Walker et al. (29), the potential for nonpoint sources of infective oocysts to contaminate source waters is difficult to assess because neither survival nor transport of oocysts in watershed environments has been examined outside of laboratory settings. Several types of exposure chambers have been developed and tested to assess effects of environmental stresses on microorganisms in natural waters and manure slurries (21, 25). However, these are not readily adapted for studies of oocyst inactivation in solid media such as soils or animal waste material because of the large volumes of the chambers, which would require large numbers of oocysts for replication, and a lack of confidence about the degree and uniformity of exposure they could provide when filled with solid material.
Recent improvements in assay methods for oocyst viability or potential animal infectivity (7, 16) and for extracting oocysts from soil (30) have provided techniques to support field studies assessing environmental factors involved in common agricultural practices, such as storage and spreading of animal wastes in agricultural areas. Among the practices of greatest concern, especially in watersheds for municipal drinking water supplies, are those that may lead to concentration and/or dispersal of wastes from animal populations most susceptible to C. parvum infection, e.g., neonatal calves on dairy or beef farms (14), which can shed billions of oocysts during an infection (5). While the spreading of mixed wastes, i.e., feces, urine, bedding, and waste feed, as a soil amendment is widespread in dairy farming regions, the potential for water supply contamination from this practice is not well investigated.
An increasing number of reports indicate several environmental parameters that may affect the survival of oocysts in watershed environments as measured in the laboratory, e.g., temperature extremes (11, 13, 16), pH, desiccation (1, 24), and exposure to ammonia (17). Data are lacking, however, that indicate the survival dynamics of oocysts under actual field conditions. In this study a sentinel container system was developed to expose small volumes of material containing viable C. parvum oocysts to ambient stresses in soils and static piles of animal wastes with adequate replication and controls. This sentinel system was designed to contain oocysts in soil or mixed animal waste solids, to prevent release of oocysts into the environment, to equilibrate with the solute and moisture regime of the external environment, and to be easily retrievable and processed for analysis by a dye permeability viability assay. We report a 2-year study of the survival kinetics of oocysts in field settings on three New York City watershed farms where the wastes from neonatal calves were being managed as part of normal dairy operations.
MATERIALS AND METHODS
Oocyst purification.
Feces from infected 6- to 14-day-old Holstein calves were processed by continuous-flow differential density flotation, as previously described (16). After a final Percoll purification step, oocysts were washed by centrifugation three times in cold distilled water at 2,100 × g for 10 min to remove the Percoll, adjusted with distilled water to a concentration of 107 ml−1, and stored at 4°C in suspension with 100 U of penicillin G sodium ml−1, 100 μg of streptomycin sulfate ml−1, and 0.25 μg of amphotericin B ml−1. The oocysts in each lot were enumerated in a hemocytometer and tested by dye permeability assay (see below) after purification and periodically during storage.
Dye permeability assay.
The details of this assay have been described elsewhere (2, 7, 16). Further details of its applicability as an indicator of viability or potential for animal infectivity and thus the ability to reproduce have been previously discussed (26). Viable oocysts are the sum of 4′,6-diamidino-2-phenylindole-negative (DAPI−) propidium iodide-negative (PI−) oocysts and DAPI+ PI− oocysts; DAPI+ PI+ oocysts are considered inactivated. Empty oocyst shells are also counted in this assay.
Sentinel chamber.
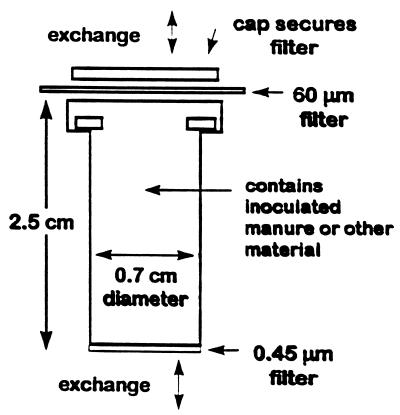
The basket of a commercially produced microfiltration system (2.5 cm long, with an internal diameter of 0.7 cm; Osmonics, Livermore, Calif.) with a nylon 0.45-μm-pore-size filter encased in one end was the principle component of the sentinel chamber. To maximize exposure and equilibration, the cap was perforated and used to secure a 60-μm nylon mesh filter (Spectra/ Mesh; Markson, Inc., Hillsboro, Oreg.) at the top end to contain solid materials (Fig. 1). The chamber was filled with solid material (such as soil or mixed animal waste), inoculated with a suspension of purified oocysts, and then placed in a natural environment (soil or animal waste pile as described below) for later recovery and analysis. The chamber was designed to contain a representative sample of moist soil or waste with a consistent manageable size to facilitate processing and analysis. Other design goals were to prevent environmental contamination by oocyst leakage, and to achieve adequate independent replicates for field experiments.
FIG. 1.
Sentinel chamber used in field experiments.
Containment.
The chamber was designed to be oriented vertically in animal waste piles and surface soil. The chamber was tested for its ability to prevent release of oocysts by testing the 0.45-μm-pore-size filter. To 10 chambers, 100-μl aliquots of purified oocysts (106 oocysts) were added. The chambers were placed in microcentrifuge tubes, and a centrifugal force of 200 × g was applied for 3 min. A 15-μl aliquot of 1% Noble agar (Sigma Chemical Co., St. Louis, Mo.) was added to each filtrate. Two 50-μl aliquots from each of the filtrate-Noble agar mixes were then placed in wells of a microscope slide, stained with Hydrofluor Combo fluorescent antibody according to the manufacturer’s instructions, and examined at a magnification of ×630. Because oocysts were to be inoculated into a solid medium and oocysts are not motile and do not replicate outside of a host, it was assumed that in the environment of an animal waste pile or surface soil, there would not be a flux of water that would allow oocyst to be transported out the 60-μm mesh secured at the top of the chamber.
Chemical equilibration.
A test of the ability of the filter membrane to equilibrate with an external source of environmentally relevant chemicals was designed by using the lethal effects of aqueous ammonia (17). Aliquots (750 μl each) of a suspension of viable oocysts in distilled water were pipetted into an empty sentinel container (1 million oocysts/container). The container without the 60-μm mesh was sealed at the top with the cap of a microcentrifuge tube. The chambers were then immersed in either commercial (Wegman’s) ammonia or distilled water as a control. In another control, 100 μl of oocysts (1 million μl−1) were exposed to 1 ml of commercial ammonia contained and sealed in a microcentrifuge tube. At 4, 8, 16, 21, and 24 h, duplicate samples were removed from chambers and tubes, washed three times in phosphate-buffered saline (PBS) (0.028 M NaH2PO4 · H2O, 0.072 M Na2HPO4, 0.145 M NaCl [pH 7.2]), and examined by the dye permeability assay.
Soil moisture equilibration.
Soil from the land spreading site used for field trials (see below) was oven dried at 105°C, sieved (2-mm mesh), and placed in a stainless steel pan (18 by 30 cm) to a depth of 5 cm. The soil was saturated with distilled water and allowed to equilibrate for 1 day at 24 ± 1°C. Sentinel chambers were tared, filled with the same soil, capped, and placed, 0.45-μm-pore-size filter down, in a shallow reservoir of distilled water to allow water to wick up and equilibrate with the soil column inside the chamber for 24 h and thus achieve approximate field capacity. After the chambers were reweighed they were inserted to the cap (0.45-μ-pore-size filter down) into soil in the pan. At intervals of approximately 24 h, sentinel chambers and samples from the surrounding wetted soil were removed randomly for gravimetric water content determination by drying at 105°C for 12 h. The bulk soil in the pan was rewetted with 200 ml of distilled water at 49.5, 146.5, and 243.5 h. The test was terminated after 333.5 h.
Temperature experiments.
Temperatures up to 55°C may be sustained for 24 h within active compost piles (27). To assess the effects of the range of ambient temperatures that may be generated in the waste piles, 1-ml suspensions of purified oocysts (106 ml−1) in distilled water were incubated in microcentrifuge tubes at 30, 35, 40, 45, and 50°C for 5 days. Duplicate samples were removed at 24-h intervals, concentrated to 0.1 ml by centrifugation, and analyzed by the dye permeability assay. Duplicate 10-μl subsamples of stained oocysts were examined microscopically, and a minimum of 100 oocysts per subsample was characterized.
Soil water potential experiments.
Desiccation has been shown to be lethal for oocysts under laboratory conditions (1, 24). However, the effects of various water potentials as a salient soil characteristic (15) on the survival of oocysts in a soil matrix have not been investigated. To assess the possible effects of various soil water potentials on oocyst inactivation, a moisture release curve for the field soil was developed by the Soil Testing Laboratory, Department of Soils, Crops, and Atmospheric Sciences, at Cornell University (by comparing soil gravimetric water content to soil water potential). Values for soil water potential were based on this soil moisture release curve and determined by the linear regression equation (r2 = 0.962) y = −5.028x + 26.002, where y is the gravimetric water content and x is the natural log of the pressure plate measurements. Differences in soil water potential attributable to hysteresis are considered negligible (15, 28). Approximately 1 g of oven-dried soil (prepared as described above for soil moisture equilibration) was added to microcentrifuge tubes and wetted by injecting distilled water to achieve a range of predetermined final soil moisture contents, which included 25 μl of water from an oocyst suspension that was added after the original wetted contents in the sealed tube had equilibrated for 24 h at 24 ± 1°C. A range of soil water contents representing 100 to 20% of the soil’s approximate field capacity, or water-holding capacity, were given as follows as percent gravimetric soil moisture content (water potential): 43.1% (−0.003 MPa), 34.5% (−0.018 MPa), 25.9% (−0.100 MPa), 17.2% (−0.580 MPa), and 8.6% (−3.20 MPa). The inoculant suspension contained 2 × 106 oocysts in 25 μl of distilled water. The tubes were sealed and incubated in the dark at 4°C for 48 h and at 25°C for 20 days. Duplicate samples at each soil water potential were removed after 2, 4, 10, and 20 days of incubation. Oocysts were extracted, as described below, and analyzed by the dye permeability assay. Duplicate 10-μl subsamples per replicate of stained oocysts were examined microscopically, and a minimum of 100 oocysts per subsample was characterized.
Sentinel chamber preparation: waste material and soils.
Wastes from neonatal calves and youngstock were collected from two calf waste piles (described below) before installing inoculated sentinel chambers. Wastes were air-dried and sieved (2-mm-diameter openings). No oocysts were detected in the dried, sieved wastes before inoculation using the oocyst extraction procedure (below) and the microscopic methods described by Walker et al. (30).
Soils were prepared as described above and added to sentinel chambers. Before wetting and inoculating the sentinel chambers, they were tared, filled, enclosed with 60-μm-pore-size Spectra Mesh filter, and reweighed. Thus, water content (and soil water potential) determinations could be made after removing the sentinel containers from their respective field sites. With the 0.45-μm-pore-size filter down, the filled sentinels were placed in a reservoir of distilled water as described above and allowed to reach their water holding capacity. Before installing sentinel chambers in waste piles and field sites, a 100-μl aliquot of purified oocyst stock (2 × 107 ml−1) was injected into the wetted contents with a syringe.
Static animal waste piles.
The experiments were conducted from November 1996 to March 1997 and from January to May 1998 (sites A and B, respectively). At each site, wastes collected periodically from neonatal and youngstock housing were piled and allowed to accumulate. Wastes at site A were collected from individual calf hutches. Wastes from the site B were collected from stalls within a barn. At site A, the pile was semienclosed by a roof and three partial walls. At site B, the pile was unconfined and open to the elements. Throughout the experiments, wastes were added to both piles as part of normal farm operations. At the beginning of the experiment at site A, the pile contained approximately 18 m3 of accumulated wastes (approximately 4 by 3 by 1.5 m [width, depth, and height, respectively]). The pile at site B contained approximately 12 m3 of accumulated wastes (roughly hemispherical in shape [4-m diameter; 1.5-m depth]). At termination of the experiments, the volume of each pile was approximately 35 m3 (4 by 4 by 2.2 m [width, depth, and height, respectively]) for site A and 31 m3 (5-m base diameter, 2.2-m depth) for site B.
Sentinel chambers contained processed wastes from the sites and were inoculated with 2 × 106 oocysts; controls contained 2 × 106 oocysts suspended in PBS for site A and distilled water for site B and were sealed in 1.5-ml microcentrifuge tubes. Both sentinel chambers and controls were buried in the manure piles using 1.2-cm-diameter flexible polyvinyl chloride piping and nylon line. The piping and line ensured that samples could be removed with minimal disturbance at sampling intervals as the stacks grew in size. The sentinel containers were laid in a trough formed by cutting away the top portion of the polyvinyl chloride piping. This ensured that sentinels and controls would be directly exposed to the surrounding wastes. Replicate sample pods, each fitted with three sentinel chambers and three controls, were installed by excavating wastes from the top to the center of each pile. Ten sets of replicate sentinel chambers and controls were installed in each pile. Directly adjacent to them were placed two temperature data loggers (HOBO devices; Onset Computer Corporation, Pocasset, Mass.) to record the temperature at 4-h intervals for the duration of the experiment. After sentinel chambers, controls, and data loggers were in place, they were covered with the waste and left undisturbed until a single set of sentinel chambers and controls was removed by sliding a single pipe (or sample pod) out of the stack. At each sampling interval, three sentinel chambers and controls were removed from the piping and transported on ice to the laboratory for extraction of oocysts and dye permeability analysis.
Volatilized ammonia measurement at site B.
Following a protocol adapted from that of Dewes (10), two 14-cm-diameter petri plates (each containing 75 ml of 0.02 M H3PO4 solution) were secured to a wire test tube rack. At an area near the center of the pile and above the site of the sentinel containers, controls, and data loggers, an excavation was made so that the test tube rack with petri plates could be placed in a level position. The rack was then covered by an inverted plastic basin, and the H3PO4 solution was allowed to trap gaseous ammonia for 6 h. The phosphoric acid solution was then removed and analyzed for the presence of ammonia by the protocol described by Weatherburn (31). At the termination of the experiment at site B, a sample of waste was taken from the interior of the pile, where sentinel chambers and controls had been, and was transported in a sealed Whirl-Pak on ice to the Cornell Soil Testing Laboratory to determine available NH3 and pH in water. The sample was extracted with 2 M KCl and analyzed by an automated phenate method.
Field spreading site.
The field was on an active dairy farm in Delaware County, N.Y., where waste spreading regularly occurred for soil amendment as part of normal farming operations. Experiment 1 began on 13 November 1996 and ended 5 March 1997. Experiment 2 took place in the same field, within 50 m of the site of experiment 1, and began on 13 January 1998 and ended 7 May 1998. Termination occurred in each case when the dairy farmer prepared the field for planting corn. The soil was a silt loam; its particle size distribution was 33.4% sand, 50.3% silt, and 16.3% clay; the soil pHw was 7.04, and the level of soil organic matter was 7.04%. Soil analysis was performed by the Cornell Soil Testing Laboratory.
Before installing the sentinel chambers and controls at the field site, surface soil from the site was air dried, sieved, and put into a plastic frame with holes (to allow drainage) in a removable bottom as well as into the sentinel chambers themselves. The soil in sentinel chambers and that in the frame were wetted separately to approximately field capacity. Controls were 1.5-ml microcentrifuge tubes containing a 1-ml suspension of 2 × 106 oocysts in PBS for the first experiment and in distilled water for the second experiment. Wetted sentinel chambers were inoculated with 2 × 106 oocysts and placed along with controls into the wetted soil in the frame. The frame was transported on ice to the field site and installed in an excavation in the surface soil. Installation entailed placing the frame in the excavation, removing the bottom and sides, and gently tamping the soil around the filled excavation to make continuous contact with the soil containing the sentinel containers and controls, which were then pressed 1 cm below the surface. Two temperature data loggers (HOBO devices; Onset Computer Corporation) were buried 2 cm below the soil surface directly adjacent to the sentinel chambers and controls.
Oocyst extraction from soil and animal waste.
A protocol described by Walker et al. (30) was used to extract oocysts from both soil and waste pile sentinel containers. First, the 0.45-μm-pore-size filter was excised with a razor blade, and the cap and Spectra/Mesh were removed. Then, the whole container was placed in a 50-ml centrifuge tube containing 10 ml of a 0.1% solution of Tween 80 in PBS. The tubes were sealed and placed on a rotary shaker at 200 rpm for 20 min. The empty containers were removed, and the suspended material was underlaid with 10 ml of a cold (4°C) sucrose (specific gravity, 1.18) solution. The tubes and their contents were centrifuged at 1,500 × g for 20 min, and 10 ml of the material at the interface between the sucrose and Tween 80-PBS solutions was removed carefully with a 20-ml syringe fitted with an 18-gauge needle. The syringe contents were transferred to another 50-ml centrifuge tube, further diluted with PBS to 50 ml, and centrifuged at 1,500 × g for 30 min. The majority of the supernatant was aspirated off, leaving 1 ml of supernatant above the pellet. The pellet was resuspended, transferred to a 1.5-ml microcentrifuge tube, and sedimented in a microcentrifuge at 11,300 × g for 1 min. The supernatant was discarded, and the pellet was resuspended in 100 μl of PBS and stained for viability as described above.
The efficiency of oocyst extraction from waste and soil samples was determined by counting extracted oocysts that had been stained with Hydrofluor antibody by using a Neubauer-Levy-Hausser counting chamber as previously described (16). Recovery from wastes was determined for four sentinel chambers removed from the waste stack at site A on the last day of sampling (day 145). The efficiency of oocyst extraction from soils was determined with triplicate 1-g soil samples maintained (by sealing in a 1.5-ml microcentrifuge tube) at a soil water potential of −0.033 MPa (based on the soil water release curve) and incubated at 24 ± 1°C for 10 days. Extraction efficiency determinations were also done on six sentinel containers that had been removed from the field spreading site on day 113 and allowed to reach a soil water content that ranged from 4.2 to 7.9% (a soil water potential that ranged from −3.60 to −7.70 MPa).
Microscopy.
All samples were examined with a Zeiss LSM-210 microscope in conventional differential interference microscopy (DIC) and epifluorescence mode as described previously (2, 16). A triple filter set (Catalog no. 61001; Chroma Technology Corp., Brattleboro, Vt.) with excitation bands at 390 to 410 nm, 485 to 510 nm, and 555 to 585 nm and emission bands at 450 to 475 nm, 510 to 550 nm, and 595 to 600 nm was used for fluorescein isothiocyanate and PI, and a separate filter combination (excitation bands at 310 to 395 nm) was used for DAPI. A Zeiss 100×/1.3 Plan-Neofluar DIC objective with 10× eyepieces was used for all microscopy procedures except for enumerations with the Neubauer-Levy-Hausser counting chamber, which were done with a Zeiss 40×/0.85 Plan-Neofluar DIC dry objective lens. A Zeiss 63×/1.40 oil Plan-Apochromat objective lens was used for the containment experiment described above. Concentrations of purified oocyst stocks were determined with a hemocytometer and a Nikon EPlan 20×/0.4 oil objective on a Nikon Labophot with 10× eyepieces.
Statistical analysis.
Except where otherwise stated, all statistical analyses of data were performed with Minitab (State College, Pa.) Statistical Software (release 8.21 accelerated.)
RESULTS
Sentinel chamber chemical and soil moisture equilibration.
Based on first-order rate kinetics, the coefficient of inactivation (K) for the aqueous suspension of oocysts in the sentinel containers that were exposed to commercial ammonia was 0.017 h−1 (r2 = 0.90). This K value was less than but not significantly different (at P = 0.05) from the coefficient of inactivation (K = 0.022 h−1; r2 = 0.99) of oocysts directly exposed to the commercial ammonia. There was no significant change in viability of oocysts in the sentinel chambers that were exposed to distilled water. The comparable K values of oocysts in the sentinel chambers and oocysts directly exposed to commercial ammonia indicated that equilibrium between the interior of the chamber and the exterior environment occurred. The rates of oocyst inactivation appeared to correspond to concentrations of ammonia of >120 and <440 mg liter−1 (17).
The sentinel chambers equilibrated with ambient soil moisture conditions of the bulk soil water. The results of the laboratory experiments indicated that change in soil water content of sentinel chambers mimicked fluctuations in bulk soils (data not shown). The gravimetric soil water contents [mean ± standard deviation] %) of three replicate sentinel chambers removed from the first field experiment on days 27 and 113 were (54.1 ± 1.8)% and (48.7 ± 1.45)%, respectively, which were somewhat greater than the external bulk soil gravimetric water content, which were (45.2 ± 0.00)% and (45.3 ± 3.05)%, respectively. The apparent greater water content of the sentinel chambers was believed to be associated with water retained in the filters and soil adhering to external parts of the container. These values of soil moisture indicated that equilibrium between the internal contents of the chambers and the external soil environment occurred in the field.
Effects of moderate temperatures.
Purified oocysts were subjected to a range of temperatures (30 to 50°C) that can occur in composting animal waste piles. Over the 5 days of the experiment, the rates of inactivation increased with each 5°C increase above 30°C (data not shown). At 30°C there was no significant change over the 5-day period. Based on the results of the dye permeability assay, coefficients of inactivation (K) (16) were (mean ± 95% confidence interval) 0.140 ± 0.041, 0.442 ± 0.071, 0.986 ± 0.270, and 3.840 ± 0.653 for exposure to 35, 40, 45, and 50°C, respectively. These K values indicated that temperatures between 35 and 50°C may inactivate 99.999% of viable oocysts in approximately 82 to 3 days, respectively.
Effects of soil water potential.
At 4°C, at least for a period of 2 days, the soil water potential of −3.20 MPa had little effect on oocyst survival. In contrast, oocysts exposed to soil water potentials of ≤−0.580 MPa, at 25°C, showed a significant decrease in viability after 2 days. Exposure to soil water potentials of −0.100 MPa for 20 days showed a significant decrease in oocyst viability, with rates of inactivation increasing with further decreases in the soil water potential (data not shown), i.e., as the soil water content decreased. The soil water potentials nearest the field capacity of the soil (>−0.100 MPa) negligibly affected potential infectivity over the 20-day period of the experiment. Even at the most negative soil water potential (−3.20 MPa) 20% of oocysts survived 20 days of exposure. There was also a 20% increase in the proportion of semipermeable DAPI+ PI− oocysts at the 10-day sampling time. Oocyst inactivation resulting from exposure to low soil water potential appeared to be a first-order process; point estimates ± 95% confidence intervals for estimated coefficients of inactivation (16) attributable to soil water potential alone at 25°C were (day−1) 0.014 ± 0.003, 0.246 ± 0.054, and 0.416 ± 0.196 for oocysts exposed to soil water potentials of −0.100, −0.580, and −3.20 MPa, respectively. The calculated time to reach 99.999% inactivation for each K value for the lowest two soil water potentials, −0.580 and −3.20 MPa, was 47 and 22 days, respectively.
Extraction efficiency and inactivation of sentinel oocysts in the animal waste piles.
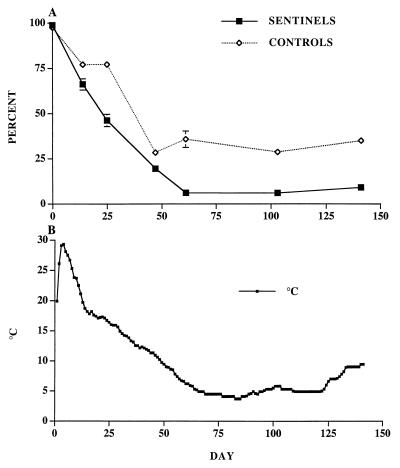
Extraction efficiency of oocysts from inoculated wastes ranged from 52.8 to 90.5%, with a mean of 73.1% and coefficient of variation of 0.21. The rate of inactivation for sentinel oocysts in both manure piles was significantly greater than that observed for control oocysts (Fig. 2 and 3). Differences in internal temperature distinguished the two piles. Site A had an initial core temperature of 30°C at the time the sentinel chambers were buried, and temperature declined steadily to less than 10°C as winter weather prevailed (Fig. 2). At site B the pile (Fig. 3) maintained a higher core temperature than at site A. Internal temperature fluctuated from 20 to 32°C, with temperatures exceeding 30°C for a sustained period of days.
FIG. 2.
Oocyst survival and temperatures recorded for the manure pile at site A (November 1996 to May 1997). (A) Viability (potential infectivity) of sentinel and control oocysts that were exposed to the interior environment of the calf manure pile as determined by the dye permeability assay. Each point represents the mean percentage sum of DAPI− PI− and DAPI+ PI− ± standard error of three replicates and two subsamples of each replicate, for a total of six subsamples with 100 oocysts characterized at random per subsample. (B) Average daily temperature of the interior of the manure pile where sentinels and controls were situated.
FIG. 3.
Oocyst survival and temperatures recorded for the manure pile at site B (January 1998 to June 1998). (A) Viability (potential infectivity) of sentinel and control oocysts that were exposed to the interior environment of the calf manure pile as determined by the dye permeability assay. Each point represents the mean percentage sum of DAPI− PI− and DAPI+ PI− ± standard error of three replicates and two subsamples of each replicate, for a total of six subsamples with 100 oocysts characterized at random per subsample. (B) Average daily temperature of the interior of the manure pile where sentinels and controls were situated.
No volatilized NH3 was trapped by phosphoric acid; analysis of the waste sample indicated that 13 mg of NH3 liter−1 was present. The pH of the waste sample in water was 9.1. This pH level indicates that based on the equation pOH = pKb + log([NH4]/[NH3]) (8), where pKb is the base disassociation constant, the NH3 concentration was in equilibrium with an NH4 concentration of ∼18 mg liter−1 and was in aqueous solution at the temperatures recorded (22).
Another principle difference between the two waste piles was indicated by the results of the dye permeability assay applied to sentinel oocysts. At site A significant increases were detected in the proportions of DAPI+ PI+ (inactivated) oocysts, and corresponding decreases in viable DAPI− PI− oocysts compared to controls (data not shown); whereas at site B the proportion of sentinel oocysts classified as empty increased significantly compared to controls, which had proportions of dye categories similar to the controls from site A. The proportions of empty oocysts in the sentinel population observed at site B reached 71% (Fig. 4).
FIG. 4.
Results of the dye permeability assay on sentinel and control oocysts from the calf manure pile at site B.
Sentinel oocysts exposed to surface soil in the field.
Recovery efficiency of oocysts from the soil at −0.033 MPa after 10 days of incubation at 24 ± 1°C was 90.1 ± 0.025%. In contrast recoveries from soil in sentinel containers that had been exposed to freeze-thaw cycles (Fig. 5B) and whose soil water potentials ranged from −3.6 to −7.7 MPa at the time of extraction ranged from 0.1 to 0.6%, for a mean value ± standard deviation of (0.33 ± 0.26)%. The density of oocysts extracted from moist soil in a sentinel container that was in equilibrium with moist field soil appeared to be similar to the density of oocysts observed and enumerated in microscopic fields at a magnification of ×1,000.
FIG. 5.
Oocyst survival and temperatures observed in surface soil experiment 1 (November 1996 to March 1997). (A) Viability (potential infectivity) of sentinel and control oocysts that were exposed to a surface soil environment as determined by the dye permeability assay. Each point represents the mean percentage sum of DAPI− PI− and DAPI+ PI− ± standard error of three replicates and two subsamples of each replicate, for a total of six subsamples with 100 oocysts characterized at random per subsample. (B) Average daily temperature of the surface soil where sentinels and controls were situated.
Results of the first field experiment indicated that inactivation kinetics of the sentinel oocysts significantly diverged from the control oocysts (Fig. 5). Significant loss of potential infectivity followed at least 13 freeze-thaw cycles recorded on days 45, 47, 52 to 55, 72, 74, 100 to 102, 103, 104, 106 to 108, 110, and 113. A similar divergence between sentinel oocysts and control oocysts was seen in the results of the second field experiment (Fig. 6), in which seven possible freeze-thaw cycle events occurred on days 29, 51, 54, 56, 57, 67, and 73. Compared to the controls the decline in potential infectivity of sentinel oocysts exposed to freezing soil alone during the second field experiment (Fig. 6) before the first thaw on day 30 appeared to be attributable to exposure to the frozen soil matrix. DAPI+ PI+ oocysts were significantly greater than empty oocysts; by contrast the most notable effect of the freeze-thaw events on the sentinel oocysts was the significantly high proportion of empty oocysts compared to the control oocysts, as illustrated in Fig. 7 for the first field experiment. Results of the second field experiment in 1998 showed a similar pattern (data not shown). Microscopic examination of samples analyzed after day 30 revealed highly distorted and fragmented oocyst walls.
FIG. 6.
Oocyst survival and temperatures observed in surface soil experiment 2 (January 1998 to May 1998). (A) Viability (potential infectivity) of sentinel and control oocysts that were exposed to a surface soil environment as determined by the dye permeability assay. Each point represents the mean percentage sum of DAPI− PI− and DAPI+ PI− ± standard error of three replicates and two subsamples of each replicate, for a total of six subsamples with 100 oocysts characterized at random per subsample. (B) Average daily temperature of the surface soil where sentinels and controls were situated.
FIG. 7.
Results of the dye permeability assay on sentinel and control oocysts from surface soil experiment 1.
DISCUSSION
The sentinel chambers used in this investigation proved effective at equilibrating with the external environment of both manure piles and surface soils. The chambers prevented release of oocysts into the environment, and effectively exposed them to ambient environmental stresses. The small sample volume allowed the use of many, appropriately sized replicates, which served as independent samples at each sampling interval. Differences in results between sentinel oocysts and control oocysts emphasize the need to use such a technique. In both environments, the differences in extent and rate of inactivation between sentinel oocysts and controls indicated the presence of environmental factors other than temperature that affected oocyst survival.
The use of the dye permeability assay (as opposed to either in vitro excystation or animal infectivity) in conjunction with the use of the sentinel container system to investigate the survival of C. parvum oocysts under field conditions such as those described in this report is practicable in view of the nature and number of samples and replicates examined. Data have been reported that support a correlation behind the various dye permeability categories and the in vitro excystation assay as well as animal infectivity (7, 16) and thus support the idea that the dye permeability assay can indicate the presence or absence of potentially infective, i.e., reproducible, oocysts extracted from environmental samples. In addition, the dye permeability assay accounts for empty oocysts, i.e., oocysts that have excysted under the environmental conditions being examined, as well as the various stages of oocyst wall permeability (e.g., DAPI+ PI− oocysts) (16). For disinfection studies, the dye permeability assay has been shown to overestimate the concentration of noninactivated oocysts among oocysts exposed to chemical disinfection tests when the assay is compared to animal infectivity (4) and thus may not be appropriate for such studies. In another disinfection study (9), both the dye permeability assay and in vitro excystation appeared to underestimate the inactivation of oocysts by 2 logs compared to the mouse infectivity assay after oocysts were exposed to an advanced UV system although the data were not shown and all 200 oocysts characterized were determined to be dead. In the same study (9) both in vitro viability assays overestimated the presence of viable (potentially infective) oocysts that were exposed to a pulsed UV system. The data for the pulsed UV system that Clancy et al. (9) reported indicated that factors in the process other than the pulsed UV radiation may have affected oocyst viability and thus may account for the disparity between the in vitro and animal assays. Jenkins et al. (17) reported data that showed a difference between results of the dye permeability assay and in vitro excystation on oocysts exposed to various low concentrations of ammonia—the dye permeability assay indicating greater viability. Their interpretation was that initially ammonia appeared to change the internal pH of the oocyst and inactivate sporozoites and the excystation process more quickly than it affected oocyst wall permeability. Analogously, UV radiation may inactivate gene expression for infectivity of oocysts without altering oocyst wall permeability or inhibiting excystation.
Although there were significant differences in the thermal behavior of the two waste piles, additional factors, such as microbial production of ammonia and other metabolites, may account for the differences between sentinel and control population decay. Ammonia is produced when animal wastes are stored (6, 10, 18). However, ammonia may remain in aqueous solution in the interior of the stack rather than volatilize as a gas. The pH of the waste (9.1) surrounding the sentinels was sufficient to maintain nonionic NH3 in equilibrium with cationic NH4+ in the liquid phase of the waste. Although volatilized NH3 was not detected, a low concentration of it, 13 mg liter−1, was extracted from the sample of waste, which, based on a regression analysis by Jenkins et al. (17), may inactivate 99.999% of viable oocysts in approximately 120 days at 25°C.
The results of the temperature experiments suggest that if manure piles can be manipulated to reach and maintain temperatures between 35 and 50°C, potential oocyst infectivity may decline significantly within 70 days. Exposure to temperatures greater than 30°C may account for the accelerated inactivation of the control oocysts observed at site B. The oocyst wall contains long-chain (16 to 21 carbons) aliphatic hydrocarbons that have melting points in the range of 18 to 60°C (3), which may account for the apparent deleterious effects of temperatures >30°C on oocysts. The high proportion of empty (excysted) oocysts observed at site B indicated that factors other than acidity (7) and temperature around 37°C (12) may have induced excystation. Such factors may include microbially produced surfactants or other surface-active compounds capable of inducing excystation, thus exposing sporozoites to a lethal environment. Further research into the characterization of these factors may lead to additional insights into the mechanisms of excystation, and the likelihood that this type of farming practice will reduce the load of infective oocysts in wastes.
Sentinel oocysts exposed to the soil environment were affected by environmental stresses significantly more than control oocysts in both the first and second field experiments. This difference indicates that exposure to the soil matrix is a factor that accelerates oocyst inactivation. The principle difference between the first and second field experiments may be attributed to the greater number of freeze-thaw cycles during the winter of 1997 than during the winter of 1998. Although the El Niño winter of 1998 was milder than the winter of 1997 (when soil temperatures reached lows of less than −5°C), the difference in temperature may not have been sufficient to affect oocyst inactivation, given results reported by Fayer and Nerad (13). Using mouse infectivity assays, they showed that oocysts suspended in water and exposed to temperatures as low as −10°C for as long as 7 days remained infective. In fact, they predicted that surface soil temperature just below freezing and insulation by a cover of snow (as was the case for both of the field trials) could sustain the survival of oocysts for weeks or months. The survival of sentinel and control oocysts in the field soil during the first 39 days of the first field experiment and the high survival percentage of the control oocysts during the entire duration of the second field trial appear to validate their prediction. The observed decline in viability of sentinel oocysts during the second field experiment, however, may also be attributable to the interaction between oocysts and the frozen soil matrix. Although the soil temperature was not <−2°C, at temperatures of >−2 and <0°C equilibration of oocysts with the frozen soil may result in either lethal desiccation caused by internal supercooled water passing through the oocyst wall to the frozen soil matrix or internal ice damage (20). As our laboratory results indicated, oocysts in soil at a water potential of −0.100 MPa at 25°C survive for many days. The soil water potentials at the field site remained close to saturation or very slightly below field capacity (0 to −0.020 MPa). Therefore, soil water potential was not a significant factor in the inactivation of sentinel oocysts exposed to the field soil environment during the time of the experiment. Freeze-thaw cycles and associated expansion and contraction of the soil matrix appear to have generated shear forces that resulted in the mechanical disruption and fragmentation of sentinel oocysts. Empty oocyst wall fragments were observed upon microscopic examination that were often highly distorted, and mangled. Reports by Anguish and Ghiorse (2) using a direct soil smear technique also indicated the presence of oocyst fragments in barnyard soil samples associated with calf feces.
Our results indicated that manure piles containing C. parvum oocysts can accelerate inactivation and thus reduce the numbers of infective oocysts before wastes are spread in the soil environment. Storing calf wastes in piles before spreading could be an effective management practice for reducing the infective oocyst load from animal agriculture in watersheds that sustain municipal water supplies. Furthermore, if storing in piles is followed by disposal on soil before periods of freeze-thaw cycling, the combination of practices may achieve multilog reduction of the proportion of oocysts capable of initiating infection in new hosts. However, spreading wastes on snow may have the opposite effect of sustaining the survival of oocysts (as the controls in our field soil experiments indicated) and positioning them for transport in surface runoff.
ACKNOWLEDGMENTS
This work was supported in part by funds from the U.S. Department of Interior through the New York State Water Resources Institute at Cornell University; the Center for Advanced Technology (CAT) in Biotechnology at Cornell, sponsored by the New York State Science and Technology Foundation, a consortium of industries and the National Science Foundation; the New York State Watershed Whole Farm Program through the New York State Water Resources Institute, and the New York City Department of Environmental Protection.
We gratefully thank D. Hinman and E. Fogarty for laboratory assistance. The expert secretarial assistance of Patti Lisk is gratefully acknowledged.
REFERENCES
- 1.Anderson B. Effect of drying on the infectivity of cryptosporidia-laden calf feces for 3- to 7-days-old mice. Am J Vet Res. 1986;47:2272–2273. [PubMed] [Google Scholar]
- 2.Anguish L J, Ghiorse W C. Computer-assisted laser scanning and video microscopy for analysis of Cryptosporidium parvum oocysts in soil, sediment, and feces. Appl Environ Microbiol. 1997;63:724–733. doi: 10.1128/aem.63.2.724-733.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anthony L C, Bowman D D, Jenkins M B, Eaglesham B S, Kachlany S C, Ghiorse W C. Abstracts of the 98th General Meeting of the American Society for Microbiology. Washington, D.C: American Society for Microbiology; 1998. Chemical composition and ultrastructure of the oocyst walls of wildtype Cryptosporidium parvum, abstr. Q-229; p. 459. [Google Scholar]
- 4.Black E K, Finch G R, Taghi-Kilani R, Belosevic M. Comparison of assays for Cryptosporidium parvum oocyst viability after chemical disinfection. FEMS Microbiol Lett. 1996;135:187–189. doi: 10.1111/j.1574-6968.1996.tb07987.x. [DOI] [PubMed] [Google Scholar]
- 5.Blewett D A. Quantitative techniques in Cryptosporidium research. In: Angus K W, Blewett D A, editors. Cryptosporidiosis. Proceedings of the First International Workshop. Edinburgh, United Kingdom: The Animal Diseases Research Association; 1989. pp. 85–95. [Google Scholar]
- 6.Buijsman E, Maas H J M, Aasman W A H. Anthropogenic NH3 emissions in Europe. Atmos Environ. 1987;21:1009–1022. [Google Scholar]
- 7.Campbell A T, Robertson L J, Smith H V. Viability of Cryptosporidium parvum oocysts: correlation of in vitro excystation with inclusion or exclusion of fluorogenic vital dyes. Appl Environ Microbiol. 1992;58:3488–3493. doi: 10.1128/aem.58.11.3488-3493.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Christian G D. Analytical chemistry. New York, N.Y: John Wiley & Sons; 1980. [Google Scholar]
- 9.Clancy J L, Hargy T M, Marshall M M, Dyksen J E. UV light inactivation of Cryptosporidium oocysts. J Am Water Works Assoc. 1998;90:92–102. doi: 10.1002/j.1551-8833.2004.tb10576.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dewes T. Effect of pH, temperature, amount of litter and storage density of ammonia emissions from stable manure. J Agric Res. 1996;1227:501–509. [Google Scholar]
- 11.Fayer R. Effect of high temperature on infectivity of Cryptosporidium parvum oocysts in water. Appl Environ Microbiol. 1994;60:2732–2735. doi: 10.1128/aem.60.8.2732-2735.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fayer R, Leek R G. The effects of reducing conditions, medium, pH, temperature, and time on in vitro excystation of Cryptosporidium. J Protozool. 1984;31:567–569. doi: 10.1111/j.1550-7408.1984.tb05504.x. [DOI] [PubMed] [Google Scholar]
- 13.Fayer R, Nerad T. Effect of low temperatures on viability of Cryptosporidium parvum oocyst. Appl Environ Microbiol. 1996;62:1431–1433. doi: 10.1128/aem.62.4.1431-1433.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Garber L, Salmon M, Hurd H, Keefe T, Schlater J. Potential risk factors for Cryptosporidium infection in dairy calves. J Am Vet Med Assoc. 1994;205:86–91. [PubMed] [Google Scholar]
- 15.Hillel D. Soil and water. New York, N.Y: Academic Press; 1971. p. 288. [Google Scholar]
- 16.Jenkins M B, Anguish L J, Bowman D D, Walker M J, Ghiorse W C. Assessment of a dye permeability assay for determination of inactivation rates of Cryptosporidium parvum oocysts. Appl Environ Microbiol. 1997;63:3844–3850. doi: 10.1128/aem.63.10.3844-3850.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jenkins M B, Bowman D D, Ghiorse W C. Inactivation of Cryptosporidium parvum oocysts by ammonia. Appl Environ Microbiol. 1998;64:784–788. doi: 10.1128/aem.64.2.784-788.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kirchmann H, Witter E. Ammonia volatilization during aerobic and anaerobic manure decomposition. Plant Soil. 1989;115:35–41. [Google Scholar]
- 19.MacKenzie W R, Hoxie N J, Proctor M E, Gradus S, Blair K A, Peterson D E, Kazmierczak J J, Fox K, Addis D G, Rose J B, Davis J P. Massive waterborne outbreak of Cryptosporidium infection associated with a filtered public water supply, Milwaukee, March and April, 1993. N Engl J Med. 1994;331:161–167. doi: 10.1056/NEJM199407213310304. [DOI] [PubMed] [Google Scholar]
- 20.Mazur P. Cryobiology: the freezing of biological systems. Science. 1970;168:939–949. doi: 10.1126/science.168.3934.939. [DOI] [PubMed] [Google Scholar]
- 21.McFeters G A, Stuart D. Survival of coliform bacteria in natural waters: field and laboratory studies with membrane-filter chambers. Appl Microbiol. 1972;24:805–811. doi: 10.1128/am.24.5.805-811.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Merck & Co., Inc. The Merck index. Whitehouse Station, N.J: Merck & Co., Inc.; 1996. [Google Scholar]
- 23.Moore A C, Herwaldt B L, Craun G F, Calderon R L, Highsmith A K, Juranek D D. Waterborne disease in the United States. 1991–1992. J Am Water Works Assoc. 1994;86:87–99. [Google Scholar]
- 24.Robertson L J, Campbell A T, Smith H V. Survival of Cryptosporidium parvum oocysts under various environmental pressures. Appl Environ Microbiol. 1992;58:3494–3500. doi: 10.1128/aem.58.11.3494-3500.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Robertson L J, Campbell A T, Smith H V. A low cost, low technology container for studying the survival of transmission stages of parasites and other pathogens in water-related environments. Water Res. 1992;27:723–725. [Google Scholar]
- 26.Robertson L J, Campbell A T, Smith H V. Viability of Cryptosporidium parvum oocysts: assessment by the dye permeability assay. Appl Environ Microbiol. 1998;64:3544. doi: 10.1128/aem.64.9.3544-3545.1998. . (Letter.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rynk R. On-farm composting handbook. Ithaca, N.Y: Northeast Regional Agricultural Extension Service; 1992. [Google Scholar]
- 28.Topp G C. Soil water hysteresis in silt loam and clay loam soils. Water Resour Res. 1971;7:914–920. [Google Scholar]
- 29.Walker M J, Montemagno C, Jenkins M B. Source water assessment and nonpoint sources of acutely toxic contaminants: a review of research related to survival and transport of Cryptosporidium parvum. Water Resour Res. 1998;34:3383–3392. [Google Scholar]
- 30.Walker M J, Montemagno C, Bryant J C, Ghiorse W C. Method detection limits of PCR and immunofluorescence assay for Cryptosporidium parvum in soil. Appl Environ Microbiol. 1998;64:2281–2283. doi: 10.1128/aem.64.6.2281-2283.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Weatherburn M W. Phenol-hypochlorite reaction for determination of ammonia. Anal Chem. 1967;39:971–974. [Google Scholar]